Chapter 111 Multilobar Resection and Hemispherectomy in Epilepsy Surgery∗
Multilobar Resection
The rationale of curative surgical procedures aiming at control of focal drug-resistant epilepsy is total inactivation (by resection or disconnection) of the epileptogenic zone (EZ), that is, the cortical region of onset and early spread of an ictal discharge.1 In this regard, the main efforts must be performed to correctly localize the EZ. Considering the dynamic features of an ictal discharge, and therefore its spatial–temporal structure,2 it is not surprising that in a number of patients the EZ may not be localized within the anatomic limits that mark the boundaries between the cerebral lobes.3 Moreover, the extension of an anatomic lesion that may correlate with seizures does not necessarily correspond to that of the EZ. Indeed, lesions limited to a single lobe may be associated with a multilobar EZ; in addition, anatomic abnormalities as wide as an entire hemisphere may be observed in patients with an EZ involving only a portion of a single lobe.
In patients with symptomatic focal epilepsy, the main problem consists of understanding the complex topographic and functional relationships between an anatomic (presumed to be epileptogenic) lesion and the EZ, and there is no general agreement concerning the strategy that best addresses this issue. Furthermore, in patients suffering from focal epilepsies with negative magnetic resonance imaging (MRI) (so-called cryptogenic epilepsies), the identification and spatial definition of the EZ must be based only on electrical and clinical findings.4
Preoperative Evaluation
Noninvasive Investigations
Collection of historical and clinical information is mandatory in the evaluation of candidates for surgical treatment of drug-resistant focal epilepsy. Age at seizure onset must be assessed, as well as seizure frequency and semeiology. Chronology of ictal symptoms must be accurately defined by carefully inquiring of both the patients and the witnesses about subjective manifestations and objective signs occurring during seizures. The possible occurrence of loss of contact must be assessed, as well as the presence of postictal deficits. This initial step may provide crucial clues to lateralization and/or gross localization of ictal onset.5
An interictal electroencephalogram (EEG) is helpful in determining the side and site of epileptiform and of slow-wave abnormalities. Long-term monitoring with scalp video-EEG recording, coupled with direct intensive surveillance of the patient allowing detailed ictal clinical examination, is mandatory when electroclinical correlates are needed.6
High-resolution MRI provides valuable anatomic information in these patients. The neuroradiologist must be aware of the electroclinical features of the patient to be evaluated to tailor the study to the individual case. In patients suspected to have a temporal lobe involvement, transverse and coronal slices are oriented parallel and perpendicular to the major hippocampal axis, respectively. Images are oriented according to the bicommissural line when electroclinical data suggest extratemporal epilepsy. Transverse double-echo, T2-weighted coronal turbo spin-echo (TSE), T2-weighted coronal TSE fluid-attenuated inversion recovery (FLAIR), and T1-weighted coronal inversion recovery are acquired in all patients, with additional sequences and slices obtained when needed.7 By these means, in more than 90% of the patients scheduled for surgery in our center (regardless of the site of resection), an anatomic lesion may be demonstrated.
In multilobar epilepsies, we often deal with highly functional areas and with cases presenting with altered anatomic patterns in eloquent regions (as are often encountered in malformations of cortical development). In these cases, functional MRI8 is particularly helpful in defining the limits of activated areas by, for instance, motor9 or speech tasks,10 thus allowing the surgeon to minimize the risks of unacceptable new neurologic deficits.11
The correlation among anatomic, EEG, and clinical data, supported by a full neuropsychologic evaluation, is then employed to formulate a coherent hypothesis as to the localization of the EZ. A high degree of correlation allows us to offer surgery to a large number of patients (currently about 70% in our center) without the need for further invasive investigations.
Invasive Investigations
When noninvasive investigations alone fail to correctly define the EZ, and/or when the EZ (or the anatomic lesion) is suspected to involve highly functional regions, invasive recording with intracranial electrodes is indicated. In many centers, subdural electrodes (arranged in strips or grids and placed through bur holes or craniotomy) are employed. In our center, stereotactic placement of intracerebral electrodes (stereoelectroencephalography, or SEEG) is preferred as the invasive EEG monitoring technique of choice, and it is required in approximately 30% of cases operated on. For each patient, the strategy of intracerebral electrode placement is tailored according to previously acquired clinical, electrical, and anatomic data. Examples of customized SEEG explorations are shown in Figs. 111-1 and 111-2. The technical details of this methodology have been reported elsewhere.12
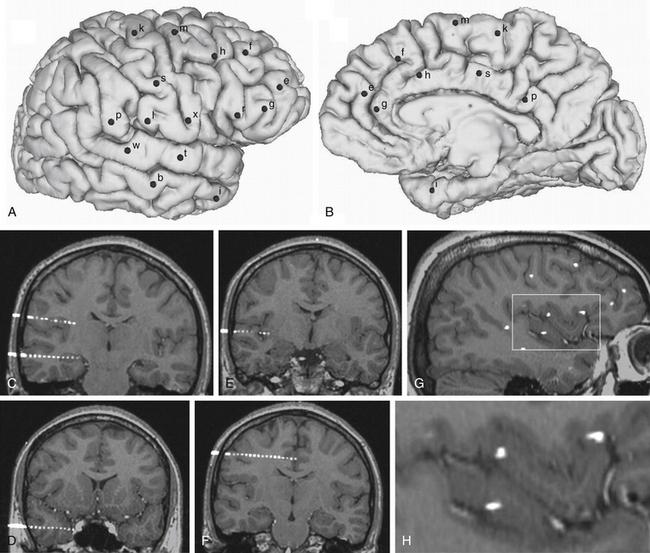
FIGURE 111-1 Example of an SEEG exploration. Coverage with intracerebral electrodes includes the right frontal and temporal lobes, as well as the rolandic and insular cortex. A brain cortical surface reconstruction of the lateral (A) and mesial (B) aspects of the right hemisphere was obtained from the 3D T1-weighted MRI structural images. (FreeSurfer software, http://surfer.nmr.mgh.harvard.edu.) Black spots, labeled by lowercase letters, indicate the surface impact points of each intracerebral electrode. C to H, Blended images of 3D T1-weighted MRI and coregistered postimplantation volumetric computed tomography, where single contacts of intracerebral electrodes are easily recognizable: electrodes b and l (C), electrode i (D), electrode t (E), electrode s (F), and a sagittal slice showing several contacts of different electrodes (G). H, The rectangular insert is magnified, where intracortical insular contacts of electrodes t, x, and r are shown.
