Movement dysfunction associated with hemiplegia
After reading this chapter the student or therapist will be able to:
1. Identify the various types of neurovascular disease.
2. Identify the atypical patterns of movement in clients with residual hemiplegia.
3. Identify significant primary and secondary body system problems (impairments) that interfere with functional movement patterns and limit ability to participate.
4. Describe a reeducation intervention strategy for improving functional movement in clients who have had a stroke.
Overview
The treatment of hemiplegia from vascular insult is controversial. Various treatment methods have been devised and advocated. Recent scientific theories have changed the focus of treatment from one of inhibition of abnormal tone and facilitation of normal movement to reeducation of control and weakness, and functional retraining. In this chapter, pathological conditions, body system problems (impairment), functional limitations, and intervention strategies for clients with hemiplegia from stroke are reviewed. Although hemiplegia from neurovascular pathological conditions is the focus of the chapter, therapists can use this information and apply it to adults with hemiplegia caused by other central nervous system (CNS) pathological conditions, such as tumor (see Chapter 25), trauma (see Chapter 24), multiple sclerosis (see Chapter 19), and demyelinating diseases (see Chapter 17). Movement components and their relationship to functional performance are used as the basis for selection of therapy techniques and training.
Epidemiology
In the United States, stroke is the third ranking cause of death—more than 137,000 people die each year—and is the leading cause of adult disability.1 The National Stroke Association estimates that 795,000 new or recurrent strokes occur each year. The incidence of stroke rises rapidly with increasing age: two thirds of all strokes occur in people older than the age of 65 years; and after the age of 55 years, the risk of stroke doubles every 10 years. With the over-50-years age group growing rapidly, more people than ever are at risk. In the United States, the incidence of stroke is greater in men than in women, and it is twice as high in blacks as in whites. Cerebral infarction (thrombosis or embolism) is the most common form of stroke, accounting for 70% of all strokes. Hemorrhages account for another 20%, and 10% remain unspecified. Stroke is the largest single cause of neurological disability. Approximately 4 million Americans are dealing with impairments and disabilities from a stroke. Of these, 31% require assistance, 20% need help walking, 16% are in long-term care facilities, and 71% are vocationally impaired after 7 years.1 One study reported that 12% of subjects have complete functional arm recovery and 38% have some dexterity 6 months after stroke. In addition, loss of leg movement in the first week after stroke and no arm movement at 4 weeks are associated with poor outcomes at 6 months.2
The three most commonly recognized risk factors for cerebrovascular disease are hypertension, diabetes mellitus, and heart disease. The most important of these factors is hypertension.3 Because high blood pressure is the greatest risk factor for stroke, human characteristics and behaviors that increase blood pressure, including increased high serum cholesterol levels, obesity, diabetes mellitus, heavy alcohol consumption, cocaine use, and cigarette smoking, increase the risk of stroke.
Ostfeld4 noted that mortality rates for stroke declined, slowly at first (from 1900 to 1950) and then more quickly (from 1950 to 1970), with a sharp drop noted around 1974. Experts have speculated that the greater use of hypertensive drugs in the 1960s and 1970s started this decline, and the creation of screening and treatment referral centers for high blood pressure may account for the marked decline in the late 1970s.
Outcome
The long-term follow-up on the Framingham Heart Study revealed that long-term stroke survivors, especially those with only one episode, have a good chance for full functional recovery.5 For people left with severe neurological and functional deficits, studies have demonstrated that rehabilitation is effective and that it can improve functional ability.6,7 It has been demonstrated that age is not a factor in determining the outcome of the rehabilitation process.8 Currently it is thought that clients should be given an opportunity to participate in the rehabilitation process, regardless of age, unless it is medically contraindicated.
The prediction of ultimate functional outcome has been hampered by the inaccuracy of commonly used predictors (medical items, income level, intelligence, functional level). Computed tomography (CT), functional magnetic resonance imaging, and regional cerebral blood flow studies are used in diagnosis and increasingly as predictors of functional recovery after stroke. Positron emission tomography and single-photon emission CT are newer techniques that are used in research centers to define areas of dysfunctional but perhaps “salvageable” tissue.2,9
Pathoneurological and pathophysiological aspectsclassification
Thrombotic infarction.
Atherosclerotic plaques and hypertension interact to produce cerebrovascular infarcts. These plaques form at branchings and curves of the arteries. Plaques usually form in front of the first major branching of the cerebral arteries. These lesions can be present for 30 years or more and may never become symptomatic. Intermittent blockage may proceed to permanent damage. The process by which a thrombus occludes an artery requires several hours and explains the division between stroke-in-evolution and completed stroke.10
Embolic infarction.
The embolus that causes the stroke may come from the heart, from an internal carotid artery thrombosis, or from an atheromatous plaque of the carotid sinus. It is usually a sign of cardiac disease. The infarction may be of pale, hemorrhagic, or mixed type. The branches of the middle cerebral artery are infarcted most commonly as a result of its direct continuation from the internal carotid artery. Collateral blood supply is not established with embolic infarctions because of the speed of obstruction formation, so there is less survival of tissue distal to the area of embolic infarct than with thrombotic infarct.2
Hemorrhage.
AV malformations are developmental abnormalities that result in a spaghetti-like mass of dilated AV fistulas varying in size from a few millimeters in diameter to huge masses located within the brain tissue. Some of these blood vessels have extremely thin, abnormally structured walls. Although the abnormality is present from birth, symptoms usually develop at ages 10 to 35 years. The hemorrhage of an AV malformation presents a pathological picture similar to that for the saccular aneurysm. The larger AV malformations frequently occur in the posterior half of the cerebral hemisphere.10
Clinical findings
The focal neurological deficit resulting from a stroke, whether embolic, thrombotic, or hemorrhagic, is a reflection of the size and location of the lesion and the amount of collateral blood flow. Unilateral neurological deficits result from interruption of the carotid vascular system, and bilateral neurological deficits result from interruption of the vascular supply to the basilar system. Clinical syndromes resulting from occlusion or hemorrhage in the cerebral circulation vary from partial to complete. Signs of hemorrhage may be more variable as a result of the effect of extension to surrounding brain tissue and the possible rise in intracranial pressure. Table 23-1 summarizes the clinical symptoms and the anatomical structures involved according to specific arterial involvement.
