CHAPTER 168 Motor Cortex Stimulation
Electrical stimulation of the nervous system has been known since antiquity to have analgesic effects. Intermittent application of electricity for the treatment of pain has a long and colorful history, culminating in the development of modern systems for the chronic administration of therapeutic electrical current.1 Following the introduction of spinal cord stimulation in 1967, electricity has been used successfully to modulate pain by stimulation of peripheral nerves and central nervous system structures, including the spinal cord, brainstem, thalamus, and most recently the cerebral cortex.
In the early 1990s, motor cortex stimulation (MCS) was introduced as a treatment for central deafferentation pain. This severe pain syndrome occurs with interruption of the spinothalamic pathway, most commonly following hemorrhagic or ischemic stroke. The primary sensory cortex was initially targeted as the final relay in the spinothalamic system; however, it was soon realized that stimulation of the motor cortex was more effective in controlling pain.2 Chronic stimulation of the precentral cortex for the treatment of pain was first introduced by Tsubokawa and colleagues in 1991.3,4 Subsequently, there has been growing corroborative evidence derived from case reports and individual case series to suggest that epidural MCS can be an effective treatment for many patients with intractable neuropathic pain. Poststroke pain, phantom limb pain, multiple sclerosis, spinal cord injury pain, postherpetic neuralgia, and neuropathic pain of the limbs or face have all been reported to respond favorably to MCS.5 Most clinical studies focus on the use of MCS in poststroke pain, for which there are few other treatments. Poststroke pain responds variably to MCS, with about 50% of patients achieving adequate relief. However, some studies have documented excellent results in the treatment of trigeminal neuropathic pain, with 75% to 100% of patients achieving good or excellent pain relief.6–10 MCS thus appears to hold promise for patients with intractable and otherwise difficult-to-treat pain syndromes.
Surgical Technique
Following Tsubokawa’s initial report,4 several studies were published using a similar technique of introducing an epidural electrode through a bur hole under local anesthetic.3,6,8,11–15 In several cases, groups that started out using a bur hole technique later switched to performing electrode placement through craniotomy,6,8,9,14 and most surgeons now perform a craniotomy either under local6–8,10,13,14,16–21 or general anesthesia.22–29 Nearly all investigators place the electrodes epidurally, although subdural placement has been described.30,31 Image-guided neuronavigation is used to precisely identify the motor cortex intraoperatively,9,20,27–29,32 and proper placement is confirmed with physiologic testing.
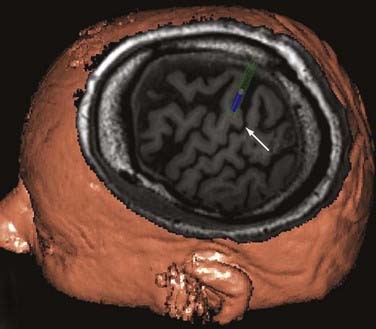
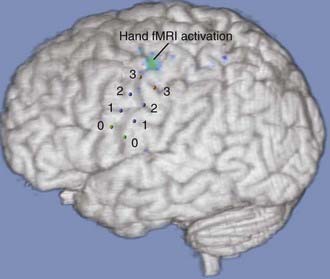
Fiducials are placed preoperatively on the scalp, and volumetric magnetic resonance imaging (MRI) is obtained. The central sulcus, sylvian fissure, and inferior and superior frontal sulci are identified. The “hand knob,” a distinctive area of motor cortex, can usually be easily identified in oblique sections (Fig. 168-1). For facial pain, the optimal target is often identified anterior to the central sulcus adjacent to or below the inferior frontal sulcus. Some investigators have used functional MRI (fMRI) to assist in identifying the appropriate areas for stimulation (Fig. 168-2). Prophylactic antibiotic medication is given along with anticonvulsant agents. The patient is placed in a supine position with a roll beneath the shoulder and the head rotated to the ipsilateral side of the pain. The target is mapped onto the contralateral scalp, and an incision centered over the central sulcus is made.
