Chapter 14 Moisturizers: Function, Formulation and Clinical Applications
MAINTENANCE OF NORMAL SKIN INTEGRITY AND WATER CONTENT
• Role of corneocytes and natural moisturizing factor
The moisture content of corneocytes is maintained by small hygroscopic compounds which have been collectively categorized under the term ‘natural moisturizing factor’ (NMF). The components of NMF include filaggrin-derived amino acids, pyrrolidone carboxylic acid (PCA), lactate, sugars, and several electrolytes (Box 14.1). If stratum corneum water content falls below a critical level, enzymatic function required for normal desquamation is impaired, leading to corneocyte adhesion and accumulation of corneocytes on the cutaneous surface. These aberrant changes correspond with the visible appearance of dryness, roughness, scaling, flaking, chafing, and fissuring.
CLINICAL IMPACT OF MOISTURIZERS
Studies evaluating the application of physiologic lipids as moisturizers suggest that these lipids can be incorporated into the formation of barrier lipids and lamellar units and do not appear to downregulate physiologic lipid production in skin. However, based on in vitro murine models, the use of physiologic lipids in moisturizers appears to require the inclusion of all three lipid components (ceramide, cholesterol, free fatty acids) in optimized concentrations, otherwise barrier recovery may be impaired (Fig. 14.1).
SIGNIFICANCE OF MOISTURIZER APPLICATION FREQUENCY
Due to the loss of applied product imposed by the continuous natural process of epidermal desquamation and limitations of product substantivity, persistent efficacy provided by moisturizer use requires repeated application on a daily basis.
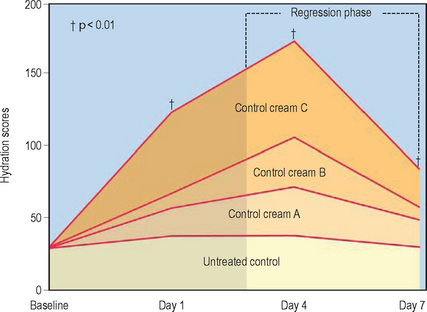
Evaluation of moisturizing properties after discontinuation of treatment with moisturizers (regression phase analysis) has demonstrated longlasting effects for several days after use of both lanolin-based and cetyl alcohol/petrolatum/polyglycerylmethacrylate-based moisturizer preparations (Fig. 14.2).
SIGNIFICANT COMPONENTS OF MOISTURIZER FORMULATIONS
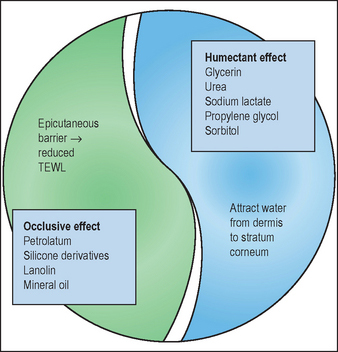
 The occlusive component retards evaporation and water loss by forming a hydrophobic film on the skin surface and within the superficial interstitium between corneocytes.
The occlusive component retards evaporation and water loss by forming a hydrophobic film on the skin surface and within the superficial interstitium between corneocytes.
 Humectant compounds attract water from the dermis and harbor water into the outer epidermal layer (‘from the inside out’). In climate conditions characterized by ambient humidity exceeding 70%, humectant compounds can also attract and trap water from the environment (‘from the outside in’). The combination of occlusive and humectant ingredients provides complementary actions in achieving and maintaining epidermal hydration and barrier function (Fig. 14.3).
Humectant compounds attract water from the dermis and harbor water into the outer epidermal layer (‘from the inside out’). In climate conditions characterized by ambient humidity exceeding 70%, humectant compounds can also attract and trap water from the environment (‘from the outside in’). The combination of occlusive and humectant ingredients provides complementary actions in achieving and maintaining epidermal hydration and barrier function (Fig. 14.3).
 Emollient ingredients, described as being capable of ‘filling the crevices’ between fragmented collections of desquamating corneocytes, are the third major component of moisturizer formulations. Emollients contribute to clinical efficacy and cosmetic elegance by imparting a smooth, soft texture to the skin surface.
Emollient ingredients, described as being capable of ‘filling the crevices’ between fragmented collections of desquamating corneocytes, are the third major component of moisturizer formulations. Emollients contribute to clinical efficacy and cosmetic elegance by imparting a smooth, soft texture to the skin surface.
