Chapter 42 Microsurgical Approaches to the Ventricular System
• Intraventricular lesions often present with nonspecific symptoms and clinical signs and can affect patients of all ages. They may grow quite large before the diagnosis is secured. Imaging studies, in concert with a detailed knowledge of the surrounding microsurgical anatomy and neural function, permit the surgeon to determine the most effective and safest approach to resection of the lesion.
• The key principles in the microsurgical resection is to approach these deep-seated lesions by the most direct route that provides the least disruption to eloquent cortex and neural function. In addition, the surgical goal includes developing the most favorable trajectory for visualization of the entire lesion, the best proximal control of arterial feeders, least disruption to the venous drainage, and minimal contamination of the cerebrospinal fluid (CSF).
• Transcortical or interhemispheric transcallosal approaches adequately access lesions throughout the entire ventricular system. Transcortical approaches are ideal in patients with hydrocephalus, and interhemispheric approaches work regardless of the ventricle size. Both approaches permit the full range of microsurgical options to enter the third ventricle through the choroidal fissure or between the fornices.
• The midline telovelar approach is a safe and effective approach to resect fourth ventricle lesions extending from the aqueduct of Sylvius to the obex, without having to split the vermis.
• Complete ventricular communication after tumor resection is an important goal. Cystic septations should be removed so that CSF spaces communicate both anatomically and physiologically. Microscopic or endoscopic inspection at the end of the procedure confirms the absence of a blood clot, residual tumor, or septations and ensures that transventricular communication has been achieved.
• Microsurgical approaches are to be tailored to the lesion location, pathological entity, and surgeon’s experience. Each approach has distinct technical advantages with associated functional risks. Endoscopic approaches can be used to achieve resection of select intraventricular lesions, most commonly colloid cysts of the third ventricle. Future advances in endoscopic instrumentation, preoperative functional imaging, and fiber tract identification will influence surgical strategies in the coming years.
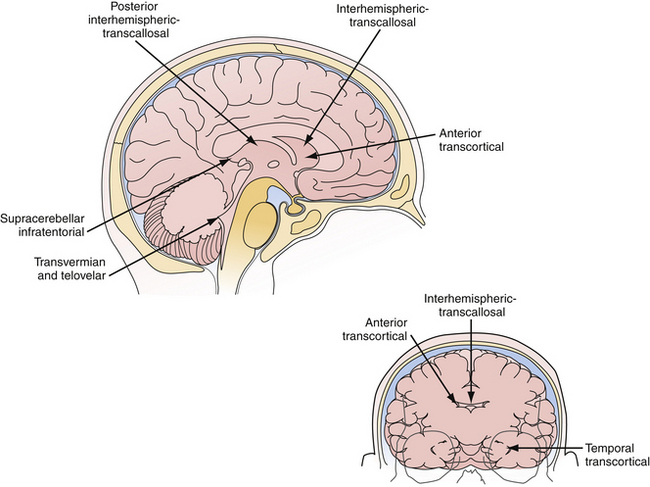
Microsurgery for ventricular tumors is both challenging for the surgeon and potentially curative for the patient. Accounting for a small percentage of all cerebral lesions,1 tumors of the ventricles present a unique clinical and elegant microsurgical experience for neurosurgeons. Intraventricular tumors can grow to a very large size, eluding diagnosis until they cause rapid decompensation from hydrocephalus or increased intracranial pressure. Surgical approaches for resection of lateral and third ventricle tumors always require the neurosurgeon to enter through the brain, either the cerebral cortex or corpus callosum. Complete resection of these lesions is often possible through either the transcortical or interhemispheric transcallosal routes. Surgical approaches to the fourth ventricle are different in that splitting of a fissure through a telovelar approach may provide access of the fourth ventricle from the aqueduct of Sylvius to the obex without sacrifice of any cerebellum (Fig. 42.1).
The age of presentation of intraventricular tumors spans from infancy to the elderly.2 Preoperative symptoms are often nonspecific; including headache (35%), weakness (25%), sensory loss (25%), nausea and vomiting (22%), dementia (18%), and visual loss (18%).2–7 Pathological entities range from slow-growing indolent lesions to aggressive malignancies. Tumors arising within the third and lateral ventricles are frequently (but not always) slow-growing and benign,4,5 whereas lesions arising from the fourth ventricle may be rapidly progressive due to cerebrospinal fluid (CSF) obstruction and brainstem signs and include both benign and malignant pathological types.
Despite these challenges, surgery for intraventricular tumors can be rewarding for the patient. When the symptoms are primarily related to the hydrocephalus resulting from the obstructive nature of these tumors, clinical improvement may be immediate. Gross total resections of low-grade lesions may be curative. Finally, the refinements in technique and equipment have improved outcomes substantially since Dandy’s first description of surgery for intraventricular tumors in 1922.8
Microsurgical Anatomy
Mastering the relevant ventricular anatomy is essential in performing microsurgical approaches to the lateral ventricles. A more detailed review of the anatomy can be found in Professor Albert L. Rhoton’s outstanding monograph on the subject, which was produced after years of study in his laboratory and operating room.9
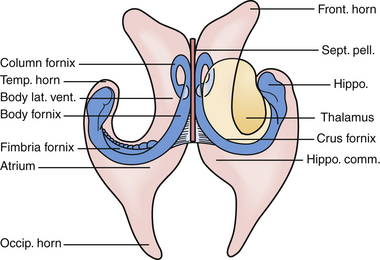
The lateral ventricles are paired C-shaped structures that are anchored by each thalamus in the middle of the brain. Each ventricle is divided into five sections. Starting anterior and superior and moving backward they are the (a) anterior horn, (b) body, (c) temporal horn, (d) atrium, and (e) occipital horn (Fig. 42.2). Each of these five sections has a floor, roof, medial wall, and lateral wall and these sections contain structures that are critical to the surgeon for navigation in the ventricles. Specifically, they are structures that either tolerate manipulation to facilitate visual access or, conversely, should not be manipulated. These relationships are important when considering either the transcortical or the transcallosal approach.
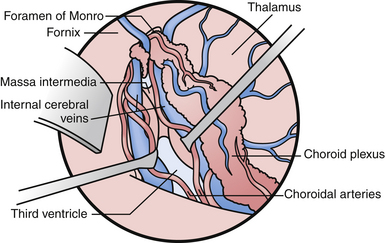
If one visualizes the ventricles in a coronal plane, the anatomical relationships are as follows. At the level of the frontal horn of the lateral ventricle, the lateral wall is composed of the oval caudate nucleus, and the medial wall is the diaphanous septum pellucidum that separates the two frontal horns. The roof is made up of the roof of the genu of the corpus callosum, and the floor is composed of the rostrum of the corpus callosum, which wraps around and tucks under the ventricles until it reaches the anterior commissural fibers. At the level of the body of the ventricle, some of the same relationships exist. The corpus callosum makes up the roof, the caudate nucleus the lateral wall, and the medial wall is again separated by the septum pellucidum. Moving posteriorly within the lateral ventricles, the floor is composed of the thalamus. In the midline of the floor lie the columns of the fornix. In the floor of the body of the lateral ventricle, sitting between the fornix and the thalamus, is the choroid plexus, which attaches to the surgically crucial structure called the choroidal fissure. Each column of the fornix also forms the curved superior and anterior borders of the foramen of Monro. Moving to the temporal horn, each column of the fornix starts as the fimbria of the hippocampi and starts in the medial wall of the temporal horn. After giving off commissural fibers, these nerve bundles continue in the inferior medial wall of the body as the fornix.10 The roof of the temporal horn is the tapetum of the corpus callosum, and the floor comprises the folded gyri of the hippocampus. Moving within the ventricle to its posterior limits we find the atrium and the occipital horn. The atrium and occipital horn form a triangular CSF cavity in the occipital lobe. Again, the tapetum of the corpus callosum covers the lateral wall and roof of the atrium. The forceps major, which is a fiber bundle that connects the two occipital lobes through the splenium of the corpus callosum, runs in the superior part of the atrium. The floor is composed of the collateral trigone and the medial wall is the calcar avis of the calcarine cortex.7
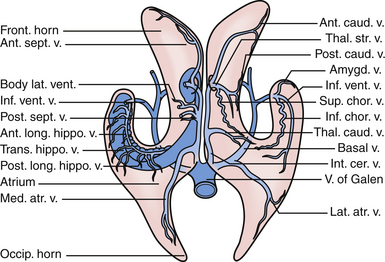
The primary arteries within the ventricles are the anterior and posterior choroidal arteries,7,10 branches of which provide the vascular supply to tumors in this region. Understanding the course of the arteries helps the surgeon choose an approach for each lesion and thus permits early proximal control, when possible, of the feeding vessels.