TABLE 23-1 
Clinical Symptoms of Vascular Lesions
| AFFECTED VESSEL | CLINICAL SYMPTOMS | STRUCTURES INVOLVED |
| Middle cerebral artery | Contralateral paralysis and sensory deficit | Somatic motor area |
| Motor speech impairment | Broca area (dominant hemisphere) | |
| “Central” aphasia, anomia, jargon speech | Parieto-occipital cortex (dominant hemisphere) | |
| Unilateral neglect, apraxia, impaired ability to judge distance | Parietal lobe (nondominant hemisphere) | |
| Homonymous hemianopia | ||
| Loss of conjugate gaze to opposite side | Optic radiation deep to second temporal convolution | |
| Avoidance reaction of opposite limbs | Frontal controversive field | |
| Pure motor hemiplegia | Parietal lobe | |
| Limb—kinetic apraxia |
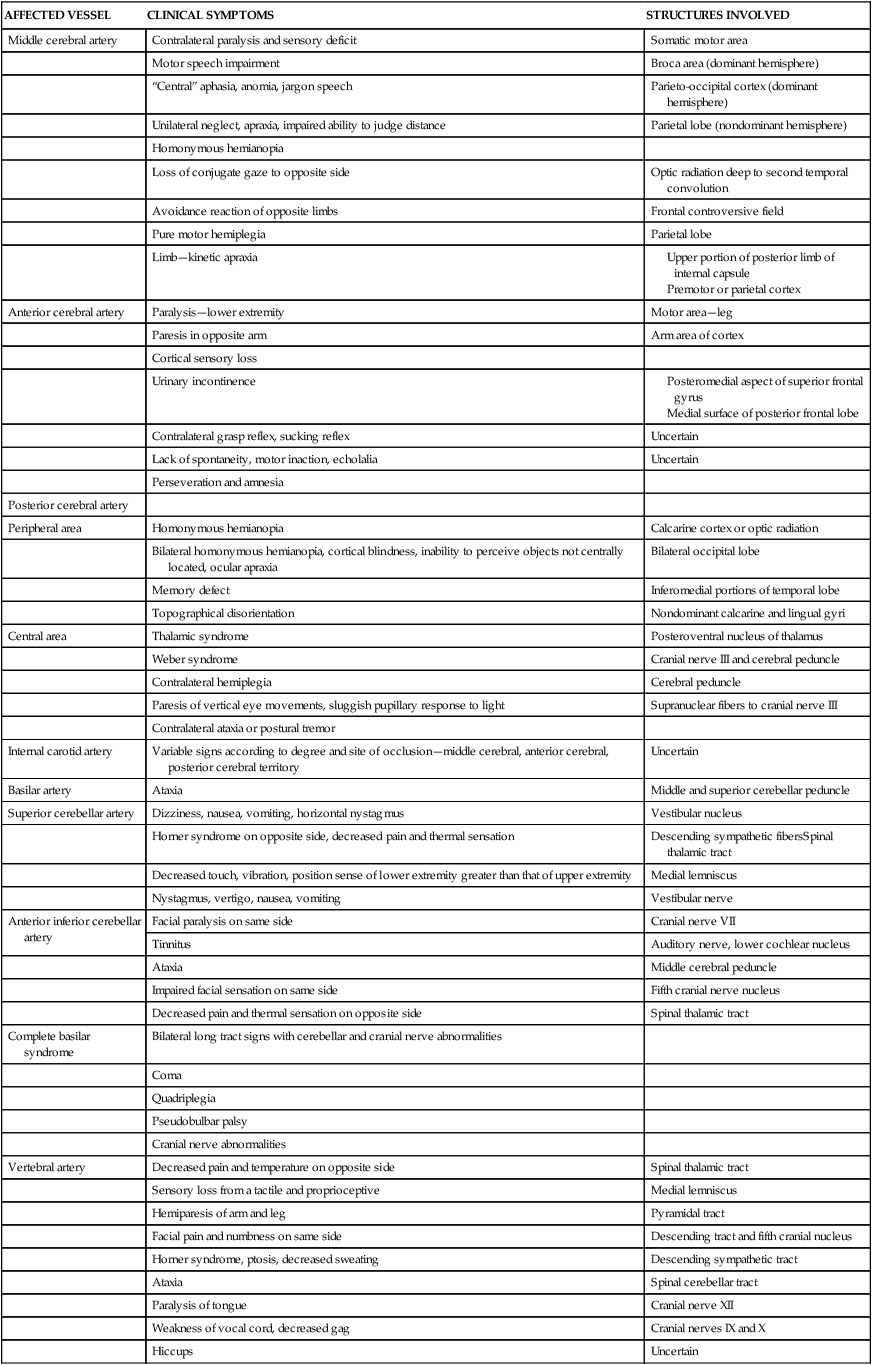
Modified from Adams RD, Victor M: Principles of neurology, New York, 1981, McGraw-Hill.
The frequencies of the three types of cerebrovascular disease—thrombosis, embolism, and hemorrhage—vary according to whether they were taken from a clinical study or from an autopsy study, but they rank in the order presented in this section. Ischemic strokes, thrombotic or embolic, account for 80% of strokes, and hemorrhagic strokes account for 20%.11 The clinical symptoms and laboratory findings for each type are condensed in Table 23-2.
TABLE 23-2 
Clinical Symptoms and Laboratory Findings for Neurovascular Disease—Ruptured Saccular Aneurysm
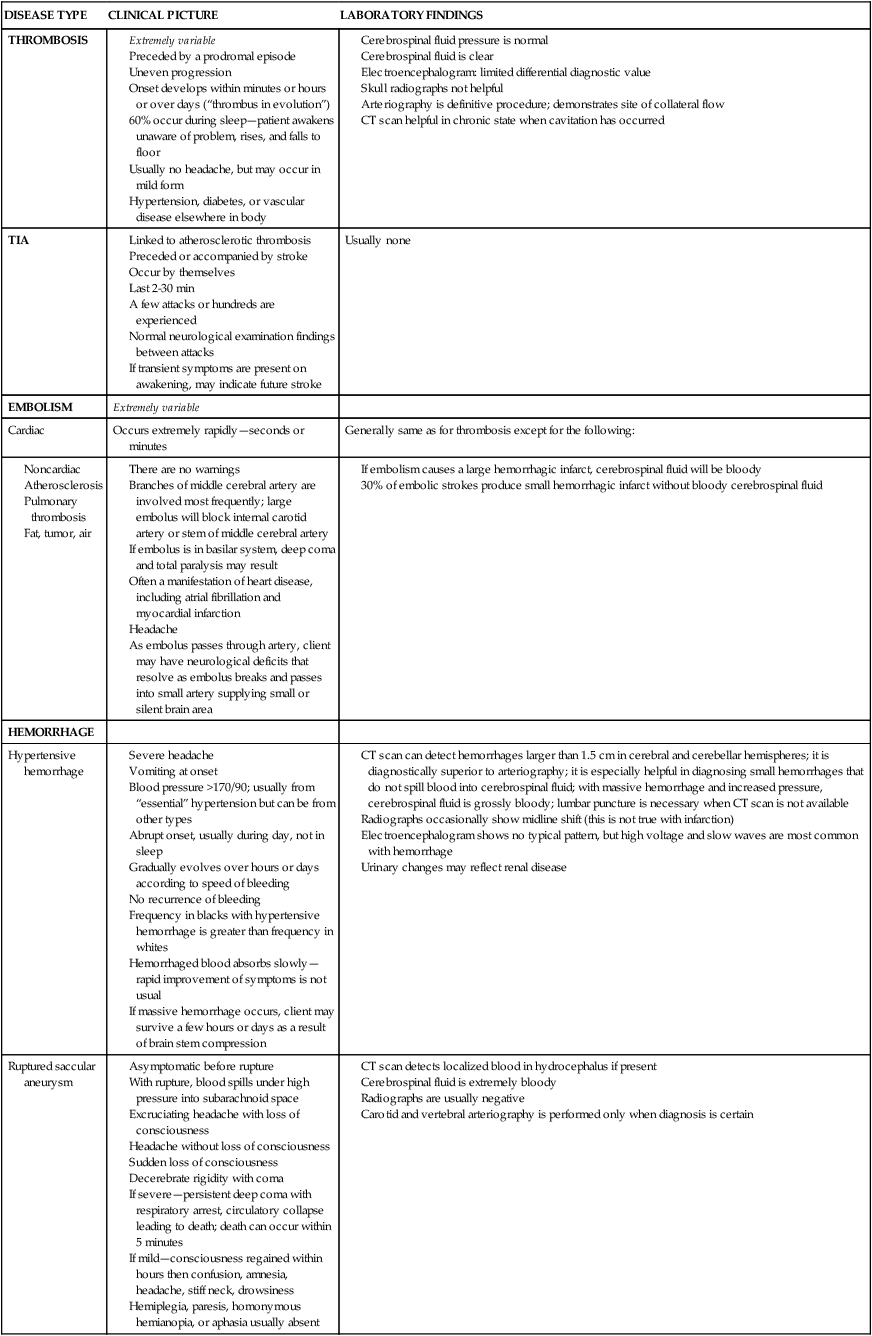
CT, Computed tomography; TIA, transient ischemic attack.
