CHAPTER 332 Mild Traumatic Brain Injury in Adults and Concussion in Sports
Definition of Concussion and Mild Traumatic Brain Injury
The terms concussion, mild traumatic brain injury, and minor traumatic brain injury are often used interchangeably, and, to date, there is no universally agreed upon definition of either. One of the more commonly used definitions for mild traumatic brain injury (mTBI) arose from a meeting of the American Congress of Rehabilitation Medicine, which characterized the injury as involving an alteration in consciousness (amnesia or confusion), less than 30 minutes loss of consciousness, or less than 24 hours of posttraumatic amnesia, with focal neurological deficits that “may or may not be transient.”1 Many other definitions of mTBI involve loss of consciousness (LOC) lasting less than 20 or 30 minutes, Glasgow Coma Scale (GCS) scores of 13 through 15, and some definitions may specify that hospitalization must not be necessary or that there are no focal neurological findings. Concussion was defined as an “immediate and transient posttraumatic impairment of neural function…because of brain stem dysfunction” by the Congress of Neurological Surgeons.2 The World Health Organization Collaborating Task Force on mTBI criteria required an acute brain injury to result from mechanical injury to the head from external physical forces and at least one of the following: LOC 30 minutes or less, focal neurological signs or seizures, posttraumatic amnesia (PTA) less than 24 hours, and/or GCS of 13 to 15 after 30 minutes.3 In 1997, the American Academy of Neurology defined concussion as “any trauma induced alteration in mental status that may or may not include a loss of consciousness.”4 The Concussion in Sport Group described the injury as “a complex pathophysiological process affecting the brain induced by traumatic biomechanical forces.”5
The Centers for Disease Control and Prevention (CDC)6 recently provided a collective and comprehensive definition for concussion and mild traumatic brain injury, using the two terms interchangeably:
Efforts have been made to classify adult mild head injury to better reflect recommended evaluative courses of action.7 In this model, all acute head injured patients are referred to as having “mild head injury,” then this group is broken into three risk groups. The “low-risk mild head injury” patients have a GCS score of 15, and no signs or symptoms of loss of consciousness, amnesia, vomiting, or diffuse headache. “Medium-risk mild head injury” patients have a GCS of 15 with one or more of the earlier described symptoms. “High-risk mild head injury” patients have a GCS of 14 or 15 and have skull fracture and/or neurological deficits. Patients with risk factors of coagulopathy, history of neurosurgical procedures, history of epilepsy, drug or alcohol consumption, or age greater than 60 are included in the high-risk group regardless of the clinical presentation. These categories are based upon the risk of intracranial hematoma requiring surgical evacuation. It is recommended that medium risk MHI patients receive a CT scan, if available, and should otherwise have a skull x-ray. All high-risk patients should have a CT scan. Table 332-1 provides descriptions of various levels of TBI severity.
| Mild TBI | GCS = 3 to 8 |
| Moderate TBI | GCS = 9 to 12 |
| Severe TBI | GCS = 13 to 15 |
Epidemiology
Estimates of mild traumatic brain injury in child and adult populations vary greatly, and have ranged from 49% to 90%8,9 of traumatic brain injury in the literature. The vast majority of patients requiring hospital admission after sustaining a head injury are those with mTBI. Studies in the 1980s cited that mTBI accounts for anywhere between 49% and 82% of traumatic brain injuries treated in hospital emergency rooms.10–14 Although many mTBI patients are seen in nonhospital settings, such as physician offices, clinics, and training rooms, ultimately about 10% will require hospitalization. The long-term morbidity and mortality should be extremely low, for few of them sustain potentially serious intracranial injury. Nonetheless, the potential for major injury is omnipresent, and an organized and consistent approach serves well to ensure proper management. It has been estimated that approximately 3% of all patients having a GCS score of 13 to 15 will ultimately need a neurosurgical procedure.
Cassidy and associates8 provide comprehensive descriptions of epidemiologic studies of mild traumatic brain injury from 1980-2000. Although estimated rates vary between studies, most studies find increased rates in males and young adults, with mixed results for race. Most common causes for mTBI across studies were motor vehicle accidents, falls, or assaults. Regarding sports- and recreation-related concussions specifically, the CDC has provided recent estimates that approximately 1.6 to 3.8 million mild traumatic brain injuries occur each year,6 and that approximately 20% of all head injuries are sports-related concussions.15 Although a daunting figure, most believe this remains an underestimate of the injury, given that many concussions go unreported. By sport, American football,16 soccer, and ice hockey are often cited as having the highest concussion rates in the United States.17,18 Per 10,000 participants, rates for concussed athletes coming to emergency departments were cited as 5.22 in basketball,19 5.2 in American football,20 4.90 in ice hockey,20 and 3.1 in soccer.20 Incidence of concussion in Australian football was 3.3 per 1000 player hours. Studies of collegiate American football players have found injury rates of 0.73,21 2.818 (high school), and 3.322,23 (college) per 1000 athlete exposures during games and practices, and one study estimated that in the United States, football players alone suffer a minimum of 1.5 concussions each year.24 Studies of soccer players found a rate of 0.6 per 1000 athlete-exposures for males and 0.4 for females in a college sample,25 and game rates of 0.57 and 0.71 per 1000 athlete exposures in high school male and female games.18 Estimates of incidence in ice hockey range from 1.5 concussions per 1000 athlete game hours in a Canadian college26 sample to 17.6 per 1000 athlete game hours in a Minnesota sample of 11 to 19 year old boys.27 Concussion rates for high school boys and girls in basketball practices were listed as 0.06 and 0.07 per 1000 exposures, respectively, and were 0.28 (boys) and 0.71 (girls) per 1000 basketball game exposures.18
In most sports, the risk of concussion is most often greater in games,16,18,21 with football players being up to 14 times more likely to sustain a concussion during a game than a contact practice.21 In cheerleading, concussions happen more often in practices than in games.16 A review of epidemiologic studies of mTBI found injury rates in sports ranging from 0.62 to 8.0 per 1000 athlete game hours, and rates of 0.1 per 1000 athlete practice or game hours for those under 15 years old and 17.1 per 100 athlete practice or game hours in a sample of senior rugby players.8
In certain sports, the position played has been linked to concussion rates. In football, linebackers, wideouts, and safeties, respectively, sustained the greatest number of concussions in one study,21 with other studies rating quarterbacks,28 running backs, and defensive secondary players being more at-risk.18,22,23 In rugby, forwards had double the risk of backs for sustaining concussions.29,30
Pathophysiology of Mild Head Injury
In a review of multiple articles from scientific literature, Giza and Hovda31 described the pathophysiology of concussion, and a more detailed description of the pathophysiology of brain injury is contained in another chapter. In brief, concussion initially results in what has been described as a “metabolic mismatch,” during which cerebral blood flow significantly decreases, yet demand for glucose increases. This is the result of a process whereby excitatory transmitters bind to N-methyl-D-aspartate (NMDA), causing depolarization of the neuron during which there is an influx of calcium and an efflux of potassium. Increased excitatory activity caused by increased extracellular K+ is then followed by diffuse depression of neuronal activity. The increased adenosine triphosphate (ATP) required by the increased activity of the sodium-potassium pump working to restore the ionic imbalance leads to increased glucose metabolism.
Although glucose metabolism initially increases postinjury, a period of hypometabolism soon follows, which has been shown to persist for up to 10 days in animal studies32 and for up to 1 month in PET studies of human TBI.33 It has been postulated the hypometabolism may be linked to the lingering postconcussion the symptoms.31 In addition, mild traumatic brain injury has been linked to decreased magnesium levels, diffuse axonal injury, persistent calcium accumulation, and alterations in neurotransmitter activity.31
Research has established that, in some instances of mTBI, both cellular and ultrastructural damage may occur. Recent experiments with radioactive tracers have shown the axonal injury may occur in a nonlethal concussive model, and that progressive axonal swelling and disturbance in axonal transport results in the hours and days following the injury.34 However, the intracellular metabolic effects of mTBI have now been more firmly recognized.
Recent autopsy studies suggest chronic traumatic encephalopathy, especially tauopathy, has been seen in case studies of modern professional football players.35,36 However, further collaborative studies will be necessary.
Signs and Symptoms of Injury
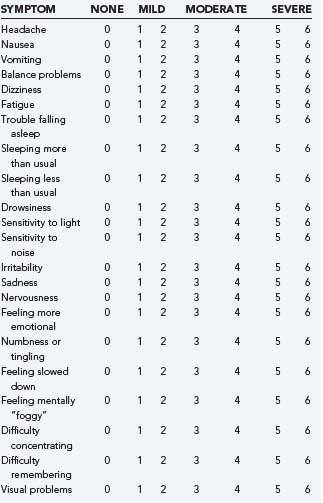
Patients with mTBI may have symptoms such as headache, nausea, vomiting, or have focal neurological deficits. Seizures or clinical signs of a skull fracture are rare, but also may occur. Concussion or mTBI may go undiagnosed or may be mismanaged in part because of the variability in the type, number, and severity of injury signs and symptoms. It is important to note that, although loss of consciousness may occur with mTBI, it often is not present, and its absence would not rule out a diagnosis of concussion. At times, patients who are experiencing postconcussion symptoms have been told they did not sustain a brain injury because they did not experience a loss of consciousness. In addition to loss of consciousness, mental status changes that may be observed following mTBI include retrograde amnesia, anterograde amnesia, disorientation, confusion, or looking “dazed.” Although more rare, occasionally tonic posturing, clonic movements, and convulsions are observed following concussion.37 Symptoms of concussion can include headache, dizziness, balance problems, phonophobia, and others. See Table 332-2 for a summary of commonly reported signs and symptoms of injury. Although it is most common for symptoms to onset immediately following trauma, some patients may not experience or notice symptoms for several minutes, hours, or sometimes days after injury. A study of concussed athletes revealed a small subsample who did not experience symptom onset until about 14 to 15 minutes after injury (mean = 14.4, SD=15.5 minutes).38 Higher postconcussion symptoms acutely (72 hours) postinjury have been related to a greater likelihood of experiencing more significant mental status changes secondary to injury, including being more likely to have experienced PTA, retrograde amnesia (RGA), and disorientation or at least 3 of the following four signs: LOC, PTA, RGA, or disorientation.39
| Signs of Injury |
Further underscoring the need to attend to all symptoms was a study that explored the subtle symptom of “feeling mentally foggy” and its relation to neurocognitive performance. In this study, concussed athletes who endorsed any degree of mental fogginess had worse performance on memory, reaction time, and processing speed measures, as well as reporting an overall higher total symptom score.40
Posttraumatic Headaches
In sports-related concussion studies, headaches have been consistently cited as the most frequently endorsed symptom.39,41–44 Studies have reported postinjury headache frequencies as high as 86%,45 and headaches are more commonly reported following mTBI than more severe brain injuries.46 Posttraumatic headaches are similar to their nontraumatic counterparts and may include, but are not limited to, tension-type, migrainous, cluster-like, and mixed.47 McCrory48 was the first to identify subtypes of exercise-related headaches, which included acute posttraumatic headache.
