Chapter 50 Multimodal Treatment of Orbital Tumors
Orbital Anatomy
Orbital Bony Anatomy
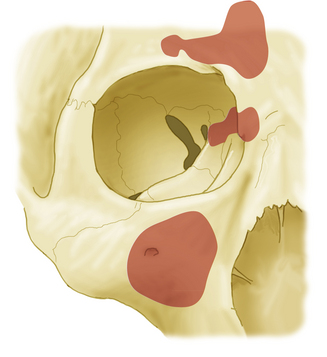
The bony orbit not only lies close to other compartments in the facial skeleton but also contains several foramina through which critical neurovascular structures pass1 (Fig. 50-1). The orbital roof is formed by the frontal bone and lesser wing of sphenoid; its superior surface is the floor of the anterior cranial fossa. The roof also lies below the frontal sinus. The orbital plate of the maxillary bone forms most of the orbital floor, in addition to the roof of the maxillary sinus; the palatine and zygomatic bones also contribute to the floor. Medially, the medial wall, also known as the lamina papyracea, forms a thin wall separating the orbit from the ethmoid sinus anteriorly and the sphenoid sinus more posteriorly, a structure through which most endoscopic approaches to the orbit can be directed. It is comprised of four bones: the maxillary, lacrimal, ethmoid, and lesser wing of sphenoid. The medial wall of the orbit also contains the foramina through which the anterior and posterior ethmoidal arteries pass. The lateral orbital wall is covered externally by the temporalis muscle and formed by the frontosphenoid process of the zygomatic bone, in addition to the greater wing of the sphenoid. The apex of the orbit is directed in a medial oblique direction and contains three critical foramina: optic canal, superior orbital fissure, and inferior orbital fissure. The optic canal bridges the intracranial space and orbit inferomedial to the anterior clinoid process while it is bordered laterally by the lesser wing of the sphenoid. The superior orbital fissure is bordered laterally by the greater wing of the sphenoid.
Muscle Cone and Annulus of Zinn
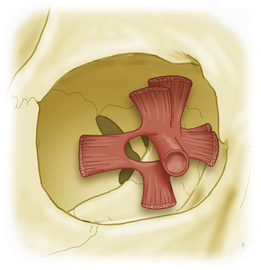
The annulus of Zinn serves as the origin of six of the seven extraocular muscles (Fig. 50-2). Superiorly, the superior rectus arises from the annulus, which at this point is fused with the dura of the optic nerve. The levator palpebrae arises medial and superior to the superior rectus muscle but remains intimately associated with it. More medial and inferior to this are the origins of the medial rectus and superior oblique muscles. Although it is firmly fused to the optic nerve dorsally, the annulus of Zinn loops widely around the nerve laterally and inferiorly, giving rise to the lateral rectus muscle, in addition to the inferior rectus. The space between the insertion sites of these two muscles is known as the oculomotor foramen. Based on this arrangement, there are evident portals of entry of neurovascular structures into the orbit: the optic canal and the superior orbital fissure.
Optic Nerve and Orbital Nerves
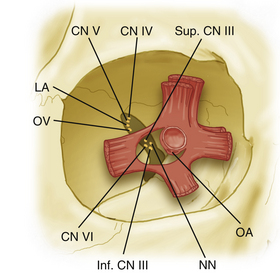
The optic nerve, throughout its entire course from the chiasm to sclera, is covered with a pial membrane (providing vascular supply) and associated subarachnoid space (Fig. 50-3). As the optic nerve enters the optic canal, the intracranial dura splits, with the outer leaf forming the orbital periosteum and the inner leaf remaining with the optic nerve. The superior orbital fissure contains the remaining cranial nerves that enter the orbital compartment. The trochlear nerve and the frontal and lacrimal branches of the trigeminal nerve enter through the superior orbital fissure above the extraocular muscles and annulus of Zinn. The remaining nerves—the oculomotor nerve (superior and inferior divisions) and the abducens nerve—pass through the superior orbital fissure and annulus of Zinn.
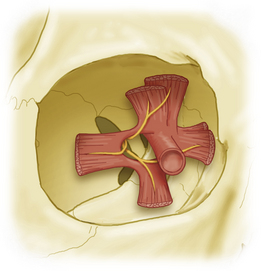
Because of this arrangement, it is evident that the optic nerve can be approached directly through the medial compartment, between the medial rectus and the levator muscles, without fear of injury to the nerve supply of any extraocular muscle (Fig. 50-4).
