Chapter E44 Minimal Access Skull Base Approaches
Endoscopic Endonasal Approaches
Historical Background
Since the 1980s, several ENT surgeons pioneered the functional endoscopic sinus surgery (FESS),1–5 thus encouraging the “pure” endoscopic endonasal approach to the sella,6 performed first by Jankowski;7 then Jho and Carrau extended this technique,8–10 followed by other teams.11–14 These experiences supported a continued structured evolution of the endoscopic technique: new skills have been transferred across subspecialties, new instruments have been designed,15–18 new image-guidance systems have been fashioned,19–24 and new surgical corridors have been defined, thus disclosing the way to the pure endoscopic approach to lesions affecting the skull base, from the frontal sinus to the C2 body.14,25–28 The shift toward minimally invasive procedures represents the evolution of previous views: Ketcham’s concept of craniofacial resection29 evolved in the concept of “endoscopic craniofacial resection,”30 and Apuzzo’s inspired guess of using the endoscope in transcranial surgery31 has been translated in Kassam’s paradigm of “360 degree surgery,” which suggests the use of an endoport to unlock intracranial lesions.32
Classification
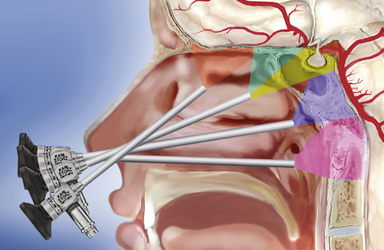
The sphenoid sinus is in the center of the skull base and the starting point for many extended endonasal approaches (Table E44.1) because critical anatomical structures, such as the internal carotid artery and the optic nerve, are identified here and then followed to other areas.14,25–27,33–36 The midline ventral skull base can be exposed from the frontal sinus to the body of C2 through specific modules: transcribriform, transtuberculum, transclival, and transodontoid25,37,38 (Fig. E44.1). Depending on the lesion extension along the sagittal plane,14,26,28 contiguous modules can be combined, and the lateral boundaries of each module are dictated by critical neurovascular structures. Lesions originating in or extending to paramedian areas, such as the cavernous sinus, the lateral recess of the sphenoid sinus, and the pterygopalatine fossa, can be approached through a transethmoid transantral transpterygoid route,39–42 and several paramedian modules have been developed to improve the endonasal exposure of the orbit, the petrous apex, the infratemporal fossa, and the jugular foramen.27,30,43–45
| Endoscopic Approach | Surgical Target |
|---|---|
| Endoscopic Endonasal Approaches | |
| Midline Approaches | |
| Transcribriform | Cribriform plate |
| Transtuberculum transplanum | Suprasellar area Third ventricle |
| Standard transsphenoidal | Sellar, suprasellar, and parasellar areas |
| Transclival | Clivus Interpeduncular and prepontine areas Prepontine area |
| Transodontoid | Craniovertebral junction Odontoid Superior cervical spine (C2 body) |
| Paramedian Approaches | |
| Supraorbital and transorbital | Orbital roof Medial intraconal space |
| Transmaxillary transpterygoid | Pterygopalatine fossa Lateral recess of the sphenoid sinus Lateral compartment of the cavernous sinus Meckel’s cave Infratemporal fossa Medial aspect of the petrous apex Jugular foramen |
| Endoscopic Transcranial Approaches | |
| Supraorbital | Anterior skull base Middle skull base |
| Retrosigmoid | Cerebellopontine angle |
Imaging Techniques
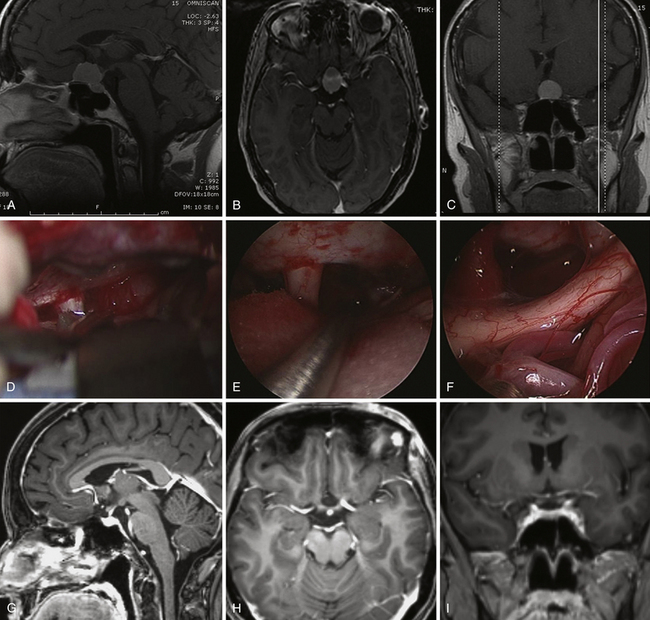
Patients are studied with both magnetic resonance imaging (MRI) and computed tomography (CT), which are complementary in delineating the individual anatomy of patients harboring skull base lesions, thus optimizing patient selection and operative procedure.35,46,47
 Identification of septal deviation, crest, or polyps claiming adequate correction during the same operative time
Identification of septal deviation, crest, or polyps claiming adequate correction during the same operative time
 Configuration of the paranasal sinuses such as pneumatization of sphenoid sinus, insertion of the sphenoid septa, presence of any Onodi (sphenoethmoid) cell48
Configuration of the paranasal sinuses such as pneumatization of sphenoid sinus, insertion of the sphenoid septa, presence of any Onodi (sphenoethmoid) cell48
 Bone dehiscence at the level of the optic and carotid prominences, dorsum sellae, and intrapetrous carotid canal
Bone dehiscence at the level of the optic and carotid prominences, dorsum sellae, and intrapetrous carotid canal
 Morphology of the olfactory cleft and insertion of the basal lamella and of the uncinate process
Morphology of the olfactory cleft and insertion of the basal lamella and of the uncinate process
 Vidian canal on axial and coronal images and its relation with the foramen lacerum
Vidian canal on axial and coronal images and its relation with the foramen lacerum
 Configuration of the craniovertebral junction
Configuration of the craniovertebral junction
 Nasopalatine line, which represents the inferior limit of the endonasal exposure
Nasopalatine line, which represents the inferior limit of the endonasal exposure
Preoperative CT angiography is used for intraoperative image guidance.
 Three-dimensional configuration, vascularity, and consistency of the lesion
Three-dimensional configuration, vascularity, and consistency of the lesion
 Relationship of the lesion with the neurovascular structures
Relationship of the lesion with the neurovascular structures
 Intactness of the arachnoidal plane and carotid sheath
Intactness of the arachnoidal plane and carotid sheath
 Infiltration of the brain, hypothalamus, or brainstem and perineural spreading
Infiltration of the brain, hypothalamus, or brainstem and perineural spreading
 Interpretation of signal from tumoral, scar, inflammatory, and adipose tissue
Interpretation of signal from tumoral, scar, inflammatory, and adipose tissue
 Critical anatomical variations, such as “kissing carotid arteries” or aneurysms
Critical anatomical variations, such as “kissing carotid arteries” or aneurysms
Surgical Technique49
Perioperative antibiotic prophylaxis with a broad-spectrum cephalosporin50 is administered half an hour before surgery and continued for 5 days. We do not usually insert any lumbar drainage. Neurophysiological monitoring of brain and nerve function allows detection of cerebral ischemia and prevention of cranial nerve injury. An optimal anesthesia and analgesia combination51 contributes to a dry field, will improve surgical precision, and can reduce complications and frustration during the surgical procedure.
Cotton pledgets soaked in diluted (1:1) povidone-iodine solution are gently slipped into the nasal cavities and the nose and upper lip are disinfected.
The procedure is performed with a rigid 0-degree endoscope, 18 cm in length, 4 mm in diameter (Karl Storz & Co., Tuttlingen, Germany), without any working channel and inserted in an irrigation shaft to keep the lens clean. A two-nostrils four-hands technique14,26,27,52,53 is recommended, with the endoscope used freehand during the whole procedure; it is held by the surgeon’s nondominant hand and, after the anterior sphenoidotomy, by the surgeon’s assistant. A 30-degree or 45-degree angled scope increases the angle of view when a more lateral view is needed, quite often in the intradural phase of the procedure.
Standard Approach
The surgical corridor between the middle turbinate and the nasal septum is enlarged by pushing the middle turbinate laterally, after topical decongestion with cotton pledgets soaked with proper anesthetics. The endoscope is moved, parallel to the nasal floor, toward the choana and angled up along the sphenoethmoid recess. After anterior sphenoidotomy, the identification of the anatomical landmarks54 over the posterior wall of the sphenoid guide the opening of the sellar floor. Dura opening is tailored to the lesion extension.
Microadenomas can be removed en bloc or piecemeal, depending on their consistency and the presence of a pseudocapsule.55 Intra- and suprasellar macroadenomas are removed systematically, starting from the bottom of the sella, because early descent of the stretched suprasellar cistern may hide tumor remnants.52 Tumor extension in the medial compartment of the cavernous sinus can be removed with atraumatic curets and suction cannulas. Cystic lesions as well as pure intrasellar craniopharyngiomas are well established indications for this technique.56
Extended Approaches
The idea of an approach extending beyond the sella should be credited to Hardy,57 and the routine use in clinical practice is due to Martin Weiss.58 This first step of the surgical procedure can be considered the common denominator of all the extended approaches. The endoscope is inserted in the right nostril, which is stretched upward while instruments run below it. Essential aspects of this stage, according to Kassam and his group,27 include the following:
 Middle turbinectomy, usually unilateral, with lateralization of the middle turbinate in the other nostril, provides the adequate room to access and manage confortably the lesion in most cases.
Middle turbinectomy, usually unilateral, with lateralization of the middle turbinate in the other nostril, provides the adequate room to access and manage confortably the lesion in most cases.
 Harvesting of the nasoseptal flap is done (see later discussion under “Reconstruction”).
Harvesting of the nasoseptal flap is done (see later discussion under “Reconstruction”).
 A wide anterior sphenoidotomy is the next step.
A wide anterior sphenoidotomy is the next step.
 A posterior septectomy permits instruments coming from the two nostrils to work without conflict.
A posterior septectomy permits instruments coming from the two nostrils to work without conflict.
 The removal of posterior ethmoidal cells completes the exposure of the posterior wall of the sphenoid sinus with its anatomical landmarks.
The removal of posterior ethmoidal cells completes the exposure of the posterior wall of the sphenoid sinus with its anatomical landmarks.
