Chapter 76 Multiple Myeloma and Other Plasma Cell Neoplasms
Epidemiology and Etiology
Radiation has been linked to the pathogenesis of MM, but radiation exposure is found in only approximately 1% of patients. In the Hiroshima and Nagasaki tumor registries, there was no sign of an excess risk of MM.1
Data regarding the role of antigenic stimulation are conflicting. Epidemiologic studies in the United States have demonstrated associations between MM and agricultural workers.2 An increasing trend for the incidence of lymphohematopoietic cancers has been associated with lifetime exposure to the chemical alachlor, a commonly used pesticide. The risk for MM gave a rate ratio of 5.66 in the highest exposure category.3 Other occupational groups associated with the development of MM include miners, workers exposed to wood dust, and sheet metal workers.4
Myeloma plasma cells express several adhesion molecules, including neural cell adhesion molecule (NCAM). Adhesion molecules are involved in homing of plasma cells to bone marrow. Although MM is a neoplasm of end-stage plasma cells, most investigators believe that myeloma stem cells exist as a self-renewing population derived from an earlier compartment. The identity of the cells responsible for the initiation and maintenance of MM remains unclear. Circulating B cells clonally related to MM plasma cells have been reported in some patients with myeloma. Data suggest that myeloma stem cells are CD138− B cells, whereas the terminally differentiated plasma cell is consistently CD138+, though this concept needs further validation. These CD138− B cells can replicate and differentiate into the malignant CD138+ plasma cells.5 Overexpression of BCL-2 and BCL-6 proteins has been seen in clinical myeloma and myeloma cell lines.6 Cytokines, including tumor necrosis factor–alpha (TNF-α), interleukin-1 (IL-1), and IL-6, play an essential role in the biology of the malignancy as well as in mediating the bony manifestations of the disease. Both IL-6 and BCL-2 have been shown to prevent apoptosis, and IL-6 has been implicated as an essential growth factor in MM.7
Pathology and Molecular Genetics
MM is distinct in that the bone marrow is involved in virtually all patients (Fig. 76-1), yet the peripheral blood shows large numbers of circulating cells in only a few patients. Adhesion molecules mediate both homotypical and heterotypical adhesion of tumor cells to either extracellular matrix (ECM) proteins or BM stromal cells. They play a critical role in disease progression. After class switching in the lymph node, adhesion molecules (e.g., CD44, VLA-4, VLA-5, LFA-1, CD56, syndecan-1, and MPC-1) mediate homing of MM cells to the BM stromal cells. Syndecan-1 is a multifunctional regulator of tumor cell growth and survival as well as of bone cell differentiation, and elevated serum syndecan-1 correlates with increased tumor cell mass, decreased metalloproteinase-9 activity, and poor prognosis.8 Syndecan-1 is shed from the surface of most MM cells, induces apoptosis, and can inhibit the growth of MM cells; it also mediates decreased osteoclast and increased osteoblast differentiation.8 Novel agents, including thalidomide, and derivatives (IMiDs), including lenalidomide, as well as the proteasome inhibitor bortezomib9,10 can target both the tumor cell and its BM microenvironment, thereby overcoming cell adhesion-mediated (CAM) conventional drug resistance. TP53 mutations are associated with more advanced myelomas and are related to the terminal phases of the disease.11 Mutations of RAS are more prevalent in MM than in other lymphoid malignant diseases. In a study using genomic DNA in 128 patients, RAS mutations were far more common in patients with aggressive plasma cell leukemias (30%) than in MM patients (9%). The RAS mutations appear to represent a late molecular lesion in the process of myeloma evolution. When levels of RAS were studied in 160 patients with newly diagnosed MM, the median survival rate for patients with mutations of N-RAS was no different from that of patients with no RAS mutations. However, patients with K-RAS mutations had significantly higher tumor burdens at diagnosis and a median survival time of 2 years versus 3.7 years for those who did not have K-RAS mutations. The RAS mutations appear to have an independent impact on the median survival rate of patients with MM.12
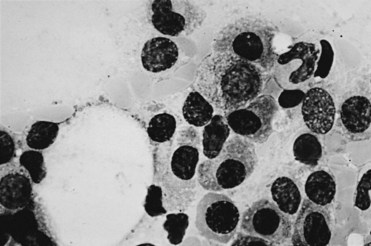
Figure 76-1 Bone marrow diagnostic for multiple myeloma. (Wright stain; original magnification ×1000.)
IgH rearrangements can be found in 75% of patients. Dysregulation of cyclin D1 can be detected in 30% of MM tumors. Cell lines that overexpress cyclin D1 have a translocation detectable into a gamma switch region that suggests an error in VDJ recombination. VH analysis of the clonal cells in MGUS showed much lower mutation frequencies than in MM. The clonogenic cell in MM likely originates from a preswitched but somatically mutated B cell. Genetic studies have demonstrated that the progression of MM from plateau phase to relapse does not involve a new B-cell clone, and progression beyond the plateau phase is not due to clonal succession.13 Advances using molecular probes for FISH have demonstrated aneuploid chromosomes where conventional cytogenetics are normal. Chromosome 13 abnormalities on metaphase cytogenetics have been associated with an unfavorable prognosis in patients with myeloma. The translocation t(11;14) results in up-regulation of cyclin D1 and is the most common translocation detected in patients with MM. Sixteen percent of patients carry the t(11;14) and have better survival and response to therapy rates.14 Immunoglobulin heavy-chain translocations are seen in 60% of patients, and these translocations are more likely to be nonhyperdiploid. Patients with light-chain myeloma never display a functional immunoglobulin heavy-chain recombination. Most patients with light-chain myeloma have one immunoglobulin heavy-chain allele with a germline configuration. The second allele is usually involved in an illegitimate recombination. Light-chain myeloma may be due to the absence of legitimate immunoglobulin heavy-chain rearrangement at the DNA level.15
Clinical Manifestations and Patient Evaluation
Multiple Myeloma and Monoclonal Gammopathy of Undetermined Significance
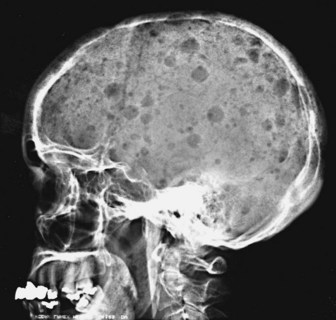
The most common problem is dealing with patients who present with bone pain. Radiographs of the spine in patients with MM frequently show osteoporosis and compression fractures. It is virtually impossible to distinguish the compression fractures associated with MM from those seen in patients with senile osteoporosis (Fig. 76-2). Spine radiographs poorly demonstrate the small lytic lesions frequently responsible for collapse of these vertebrae. All patients with back or rib pain, even with no malignant features on radiographs, should have electrophoresis of serum and urine. If a monoclonal protein is found, radiographs of the entire skeleton often demonstrate lytic lesions in the calvaria, pelvis, and long bones of the humerus and femur (Fig. 76-3).