Modified from Adams RD, Victor M: Principles of neurology, New York, 1981, McGraw-Hill.
Medical management and pharmacological considerations
Acute medical care
Thrombosis and transient ischemic attacks.
Although infarcted tissue cannot at present be restored, medical management of the acute stroke from thrombosis or TIA is geared toward improving the cerebral circulation as quickly as possible to prevent ischemic tissue from becoming infarcted tissue. Cells that have 80% to 100% ischemia will die in a few minutes because they cannot produce energy, specifically adenosine triphosphate. This energy failure results in an activation of calcium, which causes a chain reaction resulting in cell death.1 Around this area of infarction is a transitional area where the blood flow is decreased 50% to 80%. Cells in the transitional area are not irreversibly damaged.12,13
One of the newer drugs available for immediate stroke treatment is tissue plasminogen activator (t-PA) (see Chapter 36). It is approved for use within 3 hours of symptom onset but is most effective if used within the first 90 to 180 minutes. Recent studies indicate that 42% of patients who have sustained a stroke wait 24 hours before getting care, with the average being 13 hours.13 The importance of community-wide programs to increase awareness of symptoms and effectiveness of emergency medical responses is immense for this drug’s usage. The American Heart Association and the National Stroke Association are creating community campaigns to increase awareness of the medical emergency nature of stroke symptoms. These campaigns encourage people to call 911 immediately when any of the following warning signs occur:
 Sudden numbness or weakness of the face, arm, or leg, especially on one side of the body
Sudden numbness or weakness of the face, arm, or leg, especially on one side of the body
 Sudden confusion or trouble speaking or understanding
Sudden confusion or trouble speaking or understanding
 Sudden trouble walking, dizziness, loss of balance or coordination
Sudden trouble walking, dizziness, loss of balance or coordination
Anticoagulant drugs are used to prevent TIAs and may stop a stroke-in-evolution. Before anticoagulant drugs are used, an accurate differential diagnosis is necessary because of the danger of excessive bleeding if hemorrhage is present. Heparin is often used in the early stage of the stroke, and warfarin (Coumadin) or dabigatran (Pradaxa) is commonly used in the months after the stroke. Cerebral edema, if present, is managed pharmacologically during the first few days. Antiplatelet drugs such as aspirin, dipyridamole (Persantine), and sulfinpyrazone (Anturane) are used to prevent clotting by decreasing platelet “stickiness.”10
Surgical treatment (thromboendarterectomy or grafting) is used when TIAs are the result of arterial plaques. Areas accessible to and suitable for surgery include the carotid sinus and the common carotid, innominate, and subclavian arteries. Although both surgery and anticoagulant therapy are used for TIAs, Adams and Victor10 extensively reviewed the wide divergence of opinions. For clients who have had a stroke yet recovered quickly and well, medical care focuses on prevention. Prevention usually includes maintaining blood pressure and blood flow, monitoring hypotensive agents (if given), and avoiding oversedation, especially for sleep, to prevent cerebral ischemia.
Hypertensive hemorrhage.
Medical procedures for hypertensive hemorrhage parallel those for thrombosis and embolism. Surgical removal of the clot and lowering of the systemic blood pressure to decrease hemorrhage have generally not been helpful. Again, the preventive use of antihypertensive drugs in clients with essential hypertension is the soundest medical management available.10
Ruptured aneurysm.
Comatose clients are not good candidates for surgery. However, if the client survives the first few days and if the state of consciousness improves, surgical intervention, whether extracranial or intracranial, is the treatment of choice. Medical treatment consists of lowering arterial blood pressures. Bed rest for 4 to 6 weeks with all forms of exertion avoided is prescribed. Antiseizure medication may be used. Often a systemic antifibrinolysin is given to impede lysis of the clot at the site of rupture. Vasospasm, resulting in severe motor dysfunction, occurs with the use of drugs such as reserpine (Serpasil) and kanamycin (Kantrex) (see Chapter 36).
Regardless of the cause of the stroke, comatose clients are managed by (1) treatment of shock; (2) maintenance of clear airway and oxygen flow; (3) measurement of arterial blood gases, blood analysis, CT, and spinal tap; (4) control of seizures; and (5) gastric tube feeding (if coma is prolonged). Hypertensive hemorrhage is one of the most common vascular causes of coma.14
Medical management of associated problems
Spasticity.
Spasticity and its treatment constitute a major medical problem after stroke because clients complain about it, it may fluctuate, and it does not respond to one fixed treatment. The relationship between spasticity and movement after stroke is an area of continued interest for researchers. Recent studies have refuted the earlier belief that spasticity was inversely related to voluntary movement.15,16 Although therapists are more hesitant to treat spasticity now, physicians continue to treat it aggressively. Various pharmacological, surgical, and physical means are used to decrease spasticity. The pharmacological and surgical means are examined here, and therapy management is discussed later.
Peripherally acting drugs are used to block a specific link in the gamma group. Procaine blocks selectively inhibit the small gamma motor fibers, resulting in a relaxation of intrafusal fibers. The effect of procaine blocks is transient. Intramuscular neurolysis with the injection of 5% to 7% phenol has been used to destroy the small intramuscular mixed nerve branches.17 Phenol blocks relieve hypertonicity and improve function, especially when followed by an intensive course of therapy.18 It can provide relief for 2 to 12 months, and the effects have been documented to last as long as 3 years.17,18 Disadvantages of phenol use include its toxicity to tissue and the complications of pain that occasionally result.
Botulinum toxin type A (Botox) is also used to decrease the effects of hypertonicity on functional movement in hemiplegia.19–21 Local injection of the toxin into spastic muscles produces selective weakness by interfering with the uptake of acetylcholine by the motor end plate. The effect of the toxin is temporary, depends on the amount injected, and is associated with minimal side effects. Repeat injections are recommended no sooner than 12 to 14 weeks to avoid antibody formation to the toxin. Researchers report positive functional results when botulinum toxin A injections are followed by intensive muscle reeducation and appropriate splinting.22
Dantrolene sodium is used to interrupt the excitation-contraction mechanism of skeletal muscles. Trials have shown that it has reduced spasticity in 60% to 80% of clients while improving function in 40% of these clients. The side effects—drowsiness, weakness, and fatigue—can be decreased through titration of dosage. Serious side effects, including hepatotoxicity, precipitation of seizures, and lymphocytic lymphoma, have been reported when the drug has been used in high doses over a long time.17
Baclofen, in pill form, is used as a skeletal muscle relaxant to decrease spasticity. It can now be delivered intrathecally into the spinal cord with a pump that is surgically inserted into the body. It relieves spasticity with a small amount of medication (10 mg/20 mL, 10 mg/5 mL). Intrathecal baclofen has had dramatic results in cases of severe spasticity because it acts directly on the affected muscles instead of circulating in the blood. It is used for extremity spasticity that interferes with the ability to assume functional positions in patients with severe stroke, multiple sclerosis, head injury, and cerebral palsy.23
Seizures.
The highest risk for seizure after a stroke is immediately afterward; 57% of seizures occur in the first week and 88% occur within the first year.24 Seizures after thrombotic and embolic stroke are usually of early onset, whereas seizures after hemorrhagic stroke are of late onset. The management of seizures after stroke is usually with antiseizure medication. Commonly used drugs include phenytoin (Dilantin), carbamazepine (Tegretol), gabapentin (Neurontin), and divalproex (Depakote).25 Side effects that interfere with movement therapy include drowsiness, ataxia, distractibility, and poor memory.
Respiratory involvement.