Transcribriform Approach
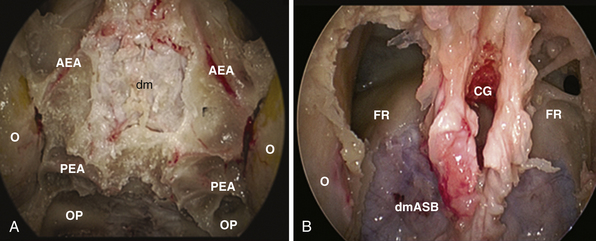
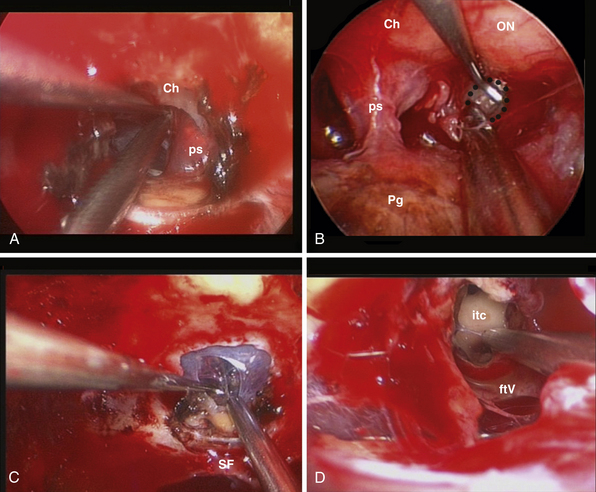
This approach is used for the management of cerebrospinal fluid (CSF) leaks,59 meningoencephaloceles, esthesioneuroblastoma,30,60 and meningiomas.61–64 It is limited by the medial orbital wall laterally, but it can be expanded along the sagittal plane, depending on the lesion extension. However, to expose the cribriform plate, the posterior limit is marked by the posterior ethmoidal artery (Fig. E44.2A). The exposure can be further expanded anteriorly, toward the frontal sinus, through a transfrontal module, which provides access to its floor and the posterior wall30 (Fig. E44.2B).
The main steps to expose the midline anterior skull base are as follows:65
 Bilateral anterior and posterior ethmoidectomy: entering the ethmoid bulla inferomedially, the lamina papyracea comes into view and should be preserved during the ethmoidectomy.
Bilateral anterior and posterior ethmoidectomy: entering the ethmoid bulla inferomedially, the lamina papyracea comes into view and should be preserved during the ethmoidectomy.
 Exposure of the frontal recess and of the anterior ethmoidal artery: opening the agger nasi air cell highlights the posterior wall of the frontal recess, which leads to the anterior ethmoid canal. The lamina papyracea is fractured with a blunt instrument and lateralization of the periorbit highlights the artery, where it leaves the orbit to enter its osseous canal. At this level, the anterior ethmoidal artery can be easily bipolar coagulated. The frontal recess guides toward the frontal sinus floor, which can be removed to enlarge the exposure anteriorly.
Exposure of the frontal recess and of the anterior ethmoidal artery: opening the agger nasi air cell highlights the posterior wall of the frontal recess, which leads to the anterior ethmoid canal. The lamina papyracea is fractured with a blunt instrument and lateralization of the periorbit highlights the artery, where it leaves the orbit to enter its osseous canal. At this level, the anterior ethmoidal artery can be easily bipolar coagulated. The frontal recess guides toward the frontal sinus floor, which can be removed to enlarge the exposure anteriorly.
 Identification and coagulation of the posterior ethmoidal artery: its bony canal runs in the ethmoidal roof horizontally and is located at an average of 8.3 mm (range 6-11 mm) anterior to the optic nerve exit from the optic canal.65
Identification and coagulation of the posterior ethmoidal artery: its bony canal runs in the ethmoidal roof horizontally and is located at an average of 8.3 mm (range 6-11 mm) anterior to the optic nerve exit from the optic canal.65
 Opening of the ethmoidal plate with bone punches or microdrill.
Opening of the ethmoidal plate with bone punches or microdrill.
In olfactory groove meningiomas, the dura is coagulated and opened along the midline. In lesions extended to the crista galli, the dura is cauterized and incised lateral to the insertion of the falx; after coagulation of the anterior falcine artery and the inferior sagittal sinus, the falx is transected and the dura is mobilized anteriorly. Further anterior extension of the lesion may require drilling of the crista galli, which can be hyperostotic or pneumatized (10% of cases).66 After internal debulking, extracapsular dissection proceeds in a lateral to medial direction; when anterior cerebral artery branches or the anterior communicating artery lie on the surface of the tumor, a piecemeal removal is performed and the last tumor remnants are carefully dissected.
In esthesioneuroblastomas extending intradurally, the olfactory bulbs are dissected from the surface of the brain and remain attached to the dura; focal areas of brain invasion are removed by suction and dissection. The olfactory nerves are then transected posteriorly and dura is removed completely; additional dural margins can be excised for frozen section analysis.60
Transtuberculum-Transplanum Sphenoidale Approach
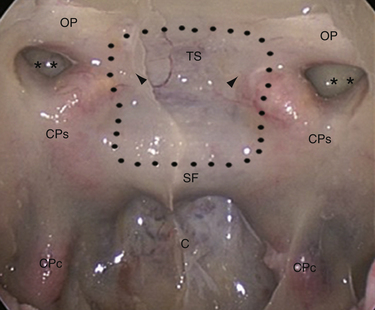
In 1980 Ed Laws, Jr., described the microscopic transsphenoidal approach to suprasellar lesions by taking down the planum sphenoidale,67 and the same approach was adapted for endoscopy.67,68 It is bordered by the optic nerve’s bony prominence, the opticocarotid recess, and the bony prominence of the parasellar segment of the internal carotid artery (Fig. E44.3).
The main aspects of this module are as follows:
 Opening of the sellar floor and drilling of the tuberculum are done in order to widen the caudal-cranial angle of exposure of the third ventricle and retrosellar area.
Opening of the sellar floor and drilling of the tuberculum are done in order to widen the caudal-cranial angle of exposure of the third ventricle and retrosellar area.
 Management of the superior intercavernous sinus with its isolation is done with two horizontal incisions and bipolar coagulation, in case of craniopharyngiomas, and by coagulation in meningiomas.
Management of the superior intercavernous sinus with its isolation is done with two horizontal incisions and bipolar coagulation, in case of craniopharyngiomas, and by coagulation in meningiomas.
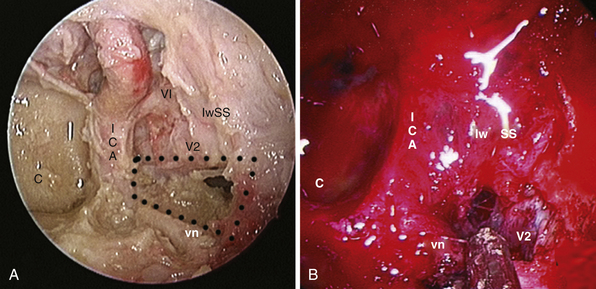
 Lateral extension of the bone window: the medial opticocarotid recess lies at the point of maximum contact between the internal carotid artery and the optic nerve and corresponds intracranially to the middle clinoid process.69 Bone removal in this critical area may be refined with a Kerrison rongeur or an ultrasonic bone curet.70
Lateral extension of the bone window: the medial opticocarotid recess lies at the point of maximum contact between the internal carotid artery and the optic nerve and corresponds intracranially to the middle clinoid process.69 Bone removal in this critical area may be refined with a Kerrison rongeur or an ultrasonic bone curet.70
 Removal of the sphenoid planum: in order to preserve olfaction, the posterior ethmoidal artery marks the anterior limit of the exposure and the rostral margin of the nasal septum (1 to 2 cm) is left intact.
Removal of the sphenoid planum: in order to preserve olfaction, the posterior ethmoidal artery marks the anterior limit of the exposure and the rostral margin of the nasal septum (1 to 2 cm) is left intact.
Once the dura has been opened, the following factors are evaluated:
In many cases, mainly cystic retrosellar lesions, removal of the whole sellar floor, flush to the clivus, provides a certain degree of pituitary mobility and the parapituitary stalk corridor could be enough to expose the prepontine cistern. When dealing with solid intrasellar lesions, a transpituitary route or additional pituitary mobilization is considered (Fig. E44.4). The pituitary gland can be mobilized on one side or reflected upward. Kassam has described an en bloc transposition.69 In order to reduce stretching of the infundibulum and the length of this step, a selective dislocation upward of the central part of the gland, through a V-shaped cut of the gland, may be considered.
Tumor removal is tailored to the lesion, following the microsurgical principles.71–74
In suprasellar supradiaphragmatic craniopharyngiomas (Fig. E44.5) the surgical strategy is guided by their relationship with the infundibulum, as has been proposed by Kassam and associates.74–76 In prechiasmatic lesions the tumor is seen immediately after dural opening, with the optic nerve and chiasm stretched superiorly. During extracapsular dissection, the distal branches of the superior hypophyseal artery, which supply the inferior aspect of the chiasm and the pars tuberalis of the pituitary stalk, should be preserved. Retrochiasmatic craniopharyngiomas can be unlocked through intra- or parapituitary stalk routes, depending on their pattern of growth. When the lesion expands in the infundibulum, the intrapituitary stalk corridor leads toward the third ventricle chamber.
 The chiasm may lie in a prefixed position, just behind the dura, which should be opened carefully above the sella without transgressing the arachnoid.
The chiasm may lie in a prefixed position, just behind the dura, which should be opened carefully above the sella without transgressing the arachnoid.
 During tumor dissection the ventricular floor should never be violated at the level of the mammillary bodies, or even more posteriorly, to avoid brainstem injury.
During tumor dissection the ventricular floor should never be violated at the level of the mammillary bodies, or even more posteriorly, to avoid brainstem injury.
 During tumor dissection along the lateral walls, adherent gliotic or invasive finger-like tumoral tissue at the level of the hypothalamus should not be violated.
During tumor dissection along the lateral walls, adherent gliotic or invasive finger-like tumoral tissue at the level of the hypothalamus should not be violated.
Retroinfundibular craniopharyngiomas can be unlocked, preserving the pituitary stalk, using the parapituitary stalk corridors and by mobilizing the gland on one side or upward, as proposed by Kassam; the critical point of this step is to avoid stretching of the pituitary stalk and retrograde cell death in the hypothalamus. When required, the surgical window can be further enlarged by drilling the dorsum sellae and removing the posterior clinoids.69
Tuberculum sellae meningiomas can be devascularized early and completely removed, together with the dura and bone involved.64,77,78 Internal debulking can be performed with radiofrequency monopolar wire electrodes (SurgiMax; Elliquence International, Hewlett, NY) and Cavitron ultrasonic aspirator.37,61 In the extracapsular dissection the pituitary gland, the pituitary stalk, and the chiasm are identified and protected with a cottonoid, with sparing of the perforators to the visual apparatus. The optic chiasm can present a neurovascular conflict with the precommunicating segment of the anterior cerebral artery.78
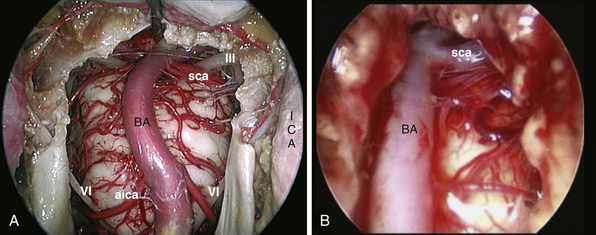
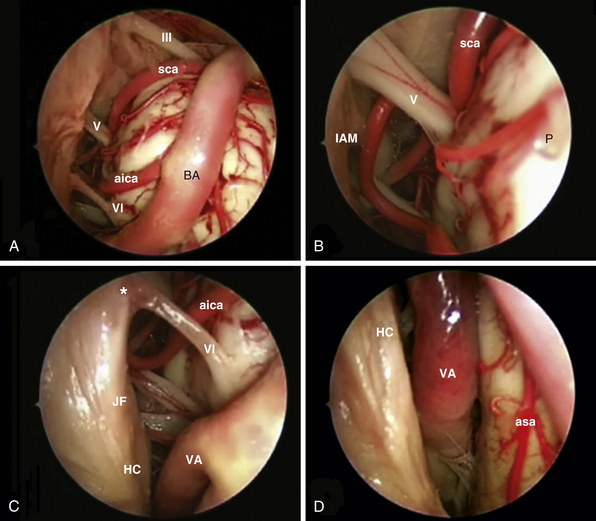
Transclival Approach
The clivus extends from the posterior clinoids to the craniovertebral junction (Fig. E44.6). Depending on the lesion extension along the cranial-caudal axis, the transclival approach is carried out across three main corridors—superior, middle, and inferior—which can be plainly combined as well.26,27,79–85 The superior corridor unlocks the retrosellar area and has been described in the previous module. The middle corridor is developed through the following steps:
 Lateral dissection of the mucosa covering the vomer: in a medial to lateral direction, the vomer-sphenoid junction, the ventral end of the palatovaginal canal, and the pterygoid canal are identified.