Although the exact mechanism(s) for posttraumatic headaches remains unclear, it has been suggested that trauma may cause posttraumatic headaches by acting as a sole triggering factor, being part of the postconcussion syndrome, triggering the first event in an otherwise susceptible patient, or happening by chance.49 Migraine and mTBI are somewhat similar physiologically in that increased extracellular potassium, intracellular sodium, calcium, and chloride occur with both mTBI and migraine. Both conditions are also linked to increased release of excitatory amino acids, including glutamate.46 It has been postulated that a migraine may make one more vulnerable to a concussion.50
Endorsement of postconcussion headache has been linked to memory dysfunction, slowed reaction time, and increased overall symptoms, as well as being more likely to have experienced AGA or any mental status change lasting longer than 5 minutes.39 Furthermore, athletes who endorsed moderate-to-severe postconcussion headaches at 1 week after injury reported a significantly greater intensity of general postconcussion symptoms and showed a trend toward lower neurocognitive scores when compared with those with mild postconcussion headaches.39 An examination of cognitive test performance and symptom reporting among concussed athletes with no headaches (no HA), nonmigrainous headaches (HA), and those exhibiting posttraumatic migraine characteristics (PTM) revealed that the PTM group performed significantly poorer on all four measures of cognitive functioning (verbal and visual memory, processing speed, and reaction time) and demonstrated increased symptoms relative to the other two groups. Those with headaches, but not meeting PTM characteristics performed better than the PTM group across all measures, and worse than the no-HA group on reaction time and total symptoms.51
Loss of Consciousness
In sports-related concussion, LOC occurs rarely, and has been cited as occurring in between 8% and 19% of sports-concussion injuries.43,39 Further, prolonged LOCs (greater than 1 or 2 minutes) is much less frequent in sports-related concussion, with most LOCs being less than a minute in duration.52 Although many studies find no differences in cognitive performance when comparing those who have sustained mTBI-related brief LOC,53,54 other studies have found that patients who lost consciousness did perform more poorly on cognitive testing55 and had a greater likelihood of a longer time to return-to-play following injury.56 A study using video analysis of motor and convulsive activities following sports-related concussion found LOC to be the only risk factor for tonic posturing following injury.37
Amnesia
Amnesia may be experienced as a loss of continuous memory functioning for events immediately preceding (retrograde amnesia) or following (anterograde amnesia) a brain injury. The duration of amnesia is documented as the time between the occurrence of injury and the time at which the individual regains normal, continuous memory functioning. In assessing retrograde amnesia, the on-field staff can ask the athlete questions regarding details occurring just before the trauma. With sports concussion, questions involving, for example, the athlete’s recollection of the hit, of the play leading up to the hit, of earlier plays in the game, and of the game score before the hit are all good ways to assess retrograde memory function. It is important to determine how far backward the retrograde amnesia stretches, both in a solid (complete forgetting) and “spotty” (patchy memories) fashion before the athlete’s recollection of the past seems continuous. Following injury, the duration of retrograde amnesia will tend to shrink over time.57
Anterograde amnesia represents the period between the time that head injury occurs and the point at which the athlete regains continuous memory functioning for events happening following head injury. Again, this amnesia may present as solid, “spotty,” or often an initial period of relatively solid amnesia, followed by spotty amnesia, then normal continuous memory functioning. On the sideline, brief anterograde amnesia can be assessed by documenting an athlete’s ability to recall three words following a brief delay. In a clinical interview and follow-up setting, a clinician can determine through an interview the athlete’s memory functioning in the hours and days after an injury. Table 332-3 contains an example of sideline assessment questions that can be used to document retrograde and anterograde amnesia.
TABLE 332-3 Acute Mental Status Testing Protocol from the University of Pittsburgh Medical Center’s Sideline Assessment
| Orientation |
Presence of either form of amnesia because of concussion has been linked to increased neurocognitive difficulties and worse symptoms following injury.52 In this study by Collins and associates, athletes with the greatest symptomatic and neurocognitive impairment at approximately day 2 postinjury were more than 10 times more likely to have experienced retrograde amnesia because of the concussion, and 4 times more likely to have experienced anterograde amnesia, although the presence of LOC was not predictive of poor outcome.
Balance Problems
Traumatic brain injury has been known to cause benign paroxysmal positional vertigo, labyrinthine concussion, perilymphatic fistulas, central vestibular disorders, endolymphatic hydrops, and cervicogenic vertigo.58 Balance difficulties following sports-related concussion have been well-documented in the literature.38,59–61
Mildest Mild Brain Injuries or “Ding” Injuries
In sports-related mTBI, as well as other causes, such as falls or motor vehicle accidents, “ding” injuries are often left unrecognized and untreated. It has been common for decades to hear individuals say they have “shaken off” mild injuries and continued with sports or other activities. At times, such injuries are not properly addressed at emergency departments or primary care offices, only to become larger problems when the mild symptoms do not resolve and an athlete is prematurely returned to play. The athlete can sustain a more severe injury or a worsening of symptoms from the mild injury as the person attempts to re-enter work and other activities of daily living, developing symptoms of postconcussion syndrome. In a groundbreaking study examining the nature of “ding” injuries, Lovell and associates62 examined a group of concussed high school athletes who had sustained AAN grade 1 concussions (e.g., transient confusion, no LOC, resolution of symptoms, and mental status changes within 15 minutes of injury). Memory scores were significantly lower than baseline at 36 hours postinjury, but had returned to baseline levels by day 6. Although athletes had claimed symptom resolution within 15 minutes of injury during the sideline evaluation, symptom scores reflected significant increases in symptoms at the 36 hour postinjury assessment that had resolved by day 6. Of the 43 subjects in the study, only 4 exhibited both asymptomatic status and baseline-equivalent neuropsychological functioning by the 36 hour postinjury follow-up.
Consistent with findings of Lovealls study, a study examining the severity of impairment between levels of grade 1 concussions revealed cognitive and symptomatic deficits for at least 4 days postinjury.63 Furthermore, when grade 1 injuries were further divided based on injury severity (<5 minutes on-field symptoms versus 5 to 15 minutes of on-field symptoms), the less severe group (<5 minutes reported symptom duration) demonstrated increased symptom reporting at the 36-hour postinjury assessment and memory dysfunction at 4 days postinjury, while the more severe group demonstrated deficits on memory testing through the final (day 7) assessment. A study assessing sideline processing speed performance in Australian rules football43 yielded similar results, with significant differences between athletes reporting less than 5 minutes of symptoms when compared with those reporting greater than 15 minutes of symptoms.
Age Effects of Sports Concussion
The number of young athletes participating in sports continues to grow, with as many as 60% of high school students participating in organized sports.64 More than 1.5 million high school and younger athletes participate in American football alone,65 and it is estimated that up to 30 million children ages 5 to 17 participate in community sponsored athletic programs.66,67
Many animal studies have provided a solid foundation to our understanding of age-related changes following mTBI. In rat studies using fluid percussion to model mild-to-moderate concussion (little to no observable structural injury), injured rats showed reduced cognitive benefit from enriched environment rearing when compared with shams raised in the same environment up to 1 month postinjury. However, if enriched environment rearing was delayed until 2 weeks postinjury, the injured rats performed more similarly to shams, though with a slightly reduced learning slope and worse performance on a delayed memory task.68 In the absence of cell death following fluid percussion brain injuries, immature rats demonstrated mild acute cognitive deficits (e.g., slowed escape latency and delayed task learning), but no storage or recall memory difficulties.69
Human studies of age effects in mild traumatic brain injury suggest that younger age may place an injured person at greater risk for slow recovery or poor acute outcome following concussion. Many of these studies have focused on sports-related head injuries. When examining time to recover based on symptom resolution and memory performance, a college athlete’s were found to recover more quickly than high school athlete’s. Memory impairment was detected for at least 1 week postinjury in a high school sample, but recovered within 24 hours in a collegiate sample.70 High school football players were found to recover more slowly than their NFL counterparts, though both groups initially demonstrated concussion-related neurocognitive decline relative to the baseline. Most professional athletes returned to baseline within 2 days, and all returned to normal by 1 week postinjury. High school football players recovered more slowly, as evidenced by longer lasting symptoms and cognitive deficits following concussion.71 Also, the extent to which postinjury scores departed from baseline were much greater in the high school versus the professional sample. In this study, concussed high school athletes were also found to have a higher rate of RGA following concussion, but did not significantly differ on the rate of LOC or disorientation. An epidemiologic study of concussion in North Carolina high school students found that being younger (e.g., ninth grade) was a moderate predictor of concussion rate.16
Epidemiologic studies of mTBI in younger populations tend to find teenagers at greater risk for injury compared with younger children.8,72,73 Also, causes of mTBI vary among age groups. For example, one study found that collision with home furnishings and fixtures were more likely to injure children younger than 7 years of age, while sports and recreational equipment were linked to the highest frequency of mTBI in those 7 to 18 years of age.72
Research examining patients with moderate to severe traumatic brain injury suggests that developmental physiologic differences may underlie age effects. The younger brain may experience more prolonged and diffuse swelling following injury,74–77 and is significantly more sensitive to glutamate (see the pathophysiology section earlier).78 Increased vulnerability to injury in children may also be linked to developmental differences in the brain, skull, and musculoskeletal systems.79 Second impact syndrome is most often recorded in persons in their teenaged years or younger,80,81 suggesting increased vulnerability in the developing brain.
