CHAPTER 371 Microsurgery of Vertebral Artery, Posterior Inferior Cerebellar Artery, and Vertebrobasilar Junction Aneurysms
Clinical Significance
Cruveilhier1 in 1829 was the first to report a vertebral aneurysm when he described a spherical aneurysm arising from the vertebral artery–PICA junction. Krayenbuhl2 was the first to show a vertebrobasilar aneurysm by positive contrast angiography in 1941. Dandy3 in 1944 described his surgical experience with treating three vertebrobasilar aneurysms. In 1947, Rizzoli and Hayes4 performed trapping of a vertebral aneurysm between two silver clips (reported in 1953), and in 1948, Schwartz5 described a suboccipital approach for direct surgical treatment of a posterior fossa aneurysm. Early surgical experiences with aneurysms in the vertebrobasilar system were met with varied success. High morbidity and mortality rates and complications plagued early neurovascular surgeons, making many reluctant to surgically treat vertebral and vertebrobasilar aneurysms aggressively. However, the introduction of the microscope ushered in new approaches to posterior circulation aneurysms.6 Drake7 was the first to report a successful large series of surgically treated vertebral and vertebrobasilar aneurysms. Subsequent advances in skull base approaches, as well as improvements in microneurosurgical technique and development of better pharmacologic brain protection, have contributed to better outcomes. A variety of surgical approaches have been developed to attack lesions at different locations in the vertebrobasilar system.8–11
Vertebral, PICA, and vertebrobasilar junction aneurysms are relatively uncommon lesions. In the study by Pia,12 posterior circulation aneurysms, including PICA and basilar aneurysms, accounted for about 8% to 9% of all intracranial aneurysms. Of these, aneurysms from the proximal vertebral artery, the vertebral artery–PICA junction, the distal PICA, and the distal vertebral artery make up 25% of all posterior circulation aneurysms and 2% of all intracranial aneurysms.12,13 More recently, the Cooperative Study of Intracranial Aneurysms and Subarachnoid Hemorrhage14 found 21 (0.8%) vertebral artery aneurysms, 4 (0.1%) vertebrobasilar junction aneurysms, and 13 (0.5%) PICA aneurysms from a total of 2672 single-site aneurysms; thus, vertebral artery, PICA, and vertebrobasilar junction aneurysms account for 1.4% of all intracranial aneurysms. In a Japanese 30-year autopsy study of 1230 consecutive cases,15 73 intracranial aneurysms were found in 57 cases (4.6%). Seven of the aneurysms were located at the vertebral artery or PICA, accounting for 9.6% of all intracranial aneurysms.
There is also a high incidence of fusiform nonsaccular aneurysms at the vertebrobasilar locations, much more frequent than in the anterior circulation. Many of fusiform aneurysms that occur at the vertebral artery are due to dissections and carry with them a high risk for bleeding and of rebleeding once hemorrhage has occurred. Yamaura and colleagues16 found vertebral dissections to account for 28% of posterior circulation aneurysms in their series. The rebleeding rate from ruptured dissecting aneurysms has been reported to be 21% to 71%,16–19 with the highest risk in the acute stage after initial bleeding. Many neurovascular surgeons believe that they cannot be left untreated. Because of their morphology, however, fusiform aneurysms, dissecting or nondissecting, often are not amenable to standard aneurysm clipping techniques. Neurovascular surgeons have been forced to devise alternative treatment strategies for these lesions. One surgical strategy has been deliberate therapeutic occlusion of the parent vertebral or basilar artery, otherwise known as proximal hunterian ligation.20–22 The advent of endovascular approaches has ushered in a new and more promising strategy for treating fusiform aneurysms.23–26 These techniques are described elsewhere in this text and involve proximal endovascular occlusion, lesion trapping, and stent reconstruction.
Indications
Although vertebral artery and vertebrobasilar aneurysms are relatively rare compared with intracranial aneurysms found at other locations, ruptured vertebral artery and vertebrobasilar aneurysms carry a high risk for rebleeding as well as high morbidity and mortality rates. Hernesniemi and colleagues27 reported fatal rebleeding in 10% of vertebrobasilar aneurysms, 3 times the number (3.4%) in their anterior circulation aneurysm group.
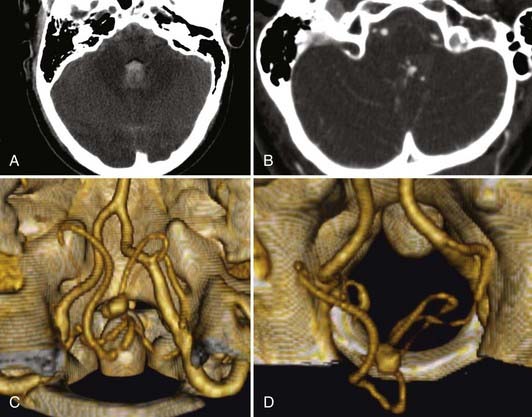
The 2-month mortality rate for untreated ruptured vertebrobasilar aneurysm in the Hernesniemi series was 63%, compared with 8% to 11% for the surgically treated group, and at 1 year, untreated vertebrobasilar aneurysms carried an 83.3% mortality rate.27 In Schievink and associates’ series of 136 aneurysmal subarachnoid hemorrhages (SAHs), the 48-hour mortality rate in ruptured vertebral artery aneurysms was noted to be 80%, compared with 23% in patients with ruptured anterior circulation aneurysms.28 For these reasons, it is generally believed that ruptured vertebral artery and vertebrobasilar aneurysms cannot be left untreated (Fig. 371-1).
Ruptured vertebral dissecting aneurysms carry a worse prognosis than saccular aneurysms of the same location. Kawagushi and associates17 reported in their series of vertebral dissecting aneurysms a 21% rebleeding rate in the first 72 hours with 100% mortality. Aoki and Sakai18 reported 30% rebleeding with 80% mortality occurring in the first few hours. Mizutani and colleagues19 reported 71% rebleeding, with the mortality of the rebleeding group being 46.7%, compared to a mortality of 8.3% in the non-rebleeding group. Some authors have advocated a conservative approach of anticoagulation and serial angiography in managing vertebral dissections. Kitanaka and associates29 managed six patients with unruptured vertebral dissections conservatively and followed them with serial angiography. There was no episode of SAH, and there was angiographic cure in four of the patients. All the patients had unruptured vertebral dissections, however, with no cases of ruptured vertebral dissections.
The timing of surgery for ruptured vertebral artery and vertebrobasilar aneurysms is still debated. The International Cooperative Study on the Timing of Aneurysm Surgery30,31 did not include enough vertebrobasilar aneurysm to make any significant conclusions about the timing of surgery for aneurysm at this location. Posterior circulation aneurysms differ from anterior circulation aneurysms in that rarely are emergent life-saving operations for hematoma evacuation necessary, whereas they more commonly are necessary with rupture of aneurysms in the anterior circulation. Ventricular drainage can be a necessary emergent procedure, however, because many patients with rupture of vertebral artery or vertebrobasilar aneurysms have SAH complicated by hydrocephalus. In the current era, early surgical or endovascular treatment of the ruptured aneurysm is generally the rule.
The high management morbidity and mortality associated with surgical treatment of ruptured aneurysms in this location have made neurovascular surgeons reluctant to treat these lesions aggressively in the acute setting. Early surgery often is confounded by a brain that is frequently more swollen with confining exposure, making the delicate perforating vessels difficult to visualize. Also, in the acute period, the aneurysm’s dome may be more friable and at greater risk for intraoperative rupture from manipulation. Although the general trend in the 1990s for anterior circulation aneurysms was early surgery, for posterior circulation aneurysms, most centers at that time adopted a protocol of delayed surgery. A few authors have advocated early surgery, however.2,32,33 The high morbidity and mortality rates associated with early rebleeding argue for early clipping of the ruptured aneurysm. In addition, with the aneurysm secured, the patient may undergo earlier and more aggressive vasospasm management. Hernesniemi and colleagues27 reported 63 patients operated on for ruptured vertebrobasilar aneurysms—36 patients in the first 6 days of SAH and 27 patients in 7 or more days after SAH. Outcomes at 1 year in the early-surgery group were 55.6% good recovery, 16.7% moderate disability, 19.4% severe disability, and 8.3% dead. Outcomes at 1 year in the late-surgery group were 59.3% good recovery, 11.1% moderate disability, 7.4% severe disability, and 22.2% dead. In an untreated group of 30 patients, 1-year outcomes were 6.7% good recovery, 6.7% moderate disability, 3.3% severe disability, and 83.3% dead. Lanzino and associates34 reported their results with a protocol for delayed surgery for vertebrobasilar aneurysms and concluded that early surgery would be preferable. Although they achieved 87% excellent or good surgical outcome and 8% surgical mortality, an additional 41% died under the delayed treatment protocol before reaching surgery. Peerless and associates32 reported an extensive series from 1959 to 1992 of 1767 patients with vertebrobasilar aneurysms. Because of their referral pattern, most of these aneurysms were operated on 14 or more days after SAH. Since 1970, these investigators operated on 206 ruptured vertebrobasilar aneurysm in 7 days or less after SAH. In the 206 early-operated patients, outcomes were 62.6% excellent, 18.9% good, 8.7% poor, and 9.7% dead. Bertalanffy and coworkers33 reported that their group operated on ruptured vertebrobasilar aneurysms in the acute stage. Of 17 vertebrobasilar aneurysms, they reported a 94% good result rate and a 5.9% mortality rate. Arguments for the timing of endovascular treatment of ruptured posterior circulation aneurysms are still in their infancy.
