Chapter 36 Multidrug-Resistant Bacteria
3 What are the risk factors for MDR infections?
Major risk factors for colonization or infection with MDR organisms are as follows:
4 How do mutations in cell wall synthesis contribute to MDR?
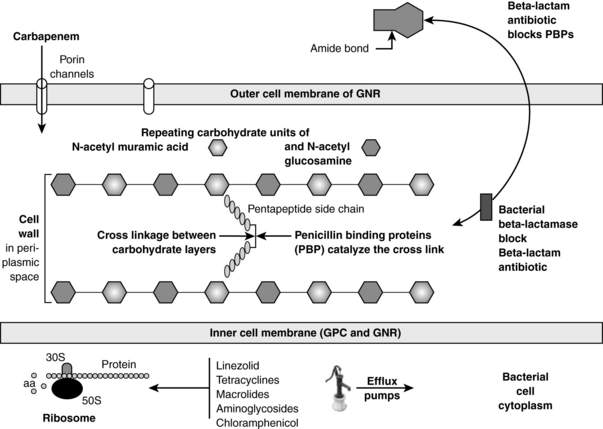
Almost all bacteria have cell walls that are located outside of the inner membrane and are composed of repeating carbohydrate units of N-acetylmuramic acid and N-acetylglucosamine. See Figure 36-1. The key structural stabilizing step is the cross-linking between the carbohydrate layers. Penicillin-binding proteins (PBP) are the bacterial enzymes that accomplish this cross-linkage. β-Lactam antibiotics (penicillins, cephalosporins, carbapenems, and monobactams) bind to PBP, inactivating them, thus interfering with the cross-linkage. Gram-positive bacteria can become MDR by mutations in the PBP such that the β-lactam cannot bind to them. Methicillin-resistant Staphylococcus aureus (MRSA), penicillin-resistant Streptococcus pneumoniae, and vancomycin-resistant enterococcus (VRE) all have evolved mutated PBP.
5 Why are β-lactamases so important in causing MDR infections?
β-Lactamases are bacterial enzymes that inactivate β-lactam antibiotics by opening the amide bond of the β-lactam ring. See Figure 36-1. These enzymes are the most common cause of MDR in GNR. The β-lactamases causing the most common MDR in the ICU include the following:
 Extended-spectrum β-lactamases (ESBL) cause resistance to most β-lactam antibiotics with the exceptions of the cephamycins (cefoxitin, cefotetan) and carbapenems. The most common bacteria carrying ESBL are Klebsiella spp. and Escherichia coli. Less commonly Enterobacter, Serratia, Morganella, Proteus, and Pseudomonas aeruginosa spp. may harbor these genes. The genes for these enzymes are carried on chromosomes or plasmids and are thus transferrable between bacteria. ESBL-producing bacteria are also often resistant to aminoglycosides and quinolones. These enzymes are usually inhibited by β-lactamase inhibitors such as clavulanic acid, sulbactam, and tazobactam.
Extended-spectrum β-lactamases (ESBL) cause resistance to most β-lactam antibiotics with the exceptions of the cephamycins (cefoxitin, cefotetan) and carbapenems. The most common bacteria carrying ESBL are Klebsiella spp. and Escherichia coli. Less commonly Enterobacter, Serratia, Morganella, Proteus, and Pseudomonas aeruginosa spp. may harbor these genes. The genes for these enzymes are carried on chromosomes or plasmids and are thus transferrable between bacteria. ESBL-producing bacteria are also often resistant to aminoglycosides and quinolones. These enzymes are usually inhibited by β-lactamase inhibitors such as clavulanic acid, sulbactam, and tazobactam.
 AmpC cephalosporinases are β-lactamases that confer resistance to cephalosporin antibiotics (including cephamycins) by Enterobacter, Nitrobacteria, Morganella, Serratia, and P. aeruginosa. In contrast to ESBL enzymes, they are most often chromosomally encoded and not transferable. Paradoxically they are inducible by third-generation cephalosporins. More recently these genes have been found on transferable plasmids but are not inducible. These enzymes are often resistant to β-lactamase inhibitors.
