Chapter 51 Milagro (Beta-Tricalcium Phosphate, Polylactide Co-Glycolide Biocomposite) Interference Screw for Anterior Cruciate Ligament Reconstruction
Introduction
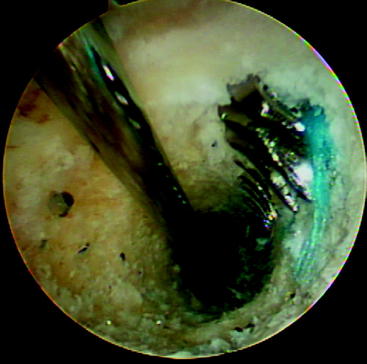
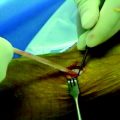
The initial interference fixation screws were made from metal and provided screw fixation for anterior cruciate ligament (ACL) reconstructions. Biodegradable interference fixation screws subsequently gained wide acceptance after their introduction in the early 1990s.1–5 The benefits of these biodegradable interference screws include reduction in the concerns previously associated with metal implants, including difficulties in postoperative imaging, reduced graft laceration during insertion, less chance of screw divergence during insertion, easier revision surgery (Fig. 51-1), and fewer problems with secondary arthritic procedures that might require the complete removal of a metal screw. In addition, the load to failure (LTF) strength of these screws is sufficient to allow for an aggressive rehabilitation program. Potential complications associated with biodegradable interference screws include the risk of screw breakage during insertion, decreased holding strength when compared with a metal alternative, and inflammatory reactions that could lead to lytic changes and cyst formation.
Poly-L-lactic acid (PLLA) is the most common material used in biodegradable interference screws. These screws offer effective graft fixation with little evidence of adverse inflammatory reactions, and many years pass before any material degradation occurs.1–8 Interference screws made of lactic acid copolymers containing dextro and levo stereoisomers subsequently became available, as did copolymers of polylactic acid and polyglycolide. The polymer degradation is more rapid with poly D, L-lactide (PDLLA) implants than pure PLLA in animal studies.9–11
Biomechanical and Biochemical Data
Implant degradation proceeds through five stages: hydration, depolymerization, loss of mass integrity, absorption, and elimination. How rapidly an implant is degraded is influenced by the polymer of which the implant is made, the degree of crystallization of that polymer, the initial mass of the polymer present (implant size), surface coverings, whether the polymer is self-reinforced, the processing technique used (machining or injection molding and sterilization technique), and the environment in which the implant is situated.12 In addition, the degradation mechanics of different polymers may differ considerably based on the hydrophilic or hydrophobic nature of the different polymers.
Degradation starts at the amorphous phase of the implant and leads to fragmentation of the material to smaller parts, which are then phagocytosed primarily by macrophages and polymorphonuclear leukocytes.13,14 The lactic acid component is broken down by hydrolysis. The resultant monomers enter the Krebs cycle and are further dissimilated into carbon dioxide and water.15 In addition to hydrolytic chain scission, glycolic acid monomers are degraded by the enzymatic activity of esterases and carboxypeptidases.16
All biodegradable materials cause some inflammatory response. The longer the degradation course, the less visible the response will be. Usually there is a mild, nonspecific tissue response with fibroblast activation and the invasion of macrophages, multinucleated foreign body giant cells, and polymorphonuclear leukocytes during the final stages of degradation. Because of the more rapid degradation associated with polyglycolic acid (PGA), there have been some foreign body reactions with varying degrees of severity ranging from mild osteolytic changes to intense granulomatous inflammatory soft tissue lesions that necessitate surgical intervention.17,18