Small case series of mainly anterior circulation aneurysms have supported earlier intervention. Van Loon and others35 reported 11 patients presenting with SAH and poor neurological grades, of which 10 were treated with endovascular techniques within 24 hours. Mean follow-up at 12 months showed 4 patients with good Glasgow Outcome Scale (GOS) scores, 2 who were moderately disabled, 3 who were severely disabled, and 2 who died as a result of uncontrollable intracranial pressure. Of the 11 ruptured aneurysms, 2 involved the basilar artery. While the timing for posterior circulation aneurysms remains under investigation, the data from the surgical literature would suggest favoring earlier intervention. Endovascular techniques allow securing of the aneurysm to prevent the high risk for rebleeding while avoiding the technical challenges of working with a swollen and injured brain. Whether early or late, there is not much question that ruptured vertebral artery, PICA, and vertebrobasilar junction aneurysms should not be left untreated except in the extenuating circumstance in which the patient is medically unstable or the treatment comes at unacceptably high risk.
The natural history of unruptured vertebral artery, PICA, or vertebrobasilar junction aneurysms without treatment is less clear, however. Nevertheless, a 1998 international cooperative study36 of 1937 unruptured intracranial aneurysms found a 13.6 relative risk for rupture in the group of patients with vertebrobasilar artery and posterior cerebral artery aneurysms that had never had a history of SAH compared with patients with intracranial aneurysms of other locations (P = .007). The follow-up study in 2003 by the International Study of Unruptured Intracranial Aneurysms37 committee evaluated 1077 patients without history of SAH, of which 1591 were treated with open surgical repair and 409 were treated by endovascular techniques. Their review found that aneurysms involving the vertebrobasilar artery, posterior cerebral artery system, and posterior communicating artery had increased 5-year cumulative rupture rates in patients without history of SAH compared with anterior circulation aneurysms of comparable sizes. Specifically, aneurysms smaller than 7 mm, 7 to 12 mm, 13 to 24 mm, and larger than 25 mm had relative rupture risks of 2.5%, 14.5%, 18.4%, and 50%, respectively, in the posterior circulation, compared with 0%, 2.6%, 14.5%, and 40%, respectively, in the corresponding anterior circulation group. These rates were equaled or exceeded by risks associated with surgical or endovascular repair of comparable lesions. In the multivariate analysis of the unruptured aneurysms treated by open surgery, worse 1-year outcomes were predicted by age greater than 50 years (relative risk of 2.4 [1.7 to 3.3], P < .0001), size greater than 12 mm (relative risk of 2.6 [1.8 to 3.8], P < .0001), and location in the posterior circulation (relative risk of 1.6 [1.1 to 2.4], P < .0001). For the endovascular cohort, poor outcome was again predicted by size greater than 12 mm (relative risk of 2.4 [1.0 to 5.9], P = .03) and location in the posterior circulation (relative risk of 2.25 [1.1 to 4.4,], P = .02). In a 2007 meta-analysis of unruptured aneurysms,38 the relative risk for rupture from an aneurysm in the posterior circulation compared with aneurysms involving the internal carotid artery, including the posterior communicating artery, was 2.5 (1.6 to 4.1). Ruptured risks based on size showed increased risk in aneurysms 5 to 10 mm, with a relative risk of 2.3 (1.0 to 5.2), larger than 10 mm with a relative risk of 2.9 (1.5 to 5.7), larger than 12 mm with a relative risk of 7.5 (3.8 to 14.9), and larger than 15 mm with a relative risk of 11.9 (5.5 to 25.8). This study also found that age older than 60 years carried an increased relative risk of 2.0 (1.1 to 3.7).
Presentation
The most frequent clinical presentation of vertebral artery, PICA, or vertebrobasilar junction aneurysms is SAH. SAH was the primary presentation for all or most aneurysms at these locations in the published series in Table 371-1. These clinical conditions of patients with SAH from ruptured vertebral artery, PICA, or vertebrobasilar aneurysms can range from relatively good condition (Hunt-Hess39 grade I or II) to poor or comatose (Hunt-Hess grade IV or V). In the report by Peerless and associates32 of 206 ruptured vertebrobasilar aneurysms (this included basilar bifurcation, basilar trunk, and posterior cerebral artery aneurysms), 51.9% presented as Hunt-Hess grade I, 30.1% presented as Hunt and Hess grade II, 10.7% presented as Hunt-Hess grade III, 4.9% presented as Hunt-Hess grade IV, and 2.4% presented as Hunt-Hess grade V. In the series by Bertalanffy and coworkers of 27 patients with vertebral artery–PICA complex aneurysms, 22 (81.5%) presented with SAH. Of these patients, 2 presented with Hunt-Hess grade I (9.1%) disease, 2 presented with Hunt-Hess grade II (9.1%), 11 presented with Hunt-Hess grade III (50%), 4 presented with Hunt-Hess grade IV (18.1%), and 3 presented with Hunt-Hess grade V (13.6%). In Yamaura and coworkers’16 series of 24 vertebral dissections, 87.5% presented with SAH, and 12.5% presented with ischemic symptoms.
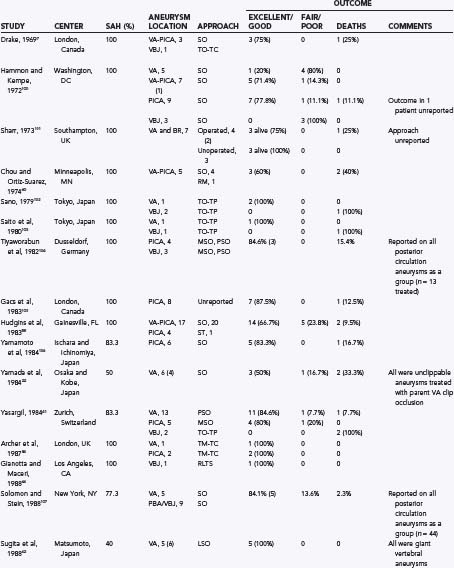
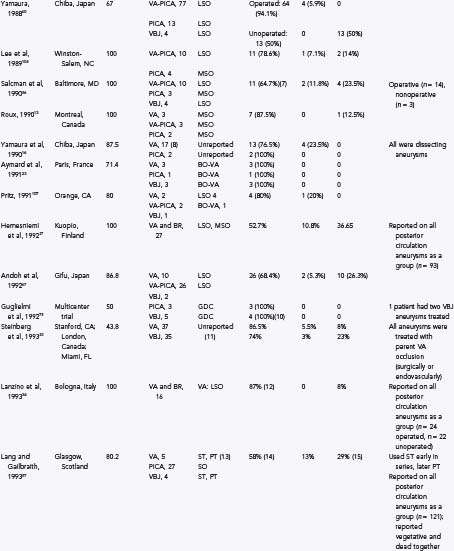
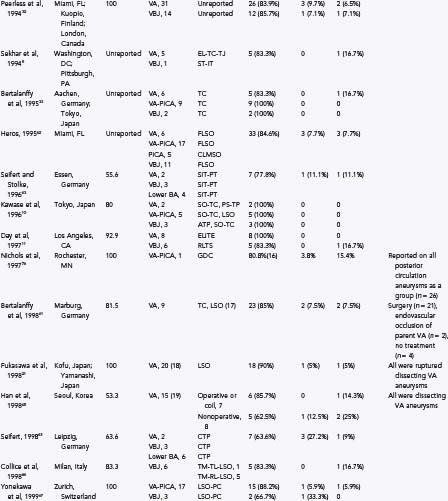
TABLE 371-1 Some Previously Reported Series of Vertebral, Posterior Inferior Cerebellar Artery, and Vertebrobasilar Aneurysms

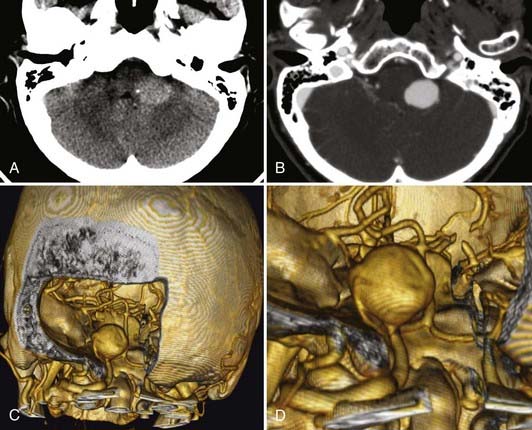
Unruptured vertebral artery, PICA, or vertebrobasilar aneurysms often present with signs and symptoms of ischemia or mass effect, such as lower cranial nerve deficits, brainstem compression, or posterior fossa symptoms. In Yamaura’s40 series of 94 vertebral artery aneurysms, 31 patients presented with unruptured aneurysms, of which 16 presented among multiple aneurysms (17%), 6 (6%) presented as mass effect, 3 (3%) presented with ischemic symptoms, and 6 (6%) presented incidentally during evaluation of unrelated disease. In Yasargil’s41 series, the patients with unruptured vertebral artery aneurysms presented with lower cranial nerve deficits (V through XII), hemiparesis, and bulbar deficits. In the series by Sugita and associates42 of giant vertebral artery aneurysms, the patients who did not present with SAH presented with lower cranial nerve deficits (dysarthria, dysphagia), cerebellar symptoms (ataxia), hemiparesis, or a combination of these (Fig. 371-2).
