CHAPTER 378 Microsurgical Management of Giant Intracranial Aneurysms
Historical Considerations
Cerebral aneurysms were not recognized as a pathologic entity until Biumi of Milan identified a ruptured aneurysm during an autopsy in 1765. Hutchinson described the first giant intracranial aneurysm in 1875, which was diagnosed by an audible bruit.1 The patient refused Hutchinson’s recommendation of carotid artery ligation. The diagnosis was confirmed 11 years later when the patient died of an aortic aneurysm and, at autopsy, a partially calcified aneurysm the size of a “bantam hen’s egg” was found in the middle fossa.
In the next century, as Standard and colleagues noted,2 the diagnosis of cerebral aneurysms improved significantly when Moniz developed cerebral angiography. Nonetheless, these lesions were still sometimes mistaken for brain tumors. In the early part of the 20th century, treatment included hunterian ligation of the internal carotid artery (ICA).3 According to Laws and Udvarhelyi,4 Dandy was the first to actually expose a cerebral aneurysm and ligate its neck by clipping.
Based on 6368 cases in a cooperative study, Locksley classified aneurysms that were 25 mm or greater as giant and observed a high rate of morbidity and mortality associated with these lesions.5 The first two significant studies of giant aneurysms were reported by Morley and Barr6 and by Bull.7 The size of the aneurysms in these series may well have been underestimated because the diagnosis was based solely on plain radiography and angiography. At that time, the natural history of these lesions was beginning to be understood, and surgical treatment was fraught with hazards. Morley and Barr concluded that “direct surgical attack on extracavernous giant aneurysms is seldom possible or successful except in the case of middle cerebral artery aneurysms.”6
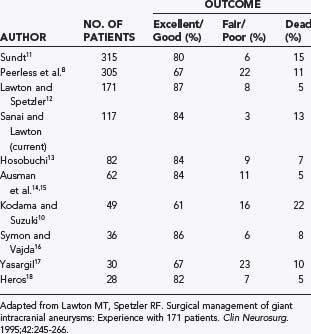
During the next several decades, several large series confirmed that significant morbidity and mortality rates were associated with these lesions. Peerless and associates reported mortality rates of 68% and 85% at 2 and 5 years, respectively, for untreated giant aneurysms, and even survivors suffered marked neurological dysfunction.8 Michel noted a 100% mortality rate at 2 years in this patient population,9 and Kodama and Suzuki reported that 75% of their untreated hospitalized patients died of subarachnoid hemorrhage (SAH).10 In contrast to this dismal natural history, several large surgical series demonstrated excellent to good outcomes in 61% to 87% of all patients treated surgically, with surgical mortality ranging from 5% to 22% (Table 378-1).8,10–18
Epidemiology and incidence
Giant aneurysms represent 2% to 5% of all intracranial aneurysms.6,19–22 As with other aneurysms, giant aneurysms have a female preponderance, although Kodama and Suzuki found an equal sex distribution.10 Most patients become symptomatic in the fourth through sixth decades of life.3,14,21,23
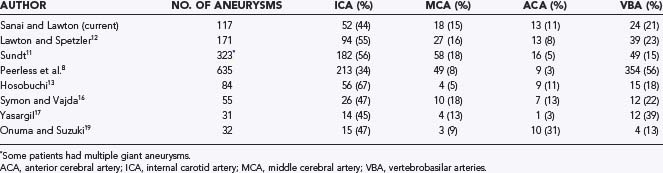
Giant aneurysms are found in all locations throughout the intracranial vasculature (Table 378-2).8,11–13,16,17,19 In general, 34% to 67% of giant intracranial aneurysms are associated with the ICA, 11% to 40% with the anterior cerebral artery and middle cerebral artery (MCA), and 13% to 56% with the vertebral and basilar arteries.3,8,11–13,16,17,19,21,24 In the senior author’s (M.T.L.) experience, posterior circulation aneurysms were treated more frequently in the group of patients with giant aneurysms (23%) than in the overall group of aneurysm patients (15%), which reflects a selection bias involving these complex aneurysms that favors microsurgery over endovascular therapy. Peerless and coauthors reported that 56% of their surgical series consisted of giant aneurysms of the vertebrobasilar system,8 a finding reflecting a referral bias to their institution.21
Pathophysiology
As early as 1930, Forbus proposed that aneurysms developed from defects in the medial layers of the vessel.25 Pathologic evaluation of giant aneurysms often demonstrates lack of a muscular layer and degeneration of the elastic laminar layers.26 Most giant aneurysms begin as saccular aneurysms located at arterial bifurcations, thus invoking hemodynamic factors in their formation. Stehbens was a proponent of acquired defects rather than congenital vessel defects contributing to the formation of cerebral aneurysms.27 Vessel weaknesses could be aggravated by flow-related stress or degenerative disease such as atherosclerosis. Some authors have postulated that giant aneurysms grow from repeated intramural hemorrhage within the aneurysm wall,20,21,28 followed by thrombus formation and neovascularization.29,30 Giant aneurysms developing de novo or from smaller aneurysms have been described.21,26,31,32 They have also been associated with underlying systemic arteriopathies.20,33 Giant aneurysms can result directly from trauma.20,33 More than one aneurysm may be present in 10% to 36% of patients.17,24,34
The morphology of giant aneurysms can be either fusiform or saccular, but their size and complexity may make it difficult to differentiate these two types. Saccular lesions most often occur at arterial bifurcations, probably the result of continuous hemodynamic stress. Fusiform or dolichoectatic lesions may result from atherosclerosis, congenital arteriopathies, or traumatic dissection.20,23,35,36 Numerous factors have been linked to the formation and rupture of aneurysms, including female sex, age, hypertension, connective tissue disease, and smoking.27,37,38
As aneurysms grow, wall tension must increase to maintain vessel integrity, as calculated from Laplace’s law. This equation relates the stress over the aneurysmal wall to the radius of the lesion.3 The same hemodynamic forces that prompt an aneurysm to grow may ultimately be responsible for its rupture.
A significant proportion of giant aneurysms have associated intraluminal thrombosis—as many as 60% in some series.3,20,21 Intra-aneurysmal thrombosis may develop in areas where blood outside the turbulent flow stream stagnates, thereby promoting thrombosis. The presence of a partially thrombosed aneurysm does not appear to lower the risk for rupture of the aneurysm.29,39
Clinical Findings
Although patients with giant intracranial aneurysms may initially be evaluated for SAH, signs and symptoms related to a mass effect develop in approximately two thirds.34,40 Only about a third of patients have SAH as the initial symptom,14,40 although Onuma and Suzuki reported that 80% of the patients in their series were initially seen because of SAH.19 As many as 50% of patients with giant fusiform aneurysms have signs of a mass effect, as opposed to 20% of patients with initial findings of SAH.36
The symptoms related to aneurysmal mass effect depend on the aneurysm’s location. In the anterior circulation, mass effect can be manifested as pain, visual field and acuity defects, and extraocular dysfunction. Dementia and mental disturbances, as well as hemiparesis and epilepsy, have also been described.20 Lownie and coworkers reported that an aneurysm had to be 3.5 cm to produce dementia and that those between 2.7 and 3.2 cm compressed the optic apparatus.41 In the posterior circulation, multiple cranial nerve dysfunctions may be present. If compression on the brainstem is significant, bulbar palsies and hemiparesis can also occur.20 The remainder of patients may have headache, syncope, sinusitis, epistaxis, confusion, cerebrovascular accidents, or symptoms related to head trauma.20,40
The risk for thromboembolism to distal vascular territories from a partially thrombosed giant aneurysm must be emphasized. In the senior author’s experience, 7% of patients with giant aneurysms initially had symptoms of distal thromboembolism, including transient ischemic attacks and stroke. Sano and associates reported that five conservatively treated patients all died of infarction.42 Naturally, only complete isolation of an aneurysm from the cerebral circulation eliminates the risk for rupture, continued enlargement, and thromboembolic phenomena.
Although SAH is less commonly associated with giant intracranial aneurysms than with small ones, it remains devastating. The annual rate of rupture of giant intracranial aneurysms is higher than that of smaller aneurysms. In several studies the annual risk for rupture correlated with increasing aneurysm size.43–45 Recent natural history studies have demonstrated an annual rupture rate for giant intracranial aneurysms of around 6%,46,47 higher than the 1% to 3% rate quoted for smaller aneurysms.12
SAH from giant aneurysms may be associated with more severe neurological deficits. According to Laplace’s law, the higher stress over the giant aneurysmal wall could result in a greater amount of SAH during rupture.2,21 As with smaller intracranial aneurysms, once SAH has occurred, more than 50% of patients die or experience significant complications.24,48 The goal is to diagnose aneurysms before they rupture or produce irreversible deficits.
Diagnosis
High-quality four-vessel cerebral angiography has long been considered the “gold standard” for the diagnosis of cerebral aneurysms; it provides information about an aneurysm’s location, anatomy, adjacent branch vessels, collateral circulation, and distal cerebral perfusion.40 It also reveals other intracranial aneurysms that might be treated simultaneously. Anteroposterior, lateral, and oblique views define the relationships between inflow and outflow vessels and the aneurysm neck. Superselective injections and manual compression techniques may clarify the anatomy or collateral blood flow. One caveat with traditional angiography is that it shows luminal filling but may fail to reveal the aneurysm’s true size if the aneurysm is thrombotic.