Fatigue is a major problem for the person with hemiplegia. This fatigability, which interferes with everyday life processes and active rehabilitation, is attributed to respiratory insufficiency resulting from paralysis of one side of the thorax. Haas and colleagues26 studied respiratory function in hemiplegia and found decreased lung volume and mechanical performance of the thorax to be significant factors, in addition to abnormal pulmonary diffusing capacity. Clients with hemiplegia consume 50% more oxygen while walking slowly (regardless of the presence or absence of orthotic devices) than that used by subjects without hemiplegia.26 The decreased respiratory output and the increased oxygen demand that result from atypical movement patterns are responsible for early fatigue in persons with hemiplegia. Treatment objectives and techniques must reflect the understanding of this respiratory problem. For clients who walk at velocities greater than 0.48 m/s, a gain in walking capacity is associated with an increased peak Vo2. Research exploring the role of exercise after stroke indicated that gains in respiratory fitness were associated with increased walking capacity. In clinical practice, therapists should remember to include standard respiratory measures and functions to evaluate the efficacy of treatment techniques.27
Cardiovascular health.
In the chronic stage of recovery, clients may have significant cardiovascular deconditioning with half the fitness levels of age-matched controls. This decrease in fitness affects the performance of daily activities and adds to these clients’ morbidity and mortality risk. This decreased fitness results in part from decreased mobility of the leg, muscular atrophy, altered muscle physiology, increased muscular fat, and altered peripheral blood flow.28,29
Fractures.
If the hemiplegic client has severe extremity or trunk weakness and relies heavily on the nonparetic extremities for function, poor balance and falls are possible. After a stroke the risk of hip fracture is greatest in the first year of recovery. Eighty percent of hip fractures occur on the paretic side and are the result of bone loss or falls. In addition, other common fracture sites are the humerus and wrist.30
Therapy intervention for a hip fracture with a hemiplegia is complicated by increased difficulty sustaining a symmetrical trunk posture over the fractured hip, decreased strength in the leg, pain, and spasticity. In addition to the loss of balance and protective mechanisms, the development of osteoporosis from disuse is a limiting factor for functional recovery after a fracture.31
Thrombophlebitis.
Thrombophlebitis may occur in the early stages of rehabilitation. Vascular changes are often premorbid. Deep vein thrombosis is caused by altered blood flow, damage to the vessel wall, and changes in blood coagulation times. The vascular changes are aggravated by the inactivity and dependent postures of the weak extremities. Deep vein thrombosis is many times more common in the weak leg.32
Complex regional pain syndrome.
Formerly known as reflex sympathetic dystrophy, complex regional pain syndrome is a chronic pain condition affecting the paretic arm or leg. The extremity pain is reported as intense and burning and may be accompanied by swelling and redness. It leads to changes in bone and skin and, if left untreated, becomes debilitating. Medical treatment includes the use of chemical sympathetic blocks and oral or intramuscular corticosteroids. The use of blocks and corticosteroids often stops the burning pain. The length of time of the relief varies from client to client. Adverse reactions from blocks and corticosteroids occur about 20% of the time33,34 (see Chapter 32).
Sequential stages of recovery from acute to adaptive phase
Evolution of recovery process
The stroke-in-evolution develops gradually with distinct demarcation of the damaged area over 6 to 24 hours. Thrombosis, the most common cause of stroke, results first in ischemia and finally in infarction. Its gradual onset has led researchers to believe that a “cure” may be found for this type of stroke. If ischemic tissue can be treated and saved before infarction occurs, the neurological damage may be reversible. Small hemorrhages also may become a stroke-in-evolution by effusing blood along nerve pathways and by attracting fluid.35 A completed stroke has a sudden onset and produces distinct, nonprogressive symptoms and damage within minutes or hours. In contrast, the TIA has a brief duration of neurological deficit and spontaneous resolution with no residual signs. TIAs vary in number and duration.
The physician decides the extent of hospitalization. The trend to hospitalize is more common today than years ago.36 However, a mild stroke or TIA may produce minimal physical and mental symptoms, and the person may not even seek medical help. Cost-containment measures in hospitals and managed care have led to decreased lengths of stay and the development of critical pathway plans to deliver services more efficiently. Critical pathways are plans that describe the duration and extent of services after a stroke. The inpatient length of stay for acute stroke is currently 2 to 4 days. After the inpatient stay, the client follows one of four pathways: he or she returns home with or without home care services, goes to a rehabilitation hospital for a 2- to 4-week stay, goes to a subacute facility to become strong enough for the rehabilitation regimen, or goes to a long-term care facility for rehabilitation or maintenance care.
Once the stroke is completed, the clinical symptoms begin to decrease in severity. A person with a stroke caused by an embolic episode may have symptoms that reverse completely in a few days; more frequently, however, improvement takes place very slowly with a marked deficit. The fatality rate is high within the first day but decreases substantially in the following months of recovery.36 Evidence from efficacy studies of rehabilitation programs that aim at improving functional performance is limited. Studies by Bamford and colleagues37 indicate that early rehabilitation intervention reduces disability and improves compensatory strategies.
The Framingham Heart Study has revealed that long-term stroke survivors have a good chance of returning to independent living. The greatest deficit in persons with hemiplegia who have recovered basic motor skills and who have returned home is in the psychosocial and environmental areas.5
Recovery of motor function
Recovery of motor function after a stroke was thought historically to be complete 3 to 6 months after onset. More recent research has shown that functional recovery from a stroke can continue for months or years.38,39 Measuring recovery is difficult because the definition of “successful” or “complete” recovery varies greatly. Duncan reports that if recovery is defined at the disability level (Barthel score greater than 90), 57% of stroke survivors have a complete recovery. However, if impairments are measured, less than 37% recover fully. And if recovery is related to prior physical functioning, less than 25% are considered completely recovered.40
The initial functional gains after the stroke are attributed to reduction of cerebral edema, absorption of damaged tissue, and improved local vascular flow. However, these factors do not play a role in long-term functional recovery. The brain damage that results from a stroke is thought to be circumvented rather than “repaired” during the process of functional recovery. The CNS reacts to injury with a variety of potentially reparative morphological processes. Two mechanisms underlying functional recovery after stroke are collateral sprouting and the unmasking of neuropathways: regeneration and reorganization.38 Research continues to provide important insights into the fundamental capabilities of the brain to respond to damage. Methods of intervention that use the environment and help the client learn lead to long-term improved recovery.
The CNS has some predictable traits in response to injury. Twitchell, in his classic study, first documented the initial loss of voluntary function.41 Although paralysis with flaccidity initially exists, there is seldom, if ever, total paralysis. He reported both an increase in deep tendon reflexes after 48 hours and the emergence of synergistic patterns of movement.41 The synergistic movement patterns of the upper extremity and lower extremity have been described in detail by many.42–44 Verbal description of a visual phenomenon often leads to differences in written and spoken communication, yet the visual array or behavioral patterns may be exactly the same.45 Synergistic patterns may not be the same as movement combinations necessary for function. Although it is stated that the leg recovers more quickly or better than the arm, a leg that is bound by an extensor synergy and that is as “rigid as a pillar” during gait has not recovered more quickly and has no better function than an arm that is flexed and held across the chest and that can only grasp in a gross pattern with no ability to release.
Although studies are investigating the exact nature of the relationship between voluntary movement and spasticity, clinical evidence demonstrates that as voluntary function increases, the dependence on synergistic movement decreases.16 With the knowledge that the CNS is capable of reacting to injury with a variety of morphological processes, we should no longer view the effect of a stroke as a fixed event. Because the brain immediately institutes neuromechanisms that reconstitute typical functions, therapy interventions should emphasize use of movement patterns on the affected side to maximize return and to help the client achieve the highest level of function.
