Mucus-controlling drug therapy
After reading this chapter, the reader will be able to:
1. Define terms that pertain to mucus-controlling drug therapy
2. Interpret the physiology and mechanisms of mucus secretion and clearance
3. Name the types of mucoactive medications and their presumed modes of action
4. Describe the medications approved for the therapy of mucus clearance disorders and their approved indications
5. Identify the contraindications to the use of mucoactive medications
6. Explain the interaction between airway clearance devices or physical therapy and mucoactive medications
Drug control of mucus: a perspective
The self-renewing, self-cleansing mucociliary escalator is a major defense mechanism of the lung. Failure of this system results in mechanical obstruction of the airway, often with thickened, adhesive secretions. A slowing of mucus transport is reported in many diseases associated with abnormal mucociliary function.1,2 Whether such slowing is due to changes in the physical properties of mucus or to decreased ciliary activity, or both, is not always clear. Mucus is found in several areas of the body, including the airways, gastrointestinal tract, and genital tract. Regardless of its location, mucus is protective, lubricating, and waterproofing, and it protects against osmotic or inflammatory changes. The mucus barrier can also entrap microorganisms, inhibiting chronic bacterial infection and biofilm formation.
Clinical indication for use
The general indication for mucoactive therapy is to reduce the accumulation of airway secretions, with concomitant improvement in pulmonary function and gas exchange and the prevention of repeated infection and airway damage. Diseases in which mucoactive therapy is indicated are those with hypersecretion or poor clearance of airway secretions, including cystic fibrosis (CF), acute bronchitis and chronic bronchitis (CB), pneumonia, diffuse panbronchiolitis (DPB), primary ciliary dyskinesia, asthma, and bronchiectasis. Not all patients with mucus retention benefit from mucoactive drug therapy; patients with adequately preserved expiratory airflow and cough usually have a better response. The use of mucoactive therapy to promote secretion clearance should be considered after therapy to decrease infection and inflammation and after minimizing or removing irritants to the airway, including tobacco smoke.3
Identification of agents
Table 9-1 presents general information about mucoactive agents that are approved, available, or commonly administered as inhaled aerosols in the United States. Table 9-2 presents similar information for other countries.4 Greater detail is given on indications, dosage and administration, hazards and side effects, and assessment of drug therapy in discussions of individual agents.
TABLE 9-1
Mucoactive Agents Available for Aerosol Administration
| DRUG | BRAND NAME | ADULT DOSAGE | USE |
| N-Acetylcysteine (NAC) | 10% Mucomyst, 20% Mucomyst | SVN: 3-5 mL | Bronchitis, efficacy not proven for any dose of NAC for any lung disease |
| Dornase alfa | Pulmozyme | SVN: 2.5 mg/ampoule, one ampoule daily* | Cystic fibrosis |
| Aqueous aerosols: water, saline | — | SVN: 3-5 mL, as ordered | Sputum induction, secretion, mobilization |
| USN: 3-5 mL, as ordered | |||
| Hyperosmolar (7%) saline | Hyper-Sal | SVN: 4 mL | Airway clearance (mucokinetics) for therapy of cystic fibrosis |
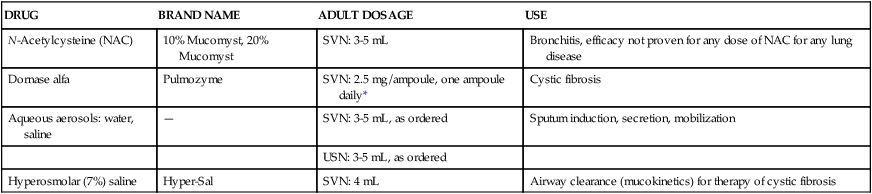
TABLE 9-2
International Pharmacopoeia Listing of Mucolytic Drugs
| AGENT | ||||||||||||||||
| COUNTRY | NAC | Amb | Brom | Carbo | Dornase | Epraz | Erdos | Eth-cys | Guaif | Letos | MESNA | Meth-cys | Sob | Stepro | Thiop | TOTAL |
| Argentina | + | + | + | − | − | − | − | − | − | − | − | − | − | − | − | 3 |
| Australia | + | − | + | − | + | − | − | − | − | − | − | − | − | − | − | 3 |
| Belgium | + | + | + | + | + | + | + | − | + | − | + | − | − | − | − | 9 |
| Brazil | + | + | + | + | + | − | − | − | − | − | − | − | + | − | − | 6 |
| Finland | + | − | + | + | − | − | + | − | + | − | − | − | − | − | − | 5 |
| France | + | + | + | + | − | − | + | − | − | + | − | − | − | − | − | 6 |
| Germany | + | + | − | + | − | − | − | − | − | − | − | − | − | − | − | 3 |
| Ireland | + | − | + | + | + | − | − | − | + | − | − | + | − | − | − | 6 |
| Italy | + | + | + | + | + | − | − | − | + | + | − | − | + | + | + | 10 |
| Japan | + | + | + | + | − | + | − | + | + | − | − | + | − | − | − | 8 |
| Netherlands | + | − | + | + | + | − | − | − | − | − | + | − | − | − | − | 5 |
| New Zealand | + | − | + | − | + | − | − | − | − | − | − | − | − | − | − | 3 |
| Russia | + | + | + | + | + | − | − | − | − | − | + | − | − | − | − | 6 |
| South Africa | + | − | + | + | − | − | − | − | + | − | + | − | − | − | − | 5 |
| Sweden | + | − | + | − | + | − | − | − | − | − | − | − | − | − | − | 3 |
| Switzerland | + | + | + | + | + | − | − | − | − | − | + | − | − | − | − | 6 |
| Taiwan | + | + | + | + | − | − | − | − | + | − | − | + | − | − | − | 6 |
| United Kingdom | − | − | − | + | + | − | − | − | − | − | − | + | − | − | − | 3 |
| U.S. | − | − | − | − | + | − | − | − | + | − | − | − | − | − | − | 2 |
| Total | 17 | 10 | 16 | 14 | 12 | 2 | 3 | 1 | 8 | 2 | 5 | 4 | 2 | 1 | 1 |
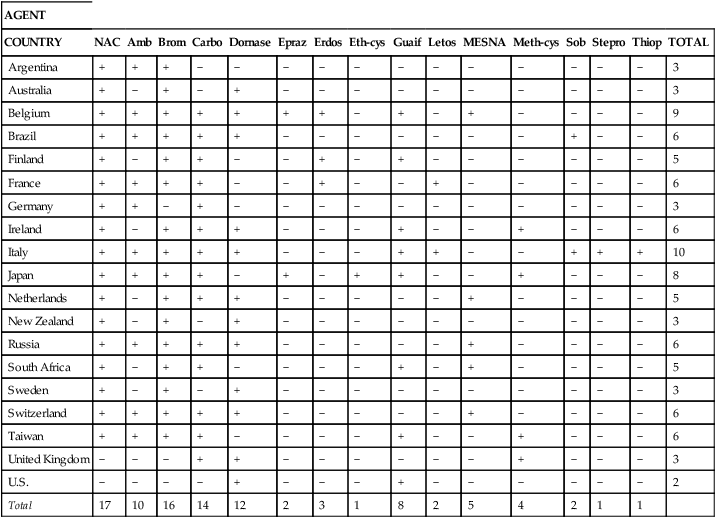
+, In pharmacopoeias (see reference); −, not in pharmacopoeias.
From Rogers DF: Mucoactive drugs for asthma and COPD: any place in therapy? Expert Opin Investig Drugs 11:15, 2002.