Computed tomography (CT) is useful in defining the outer dimensions of an aneurysm. Frequently, the aneurysm wall is thickened and calcified, which can influence the treatment strategy.20 When contrast material is administered, this eggshell border may appear as a “target sign,” with contrast material filling the center and the calcified rim appearing hyperdense.3 CT is essential in patients with SAH because it visualizes acute blood. CT also helps define the relationships between the aneurysm and skull base. CT angiography (CTA) avoids some of the risks of stroke associated with cannulation of the head and neck vessels during traditional angiography. Three-dimensional reconstructions demonstrate the aneurysm’s anatomy in exquisite detail but are not as helpful as angiography in visualizing the hemodynamics of the aneurysm. Still, angiography and CTA complement each other well and together give the neurosurgeon a clear picture of the anatomy of the aneurysm before surgery.49
Magnetic resonance imaging (MRI) offers another noninvasive technique for identifying and defining giant aneurysms. The breakdown products of blood, including deoxyhemoglobin, methemoglobin, and hemosiderin, have distinct signal characteristics on T1- and T2-weighted imaging that can be used to identify intraluminal clot. Signal flow voids, which indicate active blood flow within aneurysms, help define the filling component of an aneurysm. Magnetic resonance angiography (MRA) uses the flow void phenomenon to reconstruct an anatomic model of the vasculature.50 Even though MRI does provide information about the actual and luminal anatomy of the aneurysm, it is particularly helpful in evaluating the aneurysm’s compressive effects on adjacent brain and neural structures.51 Postoperative follow-up may be difficult with MRA, however, because of clip or coil metal artifacts.52
Management
Given the dismal natural history of giant cerebral aneurysms, a conservative or expectant approach should be considered only in patients with mitigating factors (e.g., poor medical grade, refusal of intervention). Patients with SAH should be managed according to accepted principles and protocols. In particular, we recommend aggressive control of hypertension, prompt institution of calcium channel blockers (e.g., nimodipine), and ventriculostomy if indicated.40 We recommend definitive treatment within 48 hours for ruptured aneurysms.53
Once the decision for intervention has been made, several treatment options are available in the neurosurgeon’s arsenal, including direct and indirect surgical attack, as well as endovascular procedures. Other considerations include the use of intraoperative electrophysiologic monitoring, intraoperative angiography, and specialized anesthesia techniques. Because of the potential for blood loss, all patients must have their blood typed and crossmatched, and central venous access should be available. If hypothermic circulatory arrest is being considered, a Swan-Ganz catheter should be placed for cardiac monitoring. Cerebral protective anesthetics such as isoflurane should be used. Cerebral protective agents such as barbiturates can be used during temporary vessel occlusion, and their efficacy can be monitored with electroencephalographic techniques.54,55 Intraoperative angiography may help determine whether residual luminal filling is present after the aneurysm has been clipped and can be useful in assessing the patency of bypass procedures. Furthermore, any stenosis or kinking associated with a clipped or reconstructed vessel can be confirmed immediately. Microvascular Doppler ultrasonography may be used to determine hemodynamically significant vessel stenosis. Videography with indocyanine green dye has been useful in assessing complete closure of aneurysms and the patency of parent and branch arteries, and it is faster and easier than catheter-based angiography.
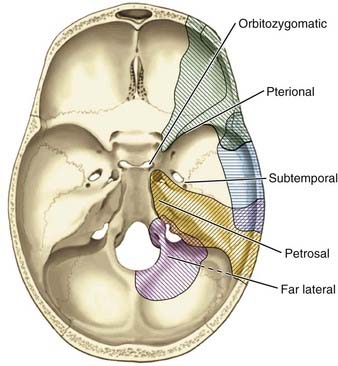
The optimal skull base approach to a giant aneurysm should offer a wide degree of exposure with full visualization of the aneurysm’s origin, outflow, associated perforators, adjacent vessels, and nearby neural structures (Fig. 378-1). The location of the aneurysm dictates the most appropriate skull base approach (Table 378-3). The basic principles of minimal brain retraction with maximal bone exposure apply. Increasingly, endovascular techniques are being combined with operative techniques to improve outcomes.56–61
TABLE 378-3 Selection of Surgical Approach Based on Location of the Giant Aneurysm
| SITE OF ANEURYSM | SKULL BASE APPROACH |
|---|---|
| Proximal internal carotid artery | Pterional, orbitozygomatic |
| Bifurcation of the internal carotid artery | Pterional, orbitozygomatic |
| Proximal anterior cerebral artery | Pterional, orbitozygomatic |
| Distal anterior cerebral artery | Pterional, orbitozygomatic, interhemispheric |
| Middle cerebral artery | Pterional, orbitozygomatic |
| Vertebral artery | Far lateral |
| Vertebrobasilar junction | Far lateral |
| Midbasilar artery | Petrosal, far lateral, orbitozygomatic |
| High basilar artery | Orbitozygomatic |
| Posterior inferior cerebellar artery | Far lateral, suboccipital |
| Anterior inferior cerebellar artery | Petrosal, far-lateral, orbitozygomatic |
| Superior cerebellar artery | Orbitozygomatic |
From Lemole GM Jr, Henn J, Spetzler RF, et al. Surgical management of giant aneurysms. Oper Tech Neurosurg. 2000;3:239-254.
Anterior Circulation Approaches
Orbitozygomatic-Pterional Approach
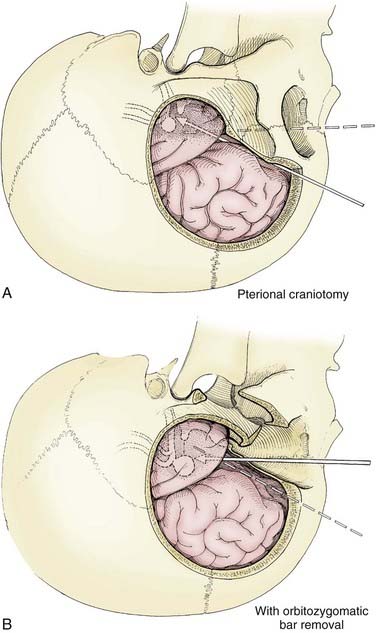
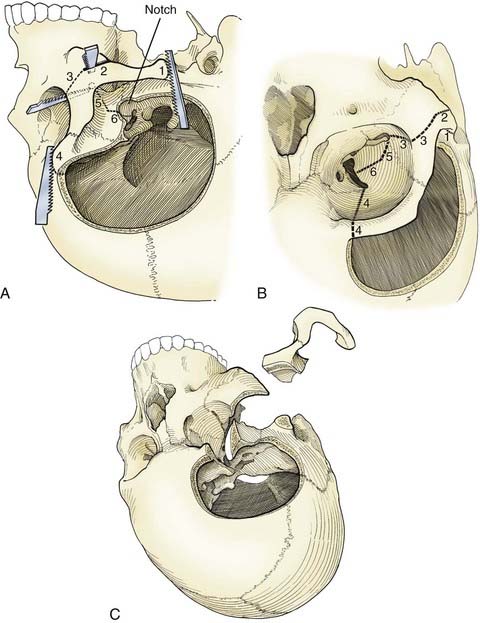
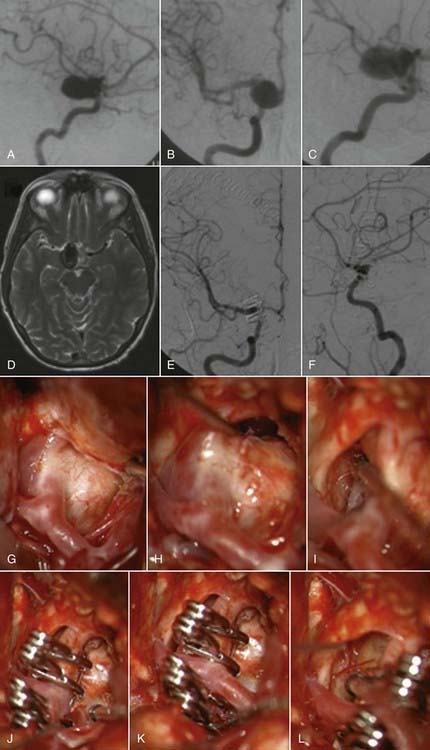
The pterional transsylvian approach is one of the most commonly used approaches to aneurysms of the anterior circulation. It provides access to the entire circle of Willis and its constituent branches. Exposure can be further enhanced by drilling the pterion and bony ridges over the floor of the frontal fossa.40 When combined with a transsylvian pterional approach, orbitozygomatic osteotomy can greatly increase the extent of exposure.62–65 Removal of the orbital rim, orbital roof, lateral wall, and zygomatic arch significantly enhances access to anterior skull base and upper clival lesions. Removing the orbitozygomatic bar provides the surgeon’s hands a wider corridor of access to the lesion and a shallower depth of field. Deep bypass procedures at the skull base are often technically difficult, and the added exposure can be essential for suturing vessels. This is particularly the case with giant MCA aneurysms that must be excised and the parent vessel reanastomosed. In addition, the orbitozygomatic approach provides a lower trajectory along the skull base, which reduces the need for cerebral retraction (Fig. 378-2).