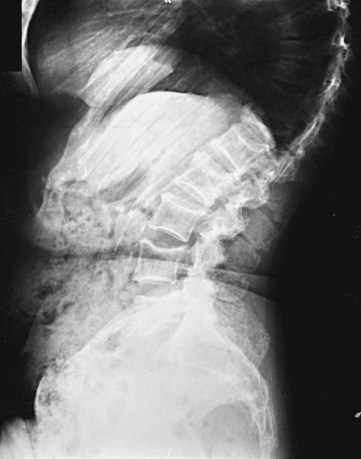
Figure 76-2 Advanced compression fractures of multiple myeloma. Note the lack of features specific for malignancy.
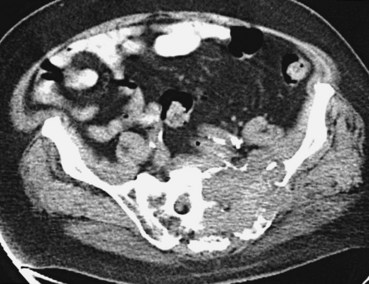
Assessment of bone disease initially requires a radiographic bone survey. Because the lesions of MM are primarily lytic, with little evidence of bony repair, radionuclide bone scans tend to be an inferior approach. In difficult cases in which osteoporosis and monoclonal gammopathy are found with no other changes, computed tomography (CT) scan or magnetic resonance imaging (MRI) of the spine and pelvis can be valuable in detecting clear-cut evidence of neoplasia (Fig. 76-4). Positron emission tomography (PET) may be useful in MM because the lytic lesions are PET avid. When 66 patients were studied with PET and compared with CT and MRI, negative PET findings reliably predicted stable MGUS.16 All patients with active myeloma had focal or diffusely positive scans, four of whom had negative full radiographic bone surveys. Extramedullary uptake was detected by PET in 23% of relapsing patients. PET also tracks response, showing a decline in lesion metabolic activity with successful intervention. It is more sensitive than other imaging techniques, and it can find additional lesions in a third of patients, which affected therapeutic decision making for a quarter of these patients.17
A second clinical problem is distinguishing MGUS from overt MM. In MGUS, the patient is expected to be asymptomatic, without evidence of anemia, hypercalcemia, or renal insufficiency. The patient should have no complaints of rib or back pain. In the absence of symptoms and with a low monoclonal protein value, regular monitoring of the level of the monoclonal protein should suffice. Patients with monoclonal gammopathies, however, should be monitored indefinitely, because the risk for transformation to a malignant plasma-proliferative process is approximately 1% per year. The risk for transformation is predicted by the initial size of the M protein peak. Patients with low monoclonal protein levels (≤0.5 g/dL) had a 6% risk for developing MM at 10 years compared with an 11% risk at 10 years for those with a 1.5-g/dL peak and a 24% risk at 10 years for those with a 2.5-g/dL peak.18
Staging of Multiple Myeloma
The most widely accepted is the International Myeloma Working Group staging system, where stage III myeloma is a β2-microglobulin level higher than 5.5 µg/mL. Stage I includes patients with a β2-microglobulin level that is less than 3.5 µg/mL and a serum albumin level that is less than 3.5 g/dL, and stage II includes all patients who do not fit stage I or stage III (Table 76-1).
TABLE 76-1 International Myeloma Working Group Staging System
| Stage | Criteria |
|---|---|
| I | β2-microglobulin <3.5 µg/mL and albumin ≥3.5 g/dL |
| II | β2-microglobulin <3. µg/mL and albumin <3.5 g/dL or β2-microglobulin = 3.5-5.5 µg/mL |
| III | β2-microglobulin ≥5.5 µg/mL |
The older staging system in clinical use is that of Durie and Salmon (Table 76-2). One problem with the Durie-Salmon staging system is that the criteria for stage I MM in this scheme are also consistent with smoldering MM and indolent MM, which do not require any form of therapy. In most clinical therapy studies, no more than 10% of patients have stage I disease, and many studies exclude patients with stage I from participation. The second difficulty is the subjective nature of interpretation of advanced lytic lesions to distinguish stage II from stage III. No specific well-defined criteria exist to ensure that all institutions use the same definition of advanced MM.
TABLE 76-2 Durie-Salmon Staging System of Multiple Myeloma
| Stage | Criteria |
|---|---|
| I | All of these required: hemoglobin >10 g/dL, Ca2+ <10.5 mg/dL, IgG <5 g/dL or IgA <3 g/dL and light-chain loss <4 g/dL |
| No lytic bone lesions | |
| II | Not fitting stage I or III |
| III | Any one of the following: hemoglobin <8.5 g/dL, Ca2+ >12 mg/dL, IgG >7 g/dL, IgA >5 g/dL, or light-chain loss >12 g/dL |
| Advanced lytic lesions | |
| IIIA | Creatinine <2 mg/dL |
| IIIB | Creatinine ≥2 mg/dL |
Response is generally assessed by the reduction in monoclonal protein; a reduction of 25% to 50% is considered a minor response and 50% to 99% an objective response. Complete eradication of the monoclonal protein in the serum and in the urine with less than 5% plasma cells in the bone marrow is considered a complete response. Patients who either did not have a bone marrow test performed or whose monoclonal protein was no longer visible but detectable by immunofixation are considered to have a near-complete response. In recipients of autologous transplants, a complete response predicts improved outcome after transplantation.19
Primary Therapy for Multiple Myeloma
Patients undergoing therapy for MM should have clinical and laboratory assessment to ensure both safety and efficacy of treatment (Table 76-3). Before each course of treatment, a complete blood count, including differential and platelets, should be done. Serum chemistries should be measured at least every 3 months or more often if clinically indicated. Concomitantly, monoclonal protein in the serum should be measured by immunoelectrophoresis or, preferably, using more sensitive immunofixation techniques and a serum free light-chain assay must be done in patients with light-chain disease. A skeletal survey should be done annually, with BM examination reserved for diagnosis and time of subsequent change in clinical status, in monoclonal Ig, or in hemogram. A bone marrow examination is typically done at diagnosis and subsequently at the time of change in clinical status (i.e., for assessment of response or at time of relapse).