Lateral dissection of the mucosa covering the vomer: in a medial to lateral direction, the vomer-sphenoid junction, the ventral end of the palatovaginal canal, and the pterygoid canal are identified.
 Drilling of the sphenoid floor: the lateral limit is the pterygoid canal, which points toward the anterior genu of the intrapetrous carotid artery.84
Drilling of the sphenoid floor: the lateral limit is the pterygoid canal, which points toward the anterior genu of the intrapetrous carotid artery.84
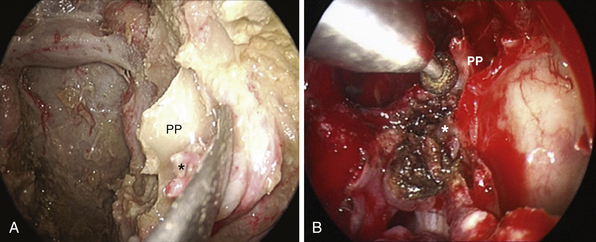
Extradural lesions, such as chordomas, have a propensity for insinuation into bone along lines of least resistance, and careful exploration at the lateral borders of the clivectomy is mandatory. At this level the abducens nerve is at risk at the level of its dural porus (Dorello’s point), which is on average situated 1 cm from the midline and 20 mm below the posterior clinoid process.86 At this level the nerve is in close relationship with the dorsal meningeal artery. Bleeding from the basilar venous plexus is managed.87
The intradural extension of chordomas and meningiomas is unlocked through a midline dura opening.81
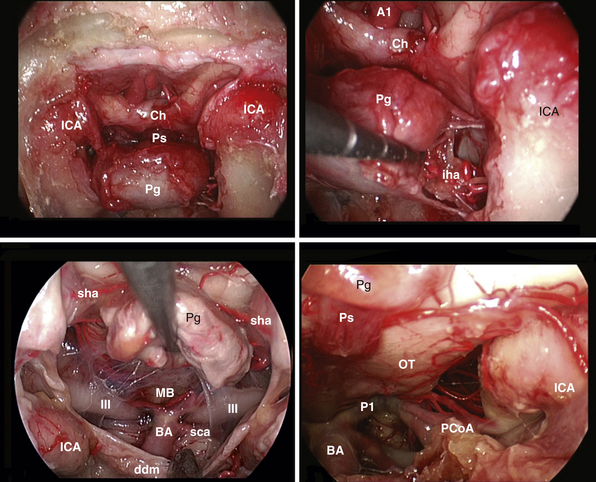
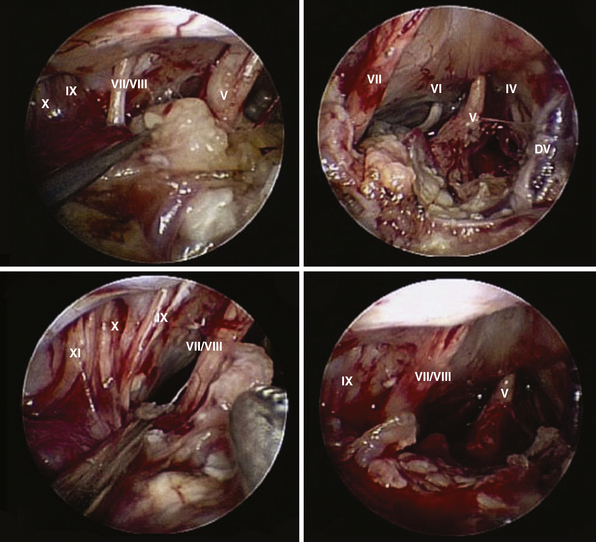
After tumor removal, intradural exploration highlights, on the midline, the pons, the medulla oblongata, and the basilar artery (Fig. E44.7). Running the endoscope above the cisternal segment of the abducens nerve, the cisternal segment of the trigeminal nerve can be followed toward its dural porus, which lies about 5 mm above and lateral to Dorello’s point. Running the endoscope below the abducens nerve, the anteroinferior cerebellar artery can be followed toward the acoustic-facial bundle and, just below it, the lower cranial nerves entering the jugular foramen, which lies about 14 mm lateral to Dorello’s point. The posteroinferior cerebellar artery or its branches can be found anterior to the lower cranial nerves.
The inferior corridor unlocks the craniovertebral junction through the following steps:
 The vomer is drilled; it should be flush with the hard palate.
The vomer is drilled; it should be flush with the hard palate.
 C-shaped incision of the mucosa of the rhinopharynx: its blood supply from the ascending and greater palatine and the ascending pharyngeal arteries should be preserved.
C-shaped incision of the mucosa of the rhinopharynx: its blood supply from the ascending and greater palatine and the ascending pharyngeal arteries should be preserved.
 Lateralization of longus colli and longus capitis muscles.
Lateralization of longus colli and longus capitis muscles.
 Identification of caudal edge of the clivus.
Identification of caudal edge of the clivus.
 Sharp disinsertion of the atlanto-occipital membrane.
Sharp disinsertion of the atlanto-occipital membrane.
 Drilling of the anterior edge of the foramen magnum.
Drilling of the anterior edge of the foramen magnum.
 Drilling of the occipital condyles: their medial one third is bounded by the hypoglossal canal. It is about 15 mm from the midline and the change from cancellous to cortical bone should be carefully appreciated during drilling. Furthermore, its opening will cause bleeding from the venous plexus surrounding the nerve.
Drilling of the occipital condyles: their medial one third is bounded by the hypoglossal canal. It is about 15 mm from the midline and the change from cancellous to cortical bone should be carefully appreciated during drilling. Furthermore, its opening will cause bleeding from the venous plexus surrounding the nerve.
 Dura opening: the tectorial membrane, attached dura, and the longitudinal part of the cruciform ligament are incised en bloc along the midline. The tectorial membrane represents the superior extension of the posterior longitudinal ligament. The basilar venous sinus should be managed when opening the dura.
Dura opening: the tectorial membrane, attached dura, and the longitudinal part of the cruciform ligament are incised en bloc along the midline. The tectorial membrane represents the superior extension of the posterior longitudinal ligament. The basilar venous sinus should be managed when opening the dura.
There are three lateral boundaries of the surgical corridor:
Transodontoid Approach
The endoscopic endonasal exposure of the craniovertebral junction and cervical spine is limited inferiorly by the projection of the nasopalatine line; it is a sagittal line tangential to the inferior edge of the nasal bones and the posterior edge of the hard palate.25,26,79,88–91 Lateral exposure is limited by the eustachian tube, which guards the parapharyngeal ICA, and by the vertebral artery (VA) and hypoglossal nerve.28
Extradural craniocervical decompression, mainly related to rheumatoid arthritis, has been performed with interesting results.28,92–95 Inherent instability of the craniovertebral junction may require posterior fusion to the occiput. Intradural extension of chordomas as well as meningiomas and even aneurysms can be treated.96,97
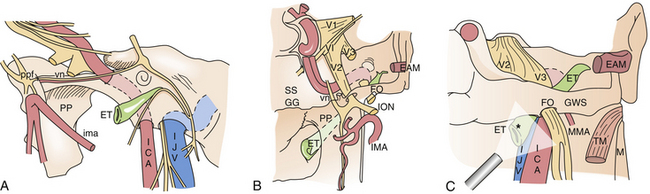
Transmaxillary Transpterygoid Approach
This approach provides direct access to lesions located or extending in the lateral compartment of the cavernous sinus, lateral recess of the sphenoid sinus, anteromedial region of Meckel’s cave, or pterygopalatine and infratemporal fossae.27,39,41,42,98–101 These compartments can represent the surgical target as well as part of the operative corridor (Fig. E44.8).
Surgical Procedure
The procedure starts with the aforesaid basic module because a wide endonasal corridor is needed to work properly and the wide anterior sphenoidotomy highlights reference landmarks, which make lateral exposure easier, such as the sphenoid floor and the paraclival segment of the ICA. The width of the nasomaxillary window and the drilling of the pterygoid process are tailored to the target area.98
The lateral recess of the sphenoid sinus is exposed through the following steps:98,102
 Retrograde uncinectomy: the free edge of the uncinate process is identified and the back-biting forceps points downward, away from the medial wall of the orbit.
Retrograde uncinectomy: the free edge of the uncinate process is identified and the back-biting forceps points downward, away from the medial wall of the orbit.
 Wide maxillary antrostomy: the nasolacrimal duct, posteriorly, and the descending palatine artery, anteriorly, must be preserved. The nasolacrimal duct is surrounded by denser bone of the lacrimal, maxillary, and inferior turbinate bones; thence if one feels increased resistance with the back-biting forceps, the bite should not be completed.
Wide maxillary antrostomy: the nasolacrimal duct, posteriorly, and the descending palatine artery, anteriorly, must be preserved. The nasolacrimal duct is surrounded by denser bone of the lacrimal, maxillary, and inferior turbinate bones; thence if one feels increased resistance with the back-biting forceps, the bite should not be completed.
 Opening of the sphenopalatine foramen.
Opening of the sphenopalatine foramen.
 Isolation and bipolar coagulation of the sphenopalatine and posterior nasal arteries: the descending palatine artery can be dissected from its bony canal and preserved to nourish a palatal flap.103
Isolation and bipolar coagulation of the sphenopalatine and posterior nasal arteries: the descending palatine artery can be dissected from its bony canal and preserved to nourish a palatal flap.103
 Identification of the ventral exit of the pterygoid canal: it is located just posterior to the sphenopalatine foramen, at the cross point between the sphenoid floor and the medial pterygoid process, which constitutes the lateral wall of the choana.84 This canal lies about 7 mm lateral to the vomerosphenoid junction, just posterior to the sphenopalatine foramen, at the junction between the sphenoid floor and the medial plate of the pterygoid process, which is the lateral wall of the choana.
Identification of the ventral exit of the pterygoid canal: it is located just posterior to the sphenopalatine foramen, at the cross point between the sphenoid floor and the medial pterygoid process, which constitutes the lateral wall of the choana.84 This canal lies about 7 mm lateral to the vomerosphenoid junction, just posterior to the sphenopalatine foramen, at the junction between the sphenoid floor and the medial plate of the pterygoid process, which is the lateral wall of the choana.
 Drilling of the sphenoid floor.
Drilling of the sphenoid floor.
 Removal of the posterior wall of the maxillary sinus up to the infraorbital nerve.
Removal of the posterior wall of the maxillary sinus up to the infraorbital nerve.
 Identification and bipolar coagulation of the terminal vessels of the maxillary artery.