The anterior choroidal artery arises from the internal carotid artery, just a millimeter or so distal to the posterior communicating artery. It exits the anterior incisural space and enters the lateral ventricle through the choroidal fissure, heading posteriorly to lie near the lateral posterior choroidal artery.7,10 The anterior choroidal artery generally supplies the choroid plexus in the temporal horn and atrium. Because the choroidal arteries pass through the choroidal fissure, opening this fissure early also facilitates proximal control of the feeding vessels.
The posterior choroidal arteries are grouped into lateral and medial divisions. The lateral posterior choroidal artery is composed of one to six branches, which arise in the ambient and quadrigeminal cisterns, typically from the posterior cerebral aratery (PCA). These branches then pierce the ventricle and pass around the pulvinar and enter through the choroidal fissure at the level of the fimbria/body of the fornix to supply the choroid plexus in the posterior temporal horn, atrium, and body of the ventricles.7 The medial posterior choroidal arteries arise as one to three branches from the PCA in the interpeduncular and crural cisterns. These arteries circumnavigate the midbrain and move to the pineal gland to enter the roof of the third ventricle and reside in the tela choroidea called the velum interpositum, adjacent to the internal cerebral veins. The medial posterior choroidal artery supplies the choroid plexus in the roof of the third ventricle and sometimes the choroid plexus of the lateral ventricle.10
The veins are useful as landmarks to help navigate in the body of the ventricle, especially in cases in which hydrocephalus is present (Fig. 42.3). There are many important veins composing the lateral and medial groups, but perhaps the best known for surgical and angiographic orientation is the thalamostriate vein, which helps orient the surgeon toward the foramen of Monro. The thalamostriate vein traverses the lateral wall of the body of the ventricle adjacent to the choroidal fissue between the caudate and thalamus. It then forms the venous angle with an acute posterior turn into the foramen of Monro to empty into the internal cerebral veins traversing the velum interpositum. The veins in the temporal horn drain into the basal vein of Rosenthal (basal vein) as it passes through the ambient cistern on each side. Veins from the atrium and occipital horn drain into the basal internal cerebral veins as well, and finally into the vein of Galen which empties into the straight sinus and torcular herophili.7
Pathological Entities
The differential diagnosis of tumors in the lateral ventricle depends on several factors: the age of the patient, location of the tumor, and specific radiological characteristics as described by computed tomography (CT), magnetic resonance imaging (MRI), and cerebral angiography.11,12 Tumors found in the lateral ventricle of children younger than 5 years of age are often choroid plexus tumors, whereas in older children they tend to be astrocytoma or ependymoma.11 Choroid plexus tumors are rare lesions with a prevalence of 0.3 per million. They comprise only about 1% of all brain tumors and occur in children with a median age of 3.5 years at the time of presentation.13,14 Choroid plexus tumors are often quite vascular and demonstrate a tumor blush on CT, MRI, and cerebral angiography. On MRI, they may possess heterogeneous signal characteristics caused by necrosis and calcification and are iso- to hypodense on T1-weighted images relative to the white matter.
The most common hypo- or isodense, nonenhancing tumor in the body or foramen of Monro is a subependymoma. In children with tuberous sclerosis (TS), these lesions are often a subependymal giant cell astrocytoma (SEGA). Roughly 6% of patients with TS develop subependymal giant cell tumors.15 In this location, they can reach a large size before being diagnosed, sometimes by unilateral ventricular obstruction.16–18 Astrocytomas can be found in any part of the ventricle as they are surrounded by white matter tracts. Astrocytoma will most frequently arise from the thalamus, where they can infiltrate. Ependymoma, when supratentorial, can be intraventricular as well as intraparenchymal.11
In adults older than 30 years of age, tumors in the atrium and trigone are most commonly meningiomas. Intraventricular meningiomas are well-circumscribed, homogeneously enhancing lesions.19 Meningiomas tend to be isodense to brain on T1-weighted images and brightly enhance with gadolinium administration. Intraventricular masses that engulf the choroid glomus, are calcified, or are demonstrated to have a choroidal artery supply on angiography are usually benign meningiomas.20,21
Outside the trigone, tumors in older patients are often either a primary or metastatic malignancy.11
Primary or metastatic malignancies present late in life with attachment to the ventricular walls and parenchymal invasion.11 Malignant intraventricular metastases include renal cell carcinoma, pulmonary adenocarcinoma, gastrointestinal carcinoma, transitional cell carcinoma, and adrenocortical carcinoma.
Central neurocytomas, which occur mostly in adults in the second to fourth decades of life, appear to attach or grow from the septum pellucidum and possess a characteristic imaging appearance.22 These lesions may be solid with cystic components and flow voids on MRI, and may contain calcifications visualized on CT.23 These benign World Health Organization (WHO) grade II lesions derive their name from the original description of a well-differentiated neural lesion that was adherent to the septum and ventricle wall.24 These tumors can be quite large on presentation. Although the natural history and long-term prognosis of these lesions are not completely understood, excision appears to be curative in those patients in whom a gross total resection is achieved. Significant blood loss and a “sticky” connection to the thalamus and third ventricle structures may complicate the attempts at a radical resection.
Cystic lesions include colloid cysts of the third ventricle and infectious lesions such as neurocysticercosis, nocardiosis, and cryptococci. Colloid cysts are covered in another chapter but at our institution they are routinely approached with a single port endoscopic resection. Other non-neoplastic processes such as cysts, sarcoidosis, xanthogranulomas (which appear to be dense on CT scans with flecks of calcification), arteriovenous malformations, and cavernous hemangiomas are also found in the lateral ventricle with geographic variation.25 In some endemic regions of the world, a new seizure or hydrocephalus associated with cystic intraventricular lesion often indicates neurocysticercosis. In our institution, the history, MRI, and serum or CSF markers may secure the diagnosis of neurocysticercosis, and these patients then receive medical therapy. Endoscopic exploration and resection are used to remove obstructive ventricular neurocysticercosis lesions when indicated by symptoms or examination.
Preoperative Planning and General Surgical Considerations
A CT scan is often the first radiological study patients undergo simply because of diagnostic ease in the surveillance workup. The presence of calcifications on the CT image may further narrow the differential diagnosis.26 Calcification may be seen in central neurocytoma, subependymal giant cell tumor, meningioma, and ependymoma.
Subsequent MRI, with and without gadolinium, reveals the precise location and extent of the lesion within the ventricle and, thus, helps guide the surgical approach. At a minimum this should include sagittal, coronal, and axial reconstructions of T1-weighted sequences (with and without gadolinium), T2-weighted images, and fluid-attenuated inversion recovery (FLAIR). These sequences are sufficient to refine the differential diagnosis and begin basic surgical planning. The role of new specialized MRI sequencing is rapidly evolving. Diffusion tensor imaging (DTI) permits reconstruction of large fiber tracts, which may contribute to surgical planning by guiding the surgical trajectory through the least disruptive pathway.27 Functional MRI (fMRI) has a role in cases in which the approach encroaches on motor or language cortex. Some authors advocate magnetic resonance spectroscopy (MRS) to characterize the chemical composition of the lesion in malignant lesions that may require a biopsy.28 However, we have found the DTI and fMRI to be more helpful in surgical planning. It is our practice to also obtain magnetic resonance angiography (MRA)and venography (MRV). These images serve several purposes. First, they permit visualization of the blood supply to the lesion. Second, MRV through the vertex allows the surgeon to tailor the placement of the craniotomy based on draining cortical veins. Finally, MRV is helpful when considering choroidal dissection to gain access to the third ventricle.29
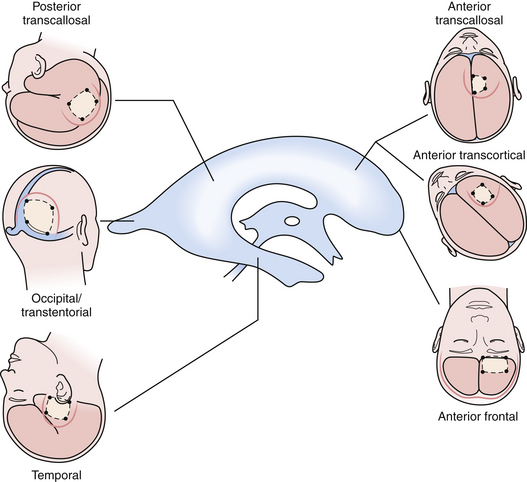
The beautiful anatomy of the lateral ventricle facilitates a wide variety of surgical approaches. The location and size of the lesion, hemispheric dominance, preoperative deficits, associated hydrocephalus, vascularity of the lesion, and experience of the surgeon all contribute to the ultimate selection of surgical approach. There is simply no class I or II data that dictates one approach is superior to another. The optimal approach facilitates the primary goal of surgery, which is gross total resection of the lesion with minimal trauma to surrounding structures and neural function. At our institution, most lateral ventricle lesions are approached through a transcortical route, a transcallosal route, or in rare cases a combination of the two. As detailed in the following sections, these routes provide excellent access to lesions within the third and lateral ventricles (Fig. 42.4).
