Chapter 85D Microwave ablation and emerging technologies for liver tumors
Overview
Hepatic malignancies are common, with more than 1 million diagnosed each year worldwide. Tumors can be primary, as with hepatocellular carcinoma (HCC) or intrahepatic cholangiocarcinoma (IHC), or they can be secondary, as with colorectal liver metastases (CLM), neuroendocrine malignancy, or metastases from other primary cancers, such as those of the breast or lung. Many patients come to medical attention with advanced disease that is not amenable to surgical therapy. Unresectable disease carries a poor prognosis, because systemic chemotherapeutic agents and radiation therapy have limited efficacy in treating many of these diseases. As a result of the limited treatment options in this particular patient population, liver-directed regional therapies have evolved that include selective internal radiation therapy (Cianni et al, 2009), transarterial chemoembolization (TACE) (Rathore et al, 2010), portal vein embolization (PVE) (Manizate et al, 2010), and ablative technologies.
Ablation is not a new concept; it has been used in medicine for more than 100 years, initially as a therapy designed for patients with unresectable lesions (Halsted, 1894); however, advancements in medicine have transformed ablation into an effective locoregional therapy with an improved perioperative profile for morbidity and mortality and options of performing the procedure using an open, laparoscopic, or percutaneous approach. It has evolved from injecting ethanol (Uflacker et al, 1986) to employing different energy sources, such as cryoablation (Iannitti et al, 1998), radiofrequency ablation (RFA) (Iannitti et al, 2002), and microwave ablation (MWA) (Simon et al, 2006) to achieve tumor destruction (see Chapters 85A through 85C). Current ablation therapies also provide the flexibility to perform procedures in an ambulatory setting to allow treatment of patients whose disease is not amenable to resection. The application of ablative technology does not necessarily preclude patients from future operations, nor does it compromise their chance for transplantation (Casaccia et al, 2009); however, complications resulting from ill-advised ablative procedures may complicate subsequent attempts at resection.
Thermoablation is an effective modality, because irreversible thermal destruction of tissue occurs when temperatures above 50° C are maintained for an interval of 4 to 6 minutes (Larson et al, 1996). Vaporization and carbonization occur when tissue temperature is raised rapidly to over 100° C. Effective ablation relies on an adequate margin around the lesion, ideally 1 cm, to ensure complete eradication of microscopic foci. Large ablation volume may require multiple applications of thermal energy.
Physics of Microwave Energy
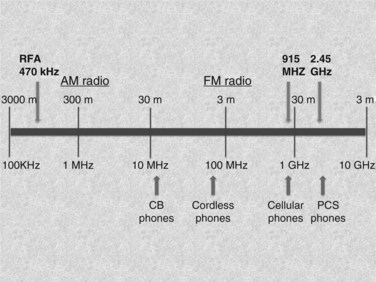
MWA that achieves heat destruction of tissue involves both active and passive heating. The active heating process of microwave energy requires the presence of dielectric molecules, such as water, to function. MWA is an excellent option for thermal ablation of solid organs, such as the liver (Brace, 2009a, 2009b). Clinical microwave energy operates at a frequency of 900 MHz to 10 GHz (Fig. 85D.1), which is much higher than radiofrequency (460 to 480 kHz). As a dipole molecule, water is affected by the applied electromagnetic field broadcasted by the microwave antenna. This property is called dielectric permittivity (ε). Water molecules orient themselves to the rapidly changing polarity of the electromagnetic field, and vibratory energy generates heat production. This process is called dielectric hysteresis. Microwaves emit nonionizing radiation for heating, and this produces homogeneous heating within the field regardless of the tissue types; this distinguishes MWA from monopolar RFA in terms of mechanism of heating and makes it a clinically superior method for ablation. The passive phase of microwave heating is by conduction of heat beyond the active heating zone and is susceptible to local tissue factors, such as heat and current sinking, which will be discussed later in this chapter.