| Category | Test |
|---|---|
| Blood | Complete blood cell count |
| Creatinine | |
| Calcium | |
| Sodium, potassium | |
| Uric acid | |
| Albumin | |
| Alkaline phosphatase | |
| Aspartate aminotransferase | |
| Serum protein electrophoresis with immunofixation | |
| Serum free light-chain assay | |
| β2-microglobulin | |
| Lactate dehydrogenase | |
| C-reactive protein | |
| Urine | Urine protein electrophoresis |
| Urine immunofixation | |
| Creatinine clearance | |
| Radiography | Skeletal survey |
| Miscellaneous | Electrocardiogram |
| Bone marrow aspirate and biopsy |
Initial Treatment
There has been a shift in the treatment paradigm in MM toward moving away from conventional chemotherapy and incorporating the use of novel agents. Thalidomide was used as a sedative and antiemetic in the 1950s, when it was withdrawn from the market due to teratogenicity. Its exact mechanism of action is unknown, but its antiangiogenic properties are thought to inhibit the growth of myeloma. Thalidomide also alters the adhesion of myeloma cells to the bone marrow stroma. Several trials using thalidomide in the management of relapsed MM have shown response rates from 30% to 45%.20 There is no clear relationship between dose and response. Although doses of up to 800 mg have been used, 50 to 200 mg is the typical dose for long-term management. The side effects of thalidomide are significant and, when combined with dexamethasone, deep vein thrombosis occurs in 16% and grade III or IV toxicities in 44% of patients.
Lenalidomide was developed as a thalidomide analog, using the structural backbone of thalidomide but with the elimination of a carbonyl group and the addition of an amine, originally to more effectively inhibit TNF-α. Several other potential mechanisms for the anti-MM effect of lenalidomide have been identified. Lenalidomide was noted to have a better side effect profile, with decreased peripheral neuropathy, somnolence, and gastrointestinal toxicity compared with thalidomide, but with greater myelosuppression. The favorable side effect profile and efficacy of lenalidomide was confirmed in two large, randomized, multicenter, double-blind, placebo-controlled studies in patients with relapsed or refractory myeloma—the MM-009 North American trial and the MM-010 European/Israeli/Australian trial.21,22 In both studies, patients were randomized to receive 25 mg of oral lenalidomide or placebo on days 1 to 21 of a 28-day cycle. All patients received dexamethasone on days 1 to 4, 9 to 12, and 17 to 20 for the first four cycles and subsequently, after the fourth cycle, only on days 1 to 4. The median time to progression was significantly longer in the lenalidomide/dexamethasone combination (MM-009: 11.1 months; MM-010: 11.3 months) compared with placebo/dexamethasone (4.7 months in both trials). Similar superiority was seen for the lenalidomide/dexamethasone combination in terms of overall response (OR) rate (MM-009: 61%; MM-010: 60.2%) compared with placebo/dexamethasone (MM-009: 19.9%; MM-010: 24%).
In a phase III trial conducted by the Eastern Cooperative Oncology Group (ECOG E4A03), 445 previously untreated patients with MM were randomly assigned to lenalidomide plus “standard” high-dose dexamethasone (40 mg/day by mouth on days 1 to 4, 9 to 12, and 17 to 20 of each cycle) versus lenalidomide plus lower-dose dexamethasone (40 mg by mouth on days 1, 8, 15, and 22 of each 28-day cycle).23 The trial was stopped prematurely by the data safety monitoring committee because mortality was increased in the high-dose dexamethasone arm. Prophylaxis against clotting with aspirin, coumadin, or subcutaneous heparin is needed when patients are treated with lenalidomide therapy.
Bortezomib represents a new class of anticancer compounds, the proteasome inhibitors. These drugs induce cell death by blocking degradation of apoptotic molecules that are normally catabolized via proteasome proteolysis. They inhibit nuclear factor kappa B (NFκB) activation by stopping the cleavage of its bound suppressor. Increased NFκB activity has been described in myeloma cells, and proteasome inhibitors seem to overcome chemotherapy resistance. A phase III study of bortezomib with dexamethasone suggested that the time to progression was prolonged in the bortezomib group. Bortezomib produces a response in approximately one-third of patients with relapsed MM; its primary toxic effects are thrombocytopenia and peripheral neuropathy.24
The bench to bedside translation of bortezomib and approval by the U.S. Food and Drug Administration (FDA) were also very rapid. NFκB was identified as a target in MM because it conferred drug resistance, modulated adhesion molecule expression on MM cells and BM stromal cells, and modulated constitutive and MM binding-induced transcription and secretion of cytokines. Phase I trials showed tolerability and early evidence of anti-MM activity. The phase II SUMMIT trial demonstrated responses in relapsed refractory MM, including complete responses, prolongation of time to progression and survival, and associated clinical benefit.25 The APEX trial, which compared dexamethasone versus bortezomib therapy of relapsed MM, was unblinded due to a statistically significant prolongation in time to progression in the bortezomib-treated cohort, forming the basis for its FDA approval extending to relapsed MM.24 With follow-up, time to progression and overall survival rates are significantly improved with bortezomib and neurologic complications manageable.