Orbitozygomatic osteotomy is usually performed after a traditional pterional craniotomy is completed.66 A series of cuts through the lateral orbit, orbital roof, and maxillary and zygomatic roots frees the orbital bar from the adjacent skull base (Fig. 378-3). This piece of bone is preserved for later reinsertion. In addition, bone surrounding the superior orbital fissure can be removed with a high-speed drill or microrongeurs. Opening the dura with its flap based inferiorly allows the dural flap to gently depress the globe inferiorly and laterally, again enhancing exposure.40 In preparation for orbitozygomatic osteotomy, we also recommend subfascial or interfascial dissection of the temporalis fascia to protect the frontalis branch of the facial nerve.66,67
The risks associated with orbitozygomatic osteotomy include periorbital bruising and swelling, pulsatile enophthalmos, injury to the frontalis nerve, orbital entrapment, diplopia from injury to an extraocular muscle or nerve, and blindness. However, the overall incidence of these complications is low. Typically, the advantages provided by the orbitozygomatic approach more than offset its theoretical risks.66,68
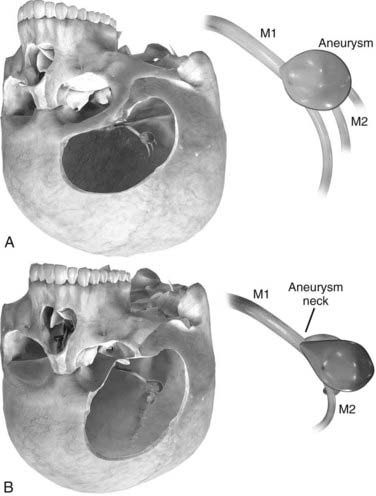
Orbitozygomatic osteotomy may be ideal for almost all giant and complex aneurysms originating from the circle of Willis, including ophthalmic, paraclinoid, superior hypophysial, posterior communicating, anterior choroidal, and ICA bifurcation aneurysms. Sometimes, additional exposure by drilling the anterior clinoid process and exposing the carotid artery in Glasscock’s triangle further enhances the exposure of certain lesions. At our institution, we also use orbitozygomatic osteotomy to approach giant and complex lesions of the MCA because the trajectory of the approach to the proximal M1 segment and the aneurysm neck is optimized (Fig. 378-4).
Posterior Circulation Approaches
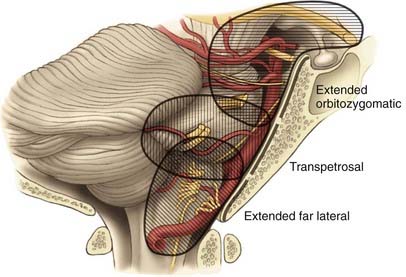
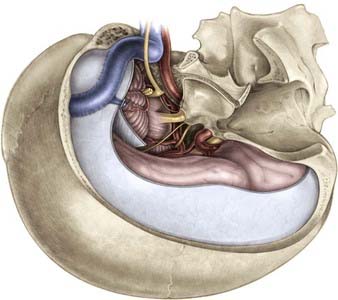
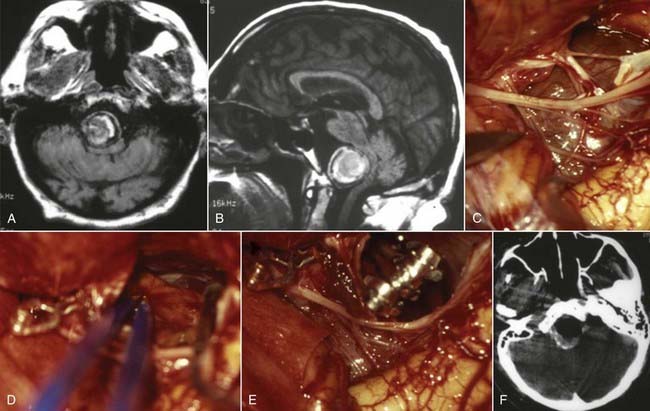
The skull base approaches used for giant aneurysms of the posterior fossa include the orbitozygomatic approach, transpetrosal approaches, and the far lateral approach. Combinations of these approaches can be used for more extensive lesions. Selection of the appropriate skull base approach depends on the aneurysm’s location (Fig. 378-5). Conceptually, the basilar artery can be divided into thirds. The orbitozygomatic approach is optimal for lesions of the upper third, and the vertebrobasilar area is best accessed with the far lateral approach. Lesions involving only the midbasilar zone may require transpetrosal or extended retrosigmoid approaches.
Orbitozygomatic Approach
The particulars of the orbitozygomatic osteotomy were described previously, but modifications can improve access to the upper basilar artery. Drilling the anterior and posterior clinoid processes and the clivus itself allows visualization down toward the midbasilar zone (Fig. 378-6).69 The orbitozygomatic approach is also useful with high-riding giant aneurysms of the basilar artery because it provides exposure of the upper interpeduncular space without requiring excessive frontal lobe traction. Important with any approach to aneurysms in this area is adequate visualization of all inflow and outflow vessels, including the bilateral posterior cerebral arteries and superior cerebellar arteries, as well as the proximal basilar artery.
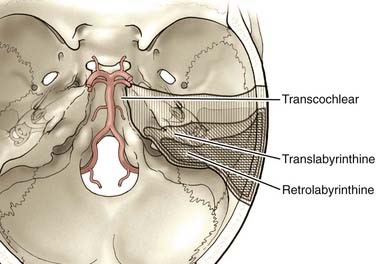
Transpetrosal Approaches
The anterior clivus and brainstem can be accessed by progressive removal of the petrous ridge. Depending on the degree of removal, the approaches can be defined as retrolabyrinthine, translabyrinthine, and transcochlear (Fig. 378-7).70–75 The amount of petrosal drilling dictates the degree of exposure of the anterior brainstem and clivus. Drilling the semicircular canals sacrifices hearing, whereas drilling the facial canal and transposition of the facial nerve may produce temporary facial nerve palsy. The expected morbidity involving cranial nerves VII and VIII with the more aggressive petrosal approaches must be weighed against the advantages of increasing exposure in the posterior fossa. At our institution, transpetrosal approaches are reserved for complex lesions of the midbasilar zone. If possible, we prefer to use the orbitozygomatic or far lateral approach or a combination to access lesions that extend into the midbasilar zone.
Far-Lateral Approach
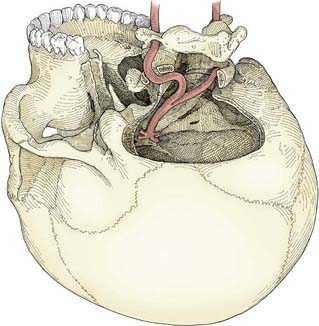
The far lateral approach provides excellent exposure from the midbasilar zone down to the intradural vertebral artery.18,55,76,77 A lateral suboccipital craniotomy is modified in three ways to create the far lateral approach: (1) the arch of C1 is removed to the sulcus arteriosus, (2) the bony rim of the foramen magnum is resected to the occipital condyle, and (3) the posterior third of the occipital condyle is removed (Fig. 378-8). Removing less than half of the occipital condyle should not affect spinal stability,78 nor should it pose a risk to cranial nerve XII as it courses through the hypoglossal canal. The extracranial vertebral artery may be freed from its course in the sulcus arteriosus and transverse foramen and transposed laterally to improve operative exposure. The far lateral approach is best used to access lesions of the vertebrobasilar junction, including giant aneurysms of the vertebrobasilar, vertebral, and proximal posterior inferior cerebellar arteries.
Combined Approaches
Several approaches can be combined to treat complex lesions involving multiple basilar zones or when anatomic complexity necessitates wider access and control (Fig. 378-9).74 A combined supratentorial and infratentorial approach merges a subtemporal craniotomy with a transpetrosal approach. It can be extended farther inferiorly if a far lateral approach is added to it.79 Sometimes, the tentorium and lateral sinus can be transected to facilitate exposure.80 As always with skull base approaches, the risks associated with extensive bony drilling are offset by minimized brain retraction.
Subtemporal Approach
Historically, the subtemporal approach was used routinely for aneurysms involving the upper basilar artery.63–65,69,70 The transsylvian pterional approach has now supplanted it to a large degree. The subtemporal approach can still be useful with posteriorly projecting upper basilar aneurysms12 and those located around the lateral edge of the tentorium.
Operative Techniques
Vascular Control
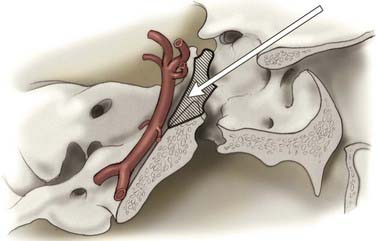
In the anterior circulation, obtaining vascular control may be routine. However, when aneurysms are located at the skull base, particularly at the point where the carotid artery exits the cavernous sinus, proximal control may be more difficult to achieve. In these instances, the aneurysm itself may obstruct the view of a proximal vessel. Alternative techniques of proximal control may be essential for giant paraclinoid, ophthalmic, and superior hypophysial artery aneurysms. In these cases, the ICA can be controlled in the neck through an additional surgical incision or along its petrous course by drilling Glasscock’s triangle.40 Intracranial exposure can be facilitated by drilling the anterior clinoid and incising the distal dural ring, thereby accessing the clinoidal ICA, which may provide enough room for a temporary clip. Endovascular balloon techniques have been used to gain proximal control of the ICA.60
Endovascular temporary balloon occlusion techniques can also be useful in the posterior circulation, particularly in the proximal basilar and vertebral artery regions.59 Distal balloon occlusion of the middle basilar artery may obstruct brainstem perforator vessels, with devastating consequences. Retrograde placement of a temporary occlusion balloon into the upper basilar artery from the anterior circulation is usually difficult.40 As with all temporary occlusion techniques, the risk for cerebral ischemia can be lessened by using cerebral protective agents, minimizing clip occlusion time, and providing alternative sources of circulation through appropriate bypass procedures, if necessary. Mild hypertension can also be induced to maximize circulation through collateral pathways.
