CHAPTER 369 Microsurgery of Distal Anterior Cerebral Artery Aneurysms
Distal anterior cerebral artery (DACA) aneurysms arise on the anterior cerebral artery (ACA) or its branches distal to the anterior communicating artery (ACoA). They are an uncommon pathologic entity, representing only 2.1% to 9.2% of all aneurysms in several large historic series.1–17 They occur most commonly at the bifurcation of the pericallosal and callosomarginal arteries, and less commonly at the site of origin of the frontopolar or orbitofrontal branches of the ACA. Typically, these aneurysms are small and broad based in conformation. In fact, in contrast to typical aneurysms in other anatomic locations, many of the ruptured DACA aneurysms are found to be less than 5 mm in diameter,12 and giant aneurysms in this region are exceedingly rare.18,19 An additional critical feature of DACA aneurysms is their common association with additional intracerebral aneurysms, occurring at rates as high as 20% to 25% in large series.7 These other aneurysms are usually found either on the middle cerebral artery (MCA) or at the bifurcations of the internal carotid artery (ICA).
DACA aneurysms are a rather heterogeneous group of aneurysms, sharing a number of diverse etiologies. These include mycotic,20 traumatic,21 and tumorous aneurysms,22 in addition to the more common spontaneous saccular aneurysm. These alternate etiologies are more commonly involved with aneurysms located more distally along the artery. Mycotic (or infectious) aneurysms are engendered through the response to septic emboli that have lodged in the vessel wall, with heart valve vegetations most often serving as the site of embolic origin. This inflammatory reaction leads to a loss of intima and elastic tissue and often involves much of the circumference of the vessel. This conformation often complicates the surgical clipping of these aneurysms. Traumatic aneurysms in the distal ACA region are thought to result from shearing forces exerted on the distal pericallosal artery at the lower level of the falx.23 Finally, aneurysms that arise from tumor emboli are most commonly observed in patients with atrial myxomas.24 The importance of recognizing these alternative etiologies lies in the fact that these aneurysms may not be amenable to classic microsurgical clipping of the neck and often require trapping with vessel sacrifice.
Relevant Anatomy
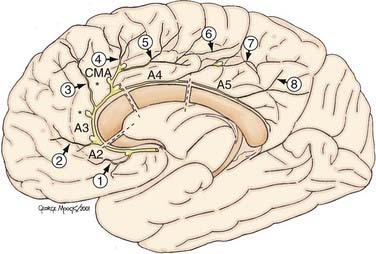
The DACA is defined as the extension of the ACA beyond the ACoA (Fig. 369-1). The DACA ascends anteriorly and superiorly from the juncture of the ACoA to the genu of the corpus callosum in the interhemispheric fissure, running between the medial aspects of the frontal lobes. Superior to the lamina terminalis, the DACA then curves around the genu of the corpus callosum and divides into the inferior pericallosal and superior callosomarginal arteries. These arteries pass over the body of the corpus callosum, where they form anastomotic connections with splenial branches of the posterior cerebral artery (PCA). The DACA provides the vascular supply for the medial surface of both hemispheres and a large segment of the anterior corpus callosum.
There have been several proposed naming schemes for the various elements of the DACA, the most accepted being the nomenclature proposed by Perlmutter and Rhoton.25 The DACA is subdivided into four segments, A2 to A5, with A1 being the segment of the ACA proximal to ACoA.25 The A2 segment is defined as the section of the artery from the ACoA to the junction of the rostrum and genu of corpus callosum. The orbitofrontal and frontopolar arteries arise from this segment. The A3 segment extends from the genu to a point where the artery makes a posterior turn above the genu. The callosomarginal artery arises from A3. The A4 segment is the posterior extension of the artery from A3 and extends to a point bisected by the coronal suture. The A5 segment then extends distally to include the anastomoses with the splenial arteries.
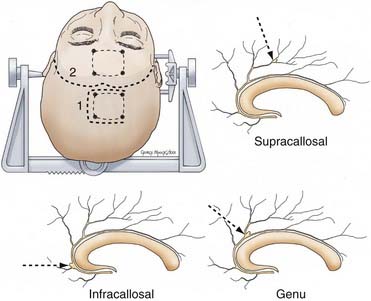
The eight cortical branches of the distal ACA include the orbitofrontal and frontopolar from A2; anterior middle, posterior internal frontal, and callosomarginal from A3; paracentral from A4; and superior and inferior parietal arteries from A5. The largest branch of the DACA, the callosomarginal artery (CMA) is the most common site of DACA aneurysms.25 The CMA gives rise to a variable number of cortical branches; most commonly, the middle internal frontal artery, followed by posterior and anterior internal frontal arteries and the paracentral artery; and less commonly, the superior parietal artery. The frontopolar artery is the second most common site of DACA aneurysms.