Concerns about implants composed of pure PGA have led to the development of PGA copolymers that still have a more rapid rate of absorption compared with PLLA implants, but the literature supports their use with excellent clinical results. Lajtai et al19,20 reported good results with a lactide/glycolide copolymer screw (85/15, D, L lactide/glycolide). Using magnetic resonance imaging (MRI scans), the screw was shown to remain intact for 4 months and then disappear by 6 months. Five years after implantation, the screw was completely reabsorbed and evidently replaced with new bone. Morgan et al21 evaluated a PLLA interference screw removed en bloc from a patient 2.5 years after insertion. The histological examination and molecular weight measurements showed a 75% decrease in the molecular weight of the screw with implant fragmentation and new bone formation adjacent to the screw. This dramatically contrasted with the MRI evaluation of the patient, which showed the presence of a clear screw outline. MRI evaluations of PLLA screw show no evidence of any progressive absorption 4 years after implantation.22 However, a recent computed tomography (CT) evaluation of patients who underwent patellar tendon autograft ACL reconstruction using PLLA screws at least 7 years earlier demonstrated complete removal of the PLLA screws without any significant bone ingrowth into the screw site.23
Clinical Information
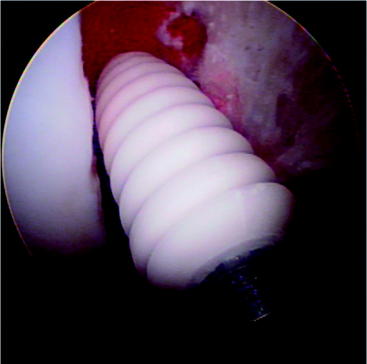
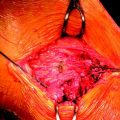
The Milagro screw can be used for femoral or tibial fixation for soft tissue or bone–tendon–bone (BTB) autografts or allografts (Fig. 51-2). It is available in various diameters from 7 to 12 mm and in 23-, 30-, and 35-mm lengths. The Milagro screw is made from a polymer composite, Biocryl Rapide. As previously mentioned, this material consists of 30% osteoconductive ß-TCP and 70% PLGA. The poly (lactide-co-glycolide) copolymer is composed of 15% PGA and 85% PLLA. This ratio of PGA to PLLA was chosen following animal studies to allow for a faster yet controlled absorption. This copolymer does not contain any of the D-isomer of lactic acid.
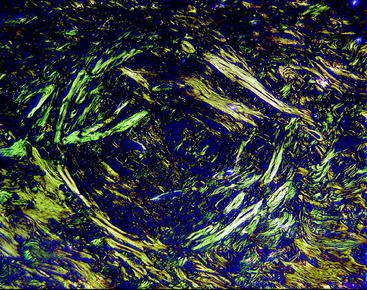
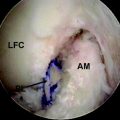
This material was recently evaluated in the lateral femoral cortex of mature beagle dogs.24 Rods of either Biocryl Rapide or PLLA measuring 3 × 10 mm were inserted into defects in the cortex and evaluated at intervals up to 24 months. Histological evaluation at intervals looked for reabsorption of the material and cracks, cell infiltrations, erosions, and fragmentation of the implants. At 24 months postimplantation, clear differences existed between the PLLA rods and the Biocryl Rapide rods (Fig. 51-3). The reabsorption of the Biocryl Rapide was nearly complete at 24 months, and radiographic bridging was observed at the Biocryl Rapide sites but not at the PLLA sites. No evidence of inflammatory reaction or cellular necrosis was observed. By 24 months, the entire cross-section of the Biocryl Rapide test rods was absorbed and replaced by normal bone or bone plus fibrous tissue or adipose tissue. The circular orientation of the new bone was seen under polarized light.
Pearls
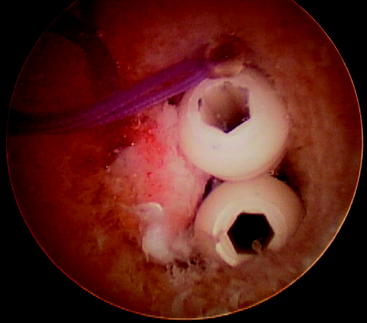
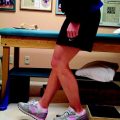
Once the tibial screw is in place, tug on the graft sutures to demonstrate that there is good tension on the graft and no movement of the graft in the joint. If graft movement is observed, the graft should be retensioned and a second screw stacked beside the first to achieve secure fixation (Fig. 51-4).
1 Barber FA, Elrod BF, McGuire DA, et al. Preliminary results of an absorbable interference screw. Arthroscopy. 1995;11:537-548.
2 Marti C, Imhoff AB, Bahrs C, et al. Metallic versus bioabsorbable interference screw for fixation of bone-patellar tendon-bone autograft in arthroscopic anterior cruciate ligament reconstruction. A preliminary report. Knee Surg Sports Traumatol Arthrosc. 1997;5:217-221.