When all other forms of vascular control prove insufficient, hypothermic circulatory arrest can be considered. Circulatory arrest permits complete vascular control, and the risk for intraoperative rupture of the aneurysm is eliminated. Hypothermic circulatory arrest can facilitate the surgical technique by allowing more aggressive manipulation of the aneurysm dome without fear of rupture. Absence of blood flow greatly facilitates debulking of the aneurysm and clip reconstruction. Deep hypothermia reduces the brain’s metabolic requirements for oxygen,54 but during circulatory arrest, adequate cerebral protection with barbiturates is imperative. The risks related to hypothermic circulatory arrest are well described and include coagulopathies associated with hypothermia81 and those associated with hemodilution after blood products and intravenous fluids are replaced.82 Systemic heparinization is needed to place the percutaneous bypass catheter required for bypass procedures, but heparin can subject patients to hemorrhagic complications.83 Finally, even when cerebral protective agents and hypothermia are used, the total duration of circulatory arrest must be minimized to avoid ischemic complications. Hypothermic circulatory arrest is associated with a morbidity rate of 13% and a mortality rate of 8%.84 These numbers, in part, reflect the complexity of aneurysms that require hypothermic circulatory arrest for adequate vascular control.
Surgical Techniques for Clipping
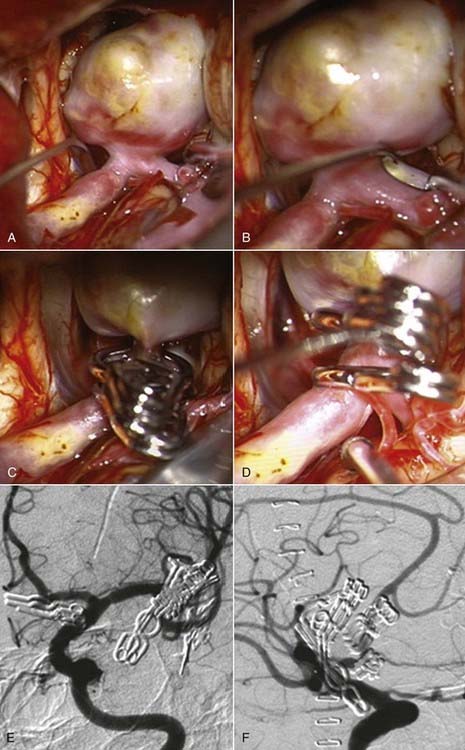
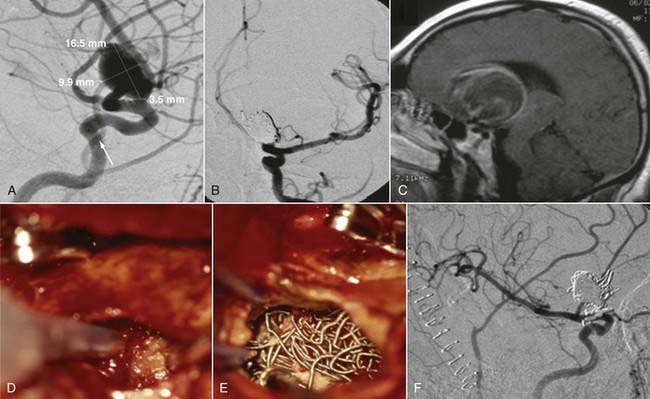
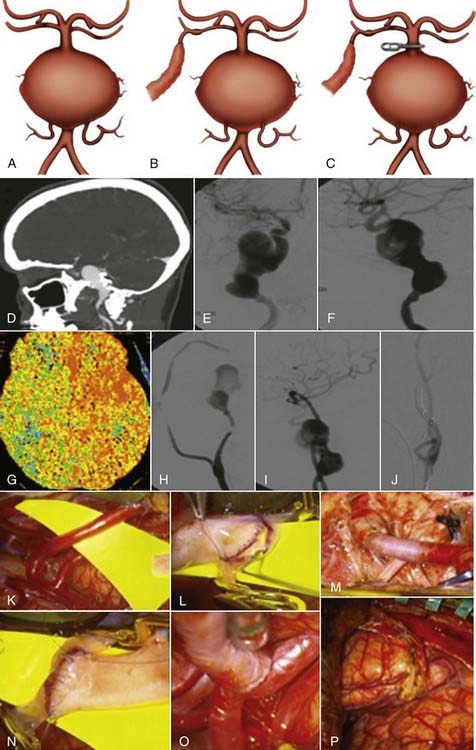
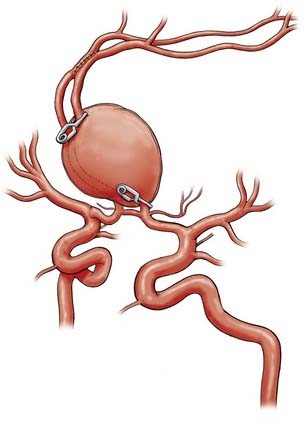
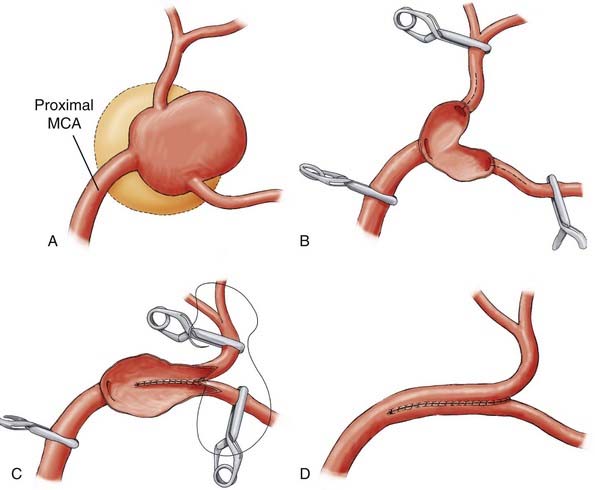
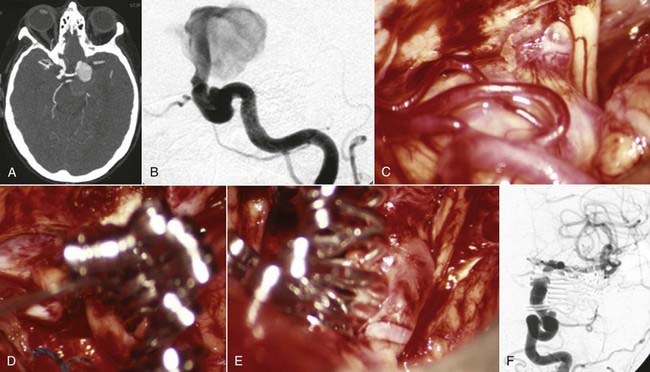
Successful clipping of a giant aneurysm depends on the anatomic complexity and morphologic features of the lesion. Successful clipping of saccular aneurysms typically requires a favorable and well-defined aneurysm neck. The clip is positioned to bring the vessel walls together at the neck of the aneurysm while the patency of the parent vessel is preserved. Fusiform or dolichoectatic giant aneurysms have no definable neck, and efferent vessels may arise at various points along the aneurysm.35 Simple clipping of these lesions is often impossible, and clip reconstruction techniques are frequently necessary to exclude these aneurysms from the circulation. Creation of a fenestration tube is one technical option useful for redirecting blood flow after occlusion of the aneurysm (Fig. 378-10). In addition, several smaller clips placed in tandem can be a better solution than placing one long clip (Fig. 378-11). With thrombotic aneurysms, clip placement needs to be combined with thrombectomy to relieve the mass effect (Fig. 378-12). Other aneurysms must be excised altogether because of their mass effect, thereby adding another layer of complexity to the reconstruction (Fig. 378-13). In these instances, clips must be used to reconstruct the vessel lumina to connect all inflow and outflow vessels. These techniques require an exquisite comprehension of vascular anatomy and spatial creativity.
The technical aspects of direct clipping of giant aneurysms must not be overlooked. Aneurysm clips have mechanical limitations. Notably, the lowest closing force along the clip is located at its tip.3 Clips may also be stacked on top of one other to guard against clip migration or slippage.69,70 Aneurysms with atherosclerotic changes within their walls may prevent clips from closing completely. The neurosurgeon must then be careful to not loosen plaque into the parent vessel, where it can embolize to distal vascular territories.16 In such cases, some authors use crushing instruments to make the aneurysm necks more malleable.85
Once a clip has been placed, the neurosurgeon should inspect the parent vessel and any associated neural and vascular structures. Hemodynamically significant stenoses may result if vessels kink after clips are placed.21 The neurosurgeon must also ascertain that no small perforating vessels are caught within the clip blades, particularly brainstem and thalamic perforators associated with basilar tip aneurysms or lenticulostriate perforators associated with ICA bifurcation and proximal MCA aneurysms. Vessel patency and blood flow can be assessed intraoperatively with micro-Doppler probes, angiography, or indocyanine green dye.