The combination of bortezomib, lenalidomide, and dexamethasone has been studied in the relapsed/refractory and up-front setting. In the up-front setting, 66 patients received eight 3-week cycles of bortezomib 1 to 1.3 mg/m2 (days 1, 4, 8, and 11), lenalidomide 15 to 25 mg (days 1 to 14), and dexamethasone 40 or 20 mg (days 1, 2, 4, 5, 8, 9, 11, and 12). Responding patients proceeded to maintenance or transplant. Phase II dosing was determined to be bortezomib 1.3 mg/m2, lenalidomide 25 mg, and dexamethasone 20 mg. The rate of partial response or better was 100% in both the phase II population and overall, with 74% and 67% achieving a very good partial response or better. Adverse cytogenetics had no effect on the rate of response or progression-free survival.26
Stem Cell Transplantation
The use of alkylating agents (melphalan, cyclophosphamide, busulfan) in a higher-than-conventional dose followed by infusion of autologous stem cells as part of management is considered the standard of care. In one prospective randomized study, the probability of event-free survival for 5 years was 28% in the transplant group and 10% in the conventional-dose group.27 The estimated rate of survival for 5 years was 52% in the high-dose group and 12% in the conventional-dose group (p = .03). The UK Medical Research Council, in a prospective randomized study, demonstrated a higher rate of complete response, 44%, versus 8% with conventional therapy, and an improved overall survival time of 54.1 months versus 42.3 months.28 Patients with sensitive disease and who are less heavily pretreated have the most favorable outcomes.
In 162 patients who had allogeneic BMT for MM, the European Group for Blood and Marrow Transplantation (EBMT)29 reported a 32% survival rate at 4 years and a 28% rate at 7 years. In the 72 patients (44%) who achieved complete remission after BMT, the overall progression-free survival rate was 34% at 6 years. Only nine patients, however, remained in complete remission more than 4 years after allogeneic BMT. With allogeneic BMT, some of the decreased survival time can be attributed to peritransplantation mortality, approaching 40% in some series, because of graft-versus-host disease and other complications of the procedure itself.
In a prospective randomized study, sequential tandem autologous transplantation demonstrated superior survival rates compared with single stem cell transplantation. Drawbacks of this study included conditioning that does not conform to current standards and the fact that the single-transplant group achieved a median survival time of only 48 months, which is shorter than survival times in many other reported studies.30 The second transplantation is normally completed within 6 months of the first.
The principal characteristics that determine eligibility for HCT are performance status and/or the presence and severity of certain comorbid conditions. Among patients with symptomatic standard-risk MM who are not candidates for autologous HCT, we recommend a regimen consisting of Velcade (bortezomib), melphalan, and prednisone (VMP) or melphalan, prednisone, and Revlimid (lenalidomide) (MPR) or lenalidomide plus low-dose dexamethasone. In a prospective phase III randomized trial comparing VMP with melphalan and prednisone (MP) in the treatment of 682 older adults (median age 71 years) with newly diagnosed MM31 at a median follow-up of 16.3 months, patients treated with VMP had a significantly longer median time to progression (24 months vs. 17 months) and higher rates of overall survival (87% vs. 78%) when compared with those treated with MP. Combined with prior data, these results led to the approval of VMP by the United States FDA for the initial treatment of MM.
Maintenance Therapy
Novel therapies are now being evaluated to prolong progression-free survival (PFS) rates. For example, the 3-year median and overall PFS times were prolonged by the use of thalidomide following transplantation. Thalidomide with or without prednisone has been evaluated as maintenance. Because lenalidomide is an oral drug with fewer side effects, it is now under evaluation as maintenance treatment. In a phase III study to determine the efficacy and safety of lenalidomide in combination with melphalan and prednisone (MPR) in elderly patients with newly diagnosed MM, the PFS rate was significantly improved in patients who received MPR followed by lenalidomide maintenance compared with those who received MP followed by placebo maintenance.32 A phase III intergroup study of lenalidomide versus placebo maintenance therapy following single autologous stem cell transplant (CALGB 100104) has completed accrual. Preliminary results indicate that maintenance therapy with lenalidomide, when compared to placebo, will prolong time to disease progression. There is an estimated 58% reduction in the risk of disease progression with lenalidomide.
Supportive Treatment
Newer supportive modalities are useful in the management of patients with MM. Although chemotherapy can destroy the malignant clone and may prevent progression of bony disease, it does not lead to remineralization or recalcification of previously involved bone. Monthly infusions of pamidronate or zoledronic acid33 for 9 consecutive months reduced the proportion of patients who had any skeletal events from 41% to 24%. Patients who received pamidronate had significant decreases in bone pain and an improved quality of life. Pamidronate or zoledronic acid has a significant palliative effect in patients with bone pain secondary to metastasis. Bisphosphonates act by inhibiting bone resorption. They have a major effect on osteoclasts and reduce bone-resorbing cytokine production. Zoledronic acid is 100 times more potent than the bisphosphonate most commonly administered orally, alendronate. These agents reduce the frequency of skeletal-related events, including the need for irradiation for bone pain, pathologic fracture, and spinal cord compression. The toxic effects of bisphosphonates include an increase in the serum creatinine level, proteinuria, and osteonecrosis of the jaw. Vertebroplasty is high-pressure injection of low-viscosity material into the vertebral body, designed to stabilize painful vertebral compression fractures. It can be done on an outpatient basis by an interventional radiologist. Its risks include leak of the material and encroachment on a nerve root.
Solitary Plasmacytoma
Solitary plasmacytoma, which accounts for 5% to 10% of all plasma cell dyscrasias, is characterized by the presence of a plasmacytoma in the absence of multiple osteolytic lesions or other features of MM.34 Approximately two thirds of the cases are solitary plasmacytomas of the bone, which most commonly involve the axial skeleton, while the remaining one-third are extramedullary plasmacytomas, most frequently presenting in the upper aerodigestive tract. Progression to MM is more likely to occur in plasmacytoma of the bone than in extramedullary plasmacytoma.35 The 5-year and 10-year rates of progression to MM in patients with solitary plasmacytoma of the bone are 30% to 50% and 70% to 90%, respectively, whereas around 10% to 35% of patients with extramedullary plasmacytoma eventually progress to MM.36,37
Definitive radiation therapy is considered standard therapy for patients with solitary plasmacytoma. Retrospective series using doses ranging from 35 to 60 Gy showed a more than 80% local control rate.36–46 In the largest series to date reported by the European Multicenter Rare Cancer Network, which included 258 patients with solitary plasmacytoma (206 with solitary plasmacytoma of the bone and 52 with extramedullary plasmacytoma), the median dose of radiation therapy was 40 Gy (range, 20 to 66 Gy).41 At a median follow-up time of 56 months, 14% of patients developed a local recurrence (median, 20 months). The 10-year probability of progression to MM was 72% for solitary plasmacytoma of the bone and 36% for extramedullary plasmacytoma.
