CHAPTER 31 Minimally Invasive Approach to Frontal Fossa and Suprasellar Meningiomas 
INTRODUCTION
During the last two decades, as a result of advances in low-profile instrumentation, surgical navigation, and endoscopy, keyhole surgical routes have been increasingly used to approach a wide spectrum of brain and skull-base tumors. For frontal fossa and suprasellar meningiomas, the extended transsphenoidal and the supraorbital “eyebrow” craniotomies are now often used for their removal.1–20 Although these two approaches traverse entirely different anatomic territories to reach the same intracranial target and have markedly different closure requirements, they are similar in their technically demanding nature, requiring an ability to maneuver through a narrow operative corridor. The transsphenoidal approach, which obviates brain retraction, minimizes optic apparatus manipulation, and allows early identification of the pituitary gland and infundibulum, has been shown to be effective for smaller midline meningiomas.1,2,6,7,9,16 It is increasingly performed via an endonasal approach with microscopic and endoscopic assistance1,2,7,9,13,14,16 or by a purely endoscopic approach.6,15 The two major drawbacks of the endonasal approach are (1) limited access to lesions lateral to the supraclinoid carotid arteries and optic nerves and (2) challenges in achieving an effective skull-base closure. Supraorbital keyhole craniotomy is also performed with minimal or no brain retraction and allows excellent access to a broader region within the frontal fossa and suprasellar area.3,4,8,11,12,17–20 The minimal scalp tissue and muscle dissection required promote a less painful recovery compared to standard craniotomies.3,17–20 The major drawback of this approach is the potential for limited maneuverability given the small craniotomy, which typically measures 15 to 20 mm by 25 to 30 mm. Herein we describe the use of the endonasal and supraorbital keyhole approaches for removing frontal fossa and suprasellar meningiomas, including a discussion of selection criteria for their use, their respective limitations, potential pitfalls, success rates, and complications.
EPIDEMIOLOGY AND INCIDENCE
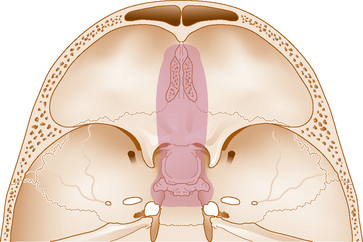
Meningiomas of the anterior cranial fossa arise in various locations and comprise approximately 12% to 22% of all intracranial meningiomas.21–23 They are commonly divided into two major subgroups: olfactory groove meningiomas that arise over the cribriform plate and frontosphenoid suture and suprasellar meningiomas, the most common of which are midline tuberculum sellae meningiomas. Other suprasellar meningiomas may arise from the diaphragma sellae itself or from the arachnoidal attachments along the anterior and posterior clinoids. Some frontal fossa meningiomas span both the olfactory groove and tuberculum sellae territories and are not easily classified.23,24 Most olfactory groove meningiomas become rather large before producing symptoms (often >3 cm in size) such as headache, personality changes, anosmia, or seizures. Large olfactory groove meningiomas that extend posteriorly to the posterior planum may encroach upon the optic apparatus producing visual loss.24–26 In contrast, most tuberculum sellae and other suprasellar meningiomas are diagnosed at a smaller size as a result of visual loss from chiasmal or optic nerve compression.27–30 With progressive growth, tuberculum sellae meningiomas develop lateral and superior extensions, often resulting in vascular encasement, cavernous sinus invasion, and optic canal invasion. Larger tumors typically, greater than 3 or 4 cm in diameter, often encase the anterior cerebral artery complex.
HISTORY AND EVOLUTION OF MICROSURGERY FOR FRONTAL FOSSA AND SUPRASELLAR MENINGIOMAS
Traditional Approaches
Many macrosurgical and microsurgical series for removal of olfactory groove and suprasellar meningiomas have been published since the first reports of removal of an olfactory groove meningioma by Durante in 1884 and of a suprasellar meningioma by Cushing and Eisenhardt in 1938.31,32 In most series over the last two decades, olfactory groove and suprasellar meningiomas have been approached through several routes including bifrontal, unilateral frontal, pterional, and fronto-pterional.22–30,33–40 In microsurgical series of olfactory groove meningiomas, high rates of total tumor removal ranging from 85% to 100% are reported with both pterional and bifrontal routes with relatively low morbidity.24–26 The recurrence rate ranges from 5% to 41% over 10 years and appears to be predominantly related to subtotal removal (Simpson grade 2 or higher) in the floor of the anterior cranial fossa, and the underlying ethmoid or sphenoid sinus area.25,26,33,37,41,42
Achieving complete removal of suprasellar meningiomas is often more problematic because of tumor extensions along the optic apparatus, hypothalamic–pituitary axis, anterior circle of Willis, and cavernous sinus. In recent series, total resection of suprasellar meningiomas has ranged from 67% to 100% with rates of visual decline ranging from 0 to 20% and mortality ranging from 0 to 8.7%, respectively.22,23,27–30,34–36,38,39 Even with technically demanding approaches such as extradural clinoidectomy for optic nerve decompression, visual worsening of up to 10% has been reported.30,38