Diagnostic Evaluation
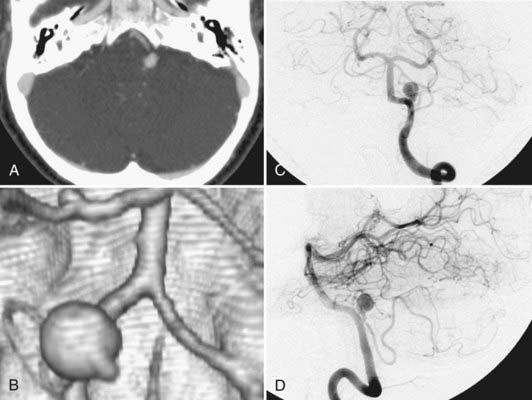
SAH in these patients is diagnosed first clinically, by history and examination, followed by confirmation with computed tomography (CT). As with the diagnostic evaluation for other forms of SAH, a CT scan that does not reveal hemorrhage must be followed by a lumbar puncture (except when contraindicated) with analysis of cerebrospinal fluid for the presence of blood. At our institution, all patients with SAH are evaluated with a computed tomographic angiogram (CTA) using 0.625-mm cuts on a 64-section multidetector. These thin-slice CT images are then used to reconstruct a three-dimensional computer model that allows for preoperative planning. The ease and low risks associated with CTA have made this technology our first line in evaluating patients with SAH. In a study of 29 patients with SAH by Pozzi-Mucelli and others,43 64-channel multidetector CTA had a sensitivity of 92.8% (26 of 28 aneurysms) and a specificity of 100% in detecting aneurysms, compared with conventional digital subtraction angiography. With the two false-negative results, the aneurysms were 1 to 2 mm in size. McKinney and colleagues44 evaluated 41 patients with 64-channel multidetector CTA and found 92.3% sensitivity in detecting aneurysms smaller than 4 mm and 100% sensitivity in locating aneurysms 4 mm and larger. The diagnosis of vertebral dissection relies on the angiographic findings of a string sign, rosette sign, pearl and string sign, tapered narrowing, occlusion, double lumen, or pseudoaneurysm.45 With angiography continuing to be our “gold standard,” suspicious cases of SAH in which no aneurysm or dissection is detected by 64-channel CTA are followed up with a conventional four-vessel angiogram with rotational angiography and three-dimensional reconstructions as needed.
A neuroradiologic review of CT scans46 of 44 ruptured PICA aneurysms showed that 95% were associated with radiologic hydrocephalus and 95% were associated with intraventricular hemorrhage (IVH). Supratentorial SAH was present in 70% of patients (isolated posterior fossa SAH occurred in only 30% of patients). In the series of 36 vertebral artery aneurysms reported by Andoh and colleagues,47 18 were ruptured saccular vertebral artery aneurysms, 10 were ruptured fusiform vertebral artery aneurysms, 5 were ruptured dissection aneurysms, and 3 were unruptured dissection aneurysms. In the subgroup of ruptured saccular vertebral artery aneurysms (n = 18), 78% (n = 14) showed CT evidence of IVH in addition to diffuse SAH in the basal cisterns, and 44% (n = 8) showed radiographic evidence of hydrocephalus. In the subgroup of ruptured fusiform vertebral artery aneurysms (n = 10), 90% (n = 9) had CT evidence of IVH in addition to diffuse SAH, and 30% (n = 3) showed hydrocephalus. In the subgroup of ruptured dissecting aneurysms (n = 5), all demonstrated IVH with diffuse SAH. In the unruptured dissecting aneurysm group (n = 3), 2 patients had pontine infarcts, and 1 had occipital lobe infarcts.
Preoperative Evaluation
The orientation and projection of the neck and dome are important considerations when planning for surgical clipping. The surgical approach to the aneurysm should be planned such that the neck is encountered before the dome. The size of a vertebrobasilar aneurysm can influence treatment strategy. For example, giant vertebrobasilar aneurysms can be difficult to treat, and many of them are not amenable to standard surgical clipping. Drake and others20,48,49 reported that clipping was impossible in 66% of their 354 giant vertebrobasilar aneurysms. Additionally, giant aneurysms of the posterior circulation have been associated with significantly worse outcomes.48,50,51 The finding of a giant aneurysm on angiography may necessitate further evaluation by CTA or magnetic resonance angiography. In more than 50% of cases of giant vertebral artery aneurysms, there are varying degrees of intraluminal thrombosis, and the angiographic opacity may not show the full size of the aneurysm appreciated on CT scan.48,52–55
Preoperative planning should also include careful attention to angiographic details, such as whether the PICA is reduplicated; whether the contralateral artery is present; whether the PICA territory is supplied by an alternative vessel (such as the anterior inferior cerebellar artery); and to what degree the posterior cerebral arteries are being supplied through the posterior circulation (i.e., whether there are fetal posterior cerebral arteries, and if not, what are the sizes of the posterior communicating arteries).56 The angiographic size of the posterior communicating artery has been found to be associated significantly with outcome in patients who have unclippable vertebrobasilar artery aneurysms treated by vertebral artery or basilar occlusion. The presence of a small posterior communicating artery was associated with worse outcome in these cases; the presence of two small posterior communicating arteries was associated with an even worse outcome.20,57
Technique
For vertebral artery, PICA, and vertebrobasilar aneurysms, various surgical approaches have been described. Most authors have used the lateral suboccipital or the far-lateral suboccipital approaches for vertebral artery, vertebral artery–PICA, and vertebrobasilar junction aneurysms; they have used midline suboccipital or paramedian suboccipital approaches for more peripheral PICA aneurysms (see Table 371-1). Subtemporal,34,58,59 retromastoid,60 pterional,59 extreme lateral-transcondylar-transjugular,8 subtemporal-infratemporal,8 transcondylar,33,61 combined lateral-medial suboccipital,62 supra/infratentorial-posterior transpetrosal,63 suboccipital transcondylar,9 presigmoid transpetrosal,9 anterotranspetrosal,9 extreme-lateral inferior transtubercular exposure,11 retrolabrinthine transsigmoid,11,64 combined transpetrosal,65 transmastoid-translabyrinthine (transsigmoid) and lateral suboccipital,66 transmastoid-retrolabyrinthine (retrosigmoid) and lateral suboccipital,66 and lateral suboccipital and partial condylectomy without laminectomy67 approaches also have been used.
Each approach has advantages and disadvantages. Some standard surgical approaches are hampered by a long working distance and by having to work around the brainstem and through the lower cranial nerve rootlets. For lesions located in the midline, medullary retraction or manipulation can occur, with the risk for stretching lower cranial nerve rootlets. Some approaches can be hazardous at times, especially in situations in which the aneurysm dome is encountered before the neck is fully visualized. The anterior midline approaches (transfacial or transoral transclival) are hampered by high rates of complications, particularly cerebrospinal fluid leak and meningitis. A review of the literature68 found a 50% incidence of cerebrospinal fluid leak, meningitis, or both in all published series using transclival approaches for aneurysms.
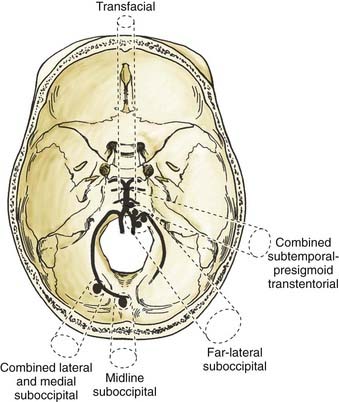
Based on the clinical experience at our institution, we use a handful of surgical approaches to gain access to various lesions of the vertebral artery, PICA, and vertebrobasilar junction. Figure 371-3 depicts the approaches we use. For distal peripheral PICA aneurysms located in the tonsillomedullary segment, we have used the combined lateral and medial suboccipital approach.62,69 For PICA aneurysms in the segment distal to the cerebellotonsillar and cortical segments, we have used a standard midline suboccipital craniectomy extending through the foramen magnum.62 We have used the far-lateral suboccipital approach described by Heros62,70 for most vertebral artery, proximal segment PICA, and vertebrobasilar junction aneurysms.69 In certain circumstances with midline vertebrobasilar junction aneurysms, we have used the transfacial transclival approach (Fig. 371-4).71 In unusually high vertebrobasilar junction aneurysms, we have used the combined subtemporal-presigmoid transtentorial approach.72
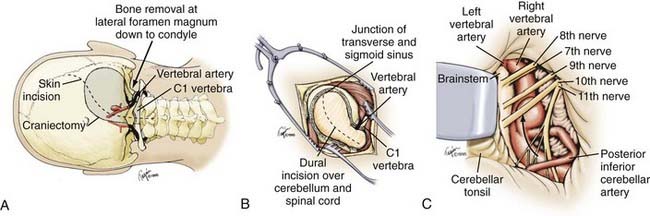
Far-Lateral Suboccipital Approach
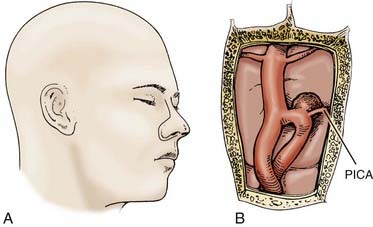
For most vertebral, vertebral artery–PICA, and vertebrobasilar junction aneurysms, we use a modification of the far-lateral suboccipital described previously in detail by Heros (Fig. 371-5).62,70 Briefly, the patient is placed in the straight lateral position with the operative side upward and the head higher and angled slightly toward the ipsilateral shoulder. An S-shaped skin incision is made from the level of the superior aspect of the pinna, medial to the mastoid, down to the C2 spinous process. The muscular attachments and soft tissues are dissected using standard electrocautery technique, and the subperiosteum is exposed with sharp dissection, paying careful attention so as not to injure the vertebral artery. A teardrop craniectomy is fashioned from the transverse-sigmoid sinus junction to beyond the midline and through the foramen magnum, followed by a C1 laminectomy. Generous bone removal from the foramen magnum is crucial and should extend from the occipital condyle laterally to the entry of the vertebral artery into the dura superiorly. The dural exposure should follow a gentle curve, after which point the microscope is brought into the field, bringing the vertebral artery immediately into view. The cerebellar tonsil and caudal hemisphere are retracted gently superiorly and medially, revealing the origins of the PICA. The vertebrobasilar junction can be reached by following the vertebral artery rostrally through a window formed by the 9th and 10th cranial nerves superiorly, the 11th nerve inferiorly, and the medulla medially. An alternative window above it is formed by the 7th and 8th cranial nerves superiorly, the 9th and 10th cranial nerves inferiorly, and the medulla medially. This window is smaller, but it may be necessary in cases of a high vertebrobasilar junction. Heros also suggests that, in certain instances, it may necessary to direct the line of vision through the upper window while working through the wider space provided by the lower window.62,70
Transfacial Transclival Approach