Alternative Occlusion Techniques
Because of their size, location, or morphologic features, certain giant aneurysms cannot be clipped directly. The neurosurgeon must then consider other options, which include proximal parent artery occlusion, distal parent artery occlusion, and trapping, with or without bypass.40,86 Aneurysm trapping excludes the aneurysm from the cerebral circulation, whereas parent artery occlusion redirects or reduces blood flow through the aneurysm to promote thrombosis and minimize its risk for rupture. These latter techniques are inferior to direct clipping and are usually considered only for aneurysms that fail direct clipping.
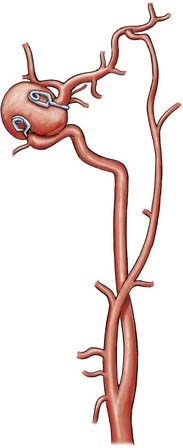
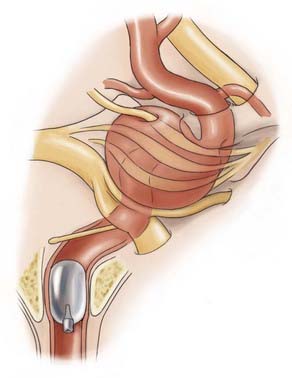
Parent artery occlusion can be either proximal or distal to the aneurysm. Hunterian ligation of parent vessels harboring giant aneurysms is a viable surgical alternative (Fig. 378-14).87 These techniques are particularly useful for aneurysms with no definable necks, such as fusiform or dolichoectatic aneurysms. Surgical alteration of blood flow dynamics through these lesions may result in thrombosis and resolution,88–90 but spontaneous revascularization of a previously thrombosed aneurysm is possible.91–93 Collateral blood flow upstream of the parent vessel occlusion must be available through the circle of Willis, leptomeningeal connections, or surgical augmentation. In the latter case, numerous bypass procedures may be able to provide blood flow to the distal circulation (Fig. 378-15). Preoperatively, collateral circulation can be assessed by cerebral angiography and temporary balloon occlusion tests to determine neurological status and cerebral blood flow. None of these studies, however, are completely reliable in ascertaining which patients will tolerate permanent vessel occlusion without a bypass.24,85,87,94
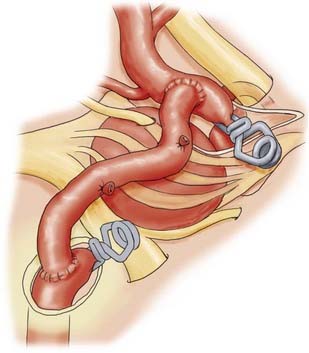
Revascularization procedures and bypass techniques must be used when the collateral circulation is insufficient to replace blood flow after permanent vessel occlusion.13,95,96 Various bypass techniques have been described for both the anterior and posterior circulations. Sometimes, altering hemodynamics with a bypass alone may result in aneurysm thrombosis and resolution.88,90 In many cases, bypass procedures involve supplying the intracranial circulation through a mobilized pedicle of the external carotid circulation. The superficial temporal arteries and occipital arteries have been used in this manner to supply blood to branches of the MCA, posterior cerebral artery, and superior cerebellar artery (Fig. 378-16).14,15 With these techniques, the neurosurgeon must be aware of mismatches in blood flow because some bypass techniques are limited by the small caliber of donor vessels. For example, if the ICA is to be sacrificed, superficial temporal artery–to-MCA bypass may not provide enough compensatory blood flow. High-flow bypasses can be created by using interposition saphenous vein grafts,88,97 or “double-barrel” bypasses can connect the anterior and posterior branches of the superficial temporal artery to two recipient vessels.40 Likewise, interposition vein grafts can be used to bypass diseased segments of large-caliber vessels (Fig. 378-17), such as petrous-to-supraclinoid bypass procedures of the ICA. The radial artery can be used as an arterial interposition graft.98 Finally, certain vascular territories within the brain are amenable to revascularization via in situ anastomoses, particularly the paired anterior cerebral and posterior inferior cerebellar arteries (Fig. 378-18). In this situation, blood flow distal to the trapped aneurysm can be augmented with a side-to-side anastomosis from the contralateral circulation. The anterior temporal branch of the MCA can sometimes be mobilized to bypass diseased segments just distal to it.
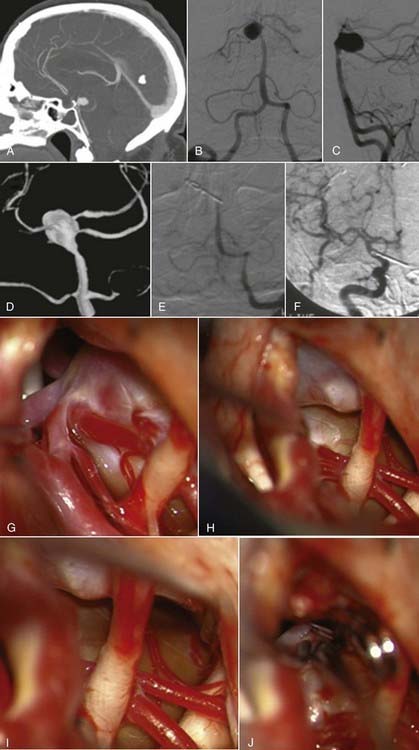
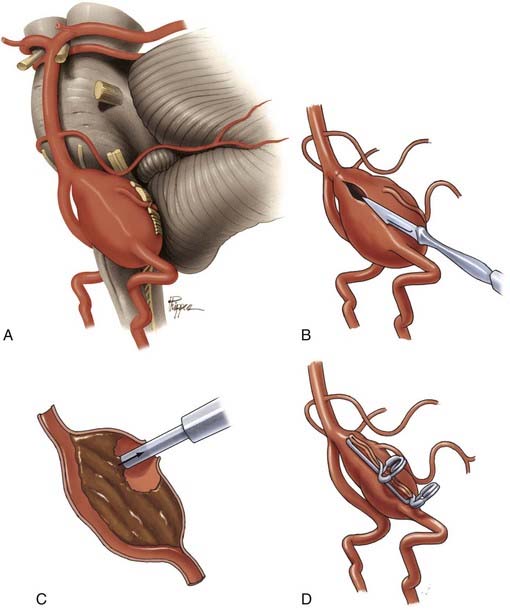
Aneurysmectomy may be considered for giant intracranial aneurysms involving vessels that can be mobilized and reanastomosed after excision of the aneurysm (Fig. 378-19), such as giant aneurysms involving the MCA bifurcation.99,100 Excision is precluded if the aneurysm directly involves significant perforators or outflow vessels.
When an aneurysm can be clipped directly or trapped, it may be possible to reduce the mass effect of the aneurysm by puncturing it21 or by opening the aneurysm and removing associated thrombus. When an aneurysm cannot be excluded from the circulation, it may not be possible to debulk it. However, some aneurysms that can be controlled completely with temporary clips can be opened, thrombectomized, and closed to reduce any mass effect (Fig. 378-20).41,101 In other circumstances, clip reconstruction can be combined with thrombectomy of the aneurysm (Fig. 378-21).
Endovascular Treatment
Endovascular techniques, which include the use of detachable balloons, embolic polymers, and detachable thrombogenic coils,2,102–104 have been used to occlude giant intracranial aneurysms directly and to exclude them from the cerebral circulation. A combination of stents and detachable coils can also induce thrombosis of the aneurysm.2,105 Significant improvements have made it possible to exclude some aneurysms completely from the cerebral circulation with endovascular techniques alone. Frequently, the lesions most amenable to endovascular treatment are also those that are best treated by surgical techniques, namely, those with well-defined, small, narrow necks.2,106
Unfortunately, occlusion is incomplete in a considerable percentage of endovascular treatments.2,106,107 Even if a significant portion of an aneurysm’s lumen is embolized or thrombosed, the patient is still subject to risk for SAH from that lesion.2,103 In fact, endothelialization of the aneurysm’s orifice does not always follow coil occlusion.108 Furthermore, follow-up studies have demonstrated refilling and recurrence of aneurysms thought to have been completely occluded.2
There are a number of hypotheses about how the lumen of an aneurysm becomes recanalized. The water hammer hypothesis proposes that the pulsatile hemodynamic blood flow compacts the thrombus and coil mass within the lumen, impacts them up toward the dome, and allows filling around the neck. Other mechanisms might include recanalization of a blood flow channel around the outer surface of the coil and thrombus mass next to the aneurysmal wall.2 However, recurrence rates in large surgical series are low,109 and endovascular techniques must be measured against this standard when their overall efficacy is assessed. One must weigh the risks associated with the procedure itself against the natural history of partially treated or recurrent aneurysms in determining the morbidity and mortality associated with either surgical or endovascular procedures.