Gender Effects with mTBI
In many studies, mTBI has been documented as occurring more frequently in males.8,82,83 Despite a growing body of literature examining gender differences in recovery from mTBI, the clinical picture remains unclear. A meta-analysis examining sex differences in outcome following TBI found women performed worse than men on 85% of measured variables, with men performing worse on only auditory symptoms.84 Female gender has been associated with significantly greater endorsement of postconcussion symptoms acutely postinjury in mTBI44,85–87 and general trauma85 patients, and in symptom reporting at baseline (preinjury) testing.44
For example, a study comparing the neurocognitive and symptom reporting in concussed collegiate males and females found no difference in postinjury cognitive performance or symptom reporting at day 2 or 8 assessments. Although there were no between-subject multivariate effects of sex found in this study, univariate posthoc analyses revealed concussed females performed worse on visual memory, and concussed males more often reported higher symptom scores of sadness and vomiting. Broshek and coworkers88 found that a sample of high school and collegiate women demonstrated worse simple and complex reaction times, processing speed, and symptom scores when compared with their male counterparts. Another study of collegiate athletes participating in six sports found that women were at increased risk for sustaining a concussion during games (9.5% for women versus 6.4% for men). This same study cited women’s soccer players as having the highest concussion rate and women’s lacrosse for having the highest inherent risk for concussion during a game.89 Regarding differences in psychological responses to injury, women tend to report more concern about the impact of injury on their health, while men report more pressure to play through an injury.90
Other Influencing Factors on Response to or Recovery from mTBI
The idea of cognitive reserve has received attention for its potential role in quicker recovery from brain injury, including mTBI. Patients that score lower on IQ testing have been found more likely to experience persistent postconcussion syndrome (PCS) following brain injury,91 though those with higher IQ scores were found in one study to be more likely to endorse at least three ICD-10 postconcussion symptoms acutely (within about 5 days) following brain injury.85 A study of collegiate football players revealed that athletes with a self-reported diagnosed learning disability and a history of multiple concussions were found to perform more poorly on baseline testing than did those with a history of multiple concussions without a diagnosis of a learning disability.28
Psychiatric difficulties, both before and after brain injury, have been linked to risk of poor outcome. Acute posttraumatic stress85 and posttraumatic stress disorder (PTSD)92 have been related to high postconcussion symptom endorsement. Preinjury histories of affective or anxiety disorders85,91 and other psychiatric disorders91 have been associated with greater acute85 and persistent91 postconcussion symptoms. Ruff and colleagues93 suggested, through several case studies, that specific premorbid emotional and personality characteristics potentially place people at increased risk of poor outcome from mTBI. This “miserable minority” was characterized as being overachieving, dependent, perfectionistic, grandiose, having borderline personality characteristics, and/or narcissistic features.
Effects of Cumulative mTBI
Because athletes often have repetitive exposures to contact, much of the literature about the potential cumulative nature of mild head injury has arisen from research on concussed athletes. Evidence of neurocognitive and neurological impairment associated with multiple injuries has been well documented in studies of boxers.94,95 In the literature, there have been mixed results regarding cumulative effects of concussion, but there is an overall suggestion that sustaining multiple concussions may be additive. Several studies have reported no differences in acute postinjury neurocognitive and symptom scores between male athletes reporting no prior history of concussion and those reporting generally one or two prior injuries.71,96 Sustaining an initial concussion has been shown to increase the likelihood of being injured by a second concussion in that same season by threefold in high school and college football players,45 and having a prior history of concussion was linked to a more than twofold elevation in concussion risk in a statewide sample of high school athletes.16 Athletes with two prior concussions were found in one study to have slower recovery times.97 While Collins and associates28 and Moser and colleagues98,99 found evidence of lower baseline scores in athletes with prior concussions, two similar studies did not observe these chronic cognitive deficits in multiple concussed athletes.100,101 Athletes with at least two prior concussions report more symptoms at baseline evaluations than do athletes with no concussion history.28,102 When comparing athletes with no prior concussions to those with three or more, multiple concussed athletes were: (1) found to perform worse on acute postinjury memory testing, (2) more likely to demonstrate a major decline in memory performance from baseline, (3) 6 times more likely to have demonstrated on-field AGA, and (4) more likely to demonstrate 5 or more minutes of mental status changes.102 A similar study103 found that athletes with three or more prior concussions were at increased risk of experiencing loss of consciousness, anterograde amnesia, and confusion because of a subsequent concussion.
A Danish study examining all male hospital admissions for head injury from 1979 to 1993 revealed that cognitive dysfunction (as defined by performance on a cognitive ability test given to those who sign up for the draft) was present in adults who had sustained one concussion at ages 12 to 17, who had sustained two concussions (either both before age 12 or one before and one after age 12), who had sustained a cranial fracture between ages 12 and 17, and who had been hospitalized at least 12 days following a head injury.104 A study of Dutch amateur soccer players found an inverse relationship between the number of concussions and worse performance on tests of attention, memory, and processing speed.105
Recent survey studies of retired professional football players have found evidence of increases in self-reported mild cognitive impairment106 and depression107 in those who sustained multiple concussions over the course of their career when compared with those who did not report prior concussions. Retired players reporting three or more prior concussions were 3 times more likely to report having been diagnosed with depression and to report significant memory problems when compared with retired players with no prior history of concussion. These multiple concussed athletes were also 5 times more likely to have received a diagnosis of mild cognitive impairment and demonstrated, on average, an earlier onset of Alzheimer’s disease (though no increase in AD diagnoses). Those with a history of one or two prior concussions were 1.5 times more likely to report a diagnosis of depression versus those players with no prior injuries.
Second Impacts Following Mild Traumatic Brain Injury
Immediately after a mild brain injury, there is believed to be an increased period of vulnerability to a second insult. Potential for cell death and irreversible neuronal injury may increase during the hyperglycolytic phase when glucose demand is high, but cerebral blood flow has decreased.31 Also, increased intracellular calcium and alterations to excitatory or inhibitory neurotransmission through a second injury may also lead to cell death through varied mechanisms.31 In rare but documented cases, this autodysregulation of the cerebral blood supply is believed to lead to vascular engorgement, diffuse cerebral swelling, increased intracranial pressure, brain herniation, coma, and permanent brain damage or death, known as second-impact syndrome.81 Although this diagnosis remains somewhat controversial, cases of suspected second-impact syndrome have been documented and noted to occur primarily in adolescent athletes,108 which is thought to reflect the increased vulnerability of the developing brain. It is difficult, if not impossible, to define the exact period of increased vulnerability to repeated blows, as each of these cellular dysfunctions occur in different time frames, and resolution of dysfunction may vary based on severity of injury and may vary across people. In an effort to examine the period at which the brain is most vulnerable to mTBI, Tavazzi and coworkers109 subjected rats to a second mTBI at 1, 2, 3, 4, and 5 days following an initial injury. Interestingly, oxidative and nitrosative stresses were most pronounced at day 3, and appeared to have returned to control levels by day 5.
Neuropsychological Functioning Following mTBI
Because concussion affects the brain in a diffuse manner, the neuropsychological profile of a concussed patient can be variable. In both adult and adolescent mTBI, neuropsychological deficits may include difficulties in memory,28,63,40,110 attention,111 reaction time,40,110 and processing speed.28,40,43,110 The duration of these neurocognitive changes varies greatly between individuals. In assessment of mTBI, a clinician should attend to both the symptom status and neurocognitive functioning of the recently concussed patient, as well as patients who report chronic, persisting symptoms of mTBI (e.g., postconcussion syndrome). Using neuropsychological testing in addition to symptom reporting most accurately discriminates between concussed and unconcussed athletes.112,113 In the field of sports-related mTBI, two summary statements from the Concussion in Sport Group following international conferences in Vienna5 and Prague114 supported the use of neurocognitive testing in concussion management and return-to-play decisions. The first summary statement described neurocognitive testing as “the cornerstone of concussion evaluation,” and the second statement encouraged use of neurocognitive testing as an “aid to clinical decision making.”
In some individuals, symptoms may resolve more quickly than neurocognitive dysfunction, and this has been demonstrated or suggested in multiple studies.59,113,115–117 The combination of symptom reporting and neurocognitive testing has been shown to correctly identify the greatest percentage (93%) of concussed athletes versus using symptom report (65% correctly identified) or neurocognitive testing (83% correctly identified) alone.113 Research has documented a tendency for athletes to underreport their symptoms, likely with the intention of returning to play more quickly,116 which highlights the need for return-to-play decisions to be made using more than symptom information. Given this tendency in athletes, it can then be extrapolated that other individuals with mTBI may be motivated to minimize symptoms to return to work, to school, or to competitive or exertional recreational pursuits.