For rare vertebral artery or vertebrobasilar aneurysms with midline locations, we have used a modification of the transfacial approach reported by deFries and colleagues73 and have described it in detail previously (see Fig. 371-4).71 Briefly, a lumbar drain should be placed if ventriculostomy is not already present. The patient should be placed in a supine position. A Doppler probe should be used at the beginning and throughout the procedure to locate and preserve the left facial artery. A skin incision is made from the glabella around the right lateral alar margin to the piriform aperture. Osteotomy of the nasal bones and disarticulation of the septal cartilage from the ethmoid allow for reflection of the nose laterally. The medial wall of one or both maxillary sinuses, the bony septum, the turbinates, the ethmoid air cells, and the floor of the sphenoid sinuses should be removed. A large triangular exposure of the clivus is revealed by a midline incision into the retropharyngeal mucosa. A rectangular window of about 2 cm superior-inferior by 1.2 to 2.5 cm left-right is draggled into the anterior clivus, with lateral margins determined superiorly by preoperative magnetic resonance imaging assessment of the carotid arteries and inferiorly by the hypoglossal canals. A curet may be useful after drilling to finish bone removal. Opening of the dura should reveal the basilar and vertebral arteries. The dural exposure should be generous to allow for proximal and distal control of the vessels as needed. A variety of clip appliers have been developed with a long shank and angulation to reach the clivus by this route.74,75
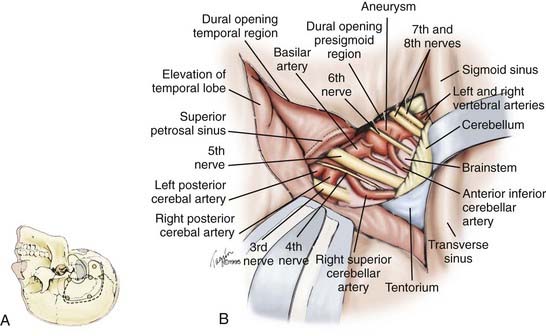
Combined Subtemporal-Presigmoid Transtentorial Approach
Rarely, for vertebrobasilar junction aneurysms that are relatively high in location, we use a combined subtemporal-presigmoid transtentorial approach, which we have described previously (Fig. 371-6).72 The patient should be placed in a supine position with the head rotated in the straight lateral position, elevated, and tilted slightly backward. When this is not possible from the supine position, the patient alternatively can be placed in the lateral position with the ipsilateral shoulder retracted. A U-shaped skin incision is made just anterior to the tragus at the level of the zygoma, circling above the pinna and descending behind the pinna to a point about 1.5 to 2 cm behind (medial to) the mastoid. The temporal muscle and the insertion of sternocleidomastoid muscle are reflected to reveal the mastoid. Bur holes are placed such that the dura over the transverse sinus and the sigmoid-transverse junction can be evaluated. If the dura can be separated easily, a small combined temporal-suboccipital (retrosigmoid) craniectomy is fashioned. If the dura does not separate easily, it is safer to fashion a small subtemporal craniotomy initially, separate the transverse and upper portions of the sigmoid sinuses carefully under direct vision, and then proceed with the suboccipital craniotomy as a separate bone flap. A complete mastoidectomy is drilled along with extensive removal of the posterior-superior petrous pyramid anteriorly to, but not exposing, the facial canal and the lateral and posterior semicircular canals. The sigmoid sinus should be skeletonized down to the beginning of the jugular bulb. A linear dural incision is made parallel to the floor of the middle fossa anteriorly and to the transverse sinus posteriorly. A vertical dural incision is made to the presigmoid region continuing up toward the tentorium. The temporal lobe is elevated gently, and under direct vision of the tentorium from above and below, the superior petrosal sinus is divided. The tentorium is divided in a direction parallel to the posterior aspect of the petrous pyramid, toward the incisura. As the incisura is approached, careful attention should be directed so as not to injure the fourth cranial nerve.
Combined Lateral and Medial Suboccipital Approach
For distal peripheral PICA aneurysms located in the tonsillomedullary segment, we have use the combined lateral and medial suboccipital approach.62,69 The patient position, skin incision, and craniectomy are as described for the far-lateral suboccipital approach, with bone removal extending well past midline in the inferior aspect of the occipital bone and the foramen magnum as well as the arch of C1. Proximal control of the PICA with temporary clips is gained from a lateral approach. Then, in most instances, we retract the tonsils upward, medially, or laterally. After temporary clipping of the PICA distal to its medullary branches, the aneurysm is approached, inspected, and clipped.
Midline Suboccipital Approach
For PICA aneurysms in the segments distal to the tonsillomedullary segment (the cerebellotonsillar and cortical segments), we have used a standard midline suboccipital craniectomy extending through the foramen magnum.62 The cerebellar tonsil can be resected with subpial dissection to the aneurysm, or both tonsils can be retracted gently laterally to approach the aneurysm between them.
Anterior Petrosectomy Approach
The Kawase approach for anterior petrosectomy has also been used by other surgeons to access the vertebrobasilar junction. This approach has been previously described in detail.76 Briefly, the approach uses a frontotemporal craniotomy with supplementary zygomatic root osteotomy. Intraoperative cerebrospinal fluid drainage through a lumbar drain is used to facilitate brain relaxation and minimization of brain retraction. The temporal lobe dura is then elevated in a posterior-to-anterior direction in order to minimize stretch injury to the greater superficial petrosal nerve (GSPN) branch of the facial nerve. The middle meningeal artery is sacrificed at the foramen spinosum, and the petrous carotid (C2) segment is exposed in Glassock’s triangle. Just posterior to the trigeminal ganglion and V3 branch is often a dehiscence in the bony middle fossa floor, exposing the horizontal portion of the petrous segment of the internal carotid artery. The trajectory of the internal auditory canal can be identified below the meatal plane by either tracing the GSPN posteriorly to the geniculate ganglion to identify the origin of the lateral labyrinthine segment of the facial nerve or bisecting the 120-degree angle formed between the GSPN and the arcuate eminence. The internal auditory canal is unroofed from lateral to medial, and the bone of Kawase’s triangle (quadrilateral) is resected to the depth of the inferior petrosal sinus to expose the dura of the posterior fossa. More extradural exposure can be obtained by resecting the lateral aspect of the clivus and by packing the inferior petrosal sinus. The temporal lobe dura is then opened with a T-shaped incision: the first incision is along the undersurface of temporal lobe, and the second perpendicular incision is along the floor of the middle fossa toward the posterior fossa dura. At the level of Kawase’s triangle, the superior petrosal sinus is sectioned between two titanium clips, and the tentorium cerebelli is sectioned into the incisura so that the posterior fossa dura may be opened. This maneuver facilitates visualization of the basilar artery from the posterior clinoid process and oculomotor nerve above to the level of the facial nerve below. After securely clipping the aneurysm, the dura is closed in a watertight fashion using fascial graft, and the wound is closed in layers in the typical fashion.
Posterior Transpetrosal Approach
Although not regularly used at our institution, the posterior transpetrosal approach is another published method to access aneurysms of the basilar trunk and vertebrobasilar junction, and the procedure has been published elsewhere in detail.77 Briefly, Seifert and Stolke report using a semisitting position, with brainstem auditory and somatosensory evoked potentials for electrophysiologic monitoring.77 An L-shaped incision is made over the temporal region above the ear, curving posteriorly and finishing parallel and slightly below the mastoid process. The temporal and suboccipital musculature is incised and retracted with the scalp to expose the temporobasal and lateral suboccipital skull with the mastoid process. A combined supratentorial and infratentorial craniotomy is performed, and a high-speed drill is used to expose the sigmoid sinus. A radical posterior petrosectomy is then performed to expose the presigmoid dura from the superior petrosal sinus to the level of the jugular bulb. Great care is taken to preserve the integrity of the semicircular canals during the extensive drilling of the posterior petrous bone. Upon completion of the extradural bone work, the temporal dura is incised parallel to the transverse sinus and the floor of the temporal fossa. Then, the presigmoid dura in Trautmann’s triangle, which covers the posterior fossa, is incised up to the superior petrosal sinus. The superior petrosal sinus is then transected between two titanium clips. The temporal lobe is slightly elevated to allow the tentorium to be transected parallel to the petrous bone in the direction of the trochlear nerve. The vein of Labbé must be identified and preserved during elevation and incision of the tentorium. Elevation of the remaining portion of the tentorium and the sigmoid sinus will allow exposure of the basilar artery from the upper clival and juxtaclival regions down to the level of the vertebrobasilar junction. After placing the aneurysm clip, the dura is then closed in a watertight fashion using a fascial graft augmented by fibrin glue. The bone flap is repositioned and secured with a titanium microplating system, and the wound is closed in layers in the typical fashion.
Endovascular Approaches
The advent of endovascular techniques has added a new treatment strategy to the armamentarium of the neurovascular surgeon in the management of intracranial aneurysms (Fig. 371-7). The technical details of different endovascular approaches are discussed elsewhere. The use of endovascular approaches in treating ruptured and unruptured intracranial aneurysms has greatly increased since the initial introduction of the Guglielmi detachable coil in 1991.78 Aside from the advantage of endovascular treatments being less invasive, the widely debated International Subarachnoid Aneurysm Trial in 2002 suggested they may have better outcomes than surgical clipping of ruptured aneurysms.79,80 Most aneurysms entered in this trial were anterior circulation lesions because many of the posterior circulation lesions were treated by endovascular techniques and were not included in the randomization.