Endovascular techniques have their own complications. Embolization and infarction from cerebral catheterization are risks.58,60 Coil migration into a parent vessel lumen with subsequent thrombosis or embolization is a rare but real risk.2 Moreover, a partially treated, coiled aneurysm poses a greater surgical challenge to the neurosurgeon than if no endovascular intervention had been undertaken.
Endovascular techniques are often inadequate to address the mass effect of a giant aneurysm, the probable manifestation in most patients. Sacrifice of the parent vessel with detachable endovascular balloons can promote thrombosis and resolution of the aneurysm. Endovascular studies have demonstrated resolution of mass effect symptoms with aneurysmal coiling. Coil occlusion of the aneurysm may reduce the hemodynamic pulsations of the aneurysm on surrounding neural structures.102,103,110 Nonetheless, deterioration after coiling procedures and signs of worsening mass effect have been described.110,111
Endovascular techniques have revolutionized the treatment of giant aneurysms of the cavernous sinus. Direct surgical approaches through the cavernous sinus can be associated with significant morbidity,85 and endovascular coiling of the lesion or sacrifice of the parent vessel by balloon occlusion, if tolerated, has reduced the need for such aggressive surgery.112,113 Furthermore, the need to completely occlude aneurysms outside the subarachnoid space is less pronounced because the morbidity and mortality associated with their rupture are much lower.
Endovascular techniques are rapidly becoming an indispensable adjunct to microsurgical treatment of giant cerebral aneurysms. Proximal or distal parent vessel occlusion can be performed with detachable endovascular balloons if an amenable vascular territory is involved (Fig. 378-22).2,61,104 Likewise, proximal vascular control is sometimes readily achieved with endovascular balloon occlusion techniques.56,58,60 The results are impressive when endovascular techniques are combined with direct surgical treatment of giant cerebral aneurysms.57,59,114 For example, endovascular balloon occlusion has been combined with suction decompression of the aneurysm to facilitate surgical manipulation and clipping of paraclinoid aneurysms.56,58,60 Undoubtedly, as endovascular technology advances, less invasive surgical and endovascular options will be applied to the treatment of these problematic lesions.
Barrow DL, Alleyne C. Natural history of giant intracranial aneurysms and indications for intervention. Clin Neurosurg. 1995;42:214-244.
Connolly ESJr, Choudhri TF, Mack WJ, et al. Influence of smoking, hypertension, and sex on the phenotypic expression of familial intracranial aneurysms in siblings. Neurosurgery. 2001;48:64-68.
Connolly ESJr, Solomon RA. Hypothermic cardiac standstill for cerebral aneurysm surgery. Neurosurg Clin N Am. 1998;9:681-695.
Drake CG. The treatment of aneurysms of the posterior circulation. Clin Neurosurg. 1979;26:96-144.
Drake CG. Giant intracranial aneurysms: experience with surgical treatment in 174 patients. Clin Neurosurg. 1979;26:12-95.
Drake CG, Peerless SJ. Giant fusiform intracranial aneurysms: review of 120 patients treated surgically from 1965 to 1992. J Neurosurg. 1997;87:141-162.
Drake CG, Peerless SJ, Ferguson GG. Hunterian proximal arterial occlusion for giant aneurysms of the carotid circulation. J Neurosurg. 1994;81:656-665.
Higashida RT, Halbach VV, Dowd C, et al. Endovascular detachable balloon embolization therapy of cavernous carotid artery aneurysms: results in 87 cases. J Neurosurg. 1990;72:857-863.
House WF. Translabyrinthine approach. In: House WF, Luetje CM, editors. Acoustic Tumors. Baltimore: University Park Press; 1979:43-87.
Juvela S, Porras M, Poussa K. Natural history of unruptured intracranial aneurysms: probability and risk factors for aneurysm rupture. Neurosurg Focus. 8(5), 2000.
Lawton MT, Daspit CP, Spetzler RF. Technical aspects and recent trends in the management of large and giant midbasilar artery aneurysms. Neurosurgery. 1997;41:513-520.
Lawton MT, Raadzens PA, Jacobowitz R, et al. Hypothermia-induced circulatory arrest in neurovascular surgery: evolving indications and predictors of patient outcome. J Neurosurg. 1997;86:401A.
Lawton MT, Spetzler RF. Surgical management of giant intracranial aneurysms: experience with 171 patients. Clin Neurosurg. 1995;42:245-266.
Lawton MT, Spetzler RF. Surgical strategies for giant intracranial aneurysms. Acta Neurochir Suppl. 1999;72:141-156.
Lawton MT, Spetzler RF. Surgical strategies for giant intracranial aneurysms. Neurosurg Clin N Am. 1998;9:725-742.
Malis LI. Surgical resection of tumors of the skull base. In: Wilkins RH, Rengachary SS, editors. Neurosurgery. New York: McGraw-Hill; 1985:1011-1021.
Peerless SJ, Ferguson GG, Drake CG. Extracranial-intracranial (EC/IC) bypass in the treatment of giant intracranial aneurysms. Neurosurg Rev. 1982;5:77-81.
Sekhar LN, Bucur SD, Bank WO, et al. Venous and arterial bypass grafts for difficult tumors, aneurysms, and occlusive vascular lesions: evolution of surgical treatment and improved graft results. Neurosurgery. 1999;44:1207-1223.
Sekhar LN, Heros RC. Origin, growth, and rupture of saccular aneurysms: a review. Neurosurgery. 1981;8:248-260.
Spetzler RF, Hadley MN. Protection against cerebral ischemia: the role of barbiturates. Cerebrovasc Brain Metab Rev. 1989;1:212-229.
Spetzler RF, Selman W, Carter LP. Elective EC-IC bypass for unclippable intracranial aneurysms. Neurol Res. 1984;6:64-68.
Standard SC, Ahuja A, Guterman LR, et al. Balloon test occlusion of the internal carotid artery with hypotensive challenge. AJNR Am J Neuroradiol. 1995;16:1453-1458.
Standard SC, Guterman LR, Chavis TD, et al. Endovascular management of giant intracranial aneurysms. Clin Neurosurg. 1995;42:267-293.
Vishteh AG, Crawford NR, Melton MS, et al. Stability of the craniovertebral junction after unilateral occipital condyle resection: a biomechanical study. J Neurosurg. 1999;90:91-98.
Yasargil MG. Giant intracranial aneurysms. In: Yasargil MG, editor. Microneurosurgery II: Clinical Considerations, Surgery of the Intracranial Aneurysms and Results. New York: Thieme-Stratton; 1984:296-304.
Zabramski JM, Kiris T, Sankhla SK, et al. Orbitozygomatic craniotomy. Technical note. J Neurosurg. 1998;89:336-341.
1 Hutchinson J. Aneurysms of the internal carotid within the skull diagnosed 11 years before patient’s death: spontaneous cure. Trans Clin Soc (London). 1875;8:127.
2 Standard SC, Guterman LR, Chavis TD, et al. Endovascular management of giant intracranial aneurysms. Clin Neurosurg. 1995;42:267-293.
3 Atkinson JLD, Piepgras DG. Giant aneurysms: supratentorial. In: Carter LP, Spetzler RF, editors. Neurovascular Surgery. New York: McGraw-Hill; 1995:815-828.
4 Walker AE, Laws ER, Udvarhelyi GB, editors. The Genesis of Neuroscience. Park Ridge, IL: American Association of Neurological Surgeons, 1998.
5 Locksley HB. Natural history of subarachnoid hemorrhage, intracranial aneurysms and arteriovenous malformations: based on 6368 cases in the cooperative study. J Neurosurg. 1966;25:219-239.
6 Morley TP, Barr HW. Giant intracranial aneurysms: diagnosis, course, and management. Clin Neurosurg. 1969;16:73-94.
7 Bull J. Massive aneurysms at the base of the brain. Brain. 1969;92:535-570.
8 Peerless SJ, Wallace MC, Drake CG. Giant intracranial aneurysms. In: Youmans JR, editor. Neurological Surgery: A Comprehensive Reference Guide to the Diagnosis and Management of Neurological Problems. Philadelphia: Saunders; 1990:1742-1763.
9 Michel WF. Posterior fossa aneurysms simulating tumours. J Neurol Neurosurg Psychiatry. 1974;37:218-223.
10 Kodama N, Suzuki J. Surgical treatment of giant aneurysms. Neurosurg Rev. 1982;5:155-160.
11 Sundt TMJ. Results of surgical management. In: Sundt TMJ, editor. Surgical Techniques for Saccular and Giant Intracranial Aneurysms. Baltimore: Williams & Wilkins; 1990:19-23.
12 Lawton MT, Spetzler RF. Surgical management of giant intracranial aneurysms: experience with 171 patients. Clin Neurosurg. 1995;42:245-266.
13 Hosobuchi Y. Giant intracranial aneurysms. In: Wilkins RH, Rengachary SS, editors. Neurosurgery. New York: McGraw-Hill; 1985:1404-1414.
14 Ausman JI, Diaz FG, Sadasivan B, et al. Giant intracranial aneurysm surgery: the role of microvascular reconstruction. Surg Neurol. 1990;34:8-15.
15 Ausman JI, Diaz FG, Vacca DF, et al. Superficial temporal and occipital artery bypass pedicles to superior, anterior inferior, and posterior inferior cerebellar arteries for vertebrobasilar insufficiency. J Neurosurg. 1990;72:554-558.