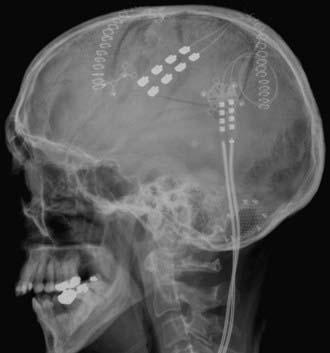
When the appropriate cortical target has been confirmed, the diagnostic grid is removed, and a 4-contact paddle electrode is positioned over the motor cortex, parallel to the central sulcus. Some investigators have described placement of the electrode perpendicular to the central sulcus, with the two central contacts of the electrode array over the target point in the motor cortex. A second electrode may be placed posteriorly over the central sulcus to allow for transsulcal stimulation and increased programming options (Fig. 168-3). Each electrode is sutured to the outer layer of the dura. If an externalized trial is to be performed, the lead wire is tunneled out through a separate stab incision, and the craniotomy bone flap is secured.
Stimulation Parameters
There is tremendous variation in reported stimulation parameters for MCS. Pain relief can occur at amplitudes from 0.5 to 10 V, rates from 5 to 130 Hz, and pulse widths from 60 to 450 microseconds.33 Although most studies have used rates of about 40 Hz, others have found higher rates to be necessary in some cases. There is also no agreement on whether wide or narrow pulse widths provide more effective stimulation. Amplitudes have in many cases been empirically chosen, whereas other investigators base stimulation amplitude on a percentage of motor threshold.
Pain relief is most commonly achieved at amplitudes of 6 V or less, with average amplitudes of 5 V or less in most studies. Amplitudes greater than 6 V are more likely to be associated with seizures during programming, with seizures commonly induced at amplitudes approaching 9 V.34
Many investigators have noted that MCS frequently produces a period of poststimulus pain relief that can range from minutes to hours. Thus, most publications report the use of a cycling mode of stimulation, with 10 minutes to 3 hours on stimulation followed by 15 minutes to 6 hours off stimulation. In one study, switching from a continuous to cycling mode (in addition to other programming changes) may have contributed to improvement in pain relief.34
Indications and Results
Central Deafferentation Pain
MCS was originally developed in response to the lack of effective treatments for central deafferentation pain. In Tsubokawa’s first published report, 5 of 12 patients had complete resolution of their pain at 1 year of follow-up, with another 3 exhibiting significant decrease in pain.3 These encouraging early results led many other investigators to pursue this technique for treatment of this otherwise difficult-to-treat condition.9,11,12,17,22,23,28,29,35–38
Nguyen and colleagues9 reported 13 patients with central deafferentation pain from ischemic and hemorrhagic stroke as well as trauma (1 case) and abscess (1 case). Ten patients (77%) had “significant improvement,” classified as more than 40% reduction in pain. In a large series of 31 patients with poststroke pain (3 bulbar, 20 thalamic, and 8 suprathalamic), Katayama and associates17 obtained “satisfactory” pain relief in 15 (48%) with follow-up periods of more than 2 years. In 20 patients with central neuropathic pain, including 16 with poststroke pain, Mertens and colleagues achieved “excellent” pain relief in 25% and “good” pain relief in 35%. Other series have generally corroborated these results, with about 50% to 60% of patients achieving significant pain relief.
Trigeminal Neuropathic Pain
Trigeminal neuropathic facial pain is a syndrome of severe, constant facial pain related to disease of, or injury to, the trigeminal nerve or ganglion. Causes of trigeminal neuropathic pain can include injury from sinus or dental surgery, skull or facial trauma, or intentional destruction for therapeutic reasons (deafferentation), as well as intrinsic pathology of any part of the trigeminal system.39 Patients who fail surgical treatment for trigeminal neuralgia may sometimes develop trigeminal neuropathic pain (also called trigeminal deafferentation pain),39 for which there are few, if any, effective treatments. Few significant advances have occurred in pharmacologic therapy, and anticonvulsant and antidepressant medications remain the mainstay of treatment. Deep brain stimulation of well-defined targets in the sensory thalamus and periaqueductal or periventricular gray matter has had generally disappointing results.40
MCS has shown some promise in the treatment of trigeminal neuropathic pain, beginning with the 1993 publication by Meyerson and colleagues.6 In a group of 10 patients with different types of neuropathic pain, all 5 patients with trigeminal neuropathic pain obtained between 60% and 90% pain relief at 8 to 28 months. A follow-up study by Herregodts and associates41 showed 50% to 100% reduction in visual analog scale pain scores in 4 of 5 patients with trigeminal neuropathic pain.