Identification and bipolar coagulation of the terminal vessels of the maxillary artery.
 Identification of the base of the pterygoid process (Fig. E44.9).
Identification of the base of the pterygoid process (Fig. E44.9).
 Identification of the foramen rotundum.
Identification of the foramen rotundum.
 Drilling of the medial pterygoid plate: it should be performed in a caudal-to-rostral direction and perpendicular to the clivus,39,41 up to the vidian canal, which is drilled in its inferomedial aspect while the vidian artery is highlighted and can be followed toward its origin from the anterior genu of the ICA.27,84
Drilling of the medial pterygoid plate: it should be performed in a caudal-to-rostral direction and perpendicular to the clivus,39,41 up to the vidian canal, which is drilled in its inferomedial aspect while the vidian artery is highlighted and can be followed toward its origin from the anterior genu of the ICA.27,84
Meningoencephaloceles and CSF leaks can be reconstructed through this wide exposure.98,101 Soft tissue lesions extended in the lateral compartment of the cavernous sinus, such as adenomas and chordomas, can be removed using atraumatic angled suction cannulas and curets through a quadrangular area bounded superiorly by V2, inferiorly by the vidian nerve, and posteriorly by the parasellar and paraclival carotid artery13,36,40,99,104,105 (Fig. E44.10). Maintaining the dura opening and dissection below the level of V2 avoids damaging the abducens nerve running toward the superior orbital fissure.106 Furthermore, the inferolateral trunk and its branches to the cranial nerves lie in a deeper plane, which is preserved through the endonasal route.107 Meckel’s cave can be reached through this route, thus providing another strategy to deal with schwannomas extending in this area27,44,106 or to follow perineural tumor spreading when dealing with the adenoidocystic carcinoma or other sinonasal malignant tumors.
The petrous apex is limited by the internal carotid artery and the bony labyrinth anteriorly, Dorello’s point (dural porus of the abducent nerve) and the posterior cranial fossa posteriorly, Meckel’s cave and the middle cranial fossa superiorly, and the jugular bulb and inferior petrosal sinus inferiorly. It is situated deep to the vertical and horizontal internal carotid artery and can be approached at the level of the clival recess between the midclival bone and the paraclival segment of the ICA.
Extradural lesions of the medial petrous apex can expand into the sphenoid sinus, thus creating by themselves a surgical window between the dura and the carotid artery at the level of the clival recess; in such cases, after evacuation, the tumor bed can be marsupialized in the sphenoid sinus.108 If the lesion does not remodel the petrous apex, the endonasal exposure requires isolation and lateralization of the internal carotid artery. Carotid artery manipulation may be associated with postoperative unilateral rhinorrhea due to dissection of the sympathetic supply to the nose, which may be misinterpreted as a CSF leak.
 Fat dissection must proceed on a superficial plane in a lateral to medial direction, thus highlighting the blood vessels, which lie ventral to the nervous structures. Distal branches of the internal maxillary artery are often encountered before the main artery is identified and coagulated, namely, the descending palatine artery. Usually the posterior superior alveolar artery and infraorbital artery run proximally to the descending palatine, pharyngeal, vidian, and sphenopalatine arteries.
Fat dissection must proceed on a superficial plane in a lateral to medial direction, thus highlighting the blood vessels, which lie ventral to the nervous structures. Distal branches of the internal maxillary artery are often encountered before the main artery is identified and coagulated, namely, the descending palatine artery. Usually the posterior superior alveolar artery and infraorbital artery run proximally to the descending palatine, pharyngeal, vidian, and sphenopalatine arteries.
 The neural structures lie deep to the vascular structures. The maxillary nerve can be easily identified at the infraorbital incisure, which borders the limit between the pterygopalatine and infratemporal fossa, pointing toward the foramen rotundum.
The neural structures lie deep to the vascular structures. The maxillary nerve can be easily identified at the infraorbital incisure, which borders the limit between the pterygopalatine and infratemporal fossa, pointing toward the foramen rotundum.
Access to the infratemporal fossa is gained by enlarging the nasomaxillary window: the medial and posterolateral walls of the antrum are removed;27,44,45,109 lateral exposure may be further increased by removing the piriform aperture, and inferior extension can be obtained by removing the posterior one half of the inferior turbinate; depending on tumor extension, the pterygoid plates, the torus tubarius, and the middle one third of the eustachian tube can be removed. When dealing with sinonasal malignancies the maxillary, mandibular, and vidian nerves must be inspected for perineural tumor spreading and sacrified when necessary.
The eustachian tube is the key landmark to follow lesions extending toward the jugular foramen; the clinical application of this module must be reserved for highly experienced skull base surgeons.110 Proficiency with the three-dimensional relationship between the eustachian tube and the internal carotid is mandatory for control and injury avoidance. The eustachian tube is about 3 to 4 cm long (adult) and has an S-shaped configuration; its bony portion, which makes up about one third of the tube, is lateral to the carotid canal and superior to the jugular fossa. Its cartilaginous part is attached to the skull base at the level of the sulcus tubae, posteromedial to the foramen ovale and spinosum, and anterolateral to the foramen lacerum and the carotid canal.
Reconstruction
After tumor removal and careful hemostasis, the reconstruction of the osteodural defect is tailored to the entity of CSF leakage.103,111–114 The latter is related to the degree of subarachnoid dissection.
The reconstruction technique comprises the following main steps:111,115
 The intradural deadspace is filled with a thin layer of fibrin glue (Tisseel, Baxter, Vienna, Austria) to create a first barrier to CSF leakage.116,117
The intradural deadspace is filled with a thin layer of fibrin glue (Tisseel, Baxter, Vienna, Austria) to create a first barrier to CSF leakage.116,117
 The osteodural defect is reconstructed extradurally with a foil of collagen-derived dural substitute, which exceeds the bone defect, held in place with a fragment of bone substitute (Lactosorb, Walter Lorenz Surgical, Inc., Florida), which fits the bone opening.111,115
The osteodural defect is reconstructed extradurally with a foil of collagen-derived dural substitute, which exceeds the bone defect, held in place with a fragment of bone substitute (Lactosorb, Walter Lorenz Surgical, Inc., Florida), which fits the bone opening.111,115
 The whole skull base defect is then covered with a vascularized nasoseptal flap.
The whole skull base defect is then covered with a vascularized nasoseptal flap.
The vascularized flap is designed preoperatively and obtained during the basic module of the approach through the following steps:112,113,118,119
 Sagittal incision is made at the junction between the floor of the nasal cavity and the nasal septum (anterior to the soft palate, which can be palpated with the instrument).
Sagittal incision is made at the junction between the floor of the nasal cavity and the nasal septum (anterior to the soft palate, which can be palpated with the instrument).
 Mucosal incision is made over the sphenoid rostrum, just below the level of the sphenoidal rostrum, in a lateral to medial direction.
Mucosal incision is made over the sphenoid rostrum, just below the level of the sphenoidal rostrum, in a lateral to medial direction.
 Sagittal septal incision is made about 1.5 cm below the roof of the nasal cavity and this incision is connected with the first sagittal incision.
Sagittal septal incision is made about 1.5 cm below the roof of the nasal cavity and this incision is connected with the first sagittal incision.
 Anterior vertical septal incision is made to join the two sagittal incisions (posterior to the projection of the most anterior aspect of the inferior turbinate over the septum).
Anterior vertical septal incision is made to join the two sagittal incisions (posterior to the projection of the most anterior aspect of the inferior turbinate over the septum).
 Dissection of the flap from the nasal septum with a sharp instrument is performed in an anterior to posterior direction.
Dissection of the flap from the nasal septum with a sharp instrument is performed in an anterior to posterior direction.
 The flap is kept in the rhinopharynx or in the homolateral maxillary sinus.
The flap is kept in the rhinopharynx or in the homolateral maxillary sinus.
During positioning of the flap, stretching of the vascular pedicle should be avoided; the perichondral-periosteal surface should be over the nude bone. A thin layer of fibrin glue is put only over the borders of the flap. The reconstructive surface can be widened, applying free mucoperichondrium from the middle turbinate or other pedicled flaps.103,120–122
Current data support the value of the vascularized flap in reducing the CSF leakage rate;75 therefore, an optimal reconstruction of the skull base should address the main factors having a negative impact on flap healing.123
In case of postoperative CSF leak, a bedside endoscopic examination is performed and, after confirmation of the correct positioning of the reconstruction material, the point of leakage is identified and fibrin glue is gently injected into the path of the leak. In our experience the thrombin solution can be diluted (1:1) with sterile saline in order to strengthen its adhesive property. Repeated applications are usually required.116 In case of failure, during surgical redo, different strategies are used, depending on the degree of integration of the autologous materials such as the nasoseptal flap and the turbinal mucoperichondrium. In patients without a history of previous irradiation, the autologous tissues are usually partially integrated; in such cases the site of leakage is identified, scarified, and closed. When complete removal of the previous reconstruction is required, the flap, if still vital, can be used again and other materials such as abdominal fat are used to reinforce and to buttress the reconstruction; in such cases a lumbar drainage tube is positioned for 5 days.
Complication Avoidance
The critical points to prevent complications are as follows:
 Accurate patient selection depending on the lesion characteristics and surgeon’s experience
Accurate patient selection depending on the lesion characteristics and surgeon’s experience
 Training in both microsurgical and endoscopic techniques
Training in both microsurgical and endoscopic techniques
 Knowledge of the endoscopic equipment and dedicated instruments
Knowledge of the endoscopic equipment and dedicated instruments
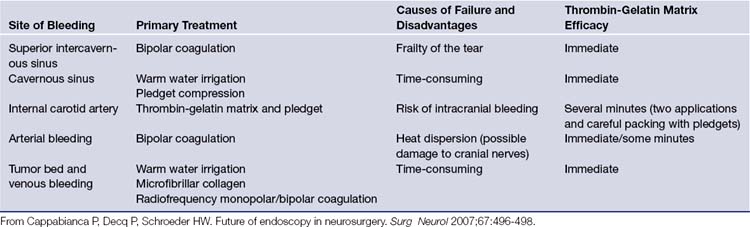
 Hemostatic materials and techniques124–127 (Table E44.2) and mastering of emergency procedures to manage severe postoperative complications such as orbital hematoma (lateral canthotomy plus inferior cantholysis and endoscopic medial orbital decompression)
Hemostatic materials and techniques124–127 (Table E44.2) and mastering of emergency procedures to manage severe postoperative complications such as orbital hematoma (lateral canthotomy plus inferior cantholysis and endoscopic medial orbital decompression)
 Knowledge of reconstruction materials and techniques
Knowledge of reconstruction materials and techniques
 Prevention of infection through prophylactic perioperative antibiotics, frequent irrigation of the surgical fields, and optimal reconstruction
Prevention of infection through prophylactic perioperative antibiotics, frequent irrigation of the surgical fields, and optimal reconstruction
Transcranial Endoscopic Approaches
The concept of “keyhole surgery” was first introduced by Yasargil128 and then developed by Perneczky, who overcame the limits of the microscopic visualization by introducing the endoscope as a further visualizing tool during microsurgical procedures and reported early encouraging results of endoscope-assisted approaches to intracranial lesions.129–131 The endoscope, introduced through limited surgical corridors, enhances visualization of structures that otherwise would be hidden by the operating microscope (endoscope-assisted technique), or it can be used as the sole visualizing tool during the whole procedure (endoscope-controlled technique). Different techniques of image fusion between endoscope and microscope have been proposed over the years;132 however, the two devices tend to exclude each other.
