Mechanisms of Action of Anti-Cancer Antibodies
Signaling Perturbation
ADCC
CDC
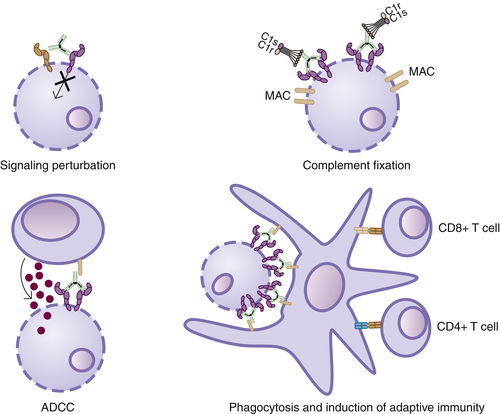
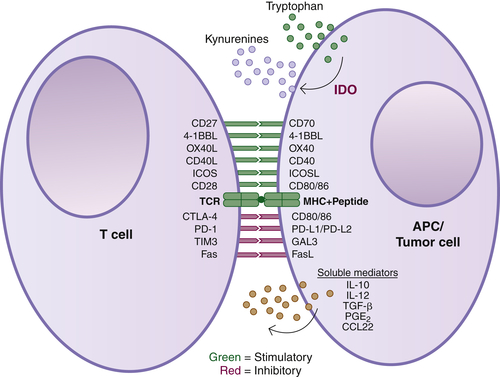
Induction of Adaptive Immunity
Table 50-1
FDA-Approved Antibodies Used in Oncology
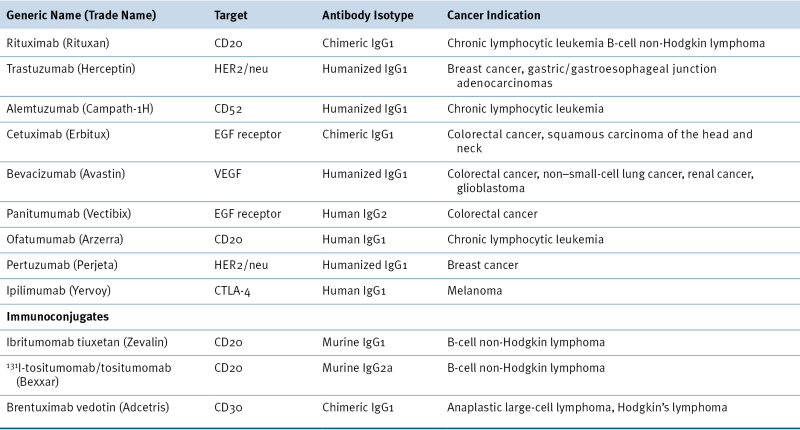
EGF, Epidermal growth factor; FDA, U.S. Food and Drug Administration; VEGF, vascular endothelial growth factor.
Antibodies Targeting Solid Tumors
Antibodies Targeting Hematological Malignancies
Antibodies Targeting Immune Cells
Antibodies Targeting Angiogenesis
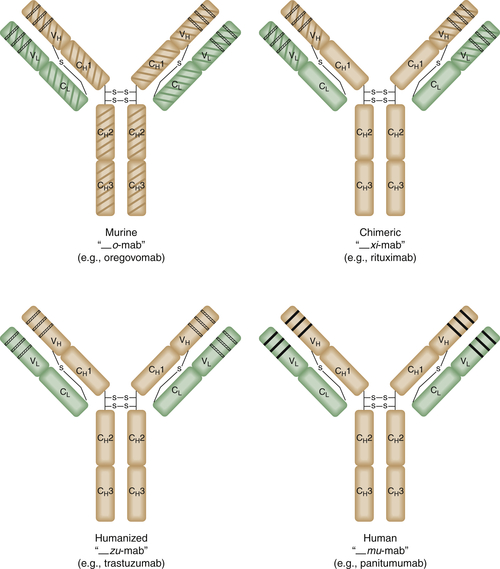
Antibody Engineering
Summary and Future Directions
1. Precise determination of the diversity of a combinatorial antibody library gives insight into the human immunoglobulin repertoire . Proc Natl Acad Sci U S A . 2009 ; 106 : 20216 – 20221 .
