Chapter 240 Mumps
Epidemiology
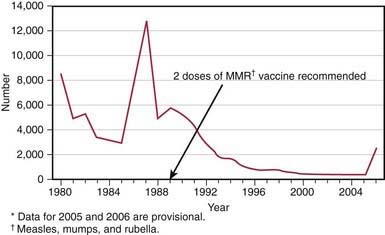
In the prevaccine era, mumps occurred primarily in young children between the ages of 5 and 9 yr and in epidemics about every 4 yr. Mumps infection occurred more often in the winter and spring months. In 1968, just after the introduction of the mumps vaccine, 185,691 cases were reported in the USA. Following the recommendation for routine use of mumps vaccine in 1977, the incidence of mumps in young children fell dramatically (Fig. 240-1), the disease occurring instead in older children, adolescents, and young adults. Outbreaks continued to occur even in highly vaccinated populations as a result of to vaccine failure and also of undervaccination of susceptible persons. After implementation of the 2-dose recommendation for the measles-mumps-rubella (MMR) vaccine for measles control in 1989, the number of mumps cases declined further. During 2001-2003, <300 mumps cases were reported each year. In 2006, the largest mumps epidemic in the last 20 years occurred in the USA. A total of 6584 cases occurred, 85% of them in 8 Midwestern states. Twenty-nine percent of the cases occurred in patients 18-24 yr old, most of whom were attending college. An analysis of 4039 patients with mumps seen in the first 7 months of the epidemic indicated that 63% had received >2 doses of the MMR vaccine.
Clinical Manifestations
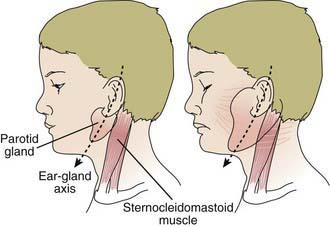
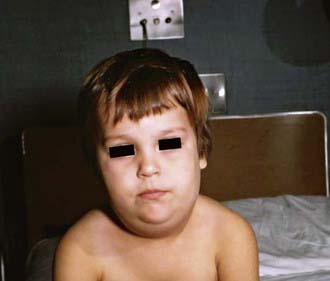
The incubation period for mumps ranges from 12 to 25 days but is usually 16-18 days. Mumps virus infection may result in clinical presentation ranging from asymptomatic or nonspecific symptoms to the typical illness associated with parotitis with or without complications involving several body systems. The typical patient presents with a prodrome lasting 1-2 days and consisting of fever, headache, vomiting, and achiness. Parotitis then appears and may be unilateral initially but becomes bilateral in about 70% of cases (Fig. 240-2). The parotid gland is tender, and parotitis may be preceded or accompanied by ear pain on the ipsilateral side. Ingestion of sour or acidic foods or liquids may enhance pain in the parotid area. As swelling progresses, the angle of the jaw is obscured and the ear lobe may be lifted upward and outward (Figs. 240-2 and 240-3). The opening of Stensen duct may be red and edematous. The parotid swelling peaks in approximately 3 days, then gradually subsides over 7 days. Fever and the other systemic symptoms resolve in 3-5 days. A morbilliform rash is rarely seen. Submandibular salivary glands may also be involved or may be enlarged without parotid swelling. Edema over the sternum due to lymphatic obstruction may also occur.
Complications
Azimi PH, Cramblett HG, Haynes RE. Mumps meningoencephalitis in children. JAMA. 1969;207:509-512.
Centers for Disease Control and Prevention. Update: mumps outbreak—New York and New Jersey, June 2009–January 2010. MMWR Morb Mortal Wkly Rep. 2010;59:125-128.
Centers for Disease Control and Prevention. Mumps epidemic—United Kingdom, 2004–2005. MMWR Morb Mortal Wkly Rep. 2006;55:173-178.
Centers for Disease Control and Prevention. Mumps epidemic—Iowa, 2006. MMWR Morb Mortal Wkly Rep. 2006;55:366-368.
Centers for Disease Control and Prevention. Update: multistate outbreak of mumps—United States, January 1–May 2, 2006. MMWR Morb Mortal Wkly Rep. 2006;55:559-564.
Centers for Disease Control and Prevention. Notice to readers: updated recommendations of the Advisory Committee on Immunization Practices (ACIP) for the control and elimination of mumps. MMWR Morb Mortal Wkly Rep. 2006;55:629-630.
Centers for Disease Control and Prevention. Updated recommendations for isolation of patients with mumps. MMWR Morb Mortal Wkly Rep. 2008;57:1103-1105.
Cohen C, White JM, Glynn JR, et al. Vaccine effectiveness estimates 2004–2005 mumps outbreak, England. Emerg Infect Dis. 2007;13:12-17.
Davidkin I, Jakinen S, Paananen A, et al. Etiology of mumps-like illness in children and adolescents vaccinated for measles, mumps and rubella. J Infect Dis. 2005;1991:719-723.
Dayan GH, Quinlisk MP, Parker AA, et al. Recent resurgence of mumps in the United States. N Engl J Med. 2008;358:1580-1589.
Endo A, Izuni H, Miyashita M, et al. Facial palsy associated with mumps parotitis. Pediatr Infect Dis J. 2001;20:815-816.
Harling R, White JM, Ramsay ME, et al. The effectiveness of the mumps component of the MMR vaccine: a case control study. Vaccine. 2005;23:4070-4074.
Horel L, Amir J, Reish O, et al. Mumps arthritis in children. Pediatr Infect Dis J. 1990;9:928-929.
Kanra G, Kara A, Cengiz AB, et al. Mumps meningoencephalitis effect on hearing. Pediatr Infect Dis J. 2002;21:1167-1169.
Kupers TA, Petrich JM, Holloway AW, et al. Depression of tuberculin hypersensitivity by live attenuated mumps virus. J Pediatr. 1970;76:716-721.
Rubin SA, Li Q, Audet SA, et al. Antibody induced by immunization with the Jeryl Lynn mumps vaccine strain effectively neutralizes a heterologous wild-type mumps virus associated with a large outbreak. J Infect Dis. 2008;198:508-515.
Ternavasio-de la Vega HG, Boronat M, Ojeda A, et al. Mumps orchitis in the post-vaccine era (1967–2009). Medicine. 2010;89:96-116.
Thompson JA. Mumps: a cause of aqueductal stenosis. J Pediatr. 1979;94:923-924.