Minimally Invasive “Keyhole” Approaches
As Wilson stated more than 35 years ago “the ideal exposure is one that is large enough to do the job well, while preserving the integrity of as much normal tissue as possible.”43 With the development of frameless surgical navigation, endoscopy, as well as lower profile microsurgical instruments and aspirators, this “keyhole” concept has been increasingly used for removing a wide spectrum of intracranial lesions, including meningiomas.44 This concept is based on the observation that with a small craniotomy, the available exposure expands as the distance from the bony opening increases, so that with an optimally placed craniotomy, exposure is more than adequate (Fig. 31-1). These advances in keyhole surgery have resulted in a shift away from traditional larger anterior and anterolateral skull-base approaches such as the pterional, bifrontal, and orbitozygomatic craniotomies and the midface degloving transsphenoidal approach. Likewise, there is waning enthusiasm for the transpetrosal approach for petroclival tumors and a resurgence of the retrosigmoid craniotomy and endonasal transclival approach to reach such tumors.36,37,45,46
Supraorbital approach
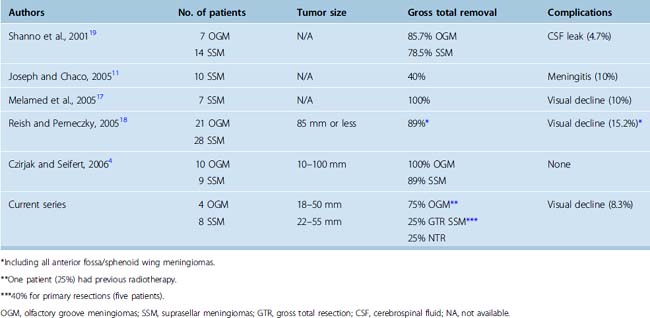
The supraorbital approach has evolved from more traditional frontal and frontotemporal approaches.47–50 The small craniotomy with an incision directly through the eyebrow obviates problems with mastication and temporalis wasting because of limited temporal muscle dissection and retraction. More importantly, placement of the craniotomy immediately above the orbital roof and along the floor of the frontal fossa affords access with minimal brain exposure and brain retraction.3,18 As recently demonstrated in an anatomic study by Figueiredo and colleagues using a slightly larger (30 mm) supraorbital craniotomy than is typically possible through an eyebrow incision, the area of exposure was similar between the supraorbital and traditional craniotomies, although it affords narrower angles of exposure.51 However, even with the narrower angles of horizontal and vertical exposure using the supraorbital craniotomy, many lesions of the frontal fossa and parasellar area are readily accessible as shown by Perneczky and others.3,4,18,44 Similar rates of total or near total resection of anterior cranial fossa meningiomas using the supraorbital approach have been reported when compared to traditional transcranial approaches (Table 31-1), ranging from 86% to 100% by the supraorbital route for olfactory groove meningiomas,4,18,19 and from 40% to 100% for suprasellar meningiomas.4,11,17–19 In these series, incomplete resection was typically attributed more to tumor invasiveness as opposed to inadequate exposure.4,11,17–19
Extended endonasal transsphenoidal approach
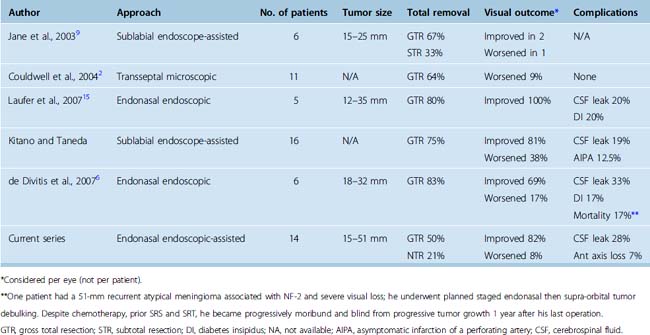
The transnasal approach is also considered a keyhole approach with the exposure expanding from the piriform aperture for the sublabial transsphenoidal approach or from the nostril for the endonasal approach. The obvious advantage of these approaches for midline frontal fossa and parasellar meningiomas is that the nasal cavity and ethmoid and sphenoid sinuses provide a direct route to the tumor without the need for brain retraction. In 1987, Weiss described the microscopic extended approach for the treatment of supradiaphragmatic craniopharyngiomas.52 Subsequent modifications of this approach have been described by multiple groups including the endonasal endoscope-assisted method and the fully endoscopic endonasal method for removal of suprasellar and olfactory groove meningiomas.1,2,5–7,9,10,14–16,53,54 In several recent reports, the extended transsphenoidal approach for tuberculum sellae meningiomas has yielded total or near total removal rates of 57% to 85% with tumor sizes ranging from 12 to 37 mm (Table 31-2).2,6,9,14–16 Transsphenoidal removal of tuberculum sellae meningiomas appears to result in similar visual recovery rates and visual worsening rates compared to transcranial approaches, although in one recent report by Kitano and colleagues, visual recovery was better by the transsphenoidal route.6,14,15 Visual improvement has ranged from 33% to 100%, with most reports noting greater than 50% visual improvement while visual decline has ranged from 0 to 38%, although visual worsening is less than 20% in most reports.2,6,7,15 The most common complication associated with the transsphenoidal or endonasal removal of suprasellar meningiomas has been inadequate skull-base repair and postoperative cerebrospinal fluid (CSF) leaks. In recent series of endonasal endoscopic and transsphenoidal microscopic approaches, the rates of postoperative CSF leak have ranged from 0 to 33%, although they have been decreasing in recent years.2,6,9,14–16
Regarding endonasal removal of olfactory groove meningiomas, there is relatively limited outcome data to date. However, this is becoming a more frequently utilized route for removal of these tumors at specialized centers with considerable endoscopic expertise.10,53,54 As with endonasal removal of suprasellar meningiomas, the most common complication using this approach is postoperative CSF leak.