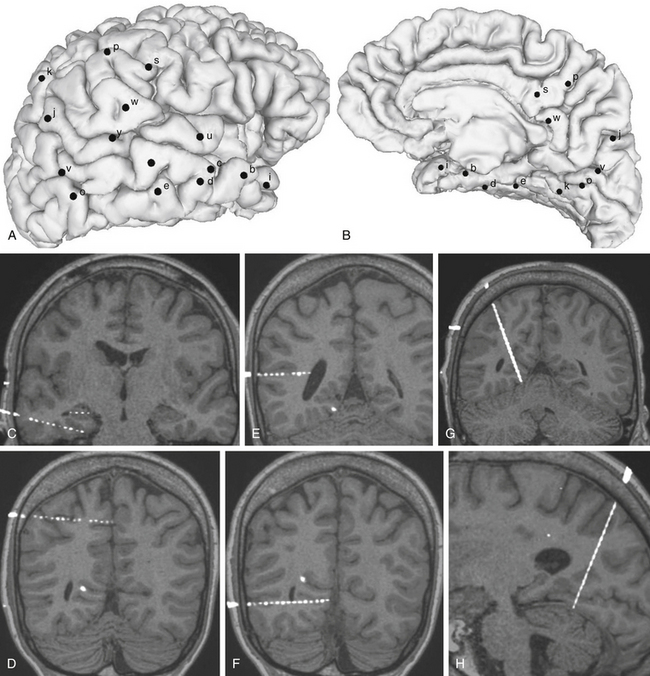
FIGURE 111-2 Example of an SEEG exploration. Coverage with intracerebral electrodes includes the right temporal, occipital, and parietal lobes. A brain cortical surface reconstruction of the lateral (A) and mesial (B) aspects of the right hemisphere was obtained from the 3D T1-weighted MRI structural images. (FreeSurfer software, http://surfer.nmr.mgh.harvard.edu.) Black spots, labeled by lowercase letters, indicate the surface impact points of each intracerebral electrode. C to H, Blended images of 3D T1-weighted MRI and coregistered postimplantation volumetric computed tomography, where single contacts of intracerebral electrodes are easily recognizable: electrode d and intrahippocampal contacts of electrode c (C), electrode p (D), electrode y (E), electrode o (F), and MRI slices coplanar with electrode k (G and H).
Surgical Decision-Making
The larger the epileptogenic cortex that needs to be removed, the harder the compromise that must be reached between the desire to cure each patient and the fear of creating new neurologic or neuropsychologic damage. The well-known concept of critical mass13 does apply to epilepsy surgery, and the decision on the extent of the EZ in multilobar partial epilepsies raises several particular diagnostic and therapeutic problems. In surgical planning, several questions can be considered that have no standard solutions and could have different answers depending on the characteristic of each patient and the attitude, feeling, and experience of the epilepsy surgery team. These issues concern the criteria for identifying the actual epileptogenic cortex. For instance, the following are challenging and not yet fully addressed questions: Should only the cortical areas that are affected by the ictal discharges at the onset of the seizures be removed? How long is the ictal onset? If the initial clinical symptom occurs several seconds after the onset of the electrical discharge, is removal of the symptomatogenic cortex mandatory,14 or will removal of only the initially affected cortical region be sufficient? What is the risk of removing the entire epileptogenic cortex? What kind of neurologic or neuropsychologic deficits can be induced? Are all of them predictable?
Anesthetic Considerations
Resective surgery for treatment of focal epilepsy does not require anesthetic approaches substantially differing from those commonly employed in other intracranial procedures. In our center, resections are performed under general anesthesia, with the patient positioned according to the site of surgery. Prophylactic antibiotics (1-2 g of cephamezine given intravenously) are given at anesthesia induction. Candidates for surgical treatment of severe partial epilepsy are commonly in optimal physical condition, and their interictal intracranial pressure (ICP) is not elevated.15 Careful anesthesiologic management (mostly hyperventilation, diuretics, or barbiturates) can be helpful when the volume of the exposed brain suddenly increases. This transitory increase in ICP may suggest the occurrence of an epileptic seizure,16 the clinical symptoms of which are masked by anesthesia. Except for this infrequent, transitory, and easily controlled incident, no other major intraoperative complications are usually expected in epileptic patients.
Surgical Technique
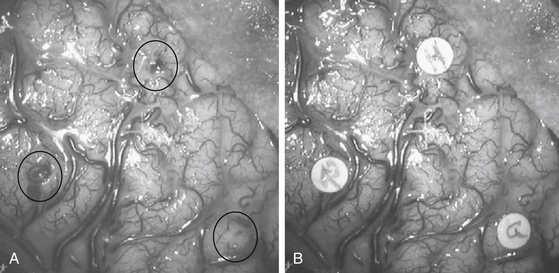
As compared to classic approaches for unilobar epilepsies, those adopted for multilobar excisions are tailored according to the site and extension of the region involved in the procedure. The supine position is usually preferred even when surgery is performed on the posterior portions of the hemisphere, with the operating table being tilted and the head rotated to obtain comfortable access to the surgical field. The prone position is used mainly when surgery involves the mesial aspects of the parietal or occipital lobes. Both skin incision and bone flap are designed to fully expose the region to be removed, as well as the surrounding cortical areas. In some instances, when a two-step procedure is likely, the surgeon must take into account the possible subsequent step when fashioning skin and bone flaps. In patients who have been previously evaluated by SEEG, the tracks of electrodes can be easily identified on the bone surface, and they are used as landmarks to plan the extent of the bone flap. While opening the dura mater, if an SEEG has been previously performed, care should be taken to separate the adhesions between the inner aspect of the dura and the cortical surface at the electrode entry points. Once the dura is opened, it is useful to match the vascular surgical anatomy with the stereoscopic angiograms obtained preoperatively, which provide an exquisite three-dimensional (3D) view of the sulcal and gyral pattern of the region.17 Careful inspection of the exposed cortex enables the surgeon to identify the entry points of intracerebral electrodes, which are used to draw the borders of resection (Fig. 111-3).
With the aid of the surgical microscope, resection is accomplished according to a few simple rules:
• Vessels that cross the region of resection but are tributaries of, or draining from, regions outside its borders must be identified on the stereoscopic angiograms, isolated, and left intact.