2. FcRn: the neonatal Fc receptor comes of age . Nat Rev Immunol . 2007 ; 7 : 715 – 725 .
3. Continuous cultures of fused cells secreting antibody of predefined specificity . Nature . 1975 ; 256 : 495 – 497 .
4. Development of human anti-murine antibody (HAMA) response in patients . Immunol Cell Biol . 1990 ; 68 : 367 – 376 .
5. Therapeutic antibodies: successes, limitations and hopes for the future . Br J Pharmacol . 2009 ; 157 : 220 – 233 .
6. Molecular, biologic, and pharmacokinetic properties of monoclonal antibodies: impact of these parameters on early clinical development . J Clin Pharmacol . 2007 ; 47 : 553 – 565 .
7. Expression and regulation of immunoglobulin heavy chain gene transfected into lymphoid cells . EMBO J . 1983 ; 2 : 1373 – 1378 .
8. Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains . Proc Natl Acad Sci U S A . 1984 ; 81 : 6851 – 6855 .
9. Replacing the complementarity-determining regions in a human antibody with those from a mouse . Nature . 1986 ; 321 : 522 – 525 .
10. Selecting and screening recombinant antibody libraries . Nat Biotechnol . 2005 ; 23 : 1105 – 1116 .
11. Human antibodies from transgenic animals . Nat Biotechnol . 2005 ; 23 : 1117 – 1125 .
12. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer . J Clin Oncol . 2007 ; 25 : 1658 – 1664 .
13. The EGFR family and its ligands in human cancer. Signalling mechanisms and therapeutic opportunities . Eur J Cancer . 2001 ; 37 ( suppl 4 ) : S3 – S8 .
14. Trastuzumab—mechanism of action and use in clinical practice . N Engl J Med . 2007 ; 357 : 39 – 51 .
15. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets . Nat Med . 2000 ; 6 : 443 – 446 .
16. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene . Blood . 2002 ; 99 : 754 – 758 .
17. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma . J Clin Oncol . 2003 ; 21 : 3940 – 3947 .
18. Impact of FcγRIIa-FcγRIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan . J Clin Oncol . 2009 ; 27 : 1122 – 1129 .
19. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer . J Clin Oncol . 2008 ; 26 : 1789 – 1796 .
20. de Haas M. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype . Blood . 1997 ; 90 : 1109 – 1114 .
21. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R . J Biol Chem . 2001 ; 276 : 6591 – 6604 .
22. Complement-mediated lysis by anti-CD20 mAb correlates with segregation into lipid rafts . Blood . 2003 ; 101 : 1045 – 1052 .
23. Complement activation determines the therapeutic activity of rituximab in vivo . J Immunol . 2003 ; 171 : 1581 – 1587 .
24. A polymorphism in the complement component C1qA correlates with prolonged response following rituximab therapy of follicular lymphoma . Clin Cancer Res . 2008 ; 14 : 6697 – 6703 .
25. Ofatumumab, a novel anti-CD20 monoclonal antibody for the treatment of B-cell malignancies . J Clin Oncol . 2010 ; 28 : 3525 – 3530 .
26. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas . Blood . 2004 ; 104 : 1793 – 1800 .
27. Ofatumumab monotherapy in rituximab-refractory follicular lymphoma: results from a multicenter study . Blood . 2012 ; 119 : 3698 – 3704 .
28. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia . J Clin Oncol . 2010 ; 28 : 1749 – 1755 .
29. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs . Nature . 1998 ; 392 : 86 – 89 .
30. The immune contexture in human tumours: impact on clinical outcome . Nat Rev Cancer . 2012 ; 12 : 298 – 306 .
31. Antitumor monoclonal antibodies enhance cross-presentation of cellular antigens and the generation of myeloma-specific killer T cells by dendritic cells . J Exp Med . 2002 ; 195 : 125 – 133 .