Craniotomy Flap and Cortical Incisions
To enter the ventricle directly, we employ a simple technique of placing a ventricular catheter by frameless stereotactic guidance, or through a freehand pass to enter the ventricle. Once the trajectory to the ventricle is determined, a small cortical incision is made and followed down to the catheter in the ventricle. The ependyma is opened with nonstick or irrigating bipolar electrocautery, and the ventricle is entered. A cortical aperture is made using flat microinstruments or microsuction to develop a subcortical path to the lesion. Very little of the subcortical white matter is removed, and flat malleable brain retractors or a microsurgical speculum is placed to increase visibility. The goal is not necessarily to make the smallest cortical incision but to make the most appropriate one. Transcortical surgery has the advantages of wide avenues and a maneuverable microsurgical field.30
There are limitations to the transcortical approach. This approach may not provide optimal visualization of the contralateral ventricle due to the linear trajectory, which may obscure lesions sitting in a location out of the line of sight of the microscope. The transcortical approach may also predispose the patient to postoperative seizures. The incidence of postoperative seizures ranges in the literature from 5% to 70%,2,3,31–34 which is higher than in transcallosal approaches when no cortisectomy is performed (0-10%). However, our experience with seizure activity after the transcortical approach parallels that of the transcallosal approach. Specifically, the postoperative seizure rate with very small corticectomy (<15 mm) and prophylactic anticonvulsant treatment for 1 week has successfully lowered the immediate postoperative seizure incidence to the 5% to 7% range.
Anterior Transcortical Approaches
Tumors in the frontal region are primarily astrocytoma, subependymal giant cell astrocytoma, ependymoma, and central neurocytoma. Tumors of the frontal horn can become very large and cause obstruction of the foramen of Monro with ventricular dilation. The transcortical middle frontal gyrus approach is an excellent route for the excision of tumors in the ipsilateral anterior horn of the lateral ventricle, the anterior body of the lateral ventricle, and the anterior third ventricle. Tumors that extend inferiorly from the lateral ventricle into the third ventricle and require an interforniceal or subchoroidal exposure for removal, can be approached using either a transcortical or a transcallosal route.10 In patients with small ventricles, tumor in both lateral ventricles, or tumor in the body of the lateral ventricle, the transcallosal approach is employed.
The patient is placed in the supine position with the head elevated 10 to 30 degrees. A free 3 × 4-cm bone flap is placed over the central portion of the middle frontal gyrus. The flap is based on the coronal suture, with the medial border off the midline and the anterior border at least 2 cm anterior to the coronal suture and the posterior border about 2 cm behind. The dura is opened in a cruciate or box-shaped fashion and a ventricular catheter is placed into the frontal horn. This is followed by microsurgical cleavage of the white matter until the ependymal lining is broached. A small ⅜-inch retractor or speculum retractor is used. After the anatomy of the lateral ventricle is visualized through the microscope, regardless of the approach, the anatomy is similar. Once inside the ventricle, surgical landmarks provide orientation. The most important landmarks are the foramen of Monro, the thalamostriate vein, the choroid plexus, and the fornix. Cotton paddies are placed over the foramen and around the lesion to minimize the circulation of blood products and debris during tumor resection.
Lesions within the third ventricle may be accessed from the transcortical approach. If the foramen is enlarged from hydrocephalus or the lesion itself, no additional dissection is necessary. If not, additional access to the third ventricle is made possible by a transchoroidal or suprachoroidal, trans–velum interpositum dissection.35 To achieve this exposure, the choroidal fissure is opened between the fornix and the thalamus.36 The suprachoroidal or transchoroidal approach divides the taenia of the fornix between the fornix and choroid plexus. The subchoroidal approach divides the taenia thalami leaf of the tela choroidea between the choroid plexus and the thalamus. The latter approach is more commonly associated with direct injury to the thalamostriate vein and thalamus. Rhoton’s elegant lifetime work has been captured in his collected works in Neurosurgery and is arguably the most complete work on the subject.9,37 In his ventricular surgery experience, he has demonstrated that the transchoroidal or suprachoroidal approach, opening the fissure through the taenia fornix, is both safe and effective.18 Although we have safely opened the fissure through the “subchoroidal” approach on the taenia thalami side, the risk of thalamic damage is quite real and can be devastating. In contrast, opening on the taenia fornix side in which a unilateral forniceal injury occurs often results in no permanent memory loss (Fig. 42.6). Regardless, these aforementioned considerations are important but must be applied to the specific anatomy at the time of the intraventricular dissection. Complications from injury to the surrounding structures result in hemiparesis, mutism, amnestic syndromes, and confusion.38 In general, these risks are minimized by a suprachoroidal approach. However, maintaining the integrity of both fornices is important for preservation of memory function. The fornix, which has been reported to carry as many as five times the number of axons as the optic tract,39 links the hippocampal formation (including the hippocampus proper, dentate gyrus, parahippocampal gyrus, and subiculum) with the septal nuclei, mammillary bodies, hypothalamus, and thalamic nuclei. Visualization of the fornix may be obscured by lesions based on the septum pellucidum, such as central neurocytomas. Patient dissection and meticulous technique here will be rewarded with a favorable postoperative outcome.
Although the precise method of tumor resection is determined on a case-by-case basis, we adhere to certain general principles in all cases. Prior to entering the lesion, attention must be directed first toward any feeding vessels entering the tumor. Once these are coagulated and divided, the tumor capsule itself may be coagulated and incised. The tumor is then internally debulked with ultrasonic aspiration. Early in the dissection, frozen specimens are collected for pathological examination. Care is taken to achieve complete hemostasis at regular intervals. As the excision proceeds, the surgeon may periodically reorient using surrounding anatomical landmarks and neuronavigation. It is critically important to preserve the integrity of the fornices, caudate, thalamus, and normal vascularity to avoid postoperative deficits.
Approach to the Temporal Horn
The transcortical approach is the primary method with which to remove tumors in the temporal horn extending from the temporal tip back to the ambient cistern. The temporal horn, however, is the least likely of the five ventricular regions to harbor a tumor.6,40
The scalp incision is performed so that bony landmarks, which include the zygoma, the asterion, and the pterion, can be visualized. A craniotomy to the floor is performed, but care is taken to avoid the middle ear; ear cells that are encountered need to be occluded.41 Anatomically, the structures that must be respected during removal of temporal horn tumors include the medial structures such as the hippocampus and its projections, temporal stem, PCA, anterior choroidal artery, and the posterior brainstem. Superiorly, one must avoid the optic tract, Meyer’s loop, and arcuate fasciculus.