Endoscopic Supraorbital Approach
 Anterior skull base meningioma extending beyond the midpoint of the orbit130,131,134,135
Anterior skull base meningioma extending beyond the midpoint of the orbit130,131,134,135
 Tuberculum sellae or diaphragma sellae meningioma or craniopharyngioma extending beyond to the supraclinoidal internal carotid artery
Tuberculum sellae or diaphragma sellae meningioma or craniopharyngioma extending beyond to the supraclinoidal internal carotid artery
Surgical Technique
 Elevated about 15 degrees above the thorax, to improve venous drainage
Elevated about 15 degrees above the thorax, to improve venous drainage
 Hyperextended about 15 degrees to improve gravity-related slumping of the frontal lobe
Hyperextended about 15 degrees to improve gravity-related slumping of the frontal lobe
The technique then proceeds along the following steps:
 The eyebrow is not shaved but is carefully disinfected.
The eyebrow is not shaved but is carefully disinfected.
 A small cross-hatch is made across the skin incision for realignment of the edges at the time of closure.
A small cross-hatch is made across the skin incision for realignment of the edges at the time of closure.
 The scalpel runs parallel to the hair follicles, which are directed upward obliquely, to prevent their damage. Skin incision is made between the supraorbital incisure and the lateral end of the eyebrow.
The scalpel runs parallel to the hair follicles, which are directed upward obliquely, to prevent their damage. Skin incision is made between the supraorbital incisure and the lateral end of the eyebrow.
 Fibers of the frontalis and the orbicularis oculi muscles are longitudinally divided just above the orbital rim.
Fibers of the frontalis and the orbicularis oculi muscles are longitudinally divided just above the orbital rim.
 Subperiosteal dissection of the frontal muscle is performed upward.
Subperiosteal dissection of the frontal muscle is performed upward.
 Subperiosteal disinsertion of the temporal muscle.
Subperiosteal disinsertion of the temporal muscle.
 Identification of the keyhole, the hole made posterior to the lateral rim of the orbit.
Identification of the keyhole, the hole made posterior to the lateral rim of the orbit.
 Lateral subperiosteal dissection of the temporal muscle should be limited to about 1.5 to 3 cm behind the keyhole, and the galeal-periosteal layer can be cut to increase bone exposure.
Lateral subperiosteal dissection of the temporal muscle should be limited to about 1.5 to 3 cm behind the keyhole, and the galeal-periosteal layer can be cut to increase bone exposure.
 Positioning of the the frontobasal bur hole.
Positioning of the the frontobasal bur hole.
 Craniotomy (about 1.5 × 3 cm) is performed.
Craniotomy (about 1.5 × 3 cm) is performed.
 The inner bone edge above the orbital rim is drilled while the frontopolar dura is protected, not retracted, with a spatula.
The inner bone edge above the orbital rim is drilled while the frontopolar dura is protected, not retracted, with a spatula.
 The dura is opened in a C-shaped fashion, reflected over the orbital rim, and covered with a wet cotton pledget.
The dura is opened in a C-shaped fashion, reflected over the orbital rim, and covered with a wet cotton pledget.
 Opening of the chiasmatic, carotid, and depending on the lesion extension, sylvian fissure to release CSF.
Opening of the chiasmatic, carotid, and depending on the lesion extension, sylvian fissure to release CSF.
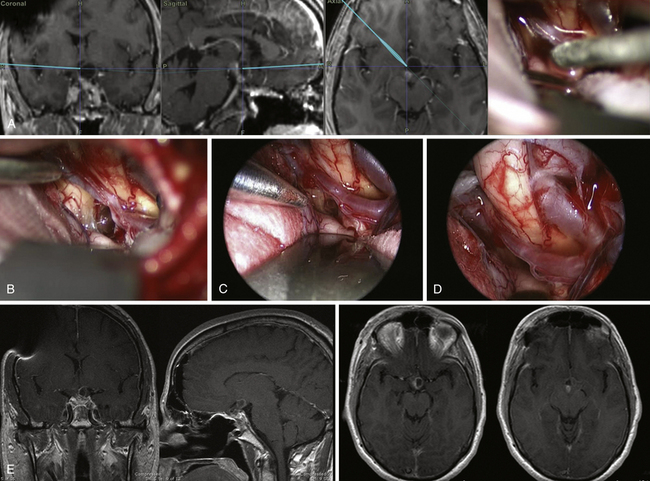
In patients with tuberculum sellae and diaphragma sellae meningiomas (Fig. E44.11) close inspection of the optic nerve when entering the canal is mandatory; optic canal decompression or intradural drilling of the anterior clinoid process can be performed with an ultrasonic bone curet.136
When dealing with craniopharyngiomas extending beyond the supraclinoidal ICA, the well-known multiple corridors can be used to approach the lesion137 (Fig. E44.12).
After tumor removal, the dura is closed in watertight fashion and the frontal sinus is properly managed, if opened; the bone flap should be carefully approximated medially while the bur hole can be covered with the titanium plates. Closure is accomplished in three layers: periosteal-galeal layer, subcutaneous tissue, and skin.
Complication Avoidance
Watertight dura closure is mandatory; however, the dura may retract and a patch may be required; special attention has to be paid when the frontal sinus is opened. During skin incision the periosteal-galeal layer can be preserved and used as a vascularized flap.133 Extension of the skin incision beyond the lateral corner of the eyebrow may be associated with transient facial weakness and should be avoided. Targeted temporary traction of the muscles overcomes this problem.
During the intradural step, the closer the approach comes to the midline, the higher is the risk of damaging the olfactory nerve during lifting of the frontal lobe. Anosmia has also been reported in traditional microsurgical approaches and early endoscopic identification of the olfactory nerve allows early dissection, or a more reasonable step may be reinforcement by injecting a film of fibrin glue along its course.
Endoscopic Retrosigmoid Approach
The microsurgical retrosigmoid approach is a well-established and highly effective technique for both lesion removal and microvascular decompression. During surgery for vestibular schwannomas, the need to improve exposure of the lateral internal acoustic canal has driven the introduction of the endoscope-assisted technique;138,139 the endoscopic visualization has been advantageous in the following cases:
 During removal of vestibular schwannomas, to assist the removal of the intrameatal lesion extension and identify open mastoid air cells at the level of the petrous bone
During removal of vestibular schwannomas, to assist the removal of the intrameatal lesion extension and identify open mastoid air cells at the level of the petrous bone
 During microvascular decompression of the trigeminal nerve, to prevent displacement of the offending vessel during cerebellar retraction and to inspect the sensory root at the level of Meckel’s cave140
During microvascular decompression of the trigeminal nerve, to prevent displacement of the offending vessel during cerebellar retraction and to inspect the sensory root at the level of Meckel’s cave140
 During microvascular decompression of the cranial nerve VII/VIII bundle, to inspect the medial face of the root entry zone138,141
During microvascular decompression of the cranial nerve VII/VIII bundle, to inspect the medial face of the root entry zone138,141
Recently, the pure endoscopic technique has been applied to well-selected avascular lesions such as epidermoids142 and arachnoid cysts and during microvascular decompression procedures.
Surgical Technique
 The ipsilateral shoulder of the patient is elevated with a pillow and should not be in the way of the surgeon’s dominant hand.
The ipsilateral shoulder of the patient is elevated with a pillow and should not be in the way of the surgeon’s dominant hand.
 The patient is secured to the table with adhesive tape, in order to further rotate the operative field away from the surgeon when dealing with medial lesion extension.
The patient is secured to the table with adhesive tape, in order to further rotate the operative field away from the surgeon when dealing with medial lesion extension.
The main steps of the procedure are as follows:
 Lateral suboccipital craniotomy and exposure of the borders of the transverse and sigmoid sinus
Lateral suboccipital craniotomy and exposure of the borders of the transverse and sigmoid sinus
 Introduction of the endoscope between the lateral surface of the cerebellar hemisphere and the petrous bone
Introduction of the endoscope between the lateral surface of the cerebellar hemisphere and the petrous bone
After arachnoid dissection and CSF drainage, the endoscope highlights the acoustic facial bundle as the first surgical landmark.143 The whole cerebellopontine angle can be unlocked through the following specific working corridors, above and below the acoustic-facial nerve bundle:
 The superior corridor is bordered by the tentorium and the trochlear nerve superiorly and by the acoustic-facial bundle, inferiorly. The superior petrosal vein (Dandy’s vein) comes into view and can usually be preserved, aside from its configuration. Running the endoscope along this corridor, the trigeminal nerve is highlighted from the entry point to its dural porus. Running the endoscope between the tentorium and the trigeminal nerve allows a closeup view of the interpeduncular cistern, trochlear nerve, and superior cerebellar artery. Running the endoscope below the trigeminal nerve, the anteroinferior cerebellar artery, the sixth cranial nerve, and its dural porus (Dorello’s point) are visualized.
The superior corridor is bordered by the tentorium and the trochlear nerve superiorly and by the acoustic-facial bundle, inferiorly. The superior petrosal vein (Dandy’s vein) comes into view and can usually be preserved, aside from its configuration. Running the endoscope along this corridor, the trigeminal nerve is highlighted from the entry point to its dural porus. Running the endoscope between the tentorium and the trigeminal nerve allows a closeup view of the interpeduncular cistern, trochlear nerve, and superior cerebellar artery. Running the endoscope below the trigeminal nerve, the anteroinferior cerebellar artery, the sixth cranial nerve, and its dural porus (Dorello’s point) are visualized.
 The inferior corridor is bordered by the acoustic-facial bundle superiorly and by the hypoglossal nerve inferiorly. The lower cranial nerves entering the jugular foramen and the posteroinferior cerebellar artery are highlighted. The latter can be followed toward its origin from the vertebral artery.
The inferior corridor is bordered by the acoustic-facial bundle superiorly and by the hypoglossal nerve inferiorly. The lower cranial nerves entering the jugular foramen and the posteroinferior cerebellar artery are highlighted. The latter can be followed toward its origin from the vertebral artery.
The endoscopic technique allows optimal removal of avascular lesions extending along several corridors such as epidermoids142 (Fig. E44.13) and several reports concerning endoscope-assisted microvascular decompression underline the ability of the endoscope to highlight nerve conflicts hidden with the microsurgical view. Furthermore, the endoscope may ease the treatment of complex neurovascular conflicts when transposition of the vessel is required.
Complication Avoidance
Concerning postoperative CSF leakage, the current rate for microsurgical techniques is 2% to 2.7%. Although reported endoscopic surgical series addressing this aspect are still inadequate,144,145 it seems evident that the endoscopic dynamic closeup view increases the opportunity to identify opened cells at the level of the meatus and of the mastoid.