In sports concussion, brief computerized neuropsychological testing has become widely used and generally well accepted as part of a concussion management protocol. This method of testing has allowed for large-scale group baseline testing of sports teams, reduces the practice effects associated with many traditional neuropsychological tests, is more sensitive at detecting subtle changes in reaction time, and reduced the cost associated with test administration and interpretation (e.g., neuropsychologist or psychometrician not needed to administer and score baseline testing). In addition to being accepted at the high school and collegiate levels, baseline and postinjury computerized cognitive testing as part of a concussion management program has been mandated in the National Football League71,118 and National Hockey League,119 and is voluntarily used in many other professional and elite sports organizations.
Studies in hospitalized mTBI85 and general trauma85 patients have found that patients who endorsed three or more ICD-10 postconcussion symptoms were more likely to demonstrate slowed processing speed and reaction time on neuropsychological testing. Examination of the symptoms and attentional performances in combat veterans diagnosed with PTSD111 with and without a history of exposure to mTBI through blast injury found attentional deficits on cognitive testing and were more likely to meet DSM-IV criteria for attention-deficit hyperactivity disorder (ADHD) in the absence of a lifetime diagnosis of the disorder.
Recovery from mTBI
Although sports-related concussion was historically believed to be a benign injury with a typical recovery period of minutes to days, current literature is showing that, like other types of mTBI, recovery from sports-related concussion is highly individualized, and most often takes longer than previously believed. The time to recover from concussion among high school athletes ranges, on average, from 7 to 14 days, although functional impairment from sports-related or other mild traumatic brain injury may persist well beyond that time period.70,120,123 A study of collegiate athletes of mixed gender and sports found that only 42% had recovered by day 2, with 30% of athletes still experiencing symptoms and/or cognitive decline from baseline at 8 days.124
Neurosurgical Assessment and Management of mTBI
CT scanning is the usual radiologic method of choice when assessing the mTBI patient. While there has been a traditional debate regarding indications for obtaining a CT in mTBI patients, current practices are generally liberal in its use. Those having a history of loss of consciousness and amnesia or any abnormality on the neurological examination have usually been routinely scanned. While skull x-rays are now infrequently employed, in the presence of a known skull fracture, the risk of intracranial hemorrhage is higher than without (6.4% versus 0.65%, respectively).125 The indications for CT vary, but generally include an abnormal neurological examination, known skull fracture, history of loss of consciousness, nausea and vomiting, intoxication, age greater than 60 years, trauma above the clavicle, and GCS score less than 14.126,127 Patients who have a deterioration in the neurological examination and those on anticoagulation are two common examples of those having need for initial and subsequent CT scan studies.
Many physicians follow a set of criteria for selecting which patients should receive a head CT following mTBI. The two most well known rules are the Canadian CT head rule128 and the New Orleans criteria.127 The Canadian CT Head Rule (CCR) was derived from a study of 3121 patients presenting to 10 Canadian hospitals with a GCS score of 13 to 15. The authors derived a list of five high-risk features, which identified all patients in need of intervention (100% sensitive), while requiring only 32% of the population to receive a CT scan under the rule, and two medium-risk factors that would indicate a CT scan for 54% of the population, with a sensitivity of 98.4% and specificity of 49.6%. This clinical decision rule indicates mandatory CT scanning for anyone having one or more of the high-risk criteria: (1) GCS score less than 15 at 2 hours postinjury; (2) suspected open or depressed skull fracture; (3) any sign of basal skull fracture (e.g., hemotympanum, “raccoon” eyes, CSF otorrhea/rhinorrhea, Battle’s sign); (4) two or more episodes of vomiting; and (5) patient is 65 years of age or older. Patients in the medium-risk category may have “clinically important lesions” that would not require intervention via neurosurgery. Patients who either have (1) greater than 30 minutes of retrograde amnesia or (2) were injured via a “dangerous mechanism” (e.g., motor vehicle accident versus pedestrian, ejection from motor vehicle, fall from greater than 3 feet or down five or more stairs) are identified as medium risk, where CT scan or close observation is recommended based upon availability of resources (please see Steill and associates for a more comprehensive discussion).128
Like the CCR, the New Orleans criteria (NOC)127 for computed tomography in mTBI was developed through study of two samples (N = 520 and 909, exploratory and confirmatory, respectively) of hospital emergency department patients having a GCS of 15 and mTBI with LOC or amnesia for the event and an unremarkable brief neurological examination. The seven criteria identified as being associated with positive CT scans following mTBI were older than 60 years and had the presence of a headache, vomiting, intoxication, anterograde amnesia, posttraumatic seizure, and physical evidence of trauma above the clavicles.
General Management of mTBI
Early identification and management of mTBI is of utmost importance in proper management of the injury. Whether the injury is a sports-related concussion first diagnosed on the sidelines by an athletic trainer or an injury evaluated by an EMT following a motor vehicle accident, it is important to thoroughly evaluate the injured person, noting all signs and symptoms, not just headache or loss of consciousness. As the nature of mTBI signs and symptoms and the recovery from the injury will vary among individuals,5,129 it will be important to structure the management plan to address the relevant emotional, social, medical, cognitive, and educational/occupational needs of the injured person.129 Whether a working adult or young student has sustained an mTBI, postconcussion symptoms may prohibit or impair one’s ability to perform optimally in the occupational or academic setting. With concussed students, even slight changes to their memory functioning may result in significant declines in grades or testing and may inhibit learning new information that will be needed as a foundation for learning in the future. Therefore, accommodations should be recommended based on the student’s level of symptoms and cognitive dysfunction. Kirkwood and associates129 provide a good discussion of considerations to be made when accommodating students with mTBI. Adults with mTBI may need to reduce work hours, request a temporary change to heavy or risky job duties, and take increased breaks to control symptom severity. For the physician faced with the task of mTBI assessment, diagnosis, and management, there are many good resources available, including the recently released and publicly available physician’s toolkit published by the Centers for Disease Control and Prevention called “Heads Up: Brain Injury in Your Practice.”6
Special Considerations with Concussed Athletes
Education
Given that athletic competitions can be fast-paced events, and mTBIs can range in severity, it is important that all persons involved in athletic teams are educated about identification and management of sports-related brain injuries. Often, due to the excitement of the game, to symptoms perceived as minor, or to a concentrated effort to hide or minimize symptoms of concussion, athletes may not self-report injuries at the moment they occur. At times, teammates, athletic trainers, team physicians, or coaches may be the first to notice changes to the athlete’s mental status, demeanor, or behavior that may indicate concussion. Furthermore, a staff and team that is well educated about the potential dangers of concussion and premature return to play may be less likely to tell the injured athlete to “shake it off” or that the injury was “just a ding.” Although many school-based organizations are increasingly placing greater importance on concussion education, given that up to 30 million youth participate in community-based sports each year, it is also important to educate the volunteers and parents who tend to become coaches for these teams.130
Most sports organizations have guidelines for return-to-play following injuries. Radelet and associates130 noted that in community youth sports, despite the presence of guidelines, coaches often used their own judgment to return players to the field, ignoring available guidelines, placing athletes at greater risk for the consequences of premature return to play. Therefore, educating staff, players, and parents about injury procedures on a regular basis, and establishing consequences for failures to follow them can be one important step in reducing risk.
Sideline Assessment
Any athlete suspected of experiencing a concussion should be immediately removed from play and evaluated by appropriately trained staff. Sideline assessments are usually performed by an athletic trainer or by a team physician when available. Certainly, before concussion assessment, the athlete should be evaluated to rule out more severe central nervous system injuries. Thus one must first evaluate for level of consciousness and the ABCs (airway, breathing, circulation). Conditions such as very severe or progressively worsening headaches, positive findings on neurological examination, vomiting, or rapidly declining mental status may indicate a more life-threatening injury (e.g., subdural hematoma, intracranial bleed), and should warrant immediate transport to the emergency department.131 Evaluation of concussion should include a complete evaluation of signs and symptoms, including mental status testing. There are many sideline evaluation assessment methods available, including the Standardized Assessment of Concussion (SAC)132 and the Sport Concussion Assessment Tool (SCAT).114 Table 332-4 contains the acute mental status testing portion of the University of Pittsburgh Medical Center’s sideline concussion evaluation.