Physiology of the mucociliary system
Source of airway secretions
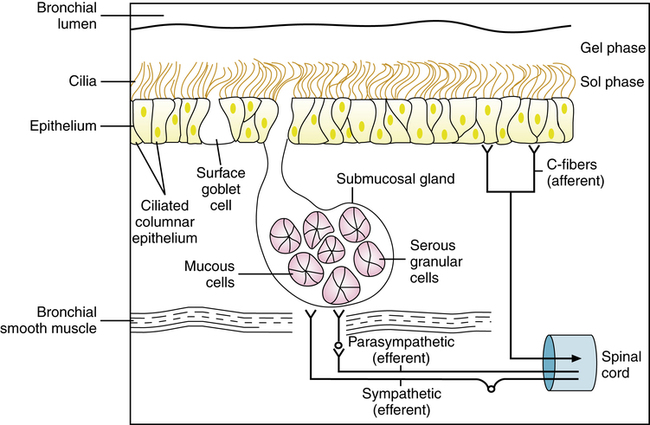
The conducting airways in the lung and the nasal cavity to the oropharynx are lined by a mucociliary system, illustrated diagrammatically in Figure 9-1. The secretion lining the surface of the airway is called mucus and has been described as having two phases: (1) A gel layer (0.5 to 20 μm) is propelled toward the larynx by the cilia and floats on top of (2) a watery periciliary layer (7 μm, the height of a fully extended cilium).3 Cells responsible for secretion in the airway and the source of components found in respiratory mucus have been summarized by Basbaum and associates.5 Although there are many cell types in the mammalian airway, the essential secretory structures of the mucociliary system are the following:
Submucosal glands are found in cartilaginous airways. These mucus-producing cells are not found beyond the distal airways. Mucus secreted by surface epithelial cells and glands in the airway provide for basic protection of the respiratory tract, including humidification and warming of inspired gas, mucociliary transport of debris, waterproofing and insulation, and antibacterial activity.3
Terminology
There has been confusion regarding the nomenclature used to classify mucoactive medications. Although some authors have used “mucolytic” as a generic term for these agents, most of these medications are thought to mobilize secretions by mechanisms other than by the direct “thinning” of mucus. For example, although the mucociliary transportability of sputum may be improved by reducing sputum viscosity while preserving elasticity, the ability to clear secretions via cough seems to be greater with increased sputum viscosity and decreased adhesivity. Knowledge of mucus properties has given us tools to understand better the mechanisms of airway disease and mucoactive therapy. The currently accepted terminology is defined in the Key Terms and Definitions list at the start of this chapter. For more details on current terminology and its evolution, see Reid and Clamp,6 Basbaum,7 and King and Rubin.8
Surface epithelial cells
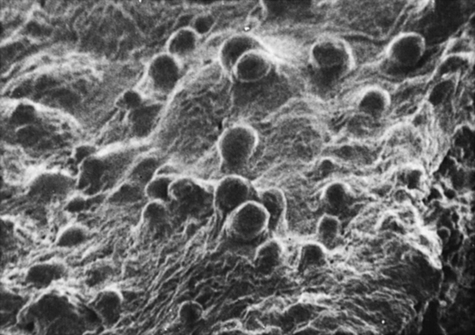
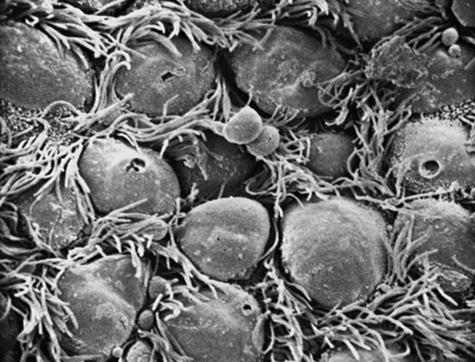
The surface of the trachea and bronchi includes primarily ciliated cells and goblet cells, at a ratio of approximately 5:1. There are more than 6000 goblet cells per square millimeter of normal airway mucosa. Goblet cells do not seem to be directly innervated in human lung, although they respond to irritants by increasing the production of mucus. Figures 9-2 and 9-3 show scanning electron micrographs of the mucus lining (Figure 9-2) and of the bronchiolar surface with the mucus stripped away (Figure 9-3). In addition to ciliated and goblet cells, microvilli, which may have a reabsorptive function, can be seen in Figure 9-3.
Submucosal mucous glands
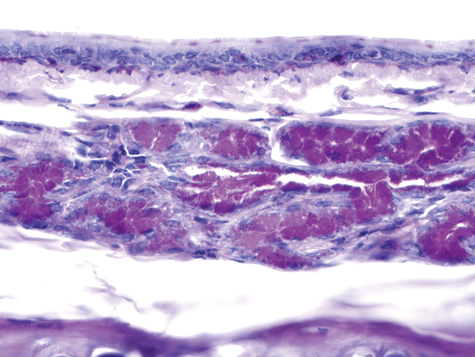
Two types of cells, mucous and serous, are found in the glands. Figure 9-4 shows a section of the ferret airway stained for mucin, with the surface mucous (goblet) cells and the submucosal gland serous and mucous cells identified. Secretions from the serous and mucous cells mix in the submucosal gland and are transported through a ciliated duct onto the airway lumen.
Ciliary system
Droplets of mucus from the secretory cells form plaques in the distal, nonciliated airway, and these coalesce into a continuous layer in the more proximal ciliated airway. Mucociliary transport results from the movement of the mucus gel by the beating cilia. There are approximately 200 cilia on each cell. Cilia are about 7 μm in length in larger airways and shorten to 5 μm or slightly less in smaller bronchioles. Luk and Dulfano9 examined the ciliary beat frequency on biopsy samples from various tracheobronchial regions and found rates of 8 to 18 Hz (Hz = 1 cycle/sec) at 37° C. A ciliary beat is composed of an effective (power) stroke and a recovery stroke with about a 1:2 ratio. In the effective stroke, the cilium moves in an upright position through a full forward arc, to contact the underside of the mucus layer and propel it forward. In the recovery stroke, the cilium swings back around to the starting point near the cell surface, to avoid pulling secretions back. Cilia beat in a coordinated or metachronal wave of motion to propel airway secretions. A functional surfactant layer lies at the tips of the cilia and separates the periciliary fluid from the mucus gel. This layer allows the cilia to transmit kinetic energy effectively to the mucus without becoming entangled. This layer also facilitates mucus spreading as a continuous layer and prevents water loss from the periciliary fluid. The properties of cilia are described in detail in an earlier review.10
Factors affecting mucociliary transport
• Chronic obstructive pulmonary disease (COPD) and CF
• Airway drying (e.g., with the use of dry gas for mechanical ventilation)
• Endotracheal suctioning, airway trauma, and tracheostomy
• Atmospheric pollutants (SO2, NO2, ozone) and allergens—these may increase transport, especially at low concentration, but at higher, toxic concentrations or with prolonged exposure, these decrease transport rates
Table 9-3 summarizes the effects of drug groups commonly used in respiratory care on ciliary beat, mucus output, and overall transport.
TABLE 9-3
Effects of Various Drug Groups on Mucociliary Clearance
| DRUG GROUP | CILIARY BEAT | MUCUS PRODUCTION | TRANSPORT |
| β-adrenergic agents | Increase | Increase* | ± |
| Cholinergic agents | Increase | Increase† | Increase |
| Methylxanthines | Increase | Increase† | ± |
| Corticosteroids | None | Decrease† | None |
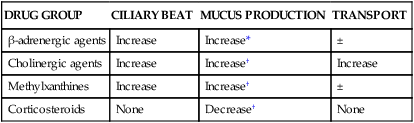
*Data from Wanner A: Clinical aspects of mucociliary transport, Am J Respir Dis 116:73, 1977.
†Data from Iravani J, Melville GN: Mucociliary activity in the respiratory tract as influenced by prostaglandin E, Respiration 32:305, 1975.
Food intake and mucus production
A common belief is that drinking dairy milk increases the production of mucus and congestion in the respiratory tract. Respiratory care personnel may be asked for advice on withholding milk from children with colds, respiratory infections, or chronic respiratory conditions such as CF. Pinnock et al.11 inoculated 60 healthy subjects with rhinovirus-2 in a study designed to answer the following question: Does milk make mucus? Milk intake ranged from 0 to 11 glasses a day. The investigators reported no association between milk and dairy product intake and upper or lower respiratory tract symptoms of congestion or nasal secretion weight. There was a trend for cough to be “loose” with increasing intake of milk, although this was not significant. None of the subjects were allergic to cow’s milk. The investigators concluded that the data do not support the withholding of milk or the belief that milk increases respiratory tract congestion.
