Multinodular Goiter
Basic Aspects
Definition and Clinical Manifestation
Benign nodular thyroid disease is a heterogeneous thyroid disorder that is highly prevalent in iodine-deficient areas. On a very general basis, it can be divided into solitary nodular and multinodular thyroid disease. Histologically, benign thyroid nodules are distinguished (1) as encapsulated lesions (true adenomas) or adenomatous nodules that lack a capsule, and (2) by morphologic criteria according to the World Health Organization (WHO) classification.1 On functional grounds, nodules are classified as cold, normal, or hot, depending on whether they show decreased, normal, or increased uptake on scintiscan. Approximately 50% to 85% of all nodules are cold, up to 40% are scintigraphically indifferent, and about 10% are hot,2,3 although the prevalence will vary geographically with the iodine supply and with the clinical setting.
Classification of thyroid nodules on the basis of results from clonality studies implies that most thyroid nodules are “true” thyroid tumors compared with polyclonal hyperplastic nodules. Traditionally, only thyroid adenomas are considered true tumors, on the basis of an exclusive histologic definition—the presence of a capsule and a growth pattern that are different from the surrounding normal parenchyma in an otherwise normal thyroid gland. Strict histologic criteria for an adenoma and its differentiation from hyperplastic thyroid nodules or adenomatous nodules (without a capsule) are, however, difficult to obtain in the frequent presence of goiter or thyroiditis. The biological basis for separating hyperplastic thyroid nodules from true tumors, therefore, should also depend on their clonality.4 Because many thyroid nodules without a capsule (adenomatous nodules) are monoclonal, a mixed functional and molecular definition of true thyroid tumors, as outlined in Fig. 16-1, appears objective and consistent. In contrast to solitary nodular thyroid disease, which has a more uniform clinical, pathologic, and molecular picture, multinodular nontoxic goiter (MNG) and multinodular toxic goiter (MNTG) make up a mixed group of nodular entities. Thus a combination of hyper-, hypo-, or normally functioning thyroid lesions usually is found within the same thyroid gland. The overall balance of functional properties of individual thyroid nodules within a multinodular goiter ultimately determines the functional status in the individual patient, which may be seen as euthyroidism (normal thyroid-stimulating hormone [TSH] and free thyroid hormone levels), subclinical hyperthyroidism (low or suppressed TSH and normal free thyroid hormone levels), or overt hyperthyroidism (suppressed TSH and elevated free thyroid hormone levels). The term MNG is applied in the first scenario, and MNTG refers to the latter situations. It is important to emphasize that this functional characterization is not stationary, but patients with MNTG usually have a history of long-standing MNG.5 In general, development of MNG proceeds in two phases: global activation of thyroid epithelial cell proliferation (e.g., as the result of iodine deficiency or other goitrogenic stimuli) leading to goiter, and a focal increase in thyroid epithelial cell proliferation causing thyroid nodules. So far, the most common stimulus for local proliferation is a somatic mutation, as detailed in the following section.
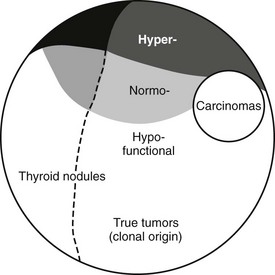
FIGURE 16-1 Classification of thyroid nodules on the basis of results from clonality studies. Such studies imply that most thyroid nodules are “true” thyroid tumors compared with polyclonal hyperplastic nodules. Traditionally, only thyroid adenomas are considered true tumors. This is based solely on a histologic definition—the presence of a capsule and a growth pattern that is different from the surrounding normal parenchyma in an otherwise normal thyroid gland.
Environment Versus Heredity
Until recently, nontoxic goiter was regarded mainly as a consequence of iodine deficiency. However, a number of goitrogens6–8 and cigarette smoking are important environmental risk factors in the origin of nontoxic goiter. The impact of smoking, most likely mediated by thiocyanate, which competitively inhibits the iodide transport into the thyroid, has been extensively studied.6,9 Additional etiologically important factors consist of gender,10,11 age,12 and increased body mass index (BMI).13 The effect of a certain goitrogen is influenced by the degree of iodine sufficiency and therefore varies regionally and interindividually. However, it is most likely that interactions between environmental factors and individual genetic determinants ultimately determine the onset of the goiter.10 Nontoxic goiter appearing early in life, often clustering in families, suggests strong genetic susceptibility, whereas environmental determinants are more likely to have additive or triggering effects. However, in an individual, it may be impossible to evaluate the relative contribution of genetic predisposition and a multitude of potential environmental factors.
In contrast to sporadic goiters, caused by spontaneous recessive genomic variations, most cases of familial goiter present an autosomal dominant pattern of inheritance, indicating predominant genetic defects.14–16 Gene-gene interactions or various polygenic mechanisms (i.e., synergistic effects of several variants or polymorphisms) could increase the complexity of the pathogenesis of nontoxic goiter and offer an explanation for its genetic heterogeneity. A strong genetic predisposition is indicated by family and twin studies. Thus, children of parents with goiter have a significantly higher risk for developing goiter compared with children of nongoitrous parents.17 The high incidence in females and the higher concordance in monozygotic than in dizygotic twins also suggest a genetic predisposition.10 Moreover, preliminary evidence reveals a positive family history for thyroid disease in those who have postoperative relapse of goiter, which can occur from months to years after surgery.18,19
The development of nontoxic goiter is most likely a continuous process that starts with thyroid hyperplasia. Therefore, defects in genes that play an important role in thyroid physiology and thyroid hormone synthesis could predispose to the development of goiter, especially in cases of borderline or overt iodine deficiency. Such defects could lead to dyshormonogenesis as an immediate response, thereby indirectly explaining the nodular transformation of the thyroid as a late consequence of dyshormonogenesis, as a form of maladaptation.12 Genes that encode the proteins involved in thyroid hormone synthesis, such as the thyroglobulin gene (TG gene), the thyroid peroxidase gene (TPO gene), the sodium-iodide symporter gene (SLC5A5), the Pendred syndrome gene (SLC26A4), the TSH receptor gene (TSH-R gene), the iodotyrosine deiodinase gene (DEHAL1), and the thyroid oxidase 2 gene (THOX2), are convincing candidate genes in familial euthyroid goiter. Originally, several mutations in these genes were identified in patients with congenital hypothyroidism. However, in cases of less severe functional impairment that can still be compensated, the contribution of variants of these genes in the development of nontoxic goiter is possible. Moreover, in case of mutations in the TG gene,20,21 SLC26A4,22,23 and SLC5A5,24,25 patients with nontoxic goiter have also been identified.
Linkage Studies
To identify novel susceptibility loci, as well as to account for the coinheritance of different genomic regions, and to further improve the understanding of the genetic mechanisms that contribute to the development of nontoxic familial goiter, linkage analyses have been performed. A genome-wide linkage analysis has identified a candidate locus, MNG1 on chromosome 14q31, in a large Canadian family with 18 affected individuals.15 This locus was confirmed in a German family with recurrent euthyroid goiters.26 A dominant pattern of inheritance with high penetrance was assumed in both investigations. Moreover, a region on 14q31 between MNG1 and the TSH-R gene was identified as a potential positional candidate region26 for nontoxic goiter. However, in the earlier study by Bignell et al.,15 the TSH-R gene was clearly excluded. Furthermore, an X-linked autosomal dominant pattern and linkage to a second locus MNG2 (Xp22) was identified in an Italian pedigree with nontoxic familial goiter.27 To identify additional candidate regions, the first extended genome-wide linkage analysis was performed to detect susceptibility loci in 18 Danish, German, and Slovakian euthyroid goiter families.14 Assuming genetic heterogeneity and a dominant pattern of inheritance, four novel candidate loci on chromosomes 2q, 3p, 7q, and 8p were identified. An individual contribution was attributable to four families for the 3p locus and to one family for each of the other loci, respectively. On the basis of previously identified candidate regions and established environmental factors, nontoxic goiter can be defined as a complex disease (Fig. 16-2). However, for the first time, a more prevalent putative locus, present in 20% of the families investigated, was identified.14
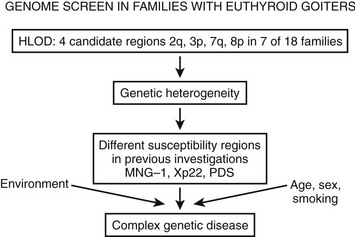
FIGURE 16-2 The identification of different susceptibility loci together with established environmental risk factors suggests that nontoxic goiter should be characterized as a complex disease. HLOD is the calculation of LOD score (logarithm of odds score) with respect to genetic heterogeneity.
The candidate region on 3p14 suggests a dominant pattern of inheritance for goiter. However, whereas linkage studies are suitable for the detection of candidate genes with a strong effect, it is possible to miss weak genetic defects of first-line candidate gene variants or of novel genes by linkage studies. Moreover, it is conceivable that the sum of several weak genetic variations in different genomic regions could lead to goiter predisposition. Therefore, the widely accepted risk factors such as iodine deficiency, smoking, old age, and female gender are likely to interact with and/or trigger the genetic susceptibility. In the future, loci identified by linkage analysis and/or association studies could reveal important genetic risk factors for familial nontoxic goiter. Further narrowing down the candidate regions and performing association studies with SNP markers in additional families and especially in case control association studies is necessary to identify the specific candidate genes for hereditary nontoxic goiter.
Mutagenesis as The Cause of Nodular Transformation Leading to Multinodular Goiter
Most goiters become nodular with time. From animal models of hyperplasia caused by iodine depletion,28 we have learned that besides an increase in functional activity, a tremendous increase in thyroid cell number occurs. These two events very likely orchestrate a burst of mutation events. It is known that thyroid hormone synthesis goes along with increased H2O2 production and free radical formation,29 which may damage genomic DNA and cause mutations. Together with a higher spontaneous mutation rate, a higher replication rate often will prevent mutation repair and increase the mutation load of the thyroid, thereby also randomly affecting genes crucial for thyrocyte physiology. Mutations that confer a growth advantage (e.g., TSH-R or Gsα protein mutations) very likely initiate focal growth. Hence, autonomously functioning thyroid nodules (AFTNs) are likely to develop from small cell clones that contain advantageous mutations, as shown for the TSH-R in “hot” microscopic regions of euthyroid goiters.30
Epidemiologic studies, animal models, and molecular/genetic data outline a general theory of nodular transformation. Based on the identification of somatic mutations and the predominant clonal origin of AFTNs and cold thyroid nodules (CTNs), the following sequence of events could lead to thyroid nodular transformation that occurs in three steps, as outlined in Fig. 16-3.17 First, iodine deficiency, nutritional goitrogens, or autoimmunity may cause diffuse thyroid hyperplasia. Then, at this stage of thyroid hyperplasia, increased proliferation together with possible DNA damage due to H2O2 action causes a higher mutation load (i.e., a higher number of cells bearing mutations). Some of these spontaneous mutations confer constitutive activation of the cyclic adenosine monophosphate (cAMP) cascade (e.g., TSH-R and Gsα mutations), which stimulates growth and function. Finally, in a proliferating thyroid, growth factor expression (e.g., insulin-like growth factor 1 [IGF-1], transforming growth factor β [TGF-β], or epidermal growth factor [EGF]) is increased. As a result of growth factor co-stimulation, most cells divide and form small clones. After increased growth factor expression ceases, small clones with activating mutations further proliferate if they can achieve self-stimulation. They thus can form small foci, which may develop into thyroid nodules. This mechanism may explain AFTNs by advantageous mutations that initiate growth and function of the affected thyroid cells, as well as CTNs by mutations that stimulate proliferation only (e.g., ras mutations, other mutations in the RAS/RAF/MEK/ERK/MAP cascade). Moreover, nodular transformation of thyroid tissue due to TSH-secreting pituitary adenomas31 and nodular transformation of thyroid tissue in Graves’ disease32 and in goiters of patients with acromegaly33 could follow a similar mechanism, because thyroid pathology in these patients is characterized by early thyroid hyperplasia.