Graduated Return-to-Play Guidelines
Previously, the postconcussion return-to-play decision was commonly based on recommendations from one of the over 20 available concussion management guidelines.133,134 However, due to the variability among guidelines and the lack of an empirical basis for guideline-based restrictions from play, the guideline system often created confusion and debate among practitioners. The Concussion in Sport (CIS) group, in a groundbreaking meeting that brought together concussion experts from a variety of disciplines to share research, ideas, and clinical experiences, published an agreement statement that introduced a new approach to concussion management to replace the “one size fits all” grading scale guidelines with a more individualized approach.5
Based on the CIS group recommendations, athletes should be restricted from physical exertion while experiencing postconcussion symptoms. Once symptom-free status has been obtained, athletes should gradually return to activity via increasing levels of noncontact, nonrisk physical exertion. When the athlete has demonstrated asymptomatic status with heavy physical exertion, he or she is typically returned to practice or controlled contact (e.g., hitting the sleds in football, light heading drills in soccer) before returning to competition. If at any point in the increasing exertional progression the athlete experiences a re-emergence of postconcussion symptoms he or she should rest again until asymptomatic for at least 24 hours, then return to the progression at a lower level. Recommendations for such a progression in soccer and hockey have been published by Johnston and colleagues.135
Neuropsychological Testing
Within the past decade, brief neuropsychological testing has become increasingly used as part of a comprehensive concussion management protocol. Ideally, athletes receive a baseline neurocognitive test before beginning contact sports activity. Once a concussion is sustained, follow-up testing can be used to determine the extent of cognitive changes because of injury, as well as help to determine when cognitive functioning returns to normal, thus providing an additional measure of recovery beyond relying on athlete’s self-reporting of symptoms. Computerized tests are often used because they provide a means through which to test athletes in a group setting, thus minimizing staffing and time demands. In addition, computerized testing platforms often provide multiple forms of tests and randomized stimuli to reduce potential practice effects. Computerized tests have been shown to demonstrate adequate sensitivity and specificity,112 reliability,136 and validity.137,138 The utility of neuropsychological testing as one part of a comprehensive concussion management program has been supported in two international symposia on concussion in sports,114,139 and deficits in neurocognitive functioning following sports-related concussion have been well documented.*
Prevention
Although there is no way to completely prevent concussions in sports, several theories and studies have suggested ways in which to potentially reduce risk of injury. Protective equipment that is properly fitted and undamaged is essential for safe sports participation. Studies examining concussions sustained in professional football revealed that many concussive blows occurred to the face or side of the helmet of the struck player (cite). Presently, football helmet manufacturers have worked toward designing helmets that may reduce the risk of concussion. An observational study142 examining the Riddell Revolution helmet versus “helmet-as-usual” in 17 Pennsylvania high schools over three football seasons examined incidence rates and recovery times. The study found a significant difference in incidence rate (5.3% for Revolution versus 7.6% for other helmets), with a relative risk reduction of 31% and an absolute risk reduction of 2.3%. There were no other differences observed (e.g., on-field markers, immediate symptoms, cognitive testing, days to recover). Studies of helmet use in a nonsports population found a lower GCS score on admission in motorcyclists with mild head injuries who did not wear helmets than those who were wearing helmets at the time of their injuries,83 as well as a higher overall incidence of TBI in injured motorcyclists not wearing helmets.143
There have also been suggestions that mouthguard use may reduce the incidence or severity of sports-related concussion, although the scientific literature has been mixed.19–21,144–146 Studies of NCAA basketball players19 and South African rugby players144 revealed no difference in the incidence of sports-related concussions between players wearing and not wearing mouthguards at the time of injury. The type of mouthguard used in NCAA Division 1-A football players was not found to effect the concussion rate or grade of concussion sustained.21 In acute postinjury assessment, mouthguard use was not associated with reduced neurocognitive or symptomatic impairment when scores were compared with athletes who were not wearing a mouthguard at the time of the injury.147 If mouthguards may indeed reduce incidence or severity of concussion, the effect is likely linked to posterior thickness of the guard that serves to create increased space between the condyles of the temporomandibular joint, thus reducing the overall force transmitted to the occiput or head.141 Cadaver studies suggest that use of a mouthguard reduced the amplitude of the intracranial pressure wave and decreased bone deformation.146
Changing team attitudes may also aid in injury prevention. A study of injuries in youth community sports hypothesized that injury rates may be lower in some sports when teams emphasize skills acquisition over competitiveness.130
Postconcussion Syndrome
A major concern with mTBI of all causes is the possibility for developing postconcussion syndrome (PCS). The Diagnostic and Statistical Manual of Mental Disorders148 defines postconcussional disorder as occurring following a significant cerebral concussion with evidence of cognitive difficulties on testing (e.g., memory, attention) and at least three common concussion symptoms that occur for at least 3 months. The symptoms must onset or worsen because of the head trauma; cause significant impairment in social, occupational, or academic functioning; and cannot be better accounted for by another mental disorder. Although postconcussion syndrome cannot be officially diagnosed until symptoms have persisted for at least 3 months, clinical interventions for more chronic symptoms can be initiated much sooner and may provide for better outcomes.85
Although cognitive difficulties associated with mTBI have been found to generally resolve within 1 to 3 months,86,149,150 up to 64% of people who have experienced mTBI may continue to report three or more symptoms at 3 months postinjury.151 In a New York University sample, approximately 10% of patients who presented to the emergency department with mTBI continued to complain of postconcussion symptoms at 1 year postinjury, and about the same percentage had not returned to work at the 1-year mark.152 Preexisting psychiatric disorder may place an individual at greater risk for developing PCS.93,152–154 Alternatively, individuals with PCS following mTBI have been found more likely to experience depression, anxiety, and acute posttraumatic stress than those with mTBI who do not experience PCS.155,156 A study of “acute PCS” in mTBI and non-mTBI trauma cases revealed that the acute PCS diagnosis (endorsement of 3 or more ICD-10 symptoms) was related to female gender, higher full scale IQ, slowed information processing speeds and reaction times, greater acute stress symptoms, and prior affective or anxiety disorder in both patients with mTBI and general trauma controls. Level of pain was positively associated with PCS in mTBI patients and negatively associated with PCS in general trauma patients.85 Severity of PCS has not been found to be related to the severity of the mTBI that caused the injury,111,157 nor has the severity of the trauma been related to persistence of PCS past a 1-year period.157
Neuroimaging and Mild Traumatic Brain Injury
In studies comparing concussed athletes to matched controls, increased activation that is diffuse in nature has been found in fMRI studies.158,159 Even in absence of attenuated neurocognitive performance (accuracy and speed measures), athletes with concussions show changes in functional activation when compared with noninjured controls159,160 and personal preinjury scans.159 In the study by Jantzen and associates,159 concussed participants showed increased amplitude and extent of activation, chiefly in the bilateral inferior-superior parietal region, dorsolateral regions, and frontal cortex while performing a working memory task. Adult athletes who had experienced mild traumatic brain injury 1 to 14 months before completing fMRI working memory tasks showed weaker activation changes in the right middorsolateral prefrontal cortex (DLPFC) compared with controls when completing verbal and visual working memory tasks.158 Extent of postconcussion hyperactivation observed has been associated with time to recovery.161 An inverse relation between symptom severity and P300 amplitudes has been demonstrated in concussed athletes.162 When comparing depressed and nondepressed athletes who have sustained mTBI, Chen and associates163 found differences in functional activation despite a lack of difference in task performance.
In general samples of adults sustaining mTBI, differences in functional activation during working memory tasks have been observed between injured and control populations in positron emission tomography studies.123,164 SPECT scans conducted 3 months postinjury in adult mild to moderate TBI165 and pediatric mild TBI166 samples demonstrated functional abnormalities that were associated with lingering postconcussion symptoms. Mild traumatic brain injury has also been linked to abnormalities on an electroencephalogram, which have been related to severity of the concussion.162,167,168
Aubry M, Cantu R, Dvorak J, et al. Summary and agreement statement of the 1st international symposium on concussion in sport, Vienna. Clin J Sport Med. 2002;12:6-11.
Aubry M, Cantu R, Dvorak J, et al. Summary and agreement statement of the first international conference on concussion in sport, Vienna 2001. Br J Sports Med. 2002;36:6-10.
Bailes JE, Cantu RC. Head injury in athletes. Neurosurgery. 2001;48:26-45.
Borczuk P. Predictors of intracranial injury in patients with mild head trauma. Ann Emerg Med. 1995;25:731-736.
Cantu RC. Second-impact syndrome. Clin Sports Med. 1998;17:37-44.
Centers for Disease Control and Prevention. National Center for Injury Prevention and Control. Heads up: brain injury in your practice. http://www.cdc.gov/ncipc/tbi/Physicians_Tool_Kit.htm, 2007. Accessed 24 Feb 2010
Chen JK, Johnston KM, Collie A, et al. A validation of the post concussion symptom scale in the assessment of complex concussion using cognitive testing and functional MRI. J Neurol Neurosurg Psychiatry. 2007;78:1231-1238.
Chen JK, Johnston KM, Frey S, et al. Functional abnormalities in symptomatic concussed athletes: an fMRI study. Neuroimage. 2004;22:68-82.
Chen SH, Kareken DA, Fastenau PS, et al. A study of persistent post-concussion symptoms in mild head trauma using positron emission tomography. J Neurol Neurosurg Psychiatry. 2003;74:326-332.
Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train. 2001;36:228-235.
Guskiewicz KM, Marshall SW, Bailes J, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57:719-726.
Guskiewicz KM, Marshall SW, Bailes J, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39:903-909.
Guskiewicz KM, McCrea M, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA concussion study. JAMA. 2003;290:2549-2555.
Haydel MJ, Preston CA, Mills TJ, et al. Indications for computed tomography in patients with minor head injury. N Engl J Med. 2000;343:100-105.
Iverson GL, Brooks BL, Lovell MR, et al. No cumulative effects of one or two prior concussions. Br J Sports Med. 2006;40:72-75.
Jantzen KJ, Anderson B, Steinberg FL, et al. A prospective functional MR imaging study of mild traumatic brain injury in college football players. AJNR Am J Neuroradiol. 2004;25:738-745.
Jordan BD, Relkin NR, Ravdin LD. Apolipoprotein E4 associated with chronic traumatic brain injury in boxing. JAMA. 1997;278:136-140.
Kirkwood MW, Yeates KO, Wilson PE. Pediatric sport-related concussion: a review of the clinical management of an oft-neglected population. Pediatrics. 2006;117:1359-1371.
Lovell MR, Collins MW, Iverson GL, et al. Grade 1 or “ding” concussions in high school athletes. Am J Sports Med. 2003;32:1-8.
Lovell MR, Pardini JE, Welling JS, et al. Functional brain abnormalities are related to clinical recovery and time to return to play in athletes. Neurosurgery. 2007;61:352-360.
McAllister TW, Sparling MB, Flashman LA, et al. Differential working memory load effects after mild traumatic brain injury. Neuroimage. 2001;14:1004-1012.
McCrory P, Johnston K, Meeuwisse W, et al. Summary and agreement statement of the 2nd international conference on concussion in sport, Prague, 2004. Clin J Sport Med. 2005;15:48-57.
Omalu BI, DeKosky ST, Hamilton RL, et al. Chronic traumatic encephalopathy in a National Football League player: part II. Neurosurgery. 2006;59:1086-1092.
Pellman EJ, Lovell MR, Viano DC, et al. Concussion in professional football: neuropsychological testing—part 6. Neurosurgery. 2004;55:1290-1305.