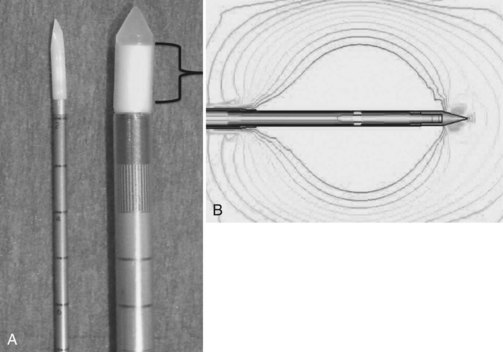
The MWA systems used in the United States today are 915 MHz, 2.45 GHz, and 9.2 GHz; however, only the 915-MHz and 2.45-GHz systems are commercially available for tumor ablation. The 915-MHz and 2.45-GHz systems are more effective for large tissue destruction than monopolar RFA, and the 915-GHz system is currently under investigation for ablating lesions in the breast. Microwave energy can be generated through a magnetron or solid-state amplifier (Brace, 2009a, 2009b), and the antenna broadcasts the electromagnetic energy to the target tissue. The coaxial cable consists of an inner and outer conductor, and dielectric material is placed between the two layers. At its tip, the outer conductor is stopped to expose the inner conductor for broadcasting the microwave energy (Fig. 85D.2). This inner conductor is covered in a ceramic pointed tip for insertion into the tissue, and microwave energy can pass freely through the ceramic. The power loss is influenced by power output, cable diameter, and antenna design. Given its broadcasting characteristic, the microwave system does not require a grounding pad.
Large ablative volume can be created by increasing the power and duration of the application. Several factors influence the final ablative size, including water content of the tissue, power output at the generator, power loss at the cable, antenna design, formation of clustered antennae, and the number of applications. Various tissue types exhibit different responses to microwave energy, and liver tissue usually exhibits a noticeable response to MWA (Hines-Peralta et al, 2006). Surgeons must understand the relationship between the different properties of tissue and the energy modality used to achieve successful ablation.
Local Tissue Factors That Affect Thermoablation
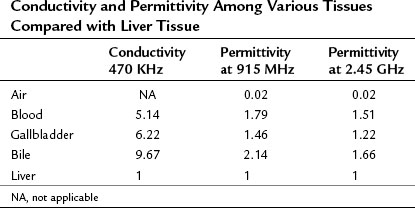
Coagulation necrosis of tissue is proportional to energy deposition minus heat loss (Goldberg et al, 2000). Hence, local tissue factors such as blood flow and tissue temperature affect energy deposition. Close proximity to blood vessels causes diversion of the current and decreases the amount of energy generated from the current; this is termed current sinking. The final ablative size is affected, because radiofrequency relies on heat transfer from the tip of the applicator, and the cooling from the vessel shunts the heat energy away; this phenomenon is termed heat sinking. Microwave technology also encounters such limitations but to a much lesser extent (Stauffer et al, 2003), because the propagation of microwave energy relies on the dielectric permittivity of the tissue, and it stays fairly constant along the broadcasted electromagnetic field (Table 85D.1). It can overcome the effect of current and heat sinking within its field and result in deeper heat penetration and more uniform ablative size. Outside the active heating zone, heat is propagated by conduction and is therefore susceptible to local factors such as heat sinking.
Another consideration is tissue desiccation and charring by the applicator during the heating process. Both conditions can significantly decrease the final ablative volume for RFA, because it requires a complete electric circuit to function. Acting as electric insulators, the resultant increase of impedance decreases the amount of current deposition and thereby decreases heat production. Moreover, if the target temperature is reached rapidly, above 100° C, the intracellular content vaporizes and carbonizes. The gas formation acts as an electric insulator that increases the impedance and hinders the heat diffusion (Dadd et al, 1996); both decrease the amount of current deposition and result in a less uniform ablative zone. Unlike radiofrequency, microwaves broadcast energy throughout the electromagnetic field and can achieve high power density uniformly, which results in deeper heat penetration and a more consistent ablative size.