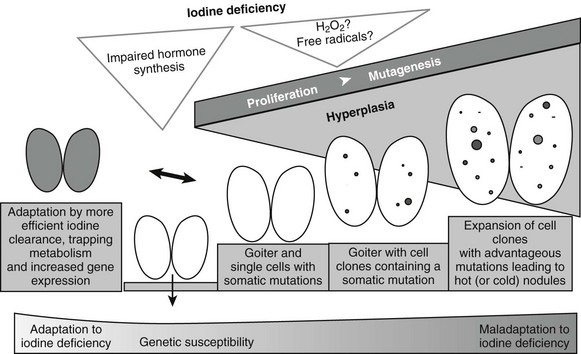
FIGURE 16-3 Hypothesis for thyroid nodular transformation. The starting point for the development of the multinodular nontoxic goiter (MNG) is hyperplasia induced by goitrogenic stimuli (e.g., iodine deficiency). Iodine deficiency increases mutagenesis directly (production of H2O2/free radicals) or indirectly (proliferation and increased number of cell divisions). Subsequently, hyperplasia forms cell clones. Some of them contain somatic mutations of the TSH-R, leading to autonomously functioning thyroid nodules (filled circles), or they contain mutations that lead to dedifferentiation and therefore cold thyroid nodules or cold adenomas (open circles).
As an alternative to the increase in cell mass, and as illustrated by those individuals who do not develop a goiter when exposed to iodine deficiency, the thyroid might also adapt to iodine deficiency without extended hyperplasia.34 Although the mechanism that allows this adaptation is poorly understood, data from a mouse model suggest an increase of mRNA expression of TSH-R, sodium iodine symporter (NIS), and TPO in response to iodine deficiency, which might be a sign of increased iodine turnover in the thyroid cell in iodine deficiency.35 Moreover, expansion of the thyroid microvasculature, caused by upregulation of vascular endothelial growth factor and other proangiogenic factors, is an additional mechanism that might help the thyroid to adapt to iodine deficiency.36
Oxidative Stress as The Downside of Thyroid Hormone Synthesis
Besides being a substrate in the hormone synthesis, H2O2 could be a major source of free radicals and reactive oxygen species (ROS). Because these molecules can cause substantial damage to a cell and impair normal function, thyroid epithelial cells are likely to have a potent defense mechanism to counterbalance potential damage mediated by free radicals. It has been shown that antioxidant enzymes, such as glutathione peroxidases (GPXs) or TPO, are upregulated during thyroid hormone synthesis.37 GPX3 has been suggested to directly interfere with thyroid hormone synthesis by affecting the concentration of H2O2.38 If antioxidant defense is not effective enough, excessive damage (e.g., peroxidation) should be detectable in lipids, DNA, and proteins of thyroid epithelial cells.
In thyrocytes, H2O2-mediated cytotoxicity appears to be dose dependent, requiring only low concentrations to result in thyroid cell apoptosis rather than necrosis, which could function as a barrier for tumorigenesis.39 Furthermore, findings in the thyroid glands of male Wistar rats suggest that the predominant cytotoxic response to oxidative stress might differ depending on the functional state of the gland.39 Moreover, ample evidence for excessive oxidative DNA damage has been found in the thyroid gland.40 Does this affect the spontaneous mutation rate (SMR) in the thyroid at large? It is most interesting to note that a strikingly high SMR was found in the thyroid gland of mice41: with an 8- to 10-fold higher number compared with liver, the thyroid stands out from many other tissues. Indeed, the SMR in mouse thyroid glands without any experimental mutagenic challenge shows values that usually are found only in other organs (e.g., liver) of animals treated with mutagens like ethyl nitrosourea or benzo[a]pyrene.42 The above data point to a connection between thyroid hormone metabolism, oxidative DNA damage, and SMR in the normal thyroid gland that may represent the basis for frequent tumorigenesis.
On top of this generally high mutation rate in the normal thyroid gland, environmental and lifestyle factors may add to the pool of DNA damage and increase mutagenesis and tumor initiation. Tobacco smoke, as previously mentioned,9 because it is one of the prime suspects in thiocyanate-induced blocking of iodine transport into the thyrocyte, could lead to intracellular iodine deficiency.
As outlined in the aforementioned, H2O2-mediated ROS generation is very likely to be an important starting point for thyroid tumor development (Fig. 16-4). Because iodine and H2O2 act as co-substrates in thyroid hormone synthesis, changes in iodine concentration are very likely to affect the H2O2 concentration. In fact, generation of H2O2 is inhibited by iodide in vivo and in vitro.43 H2O2 generation—which is mandatory for the organification of iodine—is, moreover, stimulated by TSH (in contrast to many other aspects of thyroid hormone synthesis, it is unclarified whether cAMP is the second messenger in H2O2 generation), which increases the expression of genes important for thyroid hormone synthesis (e.g., NIS, TPO).29 Low iodine levels and markers of increased thyroid functionality suggest activation of H2O2 generation, which could result in DNA damage and somatic mutations.44 Consequently, low iodine and high H2O2 levels should activate antioxidative defenses, which should be detectable in the cellular regulation of enzymes involved in the defense against oxidative stress.
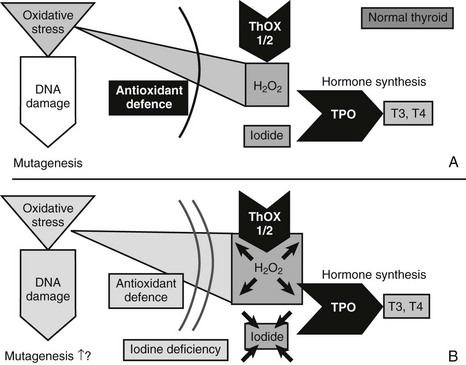
FIGURE 16-4 Mechanisms that might cause mutagenesis in the thyroid gland. The figure shows the key molecules involved in those parts of thyroid hormone synthesis which—in conditions of iodine and most likely also selenium deficiency—lead to oxidative stress, DNA damage, and possibly mutagenesis. ROS, Reactive oxygen species; THOX, thyroid oxidase; TPO, thyroid peroxidase. A, In the normal thyroid gland, the enzymes THOX1 and THOX2 generate H2O2, and TPO transfers oxidized iodine to tyrosyl residues of thyroglobulin, the precursor for T3 and T4 synthesis. H2O2 is, however, a source of ROS, which—with, other oxidative stress—can cause DNA damage. Normally, antioxidant defense could prevent oxidative stress and DNA damage. Selenoproteins like glutathione peroxidase 3 are part of the defense. B, Conditions of iodine deficiency increase levels of H2O2 and might increase the amount of oxidative stress and DNA damage. Additional selenium deficiency decreases selenoproteins and thereby could weaken antioxidative defense, which exacerbates oxidative stress and DNA damage.
Indeed, a higher expression of mRNA for superoxide dismutase 3 (SOD3)—the extracellular SOD isoform that preferentially acts in the lumen, where H2O2 is generated—is detected during experimental iodine deficiency in mice.35 Moreover, oxidative stress and antioxidant defenses are enhanced in hyperplastic and involuting glands.45
Differential expression of additional antioxidant enzymes40 underlines the importance of the antioxidant defense in the iodine-deficient thyroid gland. Moreover, glutathione peroxidases are selenium proteins. It therefore is very likely that selenium deficiency could impair the antioxidant defense and exacerbate oxidative stress (see Fig. 16-4). An increased oxidative burden in the thyroid gland through iodine deficiency is also suggested by results of the comet assay with repair-enzyme protocols to detect oxidative DNA damage.35 As a consequence, early molecular conditions for nodular and tumor transformation in the thyroid gland consist of a sequence of molecular events that include oxidative stress and DNA damage as the triggers for somatic mutations. The oxidative burden is already detectable in the normal thyroid gland and is very likely to be linked to hormone synthesis and H2O2 production. Additionally, environmental conditions (e.g., iodine deficiency) have the potential to aggravate this situation. In general, any external factor (e.g., smoking) that increases oxidative stress, causes DNA damage, or increases the SMR might aggravate the risk for tumor genesis. Also, any factor that increases proliferation (e.g., goitrogens) in all likelihood shortens the time to development of a detectable thyroid tumor.
Hot Thyroid Nodules
Somatic point mutations that constitutively activate the TSH-R were first identified by Parma and coworkers in hyperfunctioning thyroid adenomas.46 However, in different studies, the prevalence of TSH-R and Gsα mutations in autonomously functioning thyroid nodules has been reported to vary from 8% to 82% and from 8% to 75%, respectively.46–49 Available studies differ in the extent of mutation detection and in screening methods. A comparison with respect to the obvious differences between studies has been done elsewhere.50,51 A comprehensive study using the more sensitive denaturing gradient gel electrophoresis52,53 revealed a frequency of 57% TSH-R mutations and 3% Gsα mutations in 75 consecutive, autonomously functioning thyroid nodules.54 These results raise the question of the molecular origin of TSH-R and Gsα mutation-negative nodules. A possible answer is given by clonal analysis of these AFTNs, which demonstrates a predominant clonal origin of thyroid nodules and implies a neoplastic process driven by genetic alteration.
In addition to the intracellular signaling network that is connected to the TSH-R, the extracellular action of different growth factors enhances the complexity of the signal flux into the thyroid cell. Growth factors like IGF-1, EGF, TGF-β, and fibroblast growth factor (FGF) stimulate growth and dedifferentiation of thyroid epithelial cells.55 Studies focused on insulin and IGF show a permissive effect of insulin and IGF-1 on TSH signaling56,57 as well as a cooperative interaction of TSH and insulin/IGF-1.58 Other studies suggest inactivation of TGF-β signaling in AFTNs due to constitutively activated TSH-R (e.g., resulting from TSH-R mutations).59 This assumption is supported by the finding of decreased expression of TGF-β1 mRNA after TSH stimulation of thyrocytes.60 Because TGF-β1 has been shown to inhibit iodine uptake, iodine organification, and thyroglobulin expression,61 as well as cell proliferation in different cell culture systems,62,63 these findings suggest that inactivation of TGF-β signaling is a major prerequisite for increased proliferation in AFTNs.64 Signal modulation of the TSH-R that would define the cause of AFTNs and the clinical phenotype therefore could take part at a number of stages and very likely involve genetic/epigenetic, gender-related, and environmental factors.
