59 Minimally Invasive Spinal Surgery (MISS) Techniques for the Decompression of Lumbar Spinal Stenosis
KEY POINTS
Introduction
Minimally invasive foraminotomies and discectomies have gained increasing popularity among spine surgeons for the treatment of herniated intervertebral discs. The minimally invasive miscroendoscopic discectomy has been particularly attractive for its small skin incision, gentle tissue dissection and excellent visualization. The technique has been shown to provide symptomatic relief equivalent to that achieved with open discectomy, with significant reductions in operative blood loss, postoperative pain, hospital stay, and narcotic usage. The evolution of minimally invasive techniques has led to safe and effective applications for the treatment of LSS. The minimally invasive percutaneous microendoscopic approach for bilateral decompression of lumbar stenosis via a unilateral approach has been described for the treatment LSS and has been shown to offer a similar short-term clinical outcome as compared to open techniques with a significant reduction in operative blood loss, postoperative stay, and use of narcotics.1 This chapter reviews the pathophysiology of LSS; explains the rationale, indications and surgical techniques for minimally invasive LSS surgery; and presents our 4-year outcomes data.
Pathophysiology
The clinical entity of lumbar spinal stenosis is one that is familiar to almost all physicians who treat the elderly. From a pathophysiological perspective, lumbar stenosis typically results from a complex degenerative process that leads to compression of neural elements from a combination of ligamentum flavum hypertrophy, preexisting congenital narrowing (e.g., trefoil canal), intervertebral disc bulging or herniation, and facet thickening with arthropathy of the capsule soft tissues.2–4 With the unparalleled recent advances in imaging, it has become evident that the majority of these changes and thereby the neurological compression are typically seen at the level of the interlaminar window.2,5 These pathological changes are thus thought to be responsible for the clinical symptoms of lumbar stenosis. For these patients, the sine qua non feature of the history is low back and proximal leg pain that worsens with standing and walking and is alleviated by sitting and bending forward. Whether this phenomenon occurs as a result of direct neural compression or from secondary vascular ischemia of the nerve roots remains unclear. Nevertheless, this presentation of neurogenic claudication, anthropoid posture, and low back pain is becoming increasingly common as our population demographic grows older and older. Indeed, stenosis of the lumbar canal is now the most common indication for surgery of the spine for patients over the age of 65 years.6–9 This peak incidence in the geriatric population makes the surgical treatment of spinal stenosis particularly difficult because these patients are at significantly increased surgical risk because of their often poor medical condition.
Treatment Options and Guidelines
The traditional treatment of lumbar stenosis usually entails an extensive resection of posterior spinal elements such as the interspinous ligaments, spinous processes, bilateral lamina, portions of the facet joints and capsule, and the ligamentum flavum. Additionally, wide muscular dissection and retraction usually are required to achieve adequate surgical visualization. These classical operations of a wide decompressive laminectomy, medial facetectomy, and foraminotomy have been used for decades with a variable degree of success.8–11 Such extensive resection and injury of the posterior osseous and muscular complex can lead to significant iatrogenic pain, disability, and morbidity. Loss of the midline supraspinous–interspinous ligament complex can lead to a loss of flexion stability, thereby increasing the risk of delayed spinal instability.12,13 Extensive laminectomy can also be associated with significant operative blood loss as well as prolonged postoperative pain and weakness secondary to the extensive surgical dissection and muscle detachment. Such iatrogenic injury can lead to paraspinal muscle denervation and atrophy, which may correlate with an increased incidence of “failed back syndrome” and chronic pain.14,15 Because patients with lumbar stenosis are usually elderly and often medically frail, this delayed recovery can often result in significant morbidity. Deep venous thrombosis, pulmonary embolism, pulmonary atelectasis, pneumonia, urinary tract infections, ileus, and narcotic dependency are but some of these potentially devastating sequelae.