Several studies have evaluated optimal radiation doses for solitary plasmacytoma,36,38–42,45 although most have failed to show a significant dose-response relationship.36,38–42,45 In the study by the European Multicenter Rare Cancer Network,41 after adjusting the radiation dose to a biologically equivalent dose (BED) of 2 Gy per fraction, no dose-response relationship was observed for doses of more than 30 Gy. Of the 244 patients treated with radiation therapy, 2 (6%) of 32 patients treated with 30 Gy or less developed local failure compared with 27 (13%) of 212 patients treated with more than 30 Gy. When limiting the analysis to tumors of 4 cm or larger, there continues to be a lack of a significant dose-response relationship beyond 30 Gy. Other smaller series also failed to show significant differences in local control rates with doses of less than 30 Gy versus higher doses.36,39,45 A meaningful evaluation of optimal radiation dose may be limited by the retrospective nature and the small patient numbers in the available studies. One study from France,40 however, did show that a dose of more than 45 Gy to the target volume is associated with an improved local control rate. This study included 17 patients with extramedullary plasmacytoma of the head and neck region. The 5-year local control rate was 100% for the nine patients who received a dose of 45 Gy or more but only 50% for the eight patients who received a dose of less than 45 Gy (p = .034). In a study from Turkey that included 80 patients with solitary plasmacytoma,38 on multivariable analysis, doses of 50 Gy or less were associated with a significantly lower PFS rate (hazard ratio [HR], 2.3; p = .04). Although data supporting the use of higher doses are limited, most centers recommend doses of 45 to 50 Gy in 1.8- to 2-Gy daily fractions as definitive treatment for patients with solitary plasmacytoma.
Although the optimal radiation treatment volume has not been specifically addressed for solitary plasmacytoma, it is generally acceptable to include the gross disease plus a margin of 2 cm. Patients with extramedullary plasmacytoma, especially of the head and neck region, may benefit from treatment with intensity-modulated radiation therapy (IMRT) techniques to limit doses to normal structures and to preserve the quality of life.36 For these patients, most series also included clinically uninvolved regional nodes in the treatment volume. In the European Multicenter Rare Cancer Network study, however, which also included 52 patients with extramedullary plasmacytoma,41 the planning radiation treatment volume was limited to the radiographically visible gross tumor volume plus a margin; no attempt was made to cover regional lymph nodes. At a median follow-up time of 54 months, no regional nodal relapses were observed.
Investigators have explored factors predicting for local recurrence and progression to MM in patients with solitary plasmacytoma. Findings on the impact of tumor size on local control rates have been conflicting. Tsang and colleagues45 showed that the 8-year local control rate was 100% for tumors of less than 5 cm as compared with 38% for tumors of 5 cm or more in diameter (p <.01). Dagan and associates36 reported local control rates of 100% and 79%, respectively, for tumors of 5 cm or less versus tumors larger than 5 cm, although the difference did not reach statistical significance (p = .09).36 Others, however, have failed to show a significant relationship between tumor size and local control rates.38,42
The anatomic location of the tumor can also influence local control rates. Knobel and associates42 demonstrated a trend to higher 10-year local control rates for presentation in vertebral bodies versus other sites (89% vs. 78%; p = .07). The previously described Turkish study showed that surgical resection prior to radiation therapy is associated with an improved progression-free survival rate, although in the larger study by Ozsahin and colleagues,41 complete or partial resection prior to radiation therapy had no impact on local control rates. The same study showed that surgery alone for solitary plasmacytoma is clearly inadequate: 7 of 9 patients (78%) treated with surgery without radiation therapy relapsed locally, as compared with 29 of 248 patients (12%) who received radiation therapy. Other factors that have been associated with inferior prognosis include older age at diagnosis38,41,42 and persistence of myeloma protein for more than 1 year after radiotherapy.46,47
Radiation Therapy as Palliation in Multiple Myeloma
Radiation therapy represents an effective form of palliation for patients with MM with symptomatic local involvement.48,49 The main indications for palliative radiation therapy include bone pain with or without pathologic fractures, neurologic compromise including cord compression, impending cord compression, nerve root compression, or cranial nerve deficits. Patients with impending fractures of weight-bearing bones should first be evaluated by an orthopedic surgeon for consideration of surgical stabilization. Patients with compression fractures of vertebral bodies may also benefit from vertebroplasties prior to radiation therapy.49–51 For cases of cord compression with associated acute neurologic symptoms, prompt neurosurgical intervention may improve the chance of neurologic recovery, as has been demonstrated in a randomized trial for patients with metastatic cancer and cord compression. Patients with hematologic malignant diseases were not included in this trial, however. An international multi-institutional study on 172 patients with MM and cord compression found that radiation therapy without surgery resulted in improvement in motor function in 52% of patients.52 This study showed that a more gradual development of motor deficits (>7 days) was associated with a better functional outcome than rapid development of motor dysfunction (1 to 7 days). Additionally, one small series found that radiation therapy alone can be safe and effective even in the setting of spinal instability on radiographic images.53 Patients with cord compression in whom radiation therapy is offered as first-line therapy should be placed on steroids prior to initiation of radiation therapy to reduce the risk of further neurologic compromise from acute radiation-related local inflammation.