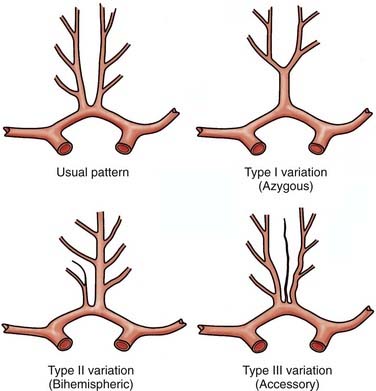
Vascular anomalies are commonly described in association with DACA aneurysms, and the most commonly observed variants in distal ACA anatomy include azygous ACA (type I variation), bihemispheric ACA (type II), and triplicate or accessory ACA (type III) (Fig. 369-2).25,26 It is thought that an association between these vascular variants and DACA aneurysms may be related to a flow disturbance that then leads to aneurysm formation. For example, up to 10% of DACA aneurysms are associated with an azygous ACA, in which the distal segments of both anterior cerebral arteries are represented by a single common vessel. The presence of these variants has significant implications, both in considering the surgical approach and in clinical outcome, because damage to the single trunk may result in bihemispheric deficits.
Clinical Presentation
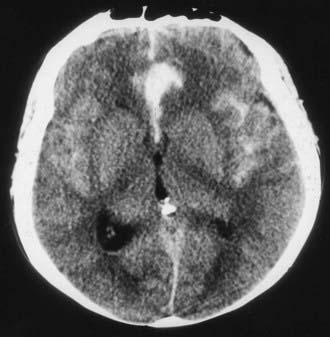
The average age at presentation of patients with DACA aneurysm is about 50 years, and there appears to be a slight female preponderance.7 Symptomatic aneurysms may present with subarachnoid hemorrhage (SAH), which is often prominent in the interhemispheric fissure across the top of the corpus callosum (Fig. 369-3), a pattern that may be confused with that from superiorly directed ACoA aneurysms. These aneurysms may also bleed into the adjacent frontal lobe and occasionally into the ventricles. Intraventricular hemorrhage generally results from extension of a frontal lobe hematoma into the ipsilateral frontal horn, rather than downward extension into the third ventricle.
Generally, patients with a ruptured DACA aneurysm present with classic findings of SAH. Additionally, intracerebral hemorrhage (ICH) is a common complication of ruptured DACA aneurysm and may be found in as many as 50% of cases.3,27 In large part resulting from this high incidence of ICH, some authors have reported an unusually high incidence of poor clinical grades in patients presenting with ruptured DACA aneurysms relative to aneurysms at other sites.2,27 One of the difficulties associated with aneurysms in this area is a lack of reliable localizing neurological findings. Nevertheless, the presence of a focal hemorrhage leading to vascular compromise and ischemia may result in memory loss, mutism, or rarely, a monoparesis in the case of a distally located clot. A hemispheric disconnection syndrome may also occur following a significant intracallosal hemorrhage.28
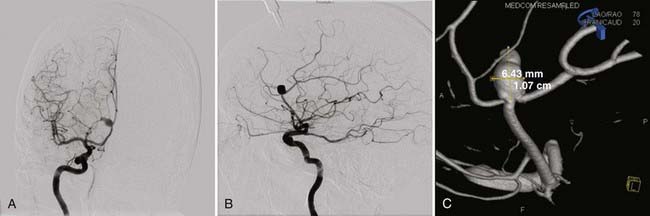
All patients with suspected ruptured DACA aneurysms should undergo four-vessel angiography in an attempt to identify the ruptured aneurysm as well as to uncover additional aneurysms (Fig. 369-4). However, it is often difficult to determine the side of origin of DACA aneurysms even with a high-quality angiogram. Magnetic resonance imaging (MRI), magnetic resonance angiography, and venography are useful in planning the skin incision and bone flap. These images also can also be used to determine the proximity of the aneurysm dome to the cingulate gyrus.
Surgical Considerations
For ruptured lesions, surgical timing and medical management are identical to those for other cerebral aneurysms. We recommend operating on ruptured aneurysms acutely and delay surgery only in patients who are so devastated neurologically that a functional recovery is unlikely. If there is an unruptured DACA aneurysm and a ruptured aneurysm at another location, some authors advocate repairing all aneurysms during one procedure to facilitate treatment of vasospasm with hemodynamic therapy.29 Our practice is to repair only the ruptured aneurysm and leave the unruptured DACA aneurysm because it requires both extending the craniotomy and further brain manipulation and operative time. We do not believe this additional risk is warranted because we have never seen an unruptured aneurysm bleed during hemodynamic therapy, intra-arterial vasodilator therapy, or angioplasty.
Preoperative Preparation
All patients are managed with normovolemia and normotension, so long as severe vasospasm is not an issue. Hydrocephalus resulting in neurological decline is treated with external ventricular drainage. However, if communicating hydrocephalus is being tolerated, intraoperative spinal drainage is preferred. Dexamethasone, gastrointestinal prophylaxis, perioperative antibiotics, and intraoperative phenytoin are administered. In good-grade disease patients with SAH and unruptured aneurysms, a loading dose of phenytoin is administered intraoperatively but not continued postoperatively. Phenytoin is continued both in poor-grade disease patients and those with risk factors for seizures such as intraparenchymal clots or other parenchymal pathology. Antifibrinolytic therapy decreases early aneurysmal rebleeding but increases ischemic deficits from vasospasm.30 Therefore, we employ this in all patients in whom surgery is delayed for any reason.