Treatment of Vertebral Dissecting and Fusiform Aneurysms
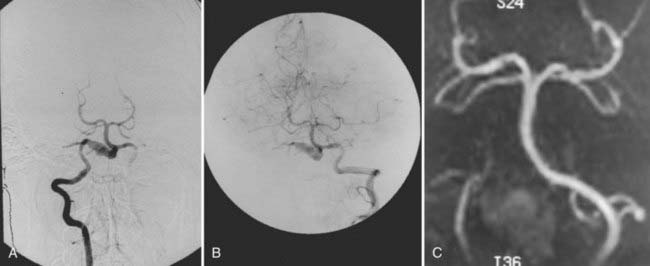
Vertebral artery dissecting aneurysms, particularly ruptured ones, carry a high risk for rebleeding in the acute period after the initial bleed and require early management. If their shape and morphology are such that direct surgical clipping is possible, we use the far-lateral suboccipital approach. This strategy has become a minor part of treatment. These aneurysms often can take on a fusiform morphology, however, and pose the same treatment dilemmas as those of fusiform vertebral artery aneurysms. Because of their shape and morphology, fusiform vertebral artery aneurysms and verbal artery dissecting aneurysms with a fusiform appearance often are not amenable to direct clip application, and neurovascular surgeons have been forced to devise alternative treatment strategies. Surgical treatments include proximal or parent artery occlusion (hunterian ligation), trapping procedures, and clip reconstructions.20–2226 Endovascular options include parent vessel occlusion, stent placement, and combinations of coil and stent therapies,23–26 in which the ipsilateral vertebral artery proximal to the aneurysm is occluded.20–22 This occlusion can be accomplished through a direct surgical approach by clipping the vertebral artery or by endovascular occlusions using balloons or coils (Fig. 371-8).23–25 Detailed temporary occlusion testing should be performed before permanent occlusion to ensure that adequate retrograde or collateral flow would supply the cerebellar territories if flow to the ipsilateral PICA is compromised. With balloon or coil proximal occlusion, we typically have used heparin to minimize thromboembolic complications. If needed, a bypass procedure can be done at the time of surgery or after an endovascular procedure. An occipital-PICA bypass can be accomplished. Alternatively, a PICA-PICA bypass can be performed to fill a PICA vessel distal to an occlusion.
Complications
A major cause of morbidity in the surgical treatment of vertebral artery, PICA, and vertebrobasilar junction aneurysms is inadvertent injury to the lower cranial nerves (9th through 12th), resulting in dysphagia, dysarthria, dysphonia, and inadequate airway protection. Some of the standard approaches use a surgical corridor through the lower cranial nerve rootlets. In addition, the variable and often tortuous course of PICA and vertebral vessels occasionally requires retraction of nerve rootlets. These manipulations of the nerve rootlets can lead to stretching injuries in these locations and require working through the lower cranial nerve rootlets, which are at particular risk for injury. At times, considerable retraction is necessary, causing injury secondary to stretching of the nerve rootlets. Additionally, the PICA is variable in its course through the lower cranial nerves and often can be quite tortuous.12,58,81,82 Injury to the lower cranial nerves can result in dysphagia, dysarthria, dysphonia, and inadequate airway protection. Occasionally, the 6th cranial nerve is located close to a high vertebrobasilar junction and can be subject to injury. Gentle retraction and sharp dissection are essential in preventing cranial nerve complications.
There is a risk for postoperative lateral medullary (Wallenberg’s) syndrome if the PICA is occluded inadvertently and in cases of deliberate occlusion of the vertebral artery for fusiform or dissecting vertebral artery aneurysms. The variable and tortuous anatomy of the PICA can make it susceptible to injury.12,58,81,82 Careful preoperative planning and meticulous attention to the complex vascular anatomy are crucial. In the case of deliberate therapeutic vertebral occlusion for fusiform or dissecting vertebral aneurysms, detailed preoperative temporary occlusion testing is essential.
Transclival approaches have been plagued by particularly high rates of complications, especially cerebrospinal fluid leak and meningitis. A review of all the published reports in the literature68 using transclival approaches for aneurysms revealed a 50% rate of cerebrospinal fluid leak, meningitis, or both. These rates remained high despite the efforts of various groups to devise techniques to prevent cerebrospinal fluid leak and meningitis, including permanent lumboperitoneal shunt,74,83 fibrin glue,74,84,85 a bone baffle,86 various oropharyngeal flaps,87–89 and various techniques for watertight dural closure.90
In Yamarua’s40 series of 94 vertebral artery, vertebral artery–PICA, PICA, and vertebrobasilar junction aneurysms, surgical complications included 6th cranial nerve palsy (3 patients, [4.4% of 68 operated cases]) and 9th or 10th cranial nerve paresis (8 patients [11.8%]; all improved except for 1 patient who had a persistent hoarse voice [1.5%]). Lateral medullary syndrome developed in 3 of the 12 patients (25%) treated with proximal vertebral occlusion for vertebral dissection. In the series by Peerless and associates32 of 45 patients with vertebral artery, vertebral artery–PICA, and vertebrobasilar junction aneurysms, lower cranial nerve deficits occurred in 14 patients (31%); in 6 patients, these were persistent (13.3%). In the series by Salcman and colleagues56 of 14 patients with vertebral artery–PICA, PICA, and vertebrobasilar junction aneurysms, complications were 6th nerve palsy (4 patients [29%]), 7th nerve palsy (3 patients [21%]), 9th and 10th nerve palsy requiring tracheotomy (4 patients [29%]), hemiparesis (2 patients [14%]), ataxia (1 patient [7%]), and meningitis (1 patient [7%]).
Outcomes
Surgical Outcome
Direct surgical treatment for vertebral artery, PICA, and vertebrobasilar junction aneurysms has improved since the 1980s as a result of several advances in operative microneurosurgery. Early neurovascular surgeons met with varied success and high rates of morbidity and mortality. Drake7 was the first to report a large series of vertebral artery, PICA, and vertebrobasilar aneurysms with good results. Since then, many authors have reported their surgical experience with these difficult lesions as well as a variety of different surgical approaches. Table 371-1 provides the surgical approaches and clinical outcomes reported from a number of authors with experience treating vertebral artery, PICA, and vertebrobasilar junction aneurysms. Although outcomes have improved as surgical treatment for these lesions has become more sophisticated, they still have not equaled those produced by surgical treatment for anterior circulation aneurysms.
Endovascular Outcome
Endovascular detachable coil embolization was designed to secure ruptured aneurysms in the acute period in which they are at highest risk for rebleeding. One of the strongest arguments against endovascular treatment is that coiling fails to meet the results produced with surgical clipping. In an eight-center prospective trial91 with 403 patients treated with endovascular detachable coil embolization for ruptured intracranial aneurysms of all locations, complete occlusion was seen in only 192 patients (47.6%). The rate of occlusion appears to be related to the size of the aneurysm and the aneurysm neck. Complete occlusion in the eight-center trial was seen in 70.8% of small aneurysms with a small neck (aneurysm ≤ 10 mm; neck ≤ 4 mm), 31.2% of small aneurysms with a wide neck, 35% of large aneurysms (11 to 25 mm), and 50% of giant aneurysms (>25 mm). In another study92 in which the aneurysm necks were measured and analyzed for radiographic outcomes in 79 aneurysms treated with detachable coil embolization, complete aneurysm occlusion was seen in 85% of small-neck aneurysms (≤4 mm) and 15% of wide-neck aneurysms. The long-term angiographic outcome can be altered by the effects of coil compaction over time, resulting in incomplete aneurysm occlusion. In a study of 63 aneurysms in 58 patients treated with coil embolization,93 follow-up angiograms showed coil compaction in 28%, and there was aneurysm growth in 11%. In a study of 45 basilar bifurcation aneurysms treated by coil embolization,94 coil compaction was seen in 38.7%, and there was a 57% recanalization rate in large aneurysms.
More recently, the largely debated International Subarachnoid Aneurysm Trial (ISAT) randomized 2143 patients with ruptured intracranial aneurysms to either surgical clipping or endovascular coiling.79,80 In this study, 1070 patients underwent surgical clipping, and 1073 were treated with endovascular coiling. As with the earlier studies, the ISAT study also showed how the surgical arm had a greater number of complete occlusions (82% versus 66%) and fewer rebleeds (0 per 1081 patient-years versus 2 per 1276 patient-years) than the endovascular arm at 1-year follow-up, with endovascular patients appearing to have better independent-living outcomes. Two months after surgery, the endovascular arm had 244 of 959 (25.4%) patients who were dependent or dead, compared with the surgical arm, which included 345 of 947 (36.4%) who were dependent or dead (relative risk, 0.698; 95% confidence interval, 0.609 to 0.801; P < .0001). At 1 year, the endovascular arm included 190 of 801 (23.7%) patients who were dependent or dead, compared with the surgical arm, in which 243 of 793 (30.6%) were dependent or dead (relative, risk 0.774; 95% confidence interval, 0.658 to 0.911; P = .0019). Of the 2143 patients in the ISAT study, only 58 aneurysms were in the posterior circulation—31 were PICA aneurysms, 17 basilar bifurcation aneurysms, 4 posterior cerebral artery aneurysms, 5 superior cerebellar aneurysms, and 1 basilar trunk aneurysm. Of this subgroup, 24 aneurysms had endovascular treatment, whereas 34 were surgically clipped. In the endovascular group, 4 patients were dependent or dead, compared with the 15 patients in the surgical group, giving a relative risk ratio of 0.38 (95% confidence interval, 0.14 to 1.00).