• Subpial dissection should be preferred, especially in mesial regions and along the main fissures, to protect vascular and extra-axial nervous structures (e.g., those in the prepeduncular cistern during mesial temporal removal).
• Suction and bipolar coagulation should be avoided to obtain as many unaltered tissue specimens as possible available for histopathologic evaluation.
Like hemispherectomy, which has progressively evolved into hemispherotomy (described later) to minimize the risks of hydrocephalus, hemosiderosis, and intraoperative blood losses, extended multilobar resections may be replaced with disconnective techniques. In our experience, disconnections have been particularly helpful in procedures involving the posterior quadrant of the hemisphere, when the EZ extends to the temporal, occipital, and parietal lobes. In these cases, we can employ a pure disconnective technique (Fig. 111-4) or otherwise combine resection with disconnection.18 Unlike other authors, when combining the two techniques, we prefer to remove the occipital and parietal lobes and to disconnect the temporal lobe, which may exert a sort of “push-up” effect if left in place, thus limiting residual brain dislocation into the resulting surgical cavity. Like other disconnective techniques, posterior hemispheric disconnections are not indicated if evolutive pathologies are suspected. A drawback of disconnective approaches is the limited availability of resected tissue, which may prevent a reliable etiologic diagnosis and restrict the access of basic scientists to pathologic specimens.
The anatomofunctional basis of morbidity in multilobar resections does not differ substantially from that of unilobar resections, and it strictly depends on the site of surgery. Morbidity may include transient motor impairment, akinesia and mutism after removal of the supplementary motor area,19 transient speech disturbances following resections involving the neocortex of the dominant temporal lobe, and contralateral superior quadrantoanopia in anterior temporal lobectomies. The risk of larger contralateral visual field defects is higher following resections in the posterior portion of the hemisphere. When removal is contiguous to the rolandic region, the risk of motor–sensory impairment is usually increased, and functional mapping by either intracerebral electrical stimulations at SEEG or functional MRI or intraoperative cortical and subcortical electrophysiologic evaluation is helpful to minimize the chances of unwanted postoperative deficits.
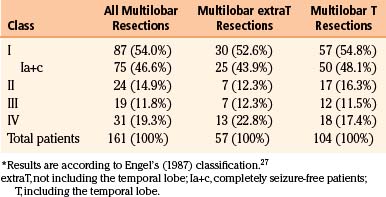
Results on seizures of multilobar surgery are far less gratifying if compared to other unilobar resections, either in our or in other centers18,20–26 (Tables 111-1 to 111-3). Slightly better results are observed in multilobar resections, including the temporal lobe. However, it must be stressed that, despite a complex epileptologic situation that could hardly be improved by further drug regimens, a substantial number of patients can be cured or improved (Engel’s27 classes I-III, approximately 80% in our series) by surgical treatment.
| Class | Patients |
|---|---|
| I | 438 (76.2%) |
| Ia+c | 365 (63.5%) |
| II | 52 (9.0%) |
| III | 45 (7.8%) |
| IV | 40 (7.0%) |
| Total patients | 575 (100%) |
Ia+c, completely seizure-free patients.
∗ Results are according to Engel’s (1987) classification.27
Table 111-3 Literature Reporting Seizure Outcome in Multilobar Resections (Publication Period 1998-2008)
| Described Population | Outcome: SF/Total (Only Multilobar) | |
|---|---|---|
| Elsharkawi et al.26 | 154 extratemporal resections | 7/15 (47%) |
| Daniel et al.18 | 13 patients with OPT epilepsy | 12/13 (92%) |
| Francione et al.22 | 10 pediatric patients with FCD | 1/2 (50%) |
| Kral et al.20 | 53 patients with FCD | 0/1 (0%) |
| Bernasconi et al.23 | 8 double cortex patients | 1/2 (50%) |
| Edwards et al.24 | 35 patients with MCD | 6/11 (55%) |
| Paolicchi et al.21 | 75 pediatric patients | 2/9 (22%) |
| Duchowny et al.25 | 31 pediatric patients | 2/5 (40%) |
FCD, focal cortical dysplasia; MCD, malformation of cortical development; SF, seizure-free; OPT, occipitoparietotemporal.
Illustrative Case
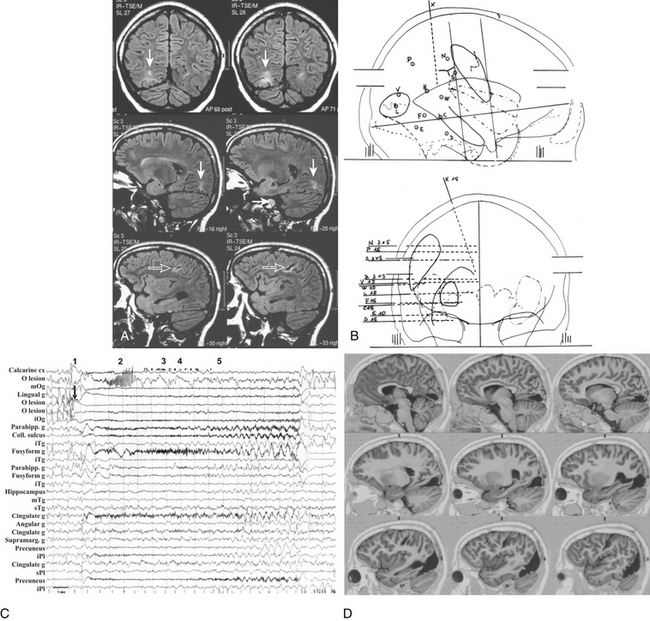
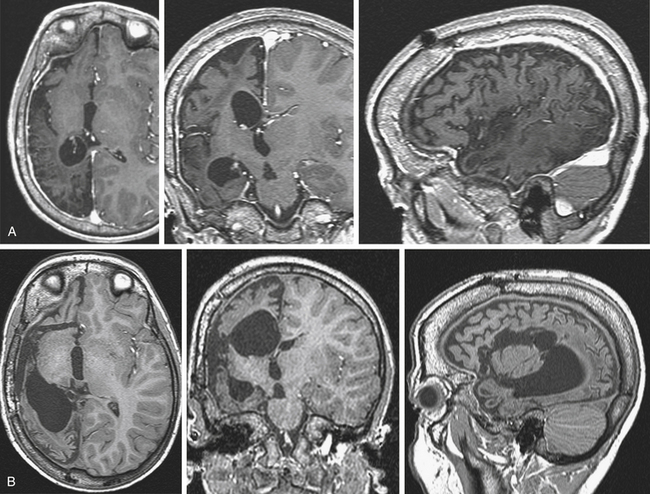
Scalp video-EEG disclosed slower background activity on the right hemisphere and interictal slow waves and spike and waves on the right temporo-occipital regions. Ictal EEG showed an early low-voltage fast activity on the right posterior leads, with late spread to the homologous contralateral regions. Brain MRI documented two separate lesions of possible ischemic nature in the right hemisphere, involving the basal occipital and the rolandic regions (Fig. 111-5A). SEEG investigation was performed to evaluate the relationships between the occipital anatomic lesion and the EZ, which could be suspected to involve also the parietal and temporal lobes on the basis of the electroclinical findings (Fig. 111-5B). No electroclinical data indicated the need to sample the rolandic lesion by intracerebral electrodes. Electroclinical correlations obtained during SEEG recordings localized the EZ in the inferior half of the occipital lobe (electrodes l and v) and in the posterior mesiobasal aspect of the temporal lobe (electrodes f and e) (Fig. 111-5C). Surgical resection of the EZ was performed (Fig. 111-5D), and histologic examination documented gliotic changes in the excised tissue corresponding to the MRI lesion. The patient was left with an expected and anticipated complete left-sided hemianopia, and she is seizure free since surgery after 3 years of follow-up.