Operative Procedure
Skin Incision and Craniotomy
The scalp incision and bone flap take into account any associated aneurysms as well as the precise location of the DACA aneurysm. For extremely proximal A2 lesions, a bicoronal, modified bicoronal, or pterional incision may be used in preparation for a pterional craniotomy with or without orbital or orbitozygomatic extension. For the usual DACA aneurysm arising from the callosomarginal artery origin in the region of the genu, we use a modified bicoronal scalp incision extending from the ipsilateral zygoma across the midline to the contralateral superior temporal line, at the hairline (Fig. 369-5). We make a parasagittal craniotomy beginning at the margin of the frontal air sinus and extending 4 to 6 cm superiorly toward the coronal suture, laterally to the superior temporal line and across the superior sagittal sinus. Some surgeons place the bur holes directly over the sagittal sinus. We prefer to place slots across the sinus so that the dura of the contralateral frontal lobe is visible. This allows one to raise the bone flap with the footplate attachment never contacting the sinus and allows one to rotate the sinus slightly to the contralateral side after opening the dura, resulting in increased exposure. Care must be taken to not occlude the sinus with this maneuver. More distal pericallosal artery aneurysms are also best approached interhemispherically (see Fig. 369-5). The exact site of the scalp incision and craniotomy is determined by the specific location of the aneurysm as well as the underlying venous anatomy. Frameless stereotactic coregistered to an MRI, magnetic resonance angiogram, or venogram can be helpful in planning the exact location of both. Attempts should be made to avoid retraction on the motor cortex, and broader-based scalp flaps that cross the midline allow flexibility to operate in front of and behind critical venous anatomy.
Clinical Series
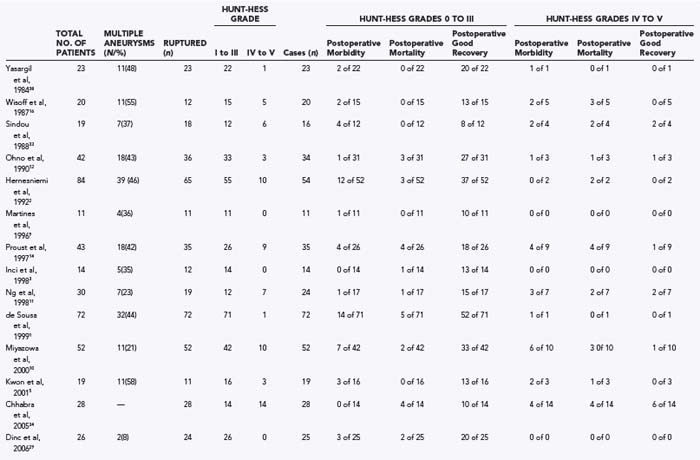
Table 369-1 summarizes the surgical results in patients with DACA aneurysms from the major reports in the English-language literature including 10 or more patients in the microsurgical era. Studies were only included if they provided data on outcome. Results of series from the premicrosurgical era are of historical interest but are not relevant to the current practice standards for these aneurysms.27,31,32 The reported surgical results are rather variable, reflecting the particular case mix of each series in relationship to the percentage of ruptured compared with unruptured aneurysms, the clinical grades of the patients at the time of surgery, and the timing of surgery.
In 1984, Yasargil published his experience with 23 patients with ruptured DACA aneurysms.17 In 16 cases, the aneurysm arose at the CMA, and in 6, it arose at the origin of the frontopolar artery. ICHs were present in 14 patients (60.9%). Thirteen patients (57%) had a good preoperative clinical grade, and 10 had a poor Hunt-Hess grade. Surgical intervention was on average delayed 3 weeks from the time of the most recent hemorrhage. In 19 cases, the aneurysm was approached through an interhemispheric route. Pterional craniotomies were used in four cases—once for an aneurysm arising very proximally at the orbitofrontal artery, and in the other cases to deal with additional aneurysms. Despite 43% of patients having a poor preoperative clinical grade, there were no deaths, and a satisfactory operative outcome was achieved in 87% of patients. The three unsatisfactory outcomes were limited to patients with poor clinical grade disease. Although this series was confounded by the fact that surgery tended to be delayed, these outstanding results established the standard for the microsurgical era.
Next, Wisoff and Flamm reported a series of 20 patients with 24 distal ACA aneurysms in 1987.16 Fourteen of these patients had ruptured aneurysms. Seven of the 12 patients (58%) with ruptured DACA aneurysms had a Hunt-Hess clinical grade of III or IV, which in each case was associated with an intracerebral or intraventricular hematoma and the subsequent development of vasospasm. All 20 patients were operated on using an interhemispheric approach. There were three perioperative deaths (15%), all in patients with grade IV disease. Two patients with poor preoperative grades suffered major morbidity. The outcome was favorable in 15 of 20 (75%) patients overall, but in only 9 of 14 patients (64%) with ruptured aneurysms.
In 1988, Sindou and associates reported a series of 19 patients with DACA aneurysms, 18 of whom had ruptured aneurysms.33 Two patients died of recurrent hemorrhage before surgery. In the 16 operated cases, there were no deaths. The overall mortality rate was 10.5%. Major postoperative morbidity occurred in 1 patient with a good clinical grade (6.3%), and some degree of postoperative morbidity occurred in 4 others. Overall, a satisfactory operative outcome occurred in 94% of patients, with a satisfactory management outcome in 16 of 19 (84%).