In the retrosigmoid approach the critical points for avoiding leaks are as follows:
Discussion
Future Developments
Research in endoscopic surgery is expanding in three main directions:
 Anatomical studies, which are the first step to evaluate the feasibility of a new approach and to learn new surgical skills
Anatomical studies, which are the first step to evaluate the feasibility of a new approach and to learn new surgical skills
 Expansion of technology and indications (better optics, dedicated instruments, integration with computerized navigation, and robotic assistance)
Expansion of technology and indications (better optics, dedicated instruments, integration with computerized navigation, and robotic assistance)
 Outcome analysis in terms of morbidity, long-term disease control, patient satisfaction, and costs
Outcome analysis in terms of morbidity, long-term disease control, patient satisfaction, and costs
Further development of the endoscopic techniques will be driven by several technological advances such as high-definition intraoperative digital imaging, three-dimensional endoscopy, chip-stick technology, integrated operating room, dedicated instruments, virtual reality systems,146 and nanotechnology.147,148 Modern integrated operating rooms provide advanced capabilities in telemedicine and live telesurgery, which will further speed the use of the endoscopic techniques. Three-dimensional endoscopy149,150 provides a sense of depth similar to that of the microscope that most neurosurgeons are familiar with. Virtual imaging will flank cadaver dissection in training laboratories and one day might guide a robot-like system in an up-to-date virtual space.16,47 Furthermore, new instruments may use nanosensors for in vivo real-time histopathological examination of lesions and may permit implantation of antineoplastic agents tailored to the patient’s tumor. International congresses on endoscopy feature a chance for interactive dialogue among surgeons, researchers, and health care leaders, providing a base for active cooperation and further developments.147
Training
 Cadaveric dissection to acquire specific anatomical knowledge, endoscopic skill, and proficiency
Cadaveric dissection to acquire specific anatomical knowledge, endoscopic skill, and proficiency
 Understanding of the basic optical and mechanical construction of the endoscopic equipment and common types of mechanical and electrical failures
Understanding of the basic optical and mechanical construction of the endoscopic equipment and common types of mechanical and electrical failures
 Competency in the assembly and dismantling of the instruments
Competency in the assembly and dismantling of the instruments
Concerning surgical experience, a gradual learning experience is mandatory to minimize complications. Kassam’s group developed a five-level training program151 to underline that surgeons should be adequately trained in basic procedures such as standard endoscopic approaches to pituitary adenomas and CSF leak reconstruction; furthermore, they should have adequate surgical volume and resources before undertaking extended approaches to the midline. In the same way, pure endoscopic transcranial approaches require specific training and careful patient selection.
Conclusion
Cushing’s early attempts to remove intra- and suprasellar lesions through the transsphenoidal route were limited by the low illumination of the surgical field.152 The advent of the binocular operating microscope provided a new standard of vision and allowed the reinassance of the transsphenoidal technique. Hardy refined the transsphenoidal approach to sellar, suprasellar, and even clival pathology and, in 1971, declared the transsphenoidal approach to be the standard of care for pituitary adenomas.57
In 1980 Edward Laws67 demonstrated the feasibility of using the transsphenoidal approach for craniopharyngiomas, and in 1977 Apuzzo31 described the successful resection of sellar tumors with suprasellar extensions using an endoscope-assisted technique. The ENT surgeons prepared the way for “pure” endoscopic endonasal approaches through the evolution of FESS. Amin Kassam guided a systematic evolution of endoscopic skull base surgery through tireless teamwork and a special dedication to skull base anatomy and to the microvascular correspondence between feeders and relevant structures and function. Technical evolution of the endoscopic equipment, dedicated instruments, and related materials favored the optimization of the endoscopic technique. Leading neurosurgical groups reported encouraging results in the endoscopic endonasal approach to tuberculum sellae meningiomas and chordomas. The value of this strategy when dealing with craniopharyngiomas is borne out by papers highlighting the technical nuances and by several outcome studies. A 360-degree exposure of the skull base can be obtained through the transcranial routes in a “pure endoscopic” or endoscope-assisted modality, depending on the case-specific situation and the team’s experience.
1. Kennedy D.W. Functional endoscopic sinus surgery. Technique Arch Otolaryngol. 1985;111:643-649.
2. Messerklinger W. Nasal endoscopy: demonstration, localization and differential diagnosis of nasal liquorrhea. HNO. 1972;20:268-270.
3. Messerklinger W. Diagnosis and endoscopic surgery of the nose and its adjoining structures. Acta Otorhinolaryngol Belg. 1980;34:170-176.
4. Stammberger H. Endoscopic endonasal surgery—concepts in treatment of recurring rhinosinusitis. Part II. Surgical technique. Otolaryngol Head Neck Surg. 1986;94:147-156.
5. Stammberger H., Posawetz W. Functional endoscopic sinus surgery. Concept, indications and results of the Messerklinger technique. Eur Arch Otorhinolaryngol. 1990;247:63-76.
6. Cappabianca P., de Divitiis O., Maiuri F. Evolution of transsphenoidal surgery. In: de Divitiis E., Cappabianca P., editors. Endoscopic Endonasal Transsphenoidal Surgery. New York: Springer; 2003:1-7.
7. Jankowski R., Auque J., Simon C., et al. Endoscopic pituitary tumor surgery. Laryngoscope. 1992;102:198-202.
8. Carrau R.L., Jho H.D., Ko Y. Transnasal-transsphenoidal endoscopic surgery of the pituitary gland. Laryngoscope. 1996;106:914-918.
9. Jho H.D., Carrau R.L. Endoscopy assisted transsphenoidal surgery for pituitary adenoma. Technical note. Acta Neurochir (Wien). 1996;138:1416-1425.
10. Jho H.D., Carrau R.L., Ko Y. Endoscopic pituitary surgery. In: Wilkins H., Rengachary S., editors. Neurosurgical Operative Atlas. Park Ridge, IL: American Association of Neurological Surgeons; 1996:1-12.
11. Cappabianca P., Alfieri A., Colao A., et al. Endoscopic endonasal transsphenoidal approach: an additional reason in support of surgery in the management of pituitary lesions. Skull Base Surg. 1999;9:109-117.
12. Cappabianca P., Alfieri A., de Divitiis E. Endoscopic endonasal transsphenoidal approach to the sella: towards functional endoscopic pituitary surgery (FEPS). Minim Invasive Neurosurg. 1998;41:66-73.
13. Frank G., Pasquini E. Endoscopic endonasal approaches to the cavernous sinus: surgical approaches. Neurosurgery. 2002;50:675.
14. Kassam A., Snyderman C.H., Mintz A., et al. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus. 2005;19:E3.
15. Cappabianca P., Alfieri A., Thermes S., et al. Instruments for endoscopic endonasal transsphenoidal surgery. Neurosurgery. 1999;45:392-395. discussion 395-396
16. Cinalli G., Cappabianca P., de Falco R., et al. Current state and future development of intracranial neuroendoscopic surgery. Expert Rev Med Devices. 2005;2:351-373.
17. Gardner P., Kassam A., Snyderman C., et al. Endoscopic endonasal suturing of dural reconstruction grafts: a novel application of the U-Clip technology. Technical note. J Neurosurg. 2008;108:395-400.
18. Prevedello D.M., Kassam A.B., Gardner P., et al. “Q-tip” retractor in endoscopic cranial base surgery. Neurosurgery. 2010;66:363-366. discussion 366-367
19. Anand V.K., Schwartz T.H., Hiltzik D.H., Kacker A. Endoscopic transsphenoidal pituitary surgery with real-time intraoperative magnetic resonance imaging. Am J Rhinol. 2006;20:401-405.
20. Asthagiri A.R., Laws E.R.Jr., Jane J.A.Jr. Image guidance in pituitary surgery. Front Horm Res. 2006;34:46-63.
21. Jagannathan J., Prevedello D.M., Ayer V.S., et al. Computer-assisted frameless stereotaxy in transsphenoidal surgery at a single institution: review of 176 cases. Neurosurg Focus. 2006;20:E9.
22. Lasio G., Ferroli P., Felisati G., Broggi G. Image-guided endoscopic transnasal removal of recurrent pituitary adenomas. Neurosurgery. 2002;51:132-136. discussion 136-137
23. Levy M.L., Nguyen A., Aryan H., et al. Robotic virtual endoscopy: development of a multidirectional rigid endoscope. Neurosurgery. 2008;62(Suppl 2):599-606.
24. Schulze F., Buhler K., Neubauer A., et al. Intra-operative virtual endoscopy for image guided endonasal transsphenoidal pituitary surgery. Int J Comput Assist Radiol Surg. 2010;5(2):143-154.
25. Cavallo L.M., Messina A., Cappabianca P., et al. Endoscopic endonasal surgery of the midline skull base: anatomical study and clinical considerations. Neurosurg Focus. 2005;19:E2.
26. Kassam A., Snyderman C.H., Mintz A., et al. Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg Focus. 2005;19:E4.
27. Kassam A.B., Gardner P., Snyderman C., et al. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus. 2005;19:E6.
28. Kassam A.B., Snyderman C., Gardner P., et al. The expanded endonasal approach: a fully endoscopic transnasal approach and resection of the odontoid process: technical case report. Neurosurgery. 2005;57:E213. discussion E213
29. Ketcham A.S., Chretien P.B., Van Buren J.M., et al. The ethmoid sinuses: a re-evaluation of surgical resection. Am J Surg. 1973;126:469-476.
30. Snyderman C.H., Pant H., Carrau R.L., et al. What are the limits of endoscopic sinus surgery? The expanded endonasal approach to the skull base. Keio J Med. 2009;58:152-160.
31. Apuzzo M.L., Heifetz M.D., Weiss M.H., Kurze T. Neurosurgical endoscopy using the side-viewing telescope. J Neurosurg. 1977;46:398-400.
32. Kassam A.B., Engh J.A., Mintz A.H., Prevedello D.M. Completely endoscopic resection of intraparenchymal brain tumors. J Neurosurg. 2009;110:116-123.
33. Aydin S., Cavallo L.M., Messina A., et al. The endoscopic endonasal trans-sphenoidal approach to the sellar and suprasellar area. Anatomic study. J Neurosurg Sci. 2007;51:129-138.
34. Catapano D., Sloffer C.A., Frank G., et al. Comparison between the microscope and endoscope in the direct endonasal extended transsphenoidal approach: anatomical study. J Neurosurg. 2006;104:419-425.
35. Cavallo L.M., Dal Fabbro M., Jalalod’din H., et al. Endoscopic endonasal transsphenoidal surgery. Before scrubbing in: tips and tricks. Surg Neurol. 2007;67:342-347.
36. Doglietto F., Lauretti L., Frank G., et al. Microscopic and endoscopic extracranial approaches to the cavernous sinus: anatomic study. Neurosurgery. 2009;64:413-421.
37. Cappabianca P., Cavallo L.M., Esposito F., et al. Extended endoscopic endonasal approach to the midline skull base: the evolving role of transsphenoidal surgery. Adv Tech Stand Neurosurg. 2008;33:151-199.
38. Prevedello D.M., Kassam A.B., Snyderman C., et al. Endoscopic cranial base surgery: ready for prime time? Clin Neurosurg. 2007;54:48-57.
39. Cavallo L.M., Messina A., Gardner P., et al. Extended endoscopic endonasal approach to the pterygopalatine fossa: anatomical study and clinical considerations. Neurosurg Focus. 2005;19:E5.
40. Frank G., Pasquini E. Endoscopic endonasal cavernous sinus surgery, with special reference to pituitary adenomas. Front Horm Res. 2006;34:64-82.
41. Magro F., Solari D., Cavallo L.M., et al. The endoscopic endonasal approach to the lateral recess of the sphenoid sinus via the pterygopalatine fossa: comparison of endoscopic and radiological landmarks. Neurosurgery. 2006;59:ONS237-ONS242.
42. Solari D., Magro F., Cappabianca P., et al. Anatomical study of the pterygopalatine fossa using an endoscopic endonasal approach: spatial relations and distances between surgical landmarks. J Neurosurg. 2007;106:157-163.