Nature of mucus secretion
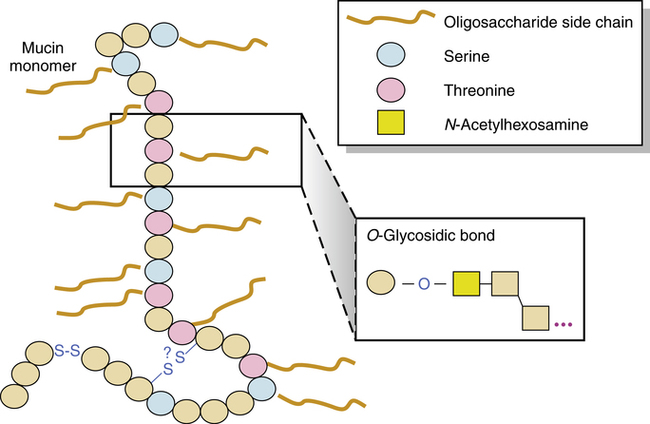
Structure and composition of mucus
The structure and major constituents of the mucus secreted by submucosal glands and surface goblet cells are pictured in Figure 9-5 and have been reviewed elsewhere.5,7,12–14a Airway mucus forms a protective barrier between the respiratory tract epithelium and the environment. Mucus is composed mainly of water and ions, with approximately 5% of the content from proteins secreted by airway cells and lipids.15–17 In health, the mucin glycoproteins are the major macromolecular component of the mucus gel. Mucins are responsible for the protective and clearance properties of mucus.18–20
There are two major classes of mucins: the secreted and the membrane-tethered mucins.21,22 Several secreted mucins (MUC2, MUC5AC, MUC5B, and MUC6) have genes that are clustered on chromosome 11p15 and contain domains with significant homology to the von Willebrand factor D domains that are sites for oligomerization.23 In sputum, MUC5AC and MUC5B are the major oligomeric mucins.24 MUC5AC apparently is produced primarily by the goblet cells in the tracheobronchial surface epithelium, whereas MUC5B is secreted primarily by the submucosal glands.25 The membrane-tethered mucins, MUC1, MUC3, MUC4, MUC12, and MUC13, contain a transmembrane domain and a short cytoplasmic domain.23 At least 12 mucin genes (MUC1, MUC2, MUC4, MUC5AC, MUC5B, MUC7, MUC8, MUC11, MUC13, MUC15, MUC19, and MUC20) have been observed at the mRNA level in tissues of the lower respiratory tract from healthy individuals.21,23,26–32
Mucus is a complex, high-molecular-weight macromolecule consisting of a mucin protein backbone to which carbohydrate (oligosaccharide) side chains are attached. At the time of this writing,22 distinct mucin proteins have been identified, but airway mucus is almost entirely composed of the MUC5AC and MUC5B secreted gel-forming mucins.27 The carbohydrate content is 80% or more of the total weight of the macromolecule. This structure is similar to a bottlebrush in appearance. This general structure of protein and attached oligosaccharide side chains is termed a glycoprotein. Mucin forms a flexible, threadlike strand 200 nm to 6 μm in length33 that is linearly cross-linked with disulfide bonds from adjacent cysteine residues. Strands may also be linked with each other by hydrogen bonding and van der Waals forces. The result is a gel that consists of a high water content (90% to 95%) organized around the structural elements and that is intensely hydrophilic and spongelike.34,35
Under normal circumstances, bonding within mucus produces low viscosity but moderate elasticity. Although mucus incorporates water during its formation, a gel acts like both a liquid and a solid. An analogy is gelatin, which is mostly water but organizes into a semisolid by its chemical structure as the liquid gels. It is important clinically that sufficient water must be available to form mucus with normal physical properties, but once formed, mucus does not readily incorporate topically applied water.36
Phospholipids are also present in the serous cell granules of the submucosal glands. When released onto the airway surface, phospholipids may serve as lubricants affecting the surface-active and adhesive properties of mucus, both of which can affect mucociliary transport function.3
In addition to the mucus gel secreted in the airway, bronchial secretions contain serum and secreted proteins, lipids, and electrolytes. Antibacterial defense in the airway is provided by mucin, secretory IgA, IgG, lysozyme, lactoferrin, defensins, and peroxidase and serine proteases. Bronchial secretions control the potentially destructive action of protease enzymes with two major antiproteases: α1-protease inhibitor and secretory leukoprotease inhibitor (sLPI), a cationic protein found in serous secretory glandular cells.3 In healthy airways, antiproteases are present in higher quantities than protease enzymes and provide a protease screen.37
Epithelial ion transport
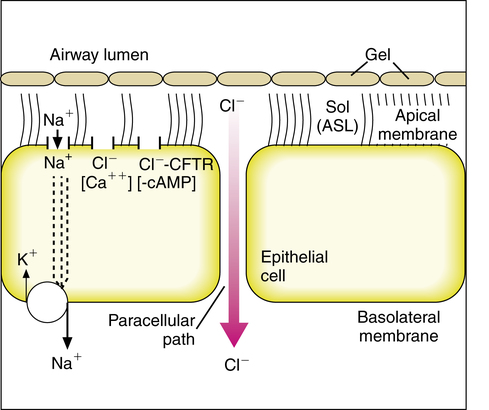
The composition and volume of the periciliary fluid layer is regulated in part by ion transport across the epithelial cells lining the airway lumen. If the periciliary layer is not approximately the height of an extended cilium, effective mucus movement cannot occur.38 Defective ion transport contributes to the cycle of retained secretions and infection seen in CF.39,40 Normal airway epithelial ion transport is illustrated in Figure 9-6.
In the basal, unstimulated state, sodium (Na+) absorption into the epithelial cell is the dominant ion exchange that absorbs liquid from the airway periciliary layer. Sodium absorption occurs as an active transport process through sodium channels (epithelial sodium channel [ENaC]) on the apical (airway) side of the cell. Sodium in the epithelial cell is pumped from the cell, driven by a sodium/potassium-ATPase pump on the basolateral membrane of the epithelial cell shown in Figure 9-6. When sodium is absorbed from the airway surface liquid, there is an accompanying absorption of chloride ions and water.41 Chloride secretion can occur through at least two different types of chloride channel in the cell apex. One channel is dependent on cyclic adenosine 3′,5′-monophosphate (cAMP), the cystic fibrosis transmembrane ion conductance regulator (CFTR) channel; the other channel is calcium activated.
Mucus in disease states
The normal clearance of airway mucus can be altered by changes in the volume, hydration, or composition of the secretion. Mucus is produced and secreted by surface goblet cells and submucosal gland cells. Water content is a function of transepithelial chloride secretion, active sodium absorption, and water transport. The composition of respiratory mucus is undergoing investigation based on structural analysis techniques.42,43
Knowledge of these features of respiratory mucus may lead to a better understanding of diseases characterized by an abnormal production of mucus, such as CB and asthma, in which there is mucus hypersecretion, and CF, in which there is decreased intact mucin. Although it had been hypothesized that patients with CF hypersecrete viscous mucus leading to airway obstruction, it has now been shown that tenacious (but not viscous) secretions that characterize CF airway disease are composed almost entirely of DNA-rich pus.44 Airway damage may predispose to bacterial infections in CB because of impaired clearance of mucus.
Sputum, or expectorated phlegm, is composed of mucus mixed with inflammatory cells, cellular debris, polymers of DNA and filamentous (F)-actin, and bacteria. Mucus is usually cleared by airflow and ciliary movement, and sputum is cleared by cough.45 Purulent, green phlegm is caused by the neutrophil-derived enzyme myeloperoxidase, indicating neutrophil activation; this sputum contains very little mucin and can be considered pus.46 Bronchial obstruction by secretions, either mucus or pus, can increase airflow resistance and lead to complete airway obstruction and atelectasis.12 Regardless of whether the airway is full of mucus or phlegm, effective airway clearance is vital to airway hygiene.