In a 1996 study by Ebel and coworkers,7 7 patients with trigeminal neuropathic pain of various etiologies were treated with motor cortex stimulation. Six of the 7 patients underwent permanent implantation, with 5 of these 6 achieving 80% or greater pain relief. Two patients subsequently lost pain relief over the course of several months, leaving 3 of 6 patients (50%) with a satisfactory result at last follow-up.
Nguyen and colleagues have published several descriptions of their surgical technique and programming approach.8,9,14 In their series, all patients with neuropathic facial pain achieved 40% to 100% pain relief.8,9,14
More recently, Brown and Pilitsis27 reported on 10 patients who underwent a trial of MCS for facial pain of various etiologies, including trigeminal neuropathic pain, postherpetic neuralgia, and central poststroke pain. All 8 patients with a peripheral neuropathic mechanism for their pain underwent placement of a permanent system after successful trial. Eighty-eight percent of these patients obtained immediate pain relief of at least 50%, and 75% experienced sustained reduction in pain at 3 to 24 months of follow-up. A review of the literature corroborated these results, showing 29 (76%) of 38 patients with neuropathic facial pain achieved at least 50% pain relief.27 All patients in the implanted cohort experienced a decrease in their medication requirements by more than 50%.
Other Indications and Overall Efficacy
MCS has been used for a number of other neuropathic pain conditions including phantom limb pain,22,23,42 spinal cord injury pain,9,29,32,43 peripheral nerve injury,6,9,22,28,29 and others. Given that only a few patients have been reported for each, no clear conclusions can be drawn regarding efficacy of MCS for these indications.
Two recent structured literature reviews have been performed to address the question of overall efficacy of MCS. In a search of the medical literature, Fontaine and colleagues44 found 244 references, 14 of which were of sufficient quality to subject to further analysis. Of 210 patients in these 14 studies, 43% achieved more than 50% pain relief, 57% had a “good postoperative outcome” (pain relief greater than 40% or 50%, depending on the study), and 30% had more than 70% pain relief. In central pain, 54% of patients achieved more than 40% to 50% pain relief, whereas for trigeminal neuropathic pain, 68% achieved more than 40% to 50% pain relief.
Another review published contemporaneously analyzed 22 articles and found that the weighted responder rate (responders defined as patients who showed a global response according to each study’s definition) was 72.6%.45 However, the selection criteria for these studies was less rigorous than the review of Fontaine and colleagues, and the definition of responder is not entirely clear. Despite these limitations, it is evident that between 30% and 60% of patients achieve more than 50% pain relief with MCS.
A single prospective study incorporating a blinded evaluation period has been performed.46 In this study, 11 patients underwent trial MCS implantation, of which 8 went on to permanent implant. These 8 patients achieved a median reduction in pain of 63.2%. Only one patient failed to achieve more than 50% pain relief. Pain scores increased to near the preoperative baseline in all patients during blinded deactivation of the stimulator. As in other studies, the presence of complete deafferentation was a poor prognostic indicator for MCS efficacy.
Safety
MCS, like any neurosurgical procedure, can be associated with risks and complications, including bleeding, infection, and neurological deficits. Although many studies have reported no adverse events with MCS,3,4,11,12,15,31,42,46,47 there have been some serious complications reported in the literature. Two epidural hematomas have been reported, one small and asymptomatic,8 the other requiring evacuation and associated with persistent dysphasia.6 One group reported two patients with devastating cerebral hemorrhages, with one patient dying from this complication and the other remaining in a persistent vegetative state.25,30 It is possible that these complications resulted from this group’s use of subdural rather than epidural placement. Infection of the hardware requiring removal or treatment with antibiotics, or both, has been reported in a number of studies.9,22,27–30,38 Some patients have also experienced wound dehiscence that resolved with surgical revision.9 Breakage of the hardware can also occur.22 Two patients in one study experienced transient postoperative neurological deficits (one speech, one motor).29
Programming of MCS systems, as well as long-term treatment with MCS, can also be associated with certain risks and side effects. Foremost among these is the risk for seizures, which have been frequently reported. These have been variously described as brief focal seizures during programming,28,29 unspecified seizures during programming,20,23,25,34 prolonged focal seizure with postictal speech arrest,7 short-lasting generalized seizures during programming17 (occurring in most patients in one study,)6 and generalized seizures with activation of the stimulator.10,38 In one recent study of intensive MCS reprogramming, the average threshold for inducing seizures was 8.9 V.34 There is at least one patient who developed severe epilepsy after long-term MCS.13 To further investigate the epileptogenic potential of chronic MCS, Bezard and associates13 undertook a study in three macaque monkeys who were implanted with MCS electrodes. They found that with stimulation at a rate of 40 Hz and a pulse width of 90 microseconds, no seizures occurred even at stimulus intensities up to 3 mA greater than the motor threshold. Higher frequencies and pulse widths induced muscle twitching at lower amplitudes and consequently also induced seizures at lower amplitudes.