Hemispherectomy and Hemispheric Disconnection
Dandy in 1928 reported resection of an entire hemisphere to treat patients with brain tumor and hemiplegia.28 This technique yielded poor results on tumor progression and was therefore discarded. Hemispherectomy for infantile hemiplegia with intractable seizures was introduced in 1950 by Krynauw,29 who removed the affected hemisphere, leaving in place only the thalamus and caudate nucleus, in 12 patients, 10 of whom had seizures that disappeared after surgery. Along with seizure freedom, patients did not experience worsening of their preoperative motor deficit; rather, a slight improvement of muscular tone was noticed in some cases, as well as of the behavioral disorders often associated with epilepsy and hemiplegia in these instances. In subsequent decades, the technique of anatomic hemispherectomy was adopted to treat patients with seizures originating from a wide hemispheric area and stabilized motor impairment, caused by several congenital and acquired etiologies. Nevertheless, occurrence of late (median 8 years postoperatively), progressive, and often fatal neurologic deterioration was observed in a number of cases with an otherwise uneventful early postoperative course.30 Postmortem evaluations showed hemosiderosis and granular ependymitis, which were ascribed to repeated subtle, persistent bleeding in the empty cavity, with blood extravasation into the open ventricular spaces.31 An additional problem was the possible occurrence of postoperative hydrocephalus. Therefore, different modifications of the original hemispherectomy were designed to avoid these complications, including (1) the Oxford modification, consisting of placing a muscle plug in the foramen of Monro to insulate the subdural space from the ventricular cavities and increasing the extradural space at the expense of the subdural space by suturing the dura to the falx and tentorium,32 and (2) hemidecortication, with removal limited to the cortex, preservation of the white matter, and the ventricles left intact.33
In their paper of 1966, Opennheimer and Griffith had advocated an alternative approach “for functionally disconnecting the abnormal hemisphere from the rest of the brain, leaving it in place.”31 Curiously, we waited until 1983 for the first report on a disconnective technique, which was named functional hemipsherectomy,34 consisting of removal of the temporal lobe and of a considerable amount of the suprasylvian region, with disconnection of the frontal and parieto-occipital remainders of the hemisphere through an intraventricular commissurotomy. This approach decreased intraoperative blood loss and the rate of postoperative hydrocephalus, with the occurrence of late hemosiderosis having been abolished. In subsequent years, different procedures of hemispheric deafferentation (hemispherotomy) were introduced, in which resection was limited and more brain was disconnected.35,36 This technique, with slight37,38 or major39 modifications, is currently the procedure of choice in most epilepsy surgery centers, with excellent surgical and functional results, and it is the main object of the next sections.
Indications
Etiologies
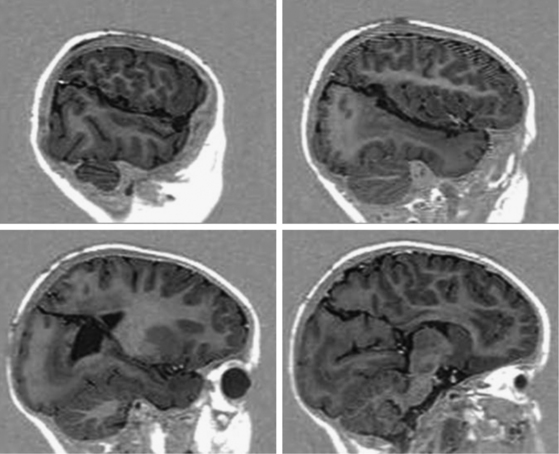
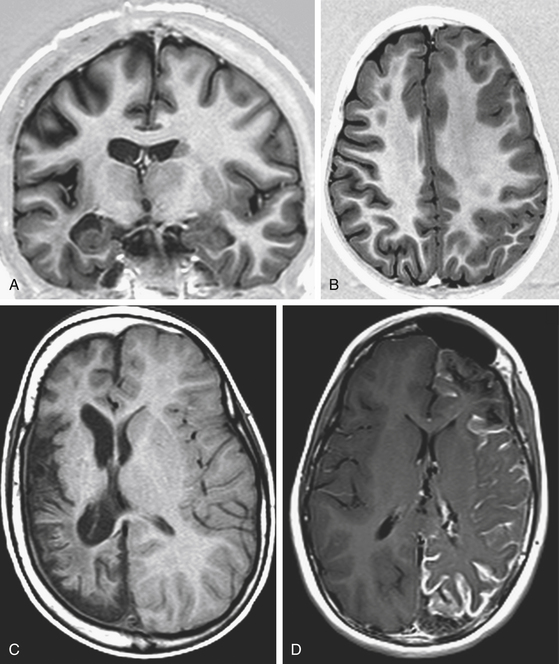
Rasmussen encephalitis is a rare, severe, acquired, immune-mediated brain disorder leading to unilateral hemispheric atrophy, associated with progressive neurologic deterioration and intractable seizures. Focal seizures originating in the affected hemisphere may be characterized in the advanced stages of the disease by epilepsia partialis continuans. Along with progressive hemispheric atrophy documented by MRI (Fig. 111-6), the patients experience increasing hemiparesis, hemianopia, cognitive deterioration, and if the dominant hemisphere is affected, speech impairment. Other acquired conditions leading to DHES are sequelae of brain trauma and of meningoencephalitic processes. Postnatal ischemic hemispheric damage is also observed, and it may be the result of complicated heart surgery.