AmpC cephalosporinases are β-lactamases that confer resistance to cephalosporin antibiotics (including cephamycins) by Enterobacter, Nitrobacteria, Morganella, Serratia, and P. aeruginosa. In contrast to ESBL enzymes, they are most often chromosomally encoded and not transferable. Paradoxically they are inducible by third-generation cephalosporins. More recently these genes have been found on transferable plasmids but are not inducible. These enzymes are often resistant to β-lactamase inhibitors.
 Carbapenemases are enzymes that can inactivate the carbapenems (meropenem, imipenem-cilastatin, ertapenem, and doripenem). These enzymes may also be able to inactivate all classes of β-lactam antibiotics and are resistant to β-lactamase inhibitors. Organisms found to carry these MDR genes include P. aeruginosa, Acinetobacter, Stenotrophomonas, Klebsiella, Serratia, Enterobacter, E. coli, and Citrobacter. In 2009, a new carbapenemase was isolated in pathogens from New Delhi, India, called the New Delhi metallo-β-lactamase-1 (NDM-1). Bacteria harboring the NDM-1 include Klebsiella, E. coli, Enterobacter, Nitrobacteria, Morganella, Providencia, Acinetobacter, and P. aeruginosa. The resistance gene is carried by a plasmid and is thus transferable between bacteria. Isolates have now been found in Europe and the United States. Transmission of aminoglycoside and quinolone resistance may be carried by other genes on the plasmid.
Carbapenemases are enzymes that can inactivate the carbapenems (meropenem, imipenem-cilastatin, ertapenem, and doripenem). These enzymes may also be able to inactivate all classes of β-lactam antibiotics and are resistant to β-lactamase inhibitors. Organisms found to carry these MDR genes include P. aeruginosa, Acinetobacter, Stenotrophomonas, Klebsiella, Serratia, Enterobacter, E. coli, and Citrobacter. In 2009, a new carbapenemase was isolated in pathogens from New Delhi, India, called the New Delhi metallo-β-lactamase-1 (NDM-1). Bacteria harboring the NDM-1 include Klebsiella, E. coli, Enterobacter, Nitrobacteria, Morganella, Providencia, Acinetobacter, and P. aeruginosa. The resistance gene is carried by a plasmid and is thus transferable between bacteria. Isolates have now been found in Europe and the United States. Transmission of aminoglycoside and quinolone resistance may be carried by other genes on the plasmid.
8 What MDR gram-positive bacteria pose the greatest risk in the ICU?
 MRSA is resistant to all penicillins and cephalosporins and often resistant to clindamycin and quinolones. Long known as a nosocomial pathogen, MRSA is now appearing as community-acquired infections. MRSA can cause pneumonias, severe abscesses with cellulitis, and bloodstream and central line infections. It is not a classic urinary pathogen, and its presence in the urine should raise the suspicion of bacteremia. Vancomycin remains the “go to” drug although resistance is emerging more commonly. For those isolates with a minimum inhibitory concentration of > 1 mcg/mL, vancomycin may not be effective, especially for endocarditis, bloodstream infections, and pneumonia.
MRSA is resistant to all penicillins and cephalosporins and often resistant to clindamycin and quinolones. Long known as a nosocomial pathogen, MRSA is now appearing as community-acquired infections. MRSA can cause pneumonias, severe abscesses with cellulitis, and bloodstream and central line infections. It is not a classic urinary pathogen, and its presence in the urine should raise the suspicion of bacteremia. Vancomycin remains the “go to” drug although resistance is emerging more commonly. For those isolates with a minimum inhibitory concentration of > 1 mcg/mL, vancomycin may not be effective, especially for endocarditis, bloodstream infections, and pneumonia.
 VRE have become increasingly common. They can be part of bowel flora and colonize the urinary tract. They can be nosocomial pathogens causing blood, urinary tract, and central catheter infections and endocarditis.
VRE have become increasingly common. They can be part of bowel flora and colonize the urinary tract. They can be nosocomial pathogens causing blood, urinary tract, and central catheter infections and endocarditis.