Powell JW, Barber-Foss KD. Traumatic brain injury in high school athletes. JAMA. 1999;282:958-963.
Raghupathi R, Graham DI, McIntosh TK. Apoptosis after traumatic brain injury. J Neurotrauma. 2000;17:927-938.
Roberts AH. Brain Damage in Boxers: a Study of the Prevalence of Traumatic Encephalopathy Among Ex-Professional Boxers. London: Pitman; 1969.
Thornbury JR, Masters SJ, Campbell JA. Imaging recommendations for head trauma: a new comprehensive strategy. AJR Am J Roentgenol. 1987;149:781-783.
van Kampen DA, Lovell MR, Pardini JE, et al. The value added of neurocognitive testing following sports-related concussion. Am J Sport Med. 2006;34:1630-1635.
1 Kay T, Harrington DE, Adams R, et al. Definition of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8:86-87.
2 Congress of Neurological Surgeons. Committee on head injury nomenclature: glossary of head injury. Clin Neurosurg. 1996;12:386-394.
3 Carroll LJ, Cassidy JD, Holm L, et al. Methodological issues and research recommendations for mild traumatic brain injury: the WHO collaborating centre task force on mild traumatic brain injury. J Rehabil Med. 2004;36:113-125.
4 Quality Standards Subcommittee. American Academy of Neurology. Practice parameter: the management of concussion in sports. Neurology. 1997;48:581-585.
5 Aubry M, Cantu R, Dvorak J, et al. Summary and agreement statement of the first international conference on concussion in sport, Vienna 2001. Br J Sports Med. 2002;36:6-10.
6 Centers for Disease Control and Prevention. National Center for Injury Prevention and Control. Heads up: brain injury in your practice. http://www.cdc.gov/ncipc/tbi/Physicians_Tool_Kit.htm, 2007. Accessed 24 Feb 2010
7 Servadei F, Teasdale G, Merry G. Defining acute mild head injury in adults: a proposal based on prognostic factors, diagnosis, and management. J Neurotrauma. 2001;18:657-664.
8 Cassidy JD, Carroll LJ, Peloso PM, et al. Incidence, risk factors, and prevention of mild traumatic brain injury: results of the WHO collaborating centre task force on mild traumatic brain injury. J Rehabil Med. 2004;43:28-60.
9 Kalsbeek WD, McLaurin RL, Harris BS, et al. The national head and spinal cord injury survey: major findings. J Neurosurg. 1980:S19-S31.
10 Annegers JF, Grabow JD, Kurland LT, et al. The incidence, causes, and secular trends of head trauma in Olmstead County, Minnesota, 1935-1974. Neurology. 1980;30:912-919.
11 Kraus JF, Nourjah P. The epidemiology of mild, uncomplicated brain injury. J Trauma. 1988;28:1637-1643.
12 Rimel RW. A prospective study of patients with central nervous system trauma. J Neurosurg Nurs. 1981;13:132-141.
13 Kraus JF, Nourjah P. The epidemiology of mild, uncomplicated brain injury. J Trauma. 1988;28:1637-1643.
14 Sosin DM, Sniezek JE, Thurman DJ. Incidence of mild and moderate brain injury in the United States, 1991. Brain Inj. 1996;10:47-54.
15 National Center for Injury Prevention and Control. Steps to prevent a serious public health problem. TBI report to congress on mild traumatic brain injury in the United States. 2003. http://www.cdc.gov/ncipc/pub-res/mtbi/mtbireport.pdf. Accessed 24 Feb 2010
16 Schulz MR, Marshall SW, Mueller FO, et al. Incidence and risk factors for concussion in high school athletes, North Carolina, 1996-1999. Am J Epidemiol. 2004;160:937-944.
17 Koh JO, Cassidy JD, Watkinson EJ. Incidence of concussion in contact sports: a systematic review of the evidence. Brain Inj. 2003;17:901-917.
18 Powell JW, Barber-Foss KD. Traumatic brain injury in high school athletes. JAMA. 1999;282:958-963.
19 Labella CR, Smith BW, Sigurdsson A. Effect of mouthguards on dental injuries and concussions in college basketball. Med Sci Sports Exerc. 2002;34:41-44.
20 Delaney JS. Head injuries presenting to emergency departments in the United States from 1990-1999 for ice hockey, soccer, and football. Clin J Sport Med. 2004;14:80-87.
21 Wisniewski JF, Guskiewicz K, Trope M, et al. Incidence of cerebral concussions associated with type of mouthguard used in college football. Dent Traumatol. 2004;20:143-149.
22 Buckley WE. Concussions in college football: an eight-year overview. J Athl Train. 1986;21:207-211.
23 Buckely WE. Concussions in college football. A multivariate analysis. Am J Sport Med. 1988;16:51-56.
24 Bailes JE, Cantu RC. Head injury in athletes. Neurosurgery. 2001;48:26-45.
25 Boden BP, Kirkendall DT, Garrett WEJr. Concussion incidence in elite college soccer players. Am J Sport Med. 1998;26:238-241.
26 Pelletier RL, Montelpare WJ, Stark RM. Intercollegiate ice hockey injuries, a case for uniform definitions and reports. Am J Sport Med. 1993;21:78-81.
27 Roberts WO, Brust JD, Leonard B. Youth ice hockey tournament injuries: rates and patterns compared to season play. Med Sci Sports Exerc. 1999;31:46-51.
28 Collins MW, Grindel SH, Lovell MR, et al. Relationship between concussion and neuropsychological performance in college football players. JAMA. 1999;82:964-970.
29 Gissane C, Jennings DC, Cumine AJ, et al. Differences in the incidence of injury between rugby league forwards and backs. Aust J Sci Med Sport. 1997;29:91-94.
30 Stephenson S, Gissane C, Jennings D. Injury in rugby league: a four year prospective survey. Br J Sports Med. 1996;30:331-334.
31 Giza CC, Hovda DA. The neurometabolic cascade of concussion. J Athl Train. 2001;36:228-235.
32 Yoshino A, Hovda DA, Kawamata T, et al. Dynamic changes in local cerebral glucose utilization following cerebral concussion in rate: evidence of a hyper- and subsequent hypometabolic state. Bran Res. 1991;561:106-119.
33 Bergsneider M, Hovda DA, Lee SM, et al. Dissociation of cerebral glucose metabolism and level of consciousness during the period of metabolic depression following human traumatic brain injury. J Neurotrauma. 2000;17:389-401.
34 Raghupathi R, Graham DI, McIntosh TK. Apoptosis after traumatic brain injury. J Neurotrauma. 2000;17:927-938.
35 Omalu BI, DeKosky ST, Hamilton RL, et al. Chronic traumatic encephalopathy in a National Football League player: part II. Neurosurgery. 2006;59:1086-1092.
36 Omalu BI, DeKosky ST, Minster RL, et al. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57:128-134.
37 McCrory PR, Berkovic SF. Video analysis of acute motor and convulsive manifestations in sport-related concussion. Neurology. 2000;54:1488-1491.
38 McCrea M, Guskiewicz KM, Marshall SW, et al. Acute effects and recovery time following concussion in collegiate football players: the NCAA concussion study. JAMA. 2003;290:2556-2563.
39 Collins MW, Field M, Lovell MR, et al. Relationship between post-concussion headache and neuropsychological test performance in high school athletes. Am J Sports Med. 2003;31:168-173.
40 Iverson GL, Gaetz M, Lovell MR, et al. Relation between subjective fogginess and neuropsychological testing following concussion. J Int Neuropsychol Soc. 2004;10:1-3.
41 Katz RT, DeLuca J. Sequelae of minor traumatic brain injury. Am Fam Physician. 1992;46:1491-1498.
42 Triplett G, Hill C, Freeman L, et al. Incidence of head injury: lasting effects among college students and working adults in the general population. Percept Mot Skills. 1996;83:1344-1346.
43 McCrory PR, Ariens M, Berkovic SF. The nature and duration of acute concussion symptoms in Australian football. Clin J Sport Med. 2000;10:235-238.
44 Lovell MR, Iverson GL, Collins MW, et al. Measurement of symptoms following sports-related concussion: reliability and normative data for the post-concussion scale. Appl Neuropsychol. 2006;13:166-174.
45 Guskiewicz KM, Weaver NL, Padua DA, et al. Epidemiology of concussion in collegiate and high school football players. Am J Sports Med. 2000;28:643-650.
46 Packard RC. Epidemiology and pathogenesis of post-traumatic headache. J Head Trauma Rehabil. 1999;4:9-21.
47 Haas DC. Chronic post-traumatic headache. In: Olesen J, Tfelt-Hansen P, Welsh KM, editors. The Headaches. New York: Raven Press; 1993:629-637.
48 McCrory P. Headaches and exercise. Sports Med. 2000;30:221-229.
49 Solomon S. John Graham senior clinicians award lecture. Post-traumatic migraine. Headache. 1998;38:772-778.
50 Gordon KE, Dooley JM, Wood EP. Is migraine a risk factor for the development of concussion. Br J Sports Med. 2006;40:184-185.
51 Mihalik JP, Stump JE, Collins MW, et al. Posttraumatic migraine characteristics in athletes following sports-related concussion. J Neurosurg. 2005;102:850-855.
52 Collins MW, Iverson GL, Lovell MR, et al. On-field predictors of neuropsychological and symptoms deficit following sports-related concussion. Clin J Sport Med. 2003;13:222-229.
53 Ruff RM, Jurica P. In search of a unified definition for mild traumatic brain injury. Brain Inj. 1999;13:943-952.
54 Iverson GL, Lovell MR, Smith SS. Does brief loss of consciousness affect cognitive functioning after mild head injury? Arch Clin Neuropsychol. 2000;15:643-648.