Predictors of recovery
Research in motor recovery shows that although motor recovery may continue after 6 months, the functional status usually remains constant, and that 86% of the variance in 6-month recovery is predictable at 1 month.46
In one study, although 58% of the patients regained independence in activities of daily living (ADLs) and 82% learned to walk, 30% to 60% of patients had no arm function.47 Initial return of movement in the first 2 weeks is one indicator of the possibility of full arm recovery. But failure to recover grip strength before 24 days was correlated with no recovery of arm function at 3 months.47 In another study that used the modified Rankin scale as the outcome measure, half of the patients recovered within 18 months with the greatest amount of recovery present at the 6-month mark. Predictors of recovery in this group included stroke severity, no previous ischemic stroke, peripheral artery disease, or diabetes.48
One problem inherent in prognostic research is the lack of a movement-based classification system. The clinical “predictors” in regression models are assumed to be static, whereas in fact they may change over time. Another problem is that there may be a lack of accuracy because of differences in researchers’ objectives.49
Classification of atypical movement patterns
Although the Guide to Physical Therapist Practice groups patients with neurological dysfunction according to pathological condition, therapy intervention rarely is directed by the diagnosis of stroke and resultant hemiplegia.50 The World Health Organization (WHO) classification system, the International Classification of Function (ICF-2), provides a structure that allows us to evaluate by health condition, impairment, or activity or participation limitation.51,52
Impairment-related classification systems for stroke are just beginning to be researched.53 Currently, atypical movement patterns in stroke are classified according to type of lesion (embolism, thrombosis, TIA) or side of weakness. The classification models make it easier for therapists to identify and define the focus of their intervention for the neurological patient. These models help us organize our interventions into two categories: (1) interventions that aim at improving relevant impairments that contribute to functional limitations and disability and (2) interventions that focus on the activity or participation limitations. The treatment interventions in this chapter try to relate limitations in activities to relevant underlying impairments.
Impairments contributing to activity and participation limitations
Impairments are the signs, symptoms, and physical findings that relate to a specific disease pathology. Schenkman and Butler were the first to apply a model of impairments to neurological physical therapy practice. Ryerson and Levit, using a similar format, specifically defined the impairment categories as primary, secondary, and composite54,55 (Box 23-1).
Patterns of recovery
In the 1970s, “neurophysiological” theories and approaches changed therapy treatment for adults with CNS lesions. The founders of these approaches described positions and patterns of trunk and extremity movement.42–44 These patterns were described in terms of spastic synergies, reflexive patterns, and position. Extremity movements were described as patterns of flexor or extensor synergies, arm and leg patterns were changeable according to the influence of tonic reflexes, and trunk position was always short on the affected side with scapular and pelvic retraction. The intervention techniques followed the descriptions and understanding of the movement problems. As knowledge from orthopedics, manual therapy, and motor control grew, therapists looked more closely at movement patterns and body position in clients with hemiplegia and expanded the categories. As early as 1982, new descriptions emerged that combined synergistic patterns and biomechanical influences on the musculoskeletal systems.56 Today, descriptions of position and patterns of movement follow the impairment categories. The composite impairment category used in this chapter has three generalized movement patterns that create one model of classification: (1) movement deficits, (2) atypical movements, and (3) undesirable compensatory patterns.54
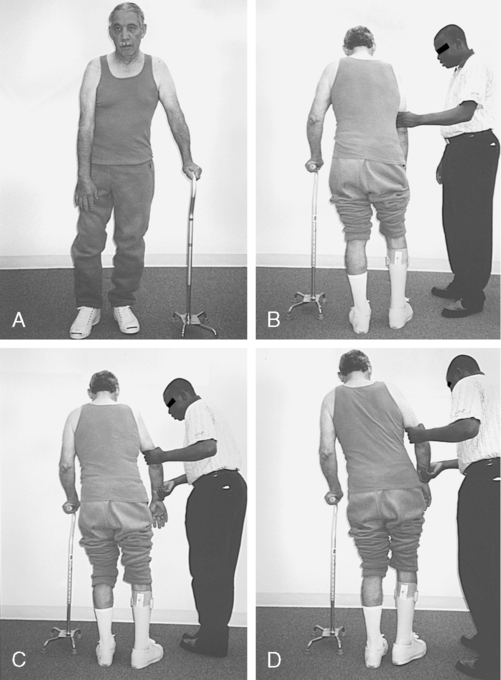
Over time, the heavy arm pulls the upper body into flexion, creating an appearance of a low shoulder. To stand and walk, a compensatory shift of the upper body onto a cane helps the client balance. This overshifting of the upper body also makes it easier for these clients to initiate stepping with the use of pelvic elevation (Figures 23-1 and 23-2).
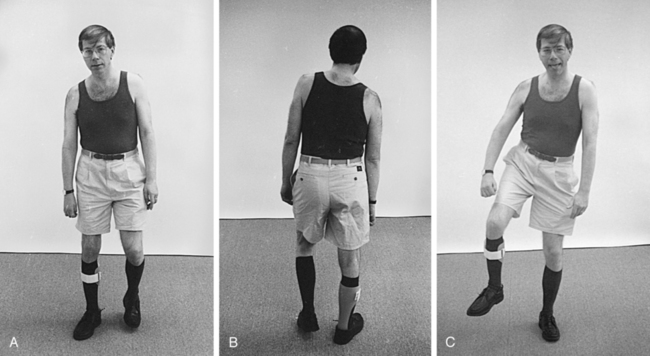
Regardless of the proximal trunk and extremity patterns, the ankle-foot and wrist-hand patterns are predictable on the basis of the amount of distal return and the effects of proximal alignment. With weakness, the ankle plantarflexes and the wrist flexes. The foot or hand rotates on the ankle or wrist according to the pattern of and amount of return of proximal movements. Finger and toe patterns (curling or fisting, clawing) follow biomechanical rules of compensation or correlation (Figure 23-3).
Although the main movement problems of stroke occur because of weakness and atypical muscle activation patterns (e.g., sequencing, initiation), other movement disturbances, such as ataxia, may occur. In clients with ataxia, the main movement problem is one of wide swings of tone and muscle activation disturbances with fewer problems of weakness. These clients demonstrate trunk instability, excessive extremity movement, and overshooting of distal targets. Voluntary extremity movements are usually present but uncoordinated (see Chapter 21).
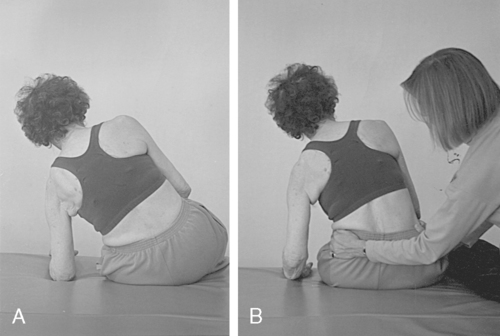
Patients who come into therapy with strongly established undesirable compensatory patterns do not respond quickly to any type of intervention. Although therapists may be tempted to train a one-sided pattern in early rehabilitation to quickly meet a stated goal, the long-term effects of learned nonuse of one side of the body include increased severity of secondary impairments and poor balance with an increased chance of falls (Figure 23-4).
Evaluation procedures
Medical evaluation
After or during the evolution of a stroke, a thorough medical examination is conducted. All systems are surveyed, with emphasis placed on the level of consciousness; mental, affective, and emotional states; communication; cranial nerves; perceptual ability; sensation; and motor function. The National Institutes of Health (NIH) Stroke Scale is often used to evaluate the level of these common impairments poststroke.57
Levels of consciousness
Scales of varying types are used to measure the client’s level of consciousness, to assess the initial severity of brain damage, and to prognosticate recovery curves. The Glasgow Coma Scale, devised by Teasdale and Jennett in collaboration with Plum,58 has been used for nontraumatic comas caused by stroke, head injury, and cardiac disease. This scale records motor responses to pain, verbal responses to auditory and visual clues, and eye opening. It assigns numerical values according to graded scales. Plum and Caronna59 and Levy and colleagues60 have also established criteria for correlating clinical signs of coma with prognosis.