9 What are the treatment options for these MDR gram positives?
 Vancomycin interferes with bacterial cell wall synthesis by blocking penicillin-binding proteins. It is active against MRSA and vancomycin-sensitive enterococci. Vancomycin-intermediate S. aureus (VISA) isolates have been found clinically, and fear remains concerning vancomycin-resistant S. aureus (VRSA) isolates.
Vancomycin interferes with bacterial cell wall synthesis by blocking penicillin-binding proteins. It is active against MRSA and vancomycin-sensitive enterococci. Vancomycin-intermediate S. aureus (VISA) isolates have been found clinically, and fear remains concerning vancomycin-resistant S. aureus (VRSA) isolates.
 Daptomycin is the first of a new class of antibiotics called lipopeptides that interfere with gram-positive bacterial cell membrane function. It has activity against MRSA, some VISA, and VRE. Daptomycin may cause myositis, and creatine phosphokinase levels need to be monitored. Daptomycin is inactivated by pulmonary surfactant and should not be used to treat pneumonia.
Daptomycin is the first of a new class of antibiotics called lipopeptides that interfere with gram-positive bacterial cell membrane function. It has activity against MRSA, some VISA, and VRE. Daptomycin may cause myositis, and creatine phosphokinase levels need to be monitored. Daptomycin is inactivated by pulmonary surfactant and should not be used to treat pneumonia.
 Linezolid is the first oxazolidinone antibiotic. It interferes with bacterial protein synthesis by binding to the 50 S ribosome. It is bacteriostatic with activity against MRSA and VRE. It can be used orally or intravenously. Linezolid can cause thrombocytopenia.
Linezolid is the first oxazolidinone antibiotic. It interferes with bacterial protein synthesis by binding to the 50 S ribosome. It is bacteriostatic with activity against MRSA and VRE. It can be used orally or intravenously. Linezolid can cause thrombocytopenia.
 Quinupristin-dalfopristin (Synercid) is a combination streptogramin antibiotic that interferes with the bacterial 50 S ribosome. It has activity against MRSA. Although it does not have activity against Enterococcus faecalis, it does work against Enterococcus faecium including VRE.
Quinupristin-dalfopristin (Synercid) is a combination streptogramin antibiotic that interferes with the bacterial 50 S ribosome. It has activity against MRSA. Although it does not have activity against Enterococcus faecalis, it does work against Enterococcus faecium including VRE.
 Ceftaroline fosamil is a new cephalosporin with activity against MRSA and penicillin-resistant S. pneumoniae. As with all cephalosporins, there is no enterococcal activity including VRE.
Ceftaroline fosamil is a new cephalosporin with activity against MRSA and penicillin-resistant S. pneumoniae. As with all cephalosporins, there is no enterococcal activity including VRE.
 Televancin is a new lipoglycopeptide antibiotic that inhibits cell wall synthesis and disrupts bacterial cell membrane function. It has activity against MRSA and enterococci but not VRE.
Televancin is a new lipoglycopeptide antibiotic that inhibits cell wall synthesis and disrupts bacterial cell membrane function. It has activity against MRSA and enterococci but not VRE.
 Older agents, such as doxycycline, may have activity against both MRSA and VRE. Trimethoprim-sulfamethoxazole (TMP-SMX) may have activity against MRSA. Clindamycin may have MRSA activity, but it is important to check for erythromycin resistance because that may predict inducible clindamycin resistance.
Older agents, such as doxycycline, may have activity against both MRSA and VRE. Trimethoprim-sulfamethoxazole (TMP-SMX) may have activity against MRSA. Clindamycin may have MRSA activity, but it is important to check for erythromycin resistance because that may predict inducible clindamycin resistance.
10 What MDR gram-negative bacteria pose the greatest risk in the ICU?
 Enterobacteriaceae that have increasing MDR include E. coli, Klebsiella, and Enterobacter. Resistance is most often conferred by ESBL, AmpC cephalosporinases, and carbapenemases. These organisms may be urinary, wound, or respiratory colonizers but also cause a wide array of nosocomial infections including pneumonia, bacteremia, and urinary tract infections. Agents with possible activity include carbapenems, aminoglycosides, tigecycline, and colistin.