55 Hickling EF, Gillen R, Blanchard EB, et al. Traumatic brain injury and post-traumatic stress disorder: a preliminary investigation of neuropsychological test results in PTSD secondary to motor vehicle accidents. Brain Inj. 1998;12:265-274.
56 Asplund CA, McKeag DB, Olsen CH. Sport-related concussion: factors associated with prolonged return to play. Clin J Sport Med. 2004;14:339-343.
57 Benson DF, Geschwind N. Shrinking retrograde amnesia. J Neurol Neursurg Psychiatry. 1967;30:539-544.
58 Reddy CC, Collins MW, Gioia GA. Adolescent sports concussion. Phys Med Rehabil Clin N Am. 2008;19:247-269.
59 Peterson CL, Ferrara MS, Mrazik M, et al. Evaluation of neuropsychological domain scores and postural stability following cerebral concussion in sports. Clin J Sport Med. 2003;13:230-237.
60 Ernst A, Basta D, Seidle RO, et al. Management of posttraumatic vertigo. Otolaryngol Head Neck Surg. 2005;132:554-558.
61 Guskiewicz KM, Perrin DH, Bansneder BM. Effect of mild head injury on postural stability in athletes. J Athl Train. 1996;31:300-330.
62 Lovell MR, Collins MW, Iverson GL, et al. Grade 1 or “ding” concussions in high school athletes. Am J Sports Med. 2003;32:1-8.
63 Lovell MR, Collins MW, Iverson GL, et al. Recovery from mild concussion in high school athletes. J Neurosurg. 2003;98:296-301.
64 National Federation of State High School Associations. NFHS 2003-2004 high school athletics participation survey. Indianapolis: National Federation of State High School Associations; 2004.
65 Mueller FO, Diehl JL. Annual Survey of Football Injury Research. Chapel Hill, NC: National Center for Catastrophic Sport Injury Research. University of North Carolina; 2004.
66 Arnheim D, Prentice W. Essentials of Athletic Training, 4th ed. Boston: WCB/McGraw; 1999.
67 Patel DR, Nelson TL. Sports injuries in adolescents. Med Clin North Am. 2000;844:983-1007.
68 Giza CC, Griesback GS, Hovda DA. Experience-dependent behavioral plasticity is disturbed following traumatic injury to the immature brain. Behav Brain Res. 2005;157:11-22.
69 Gurkoff GC, Giza CC, Hovda DA. Lateral fluid percussion injury in the developing rat causes an acute, mild behavioral dysfunction in the absence of significant cell death. Brain Res. 2006;1077:24-36.
70 Field M, Collins MW, Lovell MR, et al. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletics. J Pediatr. 2003;142:546-553.
71 Pellman EJ, Lovell MR, Viano DC, et al. Concussion in professional football: recovery of NFL and high school athletes assessed by computerized neuropsychological testing—part 12. Neurosurgery. 2006;58:263-274.
72 Rivara FP, Bergman AB, LoGerfo JP, et al. Epidemiology of childhood injuries: II. Sex differences in injury rates. Am J Dis Child. 1982;136:502-506.
73 Durkin MS, Olsen S, Barlow B, et al. The epidemiology of urban pediatric neurological trauma: evaluation of, and implications for, injury prevention programs. Neurosurgery. 1998;42:300-310.
74 Pickles W. Acute general edema of the brain in children with head injuries. N Engl J Med. 1950;242:607-611.
75 Bruce DA, Alvai A, Bilanuik L, et al. Diffuse cerebral swelling following head injuries in children: the syndrome of malignant brain edema. J Neurosurg. 1981;54:170-178.
76 Aldrich EF, Eisenberg HM, Saydjaric C, et al. Diffuse brain swelling in severely head injured children. A report from the NIH traumatic coma bank. J Neurosurg. 1992;76:450-454.
77 Lang DA, Teasdale GM, Macpherson P, et al. Diffuse brain swelling after head injury more often malignant in adults than children? J Neurosurg. 1994;80:675-680.
78 McDonald JW, Johnston MV. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res Rev. 1990;15:41-70.
79 Kirkwood MW, Yeates KO, Wilson PE. Pediatric sport-related concussion: a review of the clinical management of an oft-neglected population. Pediatrics. 2006;117:1359-1371.
80 Saunders RL, Harbaugh RE. The second impact in catastrophic contact-sports head trauma. JAMA. 1984;252:538-539.
81 Cantu RC. Second-impact syndrome. Clin Sports Med. 1998;17:37-44.
82 Whitman S, Coonley HR, Desai BT. Comparative head trauma experiences in two socioeconomically different Chicago-area communities: a population study. Am J Epidemiol. 1984;119:570-580.
83 Kelly P, Sanson T, Strange G, et al. A prospective study of the impact of helmet usage on motorcycle trauma. Ann Emerg Med. 1991;20:852-856.
84 Farace E, Alves WM. Do women fare worse? A meta-analysis of gender differences in traumatic brain injury outcome. J Neurosurg. 2000;93:539-545.
85 Meares S, Shores EA, Taylor AJ, et al. Mild traumatic brain injury does not predict acute post-concussion syndrome. J Neurol Neurosurg Psychiatry. 2008;79:300-306.
86 Ponsford J, Willmott C, Rothwell A, et al. Factors influencing outcome following mild traumatic brain injury in adults. J Int Neuropsychol Soc. 2000;6:568-579.
87 McCauley SR, Boake C, Levin HS, et al. Postconcussional disorder following mild to moderate traumatic brain injury: anxiety, depression, and social support as risk factors and comorbidities. J Clin Exp Neuropsych. 2001;23:792-808.
88 Broshek D, Kaushik T, Freeman J, et al. Sex differences in outcome following sports-related concussion. J Neurosurg. 2005;102:856-863.
89 Covassin T, Swanik C, Sachs M. Sex differences and incidence of concussions among intercollegiate athletes. J Athl Train. 2003;38:231-237.
90 Granite V, Carroll J. Psychological response to athletic injury: sex differences. J Sport Behav. 2002;25:243-259.
91 Luis CA, Vanderploeg RD, Curtiss G. Predictors of postconcussion symptom complex in community dwelling male veterans. J Int Neuropsychol Soc. 1992;9:1001-1015.
92 Bryant RA, Harvey AG. Postconcussive symptoms and posttraumatic stress disorder after mild traumatic brain injury. J Nerv Ment Dis. 1999;187:302-305.
93 Ruff RM, Camenzuli L, Mueller J. Miserable minority: emotional risk factors that influence the outcome of a mild traumatic brain injury. Brain Inj. 1996;10:551-565.
94 Jordan BD, Relkin NR, Ravdin LD. Apolipoprotein E4 associated with chronic traumatic brain injury in boxing. JAMA. 1997;278:136-140.
95 Roberts GW, Allsop B, Bruton C. The occult aftermath of boxing. J Neurol Neurosurg Psychiatry. 1990;53:373-378.
96 Iverson GL, Brooks BL, Lovell MR, et al. No cumulative effects of one or two prior concussions. Br J Sports Med. 2006;40:72-75.
97 Guskiewicz KM, McCrea M, Marshall SW, et al. Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA concussion study. JAMA. 2003;290:2549-2555.
98 Moser RS, Schatz P. Enduring effects of concussion in youth athletes. Arch Clin Neuropsychol. 2002;17:91-100.
99 Moser RS, Schatz P, Jordan B. Prolonged effects of concussion in high school athletes. Neurosurgery. 2005;57:300-306.
100 Macciocchi SN, Barth JT, Littlefield L, et al. Multiple concussions and neuropsychological functioning in collegiate football players. J Athl Train. 2001;36:303-306.
101 Guskiewicz KM, Marshall SW, Broglio SP, et al. No evidence of impaired neurocognitive performance in collegiate soccer players. Am J Sport Med. 2002;30:157-162.
102 Iverson GL, Gaetz M, Lovell MR, et al. Cumulative effects of concussion in amateur athletes. Brain Inj. 2004;18:433-443.
103 Collins MW, Lovell MR, Iverson GL, et al. Cumulative effects of concussion in high school athletes. Neurosurgery. 2002;51:1175-1181.
104 Teasdale TW, Engberg AW. Cognitive dysfunction in young men following head injury in childhood and adolescence: a population study. J Neurol Neurosurg Psychiatry. 2003;74:933-936.
105 Matser EJT, Kessels AG, Lezak MD, et al. Neuropsychological impairment in amateur soccer players. JAMA. 1999;282:971-973.
106 Guskiewicz KM, Marshall SW, Bailes J, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57:719-726.
107 Guskiewicz KM, Marshall SW, Bailes J, et al. Recurrent concussion and risk of depression in retired professional football players. Med Sci Sports Exerc. 2007;39:903-909.
108 Cantu R, Voy R. Second impact syndrome: a risk in any sport. Phys Sportsmed. 1995;23:27-36.
109 Tavazzi B, Vagnozzi R, Signoretti S, et al. Temporal window and metabolic brain vulnerability to concussions: oxidative and nitrosative stresses—part II. Neurosurgery. 2007;61:390-396.
110 Sim A, Terryberry-Spohr L, Wilson KR. Prolonged recovery of memory functioning after mild traumatic brain injury in adolescent athletes. J Neurosurg. 2008;108:511-516.
111 Trudeau DL, Anderson J, Hansen LM, et al. Findings of mild traumatic brain injury in combat veterans with PTSD and a history of blast concussion. J Neuropsychiatry Clin Neurosci. 1998;10:308-313.
112 Schatz P, Pardini JE, Lovell MR, et al. Sensitivity and specificity of the ImPACT test battery for concussion in athletes. Arch Clin Neuropsychol. 2006;21:91-99.
113 van Kampen DA, Lovell MR, Pardini JE, et al. The value added of neurocognitive testing following sports-related concussion. Am J Sport Med. 2006;34:1630-1635.