Other reported side effects from stimulation include painful stimulation of the dura mater,6,9,16 stimulation-induced dysesthesiae,8,17,48 dysarthria,22 and fatigue.22 There are two case reports of unusual side effects: impairment in a motor imagery task49 and development of a painful supernumerary phantom arm in a poststroke pain patient.50 In addition, there is a suggestion that MCS may adversely affect cognitive function, especially in older patients.51
Mechanism of Action
The exact mechanism of action of MCS has yet to be elucidated and is the subject of current study. Positron emission tomography (PET) studies demonstrate that cortical stimulation increases cerebral blood flow (CBF) in the ipsilateral thalamus, cingulate gyrus, orbitofrontal cortex, and brainstem.37,52,53 Some studies have found that regional CBF increases occur in the ipsilateral ventrolateral thalamus, which largely carries corticothalamic connections from the motor and premotor areas.37,52,53 Regional CBF increases in this site, however, are less than those seen in the anterior cingulate gyrus, insula, and brainstem. There appears to be a correlation with the extent of pain relief and the increase in cingulate blood flow.37,52,53 Activation of the brainstem periaqueductal gray area has also been suggested to play a role. The results of these CBF studies suggest that the somatosensory cortex is not activated by MCS, nor does there appear to be activation of the downstream motor pathways below the stimulating electrode. However, the exact effects seen may vary depending on the surgical technique and stimulation parameters chosen.
Conclusion
Despite the reported success with MCS for the treatment of neuropathic pain, there have been no large, controlled, prospective, randomized trials of this modality. One small, prospective, blinded trial has demonstrated outstanding efficacy. However, replication of these results is necessary. We face a situation similar to that experienced with deep brain stimulation for pain in the 1970s and 1980s. The procedure was widely used with little strong evidence for efficacy, until two prospective trials were eventually performed in the 1990s. These trials showed that deep brain stimulation for pain could be effective, but suggested that a very low percentage (13.5% to 17.8%) of patients could be proved to have clinically significant pain relief at long-term follow-up.40 The lesson learned from this study was that future trials of analgesic devices should follow structured protocols for patient selection and use uniform implantation and treatment paradigms. It is imperative that MCS be subjected to this type of scrutiny before its widespread adoption as a potential standard therapy for chronic pain.
Brown JA, Barbaro NM. Motor cortex stimulation for central and neuropathic pain: current status. Pain. 2003;104:431-435.
Brown JA, Pilitsis JG. Motor cortex stimulation for central and neuropathic facial pain: a prospective study of 10 patients and observations of enhanced sensory and motor function during stimulation. Neurosurgery. 2005;56:290-297.
Burchiel KJ. A new classification for facial pain. Neurosurgery. 2003;53:1164-1166.
Coffey RJ. Deep brain stimulation for chronic pain: Results of two multicenter trials and a structured review. Pain Med. 2001;2:183-192.
Fontaine D, Hamani C, Lozano A. Efficacy and safety of motor cortex stimulation for chronic neuropathic pain: critical review of the literature. J Neurosurg. 2009;110:251-256.
Garcia-Larrea L, Peyron R, Mertens P, et al. Electrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological study. Pain. 1999;83:259-273.
Henderson JM, Boongird A, Rosenow JM, et al. Recovery of pain control by intensive reprogramming after loss of benefit from motor cortex stimulation for neuropathic pain. Stereotact Funct Neurosurg. 2004;82:207-213.
Hosobuchi Y. Motor cortical stimulation for control of central deafferentation pain. Adv Neurol. 1993;63:215-217.