16 Symon L, Vajda J. Surgical experiences with giant intracranial aneurysms. J Neurosurg. 1984;61:1009-1028.
17 Yasargil MG. Giant intracranial aneurysms. In: Yasargil MG, editor. Microneurosurgery II: Clinical Considerations, Surgery of the Intracranial Aneurysms and Results. New York: Thieme-Stratton; 1984:296-304.
18 Heros RC. Lateral suboccipital approach for vertebral and vertebrobasilar artery lesions. J Neurosurg. 1986;64:559-562.
19 Onuma T, Suzuki J. Surgical treatment of giant intracranial aneurysms. J Neurosurg. 1979;51:33-36.
20 Pia HW, Zierski J. Giant cerebral aneurysms: a review of clinical picture, diagnosis, and management with illustrative cases. Neurosurg Rev. 1982;5:117-148.
21 Shibuya M, Sugita K. Intracranial giant aneurysms. In: Youmans JR, editor. Neurological Surgery: A Comprehensive Reference Guide to the Diagnosis and Management of Neurological Problems. Philadelphia: Saunders; 1996:1310-1319.
22 Weir B. Special aneurysms: saccular. In: Weir B, editor. Aneurysms Affecting the Nervous System. Baltimore: Williams & Wilkins; 1987:185-208.
23 Nakayama Y, Tanaka A, Kumate S, et al. Giant fusiform aneurysm of the basilar artery: consideration of its pathogenesis. Surg Neurol. 1999;51:140-145.
24 Barrow DL, Alleyne C. Natural history of giant intracranial aneurysms and indications for intervention. Clin Neurosurg. 1995;42:214-244.
25 Forbus WD. On the origin of miliary aneurysms of the superficial cerebral arteries. Bull Johns Hopkins Hosp. 1930;47:239-284.
26 Sekhar LN, Heros RC. Origin, growth, and rupture of saccular aneurysms: a review. Neurosurgery. 1981;8:248-260.
27 Stehbens WE. Etiology of intracranial berry aneurysms. J Neurosurg. 1989;70:823-831.
28 Koyama S, Kotani A, Sasaki J. Giant basilar artery aneurysm with intramural hemorrhage and then disastrous hemorrhage: case report. Neurosurgery. 1996;39:174-177.
29 Khurana VG, Wijdicks EF, Parisi JE, et al. Acute deterioration from thrombosis and rerupture of a giant intracranial aneurysm. Neurology. 1999;52:1697-1699.
30 Maruishi M, Shima K, Chigasaki H, et al. Giant intracranial aneurysm with rapid thrombus formation and intramural hemorrhage—case report. Neurol Med Chir (Tokyo). 1994;34:829-831.
31 Barth A, de Tribolet N. Growth of small saccular aneurysms to giant aneurysms: presentation of three cases. Surg Neurol. 1994;41:277-280.
32 Johnston SC, Halbach VV, Smith WS, et al. Rapid development of giant fusiform cerebral aneurysms in angiographically normal vessels. Neurology. 1998;50:1163-1166.
33 Schievink WI, Puumala MR, Meyer FB, et al. Giant intracranial aneurysm and fibromuscular dysplasia in an adolescent with alpha 1-antitrypsin deficiency. J Neurosurg. 1996;85:503-506.
34 Whittle IR, Dorsch NW, Besser M. Giant intracranial aneurysms: diagnosis, management, and outcome. Surg Neurol. 1984;21:218-230.
35 Anson JA, Lawton MT, Spetzler RF. Characteristics and surgical treatment of dolichoectatic and fusiform aneurysms. J Neurosurg. 1996;84:185-193.
36 Drake CG, Peerless SJ. Giant fusiform intracranial aneurysms: review of 120 patients treated surgically from 1965 to 1992. J Neurosurg. 1997;87:141-162.
37 Baker CJ, Fiore A, Connolly ESJr, et al. Serum elastase and alpha-1-antitrypsin levels in patients with ruptured and unruptured cerebral aneurysms. Neurosurgery. 1995;37:56-61.
38 Connolly ESJr, Choudhri TF, Mack WJ, et al. Influence of smoking, hypertension, and sex on the phenotypic expression of familial intracranial aneurysms in siblings. Neurosurgery. 2001;48:64-68.
39 Khurana VG, Piepgras DG, Whisnant JP. Ruptured giant intracranial aneurysms. Part I. A study of rebleeding. J Neurosurg. 1998;88:425-429.
40 Lawton MT, Spetzler RF. Surgical strategies for giant intracranial aneurysms. Neurosurg Clin N Am. 1998;9:725-742.
41 Lownie SP, Drake CG, Peerless SJ, et al. Clinical presentation and management of giant anterior communicating artery region aneurysms. J Neurosurg. 2000;92:267-277.
42 Sano H, Kato Y, Shankar K, et al. Treatment and results of partially thrombosed giant aneurysms. Neurol Med Chir (Tokyo). 1998;38(suppl):58-61.
43 McCormick WF, Acosta-Rua GJ. The size of intracranial saccular aneurysms. An autopsy study. J Neurosurg. 1970;33:422-427.
44 Orz Y, Kobayashi S, Osawa M, et al. Aneurysm size: a prognostic factor for rupture. Br J Neurosurg. 1997;11:144-149.
45 Wiebers DO, Whisnant JP, Sundt TMJr, et al. The significance of unruptured intracranial saccular aneurysms. J Neurosurg. 1987;66:23-29.
46 Juvela S, Porras M, Poussa K. Natural history of unruptured intracranial aneurysms: probability and risk factors for aneurysm rupture. Neurosurg Focus. 8(5), 2000.
47 Unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. International Study of Unruptured Intracranial Aneurysms Investigators. N Engl J Med. 1998;339:1725-1733.
48 Pakarinen S. Incidence, aetiology, and prognosis of primary subarachnoid haemorrhage. A study based on 589 cases diagnosed in a defined urban population during a defined period. Acta Neurol Scand. 1967;43(suppl 29):21-28.
49 Tanabe S, Ohtaki M, Uede T, et al. [Diagnosis of ruptured and unruptured cerebral aneurysms with three-dimensional CT angiography (3D-CTA).]. No Shinkei Geka. 1995;23:787-795.
50 Brugieres P, Blustajn J, Le Guerinel C, et al. Magnetic resonance angiography of giant intracranial aneurysms. Neuroradiology. 1998;40:96-102.
51 Lang EW, Steffens JC, Link J, et al. The utility of contrast-enhanced MR-angiography for posterior fossa giant cerebral aneurysm management. Neurol Res. 1998;20:705-708.
52 Anzalone N, Righi C, Simionato F, et al. Three-dimensional time-of-flight MR angiography in the evaluation of intracranial aneurysms treated with Guglielmi detachable coils. AJNR Am J Neuroradiol. 2000;21:746-752.
53 Symon L. Management of giant intracranial aneurysms. Clin Neurosurg. 1990;36:21-47.
54 Connolly ESJr. Solomon RA. Hypothermic cardiac standstill for cerebral aneurysm surgery. Neurosurg Clin N Am. 1998;9:681-695.
55 Spetzler RF, Hadley MN. Protection against cerebral ischemia: the role of barbiturates. Cerebrovasc Brain Metab Rev. 1989;1:212-229.
56 Arnautovic KI, Al-Mefty O, Angtuaco E. A combined microsurgical skull-base and endovascular approach to giant and large paraclinoid aneurysms. Surg Neurol. 1998;50:504-518.
57 Ewald C, Kuhne D, Hassler W. Giant basilar artery aneurysms incorporating the posterior cerebral artery: bypass surgery and coil occlusion—two case reports. Neurol Med Chir (Tokyo). 1998;38(suppl):83-85.
58 Fahlbusch R, Nimsky C, Huk W. Open surgery of giant paraclinoid aneurysms improved by intraoperative angiography and endovascular retrograde suction decompression. Acta Neurochir (Wien). 1997;139:1026-1032.
59 Hacein-Bey L, Connolly ESJr, Mayer SA, et al. Complex intracranial aneurysms: combined operative and endovascular approaches. Neurosurgery. 1998;43:1304-1312.
60 Ng PY, Huddle D, Gunel M, et al. Intraoperative endovascular treatment as an adjunct to microsurgical clipping of paraclinoid aneurysms. J Neurosurg. 2000;93:554-560.
61 Taki W, Nakahara I, Sakai N, et al. Large and giant middle to lower basilar trunk aneurysms treated by surgical and interventional neuroradiological methods. Neurol Med Chir (Tokyo). 1998;38:826-834.
62 Al-Mefty O. The cranio-orbital zygomatic approach for intracranial lesions. Contemp Neurosurg. 1992;14:1-6.
63 Diaz FG. Vertebrobasilar aneurysms: surgical management. Crit Rev Neurosurg. 1992;2:146-158.
64 Sano K. Temporo-polar approach to aneurysms of the basilar artery at and around the distal bifurcation: technical note. Neurol Res. 1980;2:361-367.
65 Sano K, Asano T, Tamura A. Surgical technique. In: Sano K, Tamura A, editors. Acute Aneurysm Surgery: Pathophysiology and Management. New York: Springer-Verlag; 1987:194-246.
66 Zabramski JM, Kiris T, Sankhla SK, Cabiol J, Spetzler RF. Orbitozygomatic craniotomy. Technical note. J Neurosurg. 1998;89:336-341.