Whereas conservative therapy initially is a reasonable recommendation, there will inevitably be a significant proportion of patients with progressive stenotic symptoms who will ultimately require surgical intervention. In the Maine Lumbar Spine Study group, Atlas and coworkers16 prospectively followed 97 patients over a 10-year interval. Of these, 56 were surgically treated and 41 were conservatively managed. Based on patient satisfaction assessment vehicles, 54% of surgically treated patients reported improvement versus 42% of nonsurgical patients at 10 years. In comparison, the 4-year data from the Maine Lumbar Spine Study group revealed that 70% of surgically treated patients reported a clear improvement, compared to 52% in the nonoperative group.6 This decrease in patient satisfaction indicates that surgical benefits may not be stable over the course of time because LSS is typically a chronic and progressive disease. Hurri and colleagues,7 in 1998, reported their longitudinal 12-year study of 75 patients with lumbar stenosis. Using the Oswestry index, their disability was scored over many years and could demonstrate no clear difference between those who were operated on versus those managed conservatively. From an extensive review of the literature, Turner concluded from his attempted meta-analysis that approximately 64% of surgically treated patients had a good outcome over a midterm period of follow-up (3 to 6 years). However, he also noted that delayed clinical progression and recurrence of stenosis symptoms was extremely common, thus reflecting the chronic degenerative nature of the underlying disease process.13 Thus although surgical treatment appears to have a positive effect on the natural history of LSS, clinical progression and recurrence is likely. Moreover, several subgroups have been consistently identified that are particularly prone to recurrence of symptoms. These include patients with preexisting spondylolisthesis, scoliosis, prior destabilizing laminectomies, and the presence of segmental vertebral motion on flexion–extension radiographs.17,18 According to the treatment guidelines set forth by the AANS/CNS Joint section on Disorders of the Spine and Peripheral Nerves, fusion is recommended for patients with lumbar stenosis and associated degenerative spondylolisthesis who require decompression.19 In addition, wide decompressions leading to disruption of the facet joints has also been associated with poorer outcomes.19 In light of these considerations, the need for a less invasive means of decompression without major disruption of the facet joints and muscular attachments as well as the option of fusion for the treatment for LSS is evident.
Surgical Rationale
Over the past two decades, numerous surgeons have worked toward the goal of reducing the surgical morbidity of the procedure. The ideal operation for LSS would be one that could simultaneously achieve an adequate decompression of the neural elements, while at the same time minimize damage to the posterior muscular, ligamentous, and bony complex. Because the pathological changes typically are concentrated at the level of the interlaminar space, focal laminotomy was the natural first step in the evolution of surgical procedures for LSS. By sparing most of the lamina, spinous processes, and interspinous ligamentous complex, laminotomy helped to preserve the biomechanical integrity of the spine. Aryanpur and Ducker,20 in their description of multilevel open lumbar laminotomies, reported a longitudinal good outcome rate of 79% to 85% at 2-year follow-up. As the use of the operating microscope became increasingly common among spinal surgeons, unilateral microscopic hemilaminotomy was developed as a means of sparing the contralateral musculature as well. This procedure was characterized by unilateral multifidus retraction, ipsilateral decompression, and also contralateral microscopic decompression performed under the midline bony and ligamentous structures. For this microscopic laminotomy as described by McCulloch21 and Young and colleagues,22 an 80% to 95% improvement rate was reported over a 9-month follow-up period. Despite this progressive movement toward less extensive resection of the posterior bony elements, symptomatic outcomes have remained similar regardless of the aggressiveness of the surgical procedure utilized to treat LSS.23 Indeed, in one of the only studies correlating the degree of radiographic with clinical outcome, it was observed that the satisfaction of patients with the results of surgery (e.g., Oswestry score and walking capacity) was more important in surgical outcome than the degree of decompression as seen on a postoperative CT scan.5
In the past decade, significant strides in microendoscopic visualization technology have been made. Accordingly, endoscopic-assisted procedures have become increasingly popular for the treatment of a wide range of spinal pathologies, including hyperhydrosis, herniated discs, tumors, and fractures. In the lumbar spine, microendoscopic-assisted discectomies (MEDs) have been used in treating herniated discs successfully for the last 5 years. The MED procedure has been particularly attractive for its small skin incision, gentle tissue dissection, excellent visualization, and ability to achieve results equivalent to those with open techniques. The microendoscopic decompressive laminotomy (MEDL) technique was thus developed as a synthesis of the unilateral hemilaminotomy, described earlier, and these MED techniques. The MEDL technique was initially validated in a series of cadaveric studies in which the equivalent bony decompressions were achieved via either an open or an endoscopic technique.24 Since then, it has also been studied clinically with resultant good surgical outcomes, low morbidity, and a rapid postoperative functional recovery.1 A bilateral decompression via a unilateral minimally invasive approach thus represents the next logical step in the evolution of modern surgical treatment for LSS.