43. Herzallah I.R., Germani R., Casiano R.R. Endoscopic transnasal study of the infratemporal fossa: a new orientation. Otolaryngol Head Neck Surg. 2009;140:861-865.
44. Hofstetter C.P., Singh A., Anand V.K., et al. The endoscopic, endonasal, transmaxillary transpterygoid approach to the pterygopalatine fossa, infratemporal fossa, petrous apex, and the Meckel cave. J Neurosurg. 2010;113(5):967-974.
45. Theodosopoulos P.V., Guthikonda B., Brescia A., et al. Endoscopic approach to the infratemporal fossa: anatomic study. Neurosurgery. 2010;66:196-202.
46. Castelnuovo P., Bignami M., Pistochini A., et al. Endoscopic endonasal management of encephaloceles in children: an eight-year experience. Int J Pediatr Otorhinolaryngol. 2009;73:1132-1136.
47. Gardner P.A., Kassam A.B., Rothfus W.E., et al. Preoperative and intraoperative imaging for endoscopic endonasal approaches to the skull base. Otolaryngol Clin North Am. 2008;41:215-230.
48. Fernandez-Miranda J.C., Prevedello D.M., Madhok R., et al. Sphenoid septations and their relationship with internal carotid arteries: anatomical and radiological study. Laryngoscope. 2009;119:1893-1896.
49. Cappabianca P., Cavallo L.M., Esposito I., Tschabitscher M. Transsphenoidal approaches: endoscopic. In: Cappabianca P., Califano L., Iaconetta G., editors. Cranial, Craniofacial and Skull Base Surgery. Milan: Springer-Verlag; 2010:197-212.
50. Brown S.M., Anand V.K., Tabaee A., Schwartz T.H. Role of perioperative antibiotics in endoscopic skull base surgery. Laryngoscope. 2007;117:1528-1532.
51. Cafiero T., Cavallo L.M., Frangiosa A., et al. Clinical comparison of remifentanil-sevoflurane vs. remifentanil-propofol for endoscopic endonasal transsphenoidal surgery. Eur J Anaesthesiol. 2007;24:441-446.
52. Cappabianca P., Cavallo L.M., de Divitiis E. Endoscopic Pituitary and Skull Base Surgery. Anatomy and Surgery of the Endoscopic Endonasal Approach. Tuttlingen: Endo Press; 2008.
53. Castelnuovo P., Pistochini A., Locatelli D. Different surgical approaches to the sellar region: focusing on the “two nostrils four hands technique.”. Rhinology. 2006;44:2-7.
54. Cappabianca P., Cavallo L.M., de Divitiis O., et al. Endoscopic pituitary surgery. Pituitary. 2008;11:385-390.
55. Oldfield E.H., Vortmeyer A.O. Development of a histological pseudocapsule and its use as a surgical capsule in the excision of pituitary tumors. J Neurosurg. 2006;104:7-19.
56. Cavallo L.M., Prevedello D., Esposito F., et al. The role of the endoscope in the transsphenoidal management of cystic lesions of the sellar region. Neurosurg Rev. 2008;31:55-64.
57. Hardy J. Transsphenoidal hypophysectomy. J Neurosurg. 1971;34:582-594.
58. Weiss M.H. The transnasal transsphenoidal approach. In: Apuzzo M.L.J., editor. Surgery of the Third Ventricle. Baltimore: Williams & Wilkins; 1987:476-494.
59. Locatelli D., Rampa F., Acchiardi I., et al. Endoscopic endonasal approaches for repair of cerebrospinal fluid leaks: nine-year experience. Neurosurgery. 2006;58:ONS246-ONS256.
60. Castelnuovo P.G., Delu G., Sberze F., et al. Esthesioneuroblastoma: endonasal endoscopic treatment. Skull Base. 2006;16:25-30.
61. de Divitiis E., Cavallo L.M., Cappabianca P., Esposito F. Extended endoscopic endonasal transsphenoidal approach for the removal of suprasellar tumors: part 2. Neurosurgery. 2007;60:46-58.
62. de Divitiis E., Esposito F., Cappabianca P., et al. Endoscopic transnasal resection of anterior cranial fossa meningiomas. Neurosurg Focus. 2008;25:E8.
63. Fernandez-Miranda J.C., Gardner P.A., Prevedello D.M., Kassam A.B. Expanded endonasal approach for olfactory groove meningioma. Acta Neurochir (Wien). 2009;151:287-288. author reply 289-290
64. Gardner P.A., Kassam A.B., Thomas A., et al. Endoscopic endonasal resection of anterior cranial base meningiomas. Neurosurgery. 2008;63:36-52.
65. de Notaris M., Esposito I., Cavallo L.M., et al. Endoscopic endonasal approach to the ethmoidal planum: anatomic study. Neurosurg Rev. 2008;31:309-317.
66. Lang J. Skull Base and Related Structures: Atlas of Clinical Anatomy. Stuttgart: Schattauer; 2001.
67. Laws E.R.Jr. Transsphenoidal microsurgery in the management of craniopharyngioma. J Neurosurg. 1980;52:661-666.
68. Doglietto F., Prevedello D.M., Jane J.A.Jr., et al. Brief history of endoscopic transsphenoidal surgery—from Philipp Bozzini to the First World Congress of Endoscopic Skull Base Surgery. Neurosurg Focus. 2005;19:E3.
69. Kassam A.B., Prevedello D.M., Thomas A., et al. Endoscopic endonasal pituitary transposition for a transdorsum sellae approach to the interpeduncular cistern. Neurosurgery. 2008;62:57-72.
70. Cappabianca P., Cavallo L.M., Esposito I., et al. Bone removal with a new ultrasonic bone curette during endoscopic endonasal approach to the sellar-suprasellar area. Technical note. Neurosurgery. 2010;66(Suppl 3):E118.
71. Cappabianca P., Cavallo L.M., Esposito F., de Divitiis E. Craniopharyngiomas. J Neurosurg. 2008;109:1-3. reply 3-5
72. Cavallo L.M., Prevedello D.M., Solari D., et al. Extended endoscopic endonasal transsphenoidal approach for residual or recurrent craniopharyngiomas. J Neurosurg. 2009;111:578-589.
73. de Divitiis E., Cappabianca P., Cavallo L.M., et al. Extended endoscopic transsphenoidal approach for extrasellar craniopharyngiomas. Neurosurgery. 2007;61:219-227. discussion 228
74. Kassam A.B., Gardner P.A., Snyderman C.H., et al. Expanded endonasal approach, a fully endoscopic transnasal approach for the resection of midline suprasellar craniopharyngiomas: a new classification based on the infundibulum. J Neurosurg. 2008;108:715-728.
75. Gardner P.A., Kassam A.B., Snyderman C.H., et al. Outcomes following endoscopic, expanded endonasal resection of suprasellar craniopharyngiomas: a case series. J Neurosurg. 2008;109:6-16.
76. Gardner P.A., Prevedello D.M., Kassam A.B., et al. The evolution of the endonasal approach for craniopharyngiomas. J Neurosurg. 2008;108:1043-1047.
77. de Divitiis E., Cavallo L.M., Esposito F., et al. Extended endoscopic transsphenoidal approach for tuberculum sellae meningiomas. Neurosurgery. 2007;61:229-237.
78. de Divitiis E., Esposito F., Cappabianca P., et al. Tuberculum sellae meningiomas: high route or low route? A series of 51 consecutive cases. Neurosurgery. 2008;62:556-563. discussion 556-563
79. Cavallo L.M., Cappabianca P., Messina A., et al. The extended endoscopic endonasal approach to the clivus and cranio-vertebral junction: anatomical study. Childs Nerv Syst. 2007;23:665-671.
80. de Notaris M., Cavallo L.M., Prats-Galino A., et al. Endoscopic endonasal transclival approach and retrosigmoid approach to the clival and petroclival regions. Neurosurgery. 2009;65:42-50. discussion 42-50
81. Dehdashti A.R., Karabatsou K., Ganna A., et al. Expanded endoscopic endonasal approach for treatment of clival chordomas: early results in 12 patients. Neurosurgery. 2008;63:299-307. discussion 299-307
82. Frank G., Sciarretta V., Calbucci F., et al. The endoscopic transnasal transsphenoidal approach for the treatment of cranial base chordomas and chondrosarcomas. Neurosurgery. 2006;59:ONS50-ONS57. discussion ONS50-ONS57
83. Fraser J.F., Nyquist G.G., Moore N., et al. Endoscopic endonasal transclival resection of chordomas: operative technique, clinical outcome, and review of the literature. J Neurosurg. 2010;112(5):1061-1069.
84. Kassam A.B., Vescan A.D., Carrau R.L., et al. Expanded endonasal approach: vidian canal as a landmark to the petrous internal carotid artery. J Neurosurg. 2008;108:177-183.
85. Stippler M., Gardner P.A., Snyderman C.H., et al. Endoscopic endonasal approach for clival chordomas. Neurosurgery. 2009;64:268-277.
86. Iaconetta G., Fusco M., Cavallo L.M., et al. The abducens nerve: microanatomic and endoscopic study. Neurosurgery. 2007;61:7-14. discussion 14
87. Cappabianca P., Esposito F., Esposito I., et al. Use of a thrombin-gelatin haemostatic matrix in endoscopic endonasal extended approaches: technical note. Acta Neurochir (Wien). 2009;151:69-77.
88. Abuzayed B., Tanriover N., Gazioglu N., et al. Extended endoscopic endonasal approach to the anterior cranio-vertebral junction: anatomic study. Turk Neurosurg. 2009;19:249-255.
89. Baird C.J., Conway J.E., Sciubba D.M., et al. Radiographic and anatomic basis of endoscopic anterior craniocervical decompression: a comparison of endonasal, transoral, and transcervical approaches. Neurosurgery. 2009;65:158-163.
90. de Almeida J.R., Zanation A.M., Snyderman C.H., et al. Defining the nasopalatine line: the limit for endonasal surgery of the spine. Laryngoscope. 2009;119:239-244.
91. Messina A., Bruno M.C., Decq P., et al. Pure endoscopic endonasal odontoidectomy: anatomical study. Neurosurg Rev. 2007;30:189-194.
92. Laufer I., Greenfield J.P., Anand V.K., et al. Endonasal endoscopic resection of the odontoid process in a nonachondroplastic dwarf with juvenile rheumatoid arthritis: feasibility of the approach and utility of the intraoperative Iso-C three-dimensional navigation. Case report. J Neurosurg Spine. 2008;8:376-380.
93. Leng L.Z., Anand V.K., Hartl R., Schwartz T.H. Endonasal endoscopic resection of an os odontoideum to decompress the cervicomedullary junction: a minimal access surgical technique. Spine. 2009;34:E139-E143.
94. Magrini S., Pasquini E., Mazzatenta D., et al. Endoscopic endonasal odontoidectomy in a patient affected by Down syndrome: technical case report. Neurosurgery. 2008;63:E373-E374.
95. Nayak J.V., Gardner P.A., Vescan A.D., et al. Experience with the expanded endonasal approach for resection of the odontoid process in rheumatoid disease. Am J Rhinol. 2007;21:601-606.