PATIENT SELECTION FOR KEYHOLE MENINGIOMA REMOVAL
Suprasellar Meningiomas
For tuberculum sellae and other suprasellar meningiomas, tumor size and lateral extension are the two key factors that should determine whether a transsphenoidal, supraorbital, or conventional transcranial approach is used. As our results and others suggest, most midline tuberculum sellae meningiomas less than 30 to 35 mm in diameter can be removed by either the endonasal or supraorbital routes even in the setting of a normal sized sella.1,2,5–7,9,14,15 Both approaches allow excellent access for effective optic apparatus decompression with a high rate of visual recovery. However, the endonasal approach does not allow safe access to tumor lateral to the supraclinoid carotid arteries and the optic nerves and along much of the optic canal except medially. In contrast, the supraorbital approach allows access and excellent visualization to both of these areas. Therefore, larger meningiomas greater than 30 to 35 mm in diameter, those that extend well lateral to the supraclinoid carotid arteries or those that cause vascular encasement (see Fig. 31-1) are more safely approached by a supraorbital route or traditional transcranial approach. Regarding pituitary hormonal function, given that the pituitary stalk is pushed posteriorly by tuberculum sellae meningiomas, both the endonasal and supraorbital approaches are associated with a low rate of new endocrinopathy and a high likelihood of preserving the infundibulum. Concerning skull-base closure, a supraorbital craniotomy is relatively simple to close even if the frontal air sinus is transgressed, which occurs in fewer than 10% of cases. In contrast, endonasal avin removal of a tuberculum sellae meningioma will always result in a large skull-base defect and grade 3 CSF leak.55 Although postoperative CSF leak rates for extended suprasellar and transplanum transsphenoidal approaches have been improving considerably during the last 5 years for both microscopic and endoscopic procedures, skull-base repair remains a major issue to consider carefully before embarking on endonasal meningioma removal.55–59
Olfactory Groove Meningiomas
The ideal olfactory groove meningioma to remove by a supraorbital route is one that is less than 50 to 60 mm in maximal diameter and that does not extend to the most anterior extent of the frontal fossa, particularly on the side contralateral to the approach. Provided the tumor growth is within the arc of exposure of the supraorbital route, even meningiomas up to 100 mm in maximal diameter have been removed by this route.4 However, the anterior frontal fossa recess, particularly the contralateral anterior cribriform plate area, is a relative “blind spot” for the supraorbital approach.17,51 In addition, extensive tumor invasion of the ethmoid or sphenoid sinuses may be considered a contraindication for supraorbital removal because the small craniotomy limits the ability to achieve an adequate frontal fossa floor repair should tumor removal extend into the ethmoid or sphenoid sinuses. Tumors that exhibit far anterior bilateral extension or which have extensive growth into the paranasal sinuses are probably best approached from a traditional bifrontal, pterional, or other skull-base craniotomy.24,26,40
For endonasal removal of olfactory groove meningiomas, the most important consideration is tumor size, lateral extension, and whether there is vascular encasement of the anterior cerebral complex by the tumor. The small corridor between the two orbits (22 ± 4 mm)10 afforded by the extended endonasal approach allows complete resection only for tumors medial to the mid orbit as described by Kassam.(Kassam, personal communication). In addition, if there is vascular encasement of the anterior cerebral vessels by tumor, a transcranial removal is recommended. Finally, as with the extended transsphenoidal approach for tuberculum sellae meningiomas, the challenge of achieving an effective skull-base repair and avoiding a postoperative CSF leak remains a major consideration. Given these issues, endonasal removal of olfactory groove meningiomas should be attempted only by experienced endoscopic teams.10,53,54
INSTRUMENTATION
Given the restricted visualization and reduced angles of exposure of any keyhole approach with an operating microscope, endoscopic visualization should be utilized in all cases, at least intermittently to provide a more panoramic view and to assist with tumor removal in the far recesses of the exposure. Endoscopes for keyhole surgery should include 4-mm rigid endoscopes (18 cm in length) with 0-, 30-, and 45-degree angled lenses (Karl Storz, Tuttlingen, Germany)60–64
Use of a micro-Doppler probe for vessel localization localizing is also recommended for all keyhole approaches. For example, in the supraorbital approach, after initial tumor debulking of an olfactory groove or tuberculum sellae meningiomas, localizing the anterior cerebral artery complex behind a rind of tumor with the Doppler can be very helpful. Similarly, as we have shown previously, localizing the cavernous carotid arteries before dural opening in the endonasal approach is also highly recommended and appears to reduce the risk of a cavernous carotid injury.65
SUPRAORBITAL “EYEBROW” CRANIOTOMY
Positioning
As previously described,3,18 patients are placed supine in the 3-point Mayfield head holder (Ohio Medical Instrument Co., Cincinnati, OH) and angled 20 to 60 degrees to the contralateral side based on the location and projection of tumor to the right or left. For suprasellar meningiomas, 30 degrees of head rotation is used: for olfactory groove meningiomas, 45 to 60 degrees contralateral rotation is recommended. The head is slightly retroflexed and the malar eminence is most prominent point. The lower abdomen is also prepped for a possible abdominal fat graft. The patient is registered to their preoperative surgical navigation magnetic resonance imaging (MRI) and the projection of the ipsilateral frontal sinus is then marked on the supraorbital skin surface to help define the medial limits of the craniotomy. The supraorbital notch should be palpated and noted.