Indications for Miss Decompressive Techniques
Patients with evidence of LSS in addition to spondylolisthesis, deformity, or severe degenerative disc disease may benefit from a minimally invasive transforaminal interbody fusion and percutaneous posterolateral instrumentation.19 Patients with severe spondylolisthesis or severe deformity, infection, tumor, arachnoiditis, pseudomeningocele, or cerebrospinal fluid fistula are generally not suitable candidate for MEDL. A thorough clinical and radiographic evaluation with use of dynamic flexion, extension, and lateral-bending radiographs, contrast-enhanced MRI and CT, and isotope scans should be performed to exclude these conditions. Prior surgery at the same level as the present stenotic area is also a relative contraindication. For these cases, significant epidural scarring and dense adhesions can make the MEDL technique particularly difficult with an increased risk of durotomy. However, in cases with a limited amount of decompression, a relatively intact facet complex, and little epidural scarring as shown on gadolinium-enhanced MRI, the MEDL procedure can be used to successfully achieve a repeat decompression. Redo procedures should be attempted only by surgeons who have already gained facility with the MEDL technique in a good number of simple cases. Additionally, patients undergoing redo decompression with MEDL should be clearly informed about the increased surgical risks and the possibility of conversion to an open procedure.
Surgical Technique
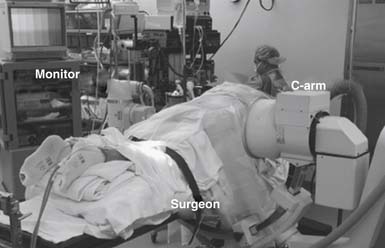
The patient is then turned into a prone position onto a radiolucent Wilson frame and Jackson table. Utmost care should be made to insure adequate padding of all pressure points, eyes, and extremities. The fluoroscopic C-arm should then be brought into the surgical field so that real-time lateral fluoroscopic images can be obtained. Ideally, this should be draped and positioned such that it can be easily swung in and out during the procedure without having to interrupt the flow of the case. The operative surgeon generally stands on the side of the approach with the C-arm and video monitors placed opposite him. However, the ultimate arrangement of the video monitor and C-arm monitor can be varied to allow for optimal ergonomic movements during the operation (Figure 59-1). Electromyographic (EMG) monitoring of motor nerve root responses can be used if desired.