Although plasma cell tumors are considered a radiosensitive entity, increasing data are available supporting the importance of adequate doses of radiation therapy to ensure durable local control and palliation. This is especially important in light of recent advances in MM therapy, resulting in longer life expectancies. In the multicenter study on patients with MM and cord compression described above,52 it was found that long-course radiation therapy with higher BED (10 fractions of 3 Gy, 15 fractions of 2.5 Gy, and 20 fractions of 2 Gy) was associated with a significantly higher chance of motor function improvement than short-course radiation therapy (1 fraction of 8 Gy, 5 fractions of 4 Gy). Motor function recovery at 1 year was found to be 76% versus 40% in patients who received long-course treatment versus short-course treatment (p = .003). In a study from Germany on 138 MM patients irradiated to 272 sites,54 pain reduction was significantly lower in patients who received less than 30 Gy compared with those who received 40 to 49 Gy (65% vs. 92%; p <.001). On multivariable analysis for pain reduction, a daily fraction of 2 Gy was superior to daily doses of 4 to 15 Gy (OR, 11; p = .027). Alcorn and associates55 reported on 74 patients with MM irradiated to 117 sites to a median dose of 30 Gy, or a BED of 39 Gy. At a median follow-up of 32 months, 26% recurred locally. On multivariable analysis, a BED of less than 39 Gy was significantly associated with increased local recurrence rates (OR, 2.8; p = .03). At our institution, commonly used radiation dose fractionation schemes for MM palliation include 37.5 Gy in 2.5-Gy fractions or 30 Gy in 10 fractions, although in patients with clearly limited life expectancies, 20 Gy in 5 fractions is considered.
3 Lee WJ, Hoppin JA, Blair A, et al. Cancer incidence among pesticide applicators exposed to alachlor in the Agricultural Health Study. Am J Epidemiol. 2004;159:373-380.
4 Fritschi L, Siemiatycki J. Lymphoma, myeloma and occupation. Results of a case-control study. Int J Cancer. 1996;67:498-503.
5 Matsui W, Huff CA, Wang Q, et al. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103:2332-2336.
6 Hideshima T, Mitsiades C, Ikeda H, et al. A proto-oncogene Bcl-6 is upregulated in the bone marrow microenvironment in multiple myeloma cells. Blood. 2010;115:3.
7 Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001;20:5991-6000.
8 Dhodapkar MV, Abe E, Theus A, et al. Syndecan-1 is a multifunctional regulator of myeloma pathobiology. Control of tumor cell survival, growth, and bone cell differentiation. Blood. 1998;91:2679-2688.
9 Hideshima T, Chauhan D, Shima Y, et al. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96:2943-2950.
10 Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071-3076.
12 Liu P, Leong T, Quam L, et al. Activating mutations of N- and K-ras in multiple myeloma show different clinical associations. Analysis of the Eastern Cooperative Oncology Group Phase III Trial. Blood. 1996;88:2699-2706.
14 Fonseca R, Blood EA, Oken MM, et al. Myeloma and the t(11;14)(q13;q32). Evidence for a biologically defined unique subset of patients. Blood. 2002;99:3735-3741.
15 Magrangeas F, Cormier ML, Descamps G, et al. Light-chain only multiple myeloma is due to the absence of functional (productive) rearrangement of the IgH gene at the DNA level. Blood. 2004;103:3869-3875.
16 Durie BG, Waxman AD, D’Agnolo A, Williams CM. Whole-body (18)F-FDG PET identifies high-risk myeloma. J Nucl Med. 2002;43:1457-1463.
17 Schirrmeister H, Buck AK, Bergmann L, et al. Positron emission tomography (PET) for staging of solitary plasmacytoma. Cancer Biother Radiopharm. 2003;18:841-845.
18 Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564-569.
19 Rajkumar SV, Fonseca R, Dispenzieri A, et al. Effect of complete response on outcome following autologous stem cell transplantation for myeloma. Bone Marrow Transplant. 2000;26:979-983.
20 Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565-1571.
21 Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133-2142.
22 Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123-2132.
23 Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma. An open-label randomised controlled trial. Lancet Oncol. 2010;11:29-37.
24 Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487-2498.
25 Richardson PG, Barlogie B, Berenson J, et al. Extended follow-up of a phase II trial in relapsed, refractory multiple myeloma. Final time-to-event results from the SUMMIT trial. Cancer. 2006;106:1316-1319.
26 Richardson P, Lonial S, Jakubowiak A, et al. Lenalidomide, bortezomib, and dexamethasone in patients with newly diagnosed multiple myeloma. Encouraging efficacy in high risk groups with updated results of a phase I/II study. ASH Annual Meeting Abstracts. 2008;112:92.
27 Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91-97.
28 Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875-1883.
30 Attal M, Harousseau JL, Facon T, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495-2502.
31 San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906-917.
32 Palumbo A, Dimopoulos MA, Delforge M, et al. A phase III study to determine the efficacy and safety of lenalidomide in combination with melphalan and prednisone (MPR) in elderly patients with newly diagnosed multiple myeloma. ASH Annual Meeting Abstracts. 2009;114:613.
33 Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma. A randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98:1735-1744.
34 Dimopoulos MA, Moulopoulos LA, Maniatis A, Alexanian R. Solitary plasmacytoma of bone and asymptomatic multiple myeloma. Blood. 2000;96:2037-2044.
35 Galieni P, Cavo M, Avvisati G, et al. Solitary plasmacytoma of bone and extramedullary plasmacytoma. Two different entities? Ann Oncol. 1995;6:687-691.
36 Dagan R, Morris CG, Kirwan J, Mendenhall WM. Solitary plasmacytoma. Am J Clin Oncol. 2009;32:612-617.
37 Galieni P, Cavo M, Pulsoni A, et al. Clinical outcome of extramedullary plasmacytoma. Haematologica. 2000;85:47-51.
38 Kilciksiz S, Celik OK, Pak Y, et al. Clinical and prognostic features of plasmacytomas. A multicenter study of Turkish Oncology Group-Sarcoma Working Party. Am J Hematol. 2008;83:702-707.
39 Bachar G, Goldstein D, Brown D, et al. Solitary extramedullary plasmacytoma of the head and neck—long-term outcome analysis of 68 cases. Head Neck. 2008;30:1012-1019.
40 Tournier-Rangeard L, Lapeyre M, Graff-Caillaud P, et al. Radiotherapy for solitary extramedullary plasmacytoma in the head-and-neck region. A dose greater than 45 Gy to the target volume improves the local control. Int J Radiat Oncol Biol Phys. 2006;64:1013-1017.