Chronic bronchitis
CB is defined clinically as daily sputum expectoration for 3 months of the year for at least 2 consecutive years, usually in a tobacco smoker or ex-smoker. In the CB airway, there is hyperplasia of submucosal glands and goblet cells. The number of goblet cells increases, and there is hypertrophy of the submucosal glands, as measured by the Reid index of gland-to-airway wall thickness ratio.47 When studied in vitro, it was found that submucosal glands from subjects with CB produce excessive amounts of mucus.48 Tobacco smoke is considered the most important predisposing factor to airway irritation and mucus hypersecretion, but other factors can include viral infections, pollutants, and genetic predisposition.12,49 It has been reported that chronic sputum expectoration is associated with a more rapid decline in lung function and, for persons with COPD, more frequent admissions to hospital.50
Asthma
Mucus hypersecretion can occur during an acute asthmatic episode or can be a chronic feature of asthma accompanying airway inflammation. Turner-Warwick and Openshaw51 reported that as many as 80% of patients with asthma report increased sputum expectoration.
In acute severe and fatal asthma, there is profound hypersecretion of highly viscous and rigid mucus leading to complete airway obstruction.52 Because most of these patients have received large amounts of β-agonist bronchodilators, sometimes even by the intravenous route, it is likely that β receptors are fully saturated. β agonists may induce the secretion of viscous mucus, so it is possible that under these circumstances the aggressive use of β agonists may contribute to airway obstruction.53
Bronchorrhea
Bronchorrhea is defined as the production of watery sputum of 100 mL or more per day. This occurs in about 9% of patients with chronic asthma.54 Some of these patients respond well to antiinflammatory therapy such as corticosteroids,55 indomethacin by aerosol,56 or macrolide antibiotics.57 These are considered mucoregulatory medications and are most effective when bronchorrhea is associated with airway inflammation. Patients with congenital fucosidosis also have a form of bronchorrhea caused by the inability of mucins to polymerize. This form of bronchorrhea does not respond to mucoregulatory therapy.58
Plastic bronchitis
Plastic bronchitis is a rare disease characterized by the formation of large gelatinous or rigid branching airway casts.59 These casts are more cohesive than those seen in ordinary mucus plugging. The casts can be spontaneously expectorated, and occasionally patients cough up large impressions of their tracheobronchial tree. Casts are often described as “pudding-like” or “toothpaste.” Many are too thick to be easily suctioned through a bronchoscope and too friable to be grasped and removed with forceps. Some patients are able to expectorate even large casts spontaneously. Not all patients expectorate casts, and this may delay the diagnosis or lead to underdiagnosis.
There is only anecdotal evidence that any specific therapy is beneficial, and this evidence has generally been provided only by single case reports. If chylous casts are present, dietary modification or thoracic duct ligation when appropriate may help. In patients with asthma or atopy and cellular casts, therapy should be directed toward treating underlying inflammation. Oral or inhaled corticosteroids and culture-directed therapy of infection have been reported to help. The 14- and 15-member macrolide antibiotics are known to be immunomodulatory and mucoregulatory drugs. We have had success using macrolides as immunomodulating agents in two patients with eosinophilic plastic bronchitis (unpublished data). Macrolides have been successfully used in other disorders, primarily DPB and CF. There is clear evidence that these effects are not related to the antimicrobial activity of the macrolide.59,60
Cystic fibrosis
CF is a chronic hereditary disease characterized by impaired function of the CFTR protein. There is chronic airway infection, often with Pseudomonas and other gram-negative organisms. There is also chronic airway inflammation, and infection and inflammation together lead to bronchiectasis, progressive pulmonary function decline, and eventually death. Although it was previously thought that there was mucus hypersecretion and airway mucus obstruction in CF, it is now known that the CF airway secretions contain very little intact mucin. The airways in CF are almost entirely filled with pus derived from neutrophil degradation. The decreased mucin in CF sputum may be related to chronic bacterial infection by Pseudomonas aeruginosa and bacterial breakdown of mucin.44
It is unclear how abnormal CFTR function leads to chronic infection and inflammation. Airway epithelia in CF show excessive absorption of sodium (Na+) compared with normal epithelia. There is a limited ability for the epithelial cells to secrete chloride (Cl−) through the chloride CFTR channels (see Figure 9-6) stimulated by cAMP. The result of excessive Na+ absorption and limited Cl− secretion may lead to decreased water and increased reabsorption of the periciliary fluid.41 Abnormal phospholipid balance in CF secretions may also increase sputum adhesion and stickiness by altering surface properties of the secretion.3 It has been shown that CF sputum is biophysically similar to bronchiectasis sputum.61
Physical properties of mucus
Adhesive forces
Adhesion refers to forces between unlike molecules. In the airway, adhesive forces refer to the attractive forces between the mucus and airway surface. Adhesion severely reduces the ability to clear secretions by airflow (cough).62 Mucokinetic agents are either abhesives, such as surfactant, which reduces the adhesivity of secretions, or agents that increase the power of airflow and cough. Mucolytics may work, in part, by severing the bonding of mucus to the epithelium, reducing inertial (frictional) adhesion.
Cohesive forces
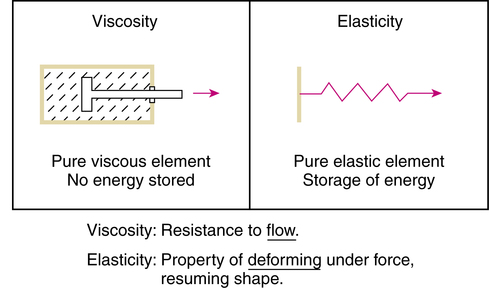
Rheology is the study of the deformation and flow of matter. The rheologic behavior of mucus describes the way it responds to forces (stress). Viscosity, or loss modulus, is the resistance of a fluid to flow. More specifically, viscosity is the proportionality constant (ratio) of applied force to rate of flow. The viscous properties of an ideal or Newtonian liquid can be described by the loss modulus, G″. Elasticity, or storage modulus, is the ability of a deformed material to return to its original shape. Ideal solids store energy during deformation, and this energy is available when the force is removed. The properties of an ideal or Hookean solid can be described by the storage modulus, G′. The complex modulus (G*) is the vectorial sum of viscosity and elasticity and is useful for describing the deformation of a pseudoplastic mucus. Figure 9-7 illustrates the concept of viscosity and of elasticity.63–65
Mucus as a viscoelastic material
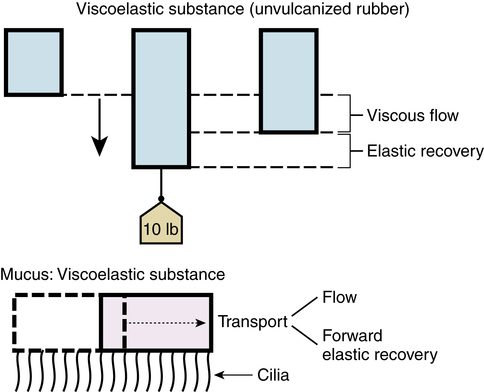
This model of gel transport is seen in Figure 9-8, using unvulcanized rubber as an example of a viscoelastic substance. If such a rubber strip is loaded on one end with a weight and allowed to hang, it would initially stretch as an elastic solid. If the applied force remains, the strip would slowly elongate because of its viscosity (i.e., the rubber would “flow”). After removing the weight, the rubber recovers most of the elastic elongation because of stored energy, but it would not recover the entire length because of flow. Mucus behaves similarly. Mucociliary transport results from flow and forward elastic recovery. For this reason, the physical properties of viscosity and elasticity are important for efficient transport of respiratory secretions. Normal mucus generally has a relatively low viscosity, and its elasticity is high enough to provide forward propulsive energy.
Spinnability (cohesivity) of mucus
The ability of mucus to be drawn out into threads was initially identified for cervical mucus; it was termed spinnability and described in the German literature as Spinnbarkeit. It was noted that this property was essential for the motility of sperm and female reproduction. The property of spinnability was subsequently studied by Puchelle and colleagues66 for respiratory mucus. They used a device to stretch mucus vertically at a constant rate and measured the spinnability of a mucus thread as the maximal length to which the thread could be drawn before breaking. In general, there was a significant and positive correlation between spinnability of mucus and its mucociliary transport rate. Spinnability was found to increase with increasing elasticity. Spinnability varied widely, however, with low viscosities, and mucus with high spinnability showed normal ciliary transport even though viscosity and elasticity were abnormally low. Spinnability and mucus transport were also found to decrease as the purulence of sputum from CB patients increased.