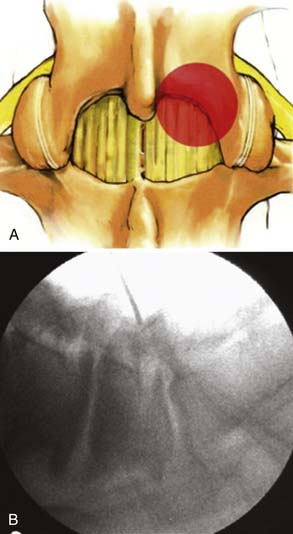
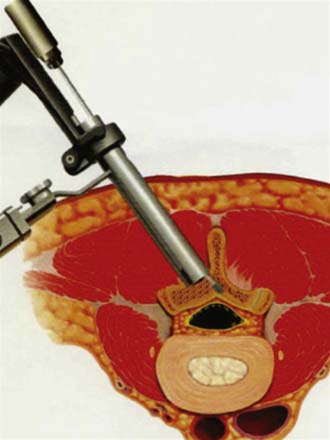
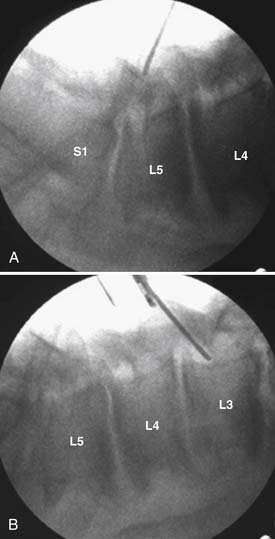
Under fluoroscopic guidance with a Steinmann pin held laterally to the patient, the approximate level of the incision is marked approximately 1 to 1.5 inches off midline on the side of the approach. This incision is marked to facilitate targeting of the laminofacet junction at the spinal level to be decompressed (Figure 59-2A). A small stab incision is then made in the middle of the marked incision through which the Steinmann pin is then passed down to the medial bony facet margin. Particular care should be made to begin this approach laterally to avoid inadvertent dural penetration. The placement of the pin is then confirmed with the fluoroscope to ensure a good working trajectory (Figure 59-2B). Although we have not routinely done so, anteroposterior radiographic images can be obtained to guarantee proper pin positioning. In our early experience with minimally invasive stenosis decompression, we decompressed the ipsilateral lateral recess before decompressing the contralateral side. For the last 4 years, we have changed our approach to contralateral decompression, a technique that has been described previously.25 Once the guidewire has been docked on the spinolaminar junction, a skin incision is made above and below the Steinmann pin for a total length of approximately 2.5 cm. The METRx (Medtronic, Sofamor-Danek, Memphis, Tenn.) microendoscopic system’s set of serial dilators (Figure 59-3) are then passed over then Steinmann pin to gently dilate the lumbar musculature and expand the lumbodorsal fascia away. Medial angulation of up to about 45 degrees is desirable at this point to ensure optimal visualization of the spinolaminar junction and ensure a proper trajectory for drilling of the anterior aspect of the lamina to the contralateral lateral recess and foramen (Figure 59-4). The 18-mm final working channel is then passed over the dilators and secured to the flexible-arm METRX retractor mounted to the table side rail. Final fluoroscopic confirmation of the working channel position is then obtained, an 18-mm working channel of adequate depth (usually 5 to 6 cm) is placed and the serial dilators are then removed. The endoscope is then attached to the tubular retractor or the operating microscope is used for the remainder of the procedure.
Bovie cautery with a long tip is then used to remove the remaining muscle and soft tissue overlying the lamina and spinolaminar junction. A long high-speed burr (e.g., AM-8, Midas Rex) is then used to core out the cancellous and deep cortical surface of the contralateral lamina, preserving the ligamentum flavum underneath and using it as a protective layer over the dura. This “intralaminar” drilling is carried out laterally to the contralateral lateral recess and foramen. Kerrison rongeurs may be used to remove the remaining laminar edge and the medial contralateral facet. After adequate contralateral bony decompression, the ligamentum flavum can be removed with use of up-angled curettes and Kerrison rongeurs.