• Occlusive ingredients
Occlusive agents are often greasy and are most effective when applied onto slightly dampened skin. Examples of occlusive ingredients include petrolatum, lanolin, mineral oil, and silicone derivatives (dimethicone, cyclomethicone) (Box 14.2). The use of lanolin is limited by odor, expense, and potential allergenicity. Mineral oil is frequently used due to its favorable texture (‘feel’), but is limited in its ability to reduce TEWL. The term ‘oil free’ implies that the formulation does not contain mineral or vegetable oils. Silicone derivatives are not greasy and when used alone impart a protective effect with limited moisturizing quality. They are often used in combination with petrolatum to impart a more cosmetically favorable texture as petrolatum alone is perceived by many consumers as ‘too greasy’.
• Humectant ingredients
Several compounds are added to moisturizers due to their humectant quality, attracting water from the dermis into the epidermis. Many humectant compounds also exhibit emollient properties when applied to skin. Examples of compounds that are commonly used in moisturizer formulations due to their humectant properties are listed in Box 14.3. It is important that a humectant agent when used as a moisturizer component be combined with an occlusive ingredient as application of a humectant alone can increase TEWL. For example, topical glycerin (glycerol) when applied alone to skin increases TEWL by 29%.
• Emollient ingredients
Emollients are frequently ‘oily’ substances that include a vast array of compounds ranging from esters to long chain alcohols. Although emolliency does not necessarily correlate with reduction in TEWL, emollient characteristics do correlate with consumer satisfaction and product preference as a smooth skin texture is expected after moisturizer application. Examples of compounds with emollient properties are listed in Box 14.4. Some compounds may be used due to their emollient qualities and compatibility with other components used in specific formulations. Unlike astringent alcohols (e.g. isopropyl alcohol, ethyl alcohol), emollient alcohols (e.g. cetyl alcohol, stearyl alcohol) are non-drying and impart a smooth texture to skin upon application. Ester-type emollients include octyl stearate, isopropyl myristate, oleyl oleate, cetearyl isononanoate, and PEG-7 glyceryl cocoate. Depending on inherent properties, emollients may be classified as protective, fatting, astringent, or dry (Box 14.4).
ESTHETIC CHARACTERISTICS AND SPECIAL MOISTURIZER ADDITIVES
• Formulation characteristics
The majority of moisturizers are formulated as lotions (oil-in-water emulsion) or creams (water-in-oil emulsion). Lotion formulations are thinner and are compatible with daytime facial use; characteristic basic components include mineral oil, propylene glycol, and water. Night creams and replenishing or ‘therapeutic’ formulations are composed of heavier lipids such as petrolatum or lanolin derivatives, mineral oil, and water. Correlation of specific formulations with ‘skin type’ is significant, as specified ingredient combinations are made more compatible with dry, normal, or oily complexions by adjusting oil : water ratios and the heaviness of occlusive ingredients and emollients. For example, dimethicone and cyclomethicone are nongreasy, noncomedogenic emollients that are commonly used in ‘oil-free’ facial moisturizers targeted for patients with ‘oily skin’. Oil-absorbent compounds such as talc or kaolin may be added to absorb excess sebum and reduce ‘facial shine’.
Bikowski J, Shroot B. Multivesicular emulsion: a novel controlled-release delivery system for topical dermatological agents. Journal of Drugs in Dermatology. 2006;5:942–946.
Del Rosso JQ. Understanding skin cleansers and moisturizers: the correlation of formulation science with the art of clinical use. Cosmetic Dermatology. 2003;16:19–31.
Draelos ZD. Moisturizers. In: Draelos ZD, editor. Cosmetics in dermatology. 2nd edn. New York: Churchill Livingstone; 1995:83–95.
Draelos ZD. Skin cleansers. In: Draelos ZD, editor. Cosmetics in dermatology. 2nd edn. New York: Churchill Livingstone; 1995:207–214.
Draelos ZD. Therapeutic moisturizers. Dermatology Clinics. 2000;18:597–607.
Fluhr J, Holleran WM, Berardesca E. Clinical effects of emollients on skin. In: Leyden JJ, Rawlings AV, editors. Skin moisturization. New York: Marcel Dekker; 2002:223–243.
Flynn TC, Petros J, Clark RE, et al. Dry skin and moisturizers. Clinics in Dermatology. 2001;19:387–392.
Gensler HL. Prevention of photoimmunosuppression and photocarcinogenesis by topical niacinamide. Nutrition and Cancer. 1997;29:157–162.