Congenital disorders include extensive Stürge-Weber syndrome, hemimegalencephaly, diffuse hemispheric nonhypertrophic cortical dysplasia, and prenatal vascular insults due to middle cerebral or internal carotid artery occlusion characteristic of infantile hemiplegia (Fig. 111-6).
Electroclinical Features
Age at seizure onset may range from early childhood to adulthood, and it depends on the nature of the underlying etiology, either congenital or acquired. Seizures may arise from any hemispheric region and are often of multifocal origin. Seizure severity and associated disability are related with their semeiology and frequency, which may be variable in the same patient according to the stage of the disease in progressive conditions, as in Rasmussen and Stürge-Weber syndromes. Drug resistance is frequent and represents a crucial, yet not exclusive, issue for surgical indication.
Ictal EEG is important in the evaluation of surgical candidates. Video-EEG recording of ictal events allows correlation of ictal electrical changes with ictal clinical behavior, and it is therefore essential in the lateralization and localization of seizures. Because of the frequent multifocality and the resulting polymorphism of seizures, video-EEG should aim to capture all seizure types to rule out possible bilateral seizure onset, which would imply poor postoperative results. Nevertheless, patients with diffuse interictal abnormalities like electrical status epilepticus during sleep may be considered for hemispherotomy if unilateral seizure onset is documented.40
Neurologic Functions
Usually, patients who are considered for hemispherotomy have a complete and stable motor impairment with associated congruent hemianopia. It is believed that in the absence of useful finger movements and when foot tapping is not possible, hemispheric deafferentation will not result in increased motor deficit.41 This has been ascribed to the role of the ipsilateral connections coming from the contralateral unaffected motor cortex.42 Patients with a progressive condition such as Rasmussen encephalitis or Stürge-Weber syndrome at an early stage may present with severe disabling seizures and preserved motor function. In such cases, the decision to impose a severe postoperative neurologic deficit should be made, balancing the actual severity of epilepsy, and the likelihood of future neurologic and cognitive deterioration with disease progression, against the postoperative abrupt onset of new neurologic deficits.
Preoperative Evaluation
Occasionally, additional information may be provided by nuclear brain imaging, such as ictal single-photon emission computed tomography and interictal positron emission tomography using 2-deoxy-2-[F-18]fluoro-d-glucose, but these techniques cannot replace anatomoelectroclinical investigations for the definition of the surgical indication.
Surgical Technique
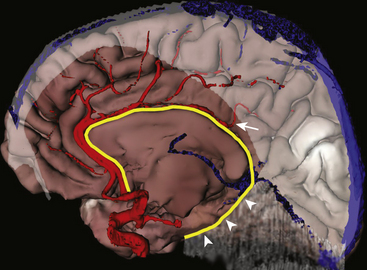
The angle between the septum pellucidum and the callosal body is identified. We prefer to perform the callosotomy from posterior to anterior, leaving behind the falcotentorial edge as the main landmark for mesial disconnection and looking for the distal branches of the pericallosal artery. This may imply that, for the first few millimeters, callosotomy is made without safe landmarks, until the distal branch of the pericallosal artery, usually directed orthogonal to the callosal longitudinal axis, is identified (Fig. 111-7). By alternating the use of a dissector and the ultrasonic aspirator adjusted at low power values, callosotomy is safely carried out 2 to 3 mm from the midline to avoid violation of the contralateral hemisphere, keeping in view the ipsilateral pericallosal artery as the main landmark. The callosal body is thicker anteriorly and at the level of the genu, creating some trouble—especially in patients with exuberant tissue, as in hemimegalencephaly. Callosotomy is continued below the genu until the junction between the A1 and the A2 tracts of the anterior cerebral artery is encountered. This is another crucial point, because no visible anatomic landmark switches a “red light” on to violation of the hypothalamus. Thus, a useful solution is to turn the disconnection plane laterally through the posterior gyrus rectus and orbital cortex paralleling the course of the lesser sphenoidal wing, which allows the incision to be stopped just anterior to the insula. The insula, according to the indications, may be separately removed by suction through arachnoidal windows opened between M2 branches; otherwise, it may be disconnected by subcortical undercutting with the aid of a large dissector. The anatomic result of this procedure is shown in Fig. 111-8.