Initial Approach
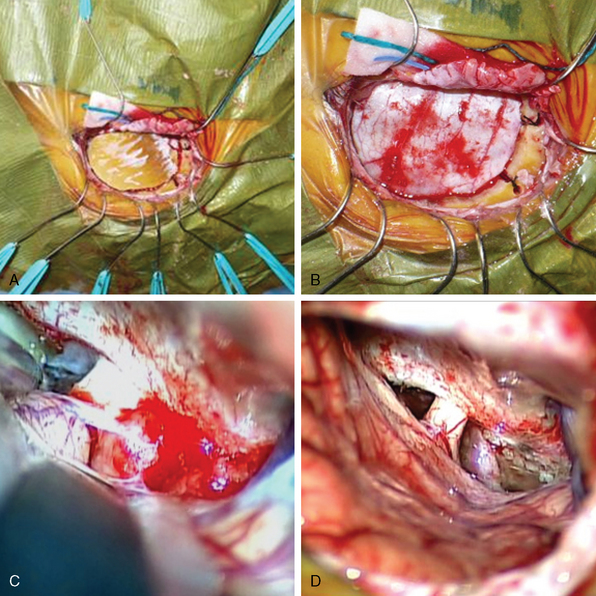
The skin incision within the eyebrow extends from just medial to the supraorbital notch and continues laterally and inferiorly beyond the eyebrow in a skin fold along the frontozygomatic process. At its most medial aspect, the skin incision remains superficial to avoid injury to the supraorbital nerve. The skin flap is elevated superiorly to gain supraorbital exposure and retracted with multiple fish hooks. A pericranial flap is then created and incised in a half moon-shaped fashion, then retracted inferiorly along the supraorbital rim area. This pericranial flap incorporates a small portion of the most superior aspect of the temporalis fascia extending just below the superior temporal line. Additional fish hooks are then used to retract inferiorly and laterally the temporalis muscle and fascia along the inferior margin of exposure to expose the keyhole below and posterior to the frontozygomatic process (Fig. 31-2).
A single burr hole is placed below the superior temporal line and posterior to the keyhole with the high-speed drill. A free supraorbital half-moon–shaped bone flap is made which does not include the orbital rim and measures approximately 15 to 20 mm by 20 to 25 mm.3,18 It is essential to drill the anterior inferior, medial inferior, and lateral inferior bone edges of the calvarium to provide the maximal angles of exposure into the frontal fossa. In addition, if there are bony protuberances along the floor of the frontal fossa in the trajectory to the parasellar area, these should be drilled as well. If the frontal sinus has been entered (which infrequently occurs), it can be sealed with bone wax at the initial opening and then more definitively repaired at the end of the procedure.
Suprasellar Meningiomas
Once the posterior tumor rind is relatively thin, using sharp dissection, gentle traction, and copious irrigation, the tumor pseudocapsule is dissected away from the ipsilateral and contralateral optic nerves, optic chiasm, supraclinoid carotid arteries, and anterior cerebral complex. Great care is taken to preserve the arachnoid plane and perforators over these key structures. The infundibulum will also begin to come into view at this point. In some instances, tumor extends into a deepened sella onto the diaphragma sella and this tumor can typically be elevated away from the diaphragma and infundibulum. In cases of firm or calcified tumor remnants that are densely adherent to the optic apparatus or anterior cerebral complex, it is best to leave such tumor behind rather than risk a serious neurovascular injury. The endoscope should be brought into use again at this point to confirm as complete a tumor removal as possible. Inspection of the optic canals should also be performed to assess for intracanalicular tumor extension. Such tumor can often be removed with drilling of the roof of the optic canal and opening the dural ring over the optic nerve. Such drilling should be performed with a 2- or 3-mm diamond bit using copious irrigation. In patients with significant preoperative visual loss, some authors advocate early optic canal opening and decompression before tumor removal to minimize chances of visual deterioration associated with optic apparatus traction.30,38
Closure
After tumor removal, the dura is closed in watertight fashion with multiple interrupted 4-0 stitches. A layer of collagen sponge (Helistat or Duragen) is placed over the dura for reinforcement of the dural closure. The anterior medial aspect of the craniotomy should be checked again for a defect into the frontal sinus, which in the great majority of cases is not present. If there is no sinus defect, the bone flap is then reapproximated with a burr hole cover placed over the lateral burr hole site and contoured to the bone anatomy; a single straight titanium plate extends from the medial bone flap edge going toward the midline and is placed under the supra-orbital nerve. The burr hole site is then covered with the superior temporalis muscle and fascia. The superior and inferior edges of the bone should be placed with minimum distance from the adjacent bone and the bone gaps further filled with collagen sponge. Some advocate for optimal cosmesis, placing the bone flap so the bone gap is placed inferiorly directly under the eyebrow incision leaving no gap superiorly in the more visible forehead area.18 The pericranial flap is then returned to its anatomic position covering the bone flap and secured to the adjacent pericranium with absorbable stitches. The scalp incision is closed with galeal and subcutaneous stitches followed by topical skin adhesive such as Dermabond (Ethicon Inc., Johnson & Johnson Co., Piscataway, NJ).