The standard descriptions of level of consciousness—normal, semistupor, stupor, deep stupor, semicoma, coma, deep coma—are categorized by objective medical data but often leave a gap in the understanding of how the client functions in life.58 This gap was closed by the creation of a scale, Levels of Cognitive Functioning, at Rancho Los Amigos Hospital. This behavioral rating scale is not a test of cognitive skill but an observational rating of the client’s ability to process information61 (see Chapter 24).
Perception
Perceptual deficits in clients with hemiplegia are complex and intimately linked to the sensorimotor deficit. Sensory integration theory has begun to establish normative values and objective data for testing and documenting perceptual deficits in children. Currently, norms and testing procedures for adults have not been standardized, but perceptual deficits have been identified in clients with hemiplegia. Common perceptual deficits found in left and right brain damage are listed in Box 23-2.
Perceptual retraining without standardized norms for the deficit is at best difficult. The soundest course currently available appears to be one that relates perceptual and motor learning rather than retraining perception in isolation (see Chapters 4 and 14).
Sensation
The presence and quality of sensory loss must be considered during the process of reeducating motor control. Although Sherrington established the principle of interdependence of sensation and movement, current researchers have refined the concept and hypothesize that sensation modifies continuing movement by providing feed-forward information, feedback, and corollary discharge. They have provided evidence that sensation is not an absolute prerequisite for movement.62
Standardized evaluations
Functional performance
During the initial interview the therapist and the client together form a list of limitations and relate them to the client’s goals and needs. The client can state his or her perceived functional limitations, or the therapist can ask the client to perform tasks. Commonly used standardized tests and scales for activity and participation limitations are listed here. Additional information can be found in Chapter 8.
Scales
The Barthel Index is one of the oldest measures of disability.63 It has excellent validity and reliability and is simple to use, but it does not discriminate at higher levels of activity.
The Motor Assessment Scale (MAS) comes from the intervention theory of Carr and Shepherd.64 Its reliability is high, it is simple to administer, and it takes only 15 minutes to perform. Although it mainly evaluates mobility skills, there is an arm and hand function section. The tests of arm function include movement patterns without tasks, and the hand function section uses object manipulation.
The Functional Independence Measure (FIM) is commonly used in rehabilitation centers, takes 45 minutes to perform, and measures ADLs, mobility, cognition, and communication.65,66 It has good to excellent reliability.
The Rivermead Mobility Index measures common mobility functions, takes 5 minutes to perform, and has been tested for reliability and validity.67
The Assessment of Motor and Process Skills (AMPS) is a standardized test that measures task-performance abilities and efficiency during instrumental ADLs (IADLs).68
Tests of motor function and balance
The Fugl-Meyer Assessment is a measure of extremity impairment severity. It is weighted, with more items measuring arm movement than leg movement. The test factors in reflexes and sensation and has good validity and reliability. It requires from 45 minutes to 1 hour to perform.69
The Berg Balance Scale is easy to administer, takes 5 to 10 minutes, and has norms specific to clients who have had a stroke.70,71
The Balance Evaluation Systems Test (BESTest and Mini-BESTest) is a balance evaluation that helps clinicians identify the impaired underlying system that is contributing to poor dynamic balance. The mini-BESTest is composed of 14 items and can be administered in 10 to 15 minutes.72,73
The Postural Assessment Scale for Stroke is a clinical balance measure that has been found to have better psychometric characteristics than the Berg Balance Scale or the balance subtest of the Fugl-Meyer test for people with severe stroke during the acute recovery phase. It has excellent reliability and validity and is easy to perform.74,75
The Functional Reach Test provides a measure of balance in standing. It measures control only during anterior (forward reach) weight shifts. Reliability is high, and the test is fast and easy to perform.76
The Wolf Motor Function Test is used to measure upper-extremity movements and functional tasks. It is a timed test, has been tested for reliability and validity, and is the assessment used in constraint-induced treatment studies.77
The Trunk Impairment Scale measures trunk movement patterns and dynamic and static sitting balance. It has high test-retest reliability and excellent concurrent validity.78
Gait
The evaluation of gait patterns includes the assessment of gait speed, a description of gait deviations, and, ideally, the assignment of a value representing the efficiency of ambulation.79 Therapists are encouraged to measure gait speed throughout the rehabilitation process to quantitatively identify improvement in walking ability.80,81
The 5-meter walk test is responsive to changes in the acute phase of recovery especially the first 5 weeks poststroke. The 10-meter walk test has excellent reliability in the chronic recovery phase and is correlated with walking parameters and endurance.82
The 2-, 6-, and 10-minute walk tests are measures of walking endurance with high reliability and validity.83
The Functional Ambulation Profile is a system that attempts to relate the temporal aspects of gait to neuromuscular and cardiovascular functioning and converts this relationship to a single numerical score.84
The Timed Up-and-Go Test measures (in seconds) the ability to rise from a chair, walk 3 meters, turn, walk, and return to a seated position. It is frequently used in geriatric populations, but there is no validity testing for people poststroke.85
Gait deviations in persons with hemiplegia have been described according to their biomechanical and kinesiological abnormalities and in terms of the loss of centrally programmed motor control mechanisms.86,87
Perry87 described common problems of the hemiplegic person’s gait as loss of controlled movement into plantarflexion at heel strike, loss of ankle movement from heel strike to midstance (resulting in loss of trunk balance and forward momentum for pushoff), and loss of the normal combination of movement patterns at the end of stance (hip extension, knee flexion, and ankle extension) and at the end of swing (hip flexion with knee extension and ankle flexion).
Knutsson and Richards86 classified the motor control problems of the hemiplegic gait into three descriptive types. Type I is characterized by inappropriate activation of the calf muscles early in the gait cycle with corresponding low muscular activity in anterior compartment muscles. In the type I activation pattern, the calf musculature is activated before the center of gravity passes over the base of support. This thrusts the tibia backward instead of propelling the body forward in a pushoff as normally occurs. The client with hemiplegia compensates for the backward thrust of the tibia by anteriorly tilting the pelvis or flexing forward at the hip. Type II consists of an absence of or severe decrease in electromyographic activity in two or more muscle groups of the involved lower extremity. This pattern of markedly decreased muscular activity results in the adoption of compensatory mechanisms to gain stability. Type III activation patterns consist of abnormal coactivation of several limb muscles with normal or increased muscular activity levels in the muscle groups of the involved side. This type of pattern results in a disruption of the sequential flow of motor activity.
The Stroke Impact Scale (SIS) and the short version, the Stroke Impact Scale–16 (SIS-16) are measures with high reliability and validity for people poststroke. The long version assesses eight domains: strength, emotion, hand function, memory, physical function and mobility, communication, ADLs, and social participation. The SIS-16 includes most of the original items in the SIS physical function and mobility domain.88,89
Evaluation of movement control
Active movement and strength
When active movement patterns in the trunk and extremities are assessed, the therapist measures both strength and control. Paralysis, weakness, and imbalanced return are determinants of strength. Initiation pattern, sequencing, and control of firing patterns are indicators of control. Weakness and paralysis after stroke have been largely ignored because of a lingering focus on spasticity. Some recent studies have shown that muscle weakness is, in fact, present and interferes with the ability to generate enough force to achieve functional performance.90–92 Motor weakness is present in 75% to 80% of clients after a stroke. There appears to be no difference in clients with left- or right-sided hemiplegia in terms of frequency or severity of weakness.93 In contrast to these studies, Landau and Sahrmann94 investigated the degree of functional impairment in strength that was a result of deficits in the contractile element of the affected muscles. Their findings from comparisons of maximal tetanic contraction of the anterior tibialis muscle suggest that maximal voluntary muscle strength was not impaired. Although recent research has moved weakness back into the impairment list, there is much more to be learned about the nature of weakness in CNS dysfunction.