Postoperatively, patients are moved to the ICU for at least one night. Varying degree of lethargy and temperature elevation above 39°C are common occurrences in the first days after hemispherotomy, and they are ascribed to aseptic meningitis. It has been reported that an external ventricular drain placed at the end of the procedure and maintained for 4 to 5 days postoperatively is helpful both in decreasing the rate of noninfectious fever and in reducing postoperative hospital stay.43 It is not clear whether external ventriculostomy can prevent postoperative hydrocephalus, whose incidence in hemispherotomy case series is, however, low.44
Technical Variations
The previously described technique reflects substantially that proposed by Villemure and Mascott in 1995,35 which subsequently underwent some slight modifications. In 1995, Schramm et al. described a similar approach for hemispherotomy, in which the so-called infrainsular window obtained by removal of T1 was replaced by a complete temporal lobectomy.36 Shimizu and Maehara in 2000 reported a “transopercular” modification of the original technique, which entailed a retroinsular approach to the temporal horn.37 Finally, in 2001, Schramm et al. proposed the keyhole hemispherotomy, which consists of a trans-sylvian approach to both the temporal horn and the lateral ventricle with minimal tissue removal.38
A different approach with identical anatomic results is the vertical parasagittal hemispherotomy adopted by Delalande et al.39 After a parasagittal frontoparietal craniotomy, the lateral ventricle is reached, following resection of an appropriate amount of frontal cortex. Callosotomy is performed, and then dissection is carried out vertically in an inferolateral direction, mainly through the white matter lateral to the basal ganglia and mesial to the insula, to achieve control of the mesial temporal structures and basal frontal lobe and thus complete the hemispheric disconnection (Fig. 111-9). Danielpour et al. proposed a modification of the vertical hemispherotomy, consisting of the interhemispheric approach to the corpus callosum without a frontal corticectomy.45
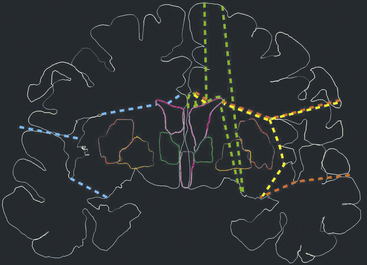
FIGURE 111-9 Schematic representation of the main technical variants of hemispherotomy. Each colored dashed line indicates the planes of disconnections on the coronal plane. The red line is the lateral peri-insular hemispherotomy,35 the yellow line is a transopercular retroinsular modification,37 the light blue line is a keyhole trans-sylvian modification,38 and the green line is a vertical hemispherotomy.39
Technique Selection
Currently, as several surgical techniques are available, the surgeon can choose the procedure considered more appropriate for single instances. We agree with Nagel et al., who advocate a customized surgical approach, tailored according to the pathology.46 For instance, techniques developed to minimize tissue removal are ideal in the presence of an atrophic brain or a wide porencephalic cavity. In these cases, the presence of wide ventricular spaces facilitates the exposure of critical anatomic landmarks and provides comfortable access to the temporal mesial structures and corpus callosum. On the other hand, in cases characterized by exuberant hemispheric size, the ventricles are often smaller than normal; periventricular anatomy, including that of the callosal body and other subcortical structures, may be distorted; and the midline is often displaced so that essential landmarks may be obscured. In these cases, enlarged perisylvian cortical resection for adequate exposure of the ventricular cavities allows the surgeon to proceed more comfortably with disconnection of the residual structures. Anatomic hemispherectomy has been recently revived for hemimegalencephaly46; it is our opinion that anatomic hemispherectomy also may be considered after a hemispherotomy that has failed to control seizures, when these are still believed to start from an imperfectly disconnected hemisphere.
Results
Outcome on Seizures
Postoperatively, a percentage of patients ranging from 74% to 90% are in Engel’s class I, that is, free of disabling seizures, following hemispherotomy surgery.38,39,41,47 Comparison of this figure with that emerging from hemispherectomy and functional hemispherectomy case series, with 52% to 78% of class I patients,48–50 suggests that disconnective techniques are superior to resective procedures in terms of seizure control.
Irrespective of the surgical technique, there is general agreement that seizure outcome strongly depends on the pathologic substrate. In a study on hemidecortication, seizure freedom was obtained in 81% of postischemic cases as opposed to 40% of patients with hemimegalencephaly, with other etiology showing intermediate results.51 In a series of patients submitted to functional hemispherectomy, class I patients with acquired, progressive, and developmental pathologies were 82%, 50%, and 31%, respectively.49 In another study reporting patients treated during a long period employing anatomic hemispherectomy, functional hemispherectomy, and hemispherotomy, the proportion of class I cases ranged from 33% in hemimegalencephaly to 71% in perinatal ischemia. The same figure emerges from the results of vertical hemispherotomy.39 Thus, etiology is an important predictor of surgical results in DHES, and because its nature can now be easily inferred from preoperative neuroimaging, it should be the main indicator of outcome when counseling patients and their families.
Functional Outcome
Usually, children with partial motor deficit experience a postoperative worsening of the symptoms, which subsequently improves with return to the baseline with time. Physiotherapy often helps the return to the preoperative motor performance. Because hemispheric surgery may be recommended to patients harboring progressive syndromes, with the aim to control disabling seizures, before a stable motor impairment has developed, the unavoidable onset of hemiplegia and hemianopia in an otherwise functionally intact patient must be anticipated and discussed with the patient and with relatives and caregivers, and a physical rehabilitation program should be planned before surgery. In patients with fully developed language, disconnection of the dominant hemisphere results in severe speech impairment, and alternative surgical strategies should be considered, with the aim to preserve language. In younger children, especially in those who have not yet completely acquired language, contralateral transfer of this function may be expected without permanent sequelae. As far as cognitive functions are concerned, it has been reported that better postoperative developmental scores are obtained in patients with shorter duration of seizures, postoperative seizure control, and higher preoperative developmental scores.39,52
Ainik A., Lehericy S., Duffau H., et al. Role of the supplementary motor area in motor deficit following medial frontal lobe surgery. Neurology. 2001;57:871-878.
Cats E.A., Kho K.H., Van Nieuwenhuizen O., et al. Seizure freedom after functional hemispherectomy and a possible role for the insular cortex: the Dutch experience. J Neurosurg. 2007;107(suppl 4):275-280.
Colombo N., Tassi L., Galli C., et al. Focal cortical dysplasias: MR imaging, histopathologic, and clinical correlations in surgically treated patients with epilepsy. AJNR Am J Neuroradiol. 2003;24:724-733.
Cossu M., Cardinale F., Castana L., et al. Stereoelectroencephalography in the presurgical evaluation of focal epilepsy: a retrospective analysis of 215 procedures. Neurosurgery. 2005;57:706-718.
Daniel R.T., Meagher-Villemure K., Farmer J.P., et al. Posterior quadrantic epilepsy surgery: technical variants, surgical anatomy and case series. Epilepsia. 2007;48:1429-1437.
Delalande O., Bulteau C., Dellatolas G., et al. Vertical parasagittal hemispherotomy: surgical procedures and clinical long-term outcomes in a population of 83 children. Neurosurgery. 2007;60(suppl 2):ONS19-ONS32.
Devlin A.M., Cross J.H., Harkness W., et al. Clinical outcomes of hemispherectomy for epilepsy in childhood and adolescence. Brain. 2003;126:556-566.
Duchowny M., Jayakar P., Resnick T., et al. Epilepsy surgery in the first three years of life. Epilepsia. 1998;39:737-743.
Edwards J.C., Wyllie E., Ruggeri P.M., et al. Seizure outcome after surgery for epilepsy due to malformation of cortical development. Neurology. 2000;55:1110-1114.
Elsharkawi A.E., Behne F., Oppel F., et al. Long term outcome of extratemporal epilepsy surgery among 154 adult patients. J Neurosurg. 2008;108:676-686.