915 MHz Versus 2.45 GHz
MWA, like RFA, can be carried out via three approaches: 1) open, 2) laparoscopic, and 3) percutaneous. The first clinical microwave system, Microtaze (Heiwa Electronics Industry, Tokyo), was developed in the 1990s. Clinical use was limited because of its 1.5-cm ablation zone (Saitsu et al, 1991, 1993), but 915-MHz and 2.45-GHz systems are currently available (Fig. 85D.3). Both systems were introduced to the United States market in 2008: the Viva Wave system (Valleylab, Boulder, CO) and the MTA microwave system (Microsulis Medical, Denmead, UK). Advancements in microwave technology and better antenna design have significantly improved ablative capabilities, and large-volume ablation can be reliably achieved.
The 2.45-GHz system is used more often in Europe and Asia, whereas the 915-mHz system is used in the United States; both are capable of producing an adequate ablation volume. The 2.45-GHz system uses a single antenna, and the 915-mHz employs a multiantenna array powered by separate generators. An advantage of using multiple antennae in different formations is to achieve various shapes and sizes of ablations (Simon et al, 2006). The microwave antenna is not composed of alloy but is coated with polytetrafluoroethylene (PTFE). It is not as rigid as the RFA metal applicator, and care should be taken during manipulation of the microwave antenna to prevent breakage at the tip of the antenna.
Clinical Results of Microwave Ablation for Hepatocellular Carcinoma (See Chapter 80)
The first open microwave application for tumor destruction was reported in the early 1990s in Japan, when Seki and colleagues (1994) reported a series of 24 patients with HCC with a 92% survival at 3 years. The application of microwave technology has since expanded to treat colorectal liver metastases and metastases from other primary tumors; this will be discussed later in this chapter.
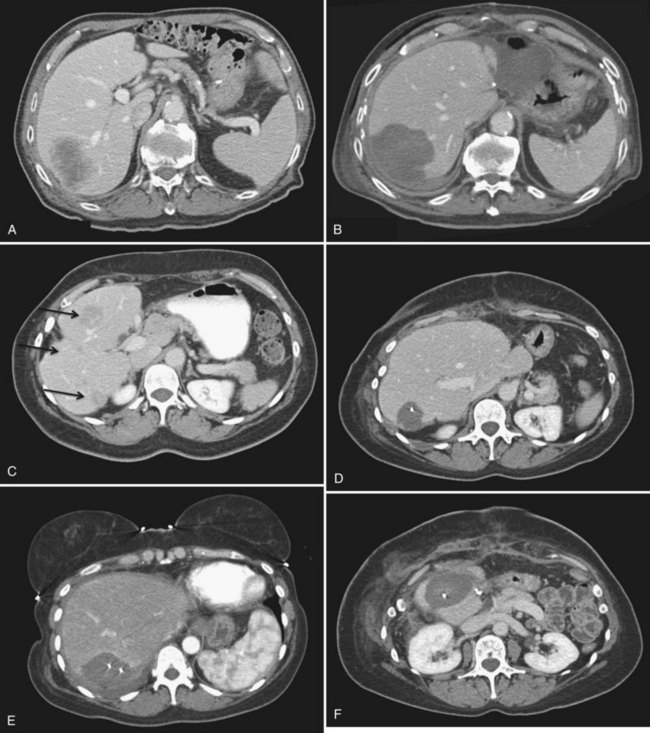
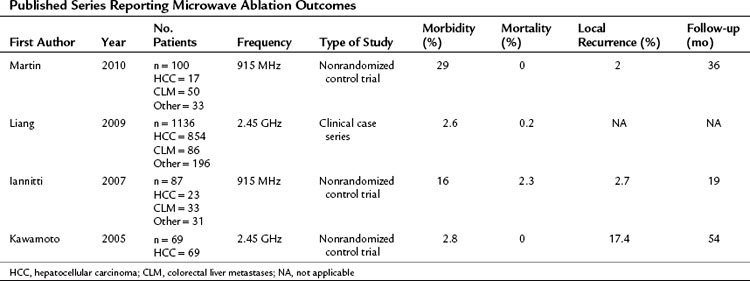
Clinical trials have evaluated the efficacy of MWA for treating HCC. In a Phase I trial that evaluated the safety of MWA, 11 patients underwent hepatoma ablation followed by resection (Simon et al, 2006). The complete destruction of viable tumor cells was confirmed by immunohistochemistry in the ablation zone. The average volume of ablation was 5.5 cm in diameter using three clustered antennae at 45 W for 10 minutes. The Phase II trial enrolled 87 patients for whom MWA was the primary treatment (Iannitti et al, 2007). In 94 ablation sessions, 224 lesions with a mean diameter of 3.6 cm were treated (Fig. 85D.4). The local recurrence rate was 2.7%, and regional recurrence was 43% during 19 months of follow-up; overall mortality rate was 2.3% with no procedure-related deaths. Major complications included two patients with postoperative fluid collections that required a drainage procedure and five patients with minor skin burn at the needle tract. This clinical trial proved that using MWA for hepatic tumors is a safe, effective treatment option with a low complication rate. Multiple series were subsequently published reaffirming the role of MWA in treating liver lesions (Table 85D.2; Kawamoto et al, 2005; Martin et al, 2009).