This last cortisectomy42 may be the safest, especially in the dominant hemisphere. This incision was described by Spencer and Collins43 in 1982 and was first used in the surgical treatment of epilepsy. It was designed to remove the hippocampus and associated structures and is therefore familiar to many neurosurgeons specializing in the treatment of epilepsy. The approach is also extremely useful for removing large, vascular lesions in either hemisphere, extending back to the brainstem. An incision can also be made in the inferior temporal gyrus as well as the occipitotemporal gyrus or collateral sulcus. Once inside the temporal horn, the choroidal fissure is identified. The fissure can be split by microsurgical technique along the taenia fimbriae, lifting the choroid plexus toward the thalamus, leaving the taeniae thalami and thalamus intact. This step permits visualization of the vascular structures to include anterior and posterior choroidal arteries, basal vein of Rosenthal, and PCA. The fissure can be split as far back as the ambient cistern. There is very little retraction in this approach and this preserves the vein of Labbé and temporal lobe. Early control of the anterior choroidal vessels can be obtained, making tumor or vascular malformation excision slightly less challenging. However, the posterior choroidal artery branches feeding a very large tumor may not be exposed until the bulk of the tumor is removed. Luders and coworkers44 have described a basal language region in the inferior temporal lobe that is best elicited by cortical stimulation. Its removal does not uniformly lead to a language deficit because it presumably represents ancillary language cortex. Our experience is similar to that of Luders and coworkers. We have also mapped this language area in several patients but have yet to record a postoperative language deficit following a ventricular tumor excision using this approach.20
The middle temporal gyrus incision provides a direct route to many middle fossa ventricular lesions. In the nondominant hemisphere, this is a very acceptable route, causing minimal morbidity. In the dominant hemisphere, the danger posed to the language cortex becomes an issue and requires refinement in technique or use of the inferior route through the collateral sulcus as described previously. Ojemann45 has meticulously detailed the individual variability of language localization to include language encoding in some individuals within 3.5 cm of the temporal tip and in gyri other than the superior temporal gyrus. Thus, in dominant hemisphere lesions, if the middle temporal gyrus approach is used, the risk of causing language deficit is most effectively prevented by cortical stimulation and mapping. Facial apraxia has also been reported in the dominant hemisphere.40 The middle temporal gyrus approach to the temporal horn of the lateral ventricle has the potential to manipulate the tracts of the visual pathways, resulting in varying degrees of temporary and permanent deficits.5,18,30,46 The optic tract can be found in the superior medial region of the temporal horn as it courses toward the lateral geniculate body. The optic radiations then head toward the calcarine cortex along the Meyer-Archambault loop in the superior and lateral aspect of the temporal horn and in the tapetum over the roof and lateral aspect of the atrium and occipital horn. Cortical incisions in the middle fossa are best placed parallel to the Meyer-Archambault loop to avoid causing a quadrantanopia or other field deficit.
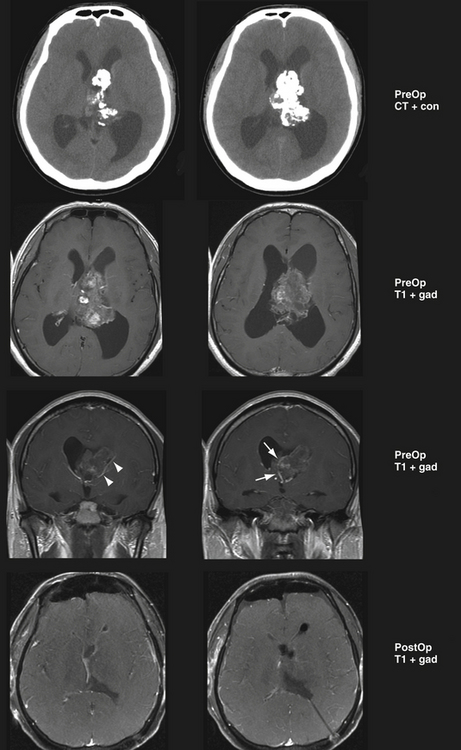
The lateral temporoparietal junction approach is applicable in patients in which the angular and supramarginal gyri are flattened by a large tumor in the nondominant atrium that lies directly beneath them. In such a case, this is the shortest and most direct route to the tumor. The advantage of this approach is that a small portion of brain needs to be traversed from the cortex to the tumor. The disadvantage of this approach is the potential for significant neuropsychological sequelae, which have been reported when manipulating the angular gyrus. It is useful for very large tumors with overlying attenuated cortex. Regardless of hemisphere, a cortical incision in the temporoparietal junction can result in a visual field deficit caused by the interruption of the optic radiations.47 A cortisectomy through the angular gyrus in the dominant hemisphere can also cause right-left confusion, digital agnosia, agraphia, and acalculia (i.e., Gerstmann syndrome).48 In the nondominant hemisphere, visual memory loss and neglect can result, which are also potentially disabling.47 However, our experience has been only with very large tumors in which there was extremely attenuated brain with little cortex covering the tumor. In these patients, taking the shortest route with least retraction after DTI analysis of the tracts yielded an improved neuropsychological outcome, perhaps because the patient’s function was already compromised and an alternative route would place those fibers at greater risk (Fig. 42.7).
Posterior Transcortical Approaches
The superior parietal route is one of the best approaches for reaching the collateral trigone, the posterior part of the body, and atrium.46,49–52 It has been used to reach vascular malformations in the trigone, as well as tumors.49,53 Postoperative cortical damage manifested by a visual field cut can occur if the medial wall of the atrium adjacent to the calcarine cortex is injured; however, the cortical incision is high enough to avoid the optic radiations in most patients. It is similar to the route taken through the cortex when placing a posterior ventricular catheter for CSF diversion. The superior parietal transcortical approach is riskier in the dominant hemisphere. The potential risks include apraxia, acalculia, visual spatial distortion, and the full-blown Gerstmann syndrome.6,54 However, this operation can be performed safely in the dominant hemisphere. A large series of the parietal approach has yielded radical tumor resection without neuropsychological sequelae.55 The vascular supply to the tumor is deep and circumnavigating the tumor may require retraction that can be deleterious to the superior parietal lobule, so preoperative embolization may be helpful in these patients. The patient is placed in the three-quarter prone position with the parietal boss in the most superior position and the face turned toward the floor. The cortical incision is performed on the long axis of the superior parietal lobule with the trajectory directed toward the atrium by frameless navigation. It is made high enough to avoid the optic radiations and posterior enough to avoid language encoded at the junction of the parietal and temporal lobes. The ventricle is entered above the junction of the body, the atrium, and the crus of the ipsilateral fornix. From a coronal plane, one can visualize the choroid plexus and fissure as a navigational landmark. The calcar avis and corpus callosum will make up the medial wall, and the pulvinar will be anterior. The collateral trigone will make up the floor. This is also an excellent approach to resect a thalamic lesion that extends into the atrium. The posterior choroidal artery can be uncovered in the choroidal fissure between the pulvinar and the crus of the fornix.7 The ventricle atrium is surrounded by subcortical white matter tracts critical for vision. Immediately below the thin layer of tapetal fibers lie the optic radiations, which leave the superior portion of the lateral geniculate nucleus to innervate the superior bank of the calcarine fissure.56 The inferior bank of the calcarine fissure receives fibers from the inferior portion of the lateral geniculate nucleus which loop forward around the temporal horn of the lateral ventricle. The fibers, which serve vision from the upper visual field, extend through the superior wall of the temporal horn of the lateral ventricle before looping laterally and inferiorly in the atrium.56 Opening the choroidal fissure at this junction will expose the internal cerebral vein running with the choroidal artery in the velum. One may also visualize the basal vein of Rosenthal or vein of Galen. Within the atrium, the glomus of the choroid plexus receives blood from the anterior and lateral posterior choroidal arteries. Approaches to the ventricle atrium therefore require careful attention to the surrounding anatomy. The atrium may be entered from parieto-occipital transcortical approaches, tailored to the cortical anatomy, draining cortical veins and periventricular white matter tracts.57 The superior parietal trajectory entails a cortisectomy between the postcentral sulcus and the parieto-occipital sulcus.54 These approaches are not without morbidity.58,59 For lesions arising from the medial surface of the atrium, such as the precuneus gyrus, a posterior interhemispheric approach may be preferred to the transcortical route.27,60 Using this approach, the brain is entered lateral to the splenium through a limited cortisectomy. Obviously, this approach may be complicated by postoperative hemanopia. A more superiorly oriented approach enters the brain lateral to the splenium from a parietal trajectory and is not associated with a high rate of visual loss.60 A lateral approach enters the trigone through the posterior temporal lobe and parietal lobe.61
Interhemispheric Transcallosal and Posterior Third Ventricle Approaches
The anterior and posterior interhemispheric transcallosal approach offers different advantages over the transcortical route. The transcallosal approach does not disrupt the cortex, and is therefore associated with a lower incidence of postoperative seizures.62 Also, the functional consequences that may be observed with transcortical approaches are largely absent.63 Although only a few centimeters of callosal division are necessary to permit ample exposure to the lateral or third ventricle,27 the anterior two thirds of the corpus callosum may be divided without clinically significant consequences.64
The anterior transcallosal approach is best suited for lesions located within the frontal horn of the lateral ventricle, the body of the lateral ventricle, and the anterior third ventricle.65 Limitations of the transcallosal approach prevent this approach from being utilized in all patients. The extent of lateral exposure within the lateral ventricle may be limited. Complications associated with transcallosal approaches to the third ventricle include hemiparesis, amnesia, and akinetic mutism.38 Section of the anterior corpus callosum is contraindicated in patients with crossed dominance, that is, patients with language lateralized to one cerebral hemisphere and dominant hand function lateralized to the other.66,67 Callosal section may result in speech and writing deficits in this patient population.