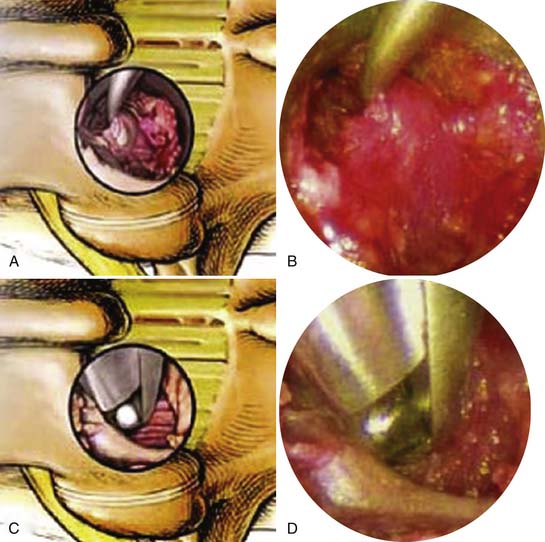
Attention is now directed toward the ipsilateral lateral recess and foramen. The tubular retractor is angled laterally toward the ipsilateral lamina-medial facet junction and drilling is carried out to thin the lamina and the medial facet complex down (Figure 59-5). With the bony edges well visualized, a small straight curette is used to scrape the inferior edge of the adjacent lamina and the medial edge of the facet complex. This exposure is then carried underneath the lamina and facet with the use of a small angled endoscopic curette (Figure 59-6 A and B). Proper placement of the curettes should be confirmed under fluoroscopy. Good dissection of the underlying flavum and dura from the bone is crucial in preventing incidental dural tears and cerebrospinal (CSF) fluid leaks. Bleeding from epidural small and edge of the flavum was controlled via long-tipped endoscopic bipolar cautery. A small angled Kerrison rongeur is then utilized to begin the laminotomy (see Figure 59-6 A to D). After adequate drilling, endoscopic Kerrison rongeurs are used to continue the removal of the lamina and medial facet. Frequent dissection with the small angled curette before biting with the Kerrison rongeur should be used to free the underlying ligament and nerve root. In this fashion, bilateral hemilaminotomies, medial facetectomies, and foraminotomies are completed (Figure 59-7).
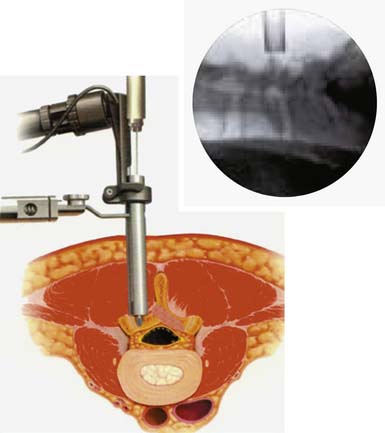
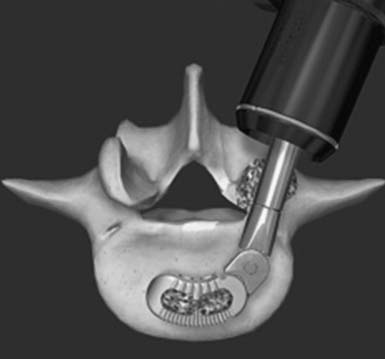
FIGURE 59-5 The tubular retractor is now angled laterally toward the ipsilateral lamina–medial facet junction.
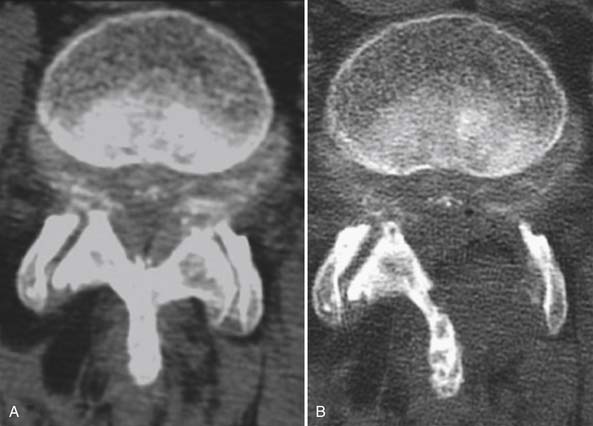
In cases of multiple adjacent level stenoses, the initial placement of the dilators and tubular retractor should be midway between the stenotic levels. In a patient with both L3-L4 and L4-L5 stenosis, for example, the working channel should be first docked on the L4 lamina and then swung caudad to decompress the L4-L5 level, and cephalad to subsequently decompress the L3-L4 level. For patients with a larger vertical distance between spinal segments, sharp incision of the lumbodorsal fascia and superoinferior translation of the working channel may also be needed. Figure 59-8 demonstrates the extent of multiple level decompression than can be obtained through repositioning of the working channel through a single incision. Figure 59-9 demonstrates a typical decompression achieved via MEDL for a representative case of lumbar stenosis.