Cold Thyroid Nodules
The term “cold” indicates reduced uptake on scintiscan. Because a histologic diagnosis typically is employed to exclude thyroid malignancy, many investigations of thyroid nodules refer only to the histologic diagnosis of thyroid adenoma. This histologic entity should not be confounded with the scintigraphically characterized entity “cold nodule,” which, like AFTNs or “warm nodules,” can appear histologically as thyroid adenomas or adenomatous nodules according to the WHO classification.1 In contrast, focal hyperplasia is not very well explained on the molecular level and has been discussed in detail elsewhere as the cause of thyroid tumors.65,66 A monoclonal origin has been detected for most cold thyroid nodules, which implies nodular development from a single mutated thyroid cell.67
With reference to their functional status (i.e., reduced iodine uptake), failure in the iodide transport system and failure of the organic binding of iodide were detected as functional aberrations of cold thyroid nodules long before the molecular components of iodine metabolism were known. Subsequently, decreased expression of the Na+/I− symporter (NIS) in thyroid carcinomas and benign cold thyroid nodules were suggested as the molecular mechanisms underlying the failure of iodide transport (reviewed in references 68 and 69). However, a defective cell membrane targeting the NIS protein is a more likely molecular mechanism accounting for the failure of iodine uptake in CTNs.68,70 The ultimate cause of this defect is currently unknown.
Compared with iodine transport, the organic binding of iodine is a multistep process with a number of protein components that still awaits final characterization.71 mRNA expression of enzymatic components (e.g., TPO, flavoproteins) and the substrate of iodination (i.e., Tg) have been quantified in CTNs without significant differences compared with normal follicular tissue.72,73 TPO, Tg, and thyroid-specific oxidases (THOX) have been successfully screened for molecular defects, especially in congenital hypothyroidism.74
Although CTNs could be regarded as a form of focal hypothyroidism, somatic mutations in enzymes that catalyze organic binding of iodine would need to exert a growth advantage on the affected cell to cause the development of a thyroid nodule. At least in the case of inactivating mutations in the TPO or THOX genes, growth advantage could result from a lack of enzyme activity, which would reduce not only thyroid hormone synthesis but also follicular iodide trapping in organic iodo compounds. Because these compounds have been shown to inhibit thyroid epithelial cell proliferation,75 reduced synthesis could have a proliferative effect. Therefore, somatic TPO or THOX mutations could be a molecular cause of CTN. However, mutations in the TPO gene have not been detected.76 A study of 40 cold thyroid adenomas and adenomatous nodules detected ras mutations in only a single case.67 Moreover, in the same set of CTNs, no point mutations in the mutational hot spots of the BRAF gene were detected.77 This is in line with the lack of BRAF mutations in benign follicular adenomas in other studies.78,79 So far, only one study has detected a single BRAF mutation in a set of 51 follicular adenomas.80 Moreover, the gene expression for approximately 10,000 full-length genes was compared between CTNs and their corresponding normal surrounding tissue.81 Increased expression of histone mRNAs and of cell cycle–associated genes like cyclin D1, cyclin H/cyclin-dependent kinase (CDK) 7, and cyclin B most likely reflects a molecular setup for increased proliferation in CTNs.82 In accordance with the low prevalence of ras mutations in CTNs,67 reduced expression of ras-MAPK (mitogen-activated protein kinase) cascade–associated genes was found, which might suggest minor importance of this signaling cascade. Furthermore, gene rearrangements unique to thyroid adenomas have recently been the focus (reviewed in reference 83). These studies led to the identification of the thyroid adenoma–associated gene (THADA) that encodes a death receptor–interacting protein.84 Although also reported for thyroid follicular carcinoma,85 the finding of loss of heterozygosity (LOH) at the TPO locus is characteristic for some CTNs (about 15%) but rather points to defects in a gene near TPO on the short arm of chromosome 2. Although the frequency of each of these DNA aberrations is rather low, together these chromosomal changes need to be considered in further elucidation of the molecular origin of CTNs.
Clinical Aspects
Epidemiologic studies of multinodular goiter are hampered by problems such as selection criteria (age and gender), influence of environmental factors (e.g., iodine intake, smoking habits), evaluation of size and morphology (palpation, ultrasound, or scintigraphy), and determination of thyroid function, and whether subjects with subclinical hyperthyroidism are categorized as euthyroid or hyperthyroid. Only thyroid nodules of at least 10 mm can be identified reliably by palpation.86 With the use of ultrasound, nodules as small as 2 mm are readily detected. It therefore is not surprising that the prevalence of nodules is increased several-fold if sonographic examination is applied, because 70% of thyroid nodules disclosed by sonography are smaller than 10 mm in diameter.86,87
Most studies have focused on middle-aged women and the elderly, whereas only a few have documented the prevalence of multinodular thyroid disease in a cross-sectional investigation of the adult population in a community. Longitudinal studies covering many years are necessary to give valid figures on incidence, etiologic risk factors, and the natural history. Such studies that take the above-mentioned problems into consideration are not available. These limitations therefore should be borne in mind when the available data are considered.87 Iodine deficiency is still the most frequent single cause of multinodular endemic goiter worldwide. Considerable regional variation exists even in nonendemic goiter areas. In the Whickham survey, 16% of the cohort had simple goiter.88 In men, the prevalence declined with age from 7% in those younger than 25 years to 4% in those older than 65 years. Among women, the frequency declined from 31% in those younger than 45 years to 12% in those older than 75 years. This finding fits the observation that lean body mass, known to decline with age, is the major determinant of thyroid size.87 Illustrating the influence of iodine intake on the epidemiology of sporadic goiter, 31 of 423 (7.3%) 68-year-olds had goiter in Jutland, Denmark (low iodine intake area), versus 2 of 100 (2%) in Reykjavik, Iceland (high iodine intake area).89
A cross-sectional study of the community in Whickham found a prevalence of hyperthyroidism of 25 per 1000 women and 2 per 1000 men in an adult population.88 Others have reported similar figures.88 The yearly incidence of hyperthyroidism (all types) varies between 0.1 and 0.2 per 1000 men and between 0.3 and 1.3 per 1000 women. As with nontoxic goiter, iodine intake is of paramount importance. In Denmark, a country with a borderline sufficient iodine intake, multinodular toxic goiter accounts for 50% of patients with hyperthyroidism, whereas Iceland, with a high iodine intake, has a lower proportion of multinodular goiter (6%) and a greater number of cases of Graves’ disease.89
Natural History
The natural history of multinodular goiter, with respect to goiter growth and function, varies and is difficult to predict in a given patient. The spontaneous growth rate in selected populations has been estimated to be up to 20% yearly90 but usually is much lower. No specific parameter exists that can predict the growth potential of multinodular goiter, which can be accurately assessed by serial yearly measurements of the size of the goiter and individual nodules by ultrasonography.91
Painful nodules are usually the result of hemorrhage into a nodule or a cyst in the goiter. The diagnosis is readily made by ultrasonographic examination and fine-needle aspiration biopsy. Such a growing painful nodule may represent thyroid malignancy and should be investigated accordingly. Multinodular goiter is not usually associated with a significantly increased risk for the development of thyroid malignancy. The risk of malignancy in thyroid nodules occurring within a multinodular goiter has not been completely clarified, but most authors find a similar frequency in uninodular and multinodular goiters.92
Patients with nontoxic multinodular goiter can become hyperthyroid or, less commonly, hypothyroid. Hyperthyroidism in such patients often develops insidiously, in contrast to that of Graves’ disease. It often begins with a prolonged period of subclinical hyperthyroidism characterized by low serum TSH and normal serum free T4 and triiodothyronine (T3) concentrations.87 This hyperthyroid state is the consequence of goiter growth and an associated increase in the mass of autonomously hormone-producing thyroid cells. Hyperthyroidism can also be the result of an increase in iodine intake from iodine-containing drugs such as disinfectants and amiodarone or from radiographic contrast agents, which, in a goiter with increased autonomous iodine metabolism, leads to the production of excessive amounts of thyroid hormone. Little is known of the incidence and the time frame for this progression from the nodular nontoxic goiter toward the nodular toxic goiter. In a large population-based cross-sectional study in an iodine-deficient area, nodular autonomy increased with age and reached 15% in elderly people.93 It appears from a few longitudinal studies87 that within 5 years, hyperthyroidism will emerge in approximately 10% of patients with a nodular goiter. In a few cases, autonomy of some of the thyroid nodules may return.
Diagnosis
Pertinent clinical signs and symptoms are given in Table 16-1, and diagnostic aspects are summarized in Table 16-2. For most, the thyroid gland does not become palpable until the volume has doubled. A visibly diffusely enlarged goiter has often reached a volume of 30 to 40 mL. Detection of nodules depends on their size, morphology, and location within the thyroid parenchyma, the anatomy of the patient’s neck, and the training of the physician. Among patients who present with a palpable nodule, approximately half have more than one lesion by sonographic examination.86 Sonography detects approximately five times as many nodules as thyroid palpation, and twice as many when only nodules larger than 2 cm are considered.86,87 Awareness, however, may depend on localization, speed of growth, and the possible pain or discomfort related to hemorrhage into a nodule (see Table 16-2).