Results
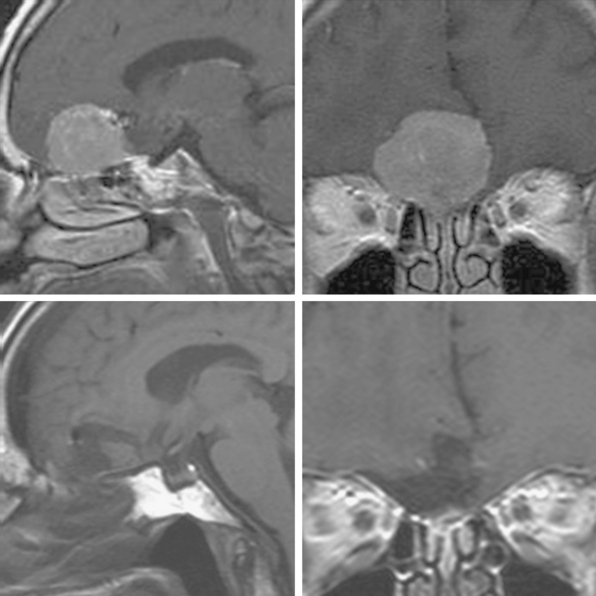
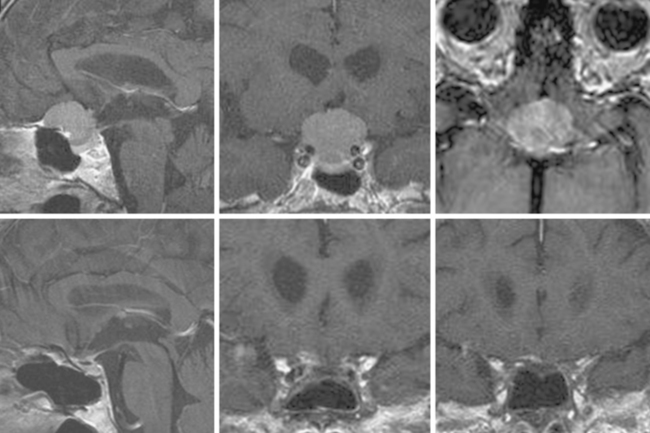
Supraorbital eyebrow craniotomy has been used in 12 patients (mean age 52 ± 9 years, 83% female), including 4 with olfactory groove meningiomas and 8 with suprasellar meningiomas. Three patients with olfactory groove meningiomas with tumor diameters ranging from 18 to 50 mm had complete tumor removal without complication; another patient with prior subtotal removal and radiosurgery had a near total tumor removal given dense adhesions of the tumor to the A-2 arterial branches (Fig. 31-3). Of the eight patients with a suprasellar meningioma, five underwent first-time surgery (Fig. 31-4). Of these five, gross total, near-total (>90% resection) and subtotal removal was accomplished in two, two, and one patient respectively. All three patients with near-total or subtotal removal had cavernous sinus invasion. One patient with near-total resection was submitted to further stereotactic radiotherapy after tumor recurrence 6 months postsurgery. In three patients with recurrent tumors and gross invasion of central skull base, the surgical goal was optic pathway decompression as part a staged treatment including further transsphenoidal tumor removal in two patients. Overall, of seven patients with preoperative visual loss, improvement was observed in four (57%) patients. One patient with a 25-mm fibrous and invasive tuberculum sella and right paraclinoid meningioma who underwent subtotal removal, experienced a delayed right inferior hemianopsia that has persisted. There was one presumed cavernous carotid artery puncture injury in a patient who had undergone two previous surgeries. Arterial bleeding was controlled with muslin gauze; a postoperative cerebral angiogram was entirely normal and the patient had no new neurologic deficits.
EXTENDED ENDONASAL APPROACH
Nasal Portion and Sphenoidotomy
As previously described, the direct endonasal approach is performed with the operating microscope and endoscopic assistance.1,7,66 For tumors projecting more to one side, the contralateral nostril is chosen to provide better tumor access. The initial approach is performed with the operating microscope. A hand-held speculum is passed into the nostril along the trajectory of the middle turbinate. A vertical mucosal incision is made at the junction of the sphenoid keel and posterior nasal septum with a bayoneted Cottle elevator by sweeping the mucosa laterally. The posterior nasal septum is then pushed across the midline with the tip of the hand-held speculum and a similar mucosal flap is elevated on the contralateral side of the sphenoid keel. This exposure should allow identification of both sphenoid ostia which should be seen at approximately the 9 to 10 o’clock and 2 to 3 o’clock positions.
Next, a short endonasal (60 or 70 mm) speculum (Mizuho America, Inc., Beverly, MA) is passed inside the hand-held speculum and the hand-held speculum is disengaged and removed in two separate halves. In most recent suprasellar endonasal cases, we use a 60-mm trapezoidal-shaped endonasal speculum (Mizuho America) that provides expanded superior visualization with the microscope and allows greater maneuverability of the endoscope and other instruments.67 To gain full visual advantage of the short speculum, and to maximize superior visualization, the most superior aspect of the sphenoid keel and soft tissue at the posterior ethmoid roof area should be removed. In addition, it is helpful to remove at least 10 to 15 mm of posterior nasal septum and lateral tissue as well so that no soft tissue or bone obstructs visualization medial to the distal speculum blades.