Enterobacteriaceae that have increasing MDR include E. coli, Klebsiella, and Enterobacter. Resistance is most often conferred by ESBL, AmpC cephalosporinases, and carbapenemases. These organisms may be urinary, wound, or respiratory colonizers but also cause a wide array of nosocomial infections including pneumonia, bacteremia, and urinary tract infections. Agents with possible activity include carbapenems, aminoglycosides, tigecycline, and colistin.
 P. aeruginosa has the greatest ability to develop resistance. It has minimal nutritional requirements, which accounts for its successful growth in many environments and its ability to colonize endotracheal tubes and urinary catheters in the ICU. P. aeruginosa may cause pulmonary, bloodstream, central line, and urinary tract infections. In the host with neutropenia, necrotic skin lesions, ecthyma gangrenosum, can occur. Resistance is mediated by ESBL, AmpC cephalosporinases, carbapenemases, efflux pumps, and outer membrane porin mutations. See Figure 36-1. No evidence exists that using two antibiotics for synergy improves outcomes or reduces emerging resistance. Antibiotics that may retain activity against MDR P. aeruginosa include colistin and, in some situations, doripenem.
P. aeruginosa has the greatest ability to develop resistance. It has minimal nutritional requirements, which accounts for its successful growth in many environments and its ability to colonize endotracheal tubes and urinary catheters in the ICU. P. aeruginosa may cause pulmonary, bloodstream, central line, and urinary tract infections. In the host with neutropenia, necrotic skin lesions, ecthyma gangrenosum, can occur. Resistance is mediated by ESBL, AmpC cephalosporinases, carbapenemases, efflux pumps, and outer membrane porin mutations. See Figure 36-1. No evidence exists that using two antibiotics for synergy improves outcomes or reduces emerging resistance. Antibiotics that may retain activity against MDR P. aeruginosa include colistin and, in some situations, doripenem.
 Acinetobacter calcoaceticus (80% of clinical isolates) and Acinetobacter baumannii have limited nutritional requirements, are resistant to many disinfectants, and can easily contaminate environmental surfaces. Approximately 25% of the healthy population are colonized. All of these factors contribute to colonization and infection in the ICU. Infections may occur in the lung, bloodstream, urinary tract, and traumatic wounds. Acinetobacter have multiple β-lactamases, loss of outer-membrane porin channels, and efflux pumps causing MDR to most β-lactams, quinolones, and aminoglycosides. Isolates may be sensitive to carbapenems, tigecycline, ampicillin-sulbactam, TMP-SMX, rifampicin, and colistin.
Acinetobacter calcoaceticus (80% of clinical isolates) and Acinetobacter baumannii have limited nutritional requirements, are resistant to many disinfectants, and can easily contaminate environmental surfaces. Approximately 25% of the healthy population are colonized. All of these factors contribute to colonization and infection in the ICU. Infections may occur in the lung, bloodstream, urinary tract, and traumatic wounds. Acinetobacter have multiple β-lactamases, loss of outer-membrane porin channels, and efflux pumps causing MDR to most β-lactams, quinolones, and aminoglycosides. Isolates may be sensitive to carbapenems, tigecycline, ampicillin-sulbactam, TMP-SMX, rifampicin, and colistin.
 Stenotrophomonas maltophilia is most often encountered in the nosocomial setting in patients with prior broad-spectrum antibiotic exposure. It is often isolated in patients with cystic fibrosis. The most common infections include bacteremias related to central venous catheters and pneumonia. Resistance is mediated through β-lactamases, efflux pumps, and outer-membrane porin channel mutations. Although TMP-SMX and ticarcillin-clavulanic acid are often effective, reports are increasing of TMP-SMX resistance. Other agents that may have activity include ceftazidime, aztreonam, minocycline, tigecycline, and ciprofloxacin.