Ohno and colleagues reported their experience with 42 patients with DACA aneurysms in 1990.12 Thirty patients presented with ruptured aneurysms, 83% with a clinical grade of I or II. Nine patients had unruptured DACA aneurysms. Importantly, only 13 patients were operated on within 48 hours of bleeding; the remainder underwent delayed operation. An interhemispheric approach was used in every case. There were two deaths (5.9%) and two poor outcomes (5.9%) in the surgical patients, with an overall satisfactory outcome in 30 of 34 patients (88%). There were two preoperative deaths, resulting in an overall satisfactory management outcome in 36 of 42 patients (85.7%). These impressive results reflect the unusually high percentage of patients presenting with a good clinical grade in this series. Of the 25 patients with grade I or II disease who had a ruptured DACA aneurysm, there was one death and one poor outcome, resulting in a satisfactory outcome in 92%. The authors concluded that excellent results were possible with early surgery in patients who presented with a favorable clinical grade.
In 1992, Hernesniemi and coworkers reported on a series of patients with DACA aneurysms, consisting of 84 patients with 92 DACA aneurysms.2 A DACA aneurysm had ruptured in 65 patients and was incidental in 19. Surgery was undertaken for 54 of the 65 ruptured aneurysms. Most of the patients were operated on within 3 days of hemorrhage (31 of 54, or 57%). Twenty-four patients (44%) had Hunt-Hess grade I or II disease before surgery, in whom there were three poor outcomes (12.5%). Thirty patients (56%) had grade III (28) or IV (2) disease, in whom there were five postoperative deaths and nine poor outcomes. In these 54 patients, the overall postoperative mortality was 5 of 54 (9.3%), and the major postoperative morbidity was 12 of 54 (22%), with a satisfactory outcome in 37 of 54 (68.5%). Thirteen of the 19 incidental aneurysms were operated on without incident. The overall postoperative mortality in this series was 5 of 67 (7.5%), and the major postoperative morbidity was 12 of 67 (17.9%), with a satisfactory outcome in 75% of the patients. Mortality and poor outcomes correlated with poor clinical grade related to the severity of the initial hemorrhage and the presence of ICH. The results reported in this large series are likely representative of the results when a significant percentage of patients present with a poor clinical grade.
Martines and coworkers also reviewed their series of 11 patients treated between 1990 and 1993 with aneurysms of the DACA submitted to neuropsychological testing.9 Notably, patients with preoperative grade IV or V disease were excluded from analysis; therefore, the preoperative grade in the 11 patients who were included was I or II. Ten patients had an aneurysm at the bifurcation of the DACA into pericallosal and callosomarginal branches, and 1 patient had an aneurysm at the frontopolar artery take-off. All were operated on by an interhemispheric approach; in 3 patients, this was combined with a pterional approach to a second aneurysm. The 11 patients were assessed preoperatively using a battery of seven psychometric tests. Five weeks after surgery, the same tests were administered. Four patients with hematoma of the cingulate gyrus presented with memory dysfunction. One patient had a preoperative mild amnesia that resolved 8 weeks after surgery. In all the other patients, no deficits were found either before or after surgery.
Proust and associates published a retrospective analysis of 43 patients with 50 DACA aneurysms in 1997.14 A DACA aneurysm had ruptured in 35 patients and was incidental in 8. An interhemispheric approach was used in most of these cases (83%). Surgery was undertaken within 2 days of admission to the neurosurgical unit but, on average, occurred 8 days from the day of bleeding. Of the 35 ruptured aneurysms, 26 were regarded as good grade (Hunt-Hess grades I to III), and 9 were regarded as poor grade (Hunt-Hess grades IV and V). There was a very high rate of premature, intraoperative aneurysmal rupture in this series (40%), which was thought to be the direct cause of the poor outcomes in all cases except those in which the poor outcome was attributed to the effects of the initial hemorrhage. Eight patients with incidental DACA aneurysms were also treated surgically. Including the results among those patients, the overall postoperative mortality rate in the 43 patients was 14%, and the major postoperative morbidity rate was 16.3%, with an overall satisfactory surgical outcome in 70%.
Inci and coworkers reported a series of 14 patients in 1998.3 All the aneurysms were approached by an interhemispheric route. In 2 patients, the aneurysm was unruptured. Early surgery was considered in patients with Hunt-Hess grade I or II disease, of whom there were 9. Thirteen of the 14 aneurysms were successfully clipped. The patient whose incidental aneurysm could not be clipped died as a result of right frontal hemorrhagic infarction attributed to excessive retraction and disruption of bridging veins. There was no major postoperative morbidity, and a satisfactory outcome was achieved in 93% of the cases.
Also in 1998, Ng and colleagues published their series of 30 patients with DACA aneurysms over the years 1970 to 1996.11 Nineteen of these cases presented with SAH, with 8 patients (42%) with good clinical grade (I or II) disease. Surgery was carried out in 25 (83%) patients. All 5 patients with unruptured aneurysms and the 8 patients with good clinical grade disease made a good recovery. In contrast, only 6 of 11 patients (55%) with poor clinical grade disease had a good outcome. The overall mortality rate in this series was 16%. This series reinforces the relationship between admission grade and postoperative outcome.
Next, de Sousa and colleagues published their large series in 1999, which included 72 patients with DACA aneurysms (65 ruptured, 7 unruptured).1 Of these patients, 32 had multiple aneurysms. Only 3 patients were operated on early; the others were treated at least 10 days after hemorrhage. Most patients (69%) with ruptured aneurysms had Hunt-Hess grade I or II disease at the time of surgery. All the aneurysms were approached by an interhemispheric route. The perioperative mortality rate was 6.9%, and the rate of major postoperative morbidity was 8.3%. A satisfactory outcome was noted in 85% of the patients. Most of the poor results occurred in patients with poor clinical grade disease at the time of surgery.
