CHAPTER 57 MULTIDISCIPLINARY MANAGEMENT OF PELVIC FRACTURES: OPERATIVE AND NONOPERATIVE HEMOSTASIS
Very few injuries are as complicated as multisystem trauma and pelvic fracture. The pelvis is a complex anatomic region. The bony pelvis affords great protection to the structures it contains. Within the pelvis are important gastrointestinal, genitourinary, vascular, and neurologic structures. The force necessary to fracture a pelvis is extreme. Therefore, every pelvic fracture must be assumed to be a high-energy injury. The proximity of the pelvis to the abdomen makes combined injuries common. Patients with pelvic fracture often have other associated injuries as well. Over 50% will have either traumatic brain injury or associated long bone fracture.
PELVIC BLEEDING: MAKING THE DIAGNOSIS
It is important to distinguish between patients with skeletally unstable pelvic fractures and patients who are hemodynamically unstable. Skeletal stability describes the bony architecture of the pelvic fracture. Hemodynamic stability describes the patient’s physiologic response. Not all patients with skeletally unstable pelvic fractures are hemodynamically unstable. In addition, patients who have skeletally stable fractures can still lose a substantial amount of blood into their retroperitoneum.
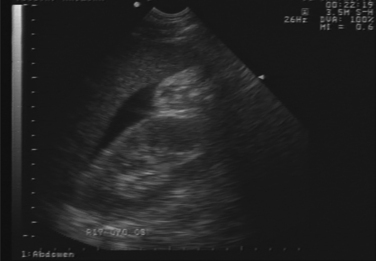
Patients with a pelvic fracture, a positive FAST, and hemodynamic instability are almost certainly best served by an immediate laparotomy. In most patients, the FAST turns positive with 200–300 cc of fluid in the abdomen (Figure 1). While free fluid could certainly be from a relatively minor intra-abdominal injury or a ruptured hollow viscus such as the bladder, diagnostic laparotomy is probably the most rapid and definitive test in patients who are hemodynamically unstable. If minor injury is found and bleeding is thought to be coming from the pelvis, abbreviated laparotomy should be performed and other plans made to control the pelvic bleeding.
PELVIC FRACTURE CLASSIFICATIONS
A number of classification schemes are available that describe the bony architecture of pelvic fractures. Probably the most commonly used scheme was described by Young and Burgess in 1986 and classifies pelvic fractures by their vector of force (Table 1). Each classification is subdivided to describe the degree of pelvic instability. The authors originally thought that this classification scheme could predict the need for transfusions. While this may actually not be the case, it is quite useful in describing fracture anatomy and guiding initial attempts at hemostasis.
| Anteroposterior Compression | |
|---|---|
| Type I | Disruption of pubic symphysis of >2.5 cm of diastasis; no significant posterior pelvic injury |
| Type II | Disruption of pubic symphysis of <2.5 cm, with tearing of anterior sacroiliac and sacrospinous and sacrotuberous ligaments |
| Type III | Complete disruption of pubic symphysis and posterior ligament complexes, with hemipelvic displacement |
| Lateral Compression | |
|---|---|
| Type I | Posterior compression of sacroiliac joint without ligament disruption; oblique pubic ramus fracture |
| Type II | Rupture of posterior sacroiliac ligament; pivotal internal rotation of hemipelvis on anterior sacroiliac joint with a crush injury of sacrum and an oblique public ramus fracture |
| Type III | Findings in type II injury with evidence of an anteroposterior compression injury to contralateral hemipelvis |
Data from Young JWR, Brumback RJ, Poka A: Pelvic fractures: value of plain radiography in early assessment and management. Radiology 160:445, 1986.
Lateral compression (LC) pelvic fractures caused by side impact generally occur after T-bone vehicular crashes or car–pedestrian collisions (Figure 2). LC fractures cause an acute shortening of the pelvic diameter. The pelvis does not open but closes down. The pelvic ligaments generally stay intact. Thus, these fractures generally do not bleed. Hemodynamic instability after a lateral compression fracture more likely results from torso injuries such as intra-abdominal bleeding or intrathoracic bleeding. There is a known association with traumatic aortic injury and LC pelvic fractures.
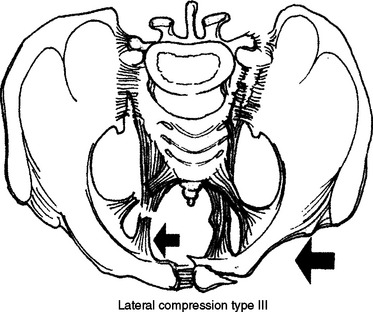
Figure 2 Lateral compression pelvic fracture.
(From Moore EE, Feliciano DV, Mattox KL: Trauma, 5th ed. New York, McGraw-Hill, 2003.)