Several authors reported their results using endovascular embolization to treat vertebral artery, PICA, and vertebrobasilar junction aneurysms. Guglielmi and others95 reported a multicenter series of posterior circulation aneurysms treated with coil embolization. Of 43 posterior circulation aneurysms, there were 5 vertebrobasilar junction aneurysms and 3 PICA aneurysms (one patient had 2 vertebrobasilar junction aneurysms). Complete occlusion of the aneurysm was seen in 2 (40%) of the 5 vertebrobasilar junction aneurysms and in 2 (66.7%) of the 3 PICA aneurysms. Clinical outcome was good in all 7 patients.
In the multicenter prospective trial from 199791 of 403 ruptured aneurysms treated with detachable coil embolization, 21 (5.2%) were vertebral artery aneurysms and 15 (3.7%) were vertebrobasilar junction aneurysms. The clinical outcomes for the 230 (57%) posterior circulation aneurysms (outcome was not reported by specific anatomic site) were 84.3% unchanged, 9.6% deterioration, and 6.1% death. Radiographic outcome was not analyzed according to aneurysm site. Nichols and colleagues96 reported in 1997 on their series of 26 posterior circulation aneurysms treated by coil embolization, 3 of which were at the vertebrobasilar junction and 1 at the origin of PICA. These authors did not report clinical outcome by anatomic site of the aneurysm, but overall outcome for the group of posterior circulation aneurysms was 80.8% excellent or good, 3.8% poor, and 15.4% dead. Follow-up angiography was performed at 6 months in 19 of the 26 patients (73.1%), and there was complete occlusion in 12 of 20 aneurysms (60%).
More recently, Peluso and coauthors97 reported endovascular treatment of 47 proximal PICA aneurysms in 46 patients. After 6 months, 38 of the initial 46 patients were alive. Six patients had died during their hospitalization—2 from procedural complications (4.3%) and 4 from poor initial SAH grade or from diffuse vasospasm. Two patients died after discharge due to medical comorbidities. Of the 38 surviving patients, 34 (89%) had a GOS score of 5, and 4 (11%) had a GOS score of 3. Of 109 patient-years of follow-up, there were no reported recurrent or primary hemorrhages. Mericle and associates98 reported a series of 31 patients with ruptured proximal PICA aneurysms. They found that 87% of the patients presenting with Hunt-Hess grade 0 to III disease had good GOS scores at 10-month follow-up. Only 50% of the patients with Hunt-Hess grade IV or V disease had good GOS scores. Sandalcioglu and others99 reported 28 vertebral artery–PICA aneurysms, of which 19 were treated with endovascular techniques. The 9 surgical cases were considered untreatable by endovascular methods. At 9-month follow-up, all survivors had complete occlusion, and 19 patients had GOS scores of 4 to 5. They reported 1 patient with procedure-related permanent morbidity and 1 patient with procedure-related mortality.
Outcome after Deliberate Artery Occlusion
Vertebral dissections and fusiform aneurysms often are not amenable to standard clipping techniques because of their shape and morphology. One strategy to treat these lesions has been proximal hunterian ligation, deliberate therapeutic occlusion of the parent vertebral artery. This strategy has been fraught with complications, however, such as thromboembolic ischemia and inadvertent occlusion of the PICA with resulting lateral medullary syndrome. Steinberg and colleagues20 reported their experience with 201 such hunterian ligations in the treatment of unclippable vertebrobasilar aneurysms. Their overall outcomes were 73% successful, 3% poor, and 24% dead. Yamada and associates22 elucidated the various complications associated with this treatment strategy, among which were failures of intra-aneurysm thrombosis and thromboembolic ischemia. Deliberate occlusion of the vertebral or basilar arteries for the treatment of unclippable aneurysms has been performed endovascularly. Aymard and coworkers23 reported their series of 21 patients with posterior circulation aneurysms treated by endovascular occlusion of unilateral or bilateral vertebral arteries. Of the 21 posterior circulation aneurysms, 3 were vertebral artery aneurysms, 1 was a PICA aneurysm, and 3 were vertebrobasilar aneurysms. All 7 had normal outcomes with radiographic cure of their aneurysms, with 1 patient suffering transient ischemia as a complication. Of the overall group of 21 posterior circulation aneurysms, 13 patients had good outcomes with radiographic cure of their aneurysms (61.9%), 2 patients died (9.5%), and 1 patient suffered a transient stroke (4.8%). The remaining patients had partial thrombosis of their aneurysms. More recently, Coert and others26 reported their series of 49 fusiform aneurysms (21 vertebral artery–PICA, 18 vertebrobasilar junction–basilar artery, and 10 posterior cerebral artery [PCA]), of which 29 presented with SAH. Mean follow-up at 33 months showed outcomes that were 59% good, 16% poor, and 24% dead. Ninety percent of the fusiform aneurysms involving the PCA had favorable long-term outcomes, compared with 60% and 39% of those involving the vertebral artery–PICA and vertebrobasilar junction–basilar artery, respectively.
Aziz KM, van Loveren HR, Tew JMJr, et al. The Kawase approach to retrosellar and upper clival basilar aneurysms. Neurosurgery. 1999;44:1225-1234.
Bertalanffy H, Sure U, Petermeyer M, et al. Management of aneurysms of the vertebral artery-posterior inferior cerebellar artery complex. Neurol Med Chir (Tokyo). 1998;38(Suppl):93-103.
Bertalanffy H, Gilsbach JM, Mayfrank L, et al. Planning and surgical strategies for early management of vertebral artery and vertebrobasilar junction aneurysms. Acta Neurochir (Wien). 1995;134:60-65.
Coert BA, Chang SD, Do HM, et al. Surgical and endovascular management of symptomatic posterior circulation fusiform aneurysms. J Neurosurg. 2007;106:855-865.
Heros RC. Aneurysms of the vertebral artery and its branches. In: Ojemann RG, Ogilvy CS, Crowell RM, editors. Surgical Management of Neurovascular Disease. Baltimore: Williams & Wilkins; 1995:291-304.
Heros RC. Lateral suboccipital approach for vertebral and vertebro-basilar artery lesions. J Neurosurg. 1986;64:559-562.
Hoh BL, Ogilvy CS. Midline approaches to cerebrovascular lesions. Operat Tech Neurosurg. 2000;3:44-52.
International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: risk of rupture and risks of surgical intervention. N Engl J Med. 1998;339:1725-1733.
International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103-110.
ISAT Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. Lancet. 2002;360:1267-1274.
ISAT Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809-817.
Iwamoto H, Kiyohara Y, Fujishima M, et al. Prevalence of intracranial saccular aneurysms in a Japanese community based on a consecutive autopsy series during a 30-year observation period: the Hisayma study. Stroke. 1999;30:1390-1395.
Kassell NF, Torner JC, Jane JA, et al. The International Cooperative Study on the Timing of Aneurysm Surgery: part 2. Surgical results. J Neurosurg. 1990;73:37-47.
Kassell NF, Torner JC, Haley EC, et al. The International Cooperative Study on the Timing of Aneurysm Surgery: Part 1. Overall management results. J Neurosurg. 1990;73:18-36.
Kawase T, Bertalanffy H, Otani M, et al. Surgical approaches for vertebro-basilar trunk aneurysms located in the midline. Acta Neurochir (Wien). 1996;138:402-410.
McKinney AM, Palmer CS, Truwit CL, et al. Detection of aneurysms by 64-section multidetector CT angiography in patients acutely suspected of having an intracranial aneurysm and comparison with digital subtraction and 3D rotational angiography. AJNR Am J Neuroradiol. 2008;29:594-602.
Ogilvy CS, Crowell RM, Heros RC, et al. Basilar and posterior cerebral artery aneurysms. In: Ojemann RG, Ogilvy CS, Crowell RM, editors. Surgical Management of Neurovascular Disease. Baltimore: Williams & Wilkins; 1995:269-290.
Peluso JP, van Rooij WJ, Sluzewski M, et al. Posterior inferior cerebellar artery aneurysms: incidence, clinical presentation, and outcome of endovascular treatment. AJNR Am J Neuroradiol. 2008;29:86-90.
Salcman M, Rigmonti D, Numaguchi Y, Sadato N. Aneurysms of the posterior inferior cerebellar artery-vertebral artery complex: variations on a theme. Neurosurgery. 1990;27:12-20.
Seifert V, Stolke D. Posterior transpetrosal approach to aneurysms of the basilar trunk and vertebrobasilar junction. J Neurosurg. 1996;85:373-379.
Seifert V. Direct surgery of basilar trunk and vertebrobasilar junction aneurysms via the combined transpetrosal approach. Neurol Med Chir (Tokyo). 1998;38(Suppl):86-92.
Sekhar LN, Kalia KK, Yonas H, et al. Cranial base approaches to intracranial aneurysm in the subarachnoid space. Neurosurgery. 1994;35:472-481.
Steinberg GK, Drake CG, Peerless FJ. Deliberate basilar or vertebral artery occlusion in the treatment of intracranial aneurysms: immediate results and long-term outcome in 201 patients. J Neurosurg. 1993;79:161-173.
Wermer MJH, Marieke JH, van der Schaaf IC, et al. Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics: an updated meta-analysis. Stroke. 2007;38:1404-1410.
Yonekawa Y, Kaku Y, Imhof HG, et al. Posterior circulation aneurysms: technical strategies based on angiographic anatomical findings and the results of 60 recent consecutive cases. Acta Neurochir Suppl (Wien). 1999;72:123-140.