In cases in which an interbody fusion is indicated, the drill is used to remove the medial aspect of the superior articular process and expose the superior and medial aspects of the pedicle and the adjoining foramen to the level of the top margin of the disc. Coagulation and division of the inferior foraminal veins allows for about 10 to 12 mm between the exiting and traversing roots at the upper margin of the disc, allowing for thorough discectomy and interbody fusion using a single intervertebral spacer is then placed and directed just across the midline using biplanar fluoroscopy (Traxis MIS TLIF set, Abbot Spine, or Concorde Cage, Depuy Spine) (Figure 59-10). Bilateral percutaneous pedicle screws and connecting rods (Pathfinder, Abbott Spine) are placed under compression to lock the interbody fusion cage and to minimize the risk of cage migration.26 A detailed discussion of a minimally invasive transforaminal lumbar interbody fusion is beyond the scope of this article.
After inspection of the thecal sac and nerve roots, hemostasis is obtained by bipolar cautery and gentle tamponade with thrombin-soaked Gelfoam pledgets. A single dose of intraoperative Cefazolin (Ancef) or vancomycin is typically employed during the procedure and a second dose can be given at the surgeon’s discretion. The area is then copiously irrigated with lactated ringers impregnated with bacitracin antibiotics. A small piece of Gelfoam soaked with methylprednisolone (Solumedrol) was typically gently placed over the laminoforaminotomy defect. Use of epidural morphine paste or similar cocktails is reasonable if there is not evidence of dural erosion or tear. Such agents may help to reduce postoperative pain and allow for more rapid recovery and ambulation. The tubular retractor and endoscope are then removed. Because the defect is typically quite small, only a limited amount of closure need be performed and a drain is not needed. A 0-Vicryl type of reabsorbable suture is used to close the lumbodorsal fascia in a figure-of-eight. Bupivacaine (Marcaine [0.25%]) is used to inject the skin edges before closure. Inverted stitches of 2-0 Vicryl suture are used to close the subcutaneous layer. A 4-0 clear Vicryl subcuticular closure is then used to carefully reapproximate the skin edges, with care taken to avoid inversion. Either Steri-Strips or Dermabond can then be used to cover the skin. Dermabond is attractive because it keeps the skin edges closely approximated for 7 to 10 days and provides a waterproof barrier. The patient can thus shower almost immediately after surgery.
Postoperative Management
Patients should be allowed to ambulate early on after surgery. Foley catheters, arterial lines, and unneeded intravenous lines should be removed as early as possible. Early evaluation and treatment by experienced physical therapists are important to begin the recovery and rehabilitation process. As most patients with LSS present with a limited capacity for ambulation, walking at the preoperative level should be encouraged. We typically have not used a rigid external orthosis after MEDL with or without TLIF and pedicle screw fixation, but use of a lumbar corset with metal stays or similar brace is reasonable for the patient’s comfort. Depending on the level of analgesic and narcotics used preoperatively, we have generally aimed to minimize usage of postoperative pain medications. We typically employ strong nonsteroidal antiinflammatory agents in nonfusion cases (e.g., celecoxib [Celebrex], rofecoxib [Vioxx]) combined with muscle relaxants (e.g., cyclobenzaprine [Flexeril], baclofen, methocarbamol [Robaxin]). Oral narcotic medications such as hydrocodone/acetaminophen (Vicodin) and oxycodone/acetaminophen (Percocet) are used primarily on a breakthrough basis. In a comparison of MEDL with open decompression, we found that LSS patients after MEDL use significantly less narcotic medication.1
Clinical Outcomes and Complications
Our rate of dural violations and CSF leak is 4% compared to previous rates of up to 16%.1 In our early experience with minimally invasive stenosis decompression, we decompressed the ipsilateral lateral recess before decompressing the contralateral side. We believe that this approach may in fact increase the risk of dural violations while drilling the contralateral side, since unprotected dura is exposed. With the technique described earlier and published previously,25 the dura is entirely protected on both sides during contralateral drilling. There were no cases of neural injury associated with the MEDL cases, and to date there have been no cases of iatrogenic or delayed spinal instability requiring fusion.