Another recent study put forth by Miyazowa and associates examined clinical outcomes of patients with ruptured DACA aneurysms.10 Fifty-two patients with ruptured DACA aneurysms were studied, with a standard policy for early surgery for Hunt-Hess grades I to IV. Univariate analysis demonstrated that clinical grade (P = .0006), size of aneurysm (P = .005), and size of ICH (P = .012) affected the outcome, whereas multiple logistic regression demonstrated that Hunt-Hess grade (P = .01) and timing of operation (P = .033) affected the outcome. Good outcome was observed in 34 patients (65%), moderate disability in 6 patients (12%), severe disability in 7 patients (14%), and death in 5 patients (9%).
In another report put forth by Kwon and colleagues in 2001,5 19 cases of DACA aneurysm were studied retrospectively. All patients were operated on using an interhemispheric approach, with intraoperative rupture developing in only 3 cases (15.8%). Fifteen of 19 patients (78.9%) had a favorable outcome, and a mortality rate of 5.3% was observed. The authors of this study suggested that the presence of preoperative ICH related to unfavorable outcome.
Chhabra and colleagues reported their experience treating a series of 28 patients over a 5-year period with ruptured DACA aneurysms that were operated on through a bifrontal basal anterior interhemispheric approach.34 Fourteen of these patients presented with Hunt-Hess grades I to III disease, and 14 with grades IV and V disease. Ten of these patients demonstrated anatomic variations of the A2 segment. In the patients with good-grade disease, there were 4 deaths; the remaining 10 patients had a good recovery. On the other hand, 4 patients with poor-grade disease died, and another 4 experienced major postoperative morbidity. The authors attributed this relatively high rate of mortality to either poor clinical grade or postoperative ischemia from vasospasm.
In 2006, Dinc and associates published their clinical series of DACA aneurysms involving 26 of 364 patients treated in their department between 1996 and 2004.29 Twenty-three of these patients were treated with an anterior interhemispheric approach and 2 with a pterional approach. There were no patients with poor-grade disease in this series. Of the operated patients, 80% had a good outcome, with a total surgical morbidity of 8%.
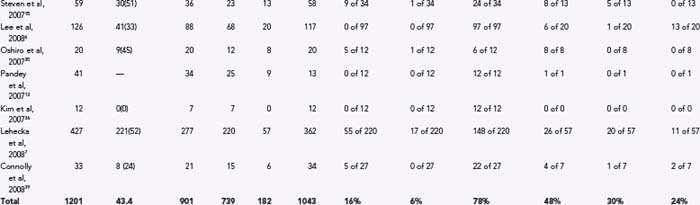
In 2007, Steven and associates published the results of their clinical series of 59 consecutively managed patients with DACA aneurysms.15 Thirty-six patients had ruptured DACA aneurysms, and 23 had aneurysms that were unruptured. In those with ruptured aneurysms, admission Hunt-Hess grade was I in 10 patients (27.8%), II in 3 patients (8.3%), III in 10 patients (27.8%), IV in 7 patients (19.4%), and V in 6 patients (16.7%). Fifty-eight patients underwent surgery. Six patients died, all with ruptured aneurysms, and 70% of survivors with ruptured aneurysms had a favorable outcome.
In their case series published in 2007, Oshiro and colleagues reviewed their experience with 20 patients with DACA aneurysms between 2000 and 2005, and excluded patients with unruptured or incidental DACA aneurysms.35 Associated ICH was present in 10 patients on admission. All patients except 1 underwent surgery through an interhemispheric approach, and all patients were operated on within 48 hours of hemorrhage. Favorable outcomes were achieved in 15 of 20 patients. One patient died secondary to infarction related to severe vasospasm, and 4 patients had poor preoperative status and severe bleeding with ICH.
In 2007, Pandey and coworkers reviewed their institutional experience in 41 consecutive patients with DACA aneurysms.13 This particular series included patients treated with endovascular embolization (28 patients, 68%) and patients treated with microsurgical clipping (13 patients, 32%). Of the patients treated with endovascular coiling, 50% had Hunt-Hess grade III or IV disease, whereas 46.2% of patients treated with clipping had elective surgery. Among the clipped patient population, 100% had successful clip ligation, and 100% of those with grades 0 to III disease had good postoperative outcomes. In contrast, the single patient with preoperative grade IV disease experienced severe postoperative disability.
In 2007, Kim and colleagues presented a series of 12 patients with DACA aneurysms who were consecutively treated using computed tomography–based neuronavigation (Brainlab, Hemistetten, Germany).36 Seven of 12 patients presented with SAH, 2 had Hunt-Hess grade II disease, and 5 had grade III disease. All aneurysms were successfully clipped without complications during surgery, and all patients had a good recovery as determined using the Glasgow Coma Scale.