Table 16-1
Clinical Signs and Symptoms of Multinodular Goiter
• Slowly growing nodular anterior neck mass
• Tracheal deviation or compression, upper airway obstruction, dyspnea
• Occasional cough and dysphagia, globulus
• Sudden pain or enlargement secondary to hemorrhage
• Superior vena cava obstruction syndrome
• Pemberton’s sign: obstruction of the thoracic inlet by the arms extended over the head
• Enlargement during pregnancy
• Gradually developing hyperthyroidism
• Iodide-induced thyrotoxicosis
Table 16-2
Diagnosis of Multinodular Goiter
• Multinodularity on examination
• Asymmetry, tracheal deviation
• Thyroid-stimulating hormone normal or decreased, free thyroxine and free triiodothyronine normal or increased, thyroglobulin elevated
• Thyroid antibodies negative in approximately 90%
• Scintigraphy with hot and cold areas
• Ultrasound finding of nodularity (nonhomogeneity); cysts and calcifications are common
• Computed tomography and magnetic resonance imaging demonstrating a nonhomogeneous mass
• Lung function testing may demonstrate impaired inspiratory capacity
• Benign cytology by fine-needle aspiration of dominant nodules
Inspection and palpation of the neck, preferably done with the patient swallowing gulps of water and with the head tilted slightly backward, may disclose anything from a single nodule in an otherwise normal nonpalpable thyroid to a large compressive multinodular gland extending retroclavicularly or into the mediastinum. However, clinical examination is associated with considerable interobserver and intraobserver variation regarding size and morphology of the thyroid.94
Laboratory Investigations
Because the transition from nontoxic to toxic goiter is part of the natural history of this disease,87 and because detection of borderline but clinically relevant hyperthyroidism requires laboratory tests, annual screening with a sensitive TSH assay is recommended. The possibility of hyperthyroidism must be considered in any goitrous patient with otherwise unexplained illness. This point is particularly true for patients with cardiac failure or arrhythmia. Subnormal serum TSH values should lead to a determination of free T4 and free T3. Even in the presence of normal serum thyroid hormone levels, suppressed serum TSH should lead to treatment, especially in the elderly.95
Calcitonin, a marker of medullary thyroid cancer (MTC) when elevated in serum, can aid in the early detection of sporadic cases of this disease. Routine determination in nodular thyroid disease has been suggested. Large-scale studies demonstrate a prevalence of MTC in the range of 0.4% to 1.37%.87,96 Studies vary with regard to the diagnostic setup and the fraction of patients with histologic verification. Basal or stimulated calcitonin levels were generally more sensitive than fine-needle aspiration biopsy (FNAB) in detecting MTC, and the routine use of serum calcitonin is recommended by most authors of these studies.87,96 However, a clear conclusion is not easy to draw from the existing data. Cost-benefit must be taken into consideration, and a high false-positive rate will result in many unnecessary thyroidectomies. It is also evident from international surveys97–100 that no consensus has been reached on this issue. More than 30% of European clinicians measure basal serum calcitonin routinely,97,99 whereas only very few in North America use this strategy except in cases of a family history of thyroid cancer.98,100
Antithyroid antibodies (TPO and TG antibodies) in serum in our opinion should be determined routinely in the workup of these patients. This recommendation is based mainly on the fact that Hashimoto’s thyroiditis may be mistaken for simple multinodular goiter, and that these antibodies may be recognized as markers of increased risk for 131I-induced hypothyroidism, as well as Graves’ disease, in patients with toxic101 and nontoxic multinodular goiter.102
131I uptake determination aids in the diagnosis of iodine contamination, ensures that uptake is adequate, and allows calculation of the 131I dose before 131I therapy is provided. However, it is used routinely in the workup of such patients only by a minority of clinicians.99,100
Diagnostic Imaging
Although not adequately investigated, it has been stated repeatedly that imaging rarely provides information that is decisive for clinical management in individual cases. Although simple and cheap, neck palpation is notoriously imprecise with regard to thyroid gland morphology and size determination.94 For this purpose, several imaging methods are available: sonography, scintigraphy, computed tomography (CT) scan, magnetic resonance (MR) imaging, and perhaps positron emission tomography (PET). Of these, sonography clearly has first priority among clinicians.97–100
Scintigraphy has little place in the anatomo-topographic evaluation of the nodular goiter, but it aids in verification of the clinical diagnosis and allows determination of the relative mass of hyperfunctioning (hot) and nonfunctioning (cold) thyroid areas. If a clinically dominant nodule is cold on scintigraphy, it should be treated as a solitary cold nodule—the risk for malignancy being the same.87,92 Nodules with high uptake by scintigraphy almost never harbor clinically significant malignancy, although exceptions have been reported. 99mTc used as tracer may result in false-positive uptake in 3% to 8% of thyroid nodules,87 and iodine isotopes are devoid of this problem. Nevertheless, comparative studies have been unable to demonstrate any clinically significant differences between the two tracers.87 Tracers like 201Thallium and 99mTc-MIBI have an increased uptake in differentiated malignant thyroid nodules, but sensitivity and specificity do not support their general use.103,104 Many disregard thyroid scintigraphy in the initial evaluation of patients with nontoxic nodular goiter.87,99,100 Nevertheless, more than two thirds of ETA members97,99 routinely use scintigraphy, and less than 25% of ATA members prefer such a strategy.98,100 Indisputable indications for scintigraphy in the setting of a nodular goiter include hyperthyroidism (to visualize hot nodules suitable for 131I therapy) and identification of a follicular neoplasm with FNAB, because warm nodules with great certainty are benign.87
Ultrasound, which is used often in Europe97,99 and less so in the United States,98,100 allows determination of total thyroid volume and individual nodule size and evaluation of regional lymph nodes, regional blood flow, nodule vascularity, and elasticity.87,105 It aids in the performance of accurate biopsies91 and is of great help in therapeutic procedures such as cyst puncture and alcohol and laser sclerosis of solid or cystic nodules.91,106–108 In the vast majority of patients, ultrasound can neither confirm nor exclude malignancy.91 For an objective determination of thyroid size, whether before therapy, such as in the dose calculation of 131I, or for follow-up post therapy, it is the technique of choice,91 although it is less useful with very large goiters.87
Computed tomography and magnetic resonance imaging are generally of little value except for evaluation of a retroclavicular or intrathoracic goiter, and for evaluation and follow-up of malignant thyroid disease. MR is thought to be more precise than CT in the anatomo-topographic evaluation of the substernal goiter.87 However, whether CT or MR is preferred probably depends on cost and availability.
In the differentiation between malignant and benign thyroid lesions, [18F]-2-deoxy-2-fluoro-D-glucose positron emission tomography (FDG-PET) may be a potentially useful tool in the evaluation of thyroid nodules with indeterminate cytologic findings. Because this method has a very low false-negative rate for the detection of malignant lesions, a number of unnecessary thyroidectomies may be avoided.109 Noteworthy, thyroid nodules detected incidentally by FDG-PET harbor cancer in 25% to 50% of cases.110
Many patients with goiter appear to have upper airway obstruction due to tracheal compression. Most often, the inspiratory component of the respiration is compromised, but this is often overlooked during a routine examination because respiratory symptoms usually are absent.87 Routine radiography of the trachea has no place in patients with a compressive goiter, because this method is too insensitive for detection of a clinically significant tracheal obstruction.87 Determination of the smallest cross-sectional tracheal area by MR imaging or CT seems more useful in this setting.87 Thus, a flow volume loop rather than tracheal imaging should be used for the evaluation of respiratory capacity in the patient with a goiter, in particular if the goiter is very large; however, this strategy is rarely used by clinicians.99,100
Fine-Needle Aspiration Biopsy (FNAB)
The possibility of thyroid malignancy should be considered in all patients with multinodular goiters, and the use of ultrasonography guidance has been shown to enhance the diagnostic efficacy of FNAB.92 If the initial FNAB is nondiagnostic owing to limited cellularity, a repeated test is advocated.111 FNAB cannot rule out malignancy but probably can reduce the risk of overlooking malignancy to below 1% and, in the worst case, could lead to delays in making the correct diagnosis. If malignancy is clinically suspected, a benign cytology should be disregarded naturally and the patient offered surgery. In subjects referred for evaluation of symptomatic multinodular nontoxic goiter and offered surgery, the incidence of carcinoma is 1% to 4%.87 This figure includes small papillary carcinomas of dubious clinical significance. In unselected patients with multinodular goiter, the prevalence of clinically important malignancy is probably less than 1%. It is important to note that hyperthyroidism does not exclude the possible presence of malignancy,87 although the risk seems inversely correlated with falling serum TSH levels.112
The examination should focus on the dominant nodule (“index nodule”) or nodules or on those that have a different consistency from other nodules within the gland.87 It has been recommended that nodules measuring less than 10 mm, detected incidentally, do not require an FNAB.113 However, Papini et al.114 demonstrated thyroid malignancy in 6% of nonpalpable lesions of 8 to 15 mm in size in multinodular goiters (9% in solitary thyroid nodules). The risk was similar in nodules smaller or greater than 10 mm. Whether carcinomas found in nodules other than the index nodule constitute clinically significant cancers or just incidental microcarcinomas remains an unsolved issue, leaving the clinician with no clear-cut guidelines for management. Sonographic features may guide the clinician to include FNAB in nodules other than the index nodule. If scintigraphy is performed, we recommend FNAB in up to two nodules, provided they are scintigraphically cold.87
In our opinion, neither diagnostic imaging nor FNAB is necessary in most patients with nodular thyroid disease if the preferred treatment is surgery. However, if a nonsurgical treatment is considered, we support the liberal use of diagnostic imaging and FNAB.87
Treatment
1. Large goiter or progressive growth of the entire gland or individual nodules
2. Signs of cervical compression
No ideal treatment is available for goiter, and no consensus has been reached.87,99,100,115 This is reflected by the fact that a third of clinicians would refrain from treatment when facing a patient with moderate discomfort due to a multinodular nontoxic goiter of 50 to 80 g in which malignancy has been ruled out.99,100 Comparative studies of available options are sparse. It is important to note that no study has evaluated health-related quality of life using a disease-specific questionnaire.116 Thus, treatment is not only a matter of goiter reduction. Patient satisfaction, the risks for hypothyroidism and goiter recurrence, and the fear of overlooking a thyroid cancer are all important issues that should be taken into account. It follows that the optimal treatment for toxic and nontoxic multinodular goiter is controversial, and at present the treatment choice must be based on individual factors.
The nontoxic and the toxic multinodular goiter should be regarded as the same disease but at different evolutionary stages. Because many of the data regarding surgery and 131I therapy apply to both conditions, the treatment options are discussed in concert. Tables 16-3 and 16-4 list effects and side effects of the various treatment options.
Table 16-3
Treatment Options for Patients With Nontoxic Multinodular Goiter
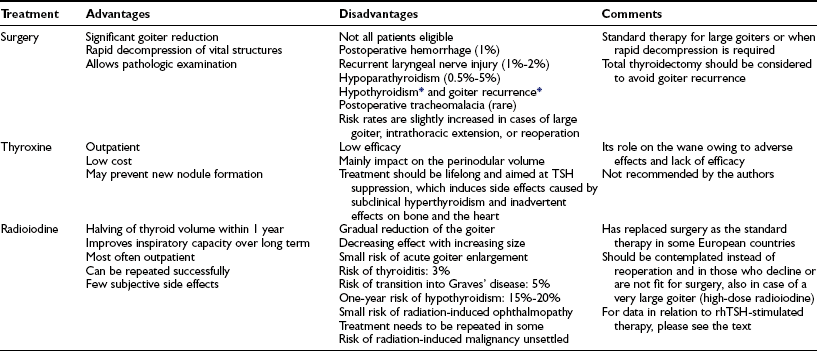
rhTSH, Recombinant human thyroid-stimulating hormone; TSH, thyroid-stimulating hormone.
*The percentage of patients affected depends on the extent of surgery.
Table 16-4
Treatment Options for Patients With Toxic Multinodular Goiter
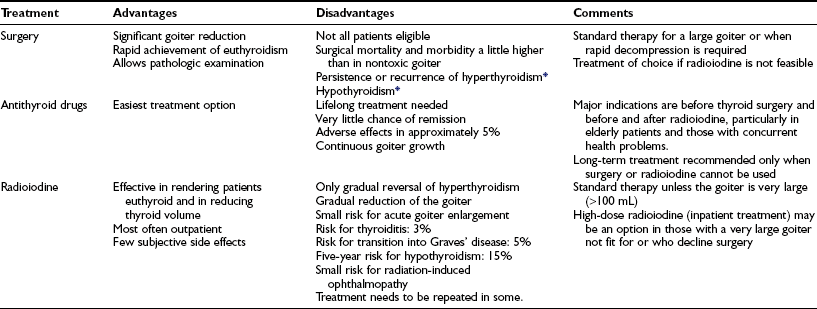
*The percentage of patients affected depends on the extent of surgery.
Antithyroid Drugs
Antithyroid drugs are indicated if the nodular goiter is complicated by coexisting hyperthyroidism. These drugs normalize thyroid function, but remission is very rare and lifelong treatment should be anticipated. Also, further goiter growth may be seen, possibly as a result of using these drugs. Antithyroid drugs are indicated before thyroid surgery to lower the operative risk and can be stopped during the immediate postoperative period.117 To reduce the risk for exacerbation of hyperthyroidism, it has been recommended to render the patient euthyroid with antithyroid drugs prior to 131I treatment. Usually, the antithyroid drug is discontinued at least 4 days before and is resumed no sooner than 3 days afterward.117 A meta-analysis, based mainly on studies of Graves’ disease, found that the use of methimazole and propylthiouracil in conjunction with 131I therapy results in a decreased remission rate.118 Whether this applies also to toxic multinodular goiter patients is unknown. One study has shown attenuation of goiter reduction if methimazole is resumed after 131I therapy despite a neutral effect on thyroid function.119
Iodine Supplementation
Iodine supplementation for treatment of goiter is used by some clinicians, particularly in Europe.99 In a placebo-controlled trial, the median volume of diffuse goiters was reduced from 29 to 18 mL, but thyroid dysfunction and antibodies appeared in 10% of patients.120 The efficacy of iodine supplementation, once a nodular goiter has developed, has only very scarcely been evaluated. In nodular goiter, iodine is no better than L-T4 suppression therapy for goiter reduction in comparative trials. But the major hindrance involving the use of iodine is the fact that a sudden increase of intake may induce thyrotoxicosis in predisposed individuals.87
Thyroid Hormone Treatment
Thyroid hormone therapy for suppression of pituitary TSH secretion has been much used in the patient with nontoxic multinodular goiter.99,100 Although L-T4 suppressive therapy is effective in reducing the volume of diffuse nontoxic goiters by up to 30%,121 few controlled studies have examined nontoxic multinodular goiter90,122 by employing sonography for objective size monitoring. In one study,90 58% of patients had a significant (>13%) decrease in thyroid volume, but regrowth was seen after discontinuation of therapy. Wesche et al.122 in a randomized trial found a median reduction of goiter volume in the 131I-treated group of 38% and 44% after 1 and 2 years, respectively; corresponding values in the L-T4–treated group were 7% and 1%, respectively, and these were nonsignificant.