Emerging Technologies
Although this chapter focuses primarily on direct decompressive techniques for LSS, posterior lumbar arthroplasty devices may provide symptomatic relief without direct decompression. The simplest posterior lumbar arthroplasty devices are those of interspinous distraction or blockade devices. These include the X-STOP (Saint Francis Medical, Alameda, Calif.), the Wallis System (Abbott Spine, Austin, Tex.), the Diam Device (Medtronics, Memphis, Tenn.) and the Coflex system (Paradigm Spine, New York, N.Y.) (Figure 59-11). These devices are placed between the bases of the spinous processes and provide mild distraction or blockade of the functional middle column at a given motion segment. As a result of this interposition, the volume and height of the spinal neural foramen and the lateral recess are maintained and/or slightly increased. Zucherman and colleagues27 reported 191 patients were treated, 100 in the X-STOP group and 91 in the control group. At every follow-up visit, X-STOP patients had significantly better outcomes in each domain of the Zurich Claudication Questionnaire. At 2 years, the X-STOP patients improved by 45.4% over the mean baseline Symptom Severity score compared with 7.4% in the control group; the mean improvement in the Physical Function domain was 44.3% in the X-STOP group and −0.4% in the control group. In the X-STOP group, 73.1% patients were satisfied with their treatment compared with 35.9% of control patients.
Additionally, biomechanical cadaveric and finite modeling studies have demonstrated an increased overall stiffness of the motion segment, presumably from middle column augmentation, as well as decreases in the stresses and loading across the posterior aspect of the intervertebral disc and annulus, as well as the facet joints themselves. Accordingly, several groups have reported benefits for use of interspinous devices in patients with degenerative mechanical pain without stenosis as well.28
1. Khoo L., Fessler R. Microendoscopic decompressive laminotomy for the treatment of lumbar stenosis. Neurosurgery. 2002;5(Suppl. 5):S146-S154.
2. Guigui P., Barre E., Benoist M., Deburge A. Radiologic and computed tomography image evaluation of bone regrowth after wide surgical decompression for lumbar stenosis. Spine. 1999;24:281-289.
3. Kleeman T.J., Hiscoe A.C., Berg E.E. Patient outcomes after minimally destabilizing lumbar stenosis decompression: the Port-Hole technique. Spine. 2000;25:865-870.
4. Simotas A.C., Dorey F.J., Hansraj K.K., Cammisa F. Nonoperative treatment for lumbar spinal stenosis: clinical and outcome results and a 3-year survivorship analysis. Spine. 2000;25:197-204.
5. Herno A., Saari T., Suomalainen O., Airaksinen. The degree of decompressive relief and its relation to clinical outcome in patients undergoing surgery for lumbar spinal stenosis. Spine. 1999;24:1010-1014.
6. Atlas S.J., Keller R.B., Robson D., Deyo R.A., Singer D.E. Surgical and nonsurgical management of lumbar spinal stenosis: four-year outcomes from the Maine Lumbar Spine Study. Spine. 2000;25:556-562.
7. Hurri H., Slatis P., Soini K. Lumbar spinal stenosis: assessment of long-term outcome 12 years after operative and conservative management. J. Spin. Dis.. 1998;11:110-115.
8. Katz J.N., Stucki G., Lipson S.J. Predictors of surgical outcome in degenerative lumbar spinal stenosis. Spine. 1999;21:2229-2233.