Engel J.Jr. Outcome with respect to epileptic seizures. In: Engel J.Jr., editor. Surgical Treatment of the Epilepsies. New York: Raven Press; 1987:553-571.
Heilbrun M.P., Lee J.N., Alvord L. Practical application of fMRI for surgical planning. Stereotact Funct Neurosurg. 2001;76:168-174.
Jonas R., Nguyen S., Hu B., et al. Cerebral hemispherectomy: hospital course, seizure, developmental, language and motor outcomes. Neurology. 2004;62:1712-1721.
Kossof E.H., Vining E.P., Pillas D.J., et al. Hemispherectomy for intractable unihemispheric epilepsy: etiology vs. outcome. Neurology. 2003;61:887-890.
Kral T., Clusmann H., Blumcke I., et al. Outcome of epilepsy surgery in focal cortical dysplasia. J Neurol Neurosurg Psychiatry. 2003;74:183-188.
Krynauw R.A. Infantile hemiplagia treated by removing one cerebral hemisphere. J Neurol Neurosurg Psychiatry. 1950;13:243-267.
Loddenkemper T., Cosmo G., Kotagal P., et al. Epilepsy surgery in children with electrical status epilepticus in sleep. Neurosurgery. 2009;64:328-337.
Paolicchi J.M., Jayakar P., Dean P., et al. Predictors of outcome in pediatric epilepsy surgery. Neurology. 2000;54:642-647.
Penfield W., Jasper H. Epilepsy and the Functional Anatomy of the Human Brain. Boston: Little, Brown; 1954.
Rasmussen T. Hemispherectomy for seizures revisited. Can J Neurol Sci. 1983;10:71-78.
Schramm J., Behrens E., Entzian W. Hemispherical deafferentation: an alternative to functional hemispherectomy. Neurosurgery. 1995;36:509-516.
Schramm J., Kral T., Clusmann H. Transsylvian keyhole functional hemispherectomy. Neurosurgery. 2001;49:891-901.
Shimizu H., Maehara T. Modification of peri-insular hemispherotomy and surgical results. Neurosurgery. 2000;47:367-373.
Villemure J.G., Daniel R.T. Peri-insular hemispherotomy in paediatric epilepsy. Child’s Nerv Syst. 2006;22:967-981.
Villemure J.G., Mascott C.R. Peri-insular hemispherotomy: surgical principles and anatomy. Neurosurgery. 1995;37:975-981.
Yasargil M.G. Topographic anatomy for microsurgical approaches to intrinsic brain tumors. In: Yasargil M.G., editor. Microneurosurgery IV A. New York: Thieme; 1994:1-114.
1. Talairach J., Bancaud J., Szikla G., et al. Approche nouvelle de la neurochirurgie de l’épilepsie: Méthodologie stéréotaxique et résultats thérapeutiques. Neurochirurgie. 1974;20(suppl 1):1-240.
2. Bancaud J., Talairach J., Bonis A., et al. Informations neurophysiopathologiques apportées par l’investigation fonctionnelle stéréotaxique (SEEG) dans les épilepsies. Rev Neurol. 1963;108:81-86.
3. Yasargil M.G. Topographic anatomy for microsurgical approaches to intrinsic brain tumors. In: Yasargil M.G., editor. Microneurosurgery IV A. New York: Thieme; 1994:1-114.
4. Quesney L.F., Gloor P. Localization of epileptic foci. Electroencephalogr Clin Neurophysiol. 1985;37(suppl):165-200.
5. Wieser H.G., Williamson P.D. Ictal semeiology. In: Engel J.Jr., editor. Surgical Treatment of the Epilepsies. 2nd ed. New York: Raven Press; 1993:161-171.
6. Munari C., Kahane P. Traitement neurochirurgical de l’épilepsie. Encycl Méd Chir (Elsevier Paris). Neurologie. 1998:14. 17-700-D-10. 1998:14
7. Colombo N., Tassi L., Galli C., et al. Focal cortical dysplasias: MR imaging, histopathologic, and clinical correlations in surgically treated patients with epilepsy. AJNR Am J Neuroradiol. 2003;24:724-733.
8. Moritz C., Houghton V. Functional MR imaging: paradigms for clinical preoperative mapping. Magn Reson Imaging Clin N Am. 2003;11:529-542.
9. Alkadhi H., Crelier G.R., Boendermaker S.H., et al. Reproducibility of primary motor cortex somatotopy under controlled conditions. AJNR Am J Neuroradiol. 2002;23:1524-1532.
10. Springer J.A., Binder J.R., Hammeke T.A., et al. Language dominance in neurologically normal and epilepsy subjects. A functional MRI study. Brain. 1999;122:2033-2045.
11. Heilbrun M.P., Lee J.N., Alvord L. Practical application of fMRI for surgical planning. Stereotact Funct Neurosurg. 2001;76:168-174.
12. Cossu M., Cardinale F., Castana L., et al. Stereoelectroencephalography in the presurgical evaluation of focal epilepsy: a retrospective analysis of 215 procedures. Neurosurgery. 2005;57:706-718.
13. Rasmussen T. Cortical resection for medically refractory focal epilepsy: results, lessons and questions. In: Rasmussen T., Marino R. Functional Neurosurgery. New York: Raven Press; 1979:253-269.
14. Lüders H.O., Engel J.Jr., Munari C. General principles. In: Engel J.Jr., editor. Surgical Treatment of the Epilepsies. 2nd ed,. New York: Raven Press; 1993:137-153.
15. Munari C., Andreoli A., Frattarelli M., et al. Activation par 1’ Amitryptiline (remarques électrocliniques à propos de 120 patients épileptiques). Rev EEG Neurophysiol. 1977;7:194-197.
16. Penfield W., Jasper H. Epilepsy and the Functional Anatomy of the Human Brain. Boston: Little, Brown; 1954.
17. Yasargil M.G. Introduction. In: Yasargil M.G., editor. Microneurosurgery IV B. New York: Thieme, 1996. : xix-xxi
18. Daniel R.T., Meagher-Villemure K., Farmer J.P., et al. Posterior quadrantic epilepsy surgery: technical variants, surgical anatomy and case series. Epilepsia. 2007;48:1429-1437.
19. Ainik A., Lehericy S., Duffau H., et al. Role of the supplementary motor area in motor deficit following medial frontal lobe surgery. Neurology. 2001;57:871-878.