Non-newtonian nature of mucus
Evaluation of mucus properties is complicated by the fact that mucus exhibits non-Newtonian rheology. In an ideal Newtonian liquid, the applied force to rate of flow remains constant with changing force. A non-Newtonian substance, such as mucus, has changing viscosity (defined as the proportionality constant of force to flow) with varying applied force (shear rate). As the shear rate increases, the apparent viscosity of mucus usually decreases. Some mucus exhibits a sudden collapse of viscous behavior at high applied stress; this is called apparent yield stress. Sputum that yields may be more easily cleared by cough.67 These changes in viscosity are consistent with rupture or change of the macromolecular chains and cross-linking network of the gel. Mucus is often thixotropic—stable at rest but becoming more fluid with applied force and then thickening again when stress is removed.
Because of its non-Newtonian behavior, evaluation of the properties of mucus and of the effect of drugs on those properties is complicated and must be performed under standardized conditions of dynamic shear rate and across the linear portion of the stress-strain curve. Otherwise, interpretation of research findings on mucus viscosity is inaccurate.43,68 Many review articles and compendia are devoted to the physiology of mucus secretion in the lung and the nature of mucus.7,9,12,16,45,69–72
Mucoactive agents
At the time of this edition, three drugs are available that have been used for administration as an aerosol, to treat abnormal pulmonary secretions: N-acetylcysteine (NAC), dornase alfa, and 7% hyperosmolar saline. The first two of these drugs are considered mucolytic in their action, disrupting disulfide bonds in mucus (NAC) or enzymatically breaking down DNA in airway secretions (dornase alfa). Inhalation of hypertonic saline as an aerosol is now recommended as part of the treatment regimen for CF. Although not an approved agent, bicarbonate solutions of 2% concentration have been instilled in 2- to 5-mL amounts into the airway to increase the pH and alter mucus bonding. Table 9-4 summarizes the spectrum of agents in current use and with the potential to improve mucus clearance.
TABLE 9-4
| DRUG | DESCRIPTION |
| Dornase alfa | Peptide mucolytic |
| P2Y2 agent (denufosol) | Increases epithelial mucus and chloride secretion |
| Hyperosmolar saline | Expectorant, hydrator |
| Dry powder mannitol | Expectorant, hydrator |
| Thymosin β4 | Peptide mucolytic |
| Surfactants | Adhesive phospholipid, mucokinetic |
| β agonists | Secretagogues and potentially mucokinetic if airflow increases |
| Macrolide antibiotics | Mucoregulatory |
| Anticholinergic agents | Mucoregulatory |
| Corticosteroids | Mucoregulatory |
| Gene therapy | Normalizes secretory cell function and mucociliary clearance |
Mucolysis and mucociliary clearance
Mucolytic agents decrease the elasticity and viscosity of mucus because the gel structure is broken down. Because elasticity is crucial for mucociliary transport, mucolytics have the potential for a negative effect on normal physiologic mucus clearance. Reduction of mucus gel to a more liquid state may facilitate aspiration of secretions with the use of suction catheters.73 Ideally, the goal should be to facilitate physiologic clearance by optimizing the viscoelasticity of mucus. The status of mucolytic agents in pulmonary disease was well reviewed at several conferences on the scientific basis of respiratory care.74–76
The therapeutic options for controlling mucus hypersecretion are outlined as follows:
Mucolytics and expectorants
Classic mucolytics reduce mucins by severing disulfide bonds or charge shielding.
N-acetyl-l-cysteine
Indications for use
As a mucolytic, NAC has been used in conditions associated with viscous mucus secretions. A second use of NAC is as an antioxidant antidote to reduce hepatic injury with acetaminophen overdose.77 The drug is given orally for this use. Despite in vitro mucolytic activity and a long history of use, there are no data showing that aerosolized NAC is effective therapy for any lung disease,78 and its use may be harmful. This harm may be due, in part, to NAC selectively depolymerizing the essential mucin polymer structure and leaving the pathologic polymers of DNA and F-actin intact. Because there are no data that show NAC to be effective for lung disease and because of the high risk of side effects, we do not recommend its use.
Dornase alfa (pulmozyme)
Dornase alfa was the first approved mucoactive agent for the treatment of CF. Dornase alfa is safe and effective in patients with more severe pulmonary disease, defined as a forced vital capacity (FVC) less than 40% of the predicted value.81 Efficacy has not yet been shown for therapy of acute exacerbations of CF lung disease or for the treatment of other chronic airway diseases.82 A small phase 1 study showed no efficacy in non-CF bronchiectasis, and there was a suggestion that the use of dornase worsened disease in this adult population.83 This worsening of disease may be due to the fact that secretions in COPD are composed primarily of mucin and related proteins,84 whereas CF airway secretions include significantly less mucin and mucus, being composed almost entirely of neutrophil-derived pus.44
Indication and use in cystic fibrosis
Dornase alfa is indicated for the management of CF, to reduce the frequency of respiratory infections requiring parenteral antibiotics and to improve or preserve pulmonary function in CF patients. The bulk and surface properties of respiratory secretions in CF are due to the presence of DNA from necrosing neutrophils present during chronic respiratory infections and to surfactant phospholipid hydrolysis by products of inflammation. In the presence of infection, neutrophils are attracted to the airways, degenerate, and release DNA, which further increases the viscosity of secretions. DNA is an extremely viscous polyanion.85,86 DNA in secretions may also contribute to reduced effectiveness of aminoglycoside antibiotics such as gentamicin. This reduced efficacy could be caused by binding of the antibiotic to the polyvalent anions of DNA.
Mode of action
The peptide mucolytic agent dornase alfa is similar in action to the proteolytic enzyme pancreatic dornase, which was approved for human use by inhalation in 1958 but is no longer available.86 Dornase alfa reduces the viscosity and adhesivity of infected respiratory secretions when given by aerosol (Figure 9-9).
When mixed with purulent sputum from subjects with CF, dornase alfa reduced the viscosity and adhesivity of the sputum.86 This reduction was associated with a decrease in the size of the DNA in the sputum. The change in sputum viscosity with the addition of dornase alfa is dose-dependent, with greater reduction occurring at higher concentrations of the drug.
Pancreatic dornase, named Dornavac by the manufacturer, the product previously removed from the market, was a bovine pancreatic DNase. This product could cause serious respiratory distress when inhaled.87 In addition, the foreign protein could generate anti-DNase antibodies and cause allergic reactions. Differences between the human and bovine pancreatic DNase I molecules in an immunogenic region imply that some of the adverse reactions to pancreatic dornase could have been caused by an immune response. Serum antibodies to DNase were found in some patients who had been given multiple doses of bovine pancreatic DNase. Adverse effects with bovine DNase may also have been caused by the presence of contaminating proteinases, trypsin and chymotrypsin, in the drug.86 Studies revealed no allergic response or serum anti-DNase antibodies with inhaled dornase alfa in healthy subjects or in patients with CF.85
Dose and administration
The aerosol product of dornase alfa is available as single-use ampoule, with 2.5 mg of drug in 2.5 mL of clear, colorless solution. The solution should be refrigerated and protected from light. The usual dose is 2.5 mg daily, delivered by one of the following tested and approved nebulizers: Hudson RCI UP-DRAFT II OPTI-NEB nebulizer with tee (Teleflex Medical, Research Triangle Park, N.C.) or the Acorn II nebulizer (Vital Signs, Totowa, N.J.) with a DeVilbiss Pulmo-Aide compressor (Sunrise Medical, Carlsbad, Calif.), or the PARI LC PLUS nebulizer (PARI Respiratory Equipment, Midlothian, Va.) with PARI Inhaler Boy compressor.88 Although other nebulizer systems may perform suitably in nebulizing dornase alfa, this should not be assumed without testing. Optimal delivery of the enzyme requires a nebulizer system capable of suitable aerosol generation (i.e., particle size and quantity). This is especially important when administering very expensive drugs or aerosol medications with a narrow therapeutic index.