Clinical Results of Colorectal Liver Metastases (See Chapter 81A)
The most common hepatic lesions result from metastatic colorectal cancer (CRC). More than 50,000 patients die from diseases related to CRC yearly, and up to 60% of patients with CRC have metastasis to other organs. Despite advances in neoadjuvant therapy, roughly 60% of the patients are not candidates for hepatic resection, which remains the single most effective therapy. The 3-year survival rate is 30% for patients undergoing open resection, and the recurrence rate at 3 years is 54% (Wagner et al, 1984; Scheele et al, 1990). The low survival rate and high recurrence rate are due to the multicentricity of the disease and the presence of systemic microscopic foci.
The clinical results of MWA for colorectal liver metastasis are encouraging. The 3-year survival and local recurrence rate from multiple clinical series are comparable with published data from open resection (Liang et al, 2006; Shibata et al, 2000; Simon et al, 2006). In a small randomized controlled trial (RCT) with 30 patients, the overall survival was similar between the hepatectomy and MWA arms (Shibata et al, 2000). The lack of a large RCT makes a direct comparison of open resection with MWA difficult, and it may be difficult to obtain institutional review board approval for such a study in the United States on ethnical grounds.
Clinical Results for Neuroendocrine Tumors and Metastasis from Other Primary Tumors (See Chapter 81B)
Lesions arising from neuroendocrine or other primary tumors should be evaluated for resection when clinically appropriate; however, curative resection is not possible in about 90% of the cases (Knigge et al, 2008). The focus of treatment should be palliative, and symptom relief can usually be achieved. Cytoreduction of the tumor can improve survival, with 5-year survival ranging from 40% to 60%. Most patients can be symptom free for 2 years (Weitz et al, 2005). Careful selection of therapeutic options combining different liver-directed modalities such as ablation, chemoembolization, selective internal radiation therapy (SIRT), and embolization may reduce the tumor burden and improve overall survival.
Microwave Ablation: Specific Complications and Future Applications
MWA heats the entire electromagnetic field, and several potential complications may arise from this modality. Technical advances have made ablation near large vessels possible, which increases the potential for thrombus in the hepatic vein. The true incidence of hepatic vein thrombus (HVT) related to MWA is unknown. We identified three such cases in 122 MWA applications, all without symptoms, and the thrombus resolved after anticoagulation. We have used a porcine model to reproduce HVT, and it appears that puncturing the hepatic vein during MWA is a risk factor (Sindram, 2011). Surprisingly, the proximity of MWA to the hepatic vein is only associated with minimal endothelial damage.
Skin burn at the needle tract during laparoscopic/percutaneous application is an MWA-specific injury that has been reported in the literature (Iannitti et al, 2007). It can occur when the power output is increased, and the outer conductor does not sufficiently shield the microwave energy (Liang et al, 2009). The risk of skin burn can be minimized by reducing the inward angulation of clustered antennae, spacing antennae 1.5 cm apart, and applying an internal cooling tip with running saline to reduce the temperature (Sindram, 2011).