41 Ozsahin M, Tsang RW, Poortmans P, et al. Outcomes and patterns of failure in solitary plasmacytoma. A multicenter Rare Cancer Network study of 258 patients. Int J Radiat Oncol Biol Phys. 2006;64:210-217.
42 Knobel D, Zouhair A, Tsang RW, et al. Prognostic factors in solitary plasmacytoma of the bone. A multicenter Rare Cancer Network study. BMC Cancer. 2006;6:118.
43 Chao MW, Gibbs P, Wirth A, et al. Radiotherapy in the management of solitary extramedullary plasmacytoma. Intern Med J. 2005;35:211-215.
44 Michalaki VJ, Hall J, Henk JM, et al. Definitive radiotherapy for extramedullary plasmacytomas of the head and neck. Br J Radiol. 2003;76:738-741.
45 Tsang RW, Gospodarowicz MK, Pintilie M, et al. Solitary plasmacytoma treated with radiotherapy. Impact of tumor size on outcome. Int J Radiat Oncol Biol Phys. 2001;50:113-120.
46 Liebross RH, Ha CS, Cox JD, et al. Solitary bone plasmacytoma. Outcome and prognostic factors following radiotherapy. Int J Radiat Oncol Biol Phys. 1998;41:1063-1067.
47 Wilder RB, Ha CS, Cox JD, et al. Persistence of myeloma protein for more than one year after radiotherapy is an adverse prognostic factor in solitary plasmacytoma of bone. Cancer. 2002;94:1532-1537.
48 Hu K, Yahalom J. Radiotherapy in the management of plasma cell tumors. Oncology (Williston Park). 2000;14:101-108. 111 discussion Oncology (Williston Park) 14:111-112, 115, 2000
49 Yaneva MP, Goranova-Marinova V, Goranov S. Palliative radiotherapy in patients with multiple myeloma. J Buon. 2006;11:43-48.
50 Diamond TH, Hartwell T, Clarke W, Manoharan A. Percutaneous vertebroplasty for acute vertebral body fracture and deformity in multiple myeloma. A short report. Br J Haematol. 2004;124:485-487.
51 Ramos L, de Las Heras JA, Sanchez S, et al. Medium-term results of percutaneous vertebroplasty in multiple myeloma. Eur J Haematol. 2006;77:7-13.
52 Rades D, Hoskin PJ, Stalpers LJ, et al. Short-course radiotherapy is not optimal for spinal cord compression due to myeloma. Int J Radiat Oncol Biol Phys. 2006;64:1452-1457.
53 Rao G, Ha CS, Chakrabarti I, et al. Multiple myeloma of the cervical spine. Treatment strategies for pain and spinal instability. J Neurosurg Spine. 2006;5:140-145.
54 Stolting T, Knauerhase H, Klautke G, et al. Total and single doses influence the effectiveness of radiotherapy in palliative treatment of plasmacytoma. Strahlenther Onkol. 2008;184:465-472.
55 Alcorn S, Mauch P, Ng A, et al. Predictors of symptomatic failure after palliative radiation therapy for multiple myeloma. Int J Radiat Oncol Biol Phys. 2009;75:S503-S504.
1 Preston DL, Kusumi S, Tomonaga M, et al. Cancer incidence in atomic bomb survivors. Part III. Leukemia, lymphoma and multiple myeloma, 1950-1987. Radiat Res. 1994;137:S68-S97.
2 Demers PA, Vaughan TL, Koepsell TD, et al. A case-control study of multiple myeloma and occupation. Am J Ind Med. 1993;23:629-639.
3 Lee WJ, Hoppin JA, Blair A, et al. Cancer incidence among pesticide applicators exposed to alachlor in the Agricultural Health Study. Am J Epidemiol. 2004;159:373-380.
4 Fritschi L, Siemiatycki J. Lymphoma, myeloma and occupation. Results of a case-control study. Int J Cancer. 1996;67:498-503.
5 Matsui W, Huff CA, Wang Q, et al. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103:2332-2336.
6 Hideshima T, Mitsiades C, Ikeda H, et al. A proto-oncogene Bcl-6 is upregulated in the bone marrow microenvironment in multiple myeloma cells. Blood. 2010;115:3.
7 Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene. 2001;20:5991-6000.
8 Dhodapkar MV, Abe E, Theus A, et al. Syndecan-1 is a multifunctional regulator of myeloma pathobiology. Control of tumor cell survival, growth, and bone cell differentiation. Blood. 1998;91:2679-2688.
9 Hideshima T, Chauhan D, Shima Y, et al. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96:2943-2950.
10 Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071-3076.
11 Portier M, Moles JP, Mazars GR, et al. p53 and RAS gene mutations in multiple myeloma. Oncogene. 1992;7:2539-2543.
12 Liu P, Leong T, Quam L, et al. Activating mutations of N- and K-ras in multiple myeloma show different clinical associations. Analysis of the Eastern Cooperative Oncology Group Phase III Trial. Blood. 1996;88:2699-2706.
13 Palumbo A, Battaglio S, Astolfi M, et al. Multiple independent immunoglobulin class-switch recombinations occurring within the same clone in myeloma. Br J Haematol. 1992;82:676-680.
14 Fonseca R, Blood EA, Oken MM, et al. Myeloma and the t(11;14)(q13;q32); evidence for a biologically defined unique subset of patients. Blood. 2002;99:3735-3741.
15 Magrangeas F, Cormier ML, Descamps G, et al. Light-chain only multiple myeloma is due to the absence of functional (productive) rearrangement of the IgH gene at the DNA level. Blood. 2004;103:3869-3875.
16 Durie BG, Waxman AD, D’Agnolo A, Williams CM. Whole-body (18)F-FDG PET identifies high-risk myeloma. J Nucl Med. 2002;43:1457-1463.
17 Schirrmeister H, Buck AK, Bergmann L, et al. Positron emission tomography (PET) for staging of solitary plasmacytoma. Cancer Biother Radiopharm. 2003;18:841-845.
18 Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564-569.