Anteroposterior compression (AP) fractures generally occur after head-on vehicular crashes or may occur after equestrian injury, typically when patients are thrown from a horse or the horse lands on them (Figure 3). With this mechanism, pelvic diameter widens and the pelvis opens. The injuries can be purely ligamentous if the sacroiliac (SI) joints rupture, even in the absence of significant bony injury. Pelvic vascular injuries are quite common. AP compression fractures have the highest chance of bleeding, and transfusion requirements are the greatest in patients with these fractures.
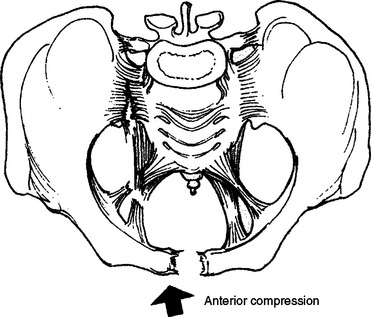
Figure 3 Anterior/posterior compression fracture.
(From Moore EE, Feliciano DV, Mattox KL: Trauma, 5th ed. New York, McGraw-Hill, 2003.)
Vertical shear (VS) injuries occur when patients land on an outstretched foot, which generally occurs after falling from a height (Figure 4). VS fractures can also occur in motorcycle crashes, particularly if patients are riding with their legs outstretched. In vertical shear fractures, the force is transmitted up the axial skeleton through the posterior pelvis. Posterior fractures and/or ligamentous ruptures are quite common. If there is a complete disruption of both the anterior and posterior elements (Malgaigne fracture), the psoas muscle pulls the hemipelvis cephalad without opposition. This may be seen on a pelvic x-ray. Vertical shear injuries have an intermediate risk of bleeding.
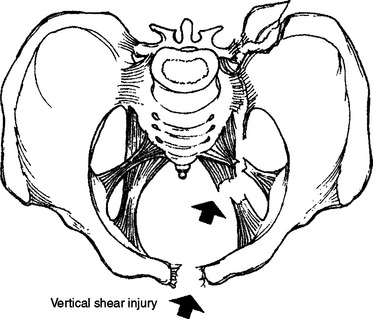
Figure 4 Vertical shear fracture.
(From Moore EE, Feliciano DV, Mattox KL: Trauma, 5th ed. New York, McGraw-Hill, 2003.)
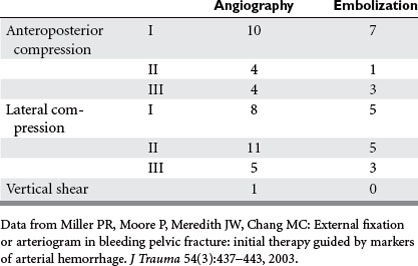
The notion that fracture anatomy could predict bleeding has been questioned recently. The group that Wake Forest reported on the use of angiography and embolization for pelvic hemostasis (Table 2). In their series, low grade AP compression fractures required angiography most often. Seventy percent of AP I injury were treated with embolization. This was substantially more common in AP II and about the same as AP III. In addition, the same was true for lateral compression fractures: nearly 70% of LC I fractures were treated without angiography and required embolization, about the same as LC II or LC III fractures.
TREATING PELVIC FRACTURE BLEEDING
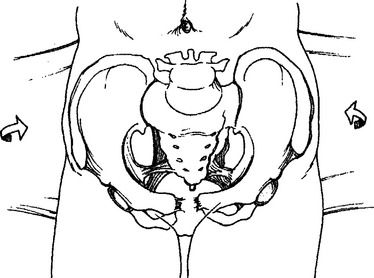
A number of devices are available to help achieve external compression. Perhaps the simplest is using a bed sheet. Ideally, the sheet is placed on the stretcher, even before the patient arrives. Alternatively, the patient can be gently lifted and the bed sheet placed underneath the patient. The bed sheet is then crisscrossed across the pelvis anteriorly and tied down (Figure 5). This low-tech technique has proven to be extraordinarily helpful. It can be especially helpful in small community emergency departments (EDs) that have limited resources and may not have sophisticated technology. We generally advise the emergency physician to tie the bed sheet down snugly but not excessively tightly. Patients can then be transported within the hospital or to a higher level of care.
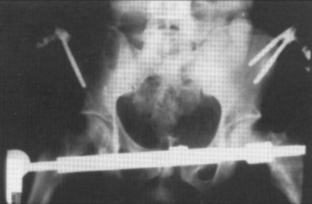
In the past, external fixation was very commonly used as a compressive device for pelvic hemostasis. External fixation is the most rigid of the external devices and closes the pelvis down definitively (Figure 6). In centers with expertise, external fixators which can be rapidly applied in the ED. In other centers, this is often placed in the operating room similar to the C clamp. In some cases, external fixation may provide definitive fracture fixation. The anterior portion of the frame can be rotated similar to the C clamp to allow access for angiography and/or laparotomy.