1 Cruveilhier J. Anatomie Pathologique de Corps Humain, Vol 2. Paris: JB Bailliere. 1829. Cited in Schwartz HG: Arterial aneurysms of the posterior fossa. J Neurosurg. 1948;5:312-316
2 Krayenbuhl H. Das Hiraneurysma. Scheiwz Arch Neurol Psychiatry. 1941;47:155-236.
3 Dandy WE. Intracranial Arterial Aneurysms. Ithaca, NY: Comstock; 1944.
4 Rizzoli HV, Hayes GJ. Congenital berry aneurysm of the posterior fossa: case report with successful operative excision. J Neurosurg. 1953;10:550-551.
5 Schwartz HG. Arterial aneurysms of the posterior fossa. J Neurosurg. 1948;5:312-316.
6 Rand RW, Jannetta PJ. Micro-neurosurgery for aneurysm of the vertebral-basilar artery system. J Neurosurg. 1967;27:330-335.
7 Drake CG. The surgical treatment of vertebral-basilar aneurysms. Clin Neurosurg. 1969;16:114-169.
8 Sekhar LN, Kalia KK, Yonas H, et al. Cranial base approaches to intracranial aneurysm in the subarachnoid space. Neurosurgery. 1994;35:472-481.
9 Sekhar LN, Estonillo R. Transtemporal approach to the skull base: an anatomical study. Neurosurgery. 1986;19:799-808.
10 Kawase T, Bertalanffy H, Otani M, et al. Surgical approaches for vertebro-basilar trunk aneurysms located in the midline. Acta Neurochir (Wien). 1996;138:402-410.
11 Day JD, Fukushima T, Giannotta SL. Cranial base approaches to posterior circulation aneurysms. J Neurosurg. 1997;87:544-554.
12 Pia HW. Classification of vertebro-basilar aneurysms. Acta Neurochir (Wien). 1979;47:3-30.
13 Roux A. Vertebro-PICA aneurysms: midline suboccipital approach and laminectomy of the atlas. Br J Neurosurg. 1990;4:113-121.
14 Locksley HB. Report on the Cooperative Study of Intracranial Aneurysms and Subarachnoid Hemorrhage: section V, part I. Natural history of subarachnoid hemorrhage, intracranial aneurysms, and arteriovenous malformations: based on 6368 cases in the cooperative study. J Neurosurg. 1966;25:219-239.
15 Iwamoto H, Kiyohara Y, Fujishima M, et al. Prevalence of intracranial saccular aneurysms in a Japanese community based on a consecutive autopsy series during a 30-year observation period: the Hisayma study. Stroke. 1999;30:1390-1395.
16 Yamaura A, Watanabe Y, Saeki N, et al. Dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. 1990;72:183-188.
17 Kawaguchi S, Sakaki T, Tsunoda S, et al. Management of dissecting aneurysms of the posterior circulation. Acta Neurochir (Wien). 1994;131:26-31.
18 Aoki N, Sakai T. Rebleeding from intracranial dissecting aneurysm in the vertebral artery. Stroke. 1990;21:1623-1631.
19 Mizutani T, Aruga T, Kirino T, et al. Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery. 1995;36:905-911.
20 Steinberg GK, Drake CG, Peerless FJ. Deliberate basilar or vertebral artery occlusion in the treatment of intracranial aneurysms: immediate results and long-term outcome in 201 patients. J Neurosurg. 1993;79:161-173.
21 Fukasawa I, Sasaki H, Nukui H. Surgical treatment for ruptured vertebral artery dissecting aneurysms. Neurol Med Chir Suppl (Tokyo). 1998;38:104-106.
22 Yamada K, Hayakawa T, Ushio Y, et al. Therapeutic occlusion of the vertebral artery for unclippable vertebral aneurysm: relationship between site of occlusion and clinical outcome. Neurosurgery. 1984;15:834-888.
23 Aymard A, Gobin YP, Hodes J, et al. Endovascular occlusion of vertebral arteries in the treatment of unclippable vertebrobasilar aneurysms. J Neurosurg. 1991;74:393-398.
24 Hodes JE, Aymard A, Gobin YP, et al. Endovascular occlusion of intracranial vessels for curative treatment of unclippable aneurysms: report of 16 cases. J Neurosurg. 1991;75:694-701.
25 Halbach VV, Higashida RT, Dowd CF, et al. Endovascular treatment of vertebral artery dissections and pseudoaneurysms. J Neurosurg. 1993;79:183-191.
26 Coert BA, Chang SD, Do HM, et al. Surgical and endovascular management of symptomatic posterior circulation fusiform aneurysms. J Neurosurg. 2007;106:855-865.
27 Hernesniemi J, Vapalahti M, Niskanen M, et al. Management outcome for vertebrobasilar artery aneurysms by early surgery. Neurosurgery. 1992;31:857-861.
28 Schievink WI, Wijdicks EF, Piepgras DG, et al. The poor prognosis of ruptured intracranial aneurysms of the posterior circulation. J Neurosurg. 1995;82:791-795.
29 Kitanaka C, Tanaka J, Kuwahara M, et al. Nonsurgical treatment of unruptured intracranial vertebral artery dissection with serial follow-up angiography. J Neurosurg. 1994;80:667-674.
30 Kassell NF, Torner JC, Jane JA, et al. The International Cooperative Study on the Timing of Aneurysm Surgery: part 2. Surgical results. J Neurosurg. 1990;73:37-47.
31 Kassell NF, Torner JC, Haley EC, et al. The International Cooperative Study on the Timing of Aneurysm Surgery: part 1. Overall management results. J Neurosurg. 1990;73:18-36.
32 Peerless SJ, Hernesniemi JA, Gutman FB, et al. Early surgery for ruptured vertebrobasilar aneurysms. J Neurosurg. 1994;80:643-649.
33 Bertalanffy H, Gilsbach JM, Mayfrank L, et al. Planning and surgical strategies for early management of vertebral artery and vertebrobasilar junction aneurysms. Acta Neurochir (Wien). 1995;134:60-65.
34 Lanzino G, Andreoli A, Limoni P, et al. Vertebro-basilar aneurysms: does delayed surgery represent the best surgical strategy? Acta Neurochir (Wien). 1993;125:5-8.
35 van Loon J, Waerzeggers Y, Wilms G, et al. Early endovascular treatment of ruptured cerebral aneurysms in patients in very poor neurological condition. Neurosurgery. 2002;50:457-465.
36 International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: risk of rupture and risks of surgical intervention. N Engl J Med. 1998;339:1725-1733.
37 International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103-110.
38 Wermer MJH, Marieke JH, van der Schaaf IC, et al. Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics: an updated meta-analysis. Stroke. 2007;38:1404-1410.
39 Hunt WE, Hess RM. Surgical risks as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28:14-20.
40 Yamaura A. Diagnosis and treatment of vertebral aneurysms. J Neurosurg. 1988;69:345-349.
41 Yasargil MG. Microneurosurgery: II. Clinical Considerations, Surgery of the Intracranial Aneurysms and Results. New York: Georg Thieme Verlag; 1984.
42 Sugita K, Kobayashi S, Tanaka Y, et al. Giant aneurysms of the vertebral artery: report of five cases. J Neurosurg. 1988;68:960-966.
43 Pozzi-Mucelli F, Bruni S, Doddi M, et al. Detection of intracranial aneurysms with 64 channel multidetector row computed tomography: comparison with digital subtraction angiography. Eur J Radiol. 2007;64:15-26.
44 McKinney AM, Palmer CS, Truwit CL, et al. Detection of aneurysms by 64-section multidetector CT angiography in patients acutely suspected of having an intracranial aneurysm and comparison with digital subtraction and 3D rotational angiography. AJNR Am J Neuroradiol. 2008;29:594-602.
45 Han DH, Kwon OK, Oh CW. Clinical characteristics of vertebrobasilar artery dissection. Neurol Med Chir Suppl (Tokyo). 1998;38:107-113.
46 Kallme DF, Palmer CS, Truwit CL, et al. Patterns of hemorrhage with ruptured posterior inferior cerebellar artery aneurysms: CT findings in 44 cases. AJR Am J Roentgenol. 1997;169:1169-1171.
47 Andoh T, Shirakami S, Nakashima T, et al. Clinical analysis of a series of vertebral aneurysm cases. Neurosurgery. 1992;31:987-993.
48 Drake CG. Giant intracranial aneurysms: experience with surgical treatment in 176 patients. Clin Neurosurg. 1979;26:12-95.
49 Peerless SJ, Wallace MC, Drake CG, et al. Giant intracranial aneurysms. In: Youmans JR, editor. Neurological Surgery, Vol 3. Philadelphia: WB Saunders; 1990:1764-1806.
50 Ogilvy CS, Carter BS. A proposed comprehensive grading system to predict outcome for surgical management of intracranial aneurysms. Neurosurgery. 1998;42:959-970.
51 Kempe LG. Aneurysm of the vertebral artery. In: Pia HW, et al, editors. Cerebral Aneurysms: Advances in Diagnosis and Therapy. Berlin: Springer-Verlag; 1979:119-120.
52 Beck DW, Boarini DJ, Kassell NF. Surgical treatment of giant aneurysm of vertebral-basilar junction. Surg Neurol. 1979;12:283-285.
53 Ganti SR, Steinberger A, McMurtry JGIII, et al. Computed tomographic demonstration of giant aneurysms of the vertebrobasilar system: report of eight cases. Neurosurgery. 1981;9:261-267.
54 Ishii R, Tanaka R, Koike T, et al. Computed tomographic demonstration of the effect of proximal parent artery ligation for giant intracranial aneurysms. Surg Neurol. 1983;19:532-540.
55 Schubiger O, Valavanis A, Hayek J. Computed tomography in cerebral aneurysms with special emphasis on giant intracranial aneurysms. J Comput Assist Tomogr. 1980;4:24-32.
56 Salcman M, Rigmonti D, Numaguchi Y, Sadato N. Aneurysms of the posterior inferior cerebellar artery-vertebral artery complex: variations on a theme. Neurosurgery. 1990;27:12-20.