L-T4 dose is often targeted toward a partially suppressed serum TSH level.99,100 The consequence is subclinical hyperthyroidism that adversely affects the skeleton and the cardiovascular system.95 Because lifelong therapy probably is needed to avoid goiter recurrence, and because the natural history of the disease involves progression toward hyperthyroidism due to autonomous function of the thyroid nodules,87 L-T4 treatment in fact is not feasible in most patients.123 Based on the aforementioned, L-T4 treatment should be abandoned on this indication.87,124,125
Surgery
The goal of surgery is removal of all thyroid tissue with a nodular appearance, usually by a unilateral hemithyroidectomy and subtotal resection of the contralateral lobe. A bilateral subtotal resection cannot be recommended. Only extremely rarely is a thoracic approach necessary. Further resection is not usually recommended if final pathologic evaluation incidentally reveals a unilateral cancer that is smaller than 1 cm. This not uncommon finding accounts for most cancers found in surgical series, a majority of which are of little if any clinical significance.126 Macroscopically normal perinodular tissue often harbors microscopic growth foci, which explains the relatively high risk for recurrence in these patients.127 In the case of a toxic nodular goiter, thyroid function is normalized more rapidly after surgery than after 131I therapy on the assumption that antithyroid drugs are not used postoperatively.87
Surgery leads to rapid decompression, resulting in improved respiratory function if affected presurgically.128 Not all patients are surgical candidates, but among those undergoing surgery, the surgical mortality rate is less than 1% in experienced centers. Disadvantages include the general risks and side effects of a surgical procedure. Specific risks include transient (6%) or permanent (2%) vocal cord paralysis, transient (6%) or permanent (5%) hypoparathyroidism, and postoperative bleeding (1%).129 Others have reported lower figures.130 Complications are related to increasing goiter size and extent of the resection.130,131 Novel techniques may reduce the operation time, the postoperative pain, and the length of the hospital stay.132,133 Postoperative tracheomalacia, necessitating intubation, may ensue in approximately 5% of patients operated for large goiters.87 A matter of concern is the apparently high prevalence (7% to 17%) of thyroid carcinomas in substernal goiters,87,134 but this seemingly high frequency probably is influenced by selection bias.
The long-term risk for hypothyroidism after subtotal resection of multinodular goiters is insufficiently described but is approximately 10% to 20%, as reported for toxic multinodular goiters,87 and is related to the extent of the resection. Recurrence of the nontoxic multinodular goiter is seen in 15% to 40% of patients on long-term follow-up.87 Postoperative use of L-T4 is preferred by many clinicians99,100 to avoid recurrence; however, based on results from randomized trials,87 the use of L-T4 generally cannot be recommended,87 nor can the use of iodine.135 A reoperation for recurrent goiter results in a threefold to 10-fold increase in risk for permanent vocal cord paralysis or hypoparathyroidism.130,131 131I therapy seems to be a favorable alternative in these cases. Goiter recurrence can be completely avoided, if a total thyroidectomy is carried out initially, in some centers with the same low rate of complications as was reported with subtotal thyroidectomy.136
Radioiodine Therapy
131I treatment is considered safe and appropriate in nearly all types of hyperthyroidism, especially that occurring in elderly patients.117 Generally, 131I is thought to carry a lower rate of complications and a lower cost than surgery.117 This fact has led a number of centers to offer 131I as the first choice of therapy for most patients. In contrast to surgery, which cures nearly all patients and normalizes hyperthyroidism within a few days,137 only 50% become euthyroid within 3 months of 131I therapy, given that antithyroid drugs are not administered.101 Twenty to 40% need additional 131I therapy, and even up to five treatment sessions will not cure all patients.101 In contrast, persistence of hyperthyroidism after surgery is rare.137
Besides being able to cure hyperthyroidism, it has long been recognized that use of 131I results in shrinkage of the thyroid gland. During the past 25 years, 131I therapy for symptomatic multinodular nontoxic goiter therefore has been introduced in a number of mainly European centers as a nonsurgical alternative to L-T4 therapy.87,99 Thyroid volume reduction is of the same magnitude in toxic and nontoxic multinodular goiters, that is, approximately 40% after 1 year101,122,138–140 and 50% to 60% after 2 years, without further reduction.101,138 Sixty percent of this decrease is seen within 3 months of initiation of therapy.101,138 In addition to relief of compressive symptoms, 131I therapy results in less tracheal compression, which improves pulmonary function, particularly the inspiratory component.139,140 Because of the often large goiters, normalization of thyroid volume, as is seen in diffuse toxic and nontoxic goiters, can rarely be achieved,87 but symptoms in most cases are considerably improved, and patient satisfaction is high.140,141 If a secondary increase in thyroid volume is seen, this should raise the suspicion of malignancy. Generally, 131I doses of 100 µCi (3.7 MBq) per gram of thyroid tissue corrected for 100% 24 hour 131I uptake have been given.101,122,138–140 However, whether such dose adjustment is worthwhile has been questioned,117,142 and fixed doses are given in a number of centers.117 Treatment can be repeated if further goiter reduction is required in a euthyroid patient.87,138
Radiation thyroiditis, seen in 3% within the first months of 131I therapy,102 is easily treated with salicylates or corticosteroids. Another complication is a Graves’-like autoimmune hyperthyroidism, which is seen in 5%. Rare cases of 131I-induced Graves’ ophthalmopathy have also been reported.87 Pretreatment presence of anti-TPO antibodies confers a significantly increased risk for this complication,87 which most likely is triggered by 131I-related release of thyroid antigens and is associated with the appearance of TSH receptor antibodies typically 3 to 6 months after 131I therapy. It also can be seen after surgical manipulation of the thyroid or after subacute thyroiditis.87 The condition is often self-limiting but may necessitate therapy.
Although early goiter enlargement caused by radiation therapy may be seen, on average 131I therapy is not followed by any significant acute thyroid enlargement.139,143 The risk for permanent hypothyroidism after 131I therapy in multinodular goiters ranges from 14% to 58% within 5 to 8 years.87,101,138 It occurs more commonly in patients with a small goiter and when anti-TPO antibodies are present.87 131I therapy, given for Graves’ disease for decades, until recently was not thought to be followed by any clinically significant increased risk for cancer death.144,145 However, a recent study questions these findings.146 Data regarding 131I therapy in multinodular goiter are sparse, and in the case of nontoxic goiter, nonexistent. In the study by Ron et al.,144 1089 patients were treated for a toxic nodular goiter, and these individuals had a 31% increase in overall cancer mortality, nearly exclusively attributable to thyroid malignancy. However, a similar pattern was seen in patients with the same disease but not treated with 131I. Hence, the disclosure of a thyroid cancer in a nodular goiter after 131I therapy raises the question, whether malignancy in a nodule was overlooked at the time of therapy.
In some European countries, 131I therapy has replaced surgery as the treatment of choice in most patients.87,99 However, the optimum treatment remains to be established, ideally through comparative randomized trials, including data on effects, side effects, costs, and patient satisfaction.
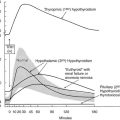
The efficacy of 131I therapy in multinodular goiter is hampered by irregular 131I uptake in the gland, and the relative goiter reduction is inversely correlated with initial goiter size.139 A high dietary iodine intake diminishes efficacy. However, recombinant human TSH (rhTSH) has the potential of increasing the 24-hour 131I uptake more than fourfold,147–149 and the effect is inversely correlated to the initial thyroid 131I uptake.147–149 Moreover, pretreatment with rhTSH causes a more homogeneous distribution of 131I within the nodular gland.150 These properties of rhTSH are ideal in the context of 131I therapy for multinodular goiter. Indeed, several studies, including two randomized, double-blinded trials,151,152 have confirmed that rhTSH, in doses from 0.1 mg to 0.9 mg and administered 24 hours before 131I therapy, improves goiter reduction by 35% to 55% within a year, when compared with conventional 131I therapy (Fig. 16-5). The impact of rhTSH pre-stimulation is most pronounced in patients with a low baseline thyroid 131I uptake.149,151,152 rhTSH-augmented 131I therapy also results in reduced tracheal compression and enhancement of the inspiratory reserve, as shown in a randomized trial.153 We have speculated that the effects of rhTSH might be mediated through factors beyond the increase in thyroid iodine uptake.151,152
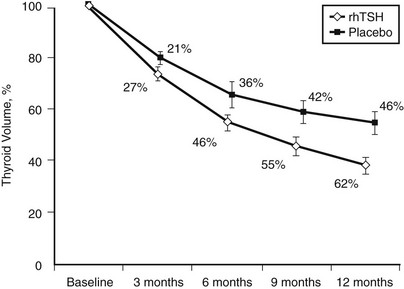
FIGURE 16-5 Effect of 131I therapy on nontoxic multinodular goiter comparing patients randomized to pretreatment with 0.3 mg recombinant human thyrotropin or placebo 24 hours before therapy is given. The y-axis represents the mean change (%) in thyroid volume. The between-group difference at 12 months was highly significant (P = .005). (Data from Nielsen et al., Arch Intern Med 2006;166:1476–1482.)
As an alternative to aiming at increased thyroid irradiation and goiter shrinkage, rhTSH allows a reduction in 131I activity while still retaining a mean goiter reduction of approximately 40% within the first 12 months.154 With this approach, extrathyroidal irradiation is diminished,155 which may render 131I therapy more attractive in young patients.
rhTSH per se, in doses from 0.3 mg to 0.9 mg, results in a transient 25% to 35% enlargement of the thyroid gland156,157; this may be potentially dangerous in patients with goiter. However, rhTSH-augmented 131I therapy is generally well tolerated, particularly when a dose of 0.1 mg or lower is used. With a higher rhTSH dose, the patient may experience cervical pain and a temporary rise in thyroid hormones within the first week after 131I therapy.149,151,152,158,159 In parallel with improved goiter reduction, a higher frequency of hypothyroidism is encountered after rhTSH-augmented 131I therapy is provided.151,152,159 When safety aspects have been further evaluated, rhTSH-augmented 131I therapy may well become a routine option in many patients with symptomatic multinodular goiter who decline surgery.
Percutaneous Interventional Therapy
Percutaneous ethanol injection therapy (PEIT) has been used for longer than a decade in solitary hot, toxic, and even cold thyroid nodules.87,108 The most convincing effect is seen in solitary thyroid cysts.108 Theoretically, PEIT can be used in multinodular goiter. Drawbacks are related to pain, risk for recurrent laryngeal nerve damage, and the possibility of extrathyroidal fibrosis complicating subsequent surgery. Interstitial laser photocoagulation was introduced recently and seems to have the same effect as PEIT with possibly fewer side effects.107 No controlled studies in multinodular goiter have examined either technique.