Clinical application and evaluation
The intent of treatment with dornase alfa is to preserve or improve lung function in CF, while reducing the frequency and severity of respiratory infections by improving secretion clearance. A reduction in use of intravenous antibiotic therapy and the need for hospitalizations has also been reported. Evaluation of drug treatment is based not only on lung function but also on a reduction in the number and severity of infectious exacerbations and the need for antibiotics and hospitalization.89
F-actin-depolymerizing drugs: gelsolin and thymosin β4
Chronic inflammation is characterized by inflammatory cell necrosis and release of polymeric DNA, filamentous actin (F-actin), and intracellular enzymes from neutrophils. These inflammatory products are present in the sputum from patients with CF. DNA and F-actin in the sputum copolymerize to form a rigid network that is entangled in the mucin gel. Peptide mucolytics degrade these filaments, while leaving the glycoprotein network relatively intact. Gelsolin, an 85-kDa actin-severing peptide, has been shown to reduce the viscosity of CF sputum in a dose-dependent manner. Similarly, thymosin β4 decreases sputum cohesivity in a dose-dependent and time-dependent manner.90,91 Thymosin β4 sequesters actin in CF sputum and decreases sputum cohesivity in vitro.90 In vitro studies have shown that administration of F-actin-depolymerizing agents along with dornase alfa results in greater reduction in sputum cohesivity and viscoelasticity than administration of either agent alone.93,94 Actin-depolymerizing agents destabilize the actin-DNA filament network and increase the depolymerizing activity of dornase alfa on the DNA filaments.
Expectorants
Iodide-containing agents
Iodide-containing agents (e.g., supersaturated potassium iodide [SSKI]) are generally considered to be expectorants. They are thought to stimulate the secretion of airway fluid. Iodopropylidene glycerol (IPG) may acutely increase tracheobronchial clearance as measured by radiolabeled aerosol in patients with CB.95 However, in a double-blinded cross-over study in subjects with stable CB, therapy with IPG failed to show any changes in pulmonary function, gas trapping, or sputum properties.96
Guaifenesin
Guaifenesin is usually considered an expectorant rather than a mucolytic. It can be ciliotoxic when applied directly to the respiratory epithelium.97 It is thought that the expectorant action of guaifenesin is mediated by stimulation of the gastrointestinal tract and not by the systemic exposure to the drug.182
Guaifenesin has been approved as an expectorant by the FDA in a bilayer extended-release tablet (Mucinex). A study suggested that as an adjunctive therapy for patients taking antibiotics with upper airway infections, guaifenesin/pseudoephedrine shortened time to relief and improved symptoms for nasal congestion and sinus headache better than placebo.183 Guaifenesin may cause nephrolithiasis.184 Neither guaifenesin nor glycerol guaiacolate has been shown to be clinically effective in randomized controlled trials for lower airway tract infections.
Dissociating solvents
Urea is a dissociating agent that can break ionic and hydrogen bonds. In mucin gels, urea disrupts the hydrogen bonds between the oligosaccharide side chains of the neighboring mucus molecules, with subsequent decrease in the physical entanglements between the molecules and decreased viscosity of the mucus. Urea may also decrease the interaction between DNA molecules. Because the mucolytic action of urea occurs only at very high concentrations of urea (3 to 8 mol/L),98,99 it is inappropriate for human use.
Oligosaccharides
Oligosaccharide side chains constitute about 80% of the mucin structure. These hydrogen bonds are weak and can be disrupted by agents such as dextran, mannitol, and lactose. The lower molecular weight fractions of dextran are primarily responsible for the mucoactive effects of dextran. Furthermore, an osmotic effect of dextran with increased hydration of the mucus could also result in improved clearance of secretions. Dextran administration via aerosol has been shown to improve tracheal mucus velocity in dogs.80
Mannitol administered by dry powder inhaler (Bronchitol) has been approved in Australia and Europe for treatment of CF and bronchiectasis. Clinical studies have shown Bronchitol to be safe and well tolerated in treating patients with CF or bronchiectasis. However, because many children with CF had a positive airway challenge with inhaled mannitol, similar to with hyperosmolar saline,185 it is important to pretreat with a short-acting bronchodilator before use.
The charged oligosaccharide heparin has a greater mucolytic and mucokinetic capacity compared with the neutral oligosaccharide dextran. Heparin may cause hydrogen bond disruption and improved ionic interactions. Aerosolized low-molecular-weight heparin shows promise in the treatment of asthma, presumably by interfering with antigen-receptor binding.100
P2y2 agonists
Chloride conductance through the Ca2+-dependent chloride channels is preserved in the CF airway. The tricyclic nucleotides UTP and ATP regulate ion transport through P2Y2 purinergic receptors, which increase intracellular calcium. UTP aerosol, alone or in combination with amiloride, increases the transepithelial potential difference and the clearance of inhaled radioaerosol.101 Denufosol is an agent that stimulates this chloride secretion pathway and is now completing clinical trials.
Denufosol has a long activity in vitro and increases mucociliary clearance in healthy subjects. In a placebo-controlled, phase 2 study with CF patients, a tiny but significant difference in forced expiratory volume in 1 second (FEV1) between some denufosol-treated patients and the control patients was found.186,187
Mucokinetic agents
Bronchodilators
β agonists increase ciliary beat frequency, but this has little effect on mucus clearance. Of greater importance is that these medications can increase expiratory airflow in individuals with an asthmatic component of airway disease.104 However, airway muscle relaxation can also decrease expiratory airflow by producing dynamic airway collapse in persons with “floppy airways,” such as those with airway malacia or bronchiectasis. β agonists are also mucus secretagogues and therefore can potentially increase mucus plugging if they increase dynamic collapse and decrease expiratory airflow.
Surface-active phospholipids
Surfactant is produced in the conducting airways and in the alveoli and is essential for mucociliary and cough clearance. A thin surfactant layer between the periciliary fluid and the mucus gel prevents airway dehydration, permits mucus spreading on extrusion from glands, and allows efficient ciliary coupling with mucus and, more importantly, ciliary release from mucus once kinetic energy is transmitted. With airway inflammation, surface-active phospholipids such as surfactant are often degraded by secretory phospholipases, and inflammatory peptides can inhibit surfactant function further. In the absence of surfactant, mucus sticks to the epithelium, rendering cough less effective. There is severe loss of surfactant in the inflamed airway of patients with CB or CF.105,106
It has been reported in randomized, double-blind, placebo-controlled, multicenter studies that surfactant aerosol improves pulmonary function and sputum transportability in patients with CB or with CF and that this effect is dose-dependent. No significant side effects were attributable to the surfactant therapy.107 As a wetting and spreading agent, surfactant also has the ability to increase the lower airway deposition of other aerosol medications, such as dornase alfa or gene therapy vectors, and may increase small particle translocation through the mucus layer.108
Mucoregulatory medications
Another approach to reducing the burden of airway secretions is to decrease hypersecretion by goblet cells and submucosal glands. Medications that decrease mucus hypersecretion are referred to as mucoregulatory agents. These medications include antiinflammatory drugs such as corticosteroids, which are effective at decreasing the inflammatory stimulus that leads to mucus hypersecretion. Aerosolized indomethacin has also been used in Japan to treat patients with DPB who have impairment secondary to mucus hypersecretion.109
Anticholinergic medications are also used extensively as mucoregulatory medications. Atropine is routinely given perioperatively to prevent laryngospasm and to decrease mucus secretion associated with endotracheal intubation. Atropine and its derivatives are mucoregulatory medications because they do not “dry” secretions or increase viscosity, but they decrease hypersecretion that is mediated through M3 cholinergic mechanisms. The quaternary ammonium derivatives of atropine, including ipratropium bromide and tiotropium, do not significantly cross the blood-airway barrier, and, as such, their use is not associated with typical systemic effects of anticholinergic medications such as flushing or tachycardia. Ipratropium bromide is widely used as a bronchodilator medication in patients with CB. Studies have also shown that the long-term use of ipratropium is associated with a reduction in the volume of mucus secretion in patients with CB.110 More specific M3 antagonists hold the promise of improved mucoregulatory efficacy of this class of medications with less risk of adverse effects.