Lee and associates published their series in 2008 including 126 patients with DACA aneurysms.6 Surgical treatment was employed in 117 cases, of which 29 were unruptured and 88 were ruptured. Endovascular occlusion was used in 9 cases in this series. The results for surgical treatment of the 117 cases were favorable in 94% of cases and unfavorable in 6% of cases. All the poor outcomes occurred in patients who had preoperative grade IV or V disease, and there was no surgical morbidity or mortality among the 29 patients with unruptured DACA aneurysms.
By far the largest series of DACA aneurysms to date was reported in 2008 by Lehecka and associates, as the combined experience of two Finnish neurosurgical centers in the treatment of 501 consecutive patients with DACA aneurysms.7 The report focuses on the 427 patients treated between 1980 and 2005. Impressively, no patients were lost to follow-up. The treatment and outcome of 277 ruptured DACA aneurysms were then compared to those of all consecutive ruptured aneurysms (n = 2243), and multivariate analysis was used to identify factors predicting 1-year outcome. DACA aneurysms were found to be smaller, more frequently associated with multiple aneurysms, and more often presenting with intracerebral hematomas than ruptured aneurysms in general. At 1-year follow-up, patients with DACA aneurysms had similar favorable outcomes (57% good recovery) as those with other ruptured aneurysms, but a lower mortality rate (13% versus 24%). Factors predicting unfavorable outcome in this series were advanced age, Hunt-Hess grade greater than III, ICH, intraventricular hemorrhage, and severe postoperative hydrocephalus.
This same group also recently published a study describing a detailed angiographic analysis in 101 patients treated between 1998 and 2007, many of whom overlapped with the clinical series presented earlier.37 Anatomic features of the digital subtraction angiogram (n = 39) or computed tomographic angiogram (n = 62) were analyzed. Of these patients, 50% had multiple aneurysms, and 7% had multiple DACA aneurysms. The 108 DACA aneurysms were found in seven different locations: 2 in frontobasal branches; 5 in the A2 segment; 19 in the A3 segment inferior to the genu of the corpus callosum, 70 anterior to the genu, 1 superior to the genu, 7 on the A4-5 segments; and 4 on distal ACA branches. Mean sizes of aneurysms were 7.4 mm (range, 2 to 35 mm) for ruptured aneurysms, and 4.2 mm (range, 1 to 9 mm) for the 41 unruptured aneurysms. Sixty-eight percent of aneurysms demonstrated a broad base, and 94% had a branch origin at the base. Anomalies of the ACA were observed in 23% of patients, including azygous ACA (4%), bihemispheric ACA (15%), and triplicated ACA (4%). This large series adds considerably to our understanding of the anatomic features of these complex aneurysms.
Baptista AG. Studies on the arteries of the brain. II. The anterior cerebral artery: some anatomic features and their clinical implications. Neurology. 1963;13:825.
Becker DH, Newton TH. Distal anterior cerebral artery aneurysm. Neurosurgery. 1979;4:495.
Chhabra R, Gupta SK, Mohindra S, et al. Distal anterior cerebral artery aneurysms: bifrontal basal anterior interhemispheric approach. Surg Neurol. 2005;64:315.
Chwajol M, Starke RM, Kim GH, et al. Antifibrinolytic therapy to prevent early rebleeding after subarachnoid hemorrhage. Neurocrit Care. 2008;8:418.
de Sousa AA, Dantas FL, de Cardoso GT, et al. Distal anterior cerebral artery aneurysms. Surg Neurol. 1999;52:128.
Dinc C, Iplikcioglu AC, Bikmaz K, et al. Distal anterior cerebral artery mirror aneurysms and middle cerebral artery aneurysms. Neurol Med Chir (Tokyo). 2006;46:438.
Eldevik OP, Gabrielsen TO. Fusiform aneurysmal dilatation of pericallosal artery: a sign of lipoma of corpus callosum. Acta Radiol Suppl. 1976;347:71.
Hernesniemi J, Tapaninaho A, Vapalahti M, et al. Saccular aneurysms of the distal anterior cerebral artery and its branches. Neurosurgery. 1992;31:994.
Inci S, Erbengi A, Ozgen T. Aneurysms of the distal anterior cerebral artery: report of 14 cases and a review of the literature. Surg Neurol. 1998;50:130.
Kim TS, Joo SP, Lee JK, et al. Neuronavigation-assisted surgery for distal anterior cerebral artery aneurysm. Minim Invasive Neurosurg. 2007;50:140.
Kuwabara S, Ishikawa S, Andoh S, et al. [Aneurysms of the distal anterior cerebral artery: report of 18 cases and review of 191 reported cases]. Neurol Med Chir (Tokyo). 1984;24:580.
Kwon TH, Chung HS, Lim DJ, et al. Distal anterior cerebral artery aneurysms: clinical features and surgical outcome. J Korean Med Sci. 2001;16:204.
Lee JW, Lee KC, Kim YB, et al. Surgery for distal anterior cerebral artery aneurysms. Surg Neurol. 2008;70:153.
Lee VH, Connolly HM, Brown RDJr. Central nervous system manifestations of cardiac myxoma. Arch Neurol. 2007;64:1115.
Lehecka M, Lehto H, Niemela M, et al. Distal anterior cerebral artery aneurysms: treatment and outcome analysis of 501 patients. Neurosurgery. 2008;62:590.
Lehecka M, Niemela M, Seppanen J, et al. No long-term excess mortality in 280 patients with ruptured distal anterior cerebral artery aneurysms. Neurosurgery. 2007;60:235.