For standard anterior transcallosal approaches to the lateral ventricle, the patient is positioned supine with the head slightly flexed. For posterior transcallosal approaches the patient is placed in the prone position. Neuronavigation assists the surgeon in defining the extent of the craniotomy based on the intraventricular target. Craniotomy may be performed on either side of the skull because the transcallosal approach permits equal access to both lateral ventricles.68 However, it is our practice to perform these craniotomies on the right side to minimize the risk of language dysfunction associated with supplementary motor syndrome, which may occur following interhemispheric retraction of the dominant hemisphere.69 Side of approach is more important when addressing third ventricle lesions to maximize a direct approach through the foramen of Monro to the lesion. We utilize neuronavigation to plan the craniotomy. As with anterior transcortical approaches, we prefer a bicoronal incision because of the wide exposure and cosmetic result.
This chapter is not intended to review pineal region tumors in detail. A more complete review can be found in Chapter 37. Lesions located in the posterior third ventricle may be difficult to approach given the complex anatomical relationships. However, the pineal region is the corridor to the posterior third ventricle.
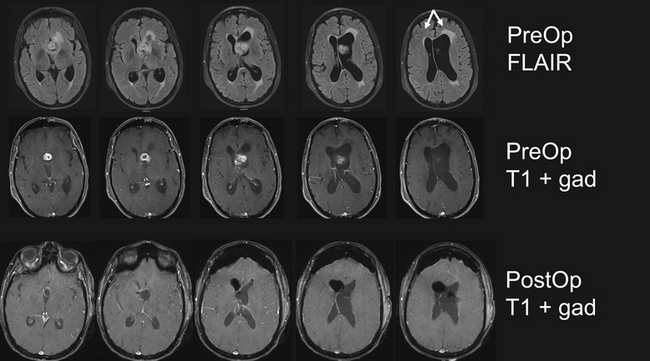
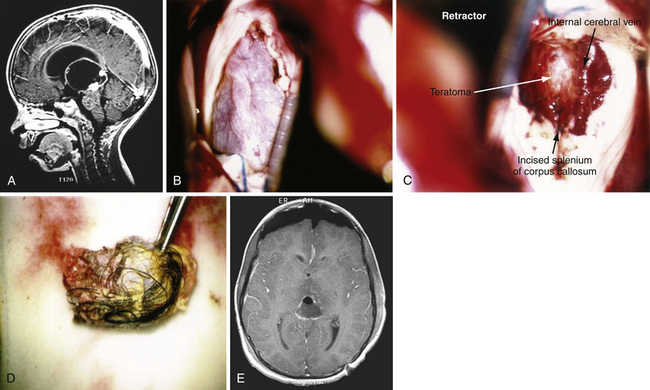
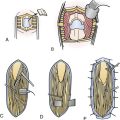
The posterior transcallosal approach through the splenium of the corpus callosum may be used to address a wide spectrum of tumors in the posterior third ventricle.4,70,71 The posterior interhemispheric route is an ideal approach for large third ventricle lesions that extend into the pineal region. There are many less bridging veins in the posterior approach compared to anterior approach, so the interhemispheric approach to the splenium is often more facile. However, one must be cautious as the trajectory through the splenium takes the surgeon close to deep drainage veins such as the vein of Galen. Despite concern about neuropsychological sequelae with a splenial incision, the patients often tolerate this remarkably well. And, when the splenium is thinned, this approach can be successfully used for both small and large lesions in children and adults (Fig. 42.8A-E).
Yaşargil advocates the median supracerebellar dissection to this region as it takes advantage of the natural corridor between the cerebellum and tentorium.27 A necessary requirement of this approach is that the angle of the tentorium must not be prohibitively steep. This dissection, also termed the infratentorial-supracerebellar approach, permits access to the velum interpositum above the pineal gland and habenular commissure. Horsley is credited with first attempting this approach for pineal region tumors.72 The approach begins with a midline occipital and suboccipital craniotomy. Careful protection of the dura overlying the torcular and transverse sinuses is required. The dura is opened below the transverse sinus. The dissection proceeds over the superior surface of the cerebellum. Where possible, lateral veins should be preserved; however, the superior vermian and the precentral cerebellar vein must be sacrificed when they are obstructing the view of the tumor. At the depth of the approach, the quadrigeminal cistern is apparent. Posterior third ventricle tumors originating from the pineal gland often project posteriorly into the quadrigeminal cistern. These lesions are immediately apparent above the quadrigeminal cistern during this dissection. Internal debulking within the tumor capsule will decompress the posterior third ventricle. Lesions within the third ventricle not arising from the pineal gland may be approached above the pineal by developing the potential space in the third ventricle with the velum interpositum being the superior border. However, this is an extremely long and deep reach through a narrow corridor.
The principal limitations to the supracerebellar infratentorial approach is the patient positioning and the deep narrow corridor in which the surgeon must work. The infratentorial supracerebellar approach is often performed in the sitting position to permit the cerebellum to sag for better visualization, although it can be performed in the prone, or Concorde position. Complications related to performing craniotomies in this position are air embolism,73,74 hemorrhage,75 tension pneumocephalus,76 and cervical flexion myelopathy.77
Broadly considered, pineal region tumors are grouped into three major pathological categories: (1) germ cell tumors, (2) primary pineal cell tumors, and (3) glial cell and all “other” tumors.73 In selected tumors that have positive markers in the serum or CSF it is worthwhile to follow these values.73 α-Fetoprotein or β-human chorionic gonadotropin levels, when elevated, can be monitored in patients with malignant germ cell components in the posterior third ventricle to assess their response to adjunctive therapies.
Combined Endoscope-Assisted Microsurgical Approach to the Lateral and Third Ventricles
The use of the neuroendoscope during intraventricular surgery is well described.78 Neuroendoscopy can be performed through the same approach as microsurgery, or through a separate approach entirely.79 Minimally invasive techniques are attractive alternatives to conventional microsurgery because they require little brain retraction. The endoscope offers superior visualization due to the proximity of the camera to the lesion and the high-intensity illumination. Endoscope-assisted resection may achieve gross total resection rates in 80% of selected cases.80,81 The principal limitations of endoscope-assisted tumor resections relate to technical limitations with the instruments. This subject will be addressed in Chapter 42.
Approach to the Fourth Ventricle
The most versatile approaches to fourth ventricle tumors are in the midline through the inferior vermis or the telovelar approach, which preserves those structures. The patient is positioned prone. The head is flexed in the Concorde position and the bed angle is adjusted so that the level of the craniotomy is even with the atrium of the heart, thereby reducing the risk of air embolism.82 A suboccipital craniotomy or craniectomy is performed, depending on surgeon preference. The posterior arch of the C1 lamina is removed after the dura is incised in Y-shaped fashion and reflected superiorly. Opening the cisterna magna affords considerable brain relaxation. Adhesions between the cerebellar tonsils are released with sharp dissection, exposing the vermis in the midline.
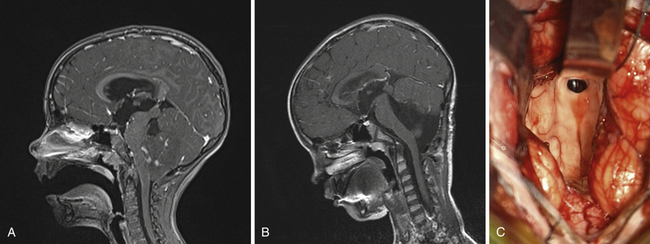
The dissection proceeds carefully to identify and protect the floor of the fourth ventricle. Once the floor has been identified under the vermian peg, our preference is to protect it with cotton paddies. Depending on the nature of the fourth ventricle lesion, the tumor may be entered first before arriving at the ventricle proper. Although splitting the vermis may be required, we have avoided that move for most tumors, including large ones, over the past decade. This is frequently the case with medulloblastomas which are midline and arise from the inferior medullary velum.83 Splitting the vermis is often undesirable in terms of side effects to the patient. The telovelar, or cerebellomedullary, approach permits the surgeon to access the fourth ventricle through the tonsillouveal sulcus.27 This approach, also performed through suboccipital craniotomy, accesses the ventricle by dividing the tela choroidea and inferior medullary velum along the lateral recess of the vermis.84 This move permits a wide opening without disturbing the vermis or the tracts from the cerebellum to the thalamus. There are few known deficits that occur from opening the tela and the velum. One can even extend the telar opening toward the foramen of Luschka, thus exposing the lateral recesses of the brainstem and cerebellum. Tumor resection proceeds with a surgical patty on the floor of the fourth ventricle protecting it and ensuring the surgeon can consistently define the plane between the tumor and floor. Once within the ventricle, delivering tumor from the aqueduct of Sylvius above permits the supratentorial ventricular system to communicate with the fourth ventricle, flooding the field with CSF. The lesion is resected with vigilance to preserve the integrity of the floor of the fourth ventricle. Very small and very large vascular tumors can be safely removed from the obex of the brainstem to the aqueduct of Sylvius using this powerful and safe approach (Fig. 42.9A-C).