The immunomodulatory and mucoregulatory properties of macrolide antibiotics have been exploited for the treatment of DPB, a chronic inflammatory airway disease with great morbidity and mortality when untreated. DPB is seen primarily in Japan and Korea. The etiology is unknown, but the disease results in chronic sinobronchitis with mucus hypersecretion and debilitation. Antibiotics and corticosteroids are ineffective for the treatment of DPB. By virtue of their immunomodulatory and mucoregulatory properties, the macrolide antibiotics have been shown to be the most effective agents for the treatment of DPB.111 The 14-member and 15-member macrolides, but not the 16-member macrolides, are also highly effective for the therapy of CF airway disease.112,113 The mechanism of action of the macrolides as mucoregulatory agents is under intensive study.114 It is anticipated that the development of macrolide medications without antibiotic properties will significantly extend the spectrum of use of these medications.
Other mucoactive agents
Antiproteases
Patients with CF have increased activity of serine proteases on the respiratory epithelial surface. Neutrophils, when activated or degenerating, release proteases such as elastase that can directly damage epithelial cells and impair airway clearance. It has been shown that neutrophil proteases cause a secretory response from submucosal glands with an increase in mucus production.115
Intravenous administration or inhalation of α1-antitrypsin suppresses the activity of neutrophil elastase and restores the bacteria-killing capacity of neutrophils. Recombinant secretory leukocyte protease inhibitor (rsLPI), when given to a small number of CF patients at a dose of 100 mg bid for 1 week, decreased neutrophil elastase and interleukin-8 in airway fluid. No significant side effects were reported.116 Although promising, numerous issues need to be resolved before these or similar agents can be used to prevent damage caused by unchecked protease activity in patients with CF.
Hyperosmolar saline
For many years, sputum induction produced by hyperosmolar saline inhalation has been used to obtain specimens for the diagnosis of pneumonia. Hypertonic saline has been used as an irritant to induce cough. Hypertonic saline (7%) can improve mucociliary transport and lung function, thought to be largely due to the acute effects of inducing cough and hydrating the airway surface fluid.189,119 In a pilot study, 58 CF subjects were randomly assigned to receive 10 mL of either 0.9% normal saline or 6% hypertonic saline twice daily by ultrasonic nebulization.117 Spirometry was performed before treatment, at the end of 2 weeks of treatment, and 2 weeks after treatment. At the end of treatment, there was a significant increase in FEV1 in the hypertonic saline group, with a return to baseline by 28 days. Despite pretreatment with 600 μg of inhaled salbutamol, several patients had an acute decrease in FEV1 after inhaling hypertonic saline. Similarly, hyperosmolar dry powder mannitol improves quality of life and pulmonary function in adult subjects with non-CF bronchiectasis and significantly improves the surface adhesivity and cough clearability of expectorated sputum.118
Studies summarized in the Cochrane Database of Systematic Reviews confirm that the long-term use of inhaled hyperosmolar saline improves pulmonary function in patients with CF,119–121 and inhaled hyperosmolar saline or mannitol is beneficial in bronchiectasis.122 Although this therapy is readily available and inexpensive, it has been reported that hypertonic saline aerosol is not as effective as dornase alfa in the therapy of CF lung disease.123 In CF, hyperosmolar saline promotes mucus clearance with improvement in pulmonary function and a decrease in respiratory tract exacerbations.119,120 It is now recommended as part of the treatment regimen for this disease. However, in a study of COPD patients, there was no improvement using hypertonic saline, but cough or bronchospasm occurred more frequently than in controls.190
Gene therapy
Gene transfer therapy represents a novel use for aerosols. Efforts in this arena have centered largely on complementary DNA transfer of the normal CFTR gene in CF patients. Gene transfer was first attempted by inserting the normal CFTR gene into a replication-defective adenovirus vector by bolus bronchoscopic delivery of the vector. An unanticipated host immune response to the vector led to reevaluation of this strategy.124
For gene transfer to be effective, the vector and its package must be nonimmunogenic, stable to shear forces during aerosolization, and safe to transfected cells. The vector should not increase cell turnover. It should either stably integrate into the progenitor (basal) cell genome or be safe and effective with repeated administration and should be able to reach the cellular target of relevance. Part of the difficulty with CF is that this cellular target has not been clearly identified as epithelial cell, goblet cell, or submucous gland, or all of these. The amount of gene and vector and persistence in the airway must also be determined for each vector and delivery system.125,126
Viral vectors that have been studied include adenoviruses, adeno-associated virus (AAV), and lentivirus. Adenoviruses naturally target the airway epithelium. AAVs are very small organisms that require a “helper” virus to replicate. These viruses are capable of site-directed insertion into DNA, reducing the risk of insertional mutagenesis (initiating cancer by activation of an oncogene or inactivation of an oncogene suppressor). Gene therapy with AAV seems to be especially promising.127 Lentiviruses are retroviruses such as human immunodeficiency virus (HIV). They are able to transfect cells that are not terminally differentiated, such as the basal or airway progenitor cell, but insertional mutagenesis is a substantial risk.128
The primary nonviral vectors studied to date have been cationic liposomes. These lipid capsules are able to form complexes with DNA and then enter cells. With the first generation of liposome vectors, the efficiency of gene transfer was poor; however, this has improved with newer systems.129,130
Using mucoactive therapy with physiotherapy and airway clearance devices
A guideline for airway clearance therapies (ACT) in CF has been published recommending ACT for all CF patients for sputum clearance, maintaining lung function, and improving quality of life. Although none of the therapies used has been shown to be superior, there may be advantages of particular therapies for individual patients. Patient preference should be considered with the anticipation that this will be associated with greater adherence to therapy.131
Many physical factors affect secretion clearance. Cephalad airflow bias is responsible for the movement of mucus in airways during normal ventilation.132–135 The narrowing of airways on exhalation increases the velocity and shearing forces in the airway, creating a cephalad airflow bias with tidal breathing. This bias is amplified during coughing, when increased transmural pressure causes the airways to fold and constrict, increasing airflow velocity even further.135
In acute airway diseases leading to ciliary dysfunction or mucus hypersecretion, or both, cough is the primary mechanism for secretion clearance from the central airways, and cephalad airflow contributes increasingly to peripheral airway clearance. Cough is one of the most common respiratory complaints of patients seeking medical attention.136 During a normal cough, the expiratory airflow increases to a maximum along with narrowing of the intrathoracic airways. The narrowing of the airways is a product of high airflows and pressure differentials across the lung. Airflow velocity varies inversely with the cross-sectional area of the airways, creating high linear velocity, increased turbulence, high shearing forces within the airway, and high kinetic energy. These forces shear secretions and debris from the airway walls, propelling them toward the central and upper airway, where they are expectorated or swallowed. In COPD, narrowing airways may close prematurely, trapping gas, reducing expiratory flow rates, and limiting the effectiveness of the cough. Directed cough or huff (forced expiration with the glottis open) is a primary maneuver in these patients to optimize secretion clearance.
Conventional chest physical therapy (CPT), consisting of directed cough with postural drainage, percussion, and vibration, produces greater expectoration than no treatment in patients with CF.137 The most effective and important part of conventional CPT is directed cough. The other components of conventional CPT add little, if any, benefit and should not be used routinely. Alternative methods of airway clearance (e.g., positive expiratory pressure, high-frequency oscillation) all seem to work as well as CPT as long as they include directed cough. The choice between CPT and alternative methods mainly depends on patient preference and the response of the individual patient to treatment.138
Gravity
Gravity is not a primary mechanism for normal mucociliary transport. In health, the viscosity of the normal mucus blanket is sufficient to resist the flow of mucus into gravity-dependent terminal bronchioles. As mentioned previously, conventional CPT results in significantly greater expectoration than no treatment in patients with CF.137 However, there is no proven benefit to adding postural drainage to CPT to patients with ineffective cough. Similarly, there is no benefit to adding postural drainage to less conventional CPT methods, such as those described subsequently.138 Postural drainage carries a risk for patients with gastroesophageal reflux or muscle weakness.