Lehecka M, Porras M, Dashti R, et al. Anatomic features of distal anterior cerebral artery aneurysms: a detailed angiographic analysis of 101 patients. Neurosurgery. 2008;63:219.
Levin HS, Goldstein FC, Ghostine SY, et al. Hemispheric disconnection syndrome persisting after anterior cerebral artery aneurysm rupture. Neurosurgery. 1987;21:831.
Maiuri F, Corriero G, D’Amico L, et al. Giant aneurysm of the pericallosal artery. Neurosurgery. 1990;26:703.
Martines F, Blundo C, Chiappetta F. Surgical treatment of the distal anterior cerebral artery aneurysms. J Neurosurg Sci. 1996;40:189.
Miyazawa N, Nukui H, Yagi S, et al. Statistical analysis of factors affecting the outcome of patients with ruptured distal anterior cerebral artery aneurysms. Acta Neurochir (Wien). 2000;142:1241.
Nakstad P, Nornes H, Hauge HN. Traumatic aneurysms of the pericallosal arteries. Neuroradiology. 1986;28:335.
Ng PY, Reilly PL, Brophy BP, et al. Distal anterior cerebral artery aneurysms: a clinical series. Br J Neurosurg. 1998;12:209.
Ohno K, Monma S, Suzuki R, et al. Saccular aneurysms of the distal anterior cerebral artery. Neurosurgery. 1990;27:907.
Oshiro S, Tsugu H, Sakamoto S, et al. Ruptured aneurysm of the distal anterior cerebral artery: clinical features and surgical strategies. Neurol Med Chir (Tokyo). 2007;47:159.
Pandey A, Rosenwasser RH, Veznedaroglu E. Management of distal anterior cerebral artery aneurysms: a single institution retrospective analysis (1997-2005). Neurosurgery. 2007;61:909.
Perlmutter D, Rhoton AL Jr. Microsurgical anatomy of the distal anterior cerebral artery. J Neurosurg. 1978;49:204.
Preul M, Tampieri D, Leblanc R. Giant aneurysm of the distal anterior cerebral artery: associated with an anterior communicating artery aneurysm and a dural arteriovenous fistula. Surg Neurol. 1992;38:347.
Proust F, Toussaint P, Hannequin D, et al. Outcome in 43 patients with distal anterior cerebral artery aneurysms. Stroke. 1997;28:2405.
Royand F, Carter P, Guthkelch N. Distal Anterior Cerebral Artery Aneurysms. New York: McGraw-Hill; 1995.
Sindou M, Pelissou-Guyotat I, Mertens P, et al. Pericallosal aneurysms. Surg Neurol. 1988;30:434.
Snyckers FD, Drake CG. Aneurysms of the distal anterior cerebral artery: a report on 24 verified cases. S Afr Med J. 1973;47:1787.
Soria ED, Paroski MW, Schamann ME. Traumatic aneurysms of cerebral vessels: a case study and review of the literature. Angiology. 1988;39:609.
Steven DA, Lownie SP, Ferguson GG. Aneurysms of the distal anterior cerebral artery: results in 59 consecutively managed patients. Neurosurgery. 2007;60:227.
Sypert GW, Young HF. Ruptured mycotic pericallosal aneurysm with meningitis due to Neisseria meningitidis infection: case report. J Neurosurg. 1972;37:467.
Wisoff JH, Flamm ES. Aneurysms of the distal anterior cerebral artery and associated vascular anomalies. Neurosurgery. 1987;20:735.
Yasargil MG, Carter LP. Saccular aneurysms of the distal anterior cerebral artery. J Neurosurg. 1974;40:218.
Yoshimoto T, Uchida K, Suzuki J. Surgical treatment of distal anterior cerebral artery aneurysms. J Neurosurg. 1979;50:40.
1 de Sousa AA, Dantas FL, de Cardoso GT, et al. Distal anterior cerebral artery aneurysms. Surg Neurol. 1999;52:128.
2 Hernesniemi J, Tapaninaho A, Vapalahti M, et al. Saccular aneurysms of the distal anterior cerebral artery and its branches. Neurosurgery. 1992;31:994.
3 Inci S, Erbengi A, Ozgen T. Aneurysms of the distal anterior cerebral artery: report of 14 cases and a review of the literature. Surg Neurol. 1998;50:130.
4 Kuwabara S, Ishikawa S, Andoh S, et al. [Aneurysms of the distal anterior cerebral artery. Report of 18 cases and review of 191 reported cases]. Neurol Med Chir (Tokyo). 1984;24:580.
5 Kwon TH, Chung HS, Lim DJ, et al. Distal anterior cerebral artery aneurysms: clinical features and surgical outcome. J Korean Med Sci. 2001;16:204.
6 Lee JW, Lee KC, Kim YB, et al. Surgery for distal anterior cerebral artery aneurysms. Surg Neurol.. 2008;70:153.
7 Lehecka M, Lehto H, Niemela M, et al. Distal anterior cerebral artery aneurysms: treatment and outcome analysis of 501 patients. Neurosurgery. 2008;62:590.
8 Lehecka M, Niemela M, Seppanen J, et al. No long-term excess mortality in 280 patients with ruptured distal anterior cerebral artery aneurysms. Neurosurgery. 2007;60:235.