Microwave technology offers superior physical properties compared with radiofrequency, and it makes ablating larger lesions in the liver possible. One series reported that large-volume ablation can be effectively and safely done with a recurrence rate of 22% for HCC lesions in sizes ranging from 3 to 7 cm (Yin et al, 2009).
Emerging Technologies
Evaluation of Surgical Device Innovation
Surgical innovation is rapid, and new technologies are developed so quickly that clinical validation is sparse. Traditionally, many surgical procedures were developed on a trial and error basis, which raised concern for patient safety during product development (Ergina et al, 2009). A framework to evaluate the safety of new devices is necessary to protect patients, but the framework must be flexible, so that regulatory bodies do not obstruct innovation. Surgeons involved in product development must have a thorough understanding of how the device works to minimize patient risk. The rapid emergence of different ablation modalities has made comparison among various technologies challenging. The introduction of a new device is usually based on case series to establish safety, followed by an RCT to prove its efficacy, before it is eventually approved by the FDA.
To critically evaluate ablation, a standardized evaluation of patient demographics that include preoperative chemotherapy, TACE, or other therapy should be recorded for a matched comparison (Barkun et al, 2009). The outcomes of ablation should be defined (local and regional recurrence, etc.) and the follow-up protocol outlined so results of different trials can be compared. Moreover, the complication profile should be uniform and based on a system similar to the Clavien-Dindo classification for surgical complications (Clavien et al, 2009). An online database should be created with dedicated personnel to report data. These measures would facilitate collaboration of different institutions and provide more reliable data for patient safety and product development.
Real-Time Three-Dimensional Virtual Reality–Guided Targeting
The advancement in ablation technology has proved that large-volume ablation is a reality, but this capacity is useless if the targeting of a lesion is inaccurate. The complete destruction of a lesion requires an adequate margin, and achievement of this margin often requires multiple applications for adequate overlap of the ablation zone. The use of 2D ultrasound has greatly enhanced the ability to identify a target lesion, but deploying the microwave requires complex coordination between interpreting the information on the monitor and positioning the applicator to track the course from the parenchyma into the tumors. This complicated process has a learning curve, and even in the hands of an expert, improper applicator position can occur. So achieving an adequate 3D overlap of the ablative zone is challenging, especially when only 2D information is available. To aid the surgeon in this process, a real-time 3D system was developed by InnerOptic (Hillsborough, NC). Using either an electromagnetic field generator with sensors or stereoscopic infrared cameras with reflectors, real-time 3D information is generated into virtual reality images and projected on a true 3D stereoscopic screen (Fig. 85D.5). This provides valuable information for targeting lesions, especially from an angle that is out of the plane of the US probe. The safety and accuracy of various procedures such as biopsy, venous catheter placement, and percutaneous ablation might be improved once this system is available commercially.
Other Ablation Modalities
Laser Interstitial Thermotherapy
The application of lasers as a source of heat is not a new concept. Unlike laser therapy used in other industries, LITT uses a slow heating source, the neodymium : yttrium aluminum garnet (Nd : YAG) diode laser. It induces thermal coagulation by emitting laser light at a wavelength of 1064 nm from a low energy source of 3 to 20 W. After insertion of the laser fiber, LITT uses a slow heating process to prevent tissue coagulation, which reduces the penetration of the laser and limits the size of the ablation. LITT requires the photon of the laser to interact with the chromophores in the tissue to generate heat; hence conditions such as carbonization and vaporization, which decrease optimum penetration, significantly decrease the size of the ablation zone (Izzo, 2003; Sturesson, 1998).