Insufflation-exsufflation
An insufflation-exsufflation (CoughAssist) device inflates the lungs with positive pressure followed by a negative pressure to simulate a cough.139 The cycle begins with an inspiratory pressure of 25 to 35 cm H2O for 1 to 2 seconds, followed by an expiratory pressure of 30 to 40 cm H2O for 1 to 2 seconds. It can be used with an oronasal mask or attached to an artificial airway. Its primary application has been in patients with neurologic muscular weakness unable to cough unassisted. Early work with COPD was suggestive of benefit, but more recent evidence is lacking in the literature.140
Active cycle of breathing and forced expiratory technique maneuver
The active cycle of breathing (ACB) technique involves a combination of breathing control (relaxed diaphragmatic breathing), thoracic expansion control (deep breaths), and forced expiration from progressively increasing lung volumes. Although often used by patients with CF, there are no controlled studies documenting benefit from this mode of airway clearance beyond the forced expiratory technique (FET) manuever.141
FET consists of a breath taken in to mid-lung volume and air squeezed out by contracting the chest wall and abdominal muscles with the mouth and glottis kept open. The huff should not be a violent or explosive exhalation.142,143 Exercise should be encouraged with patients, who often find that it is associated with requirements for shorter ACB sessions.
Autogenic drainage
Autogenic drainage (AD) aims to “optimize” airflow in the various generations of bronchi to move secretions without forced expirations.144,145 This technique incorporates staged breathing starting with small tidal breaths from expiratory reserve volume, repeated until secretions “collect” in the central airways. Patients are instructed to suppress cough, and a larger volume is taken for a series of 10 to 20 breaths, followed by a series of even larger (approaching vital capacity) breaths, followed by several huff coughs. This technique requires a great deal of patient cooperation and is recommended only for patients older than 8 years of age and patients who have a good sense of their own breathing. Some uncontrolled studies have reported similar secretion clearance with AD and CPT.141,145,146
Positive airway pressure
Positive airway pressure (PAP) techniques can be effective alternatives to CPT in expanding the lungs and mobilizing secretions. Evidence suggests that PAP therapy is more effective than incentive spirometry and intermittent positive pressure breathing (IPPB) in the management of postoperative atelectasis149,150 and as an adjunct to enhance the benefits of aerosol bronchodilator delivery.151,152 Cough, FET, and other airway clearance techniques are components of PAP therapy.153
Pursed-lip breathing is a procedure that many patients with COPD have taught themselves to relieve air trapping caused by collapse of unstable airways during expiration. The resistance at the mouth during a pursed-lip exhalation transmits back pressure to splint the airways open, preventing compression and premature closure (similar to a fixed orifice resistor). Pursed-lip breathing represents a functional predecessor to modern device-based strategies of applying positive expiratory pressure (PEP) to the airway. Expiratory positive airway pressure (EPAP) is produced as expired air passes through a fixed orifice or threshold resistor. Continuous positive airway pressure (CPAP) is also considered a form of PAP. By preventing expiratory airway collapse, PAP increases expiratory flows and related cephalad airflow bias movement of secretions and may improve the distribution of ventilation throughout the lungs, via collateral intrabronchiolar channels.154–156
Oscillation of the airway
Although the Flutter has been available since about 1994, little has been published on its efficacy.159 In one acute intervention study,160 the amount of sputum expectorated by subjects with CF was more than three times the amount expectorated with either voluntary cough or postural drainage. Another study reported that Flutter valve therapy was an acceptable alternative to conventional CPT in hospitalized patients with CF.161 However, these results have not been confirmed by other studies, the relevance of expectorated sputum volume to clinical effectiveness is unproven, and most of the more recent studies of the Flutter have not shown it to be effective for airway clearance.162–166 Device performance varies with changes in expiratory flow and angle of inclination.167 This lack of efficacy under “real life” conditions may be due to the Flutter device being very tiring to use correctly resulting in poor adherence. Similar devices such as the Acapella (Smiths Medical, St. Paul, Minn.) and the Frequencer (Dymedso, Inc., Boisbriand, Quebec, Canada) may have less technique-dependent performance, but to date any improvement has yet to be reported. We generally do not recommend the use of these devices.
Intrapulmonary percussive ventilation (IPV) of the lungs involves the use of a pneumatic device called a Percussionator (Percussionaire Corp, Sandpoint, Idaho) to treat atelectasis, enhance the mobilization and clearance of retained secretions, and deliver nebulized medications to the distal airways.168 With IPV, the patient breathes through a mouthpiece that delivers high-flow mini-bursts at rates exceeding 200 cycles/min. During these percussive bursts of gas into the airway, continuous airway pressure is maintained while the pulsatile percussive intraairway pressure progressively increases. Each percussive cycle is programmed by holding a thumb button for 5 to 10 seconds for the percussive inspiratory cycle and releasing the button for exhalation. Treatments of approximately 20 minutes are recommended by the manufacturer. Impaction pressures of 25 to 40 psig (pounds-force per square inch gauge) are delivered at a frequency ranging from less than 100 to 225 percussive cycles/min at 40 psig. Some small studies have reported comparable results with IPV and standard CPT.169–171
Chest wall compression
There are several different HFCWC systems on the market, and these all are similar in operation and probably in effectiveness. These systems consist of a large-volume, variable-frequency air pulse delivery system attached to an inflatable vest that is worn by the patient so that it extends over the entire torso. Pressure pulses that fill the vest and vibrate the chest wall are controlled by the patient and applied during exhalation or throughout the respiratory cycle. Pulse frequency is adjustable from 5 to 25 Hz, with pressure varying from 28 mm Hg at 5 Hz to 39 mm Hg at 25 Hz. However, it is generally recommended, that the oscillatory frequency be held at a single frequency between 10 Hz and 15 Hz for the duration of therapy. Theoretically, vibrations to the chest cause transient increases in airflow in the lungs, to improve gas-liquid interactions and the movement of mucus. The frequency of oscillations (cycles per second) and flow bias (inspiratory versus expiratory) seem to influence effectiveness.172,173 The vest has been reported to be effective for secretion clearance in patients with CF.174–176 Conjecture that this device has a role in lung expansion for patients other than those with CF in the acute care setting has not been confirmed, and there are risks to using this device when patients have a weak or ineffective cough.177
Future mucus-controlling agents
In the past, mucolytics were targeted only at reducing the viscosity of mucus. In the context of the normal physiology of ciliary movement, logic suggests that thicker mucus would be moved more efficiently by ciliary contact and elastic recovery than would thin, low-viscosity solutions. The conceptual analogy is that of raking water—little transport occurs. This assumes mucus clearance is being optimized physiologically. Endotracheal aspiration of secretions by suction would be easier with low-viscosity mucus. Research supports the theory that elasticity is important for mucus transport.68,170
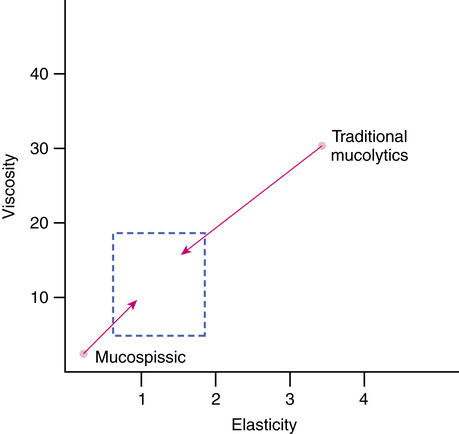
Purulent sputum has high elasticity and viscosity, and mucolytic agents such as dornase alfa might restore normal transport properties by reducing tenacity, viscosity, and elasticity. With low viscoelasticity (e.g., bronchorrhea), restructuring or cross-linking agents would increase viscosity and elasticity to improve transport. Such agents have been termed mucospissic agents.171,172 Agents with mucospissic activity include sodium tetraborate, Congo red, and tetracycline.170 The thickening effect of tetracycline can occur with oral and aerosol administration, although more so with direct aerosol. Tetracycline may bind to mucus proteins to increase viscosity and elasticity, although exact binding sites remain unclear. With adhesive sputum (CF, asthma, CB), mucokinetic agents that decrease tenacity while preserving viscoelasticity might be useful.
At present, no drugs are available in the United States as mucus-controlling agents that selectively modify viscosity or elasticity. Further investigation may produce clinically useful agents tailored to specific secretion problems, as illustrated in Figure 9-10. The first step has been to recognize the complex biophysical nature of mucus and the necessity for cross-linking to provide adequate elasticity for efficient ciliary transport. The second step has been to increase understanding of the structure of mucus and regulation of its production. Finally, a better understanding of adhesivity and cohesivity, the components of tenacity, has focused attention on control of the abnormal surface properties of mucus and sputum and their role in cough clearance.