9 Martines F, Blundo C, Chiappetta F. Surgical treatment of the distal anterior cerebral artery aneurysms. J Neurosurg Sci. 1996;40:189.
10 Miyazawa N, Nukui H, Yagi S, et al. Statistical analysis of factors affecting the outcome of patients with ruptured distal anterior cerebral artery aneurysms. Acta Neurochir (Wien). 2000;142:1241.
11 Ng PY, Reilly PL, Brophy BP, et al. Distal anterior cerebral artery aneurysms: a clinical series. Br J Neurosurg. 1998;12:209.
12 Ohno K, Monma S, Suzuki R, et al. Saccular aneurysms of the distal anterior cerebral artery. Neurosurgery. 1990;27:907.
13 Pandey A, Rosenwasser RH, Veznedaroglu E. Management of distal anterior cerebral artery aneurysms: a single institution retrospective analysis (1997–2005). Neurosurgery. 2007;61:909.
14 Proust F, Toussaint P, Hannequin D, et al. Outcome in 43 patients with distal anterior cerebral artery aneurysms. Stroke. 1997;28:2405.
15 Steven DA, Lownie SP, Ferguson GG. Aneurysms of the distal anterior cerebral artery: results in 59 consecutively managed patients. Neurosurgery. 2007;60:227.
16 Wisoff JH, Flamm ES. Aneurysms of the distal anterior cerebral artery and associated vascular anomalies. Neurosurgery. 1987;20:735.
17 Yasargil MG, Carter LP. Saccular aneurysms of the distal anterior cerebral artery. J Neurosurg. 1974;40:218.
18 Maiuri F, Corriero G, D’Amico L, et al. Giant aneurysm of the pericallosal artery. Neurosurgery. 1990;26:703.
19 Preul M, Tampieri D, Leblanc R. Giant aneurysm of the distal anterior cerebral artery: associated with an anterior communicating artery aneurysm and a dural arteriovenous fistula. Surg Neurol. 1992;38:347.
20 Sypert GW, Young HF. Ruptured mycotic pericallosal aneurysm with meningitis due to Neisseria meningitidis infection: case report. J Neurosurg. 1972;37:467.
21 Nakstad P, Nornes H, Hauge HN. Traumatic aneurysms of the pericallosal arteries. Neuroradiology. 1986;28:335.
22 Eldevik OP, Gabrielsen TO. Fusiform aneurysmal dilatation of pericallosal artery: a sign of lipoma of corpus callosum. Acta Radiol Suppl. 1976;347:71.
23 Soria ED, Paroski MW, Schamann ME. Traumatic aneurysms of cerebral vessels: a case study and review of the literature. Angiology. 1988;39:609.
24 Lee VH, Connolly HM, Brown RDJr. Central nervous system manifestations of cardiac myxoma. Arch Neurol. 2007;64:1115.
25 Perlmutter D, Rhoton ALJr. Microsurgical anatomy of the distal anterior cerebral artery. J Neurosurg. 1978;49:204.
26 Baptista AG. Studies on the arteries of the brain. II. The anterior cerebral artery: some anatomic features and their clinical implications. Neurology. 1963;13:825.
27 Snyckers FD, Drake CG. Aneurysms of the distal anterior cerebral artery: a report on 24 verified cases. S Afr Med J. 1973;47:1787.
28 Levin HS, Goldstein FC, Ghostine SY, et al. Hemispheric disconnection syndrome persisting after anterior cerebral artery aneurysm rupture. Neurosurgery. 1987;21:831.
29 Dinc C, Iplikcioglu AC, Bikmaz K, et al. Distal anterior cerebral artery mirror aneurysms and middle cerebral artery aneurysms. Neurol Med Chir (Tokyo). 2006;46:438.
30 Chwajol M, Starke RM, Kim GH, et al. Antifibrinolytic therapy to prevent early rebleeding after subarachnoid hemorrhage. Neurocrit Care. 2008;8:418.
31 Becker DH, Newton TH. Distal anterior cerebral artery aneurysm. Neurosurgery. 1979;4:495.
32 Yoshimoto T, Uchida K, Suzuki J. Surgical treatment of distal anterior cerebral artery aneurysms. J Neurosurg. 1979;50:40.
33 Sindou M, Pelissou-Guyotat I, Mertens P, et al. Pericallosal aneurysms. Surg Neurol. 1988;30:434.
34 Chhabra R, Gupta SK, Mohindra S, et al. Distal anterior cerebral artery aneurysms: bifrontal basal anterior interhemispheric approach. Surg Neurol. 2005;64:315.
35 Oshiro S, Tsugu H, Sakamoto S, et al. Ruptured aneurysm of the distal anterior cerebral artery: clinical features and surgical strategies. Neurol Med Chir (Tokyo). 2007;47:159.
36 Kim TS, Joo SP, Lee JK, et al. Neuronavigation-assisted surgery for distal anterior cerebral artery aneurysm. Minim Invasive Neurosurg. 2007;50:140.
37 Lehecka M, Porras M, Dashti R, et al. Anatomic features of distal anterior cerebral artery aneurysms: a detailed angiographic analysis of 101 patients. Neurosurgery. 2008;63:219.
38 Yasargil MG. Distal anterior cerebral artery aneurysms. In: Yarsargil MG, editor. Microneurosurgery, vol 2. New York: Georg Thieme Verlag; 1984:244-251.