Case Selection
The treatment strategy for orbital lesions is primarily determined by the nature of the lesion. An overview of the types of orbital lesions is provided in Table 50-1.2 While medical management is best suited for infectious and inflammatory processes involving the orbit, definitive surgical treatment remains the mainstay for a majority of symptomatic orbital tumors and dysthyroid orbitopathy.
| Category | Lesions |
|---|---|
| Vascular | Capillary hemangioma |
| Cavernous hemangioma | |
| Hamartoma | |
| Hemangiopericytoma | |
| Lymphangioma | |
| Nerve sheath | Neurilemomas |
| Neurofibroma (plexiform and solitary) | |
| Osseous and cartilaginous | Osteoma |
| Osteogenic sarcoma | |
| Chondroma | |
| Fibrous dysplasia | |
| Aneurysmal bone cyst | |
| Neuroepithelial | Optic nerve glioma |
| Meningioma | |
| Mesenchymal | Rhabdomyosarcoma |
| Lipoma | |
| Liposarcoma | |
| Inflammatory | Nonvasculitic |
| Vasculitic, nongranulomatous | |
| Carcinoma | |
| Cystic | |
| Other |
The diagnostic workup proceeds logically after clinical examination and primarily consists of imaging. Magnetic resonance imaging (MRI) with fat suppression provides excellent definition of orbital pathology.3,4 Fat suppression eliminates the T1 bright signal associated with orbital fat that can obscure intraorbital pathology. Contrast-enhanced MRI provides excellent soft tissue resolution and is the best modality to determine intracranial extension of orbital pathology.5,6 Computed tomography (CT) imaging provides excellent definition of orbital pathologic process and regional bone anatomy. An adequate study includes thin cuts through the orbits and shows normal orbital anatomy, including the size and position of the globe, optic nerve, and extraocular muscles. CT is superior to MRI in surgical planning because of its ability to show bony anatomy, but MRI is preferred when optic nerve involvement by the tumor or disease process must be examined.
• Is it likely benign or malignant?
• Is it confined to the muscle cone?
• Does it arise from the optic nerve, or is it medial or lateral to the optic nerve?
• Is the bony integrity of the orbit violated?
• Is the optic canal or any of the foramina or fissures enlarged or hyperostotic?
• Does the process extend from or enter the cranial cavity?
• Does the process extent from or enter the paranasal sinuses?
Surgical Approaches
Anatomically, orbital tumors can be divided into intraconal, extraconal, and intracanalicular. This distinction is made on the basis of the tumor’s relationship with the muscle cone, with intracanalicular tumors at least partially extending into the optic canal. There are two primary types of surgical approaches to the orbit: transorbital approaches (performed primarily by ophthalmologists) and extraorbital approaches (often performed by a team that includes a neurosurgeon or head and neck surgeon, as well as an ophthalmologist).7,8 This chapter focuses on the intra- and extraorbital approaches not discussed in other sections of this book. Extraorbital approaches typically employed by neurosurgeons include extended bifrontal craniotomy, orbitozygomatic craniotomy, subcranial craniotomy, and unilateral maxillectomy. As a general paradigm, lesions based anteriorly can be approached via transorbital approaches while lesions in the posterior third of the orbit are often managed by extraorbital approaches (open or endoscopic). There are instances in which a combination of approaches may be necessary.
Approaches to the Anterior Orbit
For larger lesions located in the anterior superior orbit, a superior osteotomy may be necessary. For this modification, a longer incision is made in the eyebrow, with the surgeon taking care to visualize and preserve the supratrochlear and supraorbital neurovascular bundles. The superior orbital rim can be removed with a sagittal saw or osteotomes, after which the remainder of the dissection proceeds as described earlier. The use of fine osteotomes ultimately results in thinner bone cuts (with minimal resultant bone loss) and improved cosmetic outcomes.
Approaches to the Lateral Orbit
After incision and subcutaneous dissection, the periorbita is incised along the orbital rim in a T shape. With blunt dissection, the lateral periorbita is distracted posteriorly to the posterior one fourth of the orbit to expose the lateral wall of the orbit. The periosteum and temporalis muscle are similarly freed from the bone. To perform the lateral orbital wall osteotomy, initial angled cuts are made along the lateral aspects of the superior and inferior orbital rims. These cuts are directed toward one another to result in a keystone-shaped portion of bone that is removed from the lateral wall. After this initial osteotomy, further posterior visualization is made through removal of bone with a series of rongeurs and drills. Exposure can be extended posteriorly all the way to the orbital apex. Once bone removal has been completed, an incision is made in the periorbita—avoiding the lateral rectus muscle. After entry, the lateral rectus may be retracted superiorly or inferiorly, based on the location of the lesion.