67 Salas E, Ziyal IM, Bejjani GK, et al. Anatomy of the frontotemporal branch of the facial nerve and indications for interfascial dissection. Neurosurgery. 1998;43:563-568.
68 Lawton MT, Spetzler RF. Surgical strategies for giant intracranial aneurysms. Acta Neurochir Suppl. 1999;72:141-156.
69 Drake CG. Giant intracranial aneurysms: experience with surgical treatment in 174 patients. Clin Neurosurg. 1979;26:12-95.
70 Drake CG. The treatment of aneurysms of the posterior circulation. Clin Neurosurg. 1979;26:96-144.
71 Hitselberger WE, House WF. A combined approach to the cerebellopontine angle. A suboccipital-petrosal approach. Arch Otolaryngol. 1966;84:267-285.
72 House WF. Translabyrinthine approach. In: House WF, Luetje CM, editors. Acoustic Tumors. Baltimore: University Park Press; 1979:43-87.
73 House WF, Hitselberger WE. The transcochlear approach to the skull base. Arch Otolaryngol. 1976;102:334-342.
74 Lawton MT, Daspit CP, Spetzler RF. Technical aspects and recent trends in the management of large and giant midbasilar artery aneurysms. Neurosurgery. 1997;41:513-520.
75 Malis LI. Surgical resection of tumors of the skull base. In: Wilkins RH, Rengachary SS, editors. Neurosurgery. New York: McGraw-Hill; 1985:1011-1021.
76 Hammon WM, Kempe LG. The posterior fossa approach to aneurysms of the vertebral and basilar arteries. J Neurosurg. 1972;37:339-347.
77 Sen CN, Sekhar LN. An extreme lateral approach to intradural lesions of the cervical spine and foramen magnum. Neurosurgery. 1990;27:197-204.
78 Vishteh AG, Crawford NR, Melton MS, et al. Stability of the craniovertebral junction after unilateral occipital condyle resection: a biomechanical study. J Neurosurg. 1999;90:91-98.
79 Baldwin HZ, Miller CG, van Loveren HR, et al. The far lateral/combined supra- and infratentorial approach. A human cadaveric prosection model for routes of access to the petroclival region and ventral brain stem. J Neurosurg. 1994;81:60-68.
80 Hamilton MG, Kraus GE, Daspit CP, et al. Giant aneurysms: infratentorial. In: Carter LP, Spetzler RF, editors. Neurovascular Surgery. New York: McGraw-Hill; 1995:829-850.
81 Baumgartner WA, Silverberg GD, Ream AK, et al. Reappraisal of cardiopulmonary bypass with deep hypothermia and circulatory arrest for complex neurosurgical operations. Surgery. 1983;94:242-249.
82 Morgan H, Nofzinger JD, Robertson JT, et al. Hemorrhagic studies with severe hemodilution in profound hypothermia and cardiac arrest. J Surg Res. 1973;14:459-464.
83 Doutremepuich C. Haemostasis defects following cardio-pulmonary by-pass based on a study of 1350 patients. Thromb Haemost. 1978;39:539-541.
84 Lawton MT, Raadzens PA, Jacobowitz R, et al. Hypothermia-induced circulatory arrest in neurovascular surgery: evolving indications and predictors of patient outcome. J Neurosurg. 1997;86:401A.
85 Batjer HH, Kopitnik TAJ, Purdy PD, et al. Giant intracranial aneurysms. In: Wilkins RH, Rengachary SS, editors. Neurosurgery. New York: McGraw-Hill; 1996:2361-2376.
86 Cantore GP, Santoro A. Treatment of aneurysms unsuitable for clipping or endovascular therapy. J Neurosurg Sci. 1998;42:71-75.
87 Drake CG, Peerless SJ, Ferguson GG. Hunterian proximal arterial occlusion for giant aneurysms of the carotid circulation. J Neurosurg. 1994;81:656-665.
88 Cantore G, Santoro A, Da Pian R. Spontaneous occlusion of supraclinoid aneurysms after the creation of extra-intracranial bypasses using long grafts: report of two cases. Neurosurgery. 1999;44:216-219.
89 Gerber S, Dormont D, Sahel M, et al. Complete spontaneous thrombosis of a giant intracranial aneurysm. Neuroradiology. 1994;36:316-317.
90 Yeh H, Tomsick TA. Obliteration of a giant carotid aneurysm after extracranial-to-intracranial bypass surgery: case report. Surg Neurol. 1997;48:473-476.
91 Chang SD, Marks MP, Steinberg GK. Recanalization and rupture of a giant vertebral artery aneurysm after hunterian ligation: case report. Neurosurgery. 1999;44:1117-1120.
92 Lee KC, Joo JY, Lee KS, et al. Recanalization of completely thrombosed giant aneurysm: case report. Surg Neurol. 1999;51:94-98.
93 Numagami Y, Ezura M, Takahashi A, et al. Antegrade recanalization of completely embolized internal carotid artery after treatment of a giant intracavernous aneurysm: a case report. Surg Neurol. 1999;52:611-616.
94 Standard SC, Ahuja A, Guterman LR, et al. Balloon test occlusion of the internal carotid artery with hypotensive challenge. AJNR Am J Neuroradiol. 1995;16:1453-1458.
95 Peerless SJ, Ferguson GG, Drake CG. Extracranial-intracranial (EC/IC) bypass in the treatment of giant intracranial aneurysms. Neurosurg Rev. 1982;5:77-81.
96 Spetzler RF, Selman W, Carter LP. Elective EC-IC bypass for unclippable intracranial aneurysms. Neurol Res. 1984;6:64-68.
97 Ramina R, Meneses MS, Pedrozo AA, et al. Saphenous vein graft bypass in the treatment of giant cavernous sinus aneurysms: report of two cases. Arq Neuropsiquiatr. 2000;58:162-168.
98 Sekhar LN, Bucur SD, Bank WO, et al. Venous and arterial bypass grafts for difficult tumors, aneurysms, and occlusive vascular lesions: evolution of surgical treatment and improved graft results. Neurosurgery. 1999;44:1207-1223.
99 Ceylan S, Karakus A, Duru S, et al. Reconstruction of the middle cerebral artery after excision of a giant fusiform aneurysm. Neurosurg Rev. 1998;21:189-193.
100 Hadley MN, Spetzler RF, Martin NA, et al. Middle cerebral artery aneurysm due to Nocardia asteroides: case report of aneurysm excision and extracranial-intracranial bypass. Neurosurgery. 1988;22:923-928.
101 Bojanowski WM, Spetzler RF, Carter LP. Reconstruction of the MCA bifurcation after excision of a giant aneurysm. Technical note. J Neurosurg. 1988;68:974-977.
102 Gobin YP, Vinuela F, Gurian JH, et al. Treatment of large and giant fusiform intracranial aneurysms with Guglielmi detachable coils. J Neurosurg. 1996;84:55-62.
103 Gruber A, Killer M, Bavinzski G, et al. Clinical and angiographic results of endosaccular coiling treatment of giant and very large intracranial aneurysms: a 7-year, single-center experience. Neurosurgery. 1999;45:793-803.
104 Ross IB, Weill A, Piotin M, et al. Endovascular treatment of distally located giant aneurysms. Neurosurgery. 2000;47:1147-1152.
105 Lanzino G, Wakhloo AK, Fessler RD, et al. Efficacy and current limitations of intravascular stents for intracranial internal carotid, vertebral, and basilar artery aneurysms. J Neurosurg. 1999;91:538-546.
106 Fernandez Zubillaga A, Guglielmi G, et al. Endovascular occlusion of intracranial aneurysms with electrically detachable coils: correlation of aneurysm neck size and treatment results. AJNR Am J Neuroradiol. 1994;15:815-820.
107 Klein GE, Szolar DH, Leber KA, et al. Basilar tip aneurysm: endovascular treatment with Guglielmi detachable coils—midterm results. Radiology. 1997;205:191-196.
108 Bavinzski G, Talazoglu V, Killer M, et al. Gross and microscopic histopathological findings in aneurysms of the human brain treated with Guglielmi detachable coils. J Neurosurg. 1999;91:284-293.
109 David CA, Vishteh AG, Spetzler RF, et al. Late angiographic follow-up review of surgically treated aneurysms. J Neurosurg. 1999;91:396-401.
110 Vargas ME, Kupersmith MJ, Setton A, et al. Endovascular treatment of giant aneurysms which cause visual loss. Ophthalmology. 1994;101:1091-1098.
111 Ushikoshi S, Kikuchi Y, Houkin K, et al. Aggravation of brainstem symptoms caused by a large superior cerebellar artery aneurysm after embolization by Guglielmi detachable coils—case report. Neurol Med Chir (Tokyo). 1999;39:524-529.
112 Bavinzski G, Killer M, Ferraz-Leite H, et al. Endovascular therapy of idiopathic cavernous aneurysms over 11 years. AJNR Am J Neuroradiol. 1998;19:559-565.
113 Higashida RT, Halbach VV, Dowd C, et al. Endovascular detachable balloon embolization therapy of cavernous carotid artery aneurysms: results in 87 cases. J Neurosurg. 1990;72:857-863.
114 Pluchino F, Giombini S, Broggi G, et al. Surgical management of giant anterior aneurysm. J Neurosurg Sci. 1998;42:65-69.