3 Barber FA. Tripled semitendinosus-cancellous bone anterior cruciate ligament reconstruction with bioscrew fixation. Arthroscopy. 1999;15:360-367.
4 Tuompo P, Partio EK, Jukkala-Partio K, et al. Comparison of polylactide screw and expansion bolt in bioabsorbable fixation with patellar tendon bone graft for anterior cruciate ligament rupture of the knee. A preliminary study. Knee Surg Sports Traumatol Arthrosc. 1999;7:296-302.
5 McGuire DA, Barber FA, Elrod BF, et al. Bioabsorbable interference screws for graft fixation in anterior cruciate ligament reconstruction. Arthroscopy. 1999;15:463-473.
6 Warden WH, Friedman R, Teresi LM, et al. Magnetic resonance imaging of bioabsorbable polylactic acid interference screws during the first 2 years after anterior cruciate ligament reconstruction. Arthroscopy. 1999;15:474-480.
7 Barber FA, Elrod BF, McGuire DA, et al. Bioscrew fixation of patellar tendon autografts. Biomaterials. 2000;21:2623-2629.
8 Kotani A, Ishii Y. Reconstruction of the anterior cruciate ligament using poly-L-lactide interference screws or titanium screws: a comparative study. Knee. 2001;8:311-315.
9 Sedel L, Chabot F, Christel P, et al. Biodegradable implants in orthopedic surgery. Rev Chir Orthop Reparatrice Appar Mot. 1978;64(Suppl 2):92-96.
10 Chen CC, Chueh JY, Tseng H, et al. Preparation and Characterization of biodegradable PLA polymeric blends. Biometrials. 2003;24:1167-1173.
11 Barber FA. Poly-D, L-lactide interference screws for anterior cruciate ligament reconstruction. Arthroscopy. 2005;21:804-808.
12 Daniels AU, Chang MKO, Andriano KP. Mechanical properties of biodegradable polymers and composites proposed for internal fixation of bone. J Appl Biomater. 1990;1:57-78.
13 Lam KH, Schakenrad JM, Esselbrugge H, et al. The effect of phagocytosis of poly (L-lactic acid) fragments on cellular morphology and viability. J Biomed Mater Res. 1993;27:1569-1577.
14 Tabata Y, Ikada Y. Macrophage phagocytosis of biodegradable microspheres composed of L-lactic acid/glycolic acid homo- and copolymers. J Biomed Mater Res. 1988;22:837-858.
15 Hollinger JO, Battistone GC. Biodegradable bone repair materials. Synthetic polymers and ceramics. Clin Orthop. 1986;207:290-305.
16 Williams F, Mort E. Enzyme-accelerated hydrolysis of polyglycolic acid. J Bioengin. 1977;1:231-238.
17 Böstman O, Pihlajamäki H, Partio E, et al. Clinical biocompatibility and degradation of polylevolactide screws in the ankle. Clin Orthop. 1995;320:101-109.
18 Böstman O. Osteolytic changes accompanying degradation of absorbable fracture fixation implants. J Bone Joint Surg. 1991;73B:679-682.
19 Lajtai G, Hummer K, Aitzetmuller G, et al. Serial magnetic resonance imaging evaluation of a bio-absorbable interference screw and the adjacent bone. Arthroscopy. 1999;15:481-488.
20 Lajtai G, Schmiedhuber G, Unger F, et al. Bone tunnel remodeling at the site of bio-degradable interference screws used for anterior cruciate ligament reconstruction—five year follow up. Arthroscopy. 2001;17:597-602.
21 Morgan CD, Gehrmann RM, Jayo MJ, et al. Histologic findings with a bio-absorbable anterior cruciate ligament interference screw explant after 2.5 years in vivo. Arthroscopy. 2002;18:E47.
22 Radford MJ, Noakes J, Read J, et al. The natural history of a bioabsorbable interference screw used for anterior cruciate ligament reconstruction with a 4-strand hamstring technique. Arthroscopy. 2005;21:707-710.
23 Barber FA, Dockery WD. Long term absorption of poly L-lactic acid interference screws. Arthroscopy. 2006;22:820-826.
24 Poandl T, Trenka-Benthin S, Azri-Meehan S, et al. A new faster degrading biocomposite material: long-term in-vivo tissue reaction and absorption. AANA Annual Meeting e-poster (E-09). 2005. Vancouver