Postoperative Deficits and Complication Avoidance
The postoperative visual field defect is one of the most common deficits associated with transcortical approaches in the posterior cortical and middle fossa approaches (Table 42.1). The most common deficit is a homonymous hemianopia or quadrant cut.
TABLE 42.1 Approaches to Microsurgical Resection of Ventricular Lesions: Indications and Risks
| Approach | Indication(s) | Potential Complication(s) |
|---|---|---|
| Anterior transcortical approach via frontal gyrus | Ipsilateral frontal horn, select bilateral lesions, anterior body lesions and anterior third ventricle, especially for choroidal fissure approaches | Attention deficits in either hemisphere (rare); speech apraxia or abulia in dominant hemisphere; potential collapse of cortex and subdural hygroma, seizure disorder |
| Transtemporal approach; middle temporal gyrus approach; lateral temporoparietal incision; occipitotemporal gyrus/collateral sulcus or inferior temporal gyrus |
Lesions in temporal horn extending as far forward as temporal tip and as far posterior as the ambient and crural cisterns | Language deficits in dominant hemisphere, hemiparesis, safer in nondominant hemisphere, visual field deficit, retraction injury to temporal lobe or vein of Labbé; Gerstmann’s syndrome in the dominant hemisphere–angular gyrus injury; neglect and visual field deficit Injury to vascular structures in choroidal fissue, visual field injury |
| Anterior interhemispheric transcallosal approach | Bilateral anterior horn and body of lateral ventricles, anterior third ventricle via choroidal fissure or interforniceal approach | Injury to cortical veins, hemiparesis from venous infarct or retraction, injury to pericallosal vessels, seizure disorder |
| Posterior interhemispheric transcallosal incision | Posterior third ventricle, medial surface of the atrium, and pineal region | Injury to internal cerebral or deep venous system, Gerstmann’s syndrome, retraction injury |
| Posterior transcortical approach | Atrium, occipital horn, posterior and middle portion of the body, collateral trigone, pulvinar, thalamus, corpus callosum, or quadrigeminal cistern | Homonymous field deficit, hemiparesis, neuropsychiatric deficits in dominant hemisphere |
| Cerebellum/fourth ventricle via vermian or telovelar approach | Fourth ventricle from obex to aqueduct of Sylvius | Cerebellar mutism (less with telovelar approach), cerebellar injury, brainstem or cranial nerve injury, intractable vomiting |
Note: This table provides an overview for the various approaches to microsurgical resection of ventricular lesions. Column 2 describes the indications for each approach, and column 3 describes the risks and potential complications.
Language impairment is a possibility when the tumor is based in the dominant hemisphere. In such cases approximately 10% to 30% of patients will suffer a new speech deficit or worsening of their preoperative deficit.85 Cognitive deficits and personality disorders are much more difficult to measure objectively, especially if preoperative neuropsychological studies are not conducted. It is wise to leave a small tumor remnant, especially if it is attached to critical structures such as the basal ganglia or fornices, particularly if the lesion is benign. Often, subtotal resections of intraventricular lesions are a result of the tumor histological type and its site of origin, as opposed to the surgical approach.85–87
Subdural hygroma formation is a well-recognized problem, especially in patients in whom a large tumor is associated with ventriculomegaly. These subdural fluid collections after significant decompression of the ventricles may occur following either transcortical or transcallosal approaches, with an incidence of approximately 5%.2 The cortical surface may collapse or pull away from the dura and create a hematoma or hygroma, which may eventually require the placement of a shunt. Filling the ventricles with sterile saline or lactated Ringer’s solution before the completion of the surgery and leaving both subdural and intraventricular catheters in place may reduce the incidence of hygroma and hydrocephalus. Some surgeons argue that the application of fibrin glue over the cortical surface significantly decreases the rate of subdural fluid collections.42 We prefer to position the patient with the operated side down for the first 8 hours to lessen the chance that a greater amount of cortex will pull away from the dura and skull. Treatment of this may require subdural taps, external drains, or a subdural shunt. However, most chronic subdural collections are self-limited and require simple observation.
Postoperative ventriculomegaly is common. Not all patients will require placement of a shunt. The precise incidence of shunting after resection is unpredictable and depends on a wide spectrum of parameters such as the tumor type, amount of blood and debris left in the ventricles, and other technical issues.51 The inability to “wean” a postoperative external ventricular drain or development of a recalcitrant pseudomeningocele may indicate elevated intracranial pressure requiring CSF diversion. All patients may suffer some form of meningeal irritation caused by the presence of blood products and particulate matter in the CSF. This type of chemical meningitis can best be treated by aggressive postoperative ventricular drainage, analgesic agents, and a tapered course of steroids. Occasionally patients will require permanent CSF diversion following all the microsurgical approaches to ventricular tumors. Regardless, external ventricular drainage or external subdural drainage after resection is uniformly beneficial in lessening the chance of shunt-dependent hydrocephalus. Although ventriculitis remains a risk with intraventricular surgery and external ventricular drainage, infection rates of less than 5% are achievable after surgery.85
Historically, death was usually secondary to catastrophic postoperative hemorrhage or pulmonary emboli.17,18,30,46,85–89 Nonetheless, even with our enormous advances in microsurgery, neuroanesthesia, and postoperative technology, a flexible surgical approach is important to lessen risks of morbidity and mortality. Preoperative embolization or a staged removal of a very large vascular lesion via a combined approach is preferable to a single, long, difficult procedure fraught with risks and excessive blood loss.
Postoperative Considerations
All cases are discussed at multidisciplinary tumor conferences. Adjunctive therapies are considered in cases of malignant disease, although some advocate radiotherapy in cases of subtotal resection of benign lesions, such as central neurocytomas. When applicable, adjunctive therapy may begin as early as 2 weeks following surgery. Patients may experience complications such as delayed hydrocephalus, pseudomeningocele, or subdural fluid collections.90 Although permanent CSF diversion is a known risk of these operations, we have been successful in managing patients with aggressive ventricular drainage or a lumbar drain.
Future Directions
Substantial gains in the treatment of intraventricular tumors have developed since surgery for these lesions was originally described. Early diagnosis, enhanced preoperative imaging, neuronavigation, microsurgical techniques, and endoscopic assistance improve clinical outcomes. Advances along each of these fronts will certainly lead to improved outcome. The greatest advances will likely come in the form of technical improvements in the realm of endoscopic instrumentation and targeted therapies for brain tumors. Flexible and robotic endoscopes, for example, may improve visualization of lesions within the ventricles. Flexible instruments for tumor resection may further reduce the need for brain retraction during transcortical approaches. The ventricular system invites targeted delivery of antineoplastic agents, immunotherapies, and viral vectors, which are under development.91 Additional advances in preoperative imaging and functional mapping will permit the surgeon to plan approaches around fiber tracts associated with functionally important cortical regions.37
Conclusion
Intraventricular lesions are rare lesions that require special neurosurgical consideration. Transcortical and transcallosal microsurgical approaches offer different advantages based on lesion location, size, and proximity to surrounding structures. Combined microsurgical approaches are necessary for large lesions when one approach does not permit visualization of critical landmarks (Fig. 42.10). Neuroendoscopic approaches may achieve gross total resection of carefully selected lesions. The future of intraventricular surgery holds improvements in the realm of preoperative functional imaging, DTI, and neuroendoscopy.