Katayama Y, Fukaya C, Yamamoto T. Poststroke pain control by chronic motor cortex stimulation: neurological characteristics predicting a favorable response. J Neurosurg. 1998;89:585-591.
Lima MC, Fregni F. Motor cortex stimulation for chronic pain: systematic review and meta-analysis of the literature. Neurology. 2008;70:2329-2337.
Mertens P, Nuti C, Sindou M, et al. Precentral cortex stimulation for the treatment of central neuropathic pain: results of a prospective study in a 20-patient series. Stereotact Funct Neurosurg. 1999;73:122-125.
Meyerson BA, Lindblom U, Linderoth B, et al. Motor cortex stimulation as treatment of trigeminal neuropathic pain. Acta Neurochir Suppl (Wien). 1993;58:150-153.
Nguyen JP, Lefaucheur JP, Decq P, et al. Chronic motor cortex stimulation in the treatment of central and neuropathic pain. Correlations between clinical, electrophysiological and anatomical data. Pain. 1999;82:245-251.
Nuti C, Peyron R, Garcia-Larrea L, et al. Motor cortex stimulation for refractory neuropathic pain: four-year outcome and predictors of efficacy. Pain. 2005;118:43-52.
Rossi U. The history of electrical stimulation of the nervous system for the control of pain. In: Simpson BA, editor. Pain Research and Clinical Management, Vol. 15. Amsterdam: Elsevier Science; 2003:5-16.
Saitoh Y, Kato K, Ninomiya H, et al. Primary motor cortex stimulation within the central sulcus for treating deafferentation pain. Acta Neurochir. 2003;87(suppl):149-152.
Sol JC, Casaux J, Roux FE, et al. Chronic motor cortex stimulation for phantom limb pain: correlations between pain relief and functional imaging studies. Stereotact Funct Neurosurg. 2001;77:172-176.
Tsubokawa T, Katayama Y, Yamamoto T, et al. Treatment of thalamic pain by chronic motor cortex stimulation. Pacing Clin Electrophysiol. 1991;14:131-134.
Tsubokawa T. Motor cortex stimulation for relief of central deafferentation pain. In: Burchiel KJ, editor. Surgical Management of Pain. New York: Thieme; 2002:555-564.
Velasco F, Arguelles C, Carrillo-Ruiz JD, et al. Efficacy of motor cortex stimulation in the treatment of neuropathic pain: a randomized double-blind trial. J Neurosurg. 2008;108:698-706.
1 Rossi U. The history of electrical stimulation of the nervous system for the control of pain. In: Simpson BA, editor. Pain Research and Clinical Management, Vol. 15. Amsterdam: Elsevier Science; 2003:5-16.
2 Tsubokawa T. Motor cortex stimulation for relief of central deafferentation Pain. In: Burchiel KJ, editor. Surgical Management of Pain. New York: Thieme; 2002:555-564.
3 Tsubokawa T, Katayama Y, Yamamoto T, et al. Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir Suppl (Wien). 1991;52:137-139.
4 Tsubokawa T, Katayama Y, Yamamoto T, et al. Treatment of thalamic pain by chronic motor cortex stimulation. Pacing Clin Electrophysiol. 1991;14:131-134.
5 Brown JA, Barbaro NM. Motor cortex stimulation for central and neuropathic pain: current status. Pain. 2003;104:431-435.
6 Meyerson BA, Lindblom U, Linderoth B, et al. Motor cortex stimulation as treatment of trigeminal neuropathic pain. Acta Neurochir Suppl (Wien). 1993;58:150-153.
7 Ebel H, Rust D, Tronnier V, et al. Chronic precentral stimulation in trigeminal neuropathic pain. Acta Neurochir (Wien). 1996;138:1300-1306.
8 Nguyen JP, Keravel Y, Feve A, et al. Treatment of deafferentation pain by chronic stimulation of the motor cortex: report of a series of 20 cases. Acta Neurochir Suppl (Wien). 1997;68:54-60.
9 Nguyen JP, Lefaucheur JP, Decq P, et al. Chronic motor cortex stimulation in the treatment of central and neuropathic pain. Correlations between clinical, electrophysiological and anatomical data. Pain. 1999;82:245-251.
10 Rainov NG, Fels C, Heidecke V, Burkert W. Epidural electrical stimulation of the motor cortex in patients with facial neuralgia. Clin Neurol Neurosurg. 1997;99:205-209.