Conclusions
CASE EXAMPLE
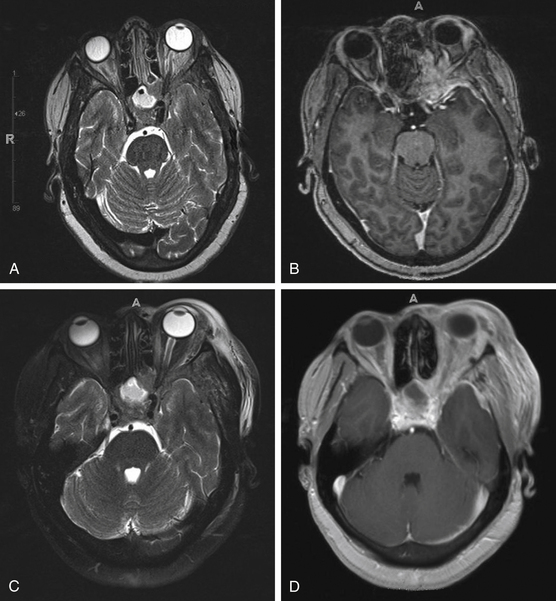
The patient is a 50-year-old female, status postmastectomy and postchemotherapy for breast cancer, who presented to our service with vision loss and motility abnormalities in her left eye. The patient was at neurologic baseline until 2 months prior to presentation, when she noted dull color perception in her left eye associated with blurring of vision. Several days after onset of her symptoms, the patient also experienced difficulty in abduction of her left eye, which prompted further workup by her oncologist. MRI (Fig. 50-5A and B) demonstrated an extra-axial enhancing mass along the floor of the anterior fossa extending into the left orbit and middle cranial fossa with encasement of the left optic nerve. On initial neuro-ophthalmologic evaluation, the patient’s exam was notable for the following: no light perception in the left eye, a left pupil that was amaurotic, and extraocular movements in the left 95% of abduction, 60% of normal elevation, 60% of normal depression, and 10% of abduction past midline. Furthermore, numbness in the left V2 distribution was noted. On orbital echography, enlargement of the left periophthalmic vein was noted.
The patient was taken to the operating room for tissue diagnosis and decompression. Via an eyebrow incision, a left-sided orbitozygomatic craniotomy was performed. Extradurally, the remaining diseased bone was drilled to decompress the superior orbital fissure and optic canal. Postoperatively, the patient was monitored in the neurocritical care unit and discharged home postoperative day 1. Postoperative MRI demonstrated adequate debulking (Fig. 50-5C and D); final pathology indicated metastatic poorly differentiated adenocarcinoma, consistent with the patient’s history of breast cancer.
Bejjani G.K., Cockerham K.P., Kennerdel J.S., Maroon J.C. A reappraisal of surgery for orbital tumors. Part I: extraorbital approaches. Neurosurg Focus. 2001;10:E2.
Cockerham K.P., Bejjani G.K., Kennerdell J.S., Maroon J.C. Surgery for orbital tumors. Part II: transorbital approaches. Neurosurg Focus. 2001;10:E3.
Darsaut T.E., Lanzino G., Lopes M.B., Newman S. An introductory overview of orbital tumors. Neurosurg Focus. 2001;10:E1.
Haik B.G., Saint Louis L., Bierly J., et al. Magnetic resonance imaging in the evaluation of optic nerve gliomas. Ophthalmology. 1987;94:709-717.
Housepian E.M. Microsurgical anatomy of the orbital apex and principles of transcranial orbital exploration. Clin Neurosurg. 1978;25:556-573.
Jakobiec F.A., Depot M.J., Kennerdell J.S., et al. Combined clinical and computed tomographic diagnosis of orbital glioma and meningioma. Ophthalmology. 1984;91:137-155.
Jakobiec F.A., Yeo J.H., Trokel S.L., et al. Combined clinical and computed tomographic diagnosis of primary lacrimal fossa lesions. Am J Ophthalmol. 1982;94:785-807.
1. Cockerham K.P., Bejjani G.K., Kennerdell J.S., Maroon J.C. Surgery for orbital tumors. Part II: transorbital approaches. Neurosurg Focus. 2001;10:E3.
2. Haik B.G., Saint Louis L., Bierly J., et al. Magnetic resonance imaging in the evaluation of optic nerve gliomas. Ophthalmology. 1987;94:709-717.
3. Housepian E.M. Microsurgical anatomy of the orbital apex and principles of transcranial orbital exploration. Clin Neurosurg. 1978;25:556-573.
4. Jakobiec F.A., Depot M.J., Kennerdell J.S., et al. Combined clinical and computed tomographic diagnosis of orbital glioma and meningioma. Ophthalmology. 1984;91:137-155.
5. Jakobiec F.A., Yeo J.H., Trokel S.L., et al. Combined clinical and computed tomographic diagnosis of primary lacrimal fossa lesions. Am J Ophthalmol. 1982;94:785-807.
6. Bejjani G.K., Cockerham K.P., Kennerdel J.S., Maroon J.C. A reappraisal of surgery for orbital tumors. Part I: extraorbital approaches. Neurosurg Focus. 2001;10:E2.
7. Rhoton A.L.Jr. The orbit. Neurosurgery. 2002;51:S303-S334.
8. Darsaut T.E., Lanzino G., Lopes M.B., Newman S. An introductory overview of orbital tumors. Neurosurg Focus. 2001;10:E1.