References
1. Hedinger, C, Williams, ED, Sobin, LH. The WHO histological classification of thyroid tumors: a commentary on the second edition. Cancer. 1989;63:908–911.
2. Belfiore, A, La Rosa, GL, La Porta, GA, et al. Cancer risk in patients with cold thyroid nodules: relevance of iodine intake, sex, age, and multinodularity. Am J Med. 1992;93:363–369.
3. Knudsen, N, Perrild, H, Christiansen, E, et al. Thyroid structure and size and two-year follow-up of solitary cold thyroid nodules in an unselected population with borderline iodine deficiency. Eur J Endocrinol. 2000;142:224–230.
4. Chan, JKC, Hirokawa, M, Evans, H, et al. Follicular adenoma. In: DeLellis RA, Lloyd RV, Heitz PU, et al, eds. WHO Classification of Tumours. Pathology and Genetics of Tumours of Endocrine Organs. Lyon: IARC Press; 2004:98–103.
5. Berghout, A, Wiersinga, WM, Smits, NJ, et al. Interrelationships between age, thyroid volume, thyroid nodularity, and thyroid function in patients with sporadic nontoxic goiter. Am J Med. 1990;89:602–608.
6. Knudsen, N, Laurberg, P, Perrild, H, et al. Risk factors for goiter and thyroid nodules. Thyroid. 2002;12:879–888.
7. Pisarikova, B, Herzig, I, Riha, J. [Inorganic anions with a potential goitrogenic effect in drinking water supply for humans and animals]. Vet Med (Praha). 1996;41:33–39.
8. Scanelli, G. [Lithium thyrotoxicosis, Report of a case and review of the literature]. Recenti Prog Med. 2002;93:100–103.
9. Brix, TH, Hansen, PS, Kyvik, KO, et al. Cigarette smoking and risk of clinically overt thyroid disease: a population-based twin case-control study. Arch Intern Med. 2000;160:661–666.
10. Brix, TH, Kyvik, KO, Hegedüs, L. Major role of genes in the etiology of simple goiter in females: a population-based twin study. J Clin Endocrinol Metab. 1999;84:3071–3075.
11. Knudsen, N, Bulow, I, Laurberg, P, et al. Parity is associated with increased thyroid volume solely among smokers in an area with moderate to mild iodine deficiency. Eur J Endocrinol. 2002;146:39–43.
12. Krohn, K, Fuhrer, D, Bayer, Y, et al. Molecular pathogenesis of euthyroid and toxic multinodular goiter. Endocr Rev. 2005;26:504–524.
13. Hansen, PS, Brix, TH, Bennedbæk, FN, et al. Genetic and environmental causes of individual differences in thyroid size: a study of healthy Danish twins. J Clin Endocrinol Metab. 2004;89:2071–2077.
14. Bayer, Y, Neumann, S, Meyer, B, et al. Genome-wide linkage analysis reveals evidence for four new susceptibility loci for familial euthyroid goiter. J Clin Endocrinol Metab. 2004;89:4044–4052.
15. Bignell, GR, Canzian, F, Shayeghi, M, et al. Familial nontoxic multinodular thyroid goiter locus maps to chromosome 14q but does not account for familial nonmedullary thyroid cancer. Am J Hum Genet. 1997;61:1123–1130.
16. Neumann, S, Bayer, Y, Reske, A, et al. Further indications for genetic heterogeneity of euthyroid familial goiter. J Mol Med. 2003;81:736–745.
17. Brix, TH, Hegedüs, L. Genetic and environmental factors in the aetiology of simple goitre. Ann Med. 2000;32:153–156.
18. Geerdsen, JP, Hee, P. Nontoxic goitre. I. Surgical complications and longterm prognosis. Acta Chir Scand. 1982;148:221–224.
19. Piraneo, S, Vitri, P, Galimberti, A, et al. Ultrasonographic surveillance after surgery for euthyroid goitre in patients treated or not with thyroxine. Eur J Surg. 1997;163:21–26.
20. Corral, J, Martin, C, Perez, R, et al. Thyroglobulin gene point mutation associated with non-endemic simple goitre. Lancet. 1993;341:462–464.
21. Gonzalez-Sarmiento, R, Corral, J, Mories, MT, et al. Monoallelic deletion in the 5′ region of the thyroglobulin gene as a cause of sporadic nonendemic simple goiter. Thyroid. 2001;11:789–793.
22. Everett, LA, Glaser, B, Beck, JC, et al. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat Genet. 1997;17:411–422.
23. Masmoudi, S, Charfedine, I, Hmani, M, et al. Pendred syndrome: phenotypic variability in two families carrying the same PDS missense mutation. Am J Med Genet. 2000;90:38–44.
24. Fujiwara, H, Tatsumi, K, Miki, K, et al. Recurrent T354P mutation of the Na+/I− symporter in patients with iodide transport defect. J Clin Endocrinol Metab. 1998;83:2940–2943.
25. Matsuda, A, Kosugi, S. A homozygous missense mutation of the sodium /iodide symporter gene causing iodide transport defect. J Clin Endocrinol Metab. 1997;82:3966–3971.
26. Neumann, S, Willgerodt, H, Ackermann, F, et al. Linkage of familial euthyroid goiter to the multinodular goiter-1 locus and exclusion of the candidate genes thyroglobulin, thyroperoxidase, and Na+/I− symporter. J Clin Endocrinol Metab. 1999;84:3750–3756.
27. Capon, F, Tacconelli, A, Giardina, E, et al. Mapping a dominant form of multinodular goiter to chromosome Xp22. Am J Hum Genet. 2000;67:1004–1007.
28. Many, MC, Denef, JF, Hamudi, S, et al. Effects of iodide and thyroxine on iodine-deficient mouse thyroid: a morphological and functional study. J Endocrinol. 1986;110:203–210.
29. Raspe, E, Dumont, JE. Tonic modulation of dog thyrocyte H2O2 generation and I− uptake by thyrotropin through the cyclic adenosine 3′,5′-monophosphate cascade. Endocrinology. 1995;136:965–973.
30. Krohn, K, Wohlgemuth, S, Gerber, H, et al. Hot microscopic areas of iodine deficient euthyroid goiters contain constitutively activating TSH receptor mutations. J Pathology. 2000;192:37–42.
31. Abs, R, Stevenaert, A, Beckers, A. Autonomously functioning thyroid nodules in a patient with a thyrotropin-secreting pituitary adenoma: possible cause–effect relationship. Eur J Endocrinol. 1994;131:355–358.
32. Studer, H, Huber, G, Derwahl, M, et al. [The transformation of Basedow’s struma into nodular goiter: a reason for recurrence of hyperthyroidism]. Schweiz Med Wochenschr. 1989;119:203–208.
33. Cheung, NW, Boyages, SC. The thyroid gland in acromegaly: an ultrasonographic study. Clin Endocrinol (Oxf). 1997;46:545–549.
34. Dumont, JE, Ermans, AM, Maenhaut, C, et al. Large goitre as a maladaptation to iodine deficiency. Clin Endocrinol (Oxf ). 1995;43:1–10.
35. Maier, J, van Steeg, H, van Oostrom, C, et al. Iodine deficiency activates antioxidant genes and causes DNA damage in the thyroid gland of rats and mice. Biochim Biophys Acta. 2007;1773:990–999.
36. Gerard, AC, Poncin, S, Caetano, B, et al. Iodine deficiency induces a thyroid stimulating hormone-independent early phase of microvascular reshaping in the thyroid. Am J Pathol. 2008;172:748–760.
37. Howie, AF, Arthur, JR, Nicol, F, et al. Identification of a 57-kilodalton selenoprotein in human thyrocytes as thioredoxin reductase and evidence that its expression is regulated through the calcium-phosphoinositol signaling pathway. J Clin Endocrinol Metab. 1998;83:2052–2058.
38. Howie, AF, Walker, SW, Akesson, B, et al. Thyroidal extracellular glutathione peroxidase: a potential regulator of thyroid-hormone synthesis. Biochem J. 1995;308:713–717.
39. Demelash, A, Karlsson, JO, Nilsson, M, et al. Selenium has a protective role in caspase-3-dependent apoptosis induced by H2O2 in primary cultured pig thyrocytes. Eur J Endocrinol. 2004;150:841–849.
40. Krohn, K, Maier, J, Paschke, R. Mechanisms of disease: hydrogen peroxide, DNA damage and mutagenesis in the development of thyroid tumors. Nat Clin Pract Endocrinol Metab. 2007;3:713–720.
41. Maier, J, van Steeg, H, van Oostrom, C, et al. Deoxyribonucleic acid damage and spontaneous mutagenesis in the thyroid gland of rats and mice. Endocrinology. 2006;147:3391–3397.
42. van Steeg, H, Mullenders, LH, Vijg, J. Mutagenesis and carcinogenesis in nucleotide excision repair-deficient XPA knock out mice. Mutat Res. 2000;450:167–180.
43. Cardoso, LC, Martins, DC, Figueiredo, MD, et al. Ca(2+)/nicotinamide adenine dinucleotide phosphate-dependent H(2)O(2) generation is inhibited by iodide in human thyroids. J Clin Endocrinol Metab. 2001;86:4339–4343.
44. Cooke, MS, Evans, MD, Dizdaroglu, M, et al. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB Journal. 2003;17:1195–1214.
45. Poncin, S, Gerard, AC, Boucquey, M, et al. Oxidative stress in the thyroid gland: from harmlessness to hazard depending on the iodine content. Endocrinology. 2008;149:424–433.
46. Parma, J, Duprez, L, Van Sande, J, et al. Somatic mutations in the thyrotropin receptor gene cause hyperfunctioning thyroid adenomas. Nature. 1993;365:649–651.
47. Führer, D, Holzapfel, HP, Wonerow, P, et al. Somatic mutations in the thyrotropin receptor gene and not in the Gs alpha protein gene in 31 toxic thyroid nodules. J Clin Endocrinol Metab. 1997;82:3885–3891.
48. Georgopoulos, NA, Sykiotis, GP, Sgourou, A, et al. Autonomously functioning thyroid nodules in a former iodine-deficient area commonly harbor gain-of-function mutations in the thyrotropin signaling pathway. Eur J Endocrinol. 2003;149:287–292.
49. Gozu, HI, Bircan, R, Krohn, K, et al. Similar prevalence of somatic TSH receptor and Gs alpha mutations in toxic thyroid nodules in geographical regions with different iodine supply in Turkey. Eur J Endocrinol. 2006;155:535–545.
50. Vassart, G. Activating mutations of the TSH receptor. Thyroid. 2004;14:86–87.
51. Krohn, K, Paschke, R. Progress in understanding the etiology of thyroid autonomy. J Clin Endocrinol Metab. 2001;86:3336–3345.
52. Garcia-Delgado, M, Gonzalez-Navarro, CJ, Napal, MC, et al. Higher sensitivity of denaturing gradient gel electrophoresis than sequencing in the detection of mutations in DNA from tumor samples. Biotechniques. 1998;24:72.
53. Trulzsch, B, Krohn, K, Wonerow, P, et al. DGGE is more sensitive for the detection of somatic point mutations than direct sequencing. Biotechniques. 1999;27:266–268.