32. Monoclonal antibodies targeted against melanoma and ovarian tumors enhance dendritic cell-mediated cross-presentation of tumor-associated antigens and efficiently cross-prime CD8+ T cells . J Immunother . 2006 ; 29 : 41 – 52 .
33. Selective blockade of inhibitory Fcgamma receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells . Proc Natl Acad Sci U S A . 2005 ; 102 : 2910 – 2915 .
34. Cetuximab ± chemotherapy enhances dendritic cell-mediated phagocytosis of colon cancer cells and ignites a highly efficient colon cancer antigen-specific cytotoxic T-cell response in vitro . Int J Cancer . 2012 ; 130 : 1577 – 1589 .
35. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity . Cancer Cell . 2010 ; 18 : 160 – 170 .
36. Effective antibody therapy induces host-protective antitumor immunity that is augmented by TLR4 agonist treatment . Cancer Immunol Immunother . 2012 ; 61 : 49 – 61 .
37. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab . Cancer Cell . 2005 ; 7 : 301 – 311 .
38. Inhibitory activity of cetuximab on epidermal growth factor receptor mutations in non small cell lung cancers . Mol Cancer Ther . 2007 ; 6 : 2642 – 2651 .
39. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer . N Engl J Med . 2009 ; 360 : 1408 – 1417 .
40. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status . J Clin Oncol . 2011 ; 29 : 2011 – 2019 .
41. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer . J Clin Oncol . 2008 ; 26 : 1626 – 1634 .
42. Structural basis for EGF receptor inhibition by the therapeutic antibody IMC-11F8 . Structure . 2008 ; 16 : 216 – 227 .
43. A phase I pharmacologic study of necitumumab (IMC-11F8), a fully human IgG1 monoclonal antibody directed against EGFR in patients with advanced solid malignancies . Clin Cancer Res . 2010 ; 16 : 1915 – 1923 .
44. Zalutumumab plus best supportive care versus best supportive care alone in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck after failure of platinum-based chemotherapy: an open-label, randomised phase 3 trial . Lancet Oncol . 2011 ; 12 : 333 – 343 .
45. Enhancement of the antitumor activity of ionising radiation by nimotuzumab, a humanised monoclonal antibody to the epidermal growth factor receptor, in non-small cell lung cancer cell lines of differing epidermal growth factor receptor status . Br J Cancer . 2008 ; 98 : 749 – 755 .
46. Nimotuzumab, an antitumor antibody that targets the epidermal growth factor receptor, blocks ligand binding while permitting the active receptor conformation . Cancer Res . 2009 ; 69 : 5851 – 5859 .
47. Bivalent binding by intermediate affinity of nimotuzumab: a contribution to explain antibody clinical profile . Cancer Biol Ther . 2011 ; 11 : 373 – 382 .
48. The emerging role of nimotuzumab in the treatment of non-small cell lung cancer . Biologics . 2010 ; 4 : 289 – 298 .
49. Strategies to target HER2/neu overexpression for cancer therapy . Drug Resist Updat . 2003 ; 6 : 129 – 136 .
50. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer . J Clin Oncol . 2002 ; 20 : 719 – 726 .
51. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2 . N Engl J Med . 2001 ; 344 : 783 – 792 .
52. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer . J Clin Oncol . 2010 ; 28 : 2698 – 2704 .
53. A phase II study of trastuzumab emtansine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer who were previously treated with trastuzumab, lapatinib, an anthracycline, a taxane, and capecitabine . J Clin Oncol . 2012 ; 30 : 3234 – 3241 .
54. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer . Breast Cancer Res Treat . 2011 ; 128 : 347 – 356 .
55. Trastuzumab emtansine for HER2-positive advanced breast cancer . N Engl J Med . 2012 ; 367 : 1783 – 1791 .
56. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex . Cancer Cell . 2004 ; 5 : 317 – 328 .
57. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models . Cancer Res . 2009 ; 69 : 9330 – 9336 .
58. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer . N Engl J Med . 2012 ; 366 : 109 – 119 .
59. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program . J Clin Oncol . 1998 ; 16 : 2825 – 2833 .
60. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma . N Engl J Med . 2002 ; 346 : 235 – 242 .
61. Monoclonal antibody therapy of chronic lymphocytic leukemia . J Clin Oncol . 2003 ; 21 : 1874 – 1881 .
62. Phase II multicenter study of human CD52 antibody in previously treated chronic lymphocytic leukemia. European Study Group of CAMPATH-1H Treatment in Chronic Lymphocytic Leukemia . J Clin Oncol . 1997 ; 15 : 1567 – 1574 .
63. Treatment of T-cell prolymphocytic leukemia with human CD52 antibody . J Clin Oncol . 1997 ; 15 : 2667 – 2672 .
64. CAMPATH-1H monoclonal antibody in therapy for previously treated low-grade non-Hodgkin’s lymphomas: a phase II multicenter study. European Study Group of CAMPATH-1H Treatment in Low-Grade Non-Hodgkin’s Lymphoma . J Clin Oncol . 1998 ; 16 : 3257 – 3263 .
65. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma . J Clin Oncol . 2012 ; 30 : 2183 – 2189 .
66. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study . J Clin Oncol . 2012 ; 30 : 2190 – 2196 .
67. Milatuzumab—a promising new immunotherapeutic agent . Expert Opin Investig Drugs . 2010 ; 19 : 141 – 149 .
68. Antiproliferative activity of a humanized anti-CD74 monoclonal antibody, hLL1, on B-cell malignancies . Blood . 2004 ; 104 : 3705 – 3711 .
69. Combining milatuzumab with bortezomib, doxorubicin, or dexamethasone improves responses in multiple myeloma cell lines . Clin Cancer Res . 2009 ; 15 : 2808 – 2817 .
70. Anti-CD74 antibody-doxorubicin conjugate, IMMU-110, in a human multiple myeloma xenograft and in monkeys . Clin Cancer Res . 2005 ; 11 : 5257 – 5264 .
71. The blockade of immune checkpoints in cancer immunotherapy . Nat Rev Cancer . 2012 ; 12 : 252 – 264 .
72. Enhancement of antitumor immunity by CTLA-4 blockade . Science . 1996 ; 271 : 1734 – 1736 .
73. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients . Proc Natl Acad Sci U S A . 2003 ; 100 : 4712 – 4717 .
74. Improved survival with ipilimumab in patients with metastatic melanoma . N Engl J Med . 2010 ; 363 : 711 – 723 .
75. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells . J Exp Med . 2009 ; 206 : 3015 – 3029 .
76. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion . Nat Med . 2002 ; 8 : 793 – 800 .
77. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer . N Engl J Med . 2012 ; 366 : 2455 – 2465 .
78. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer . N Engl J Med . 2012 ; 366 : 2443 – 2454 .
79. Prospect of targeting the CD40 pathway for cancer therapy . Clin Cancer Res . 2007 ; 13 : 1083 – 1088 .
80. Preclinical antilymphoma activity of a humanized anti-CD40 monoclonal antibody, SGN-40 . Cancer Res . 2005 ; 65 : 8331 – 8338 .
81. Phase I study of the humanized anti-CD40 monoclonal antibody dacetuzumab in refractory or recurrent non-Hodgkin’s lymphoma . J Clin Oncol . 2009 ; 27 : 4371 – 4377 .
82. The antileukemia activity of a human anti-CD40 antagonist antibody, HCD122, on human chronic lymphocytic leukemia cells . Blood . 2008 ; 112 : 711 – 720 .
83. Phase I study of the anti-CD40 humanized monoclonal antibody lucatumumab (HCD122) in relapsed chronic lymphocytic leukemia . Leuk Lymphoma . 2012 ; 53 : 2136 – 2142 .
84. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody . J Clin Oncol . 2007 ; 25 : 876 – 883 .
85. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans . Science . 2011 ; 331 : 1612 – 1616 .
86. Prognostic value of vascular endothelial growth factor expression in colorectal cancer patients . Eur J Cancer . 2000 ; 36 : 748 – 753 .