Skull-Base Reconstruction and Nasal Closure
After removal of a tuberculum sella meningioma, a large (grade 3) CSF leak results which is closed in a multilayered fashion.55 As described previously, first an abdominal fat graft is placed in the intrasellar/suprasellar dead space followed by a layer of collagen sponge placed over the fat. A piece of precisely cut titanium mesh is placed over the collagen sponge. The mesh is shaped to conform precisely to the defect and includes a small handle so that it could be maneuvered into the intrasellar extradural space in cephalad-to-caudal direction. Care is taken to trim sharp edges off the mesh and ensure that there is no encroachment on the optic canals or cavernous sinus. An additional concern in grade 3 leaks is the possibility of intra dural graft migration because there is typically a large diaphragmatic and/or dural defect through which the fat graft and collagen may migrate. Thus, in assembling the graft it is important to have portions of the fat and collagen extending out extradurally on at least two sides of the defect so that when the mesh is placed in the extradural bony defect, it holds the fat and collagen against the edges of the bony and dural defect, thereby preventing intradural graft migration. At this point, the anesthesiologist should induce a Valsalva maneuver to stress the repair. If there is seen to be copious CSF streaming around the repair or the titanium mesh becomes dislodged, it should be appropriately revised. Once the Valsalva maneuver confirms an adequate and stable repair, additional fat is placed in the sphenoid sinus against the titanium mesh and covered by a thin layer of BioGlue to hold the construct in position.68 The endonasal speculum is removed, nasal hemostasis is obtained with Surgifoam, the ipsilateral middle turbinate which is out-fractured at the beginning of the procedure, is medialized and the nasal septum is returned to the midline. No nasal packing is placed. Before awakening the patient from anesthesia, a lumbar drain is placed for CSF diversion at 5 to 10 mL/h for the first 48 hours after surgery. As described previously, however, the lumbar drain is not opened until an early postoperative CT scan is performed to document the amount of immediate postoperative pneumocephalus and to insure that the repair materials are in good position.55
Results
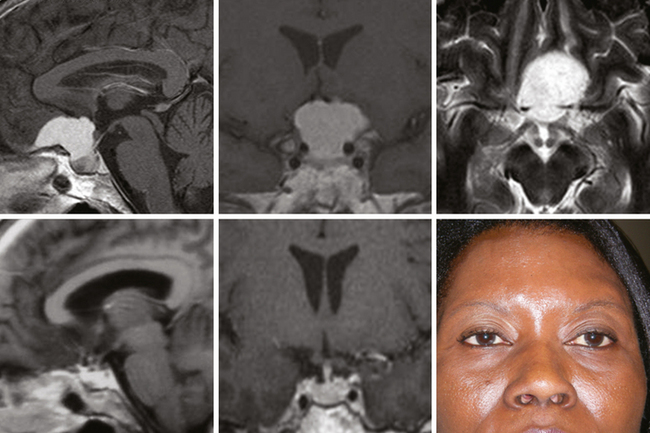
To date, 14 patients (mean age 51 ± 11 years, 71% female) have undergone extended endonasal removal of a tuberculum sellae meningioma. In 7 patients, total resection was accomplished, and no recurrence was observed in a follow up that ranged 13 to 51 months (median 24 ± 14 months). Two patients underwent endonasal tumor removal as part of a staged procedure to control recurrent tumor including one with an atypical meningioma treated with multiple operations, SRS, SRT, and chemotherapy. He has became progressively moribund and blind from progressive tumor growth one year after his last operation. The other five patients had a near total 3 (21%), or partial resection in 2 (14%) due to cavernous sinus invasion. Three patients had tumor progression on follow-up MRI and had stereotactic radiotherapy (Fig. 31-5). Regarding visual recovery, 9 of 11 (82%) patients with pre-operative impaired vision had complete recovery and only one has had a mild visual decline (8%). This 72-year-old woman sustained mild visual hemifield worsening after complete removal of a tuberculum sellae meningioma who presented with a bitemporal hemianopsia; her bitemporal defect, however, improved. Four patients (28.5%) had a postoperative CSF leak, requiring reoperation in two and lumbar drain CSF diversion in two.
[1] Cook S.W., Smith Z., Kelly D.F. Endonasal transsphenoidal removal of tuberculum sellae meningiomas: technical note. Neurosurgery. 2004;55:239-244. discussion 244–6
[2] Couldwell W.T., Weiss M.H., Rabb C., et al. Variations on the standard transsphenoidal approach to the sellar region, with emphasis on the extended approaches and parasellar approaches: surgical experience in 105 cases. Neurosurgery. 2004;55:539-547. discussion 547–50
[3] Czirjak S., Seifert G. Surgical experience with frontolateral keyhole craniotomy through a superciliary skin incision. Neurosurgery. 2001;48:145-150.
[4] Czirjak S., Seifert G.T. The role of the superciliary approach in the surgical management of intracranial neoplasms. Neurol Res. 2006;28:131-137.
[5] Cappabianca P., et al. Extended endoscopic endonasal transsphenoidal approach for the removal of suprasellar tumors: Part 2. Neurosurgery. 2007;60:46-58. discussion 58–9
[6] de Divitiis E., Cavallo L.M., Esposito F., et al. Extended endoscopic transsphenoidal approach for tuberculum sella meningiomas. Neurosurgery. 2007;61(5):229-238. ONS2
[7] Dusick J.R., Esposito F., Kelly D.F., et al. The extended direct endonasal transsphenoidal approach for nonadenomatous suprasellar tumors. J Neurosurg. 2005;102:832-841.
[8] Fernandes Y.B., Maitrot D., Kehrli P., et al. Supraorbital eyebrow approach to skull base lesions. Arq Neuropsiquiatr. 2002;60:246-250.
[9] Jane J.A., Dumont A.S., Lee Vance M. The transsphenoidal transtuberculum sellae approach for suprasellar meningiomas. Semin Neurosurg. 2003;14(3):211-218.
[10] Jho H.D., Ha H.G. Endoscopic endonasal skull base surgery: Part 1–-The midline anterior fossa skull base. Minim Invasive Neurosurg. 2004;47:1-8.
[11] Joseph V., Chacko A.G. Suprabrow minicraniotomy for suprasellar tumours. Br J Neurosurg. 2005;19:33-37.
[12] Jho H.D. Orbital roof craniotomy via an eyebrow incision: a simplified anterior skull base approach. Minim Invasive Neurosurg. 1997;40:91-97.
[13] Kitano M., Taneda M. Extended transsphenoidal approach with submucosal posterior ethmoidectomy for parasellar tumors. Technical note. J Neurosurg. 2001;94:999-1004.