The objective assessment of active movement in hemiplegia is commonly documented by therapists through the use of the Fugl-Meyer assessment scale, derived from synergistic stages as outlined by Brunnstrom,43 and is similar to a version of Bobath’s long evaluation form, which gradually builds series of selective or fractionated movement in the arm, trunk, and leg.95
When clinically assessing weakness and control of active movement patterns, the therapist analyzes and identifies the client’s patterns of posture and movement in the trunk and extremities by position (supine, side lying, sitting, and standing) and in linked combinations. Active movement control is evaluated in individual muscles, movement components, and movement sequences.54 Verbal directions or demonstrations may be necessary to help the client understand what is desired. In this phase of the evaluation, the therapist should not physically assist the client’s movement but should be prepared to prevent loss of balance.
While evaluating force production or weakness in all these categories, the therapist gathers information about sequencing movements in increasingly complex patterns, timing of muscle firing, and speed of movement. Muscle activation deficits in these categories may explain why some clients with minimal weakness do not regain spontaneous functional use of the extremities.54
Assisted movement
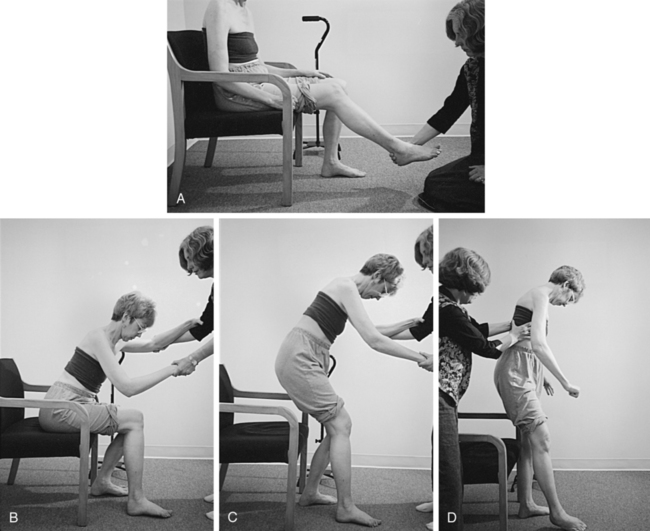
1. To correct alignment to gather additional information about strength, control, and orthopedic impairments (Figure 23-5)
2. To limit degrees of freedom of one of the joints to assess relationships between intralimb segments
3. To assist the movement of a weak muscle
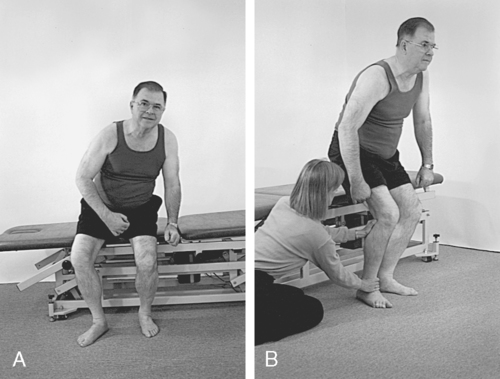
4. To block or stabilize a joint to assess the performance of a weaker muscle group or to limit the degrees of freedom of an intralimb segment54 (Figure 23-6)
Example
Step 1. Assessment of forward reach in sitting by client with left hemiplegia. Active movement patterns on left: client initiates movement proximally; shoulder flexes to 60 degrees, with internal humeral rotation; abducted, downwardly rotated scapula elevates during the movement; elbow flexes, forearm supinates to 10 degrees; wrist remains in flexion and radial deviation. Client leans trunk forward to assist with task but cannot reach arm forward to place it on table.
Step 2. Clinical judgment or hypothesis 1: Weakness of scapula and humeral external rotators prevents antigravity use of elbow extensors during forward reach. Supination of forearm comes from strong proximal initiation and use of elbow flexors to lift arm. Clinical judgment or hypothesis 2: Forearm, wrist, and hand position prevents distal initiation and biases shoulder in internal rotation, thus blocking use of elbow extensors.
Step 3. Test hypothesis 1: Therapist uses her or his hands to externally rotate the humerus to neutral and asks client to reach again. Result: Client activates elbow extension halfway through range with shoulder forward flexion and places wrist and hand on table. Clinical intervention implication: Increased control of humeral external rotation and increased control of accompanying scapular pattern are important intervention goals to regain forward reach of arm. Retrain trunk, scapular, and humeral movement patterns, with emphasis on shoulder external rotation and scapular upward rotation. Assess secondary impairments of pectoral and rotator cuff tightness (rotator cuff is shortened if scapula is in an abducted position). If result is unchanged, test hypothesis 2: Therapist supports wrist and hand with wrist splint or with his or her hand and asks client to reach again. Result: Client activates elbow extension and places the wrist and hand on the table. Clinical intervention implication: Prevention or blocking of wrist flexion limits the degrees of freedom, changes the internal rotation moment on the distal portion of the lever arm, and allows use of existing elbow extensors. Use small wrist splint during independent practice or use object to assist or preset distal segment during practice.
Tone
The evaluation of extremity tone, sometimes referred to as spasticity, continues to be an integral part of poststroke movement research and is part of the physician’s neurological examination. Over the years, leading physiologists have split into two camps over the definition of tone. During the beginning of the 20th century, tone was thought of as postural reflexes. In the 1950s the concept of tone was thought of as a state of light excitation or a state of preparedness.96 Granit97 later encouraged us to think of the relatedness of both these views. He believed that the same spinal organization is mobilized by the basal ganglia to produce both manifestations of tone: a state of preparedness and the postural reflexes.97 In the 1980s, scientists challenged the concept that what led to a spastic movement pattern was hypertonicity resulting from an exaggerated stretch reflex.98,99 A new construct emerged in the following years that acknowledged the contribution of both neural and nonneural elements to the phenomenon of “spasticity.” This newer concept of spasticity explains why the stretch reflex or tendon tap response (performed in a passive condition—during rest) is an “epiphenomenon and is not the cause of the “spastic movement problem” that interferes with movement.”100 Although the Modified Ashworth Scale is an objective measure of spasticity caused by the stretch reflex,101 it is not a measure of the functional problem that interferes with skilled movement. It is heartening to hear such discussions occurring among physiologists because therapists are also questioned about their notations of and changes in tone and they often have no objectively derived standard clinical system for measurement. The debate over tone continues, but clients with CNS dysfunction clinically display changes of muscle tone that result in longer rehabilitation stays and problematic secondary impairments.102
The response of a spastic muscle to stretch differs during passive and active movements, leading some to question the usefulness of the classic numerical test of spasticity, the Ashworth Scale. The Ashworth Scale rates the severity of tone from 1 to 5.103
As the client becomes more active, he or she uses all available movement patterns. Ryerson and Levit have described three specific situations, which in reality have overlap, wherein tone increases (see page 23 for a detailed discussion).54 This increased tone, or clinical hypertonicity, occurs in the arm and leg if the client’s trunk control is less than the demand of the task, if altered joint alignment increases the tension of the muscle, or if the voluntary movement pattern of the extremity is unbalanced and disorganized.54,104
One clinical description of increased extremity tone put forth in the 1970s is still somewhat useful today: severe hypertonicity makes coordinated movements impossible; moderate hypertonicity allows movements that are characterized by great effort, slow velocity, and abnormal coordination; slight hypertonicity allows gross movement patterns to occur with smooth coordination, but combined, selective movement patterns are uncoordinated or impossible.105
Equilibrium and protective reactions
When assessing equilibrium or balance reactions in clients with hemiplegia, the therapist remembers the distinction between equilibrium reactions and protective reactions. Equilibrium reactions should be assessed while slowly moving either the limb or trunk away from the base of support. The amount of control in the trunk and supporting limb, the size of the base of support, and the available range of motion as well as the evaluator’s handling skills affect the response (see Chapter 22).