96. Eloy J.A., Carai A., Patel A.B., et al. Combined endoscope-assisted transclival clipping and endovascular stenting of a basilar trunk aneurysm: case report. Neurosurgery. 2008;62:142-143. discussion 143-144
97. Kassam A.B., Mintz A.H., Gardner P.A., et al. The expanded endonasal approach for an endoscopic transnasal clipping and aneurysmorrhaphy of a large vertebral artery aneurysm: technical case report. Neurosurgery. 2006;59:ONSE162-ONSE165.
98. Castelnuovo P., Dallan I., Pistochini A., et al. Endonasal endoscopic repair of Sternberg’s canal cerebrospinal fluid leaks. Laryngoscope. 2007;117:345-349.
99. Cavallo L.M., Cappabianca P., Galzio R., et al. Endoscopic transnasal approach to the cavernous sinus versus transcranial route: anatomic study. Neurosurgery. 2005;56:379-389.
100. Fortes F.S., Sennes L.U., Carrau R.L., et al. Endoscopic anatomy of the pterygopalatine fossa and the transpterygoid approach: development of a surgical instruction model. Laryngoscope. 2008;118:44-49.
101. Tabaee A., Anand V.K., Cappabianca P., et al. Endoscopic management of spontaneous meningoencephalocele of the lateral sphenoid sinus. J Neurosurg. 2010;112(5):1070-1077.
102. Pasquini E., Sciarretta V., Farneti G., et al. Endoscopic treatment of encephaloceles of the lateral wall of the sphenoid sinus. Minim Invasive Neurosurg. 2004;47:209-213.
103. Oliver C.L., Hackman T.G., Carrau R.L., et al. Palatal flap modifications allow pedicled reconstruction of the skull base. Laryngoscope. 2008;118:2102-2106.
104. Ceylan S., Koc K., Anik I. Endoscopic endonasal transsphenoidal approach for pituitary adenomas invading the cavernous sinus. J Neurosurg. 2010;112:99-107.
105. D’Avella E., Tschabitscher M., Santoro A., Delfini R. Blood supply to the intracavernous cranial nerves: comparison of the endoscopic and microsurgical perspectives. Neurosurgery. 2008;62:ONS305-ONS310.
106. Kassam A.B., Prevedello D.M., Carrau R.L., et al. The front door to Meckel’s cave: an anteromedial corridor via expanded endoscopic endonasal approach—technical considerations and clinical series. Neurosurgery. 2009;64:71-82.
107. Conti M., Prevedello D.M., Madhok R., et al. The antero-medial triangle: the risk for cranial nerves ischemia at the cavernous sinus lateral wall. Anatomic cadaveric study. Clin Neurol Neurosurg. 2008;110:682-686.
108. Zanation A.M., Snyderman C.H., Carrau R.L., et al. Endoscopic endonasal surgery for petrous apex lesions. Laryngoscope. 2009;119:19-25.
109. Harvey R.J., Sheehan P.O., Debnath N.I., Schlosser R.J. Transseptal approach for extended endoscopic resections of the maxilla and infratemporal fossa. Am J Rhinol Allergy. 2009;23:426-432.
110. Zimmer L.A., Hirsch B.E., Kassam A., et al. Resection of a recurrent paraganglioma via an endoscopic transnasal approach to the jugular fossa. Otol Neurotol. 2006;27:398-402.
111. Cavallo L.M., Messina A., Esposito F., et al. Skull base reconstruction in the extended endoscopic transsphenoidal approach for suprasellar lesions. J Neurosurg. 2007;107:713-720.
112. Hadad G., Bassagasteguy L., Carrau R.L., et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116:1882-1886.
113. Kassam A.B., Thomas A., Carrau R.L., et al. Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery. 2008;63:ONS44-ONS52.
114. Tabaee A., Anand V.K., Brown S.M., et al. Algorithm for reconstruction after endoscopic pituitary and skull base surgery. Laryngoscope. 2007;117:1133-1137.
115. Leng L.Z., Brown S., Anand V.K., Schwartz T.H. “Gasket-seal” watertight closure in minimal-access endoscopic cranial base surgery. Neurosurgery. 2008;62:ONSE342-ONSE343.
116. Cappabianca P., Esposito F., Magro F., et al. Natura abhorret a vacuo—use of fibrin glue as a filler and sealant in neurosurgical “dead spaces.” Technical note. Acta Neurochir (Wien). 2010;152(5):897-904.
117. Fraioli R.E., Hirsch B.E., Kassam A.B. Fibrin sealant for control of cerebrospinal fluid otorrhea. Am J Otolaryngol. 2008;29:135-137.
118. Pinheiro-Neto C.D., Prevedello D.M., Carrau R.L., et al. Improving the design of the pedicled nasoseptal flap for skull base reconstruction: a radioanatomic study. Laryngoscope. 2007;117:1560-1569.
119. Zanation A.M., Carrau R.L., Snyderman C.H., et al. Nasoseptal flap reconstruction of high flow intraoperative cerebral spinal fluid leaks during endoscopic skull base surgery. Am J Rhinol Allergy. 2009;23:518-521.
120. Fortes F.S., Carrau R.L., Snyderman C.H., et al. Transpterygoid transposition of a temporoparietal fascia flap: a new method for skull base reconstruction after endoscopic expanded endonasal approaches. Laryngoscope. 2007;117:970-976.
121. Fortes F.S., Carrau R.L., Snyderman C.H., et al. The posterior pedicle inferior turbinate flap: a new vascularized flap for skull base reconstruction. Laryngoscope. 2007;117:1329-1332.
122. Zanation A.M., Snyderman C.H., Carrau R.L., et al. Minimally invasive endoscopic pericranial flap: a new method for endonasal skull base reconstruction. Laryngoscope. 2009;119:13-18.
123. Castelnuovo P., Mauri S., Locatelli D., et al. Endoscopic repair of cerebrospinal fluid rhinorrhea: learning from our failures. Am J Rhinol. 2001;15:333-342.
124. Cappabianca P., Briganti F., Cavallo L.M., de Divitiis E. Pseudoaneurysm of the intracavernous carotid artery following endoscopic endonasal transsphenoidal surgery, treated by endovascular approach. Acta Neurochir (Wien). 2001;143:95-96.
125. Cavallo L.M., Briganti F., Cappabianca P., et al. Hemorrhagic vascular complications of endoscopic transsphenoidal surgery. Minim Invasive Neurosurg. 2004;47:145-150.
126. Kassam A., Snyderman C.H., Carrau R.L., et al. Endoneurosurgical hemostasis techniques: lessons learned from 400 cases. Neurosurg Focus. 2005;19:E7.
127. Laws E.R.Jr. Vascular complications of transsphenoidal surgery. Pituitary. 1999;2:163-170.
128. Yasargil M.G. Introduction. In: Yasargil M.G., editor. Microneurosurgery. New York: Thieme; 1984:3.
129. Fries G., Perneczky A. Endoscope-assisted brain surgery: part 2—analysis of 380 procedures. Neurosurgery. 1998;42:226-231.
130. Perneczky A., Fries G. Endoscope-assisted brain surgery: part 1—evolution, basic concept, and current technique. Neurosurgery. 1998;42:219-224.
131. Perneczky A., Muller-Forell W., van Lindert E., Fries G. Keyhole concept in neurosurgery. Stuttgart: Thieme; 1999.
132. Kassam A., Horowitz M., Welch W., et al. The role of endoscopic assisted microneurosurgery (image fusion technology) in the performance of neurosurgical procedures. Minim Invasive Neurosurg. 2005;48:191-196.
133. Knopman J., Sigounas D., Huang C., et al. Combined supraciliary and endoscopic endonasal approach for resection of frontal sinus mucoceles: technical note. Minim Invasive Neurosurg. 2009;52:149-151.
134. King W.A., Frazee J.G., Teo C., Wackym P.A. Endoscopic treatment of cranial base lesions. In: King W.A., Frazee J.G., Salles A.A.F.D., editors. Endoscopy of the Central and Peripheral Nervous System. New York: Thieme; 1998:120-136.
135. Reisch R., Perneczky A. Ten-year experience with the supraorbital subfrontal approach through an eyebrow skin incision. Neurosurgery. 2005;57:242-255.
136. Chang H.S., Joko M., Song J.S., et al. Ultrasonic bone curettage for optic canal unroofing and anterior clinoidectomy. Technical note. J Neurosurg. 2006;104:621-624.
137. Tschabitscher M., Galzio R. Central skull base anatomy as seen through the endoscope. In: Dolenc V.V., editor. Cavernous Sinus. New York: Springer; 2008:54-57.
138. Badr-El-Dine M., El-Garem H.F., Talaat A.M., Magnan J. Endoscopically assisted minimally invasive microvascular decompression of hemifacial spasm. Otol Neurotol. 2002;23:122-128.
139. Schroeder H.W., Oertel J., Gaab M.R. Endoscope-assisted microsurgical resection of epidermoid tumors of the cerebellopontine angle. J Neurosurg. 2004;101:227-232.
140. Teo C., Nakaji P., Mobbs R.J. Endoscope-assisted microvascular decompression for trigeminal neuralgia: technical case report. Neurosurgery. 2006;59:ONSE489-ONSE490.
141. Magnan J., Caces F., Locatelli P., Chays A. Hemifacial spasm: endoscopic vascular decompression. Otolaryngol Head Neck Surg. 1997;117:308-314.
142. de Divitiis O., Cavallo L.M., Dal Fabbro M., et al. Freehand dynamic endoscopic resection of an epidermoid tumor of the cerebellopontine angle: technical case report. Neurosurgery. 2007;61:E239-E240.
143. Cappabianca P., Cavallo L.M., Esposito F., et al. Endoscopic examination of the cerebellar pontine angle. Clin Neurol Neurosurg. 2002;104:387-391.
144. Magnan J., Barbieri M., Mora R., et al. Retrosigmoid approach for small and medium-sized acoustic neuromas. Otol Neurotol. 2002;23:141-145.
145. Onizuka M., Tokunaga Y., Shibayama A., Miyazaki H. Computer-assisted neurosurgical navigational system for transsphenoidal surgery—technical note. Neurol Med Chir (Tokyo). 2001;41:565-568. discussion 569
146. Tyler-Kabara E.C., Kassam A.B., Horowitz M.H., et al. Predictors of outcome in surgically managed patients with typical and atypical trigeminal neuralgia: comparison of results following microvascular decompression. J Neurosurg. 2002;96:527-531.
147. Cappabianca P., Decq P., Schroeder H.W. Future of endoscopy in neurosurgery. Surg Neurol. 2007;67:496-498.
148. Snyderman C.H., Carrau R.L., Prevedello D.M., et al. Technologic innovations in neuroendoscopic surgery. Otolaryngol Clin North Am. 2009;42:883-890.
149. Roth J., Singh A., Nyquist G., et al. Three-dimensional and 2-dimensional endoscopic exposure of midline cranial base targets using expanded endonasal and transcranial approaches. Neurosurgery. 2009;65:1116-1128. discussion 1128-1130
150. Tabaee A., Anand V.K., Fraser J.F., et al. Three-dimensional endoscopic pituitary surgery. Neurosurgery. 2009;64:288-293.
151. Snyderman C., Kassam A., Carrau R., et al. Acquisition of surgical skills for endonasal skull base surgery: a training program. Laryngoscope. 2007;117:699-705.
152. Cushing H. Intracranial Tumours. Notes Upon a Series of Two-Thousand Verified Cases with Surgical-Mortality Percentages Pertaining Thereto. Springfield, IL: Charles C Thomas; 1932.