Despite changes in the technology, LITT usually produces a relatively small lesion of 1 to 1.5 cm. Alterations of tip design have included using sapphire-tipped laser fibers, cool-tip applicators, and applications of different quartz diffusers; however, no significant improvement in ablative volume was observed with any of these (Castren-Persons et al, 1992; Heisterkamp et al, 1997). To overcome this deficit, a multifiber system was developed that is capable of producing lesions as large as 7 cm (Izzo, 2003); however, clinical use of this system is not widespread. Case series have reported favorable survival rates of 74% and 30% at 2 and 5 years, respectively (Mack et al, 2001), but no randomized trial has compared LITT with other ablative modalities, and further investigation is needed.
High-Intensity Focused Ultrasound
HIFU was first used to treat the eye, prostate, and kidney, but further investigation in ablating liver lesions has shown some promising results. A randomized trial compared TACE with HIFU, and a combination HIFU/TACE group showed a survival benefit of 6 months over a TACE-alone group (Wu et al, 2005). Research is ongoing for the treatment of liver lesions using HIFU, and clinical series have shown HIFU to be safe and effective, even near large blood vessels (Li et al, 2007; Zhang et al, 2009).
Irreversible Electroporation
A change in cell permeability caused by pulsed electrical energy, a phenomenon called electroporation, has been known for centuries and has been studied extensively in gene therapy and electrochemotherapy. Initially regarded as an undesired side effect, irreversible electroporation (IRE) is now under active investigation for hepatic tumor ablation (Miller, 2005). IRE works by inducing cell necrosis after application of 1000 to 3000 V for a duration of milliseconds to microseconds (Padma et al, 2009). IRE is in its own class, because it does not involve thermal ablation; hence, local tissue factors such as heat or current sinking do not apply. Currently, no data for human use are available (Lencioni et al, 2010). An IRE system is commercially available now, and clinical investigation is ongoing to evaluate its safety and efficiency.
Barkun JS, et al. Evaluation and stages of surgical innovations. Lancet. 2009;374:1089-1096.
Brace CL. Microwave ablation technology: what every user should know. Curr Probl Diagn Radiol. 2009;38:61-67.
Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol. 2009;38:135-143.
Casaccia M, et al. Laparoscopic staging and radiofrequency of hepatocellular carcinoma in liver cirrhosis: a “bridge” treatment to liver transplantation. Hepatogastroenterology. 2009;56:793-797.
Castren-Persons M, et al. Laser-induced hyperthermia: comparison of two different methods. Lasers Surg Med. 1992;12:665-668.
Cianni R, et al. Selective internal radiation therapy with SIR-spheres for the treatment of unresectable colorectal hepatic metastases. Cardiovasc Intervent Radiol. 2009;32:1179-1186.
Clavien PA, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196.
Dadd JS, et al. Tissue impedance as a function of temperature and time. Biomed Sci Instrum. 1996;32:205-214.
Ergina PL, et al. Challenges in evaluating surgical innovation. Lancet. 2009;374:1097-1104.
Goldberg SN, et al. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000;174:323-331.
Halsted WS. The results of operations for the cure of cancer of the breast performed at the Johns Hopkins Hospital from June, 1889, to January, 1894. Ann Surg. 1894;20:497-555.
Heisterkamp J, et al. Heat-resistant cylindrical diffuser for interstitial laser coagulation: comparison with the bare-tip fiber in a porcine liver model. Lasers Surg Med. 1997;20:304-309.
Hines-Peralta AU, et al. Microwave ablation: results with a 2.45 GHz applicator in ex vivo bovine and in vivo porcine liver. Radiology. 2006;239:94-102.
Iannitti DA, et al. Laparoscopic cryoablation of hepatic metastases. Arch Surg. 1998;133:1011-1015.
Iannitti DA, et al. Hepatic radiofrequency ablation. Arch Surg. 2002;137:422-426.
Iannitti DA, et al. Hepatic tumor ablation with clustered microwave antennae: the US phase II trial. HPB (Oxford). 2007;9:120-124.
Izzo F. Other thermal ablation techniques: microwave and interstitial laser ablation of liver tumors. Ann Surg Oncol. 2003;10:491-497.
Kawamoto C, et al. Long-term outcomes for patients with solitary hepatocellular carcinoma treated by laparoscopic microwave coagulation. Cancer. 2005;103:985-993.