Cappabianca P., Cinalli G., Gangemi M., et al. Application of neuroendoscopy to intraventricular lesions. Neurosurgery. 2008;62(Suppl 2):575-597. discussion 597-598
Ellenbogen R.G. Transcortical surgery for lateral ventricular tumors. Neurosurg Focus. 2001;10:1-13.
Piepmeier J.M., Spencer D.D., Sass K.J., George T.M. Lateral ventricular masses. Apuzzo M.L.J., editor, Brain Surgery: Complication Avoidance and Management. 1993:581-599. New York
Rhoton A.L.Jr. The lateral and third ventricles. Neurosurgery. 2003;53:S235-S299.
Yasargil M.G., Abdulrauf S.I. Surgery of intraventricular tumors. Neurosurgery. 2008;62:SHC1029-SHC1041.
Please go to expertconsult.com to view the complete list of references.
1. Spencer D.D., Collins W., Sass K.J. Surgical management of lateral intraventricular tumors. In: Schmidek H.H., Sweet W.H., editors. Operative Neurosurgical Techniques: Indications, Methods and Results. Orlando: Grune & Stratton; 1988:583-596.
2. D’Angelo V.A., Galarza M., Catapano D., et al. Lateral ventricle tumors: surgical strategies according to tumor origin and development—a series of 72 cases. Neurosurgery. 2005;56:ONS36-ONS45.
3. Ellenbogen R.G. Transcortical surgery for lateral ventricular tumors. Neurosurg Focus. 2001;10:1-13.
4. Mutlu N., Culha M., Mutlu B., et al. Cobb’s collar and syringocele with stone. Int J Clin Pract. 1998;52:352-353.
5. Pendl G., Ozturk E., Haselsberger K. Surgery of tumors of the lateral ventricle. Acta Neurochir. 1992;116:128-136.
6. Piepmeier J.M., Sass K.J. Surgical management of lateral ventricular tumors. In: Paoletti P., Takakura K., Walker M.D., editors. Neuro-Oncology. Dordecht: Kluwer Academic Publishers; 1991:333-335.
7. Timurkaynak E., Rhoton A.L.Jr., Barry M. Microsurgical anatomy and operative approaches to the lateral ventricles. Neurosurgery. 1986;19:685-723.
8. Dandy W.E. Diagnosis, localization and removal of tumors of the third ventricle. Bull Johns Hopkins Hosp. 1922;33:188-189.
9. Rhoton A.L.Jr. The lateral and third ventricles. Neurosurgery. 2003;53:S235-S299.
10. Rhoton A.L.Jr., Fujii K., Fradd B. Microsurgical anatomy of the anterior choroidal artery. Surg Neurol. 1979:171-187.
11. Jelinek J., Smirniotopoulos J.G., Parisi J.E., Kanzer M. Lateral ventricular neoplasms of the brain: differential diagnosis based on clinical, CT, and MR findings. AJNR Am J Neuroradiol. 1990:567-574.
12. Wang A.M.P.T., Rumbaugh C.L. Lateral ventricular meningioma. Comput Radiol. 1985:355-358.
13. Janisch W., Staneczek W. Primary tumors of the choroid plexus. Frequency, localization and age [German]. Zentralbl Allg Pathol. 1989;135:235-240.
14. Wolff J.E., Sajedi M., Brant R., et al. Choroid plexus tumours. Br J Cancer. 2002;87:1086-1091.
15. Shepherd C.W., Scheithauer B.W., Gomez M.R., et al. Subependymal giant cell astrocytoma: a clinical, pathological, and flow cytometric study. Neurosurgery. 1991;28:864-868.
16. Lobato R.D .C.A., Camena J.J., et al. Subependymoma of the lateral ventricle. Surg Neurol. 1981:144-147.
17. Nagib M.G., O’Fallon M.T. Posterior fossa lateral ependymoma in childhood. Pediatr Neurosurg. 1996:299-305.
18. Roszkowski M., Drabik K., Barszcz S., et al. Surgical treatment of intraventricular tumors associated with tuberous sclerosis. Childs Nerv Syst. 1995:335-339.
19. Criscuolo G.R., Symon L. Intraventricular meningioma. A review of 10 cases of the National Hospital, Queen Square (1974-1985) with reference to the literature. Acta Neurochir (Wien). 1986;83:83-91.
20. Mani R.L., Hedgcock M.W., Mass S.I., et al. Radiographic diagnosis of meningioma of the lateral ventricle. Review of 22 cases. J Neurosurg. 1978:249-255.
21. Silver A.J., Ganti S.R., Hital S.K. Computed tomography of tumors involving the atria of the lateral ventricles. Radiology. 1982:71-78.
22. Hassoun J., Soylemezoglu F., Gambarelli D., et al. Central neurocytoma: a synopsis of clinical and histological features. Brain Pathol. 1993;3:297-306.
23. Chang K.H., Han M.H., Kim D.G., et al. MR appearance of central neurocytoma. Acta Radiol. 1993;34:520-526.
24. Hassoun J., Gambarelli D., Grisoli F., et al. Central neurocytoma. An electron-microscopic study of two cases. Acta Neuropathol. 1982;56:151-156.
25. Goergen S.K., Gonzales M.F., McLean C.A. Intraventricular neurocytoma: radiologic features and review of the literature. Radiology. 1992:787-792.
26. Nishio S., Fujiwara S., Tashima T., et al. Tumors of the lateral ventricular wall, especially the septum pellucidum: clinical presentation and variations in pathological features. Neurosurgery. 1990;27:224-230.
27. Yasargil M.G., Abdulrauf S.I. Surgery of intraventricular tumors. Neurosurgery. 2008;62:SHC1029-SHC1041.
28. Kim D.G., Choe W.J., Chang K.H., et al. In vivo proton magnetic resonance spectroscopy of central neurocytomas. Neurosurgery. 2000;46:329-333. discussion 333–334
29. Ture U., Yasargil M.G., Al-Mefty O. The transcallosal-transforaminal approach to the third ventricle with regard to the venous variations in this region. J Neurosurg. 1997;87:706-715.
30. Nagasawa S., Miyaki H., Ohta T. Transcallosal and transcortical approaches for tumors at the anterior part of the ventricle: relations between visualized and ventricular size. No Shinkei Geka (Jpn). 1997:321-327.
31. Asgari S., Engelhorn T., Brondics A., et al. Transcortical or transcallosal approach to ventricle associated lesions: a clinical study on the prognostic role of surgical approach. Neurosurgery. 2003;26:192-197.
32. Bellotti C., Pappada G., Sani R., et al. The transcallosal approach for lesions affecting the lateral and third ventricles: surgical considerations and results of a series of 42 cases. Acta Neurochir. 1991;111:103-107.
33. Kemp L.G., Blaylock R. Lateral-trigonal intraventricular tumors: a new operative approach. Acta Neurochir. 1976;35:233-242.
34. Shucart W. Anterior transcallosal and transcortical approaches. In: Apuzzo M.J., editor. Surgery of the Third Ventricle. Baltimore: Williams & Wilkins; 1987:307-325.
35. Amar A.P., Ghosh S., Apuzzo M.J. Ventricular tumors. In: Winn H.R., editor. Youmans Neurological Surgery. Philadelphia: WB Saunders; 2004:1237-1263.
36. Wen H.T., Rhoton A.L.Jr., de Oliveira E. Transchoroidal approach to the third ventricle: An anatomic study of the choroidal fissure and its clinical application. Neurosurgery. 1998;42:1205-1219.
37. Le Bihan D., Mangin J.F., Poupon C., et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534-546.
38. Apuzzo M.J., Litofsky N.S. Surgery in and around the anterior third ventricle. In: Apuzzo M.J., editor. Brain Surgery. New York: Churchill-Livingstone; 1993:541-579.
39. Greenwood J. Paraphysial cysts of the third ventricle: with report of eight cases. J Neurosurg. 1949;6:153-159.
40. Spencer D.D., Collins W., Sass K.J., editors. Surgical Management of Lateral Intraventricular Tumors. Orlando: Grune & Stratton, 1988.
41. Jun C.L., Nutik S.L. Surgical approaches to intraventricular meningiomas of the trigone. Neurosurgery. 1985:416-420.
42. Al-Yamany M., Del Maestro R.F. Prevention of subdural fluid collections following transcortical intraventricular and/or paraventricular procedures by using fibrin adhesive. J Neurosurg. 2000:406-412.
43. Spencer D.D., Collins W., editors. Surgical Management of Lateral Intraventricular Tumors. New York: Grune & Stratton, 1982.