[14] Kitano M., Taneda M., Nakao Y. Postoperative improvement in visual function in patients with tuberculum sellae meningiomas: results of the extended transsphenoidal and transcranial approaches. J Neurosurg. 2007;107:337-346.
[15] Laufer I., Anand V.K., Schwartz T.H. Endoscopic, endonasal extended transsphenoidal, transplanum transtuberculum approach for resection of suprasellar lesions. J Neurosurg. 2007;106:400-406.
[16] Laws E.R., Kanter A.S., Jane J.A.Jr, Dumont A.S. Extended transsphenoidal approach. J Neurosurg. 2005;102:825-827. discussion 827–8
[17] Melamed I., Merkin V., Korn A., et al. The supraorbital approach: an alternative to traditional exposure for the surgical management of anterior fossa and parasellar pathology. Minim Invasive Neurosurg. 2005;48:259-263.
[18] Reisch R., Perneczky A. Ten-year experience with the supraorbital subfrontal approach through an eyebrow skin incision. Neurosurgery. 2005;57:242-255. discussion 242–55
[19] Shanno G., Maus M., Bilyk J., et al. Image-guided transorbital roof craniotomy via a suprabrow approach: a surgical series of 72 patients. Neurosurgery. 2001;48:559-567. discussion 567–8
[20] Wiedemayer H., Sandalcioglu I.E., Wiedemayer H., et al. The supraorbital keyhole approach via an eyebrow incision for resection of tumors around the sella and the anterior skull base. Minim Invasive Neurosurg. 2004;47:221-225.
[21] Kallio M., Sankila L., Hakulien T., Jaaskilainen J. Factors affecting operative and excess long-term mortality in 935 patients with intracranial meningeoma. Neurosurgery. 1992;31:2-12.
[22] Rubin G., David U.B., Gornish M., Rappaport Z.H. Meningeomas of the anterior cranial floor: review of 67 cases. Acta Neurochir (Wien). 1994;129:26-30.
[23] Solero C.L., Giombini S., Morello G. Suprasellar and olfactory meningioma. report of a series of 153 personal cases. Acta Neurochir (Wien). 1993;67:181-194.
[24] Hentschel S.J., DeMonte F. Olfactory groove meningiomas. Neurosurg Focus. 2003:4.
[25] Obeid F., Al-Mefty O. Recurrence of olfactory groove meningioma. Neurosurgery. 2003;53:534-543.
[26] Turazzi S., Cristofori L., Gambini R., Bricolo A. The pterional approach for microsurgical removal of olfactory groove meningioma. Neurosurgery. 1999;45:821-826.
[27] Al-Mefty O., Holoubi A., Rifai A., Fox J.L. Microsurgical removal of suprasellar meningiomas. Neurosurgery. 1985;16:364-372.
[28] Andrews B.T., Wilson C.B. Suprasellar meningiomas: the effect of tumor location on postoperative visual outcome. J Neurosurg. 1988;69:523-528.
[29] Fahlbusch R., Schott W. Pterional surgery of meningiomas of the tuberculum sellae and planum sphenoidale: surgical results with special consideration of ophthalmological and endocrinological outcomes. J Neurosurg. 2002;96:235-243.
[30] Mathiesen T., Kihlstrom L. Visual outcome of tuberculum sellae meningiomas after extradural optic nerve decompression. Neurosurgery. 2006;59:570-576.
[31] Durante F. Estirpazione di un tumore endocranio. Arch Soc Ital Chir. 1885;2:252-255.
[32] Cushing H., Eisenhardt L. Suprasellar meningiomas. The chiasmal syndrome. In: Meningiomas: their Classification, Regional Behavior, Life History and Surgical End Results. Springfield, IL: Charles C Thomas; 1938:224-249.
[33] Black P. Aggressive surgery and focal radiotherapy in the management of meningiomas of the skull base: preservation of function with maintenance of local control. Acta Neurochir (Wien). 2001;143:555-562.
[34] Goel A., Muzumdar D., Desai K.I. Tuberculum sellae meningioma: a report on management on the basis of a surgical experience with 70 patients. Neurosurgery. 2002;51:1358-1363. discussion 1363–4
[35] Jallo G.I., Benjamin V. Tuberculum sellae meningiomas: microsurgical anatomy and surgical technique. Neurosurgery. 2002;51:1432-1439. discussion 1439–40
[36] Nakamura M., Roser F., Struck M., et al. Tuberculum sellae meningioma: Clinical outcome considering different surgical approaches. Neurosurgery. 2006;59:1019-1029.
[37] Nakamura M., Roser F., Struck M., et al. Olfactory groove meningioma: clinical outcome and recurrence rates after tumor removal through the frontolateral and bifrontal approach. Neurosurgery. 2007;60(5):844-852.
[38] Otani N., Muroi C., Yano H., et al. Surgical management of tuberculum sellae meningioma: role of selective extradural anterior clinoidectomy. Br J Neurosurg. 2006;20:129-138.
[39] Park C.K., Jung H.W., Yang S.Y., et al. Surgically treated tuberculum sellae and diaphragm sellae meningiomas: the importance of short-term visual outcome. Neurosurgery. 2006;59:238-243. discussion 238–43
[40] Spektor S., Valarezo J. Fliss D. Olfactory groove meningiomas from neurosurgical and ear, nose and throat perspectives. Approaches, techniques and outcomes. Neurosurgery. 2005;57(4):268-280. ONS Supplement 4
[41] Mathiesen T., Lindquist C., Kihlstrom L., Karlsson B. Recurrence of cranial base meningiomas. Neurosurgery. 1996;39(1):2-7.