11 Hosobuchi Y. Motor cortical stimulation for control of central deafferentation pain. Adv Neurol. 1993;63:215-217.
12 Herregodts P, Stadnik T, De Ridder F, D’Haens J. Cortical stimulation for central neuropathic pain: 3-D surface MRI for easy determination of the motor cortex. Acta Neurochir Suppl (Wien). 1995;64:132-135.
13 Bezard E, Boraud T, Nguyen JP, et al. Cortical stimulation and epileptic seizure: a study of the potential risk in primates. Neurosurgery. 1999;45:346-350.
14 Nguyen JP, Lefaucher JP, Le Guerinel C, et al. Motor cortex stimulation in the treatment of central and neuropathic pain. Arch Med Res. 2000;31:263-265.
15 Gharabaghi A, Hellwig D, Rosahl SK, et al. Volumetric image guidance for motor cortex stimulation: integration of three-dimensional cortical anatomy and functional imaging. Neurosurgery. 2005;57(ONS suppl 1):ONS-114-ONS-120.
16 Katayama Y, Tsubokawa T, Yamamoto T. Chronic motor cortex stimulation for central deafferentation pain: experience with bulbar pain secondary to Wallenberg syndrome. Stereotact Funct Neurosurg. 1994;62:295-299.
17 Katayama Y, Fukaya C, Yamamoto T. Poststroke pain control by chronic motor cortex stimulation: neurological characteristics predicting a favorable response. J Neurosurg. 1998;89:585-591.
18 Katayama Y, Yamamoto T, Kobayashi K, et al. Motor cortex stimulation for post-stroke pain: comparison of spinal cord and thalamic stimulation. Stereotact Funct Neurosurg. 2001;77:183-186.
19 Rainov NG, Heidecke V. Motor cortex stimulation for neuropathic facial pain. Neurol Res. 2003;25:157-161.
20 Sharan AD, Rosenow JM, Turbay M, et al. Precentral stimulation for chronic pain. Neurosurg Clin N Am. 2003;14:437-444.
21 Farina S, Tinazzi M, Le Pera D, Valeriani M. Pain-related modulation of the human motor cortex. Neurol Res. 2003;25:130-142.
22 Carroll D, Joint C, Maartens N, et al. Motor cortex stimulation for chronic neuropathic pain: a preliminary study of 10 cases. Pain. 2000;84:431-437.
23 Saitoh Y, Shibata M, Hirano S, et al. Motor cortex stimulation for central and peripheral deafferentation pain. Report of eight cases. J Neurosurg. 2000;92:150-155.
24 Roux FE, Ibarrola D, Lazorthes Y, Berry I. Chronic motor cortex stimulation for phantom limb pain: a functional magnetic resonance imaging study. Technical case report. Neurosurgery. 2001;48:681-687.
25 Saitoh Y, Hirano S, Kato K, et al. Motor cortex stimulation for deafferentation pain. Neurosurg Focus. 11, 2001. Article 1
26 Velasco M, Velasco F, Brito F, et al. Motor cortex stimulation in the treatment of deafferentation pain. I. Localization of the motor cortex. Stereotact Funct Neurosurg. 2002;79:146-167.
27 Brown JA, Pilitsis JG. Motor cortex stimulation for central and neuropathic facial pain: a prospective study of 10 patients and observations of enhanced sensory and motor function during stimulation. Neurosurgery. 2005;56:290-297.
28 Pirotte B, Voordecker P, Neugroschl C, et al. Combination of functional magnetic resonance image-guided neuronavigation and intraoperative cortical brain mapping improves targeting of motor cortex stimulation in neuropathic pain. Neurosurgery. 2005;56(ONS suppl 2):ONS-334-ONS-359.
29 Nuti C, Peyron R, Garcia-Larrea L, et al. Motor cortex stimulation for refractory neuropathic pain: four-year outcome and predictors of efficacy. Pain. 2005;118:43-52.
30 Saitoh Y, Kato K, Ninomiya H, et al. Primary motor cortex stimulation within the central sulcus for treating deafferentation pain. Acta Neurochir. 2003;87(suppl):149-152.
31 Tani N, Saitoh Y, Hirata M. Bilateral cortical stimulation for deafferentation pain after spinal cord injury. et al. J Neurosurg. 2004;101;:687-689.