114 McCrory P, Johnston K, Meeuwisse W, et al. Summary and agreement statement of the 2nd international conference on concussion in sport, Prague, 2004. Clin J Sport Med. 2005;15:48-57.
115 Fazio VC, Lovell MR, Pardini JE, et al. The relation between post concussion symptoms and neurocognitive performance in concussed athletes. Neuro Rehabilitation. 2007;22:207-216.
116 Pellman EJ, Lovell MR, Viano DC, et al. Concussion in professional football: neuropsychological testing—part 6. Neurosurgery. 2004;55:1290-1305.
117 Echemendia RJ, Putukian M, Makin RS, et al. Neuropsychological test performance prior to and following sports-related mild traumatic brain injury. Clin J Sport Med. 2001;11:23-31.
118 Lovell MR. Evaluation of the professional athlete. In: Bailes JE, Lovell MR, Maroon JC, editors. Sports-Related Concussion. St Louis: Quality Medical, 1999.
119 Lovell MR, Burke CJ. Concussion management in professional hockey. In: Cantu RE, editor. Neurologic Athletic Head and Spine Injury. Philadelphia: Saunders; 2000:109-116.
120 McClincy MP, Lovell MR, Pardini JP, et al. Recovery from sports concussion in high school and collegiate athletes. Brain Inj. 2006;20:33-39.
121 Alves W, Macciocchi SN, Barth JT. Postconcussive symptoms after uncomplicated mild head injury. J Head Trauma Rehabil. 1993;8:48-59.
122 Macchiochi SN, Barth JT, Alves W, et al. Neuropsychological functioning and recovery after mild head injury in collegiate athletics. Neurosurgery. 1996;39:510-514.
123 Ruff RM, Crouch JA, Troster AI, et al. Selected cases of poor outcome following a minor brain trauma: comparing neuropsychological and positron emission tomography assessment. Brain Inj. 1994;8:297-308.
124 Covassin T, Schatz P, Swanik CB. Sex differences in neuropsychological function and post-concussion symptoms of concussed college athletes. Neurosurgery. 2007;61:345-351.
125 Thornbury JR, Masters SJ, Campbell JA. Imaging recommendations for head trauma: a new comprehensive strategy. AJR Am J Roentgenol. 1987;149:781-783.
126 Borczuk P. Predictors of intracranial injury in patients with mild head trauma. Ann Emerg Med. 1995;25:731-736.
127 Haydel MJ, Preston CA, Mills TJ, et al. Indications for computed tomography in patients with minor head injury. N Engl J Med. 2000;343:100-105.
128 Stiell IG, Wells GA, Vandenheem K, et al. The Canadian CT Head Rule for patients with minor head injury. Lancet. 2001;357:1391-1396.
129 Kirkwood MW, Yeates KO, Wilson PE. Pediatric sport-related concussion: a review of the clinical management of an oft-neglected population. Pediatrics. 2006;117:1359-1371.
130 Radelet MA, Lephart SM, Rubinstein EN, et al. Survey of the injury rate for children in community sports. Pediatrics. 2002;110:e28.
131 Lovell M, Collins M, Bradley J. Return to play following sports-related concussion. Clin Sports Med. 2004;23:421-441.
132 McCrea M, Kelly JP, Kluge J, et al. Standardized assessment of concussion in football players. Neurology. 1997;48:586-588.
133 Collins MW, Lovell MR, McKeag DB. Current issues in managing sports concussion. JAMA. 1999;282:2283-2285.
134 Johnston KM, McCrory P, Mohtadi N, et al. Evidence-based review of sport-related concussion: clinical science. Clin J Sport Med. 2001;11:150-159.
135 Johnston KM, Bloom GA, Ramsay J, et al. Current concepts in concussion rehabilitation. Curr Sports Med Rep. 2004;3:316-323.
136 Iverson GL, Lovell MR, Collins MW, et al. Tracking recovery from concussion using ImPACT: applying reliable change methodology. Arch Clin Neuropsychol. 2002;17:770.
137 Iverson G, Lovell M, Collins M. Validity of ImPACT for measuring the effects of sports-related concussion. Arch Clin Neuropsychol. 17:769.
138 Iverson F, Gaetz M, Lovell M, et al. Validity of ImPACT for measuring processing speed following sports-related concussion. JCEN. 2005;27:683-689.
139 Aubry M, Cantu R, Dvorak J, et al. Summary and agreement statement of the 1st international symposium on concussion in sport, Vienna. Clin J Sport Med. 2002;12:6-11.
140 Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36:263-273.
141 Kerr L. Dental problems in athletes. Clin Sports Med. 1983;2:115-122.
142 Collins M, Lovell MR, Iverson GL, et al. Examining concussion rates and return to play in high school football players wearing newer helmet technology: a three-year prospective cohort study. Neurosurgery. 2006;58:275-284.
143 Orsay E, Holden JA, Williams J, et al. Motorcycle trauma in the state of Illinois: analysis of the Illinois Department of Public Health Trauma Registry. Ann Emerg Med. 1995;26:455-460.
144 Blignaut JB, Carstens IL, Lombard CJ. Injuries sustained in rugby by wearers and non-wearers of mouthguards. Br J Sports Med. 1987;21:5-7.
145 Stenger JM, Lawton EA, Wright JM, et al. Mouthguards: protection against shock to head, neck, and teeth. J Am Dent Assoc. 1964;69:273-281.
146 Hickey JC, Morris AL, Carlson LD, et al. The relation of mouth protectors to cranial pressure and deformation. J Am Dent Assoc. 1967;74:735-740.
147 Mihalik JP, McCaffrey MA, Rivera EM, et al. Effectiveness of mouthguards in reducing neurocognitive deficits following sports-related cerebral concussion. Dent Traumatol. 2006;23:14-20.
148 American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. text revision. 4th ed. Washington, DC: American Psychiatric Association. 2000.
149 Carroll LJ, Cassidy JD, Peloso PM, et al. Prognosis for mild traumatic brain injury: results of the WHO collaborating centre task force on mild traumatic brain injury. J Rehabil Med. 2004;36:84-105.
150 Levin HS, Mattis S, Ruff RM, et al. Neurobehavioral outcome following minor head injury: three-center study. J Neurosurg. 1987;66:234-243.
151 Boake C, McCauley SR, Levin HS, et al. Limited agreement between criteria-based diagnoses of postconcussional syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:493-499.
152 Kay T, Newman B, Cavallo M, et al. Toward a neuropsychological model of functional disability after mild traumatic brain injury. J Neurol Neurosurg Psychiatry. 1992;6:371-384.
153 Fann JR, Leonetti A, Jaffe WJ. Psychiatric illness and subsequent traumatic brain injury: a case control study. J Neurol Neurosurg Psychiatry. 2002;72:615-620.
154 Fann JR, Burlington B, Leonetti A, et al. Psychiatric illness following traumatic brain injury in an adult health maintenance organization population. Arch Gen Psychiatry. 2004;61:53-61.
155 Meares S, Shores EA, Batchelor J, et al. The relationship of psychological and cognitive factors and opioids in the development of the postconcussion syndrome in general trauma patients with mild traumatic brain injury. J Int Neuropsychol Soc. 2006;12:792-801.
156 King NS. Emotional, neuropsychological, and organic factors: their use in the prediction of persisting postconcussion symptoms after moderate and mild head injuries. J Neurol Neurosurg Psychiatry. 1996;61:75-81.
157 Hoffman D. Subtypes for postconcussional disorder [letter]. J Neuropsychiatry Clin Neurosci. 1994;6:332-333.
158 Chen JK, Johnston KM, Frey S, et al. Functional abnormalities in symptomatic concussed athletes: an fMRI study. Neuroimage. 2004;22:68-82.
159 Jantzen KJ, Anderson B, Steinberg FL, et al. A prospective functional MR imaging study of mild traumatic brain injury in college football players. AJNR Am J Neuroradiol. 2004;25:738-745.
160 McAllister TW, Sparling MB, Flashman LA, et al. Differential working memory load effects after mild traumatic brain injury. Neuroimage. 2001;14:1004-1012.
161 Lovell MR, Pardini JE, Welling JS, et al. Functional brain abnormalities are related to clinical recovery and time to return to play in athletes. Neurosurgery. 2007;61:352-360.
162 Lavoie ME, Dupuis F, Johnston KM, et al. Visual P300 effects beyond symptoms in concussed college athletes. J Contin Educ Nurs. 2004;26:55-73.
163 Chen JK, Johnston KM, Collie A, et al. A validation of the post concussion symptom scale in the assessment of complex concussion using cognitive testing and functional MRI. J Neurol Neurosurg Psychiatry. 2007;78:1231-1238.
164 Chen SH, Kareken DA, Fastenau PS, et al. A study of persistent post-concussion symptoms in mild head trauma using positron emission tomography. J Neurol Neurosurg Psychiatry. 2003;74:326-332.
165 Jacobs A, Put E, Ingels M, et al. Prospective evaluation of the Technetium-99m-HMPAO SPECT in mild and moderate traumatic brain injury. J Nucl Med. 1994;35:6942-6947.
166 Agrawal D, Gowda NK, Bal CS, et al. Is medial temporal injury responsible for pediatric postconcussion syndrome? A prospective controlled study with single-photon emission computerized tomography. J Neurosurg. 2005;102:167-171.
167 Gaetz M, Bernstein D. The current statues of electrophysiologic procedures for the assessment of mild traumatic brain injury. J Head Trauma Rehabil. 2001;6:386-405.
168 Geets W, deZegher F. EEG and brainstem abnormalities after cerebral concussion. Short term observations. Acta Neurol Belg. 1985;85:277-283.
169 Roberts AH. Brain damage in boxers: a study of the prevalence of traumatic encephalopathy among ex-professional boxers. London: Pitman; 1969.