54. Trülzsch, B, Krohn, K, Wonerow, P, et al. Detection of thyroid-stimulating hormone receptor and Gs alpha mutations in 75 toxic thyroid nodules by denaturing gradient gel electrophoresis. J Mol Med. 2001;78:684–691.
55. Van Sande, J, Parma, J, Tonacchera, M, et al. Somatic and germline mutations of the TSH receptor gene in thyroid diseases. J Clin Endocrinol Metab. 1995;80:2577–2585.
56. Dugrillon, A, Bechtner, G, Uedelhoven, WM, et al. Evidence that an iodolactone mediates the inhibitory effect of iodide on thyroid cell proliferation but not on adenosine 3′,5′-monophosphate formation. Endocrinology. 1990;127:337–343.
57. Roger, PP, Servais, P, Dumont, JE. Stimulation by thyrotropin and cyclic AMP of the proliferation of quiescent canine thyroid cells cultured in a defined medium containing insulin. FEBS Lett. 1983;157:323–329.
58. Eggo, MC, Bachrach, LK, Burrow, GN. Interaction of TSH, insulin and insulin-like growth factors in regulating thyroid growth and function. Growth Factors. 1990;2:99–109.
59. Eszlinger, M, Krohn, K, Frenzel, R, et al. Gene expression analysis reveals evidence for inactivation of the TGF-beta signaling cascade in autonomously functioning thyroid nodules. Oncogene. 2004;23:795–804.
60. Gärtner, R, Schopohl, D, Schaefer, S, et al. Regulation of transforming growth factor beta 1 messenger ribonucleic acid expression in porcine thyroid follicles in vitro by growth factors, iodine, or delta-iodolactone. Thyroid. 1997;7:633–640.
61. Taton, M, Lamy, F, Roger, PP, et al. General inhibition by transforming growth factor beta 1 of thyrotropin and cAMP responses in human thyroid cells in primary culture. Mol Cell Endocrinol. 1993;95:13–21.
62. Depoortere, F, Pirson, I, Bartek, J, et al. Transforming growth factor beta(1) selectively inhibits the cyclic AMP-dependent proliferation of primary thyroid epithelial cells by preventing the association of cyclin D3-cdk4 with nuclear p27(kip1). Mol Biol Cell. 2000;11:1061–1076.
63. Grubeck-Loebenstein, B, Buchan, G, Sadeghi, R, et al. Transforming growth factor beta regulates thyroid growth. Role in the pathogenesis of nontoxic goiter. J Clin Invest. 1989;83:764–770.
64. Krohn, K, Emmrich, P, Ott, N, et al. Increased thyroid epithelial cell proliferation in toxic thyroid nodules. Thyroid. 1999;9:241–246.
65. Derwahl, M, Studer, H. Nodular goiter and goiter nodules: Where iodine deficiency falls short of explaining the facts. Exp Clin Endocrinol Diabetes. 2001;109:250–260.
66. Studer, H, Peter, HJ, Gerber, H. Natural heterogeneity of thyroid cells: the basis for understanding thyroid function and nodular goiter growth. Endocr Rev. 1989;10:125–135.
67. Krohn, K, Reske, A, Ackermann, F, et al. Ras mutations are rare in solitary cold and toxic thyroid nodules. Clin Endocrinol. 2001;55:241–248.
68. Dohan, O, Baloch, Z, Banrevi, Z, et al. Rapid communication: predominant intracellular overexpression of the Na(+)/I(−) symporter (NIS) in a large sampling of thyroid cancer cases. J Clin Endocrinol Metab. 2001;86:2697–2700.
69. Dohan, O, De la Vieja, A, Paroder, V, et al. The sodium/iodide Symporter (NIS): characterization, regulation, and medical significance. Endocr Rev. 2003;24:48–77.
70. Tonacchera, M, Viacava, P, Agretti, P, et al. Benign nonfunctioning thyroid adenomas are characterized by a defective targeting to cell membrane or a reduced expression of the sodium iodide symporter protein. J Clin Endocrinol Metab. 2002;87:352–357.
71. Dunn, JT, Dunn, AD. Update on intrathyroidal iodine metabolism. Thyroid. 2001;11:407–414.
72. Lazar, V, Bidart, JM, Caillou, B, et al. Expression of the Na+/I− symporter gene in human thyroid tumors: a comparison study with other thyroid-specific genes. J Clin Endocrinol Metab. 1999;84:3228–3234.
73. Caillou, B, Dupuy, C, Lacroix, L, et al. Expression of reduced nicotinamide adenine dinucleotide phosphate oxidase (ThoX, LNOX, Duox) genes and proteins in human thyroid tissues. J Clin Endocrinol Metab. 2001;86:3351–3358.
74. De Vijlder, JJ. Primary congenital hypothyroidism: defects in iodine pathways. Eur J Endocrinol. 2003;149:247–256.
75. Pisarev, MA, Krawiec, L, Juvenal, GJ, et al. Studies on the goiter inhibiting action of iodolactones. Eur J Pharmacol. 1994;258:33–37.
76. Krohn, K, Paschke, R. Loss of heterozygosity at the thyroid peroxidase gene locus in solitary cold thyroid nodules. Thyroid. 2001;11:741–747.
77. Krohn, K, Paschke, R. BRAF mutations are not an alternative explanation for the molecular etiology of ras mutation negative cold thyroid nodules. Thyroid. 2004;14:359–361.
78. Xing, M, Vasko, V, Tallini, G, et al. BRAF T1796A transversion mutation in various thyroid neoplasms. J Clin Endocrinol Metab. 2004;89:1365–1368.
79. Puxeddu, E, Moretti, S, Elisei, R, et al. BRAF(V599E) mutation is the leading genetic event in adult sporadic papillary thyroid carcinomas. J Clin Endocrinol Metab. 2004;89:2414–2420.
80. Soares, P, Trovisco, V, Rocha, AS, et al. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene. 2003;22:4578–4580.
81. Eszlinger, M, Krohn, K, Berger, K, et al. Gene expression analysis reveals evidence for increased expression of cell cycle-associated genes and Gq-protein-protein kinase C signaling in cold thyroid nodules. J Clin Endocrinol Metab. 2005;90:1163–1170.
82. Krohn, K, Stricker, I, Emmrich, P, et al. Cold thyroid nodules show a marked increase of proliferation markers. Thyroid. 2003;13:569–576.
83. Bol, S, Belge, G, Thode, B, et al. Structural abnormalities of chromosome 2 in benign thyroid tumors. Three new cases and review of the literature. Cancer Genet Cytogenet. 1999;114:75–77.
84. Rippe, V, Drieschner, N, Meiboom, M, et al. Identification of a gene rearranged by 2p21 aberrations in thyroid adenomas. Oncogene. 2003;22:6111–6114.
85. Ward, LS, Brenta, G, Medvedovic, M, et al. Studies of allelic loss in thyroid tumors reveal major differences in chromosomal instability between papillary and follicular carcinomas. J Clin Endocrinol Metab. 1998;83:525–530.
86. Tan, GH, Gharib, H, Reading, CC. Solitary thyroid nodule. Comparison between palpation and ultrasonography. Arch Intern Med. 1995;155:2418–2423.
87. Hegedüs, L, Bonnema, SJ, Bennedbæk, FN. Management of simple nodular goiter: current status and future perspectives. Endocr Rev. 2003;24:102–132.
88. Vanderpump, MP, Tunbridge, WM, French, JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf). 1995;43:55–68.
89. Laurberg, P, Pedersen, KM, Vestergaard, H, et al. High incidence of multinodular toxic goitre in the elderly population in a low iodine intake area vs. high incidence of Graves’ disease in the young in a high iodine intake area: comparative surveys of thyrotoxicosis epidemiology in East-Jutland Denmark and Iceland. J Intern Med. 1991;229:415–420.
90. Berghout, A, Wiersinga, WM, Drexhage, HA, et al. Comparison of placebo with L-thyroxine alone or with carbimazole for treatment of sporadic non-toxic goitre. Lancet. 1990;336:193–197.
91. Hegedüs, L. Thyroid Ultrasound. Endocrinol Metab Clin North Am. 2001;30:339–360.
92. Tollin, SR, Mery, GM, Jelveh, N, et al. The use of fine-needle aspiration biopsy under ultrasound guidance to assess the risk of malignancy in patients with a multinodular goiter. Thyroid. 2000;10:235–241.
93. Aghini-Lombardi, F, Antonangeli, L, Martino, E, et al. The spectrum of thyroid disorders in an iodine-deficient community: the Pescopagano survey. J Clin Endocrinol Metab. 1999;84:561–566.
94. Jarløv, AE, Nygaard, B, Hegedüs, L, et al. Observer variation in the clinical and laboratory evaluation of patients with thyroid dysfunction and goiter. Thyroid. 1998;8:393–398.
95. Surks, MI, Ortiz, E, Daniels, GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228–238.
96. Elisei, R, Bottici, V, Luchetti, F, et al. Impact of routine measurement of serum calcitonin on the diagnosis and outcome of medullary thyroid cancer: experience in 10,864 patients with nodular thyroid disorders. J Clin Endocrinol Metab. 2004;89:163–168.
97. Bennedbæk, FN, Perrild, H, Hegedüs, L. Diagnosis and treatment of the solitary thyroid nodule. Results of a European survey. Clin Endocrinol (Oxf). 1999;50:357–363.
98. Bennedbæk, FN, Hegedüs, L. Management of the solitary thyroid nodule: results of a North American survey. J Clin Endocrinol Metab. 2000;85:2493–2498.
99. Bonnema, SJ, Bennedbæk, FN, Wiersinga, WM, et al. Management of the nontoxic multinodular goitre: a European questionnaire study. Clin Endocrinol (Oxf). 2000;53:5–12.
100. Bonnema, SJ, Bennedbæk, FN, Ladenson, PW, et al. Management of the nontoxic multinodular goiter: a North American survey. J Clin Endocrinol Metab. 2002;87:112–117.
101. Nygaard, B, Hegedüs, L, Ulriksen, P, et al. Radioiodine therapy for multinodular toxic goiter. Arch Intern Med. 1999;159:1364–1368.
102. Nygaard, B, Knudsen, JH, Hegedüs, L, et al. Thyrotropin receptor antibodies and Graves’ disease, a side-effect of 131I treatment in patients with nontoxic goiter. J Clin Endocrinol Metab. 1997;82:2926–2930.
103. Okumura, Y, Takeda, Y, Sato, S, et al. Comparison of differential diagnostic capabilities of 201Tl scintigraphy and fine-needle aspiration of thyroid nodules. J Nucl Med. 1999;40:1971–1977.
104. Demirel, K, Kapucu, O, Yucel, C, et al. A comparison of radionuclide thyroid angiography, (99m)Tc-MIBI scintigraphy and power Doppler ultrasonography in the differential diagnosis of solitary cold thyroid nodules. Eur J Nucl Med Mol Imaging. 2003;30:642–650.
105. Rago, T, Santini, F, Scutari, M, et al. Elastography: new developments in ultrasound for predicting malignancy in thyroid nodules. J Clin Endocrinol Metab. 2007;92:2917–2922.
106. Bennedbæk, FN, Karstrup, S, Hegedüs, L. Percutaneous ethanol injection therapy in the treatment of thyroid and parathyroid diseases. Eur J Endocrinol. 1997;136:240–250.