32 Mogilner AY, Rezai AR. Epidural motor cortex stimulation with functional imaging guidance. Neurosurg Focus. 11, 2001. Article 4
33 Bonicalzi V, Canavero S. Motor cortex stimulation for central and neuropathic pain (letter regarding Topical Review by Brown and Barbaro). Pain. 2004;108:199-200.
34 Henderson JM, Boongird A, Rosenow JM, et al. Recovery of pain control by intensive reprogramming after loss of benefit from motor cortex stimulation for neuropathic pain. Stereotact Funct Neurosurg. 2004;82:207-213.
35 Tsubokawa T, Katayama Y, Yamamoto T, et al. Chronic motor cortex stimulation in patients with thalamic pain. J Neurosurg. 1993;78:393-401.
36 Yamamoto T, Katayama Y, Hirayama T, Tsubokawa T. Pharmacological classification of central post-stroke pain: comparison with the results of chronic motor cortex stimulation therapy. Pain. 1997;72:5-12.
37 Garcia-Larrea L, Peyron R, Mertens P, et al. Electrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological study. Pain. 1999;83:259-273.
38 Smith H, Joint C, Schlugman D, et al. Motor cortex stimulation for neuropathic pain. Neurosurg Focus. 11, 2001. Article 2
39 Burchiel KJ. A new classification for facial pain. Neurosurgery. 2003;53:1164-1166.
40 Coffey RJ. Deep brain stimulation for chronic pain: results of two multicenter trials and a structured review. Pain Med. 2001;2:183-192.
41 Herregodts P, Stadnik T, De Ridder F, D’Haens J. Cortical stimulation for central neuropathic pain: 3-D surface MRI for easy determination of the motor cortex. Acta Neurochir Suppl. 1995;64:132-135.
42 Sol JC, Casaux J, Roux FE, et al. Chronic motor cortex stimulation for phantom limb pain: correlations between pain relief and functional imaging studies. Stereotact Funct Neurosurg. 2001;77:172-176.
43 Mertens P, Nuti C, Sindou M, et al. Precentral cortex stimulation for the treatment of central neuropathic pain: results of a prospective study in a 20-patient series. Stereotact Funct Neurosurg. 1999;73:122-125.
44 Fontaine D, Hamani C, Lozano A. Efficacy and safety of motor cortex stimulation for chronic neuropathic pain: critical review of the literature. J Neurosurg. 2008;110:251-256.
45 Lima MC, Fregni F. Motor cortex stimulation for chronic pain: systematic review and meta-analysis of the literature. Neurology. 2008;70:2329-2337.
46 Velasco F, Arguelles C, Carrillo-Ruiz JD, et al. Efficacy of motor cortex stimulation in the treatment of neuropathic pain: a randomized double-blind trial. J Neurosurg. 2008;108:698-706.
47 Son UC, Kim MC, Moon DE, Kang JK. Motor cortex stimulation in a patient with intractable complex regional pain syndrome type II with hemibody involvement. Case report. J Neurosurg. 2003;98:175-179.
48 Fukaya C, Katayama Y, Yamamoto T, et al. Motor cortex stimulation in patients with post-stroke pain: conscious somatosensory response and pain control. Neurol Res. 2003;25:153-156.
49 Tomasino B, Budai R, Mondani M, et al. Mental rotation in a patient with an implanted electrode grid in the motor cortex. NeuroReport. 2005;16:1795-1800.
50 Canavero S, Bonicalzi V, Castellano G, et al. Painful supernumerary phantom arm following motor cortex stimulation for central poststroke pain. Case report. J Neurosurg. 1999;91:121-123.
51 Montes C, Mertens P, Convers P, et al. Cognitive effects of precentral cortical stimulation for pain control: an ERP study. Neurophysiol Clin. 2002;32:313-325.
52 Peyron R, Garcia-Larrea L, Deiber MP, et al. Electrical stimulation of precentral cortical area in the treatment of central pain: electrophysiological and PET study. Pain. 1995;62:275-286.
53 Saitoh Y, Osaki Y, Nishimura H, et al. Increased regional cerebral blood flow in the contralateral thalamus after successful motor cortex stimulation in a patient with poststroke pain. J Neurosurg. 2004;100:935-939.