Knigge U, et al. Interventional treatment of neuroendocrine liver metastases. Surgeon. 2008;6:232-239.
Larson TR, et al. Temperature-correlated histopathologic changes following microwave thermoablation of obstructive tissue in patients with benign prostatic hyperplasia. Urology. 1996;47:463-469.
Lencioni R, et al. Hepatocellular carcinoma: new options for image-guided ablation. J Hepatobiliary Pancreat Surg. 2010;17:399-403.
Li YY, et al. Short and long term efficacy of high intensity focused ultrasound therapy for advanced hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22:2148-2154.
Liang P, et al. Evaluation of long-term therapeutic effects of ultrasound-guided percutaneous microwave ablation of liver metastases. Zhonghua Yi Xue Za Zhi. 2006;86:806-810.
Liang P, et al. Malignant liver tumors: treatment with percutaneous microwave ablation—complications among cohort of 1136 patients. Radiology. 2009;251:933-940.
Mack MG, et al. Percutaneous MR imaging-guided laser-induced thermotherapy of hepatic metastases. Abdom Imaging. 2001;26:369-374.
Manizate F, et al. Liver functional reserve estimation: state of the art and relevance for local treatments: the Western perspective. J Hepatobiliary Pancreat Surg. 2010;17:385-388.
Martin RC, et al. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol. 2009;17:171-178.
Miller L. Cancer cells ablation with irreversible electroporation. Technol Cancer Res Treat. 2005;4:699-705.
Padma S, et al. Liver tumor ablation: percutaneous and open approaches. J Surg Oncol. 2009;100:619-634.
Rathore R, et al. Phase I study of hepatic arterial infusion of oxaliplatin in advanced hepatocellular cancer: a Brown University Oncology Group study. Am J Clin Oncol. 2010;33:43-46.
Saitsu H, et al. Investigation of coagulo-necrotic therapy using microtase and liver resection of small hepatocellular carcinoma based on changes in NK activity and T cell subsets. Gan To Kagaku Ryoho. 1991;18:2615-2617.
Saitsu H, et al. Investigation of microwave coagulo-necrotic therapy for 21 patients with small hepatocellular carcinoma less than 5 cm in diameter. Nippon Geka Gakkai Zasshi. 1993;94:359-365.
Scheele J, et al. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241-1246.
Seki T, et al. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer. 1994;74:817-825.
Shibata T, et al. Microwave coagulation therapy for multiple hepatic metastases from colorectal carcinoma. Cancer. 2000;89:276-284.
Simon CJ, et al. Intraoperative triple antenna hepatic microwave ablation. AJR Am J Roentgenol. 2006;187:W333-W340.
Sindram D, et al. Collagen-elastin ratio predicts burst pressure of arterial seals created using a bipolar vessel sealing device in a porcine model. Surg Endosc. 2011;25(8):2604-2612.
Stauffer PR, et al. Phantom and animal tissues for modelling the electrical properties of human liver. Int J Hyperthermia. 2003;19:89-101.
Sturesson C. Interstitial laser-induced thermotherapy: influence of carbonization on lesion size. Lasers Surg Med. 1998;22:51-57.
Uflacker R, et al. Ablation of tumor and inflammatory tissue with absolute ethanol. Acta Radiol Diagn (Stockh). 1986;27:131-138.
Wagner JS, et al. The natural history of hepatic metastases from colorectal cancer: a comparison with resective treatment. Ann Surg. 1984;199:502-508.
Weitz J, et al. Partial hepatectomy for metastases from noncolorectal, nonneuroendocrine carcinoma. Ann Surg. 2005;241:269-276.
Wu F, et al. Advanced hepatocellular carcinoma: treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology. 2005;235:659-667.
Yin XY, et al. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: long-term outcome and prognostic factors. Cancer. 2009;115:1914-1923.
Zhang L, et al. High-intensity focused ultrasound (HIFU): effective and safe therapy for hepatocellular carcinoma adjacent to major hepatic veins. Eur Radiol. 2009;19:437-445.