Many American trauma centers have abandoned virtually all other external compressive devices and exclusively use a commercially available pelvic binder. The pelvic binder is a Velcro device that applies even direct pressure on the pelvis (Figure 7). The pressure is set by the Velcro and lace system on the anterior portion of the binder. The binders can be applied rapidly and require a minimum of expertise. The binder should be applied across the femoral trochanters, not across the lower abdomen. Correct placement of the binder can limit access to the groins for angiography. If angiography is required, a hole can be cut in the binder or the binder must be placed slightly higher.
Angiographic embolization for pelvic hemostasis has been used for over 30 years. Diagnostic pelvic angiography should be able to identify all sites of pelvic arterial injury. Embolization with Gelfoam, stainless steel coils, or both can be quite effective in achieving pelvic hemostasis. The more liberal the use of angiography, the less the yield will be. Conversely, withholding angiography until a patient is in extremis, increases the yield of angiography but is probably not the wisest patient care. Our indications for angiography are in Table 3. These were developed nearly 20 years ago and are in current use.
Data from Moore EE, Feliciano DV, Mattox KL: Trauma, 5th ed. New York, McGraw-Hill, 2003.
Some patients almost certainly benefit from an attempt at operative hemostasis. Patients that present in hemorrhagic shock and have a unilateral absence of a femoral pulse likely have injury to either the common or external iliac artery. Typically, these patients have a traumatic hemipelvectomy (Figure 8). An extreme amount of force is necessary to produce this injury, and these patients usually are in refractory shock. A direct operative approach utilizing medial visceral rotation, combined with a direct approach to the iliac arteries is potentially life saving for these patients.
Early rigid fracture fixation is ideal, and definitive reduction of the fracture fragments can be quite helpful in achieving hemostasis. However, early open reduction and internal fixation risks torrential blood loss. Percutaneous SI screw fixation can reduce the posterior pelvis with minimal blood loss. These percutaneous screws are threaded under fluoroscopic guidance and rigidly reduce the pelvis. We typically use this technique with AP II or AP III fractures. The patients must be stable enough to undergo the operative procedure. This technique is not recommended for patients in profound shock. Percutaneous SI screws allow for good reduction of the pelvic fracture, reduction of pelvic volume, and clot stabilization. Once the fracture is definitively fixed, patients can be mobilized out of bed, limiting pulmonary complications.
Rather than approaching the pelvis via a laparotomy, it is wisest to pack via a retroperitoneal approach and approach the patient through a low midline incision. The pelvic hematoma has lifted the abdominal contents up out of the pelvis (Figure 9). The lower abdominal muscles are split in the midline and the pelvic hematoma entered directly. The pelvic hematoma must be completely evacuated and the pelvis then packed. It is important to stay extraperitoneal. When the packs are applied up against the peritoneum, the peritoneum increases the tamponade effect.
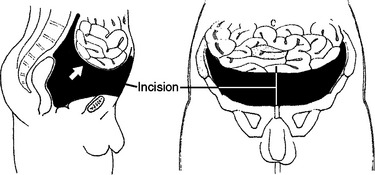
Figure 9 Low midline incision to approach pelvic hematoma.
(From Moore EE, Feliciano DV, Mattox KL: Trauma, 5th ed. New York, McGraw-Hill, 2003.)
Virtually any material can be used for pelvic packing, such as towels, gauze, or laparotomy pack. We have favored the use of homemade fibrin bandages. These mesh packs can be constructed by using fibrin sealant and a Vicryl mesh. The mesh can be folded to the desired size (Figures 10 and 11). We generally pack these deep into the pelvis and supplement hemostasis with lap pads or towels.
MANAGEMENT OF OPEN PELVIC FRACTURES
It is important to characterize the anatomy of the pelvic fracture. It can generally be accomplished with a combination of physical exam and pelvic x-ray. The pelvic fracture should be reduced using any of the compressive devices discussed previously. The entire team should be alerted, including the operating room, the orthopedic service, and the angiography suite. Even if the bleeding looks venous and/or is controlled with packing, we advocate angiography for these patients.
1 Burgess AR, Eastridge BJ, Young JWR. Pelvic ring disruptions: effective classification system and treatment protocols. J Trauma. 1990;30:848.
2 Ben-Menachem Y. Embolotherapy in pelvic trauma. In: Neal MP, Tisnado J, Chu SR, editors. Emergency Interventional Radiology. Boston: Little & Brown, 1989.
3 Scalea TM, Burgess AR. Pelvic fractures. In Moore EE, Feliciano DV, Mattox KL, editors: Trauma, 5th ed., New York: McGraw-Hill Companies, 2004.
4 Miller PR, Moore PS, Mansell E, Meredith JW, Chang MC. External fixation or arteriogram in bleeding pelvic fracture: initial therapy guided by markers of arterial hemorrhage. J Trauma. 2003;54:437-443.