Descriptive analysis of functional activities
When evaluating functional activities, the therapist assesses three phases of the movement pattern. The first phase is the initiation of the act, which includes the body segment initiating the movement, the direction of movement, and the establishment of antigravity control. Transition, the second phase, represents the point in the functional activity at which there is a switch in the muscle groups that provide antigravity control. The third phase is the completion of the activity, involving a final weight shift and the ability to maintain postural control.54
Evaluation of secondary impairments
Loss of joint range and muscle shortening
Example 1.
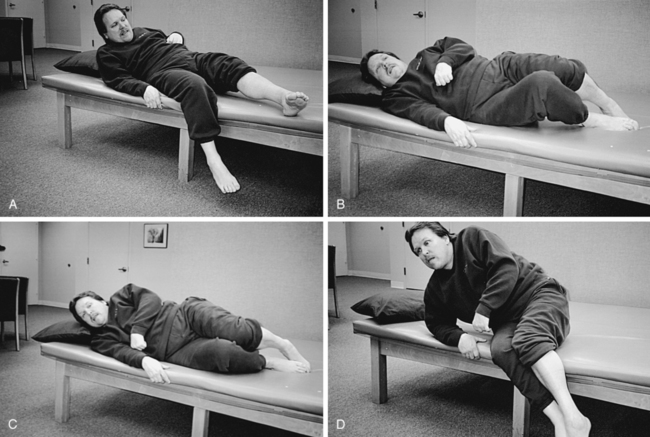
In sitting (knee bent), the client has ankle joint dorsiflexion range from 0 to 10 degrees; but in standing (knee and hip straight), ankle joint dorsiflexion range is −20 degrees. This functional loss of ankle range causes significant problems for standing and walking. Loss of ankle joint range in standing may be the result of gastrocnemius and soleus, tensor fasciae latae, or hamstring muscle tightness (Figure 23-7).
Pain
Two commonly used standardized pain measurement scales are the visual analog pain rating scale and the McGill Pain Questionnaire.106,107 These scales focus primarily on the intensity of pain but provide an objective measure of intervention effectiveness. For an in-depth discussion of the topic of pain management, see Chapter 32.
The presence of pain in hemiplegia is devastating for the client and makes movement reeducation difficult. Shoulder pain is the most frequent pain complaint after stroke.11,108 Pain must be evaluated specifically and should not be allowed to occur during intervention; the “no pain, no gain” message that is sometimes used in sports or orthopedic intervention should not be used in neurorehabilitation. Pain is an indicator that joint alignment or movements are incorrect. See Box 23-3 for general questions.
Clinical decision making and problem solving
Developing hypotheses for significant impairments
The process of motor performance evaluation results in a list of activities and related impairments. However, not all these impairments directly relate to each activity limitation of the client. The therapist, using clinical judgment, hypothesizes a causal relationship between frequently occurring impairments and activity limitation. These impairments, called significant impairments, are the ones that must be changed for measurable changes in movement and function to occur.54 The other impairments are not forgotten but are reevaluated later as improvement begins and new activity goals are chosen. The significant impairments are often used as the focus of short- and long-term goals because they are the underlying building blocks of the selected activity goal. Because functional movement depends on the linkage of trunk and extremity movements, the therapist develops hypotheses between impairments in the extremities and specific levels of trunk control to set goals that result in improved activity performance. If weakness and control deficits of the trunk, arm, and leg are treated separately, the client may see improvement in the impairments but not see a change in function (Box 23-4). (See Chapters 4 and 9 for further discussion.)
Goal setting
Functional goals
Functional goals are based on the needs and desires of the client and on the functional impairments that were identified by the therapist during the initial assessment. Functional goals should represent a significant change in the patient’s level of independence, be practical, and reflect improvement in a specific activity limitation. They state the desired function and the expected level of performance.54
Long-term goals
A long-term goal should reflect a major improvement in a primary or secondary impairment or an increase in level of performance of an existing skill. The accomplishment of a long-term goal brings the client closer to the functional goal. The time it takes to accomplish a long-term goal varies tremendously depending on the frequency of treatment and the length of time after stroke. The therapist may set many short-term goals to achieve one long-term goal. Long-term goals may be stated in functional terms, but they usually reflect a change in a primary impairment: an increase in strength, movement control, or balance.54
Short-term goals
A realistic short-term goal should be achievable quickly and should be based on the result of the patient’s response to handling during the evaluation of movement. Short-term goals should directly relate to the accomplishment of the long-term goal. There are multiple short-term goals that relate to one long-term goal. Short-term goals are compiled from the list of relevant secondary impairments or desired increases of strength or movement control. These goals are measurable but do not in and of themselves result in a functional change.54
When stated in terms of movement control rather than functional performance, these goals include the reestablishment of generalized movement patterns that link movement patterns of the trunk and extremities (Box 23-5).
Choosing intervention techniques
Controversy exists as to the means of increasing functional mobility and performance in clients who have had a stroke. One school of thought teaches compensatory patterns or hopes for some use of the affected side through task-specific practice without direct intervention for the neurological impairments. The other prevalent practice pattern is to increase functional movement patterns on the affected side to help achieve an activity goal by increasing control and strength of movement sequences of the trunk and limb through specific levels of reeducation.54,109–111
A combination of these two practices may be useful: impairment-based intervention strategies to reeducate movement and training strategies to foster desirable compensations—a functional reeducation strategy. This type of intervention includes strengthening trunk and extremity linked patterns of movement, minimizing or eliminating secondary impairments that interfere with regaining control, teaching appropriate compensations, and training the client to practice functional movement patterns in the context of daily tasks54,112 (Box 23-6). Research findings support a link between the trunk and upper extremity and the trunk and lower extremity during reaching activities.113,114 One result of this research has been to design treatment interventions that restrain trunk movements during forward reach retraining to increase control of elbow extension movement in the paretic arm.115,116
For reeducation to be effective, therapists must allow the patient to initiate the active trunk and extremity pattern, must move from assisted practice to independent practice with the assistance of appropriately selected objects or verbal cues, and must teach the patient appropriately staged practice patterns. Studies based on the “learned nonuse” phenomenon described by Taub have shown that when patients are encouraged to use the affected arm rather than receiving pessimistic messages about its potential, movement and functional use, even if limited, are possible.117,118
Regardless of intervention type used, task-performance practice or a reeducation strategy, there comes a time in the recovery process when therapists help the client select practical compensatory strategies. Compensatory strategies are taught when the client needs to function independently and cannot yet use the affected arm because of insufficient recovery or the severity of damage. To be appropriate, the strategy should incorporate the use of the involved extremities and use appropriate trunk movement patterns to maximize future return of movement. Undesirable compensations are patterns that are so asymmetrical that they fail to incorporate available movements of the affected trunk and extremities (Figure 23-8).
Although current literature generally applauds function-based techniques, therapists in clinical settings use hands-on approaches to increase muscle strength and control and to decrease impairments that block the emergence of new functional patterns.54 As research in movement science and recovery of movement increases, therapists must critically analyze research findings and judiciously integrate them with their clinical experience and judgment.