[42] Mirimanoff R.O., Linggood R.M., Ojeman R., Martuza R. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62:18-24.
[43] Wilson D.H. Limited exposure in cerebral surgery. Technical note. J Neurosurg. 1971;34:102-106.
[44] Perneczky A., Muller-Forell W., Van Lindert E., Fries G. Keyhole Concept in Neurosurgery: With Endoscopic-Assisted Microneurosurgery and Case Studies. New York: Thieme, 1999.
[45] Goel A., Muzumdar D. Conventional posterior fossa approach for surgery on petroclival meningiomas: a report on an experience with 28 cases. Surg Neurol. 2004;62:332-338. discussion 338–40
[46] Samii M., Tatagiba M., Carvalho G.A. Resection of large petroclival meningiomas by the simple retrosigmoid route. J Clin Neurosci. 1999;6:27-30.
[47] Kaplan M.J., Jane J.A., Park T.S., et al. Supraorbital rim approach to anterior skull base. Laryngoscope. 1984;94(9):1137-1139.
[48] Brock M., Dietz H. The small frontolateral approach for the microsurgical treatment of intracranial aneurysms. Neurochirurgia (Stuttg). 1978;21:185-191.
[49] Jane J.A., Park T.S., Pobereskin L.H., et al. The supraorbital approach: technical note. Neurosurgery. 1982;11(4):537-542.
[50] Yasargil M.G., Fox J.L., Ray M.W. The operative approach to aneurysms of the anterior communicating artery. Krayenbühl H., editor. Advances and Technical Standards in Neurosurgery, vol. 2. Springer-Verlag, New York, 1975;113-170.
[51] Figueiredo E.G., Deshmukh V., Nakaji P., et al. An anatomical evaluation of the mini-supraorbital approach and comparison with standard craniotomies. Neurosurgery. 59, 2006. ONS212–ONS220
[52] Weiss M.H. Transnasal transsphenoidal approach. In: Apuzzo M.L.J., editor. Surgery of the Third Ventricle. Baltimore: Williams & Wilkins; 1987:476-494.
[53] Kassam A., Snyderman C.H., Mintz A., et al. Expanded endonasal approach: the rostrocaudal axis. Part 1. Crista galli to sella turcica. Neurosurg Focus. 2005;19(1):E3.
[54] Webb-Myers R., Wormald P.J., Brophy B. An endoscopic endonasal technique for resection of olfactory groove meningioma. J Clin Neurosci. 2008;15:451-455.
[55] Esposito F., Dusick J.R., Fatemi N., Kelly D.F. Graded repair of cranial base defects and cerebrospinal fluid leaks in transsphenoidal surgery. Neurosurgery. 2007;60:295-304.
[56] Cavallo L.M., Messina A., Esposito F., et al. Skull base reconstruction in the extended endoscopic transsphenoidal approach for suprasellar lesions. J Neurosurg. 2007;107:713-720.
[57] Kitano M., Taneda M. Subdural patch graft technique for watertight closure of large dural defects in extended transsphenoidal surgery. Neurosurgery. 2004;54:653-661.
[58] Snyderman C.H., Kassam A.B., Carrau R., Mintz A. Endoscopic reconstruction of cranial base defects following endonasal skull base surgery. Skull Base. 2007;17:73-78.
[59] Tabaee A., Anand V., Brown S. Algorithm for reconstruction after endoscopic pituitary and skull base surgery. Laryngoscope. 2007;117(7):1133-1137.
[60] Cappabianca P., Alfieri A., Thermes S., et al. Instruments for endoscopic endonasal transsphenoidal surgery. Neurosurgery. 1999;45:392-395. discussion 395–6
[61] Cappabianca P., Cavallo L.M., Esposito F., de Divitiis E. Endoscopic endonasal transsphenoidal surgery: procedure, endoscopic equipment and instrumentation. Childs Nerv Syst. 2004;20:796-801.
[62] Leonhard P., Cappabianca P., de Diviitis E. The endoscope, endoscopic equipment and instrumentation. In: de Divitis E., Cappabianca P., editors. Endoscopic Endonasal Transsphenoidal Surgery. Vienna: Springer-Verlag; 2003:9-20.
[63] Prevedello D.M., Doglietto F., Jane J.A.Jr, et al. History of endoscopic skull base surgery: its evolution and current reality. J Neurosurg. 2007;107:206-213.
[64] Perneczky A., Fries E. Endoscope-assisted brain surgery: part 1 – evolution, basic concept, and current technique. Neurosurgery. 1998;42:219-224.
[65] Dusick J., Esposito F., Malkasian D. Avoidance of carotid artery injuries in transsphenoidal surgery with the Doppler probe and micro-hook blades. Neurosurgery. 60(ONS Suppl. 2), 2007. ONS-322–ONS-329
[66] Zada G., Kelly D.F., Cohan P., et al. Endonasal transsphenoidal approach for pituitary adenomas and other sellar lesions: an assessment of efficacy, safety, and patient impressions. J Neurosurg. 2003;98:350-358.
[67] Fatemi N., Dusick J.R., Malkasian D., et al. Instrumentation assessment: short trapezoidal speculums for suprasellar and infrasellar exposure in endonasal transsphenoidal surgery. Neurosurgery. 2008;62:325-329.
[68] Dusick J.R., Mattozo C.A., Esposito F., et al. Bioglue for prevention of postoperative cerebrospinal fluid leaks in transsphenoidal surgery: A case series. Surg Neurol. 2006;66:371-376. discussion 376