9. Postacchini F. Spine update: surgical management of lumbar spinal stenosis. Spine. 1999;24:1043-1047.
10. Herron L.D., Mangelsdorf C. Lumbar stenosis: results of surgical treatment. J. Spinal Disord. 1991;4:26-33.
11. Sanderson P.L., Wood P.L.R. Surgery for lumbar spinal stenosis in old people. J. Bone Joint Surg. Br.. 1993;75:393-397.
12. Tsai R.Y.C., Yang R.S., Bray R.S. Microscopic laminotomies for degenerative lumbar spinal stenosis. J. Spin. Dis.. 1998;11:389-394.
13. Tuite G.F., Stern J.D., Doran S.E. Outcome after laminectomy for lumbar spinal stenosis, part I: clinical correlations. J. Neurosurg. 1994;81:699-706.
14. See D.H., Kraft G.H. Electromyography in paraspinal muscles following surgery for root compression. Arch. Phys. Med. Rehab.. 1975;56:80-83.
15. Sihvonen T., Herno A., Paljarva L. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine. 1993;18:575-581.
16. Atlas S., Keller B., Wu Y., Deyo R., Singer D. Long-term outcomes of surgical and nonsurgical management of lumbar spinal stenosis: 8-10 year results from the Maine Lumbar Spine Study. Spine. 2005;30:936-943.
17. Caputy A., Luessenhop A. Long-term evaluation of decompressive surgery for degenerative lumbar stenosis. J. Neurosurg. 1992;77:669-676.
18. Katz J., Lipson S., Lew R. Lumbar laminectomy alone or with instrumented or noninstrumented arthrodesis in degenerative lumbar spinal stenosis. Patient selection, costs, and surgical outcomes. Spine. 1997;22:1123-1131.
19. Resnick D., Choudhri T., Dailey A. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 9: fusion in patients with stenosis and spondylolisthesis. J. Neurosurg. Spine. 2005;2:679-685.
20. Aryanpur J., Ducker T. Multilevel lumbar laminotomies: an alternative to laminectomy in the treatment of lumbar stenosis. Neurosurgery. 1990;26:429-433.
21. McCulloch J.A. Microsurgical spinal laminotomies. In: Frymoyer J.W., editor. The adult spine: principles and practice. New York: Raven Press, Ltd, 1991.
22. Young S., Veerapen R., O’Laire S.A. Relief of lumbar canal stenosis using multilevel subarticular fenestrations as an alternative to wide laminectomy: preliminary report. Neurosurgery. 1988;23(5):628-633.
23. Turner J.A., Ersek M., Herron L., Haselkorn J., Deyo R. Surgery for lumbar spinal stenosis, attempted meta-analysis of the literature. Spine. 1992;17:1-8.
24. Guiot B.H., Khoo L.T., Fessler R.G. A Minimally invasive technique for decompression of the lumbar spine. Spine. 2002;27(4):432-438.
25. Palmer S., Turner R., Palmer R. Bilateral decompression of lumbar stenosis involving a unilateral approach with microscope and tubular retractor system. J. Neurosurg. Spine. 2002;97:213-217.
26. McCaffert R., Khoo L., Perez-Cruet M. Percutaneous pedicle screw fixation of the lumbar spine using the pathfinder system. In: Perez-Cruet M., Khoo L., Fessler R., editors. An anatomic approach to minimally invasive spine surgery. St. Louis: QMP; 2006:599-614.
27. Zucherman J.F., Hsu K.Y., Hartjen C.A. A multicenter, prospective, randomized trial evaluating the X STOP interspinous process decompression system for the treatment of neurogenic intermittent claudication: two-year follow-up results. Spine. 2005;30(12):1351-1358.
28. Senegas J. Mechanical supplementation by non-rigid fixation in degenerative intervertebral lumbar segments: the Wallis system. Eur. Spine J.. 2002;11(2):S164-S169.