44. Luders H., Lesser R.P., Hahn J., et al. Basal temporal language area. Brain. 1991:743-754.
45. Ojemann G. Individual variability in cortical localization of language. J Neurosurg. 1979:164-169.
46. Gokalp H.Z., Yuceer N., Arasil E., et al. Tumours of the lateral ventricle. A retrospective review of 112 cases operated upon 1970-1997. Neurosurg Rev. 1998:126-137.
47. Gassel M.M., Davies H. Meningiomas in the lateral ventricles. Brain. 1961:605-630.
48. Gerstmann J. Zur Symptomatologie der Hirnläsionen im Übergangsgebeit der unteren Parietal—and mittleren Occipitalwindung. Nervenärzt. 1930:691-695.
49. Barrow D.L., Dawson R. Surgical management of arteriovenous malformations in the region of the ventricular trigone. Neurosurgery. 1994:1046-1054.
50. Batjer H., Samson D. Surgical approaches to trigonal arteriovenous malformations. J Neurosurg. 1987:511-517.
51. de la Torre E., Crandell D.L., Davis C.H., et al. Tumors of the lateral ventricles of the brain. J Neurosurg. 1963:461-470.
52. Raimondi A.J., Gutierrez F.A. Diagnosis and surgical treatment of choroid plexus papillomas. Childs Brain. 1975:81-115.
53. Nair S., Rout D., Menon G., et al. Medial trigonal arteriovenous malformations. Keio J Med. 2000;49:14-19.
54. Fornari M., Savoiardo M., Morello G., Solero C.L. Meningiomas of the lateral ventricles. J Neurosurg. 1981;54:64-74.
55. Sugita K., Hongo K., editors. Posterior Transcortical Approach. In Apuzzo MLJ, ed: Surgery of the Third Ventricle, Baltimore: Williams & Wilkins, 1987.
56. Kawashima M., Li X., Rhoton A.L.Jr., et al. Surgical approaches to the atrium of the lateral ventricle: microsurgical anatomy. Surg Neurol. 2006;65:436-445.
57. Abosch A., McDermott M.W., Wilson C.B. Lateral Ventricular Tumors. New York: Churchill-Livingstone; 2000.
58. Heilman K., Gonzales-Rothi L.J. Apraxia. In: Heilman K., Valenstein E., editors. Clinical Neuropsychology. New York: Oxford University Press; 1985:131-149.
59. Levin H.S., Speirs P.A. Acalculia. In: Heilman K., Valenstein E., editors. Clinical Neuropsychology. New York: Oxford University Press; 1985:97-113.
60. Rhoton A.L.Jr. The lateral and third ventricles. Neurosurgery. 2002;51:S207-S271.
61. Nagata S., Sasaki T. Lateral transsulcal approach to asymptomatic trigonal meningiomas with correlative microsurgical anatomy. Neurosurgery. 2005;56:E438-E439.
62. Appuzo M.L., Chikovani O.K., Gott P.S. Transcallosal, interfornicial approaches for lesions affecting the third ventricle: surgical considerations and consequences. Neurosurgery. 1982;10:547-554.
63. Winston K.R., Cavazzuti V., Arkins T. Absence of neurological and behavioral abnormalities after anterior transcallosal operation for third ventricular lesions. Neurosurgery. 1979;4:386-393.
64. Lucas T.H., West G.A. Corpus callosotomy: indications, surgical procedures and outcomes. In: Miller J.W., Silbergeld D.L., editors. Epilepsy Surgery: Principles and Controversies. New York: Taylor & Francis; 2006:541-551.
65. Yasargil M.G. Microneurosurgery: Microneurosurgery of CNS Tumors. Stuttgart: Thieme Verlag; 1996.
66. Geshwind N. Disconnection syndromes in animals and man. Brain. 1965;88:237-294.
67. Heilman K.M., Watson R.T., Valenstein E. Localization of lesions in neglect. In: Heilman K., Valenstein E., editors. Clinical Neuropyschology. New York: Oxford University Press; 1985:243-293.
68. Kasowski H., Piepmeier J.M. Transcallosal approach for tumors of the lateral and third ventricles. Neurosurg Focus. 2001;10:1-5.
69. von Lehe M., Schramm J. Gliomas of the cingulate gyrus: surgical management and functional outcome. Neurosurg Focus. 2009;27:E9.
70. McComb J.G., Appuzo M.L. Posterior interhemispheric retrocallosal and transcallosal approaches. In: Appuzo M.L., editor. Surgery of the Third Ventricle. Baltimore: Williams & Wilkins; 1987:611-641.
71. Yasargil M.G., von Ammon K., von Deimling A. Central neurocytoma: histopathological variants and therapeutic approaches. J Neurosurg. 1991;76:32-37.
72. Horsley V. Discussion. Proc Roy Soc Med. 1910;3:77-78.
73. Lozier A.P., Bruce J.N. Surgical approaches to posterior third ventricular tumors. In: Parsa A.T., Berger M.S., editors. Neurosurgery Clinics of North America. Philadelphia: WB Saunders; 2003:527-545.
74. Porter J.M., Pidgeon C., Cunningham A.J. The sitting position in neurosurgery: a critical appraisal. Br J Anaesth. 1999;82:117-128.
75. Seiler R.W., Zurbrugg H.R. Supratentorial intracerebral hemorrhage after posterior fossa operation. Neurosurgery. 1986;18:472-474.
76. Sloan T. The incidence, volume, absorption, and timing of supratentorial pneumocephalus during posterior fossa neurosurgery conducted in the sitting position. J Neurosurg Anesthesiol. 2010;22:59-66.
77. Haisa T., Kondo T. Midcerevical flexion myelopathy after posterior fossa surgery in the sitting position: case report. Neurosurgery. 1996;38:819-821.
78. Fries G., Perneczky A. Endoscopic-assisted brain surgery: part 2—analysis of 380 procedures. Neurosurgery. 1998;42:226-232.
79. Gore P.A., Nakaji P., Deshmukh V., Rekate H.L. Synchronous endoscopy and microsurgery: a novel strategy to approach complex ventricular lesions. J Neurosurg. 2006;105:485-489.
80. Charalampaki P., Filippi R., Welschehold S., et al. Turmors of the lateral and third ventricle: removal under endoscope-assisted keyhole conditions. Neurosurgery. 2005;57:ONS302-ONS311.
81. Cappabianca P., Cinalli G., Gangemi M., et al. Application of neuroendoscopy to intraventricular lesions. Neurosurgery. 2008;62(Suppl 2):575-597. discussion 597-598
82. Shenkin H., Goldfedder P. Air embolism from exposure of posterior cranial fossa in prone position. JAMA. 1969;210:726-727.
83. Zimmerman R.A., Bilaniuk L.T., Pahlajani H. Spectrum of medulloblastomas demonstrated by computed tomography. Radiology. 1978;41:126-137.
84. Kellogg J.X., Piatt J.H.J. Resection of fourth ventricle tumors without splitting the vermis: the cerebellomedullary fissure approach. Pediatr Neurosurg. 1997;27:28-33.
85. Piepmeier J.M., Spencer D.D., George T.M., Sass K.J. Lateral ventricular masses. Apuzzo M.L.J, editor, Brain Surgery: Complication Avoidance and Management. 1993:581-599. New York
86. Ehni G. Interhemispheric and percallosal (transcallosal) approach to the cingulate gyri, intraventricular shunt tubes, and certain deeply placed brain lesions. Neurosurgery. 1984;14:99-110.
87. Jeeves M.A., SD, Geffen G. Functional consequences in the transcallosal removal of intraventricular tumours. J Neurol Neurosurg Psychiatry. 1979:134-142.
88. Afra D .T.L., Deak G. Ependymomas extending into both lateral ventricles: CT diagnosis and operability. Zentralbl Neurochir. 1981:255-262.
89. Pascual-Castroviejo I., Villarejo F., Perez-Higueras A., et al. Childhood choroid plexus neoplasms. A study of 14 cases less than 2 years old. Eur J Pediatr. 1983:51-56.
90. Tanaka Y., Sugita K., Kobayashi S. Subdural fluid collections following transcortical approach to intra- or paraventricular tumours. Acta Neurochir. 1989;99:20-25.
91. Frazier JL, Han JE, Lim M, Olivi A. Immunotherapy combined with chemotherapy in the treatment of tumors. Neurosurg Clin North Am. 21:187-194.