87. VEGF inhibits T cell development and may contribute to tumor-induced immune suppression . Blood . 2003 ; 101 : 4878 – 4886 .
88. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer . N Engl J Med . 2006 ; 355 : 2542 – 2550 .
89. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma . J Clin Oncol . 2009 ; 27 : 740 – 745 .
90. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme . J Clin Oncol . 2007 ; 25 : 4722 – 4729 .
91. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer . N Engl J Med . 2007 ; 357 : 2666 – 2676 .
92. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer . J Clin Oncol . 2011 ; 29 : 1252 – 1260 .
93. RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer . J Clin Oncol . 2011 ; 29 : 4286 – 4293 .
94. Efficacy and safety of bevacizumab in combination with docetaxel for the first-line treatment of elderly patients with locally recurrent or metastatic breast cancer: results from AVADO . Eur J Cancer . 2011 ; 47 : 2387 – 2395 .
95. Ramucirumab (IMC-1121B): Monoclonal antibody inhibition of vascular endothelial growth factor receptor-2 . Curr Oncol Rep . 2011 ; 13 : 97 – 102 .
96. Distinct roles of VEGFR-1 and VEGFR-2 in the aberrant hematopoiesis associated with elevated levels of VEGF . Blood . 2007 ; 110 : 624 – 631 .
97. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2 . J Clin Oncol . 2010 ; 28 : 780 – 787 .
98. Targeting the tumour vasculature: insights from physiological angiogenesis . Nat Rev Cancer . 2010 ; 10 : 505 – 514 .
99. Cancer Associated Fibroblasts (CAFs) in tumor microenvironment . Front Biosci . 2010 ; 15 : 166 – 179 .
100. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha . Science . 2010 ; 330 : 827 – 830 .
101. Stromal antigen targeting by a humanised monoclonal antibody: an early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer . Onkologie . 2003 ; 26 : 44 – 48 .
102. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice . J Clin Invest . 2009 ; 119 : 3613 – 3625 .
103. Effective immunoconjugate therapy in cancer models targeting a serine protease of tumor fibroblasts . Clin Cancer Res . 2008 ; 14 : 4584 – 4592 .
104. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival . J Clin Oncol . 2011 ; 29 : 2493 – 2498 .
105. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM x anti-CD3) as a targeted cancer immunotherapy . Cancer Treat Rev . 2010 ; 36 : 458 – 467 .
106. Induction of a long-lasting antitumor immunity by a trifunctional bispecific antibody . Blood . 2001 ; 98 : 2526 – 2534 .
107. Induction of anti-tumor immunity by trifunctional antibodies in patients with peritoneal carcinomatosis . J Exp Clin Cancer Res . 2009 ; 28 : 18 .
108. Immunomonitoring results of a phase II/III study of malignant ascites patients treated with the trifunctional antibody catumaxomab (anti-EpCAM x anti-CD3) . Cancer Res . 2012 ; 72 : 24 – 32 .
109. Potent in vitro and in vivo activity of an Fc-engineered anti-CD19 monoclonal antibody against lymphoma and leukemia . Cancer Res . 2008 ; 68 : 8049 – 8057 .
110. Shortened engineered human antibody CH2 domains: increased stability and binding to the human neonatal Fc receptor . J Biol Chem . 2011 ; 286 : 27288 – 27293 .
111. Enhanced natural killer cell binding and activation by low-fucose IgG1 antibody results in potent antibody-dependent cellular cytotoxicity induction at lower antigen density . Clin Cancer Res . 2005 ; 11 : 2327 – 2336 .
112. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity . Blood . 2010 ; 115 : 4393 – 4402 .
113. Phase 1 study results of the type II glycoengineered humanized anti-CD20 monoclonal antibody obinutuzumab (GA101) in B-cell lymphoma patients . Blood . 2012 ; 119 : 5126 – 5132 .
114. A phase 1 study of obinutuzumab induction followed by 2 years of maintenance in patients with relapsed CD20-positive B-cell malignancies . Blood . 2012 ; 119 : 5118 – 5125 .