Grubauer G, Feingold KR, Elias PM. The relationship of epidermal lipogenesis to cutaneous barrier function. Journal of Lipid Research. 1987;28:746–752.
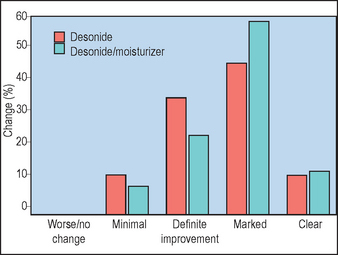
Hanifin JM, Hebert AA, Mays SR, et al. Effects of a low-potency corticosteroid lotion plus a moisturizing regimen in the treatment of atopic dermatitis. Current Therapeutic Research. 1998;59:227–233.
Imokawa G, Abe A, Jin Y, et al. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? Journal of Investigative Dermatology. 1991;96:523–526.
Jackson EM. Moisturizers: adjunct therapy and advising patients. American Journal of Contact Dermatitis. 1996;7:247–251.
Johnson AW. The skin moisturizer marketplace. In: Leyden JJ, Rawlings AV, editors. Skin moisturization. New York: Marcel Dekker; 2002:1–30.
Kirsner RS, Froehlich CW. Soaps and detergents: understanding their composition and effect. Ostomy/Wound Management. 1998;44(3A):62S-69S.
Kligman A. Regression method for assessing the efficacy of moisturizers. Cosmetics and Toiletries. 1978;93:27–32.
Lazar AP, Lazar P. Dry skin, water, and lubrication. Dermatologic Clinics. 1991;9:45–51.
Laquieze S, Czernielewski J, Baltas E. Beneficial use of cetaphil moisturizing cream as part of a daily skin care regimen for individuals with rosacea. Journal of Dermatological Treatment. 2007;18:158–162.
Loden M. Barrier recovery and influence of irritant stimuli in skin treated with a moisturizing cream. Contact Dermatitis. 1997;36:256–260.
Loden M, Andersson A-C, Lindberg M. Improvement in skin barrier function in patients with atopic dermatitis after treatment with a moisturizer cream. British Journal of Dermatology. 1999;140:264–267.
Ludwig A, Dietel M, Schafer G, et al. Nicotinamide and nicotinamide analogues as antitumor promoters in mouse skin. Cancer Research. 1990;50:2470–2475.
Mao-Qiang M, Brown BE, Wu-Pong S, et al. Exogenous non-physiologic vs physiologic lipids. Divergent mechanisms for correction of permeability barrier dysfunction. Archives of Dermatology. 1995;131:809–816.
Menon GK, Feingold KR, Elias PM. Lamellar body secretory response to barrier disruption. Journal of Investigative Dermatology. 1992;98:279–289.
Presland RB, Jurevic RJ. Making sense of the epithelial barrier: what molecular biology and genetics tell us about the functions of oral mucosal and epidermal tissues. Journal of Dental Education. 2002;66:564–574.
Proksch E, Elias PM. Epidermal barrier in atopic dermatitis. In: Bieber T, Leung DYM, editors. Atopic dermatitis. New York: Marcel Dekker; 2002:123–143.
Rawlings AV, Harding CR. Moisturization and skin barrier function. Dermatologic Therapeutics. 2004;17:43–48.
Rawlings AV, Harding CR, Watkinson A, Scott IR. Dry and xerotic skin conditions. In: Leyden JJ, Rawlings AV, editors. Skin moisturization. New York: Marcel Dekker; 2002:119–144.
Rawlings AV, Canestrari DA, Dobkowski B. Moisturizer technology versus clinical performance. Dermatologic Therapy. 2004;17:49–56.
Salka BA. Emollients. Cosmetics and Toiletries. 1997;112:101–106.
Shurer NY, Plewig G, Elias PM. Stratum corneum lipid function. Dermatologica. 1991;183:77–94.
Tabata N, O’Goshi K, Zhen XY, et al. Biophysical assessment of persistent effects of moisturizers after daily applications: evaluation of corneotherapy. Dermatology. 2000;200:308–313.
Wehr RF, Krochmal L. Considerations in selecting a moisturizer. Cutis. 1987;39:512–515.
Yamamoto A, Serizawa S, Ito M, et al. Stratum corneum lipid abnormalities in atopic dermatitis. Archives of Dermatological Research. 1991;283:219–223.
Zettersten EM, Ghadially R, Feingold KR, et al. Optimal ratios of topical stratum corneum lipids improve barrier recovery in chronically aged skin. Journal of the American Academy of Dermatology. 1997;37:403–408.