19 Rajkumar SV, Fonseca R, Dispenzieri A, et al. Effect of complete response on outcome following autologous stem cell transplantation for myeloma. Bone Marrow Transplant. 2000;26:979-983.
20 Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565-1571.
21 Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133-2142.
22 Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123-2132.
23 Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma. An open-label randomised controlled trial. Lancet Oncol. 2010;11:29-37.
24 Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487-2498.
25 Richardson PG, Barlogie B, Berenson J, et al. Extended follow-up of a phase II trial in relapsed, refractory multiple myeloma. Final time-to-event results from the SUMMIT trial. Cancer. 2006;106:1316-1319.
26 Richardson P, Lonial S, Jakubowiak A, et al. Lenalidomide, bortezomib, and dexamethasone in patients with newly diagnosed multiple myeloma. Encouraging efficacy in high risk groups with updated results of a phase I/II study. ASH Annual Meeting Abstracts. 2008;112:192.
27 Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91-97.
28 Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875-1883.
29 Gahrton G, Tura S, Ljungman P, et al. Allogeneic bone marrow transplantation in multiple myeloma. European Group for Bone Marrow Transplantation. N Engl J Med. 1991;325:1267-1273.
30 Attal M, Harousseau JL, Facon T, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495-2502.
31 San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906-917.
32 Palumbo A, Dimopoulos MA, Delforge M, et al. A phase III study to determine the efficacy and safety of lenalidomide in combination with melphalan and prednisone (MPR) in elderly patients with newly diagnosed multiple myeloma. ASH Annual Meeting Abstracts. 2009;114:613.
33 Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma. A randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98:1735-1744.
34 Dimopoulos MA, Moulopoulos LA, Maniatis A, Alexanian R. Solitary plasmacytoma of bone and asymptomatic multiple myeloma. Blood. 2000;96:2037-2044.
35 Galieni P, Cavo M, Avvisati G, et al. Solitary plasmacytoma of bone and extramedullary plasmacytoma. Two different entities? Ann Oncol. 1995;6:687-691.
36 Dagan R, Morris CG, Kirwan J, Mendenhall WM. Solitary plasmacytoma. Am J Clin Oncol. 2009;32:612-617.
37 Galieni P, Cavo M, Pulsoni A, et al. Clinical outcome of extramedullary plasmacytoma. Haematologica. 2000;85:47-51.
38 Kilciksiz S, Celik OK, Pak Y, et al. Clinical and prognostic features of plasmacytomas. A multicenter study of Turkish Oncology Group-Sarcoma Working Party. Am J Hematol. 2008;83:702-707.
39 Bachar G, Goldstein D, Brown D, et al. Solitary extramedullary plasmacytoma of the head and neck—long-term outcome analysis of 68 cases. Head Neck. 2008;30:1012-1019.
40 Tournier-Rangeard L, Lapeyre M, Graff-Caillaud P, et al. Radiotherapy for solitary extramedullary plasmacytoma in the head-and-neck region. A dose greater than 45 Gy to the target volume improves the local control. Int J Radiat Oncol Biol Phys. 2006;64:1013-1017.
41 Ozsahin M, Tsang RW, Poortmans P, et al. Outcomes and patterns of failure in solitary plasmacytoma. A multicenter Rare Cancer Network study of 258 patients. Int J Radiat Oncol Biol Phys. 2006;64:210-217.
42 Knobel D, Zouhair A, Tsang RW, et al. Prognostic factors in solitary plasmacytoma of the bone. A multicenter Rare Cancer Network study. BMC Cancer. 2006;6:118.
43 Chao MW, Gibbs P, Wirth A, et al. Radiotherapy in the management of solitary extramedullary plasmacytoma. Intern Med J. 2005;35:211-215.
44 Michalaki VJ, Hall J, Henk JM, et al. Definitive radiotherapy for extramedullary plasmacytomas of the head and neck. Br J Radiol. 2003;76:738-741.
45 Tsang RW, Gospodarowicz MK, Pintilie M, et al. Solitary plasmacytoma treated with radiotherapy. Impact of tumor size on outcome. Int J Radiat Oncol Biol Phys. 2001;50:113-120.
46 Liebross RH, Ha CS, Cox JD, et al. Solitary bone plasmacytoma. Outcome and prognostic factors following radiotherapy. Int J Radiat Oncol Biol Phys. 1998;41:1063-1067.
47 Wilder RB, Ha CS, Cox JD, et al. Persistence of myeloma protein for more than one year after radiotherapy is an adverse prognostic factor in solitary plasmacytoma of bone. Cancer. 2002;94:1532-1537.
48 Hu K, Yahalom J. Radiotherapy in the management of plasma cell tumors. Oncology (Williston Park). 2000;14:101-108. 111 discussion Oncology (Williston Park) 14:111-112, 115, 2000
49 Yaneva MP, Goranova-Marinova V, Goranov S. Palliative radiotherapy in patients with multiple myeloma. J Buon. 2006;11:43-48.
50 Diamond TH, Hartwell T, Clarke W, Manoharan A. Percutaneous vertebroplasty for acute vertebral body fracture and deformity in multiple myeloma. A short report. Br J Haematol. 2004;124:485-487.
51 Ramos L, de Las Heras JA, Sanchez S, et al. Medium-term results of percutaneous vertebroplasty in multiple myeloma. Eur J Haematol. 2006;77:7-13.
52 Rades D, Hoskin PJ, Stalpers LJ, et al. Short-course radiotherapy is not optimal for spinal cord compression due to myeloma. Int J Radiat Oncol Biol Phys. 2006;64:1452-1457.
53 Rao G, Ha CS, Chakrabarti I, et al. Multiple myeloma of the cervical spine. Treatment strategies for pain and spinal instability. J Neurosurg Spine. 2006;5:140-145.
54 Stolting T, Knauerhase H, Klautke G, et al. Total and single doses influence the effectiveness of radiotherapy in palliative treatment of plasmacytoma. Strahlenther Onkol. 2008;184:465-472.
55 Alcorn S, Mauch P, Ng A, et al. Predictors of symptomatic failure after palliative radiation therapy for multiple myeloma. Int J Radiat Oncol Biol Phys. 2009;75:S503-S504.