20. Kral T., Clusmann H., Blumcke I., et al. Outcome of epilepsy surgery in focal cortical dysplasia. J Neurol Neurosurg Psychiatry. 2003;74:183-188.
21. Paolicchi J.M., Jayakar P., Dean P., et al. Predictors of outcome in pediatric epilepsy surgery. Neurology. 2000;54:642-647.
22. Francione S., Vigliano P., Tassi L., et al. Surgery for drug-resistant partial epilepsy in children with focal cortical dysplasia: anatomical–clinical correlations and neurophysiological data in 10 patients. J Neurol Neurosurg Psychiatry. 2003;74:1493-1501.
23. Bernasconi A., Martinez V., Rosa-Neto P., et al. Surgical resection for intractable epilepsy in “double cortex” syndrome yields inadequate results. Epilepsia. 2001;42:1124-1129.
24. Edwards J.C., Wyllie E., Ruggeri P.M., et al. Seizure outcome after surgery for epilepsy due to malformation of cortical development. Neurology. 2000;55:1110-1114.
25. Duchowny M., Jayakar P., Resnick T., et al. Epilepsy surgery in the first three years of life. Epilepsia. 1998;39:737-743.
26. Elsharkawi A.E., Behne F., Oppel F., et al. Long term outcome of extratemporal epilepsy surgery among 154 adult patients. J Neurosurg. 2008;108:676-686.
27. Engel J.r. J. Outcome with respect to epileptic seizures. In: Engel J.Jr., editor. Surgical Treatment of the Epilepsies. New York: Raven Press; 1987:553-571.
28. Dandy W.E. Removal of right cerebral hemisphere for certain tumors with hemiplegia. J Am Med Assoc. 1928;90:823-825.
29. Krynauw R.A. Infantile hemiplagia treated by removing one cerebral hemisphere. J Neurol Neurosurg Psychiatry. 1950;13:243-267.
30. Laine E., Pruvot P., Osson D. Ultimate results of hemispherectomy in patients with infantile cerebral hemiatrophy productive of epilepsy. Neurochirurgie. 1964;10:507-522.
31. Oppenheimer D.R., Griffith H.B. Persistent intracranial bleeding as a complication of hemispherectomy. J Neurol Neurosurg Psychiatry. 1966;29:229-240.
32. Adams C.B. Hemispherectomy—a modification. J Neurol Neurosurg Psychiatry. 1983;46:617-619.
33. Ignelzi R.J., Bucy P.C. Cerebral hemidecortication in the treatment of infantile cerebral hemiatrophy. J Nerv Ment Dis. 1968;147:14-30.
34. Rasmussen T. Hemispherectomy for seizures revisited. Can J Neurol Sci. 1983;10:71-78.
35. Villemure J.G., Mascott C.R. Peri-insular hemispherotomy: surgical principles and anatomy. Neurosurgery. 1995;37:975-981.
36. Schramm J., Behrens E., Entzian W. Hemispherical deafferentation: an alternative to functional hemispherectomy. Neurosurgery. 1995;36:509-516.
37. Shimizu H., Maehara T. Modification of peri-insular hemispherotomy and surgical results. Neurosurgery. 2000;47:367-373.
38. Schramm J., Kral T., Clusmann H. Transsylvian keyhole functional hemispherectomy. Neurosurgery. 2001;49:891-901.
39. Delalande O., Bulteau C., Dellatolas G., et al. Vertical parasagittal hemispherotomy: surgical procedures and clinical long-term outcomes in a population of 83 children. Neurosurgery. 2007;60(suppl 2):ONS19-ONS32.
40. Loddenkemper T., Cosmo G., Kotagal P., et al. Epilepsy surgery in children with electrical status epilepticus in sleep. Neurosurgery. 2009;64:328-337.
41. Villemure J.G., Daniel R.T. Peri-insular hemispherotomy in paediatric epilepsy. Child’s Nerv Syst. 2006;22:967-981.
42. Eyre J.A., Taylor J.P., Villagra F., et al. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology. 2001;57:1543-1554.
43. Sood S., Asano E., Chugani H.T. Role of external ventriculostomy in the management of fever after hemispherectomy. J Neurosurg (Pediatrics). 2008;2:427-429.
44. Villemure J.G., Vernet O., Delalande O. Hemispheric disconnection: callosotomy and hemispherotomy. Adv Tech Stand Neurosurg. 2000;26:25-78.
45. Danielpour M., Von Koch C.S., Ojemann S.G., et al. Disconnective hemispherectomy. Pediatr Neurosurg. 2001;35:169-172.
46. Nagel S.J., Elbabaa S.K., Hadar E.J., et al. Hemispherectomy techniques. In: Luders H.O., editor. Textbook of Epilepsy Surgery. London: Informa UK; 2008:1121-1130.
47. Binder D.K., Schramm J. Transsylvian functional hemispherectomy. Childs Nerv Syst. 2006;22:960-966.
48. Vining E.P., Freeman J.M., Pillas D.J., et al. Why would you remove half a brain? The outcome of 58 children after hemispherectomy—The Johns Hopkins experience: 1968 to 1996. Pediatrics. 1997;100:163-171.
49. Devlin A.M., Cross J.H., Harkness W., et al. Clinical outcomes of hemispherectomy for epilepsy in childhood and adolescence. Brain. 2003;126:556-566.
50. Cats E.A., Kho K.H., Van Nieuwenhuizen O., et al. Seizure freedom after functional hemispherectomy and a possible role for the insular cortex: the Dutch experience. J Neurosurg. 2007;107(suppl 4):275-280.
51. Kossof E.H., Vining E.P., Pillas D.J., et al. Hemispherectomy for intractable unihemispheric epilepsy: etiology vs. outcome. Neurology. 2003;61:887-890.
52. Jonas R., Nguyen S., Hu B., et al. Cerebral hemispherectomy: hospital course, seizure, developmental, language and motor outcomes. Neurology. 2004;62:1712-1721.
53. Avanzini G Obituary. Claudio Munari (1943-1999). Clin Neurophysiol. 2000;111:951-952.
∗ In memoriam
This chapter is dedicated to our master and friend Claudio Munari. His “unique blend of intellectual provocation and human warmth”53 marked his life and affected all his pupils. His memory continuously inspires and supports our work in the Centre of Epilepsy Surgery named after him.