57 Pelz DM, Vinuela F, Fox AJ, Drake CG. Vertebrobasilar occlusion therapy of giant aneurysms: significance of angiographic morphology of the posterior communicating arteries. J Neurosurg. 1984;60:560-565.
58 Hudgins RJ, Day AL, Quisling RG, et al. Aneurysms of the posterior inferior cerebellar artery: a clinical and anatomical analysis. J Neurosurg. 1983;58:381-387.
59 Lang DA, Galbraith SL. The management outcome of patients with a ruptured posterior circulation aneurysm. Acta Neurochir (Wien). 1993;125:9-14.
60 Chou SN, Ortiz-Suarez HJ. Surgical treatment of arterial aneurysms of the vertebrobasilar circulation. J Neurosurg. 1974;41:671-680.
61 Bertalanffy H, Sure U, Petermeyer M, et al. Management of aneurysms of the vertebral artery-posterior inferior cerebellar artery complex. Neurol Med Chir (Tokyo). 1998;38(Suppl):93-103.
62 Heros RC. Aneurysms of the vertebral artery and its branches. In: Ojemann RG, Ogilvy CS, Crowell RM, editors. Surgical Management of Neurovascular Disease. Baltimore: Williams & Wilkins; 1995:291-304.
63 Seifert V, Stolke D. Posterior transpetrosal approach to aneurysms of the basilar trunk and vertebrobasilar junction. J Neurosurg. 1996;85:373-379.
64 Giannotta SL, Maceri DR. Retrolabyrinthine transsigmoid approach to basilar trunk and vertebrobasilar artery junction aneurysms: technical note. J Neurosurg. 1988;69:461-466.
65 Seifert V. Direct surgery of basilar trunk and vertebrobasilar junction aneurysms via the combined transpetrosal approach. Neurol Med Chir (Tokyo). 1998;38(Suppl):86-92.
66 Collice M, Arena O, D’Aliberti C, et al. Transbasal approaches to aneurysms of the vertebro-basilar junction. J Neurosurg Sci. 1998;42:81-86.
67 Yonekawa Y, Kaku Y, Imhof HG, et al. Posterior circulation aneurysms: technical strategies based on angiographic anatomical findings and the results of 60 recent consecutive cases. Acta Neurochir Suppl (Wien). 1999;72:123-140.
68 Hoh BL, Ogilvy CS. Midline approaches to cerebrovascular lesions. Operat Tech Neurosurg. 2000;3:44-52.
69 Ogilvy CS, Barker FGII, Joseph MP, et al. Surgical treatment of vertebral and posterior inferior cerebellar artery aneurysms. Neurosurg Clin N Am. 1998;9:851-860.
70 Heros RC. Lateral suboccipital approach for vertebral and vertebro-basilar artery lesions. J Neurosurg. 1986;64:559-562.
71 Ogilvy CS, Barker FGII, Joseph MP, et al. Transfacial transclival approach for midline posterior circulation aneurysms. Neurosurgery. 1996;39:736-741.
72 Ogilvy CS, Crowell RM, Heros RC, et al. Basilar and posterior cerebral artery aneurysms. In: Ojemann RG, Ogilvy CS, Crowell RM, editors. Surgical Management of Neurovascular Disease. Baltimore: Williams and Wilkins; 1995:269-290.
73 deFries HO, Deeb ZE, Hudkins CP. A transfacial approach to the nasal-paranasal cavities and anterior skull base. Arch Otolaryngol Head Neck Surg. 1988;114:766-769.
74 Crockard HA, Koksel T, Watkin N. Transoral transclival clipping of anterior inferior cerebellar artery aneurysms using new rotational applier: technical note. J Neurosurg. 1991;75:483-485.
75 Sano K. A multipurpose all-angle clip applier for aneurysm surgery: technical note. J Neurosurg. 1980;53:260-261.
76 Aziz KM, van Loveren HR, Tew JMJr, et al. The Kawase approach to retrosellar and upper clival basilar aneurysms. Neurosurgery. 1999;44:1225-1234.
77 Seifert V, Stolke D. Posterior transpetrosal approach to aneurysms of the basilar trunk and vertebrobasilar junction. J Neurosurg. 1996;85:373-379.
78 Guglielmi G, Vinuela F, Sepetka I, Macellari V. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: preliminary clinical experience. J Neurosurg. 1991;75:8-14.
79 ISAT Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. Lancet. 2002;360:1267-1274.
80 ISAT Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809-817.
81 Lister JR, Rhoton ALJr, Matsushima T, Peace D. Microsurgical anatomy of the posterior inferior cerebellar artery. Neurosurgery. 1982;10:170-199.
82 Shrontz C, Dujovny M, Ausman JI, et al. Surgical anatomy of the arteries of the posterior fossa. J Neurosurg. 1986;65:540-544.
83 Crockard HA, Bradford R. Transoral transclival removal of a schwannoma anterior to the craniocervical junction: case report. J Neurosurg. 1985;62:293-295.
84 Archer DJ, Young S, Uttley D, et al. Basilar aneurysms: a new transclival approach via maxillotomy. J Neurosurg. 1987;67:54-58.
85 Hadley MN, Martin MA, Spetzler RF, et al. Comparative transoral dural closure techniques: a canine model. Neurosurgery. 1988;22:392-397.
86 Bonkowski JA, Gibson RD, Shape L. Foramen magnum meningioma: transoral resection with a bone baffle to prevent CSF leakage. case report. J Neurosurg. 1990;72:493-496.
87 Hayakawa T, Kamikawa K, Ohnishi T, Yoshimine T. Prevention of postoperative complications after a transoral transclival approach to basilar aneurysms: technical note. J Neurosurg. 1981;54:699-703.
88 Litvak J, Sumners TC, Barron JL, et al. A successful approach to vertebrobasilar aneurysms: technical note. J Neurosurg. 1981;55:491-494.
89 Hayakwa T, Yamada K, Yoshimine T. Transoral transclival approach: anatomical and technical notes. No Shinkei Geka. 1989;17:609-614.
90 Guity A, Young PH. A new technique for closure of the dura following transsphenoidal and transclival operations: technical note. J Neurosurg. 1990;72:824-828.
91 Vinuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 1997;86:475-482.
92 Zubillaga AF, Guglielmi G, Vinuela F, Duckwiler GR. Endovascular occlusion of intracranial aneurysms with electrically detachable coils: correlation of aneurysm neck size and treatment results. AJNR Am J Neuroradiol. 1994;15:815-820.
93 Hope JK, Byrne JV, Molyneux AJ. Factors influencing successful angiographic occlusion of aneurysms treated by coil embolization. AJNR Am J Neuroradiol. 1999;20:391-399.
94 Bavinkski G, Killer M, Gruber A, et al. Treatment of basilar artery bifurcation aneurysms by using Guglielmi detachable coils: a 6-year experience. J Neurosurg. 1999;90:843-852.
95 Guglielmi G, Vinuela F, Duckwiler G, et al. Endovascular treatment of posterior circulation aneurysms by electrothrombosis using electrically detachable coils. J Neurosurg. 1992;77:515-524.
96 Nichols DA, Brown RDJr, Thielen KR, et al. Endovascular treatment of ruptured posterior circulation aneurysms using electrolytically detachable coils. J Neurosurg. 1997;87:374-380.
97 Peluso JP, van Rooij WJ, Sluzewski M, et al. Posterior inferior cerebellar artery aneurysms: incidence, clinical presentation, and outcome of endovascular treatment. AJNR Am J Neuroradiol. 2008;29:86-90.
98 Mericle RA, Reig AS, Burry MV, et al. Endovascular surgery for proximal posterior inferior cerebellar artery aneurysms: an analysis of Glasgow Outcome Score by Hunt-Hess grades. Neurosurgery. 2006;58:619-625.
99 Sandalcioglu IE, Wanke I, Schoch B, et al. Endovascularly or surgically treated vertebral artery and posterior inferior cerebellar artery aneurysms: clinical analysis and results. Zentralbl Neurochir. 2005;66:9-16.
100 Hammon WM, Kempe LG. The posterior fossa approach to aneurysms of the vertebral and basilar arteries. J Neurosurg. 1972;37:339-347.
101 Sharr MM. Vertebrobasilar aneurysms: experience with 27 cases. Eur Neurol. 1973;10:129-143.
102 Sano K. Basilar artery aneurysms: transoral-transclival approach. In: Pia HW, Langmaid C, Zierski J, et al, editors. Cerebral Aneurysms: Advances in Diagnosis and Therapy. New York: Springer-Verlag; 1979:326-328.
103 Saito I, Takahashi H, Joshita H, et al. Clipping of vertebrobasilar aneurysms by the transoral transclival approach. Neurol Med Chir (Tokyo). 1980;20:753-758.
104 Tiyaworabun S, Wanis A, Schirmer M, et al. Aneurysms of the vertebro-basilar system: clinical analysis and follow-up results. Acta Neurochir (Wien). 1982;63:221-229.
105 Gacs G, Vinuela F, Fox AJ, et al. Peripheral aneurysms of the cerebellar arteries: review of 16 cases. J Neurosurg. 1983;58:63-68.
106 Yamamota I, Tsugane R, Ohya M, et al. Peripheral aneurysms of the posterior inferior cerebellar artery. Neurosurgery. 1984;15:839-845.
107 Solomon RA, Stein BM. Surgical approaches to aneurysms of the vertebral and basilar arteries. Neurosurgery. 1988;23:203-208.
108 Lee KS, Gower DJ, Branch CL, et al. Surgical repair of aneurysms of the posterior inferior cerebellar artery: a clinical series. Surg Neurol. 1989;31:85-91.
109 Priz MB. Evaluation and treatment of aneurysms of the vertebral artery: different strategies for different lesions. Neurosurgery. 1991;29:247-256.