107. Døssing, H, Bennedbæk, FN, Hegedüs, L. Effect of ultrasound-guided interstitial laser photocoagulation on benign solitary solid cold thyroid nodules—a randomised study. Eur J Endocrinol. 2005;152:341–345.
108. Bennedbæk, FN, Hegedüs, L. Treatment of recurrent thyroid cysts with ethanol: a randomized double-blind controlled trial. J Clin Endocrinol Metab. 2003;88:5773–5777.
109. Sebastianes, FM, Cerci, JJ, Zanoni, PH, et al. Role of 18F-fluorodeoxyglucose positron emission tomography in preoperative assessment of cytologically indeterminate thyroid nodules. J Clin Endocrinol Metab. 2007;92:4485–4488.
110. Kang, KW, Kim, SK, Kang, HS, et al. Prevalence and risk of cancer of focal thyroid incidentaloma identified by 18F-fluorodeoxyglucose positron emission tomography for metastasis evaluation and cancer screening in healthy subjects. J Clin Endocrinol Metab. 2003;88:4100–4104.
111. Baloch, Z, Livolsi, VA, Jain, P, et al. Role of repeat fine-needle aspiration biopsy (FNAB) in the management of thyroid nodules. Diagn Cytopathol. 2003;29:203–206.
112. Haymart, MR, Repplinger, DJ, Leverson, GE, et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab. 2008;93:809–814.
113. Tan, GH, Gharib, H. Thyroid incidentalomas: management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann Intern Med. 1997;126:226–231.
114. Papini, E, Guglielmi, R, Bianchini, A, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87:1941–1946.
115. Bhagat, MC, Dhaliwal, SS, Bonnema, SJ, et al. Differences between endocrine surgeons and endocrinologists in the management of non-toxic multinodular goitre. Br J Surg. 2003;90:1103–1112.
116. Watt, T, Grønvold, M, Rasmussen, AK, et al. Quality of life in patients with benign thyroid disorders. A review. Eur J Endocrinol. 2006;154:501–510.
117. Weetman, AP. Radioiodine treatment for benign thyroid diseases. Clin Endocrinol (Oxf). 2007;66:757–764.
118. Walter, MA, Briel, M, Christ-Crain, M, et al. Effects of antithyroid drugs on radioiodine treatment: systematic review and meta-analysis of randomised controlled trials. BMJ. 2007;334:514.
119. Bonnema, SJ, Bennedbæk, FN, Gram, J, et al. Resumption of methimazole after 131I therapy of hyperthyroid diseases: effect on thyroid function and volume evaluated by a randomized clinical trial. Eur J Endocrinol. 2003;149:485–492.
120. Kahaly, G, Dienes, HP, Beyer, J, et al. Randomized, double blind, placebo-controlled trial of low dose iodide in endemic goiter. J Clin Endocrinol Metab. 1997;82:4049–4053.
121. Perrild, H, Hansen, JM, Hegedüs, L, et al. Triiodothyronine and thyroxine treatment of diffuse non-toxic goitre evaluated by ultrasonic scanning. Acta Endocrinol (Copenh). 1982;100:382–387.
122. Wesche, MF, Tiel-Van Buul, MM, Lips, P, et al. A randomized trial comparing levothyroxine with radioactive iodine in the treatment of sporadic nontoxic goiter. J Clin Endocrinol Metab. 2001;86:998–1005.
123. Fast, S, Bonnema, SJ, Hegedüs, L. The majority of Danish non-toxic goitre patients are ineligible for Levothyroxine suppressive therapy. Clin Endocrinol (Oxf). 2008;69:653–658.
124. Hegedüs, L. The thyroid nodule. N Engl J Med. 2004;351:1764–1771.
125. Cooper, DS, Doherty, GM, Haugen, BR, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109–142.
126. Ito, Y, Uruno, T, Nakano, K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid. 2003;13:381–387.
127. Hegedüs, L, Nygaard, B, Hansen, JM. Is routine thyroxine treatment to hinder postoperative recurrence of nontoxic goiter justified? J Clin Endocrinol Metab. 1999;84:756–760.
128. Pradeep, PV, Tiwari, P, Mishra, A, et al. Pulmonary function profile in patients with benign goiters without symptoms of respiratory compromise and the early effect of thyroidectomy. J Postgrad Med. 2008;54:98–101.
129. Erickson, D, Gharib, H, Li, H, et al. Treatment of patients with toxic multinodular goiter. Thyroid. 1998;8:277–282.
130. al Suliman, NN, Ryttov, NF, Qvist, N, et al. Experience in a specialist thyroid surgery unit: a demographic study, surgical complications, and outcome. Eur J Surg. 1997;163:13–20.
131. Thomusch, O, Machens, A, Sekulla, C, et al. Multivariate analysis of risk factors for postoperative complications in benign goiter surgery: prospective multicenter study in Germany. World J Surg. 2000;24:1335–1341.
132. Morrissey, AT, Chau, J, Yunker, WK, et al. Comparison of drain versus no drain thyroidectomy: randomized prospective clinical trial. J Otolaryngol. 2008;37:43–47.
133. Youssef, T, Mahdy, T, Farid, M, et al. Thyroid surgery: Use of the LigaSure Vessel Sealing System versus conventional knot tying. Int J Surg. 2008 May 23.
134. Torre, G, Borgonovo, G, Amato, A, et al. Surgical management of substernal goiter: analysis of 237 patients. Am Surg. 1995;61:826–831.
135. Feldkamp, J, Seppel, T, Becker, A, et al. Iodide or L-thyroxine to prevent recurrent goiter in an iodine-deficient area: prospective sonographic study. World J Surg. 1997;21:10–14.
136. Pappalardo, G, Guadalaxara, A, Frattaroli, FM, et al. Total compared with subtotal thyroidectomy in benign nodular disease: personal series and review of published reports. Eur J Surg. 1998;164:501–506.
137. Porterfield, JR, Jr., Thompson, GB, Farley, DR, et al. Evidence-based management of toxic multinodular goiter (Plummer’s disease). World J Surg. 2008;32:1278–1284.
138. Nygaard, B, Hegedüs, L, Gervil, M, et al. Radioiodine treatment of multinodular non-toxic goitre. Br Med J. 1993;307:828–832.
139. Bonnema, SJ, Bertelsen, H, Mortensen, J, et al. The feasibility of high dose iodine 131 treatment as an alternative to surgery in patients with a very large goiter: effect on thyroid function and size and pulmonary function. J Clin Endocrinol Metab. 1999;84:3636–3641.
140. Huysmans, DA, Hermus, AR, Corstens, FH, et al. Large, compressive goiters treated with radioiodine. Ann Intern Med. 1994;121:757–762.
141. Bonnema, SJ, Nielsen, VE, Hegedüs, L. Long-term effects of radioiodine on thyroid function, size and patient satisfaction in non-toxic diffuse goitre. Eur J Endocrinol. 2004;150:439–445.
142. Jarløv, AE, Hegedüs, L, Kristensen, LØ, et al. Is calculation of the dose in radioiodine therapy of hyperthyroidism worth while? Clin Endocrinol (Oxf). 1995;43:325–329.
143. Nygaard, B, Faber, J, Hegedüs, L. Acute changes in thyroid volume and function following 131I therapy of multinodular goitre. Clin Endocrinol (Oxf). 1994;41:715–718.
144. Ron, E, Doody, MM, Becker, DV, et al. Cancer mortality following treatment for adult hyperthyroidism. Cooperative Thyrotoxicosis Therapy Follow-up Study Group. JAMA. 1998;280:347–355.
145. Franklyn, JA, Maisonneuve, P, Sheppard, M, et al. Cancer incidence and mortality after radioiodine treatment for hyperthyroidism: a population-based cohort study. Lancet. 1999;353:2111–2115.
146. Metso, S, Auvinen, A, Huhtala, H, et al. Increased cancer incidence after radioiodine treatment for hyperthyroidism. Cancer. 2007;109:1972–1979.
147. Huysmans, DA, Nieuwlaat, WA, Erdtsieck, RJ, et al. Administration of a single low dose of recombinant human thyrotropin significantly enhances thyroid radioiodide uptake in nontoxic nodular goiter. J Clin Endocrinol Metab. 2000;85:3592–3596.
148. Nielsen, VE, Bonnema, SJ, Boel-Jørgensen, H, et al. Recombinant human thyrotropin markedly changes the 131I kinetics during 131I therapy of patients with nodular goiter: an evaluation by a randomized double-blinded trial. J Clin Endocrinol Metab. 2005;90:79–83.
149. Albino, CC, Mesa, CO, Jr., Olandoski, M, et al. Recombinant human thyrotropin as adjuvant in the treatment of multinodular goiters with radioiodine. J Clin Endocrinol Metab. 2005;90:2775–2780.
150. Nieuwlaat, WA, Hermus, AR, Sivro-Prndelj, F, et al. Pretreatment with recombinant human TSH changes the regional distribution of radioiodine on thyroid scintigrams of nodular goiters. J Clin Endocrinol Metab. 2001;86:5330–5336.
151. Bonnema, SJ, Nielsen, VE, Boel-Jørgensen, H, et al. Improvement of goiter volume reduction after 0.3 mg recombinant human thyrotropin-stimulated radioiodine therapy in patients with a very large goiter: a double-blinded, randomized trial. J Clin Endocrinol Metab. 2007;92:3424–3428.
152. Nielsen, VE, Bonnema, SJ, Boel-Jørgensen, H, et al. Stimulation with 0.3-mg recombinant human thyrotropin prior to iodine 131 therapy to improve the size reduction of benign nontoxic nodular goiter: a prospective randomized double-blind trial. Arch Intern Med. 2006;166:1476–1482.
153. Bonnema, SJ, Nielsen, VE, Boel-Jørgensen, H, et al. Recombinant human thyrotropin stimulated radioiodine therapy of large nodular goiters facilitates tracheal decompression and improves inspiration. J Clin Endocrinol Metab. 2008;93:3981–3984.
154. Nieuwlaat, WA, Huysmans, DA, Van Den Bosch, HC, et al. Pretreatment with a single, low dose of recombinant human thyrotropin allows dose reduction of radioiodine therapy in patients with nodular goiter. J Clin Endocrinol Metab. 2003;88:3121–3129.
155. Nieuwlaat, WA, Hermus, AR, Ross, HA, et al. Dosimetry of radioiodine therapy in patients with nodular goiter after pretreatment with a single, low dose of recombinant human thyroid-stimulating hormone. J Nucl Med. 2004;45:626–633.
156. Nielsen, VE, Bonnema, SJ, Hegedüs, L. Transient goiter enlargement after administration of 0.3 mg of recombinant human thyrotropin in patients with benign nontoxic nodular goiter: a randomized, double-blind, crossover trial. J Clin Endocrinol Metab. 2006;91:1317–1322.
157. Nielsen, VE, Bonnema, SJ, Hegedüs, L. Effects of 0.9 mg recombinant human thyrotropin on thyroid size and function in normal subjects: a randomized, double-blind, cross-over trial. J Clin Endocrinol Metab. 2004;89:2242–2247.
158. Cohen, O, Ilany, J, Hoffman, C, et al. Low-dose recombinant human thyrotropin-aided radioiodine treatment of large, multinodular goiters in elderly patients. Eur J Endocrinol. 2006;154:243–252.
159. Silva, MN, Rubio, IG, Romao, R, et al. Administration of a single dose of recombinant human thyrotrophin enhances the efficacy of radioiodine treatment of large compressive multinodular goitres. Clin Endocrinol (Oxf). 2004;60:300–308.