Stenotrophomonas maltophilia is most often encountered in the nosocomial setting in patients with prior broad-spectrum antibiotic exposure. It is often isolated in patients with cystic fibrosis. The most common infections include bacteremias related to central venous catheters and pneumonia. Resistance is mediated through β-lactamases, efflux pumps, and outer-membrane porin channel mutations. Although TMP-SMX and ticarcillin-clavulanic acid are often effective, reports are increasing of TMP-SMX resistance. Other agents that may have activity include ceftazidime, aztreonam, minocycline, tigecycline, and ciprofloxacin.
11 What are the treatment options for these MDR gram negatives?
 Carbapenems should remain active against ESBL and AmpC-producing bacteria. Rates of P. aeruginosa, Acinetobacter, and S. maltophilia resistance are increasing.
Carbapenems should remain active against ESBL and AmpC-producing bacteria. Rates of P. aeruginosa, Acinetobacter, and S. maltophilia resistance are increasing.
 Colistin is polymyxin E, a cationic polypeptide antibiotic that binds to the lipopolysaccharides of the GNR outer membrane to disrupt it and cause cell death. It has been used successfully intravenously and as an aerosol to treat MDR Acinetobacter. It may be used for MDR P. aeruginosa and carbapenemase-producing Enterobacteriaceae. Serratia, Proteus, and S. maltophilia are resistant. Nephrotoxicity and neurotoxicity are the major toxicities.
Colistin is polymyxin E, a cationic polypeptide antibiotic that binds to the lipopolysaccharides of the GNR outer membrane to disrupt it and cause cell death. It has been used successfully intravenously and as an aerosol to treat MDR Acinetobacter. It may be used for MDR P. aeruginosa and carbapenemase-producing Enterobacteriaceae. Serratia, Proteus, and S. maltophilia are resistant. Nephrotoxicity and neurotoxicity are the major toxicities.
 Tigecycline is a parenteral derivative of minocycline. It may have activity against ESBL and carbapenemase-producing Enterobacteriaceae, S. maltophilia, and Acinetobacter. P. aeruginosa and Proteus are resistant. Because of low urinary concentrations, it should not be used to treat urinary tract infections.
Tigecycline is a parenteral derivative of minocycline. It may have activity against ESBL and carbapenemase-producing Enterobacteriaceae, S. maltophilia, and Acinetobacter. P. aeruginosa and Proteus are resistant. Because of low urinary concentrations, it should not be used to treat urinary tract infections.
12 Control of MDR bacteria
Key Points Multidrug-Resistant Bacteria
1. GNR and GPC MDR bacteria are becoming an increasingly greater problem.
2. Increasing bacterial resistance is caused by excessive antibiotic usage both by physicians and in animal feeds.
3. Before initiating antibiotic therapy it is vital to differentiate between colonization and infection.
4. Many new agents exist with activity against MDR staphylococcus and enterococcus.
5. Because of the lack of new agents with activity against MDR GNR, older drugs such as colistin will be required more often.
1 Garnacho-Montero J., Amaya-Villar R. Multiresistant Acinetobacter baumannii infections: epidemiology and management. Curr Opin Infect Dis. 2010;23:332–339.
2 Hoa J., Tambyaha P.A., Paterson D.L. Multi-resistant gram-negative infections: a global perspective. Curr Opin Infect Dis. 2010;23:546–553.
3 Kumarasamy K.K., Toleman M.A., Walsh T.R., et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597–602.
4 Niederman M.S., Craven D., Bonten M.J., et al. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416.
5 Nikaido H. Multidrug resistance in bacteria. Annu Rev Biochem. 2009;78:119–146.
6 Overdevest I., Willemsen I., Rijnsburger M., et al. Extended-spectrum β-lactamase genes of Escherichia coli in chicken meat and humans, the Netherlands. Emerg Infect Dis. 2011;17:1216–1222.
7 Roberts R.R., Hota B., Ahmad I., et al. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis. 2009;49:1175–1184.
8 Toleman M.A., Bennett P.M., Bennett D.M.C., et al. Global emergence of trimethoprim/sulfamethoxazole resistance in Stenotrophomonas maltophilia mediated by acquisition of sul genes. Emerg Infect Dis. 2007;13:556–565.