Microbiology, Sterilization, and Infection Control
I Classification of Microorganisms by Cell Type
II Eukaryotic Cell Structure (Figure 3-1)
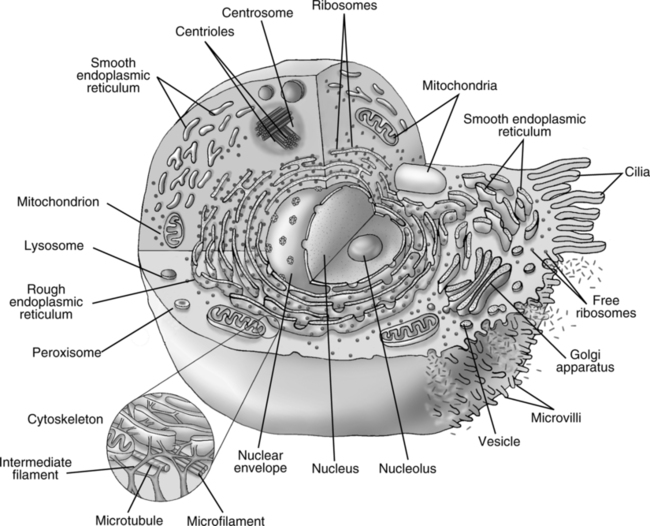
1. Cell membrane: Complex lipoprotein structure.
2. Cell wall: Rigid to moderately rigid polysaccharide structure.
1. Endoplasmic reticulum: System of large sacs and smaller tubules responsible for macromolecular transport.
a. Smooth ER (without attached ribosomes): Involved in lipid and steroid synthesis.
b. Rough ER (with attached ribosomes): Involved in protein synthesis.
2. Mitochondria: Responsible for production of adenosine triphosphate and aerobic metabolism (Krebs’ cycle and electron transport chain); seen in animal cells.
3. Chloroplasts: Contain pigments, starches, and enzymes used in photosynthesis; seen in plant cells.
4. Ribosomes: Free or attached to ER; responsible for protein synthesis.
5. Lysosomes: Contain proteolytic enzymes for metabolism of ingested organic material.
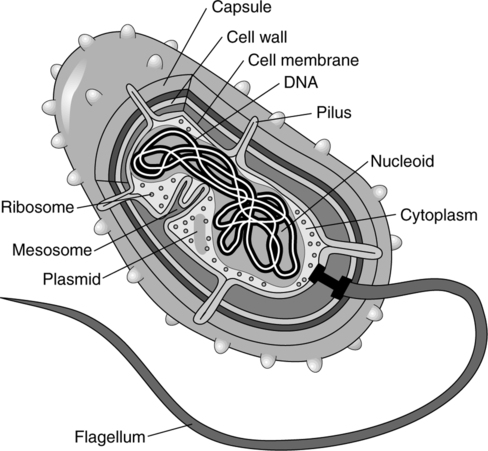
III Prokaryotic Cell Structure (Figure 3-2)
1. Cell membrane or plasma membrane
b. The inner layer beneath the cell wall.
c. Acts as osmotic barrier and is the site of some enzyme activity.
2. Cell wall: Moderately rigid to very rigid structure
3. Cell wall in gram-positive bacteria
b. Murein network: Peptide chains attached to larger polysaccharide chains
c. Stains violet on Gram stain because of peptidoglycan in the cell wall
4. Cell wall in gram-negative bacteria: three layers
a. The inner layer is a mucopeptide.
b. The middle layer is lipopolysaccharide.
c. The outer layer is a lipoprotein.
d. Stains pink on Gram stain with counter-safranin stain because of lack of peptidoglycan.
1. No distinct nucleus; no separation from the cytoplasm.
2. The cell may contain one or more regions of nuclear material called nucleoids.
4. Chromosomes exist freely in cytoplasm; may be circular or attached to the cell membrane.
2. Mitochondria are absent. Aerobic metabolic enzymes are present in the form of multienzyme complexes; they are attached to the cell membrane or other internal membranes.
3. Chloroplasts are absent. Photosynthetic enzymes and pigments are present in special arrangements; these are not separated from the cytoplasm by a membrane.
4. Ribosomes: Slightly smaller than eukaryotic ribosomes; exist freely in cytoplasm.
5. Mesosome: They are found in the cell membrane and allow for attachment of DNA in cell division. They are found chiefly in gram-positive forms.
1. They are the intermediate form of the organism that develops in response to adverse environmental conditions.
2. They will regenerate to a vegetative cell when conditions improve.
3. They are notably produced by the aerobic genus Bacillus and the anaerobic genus Clostridium, and Sporosarcina.
4. They resist adverse environmental conditions of dryness, heat, and poor nutrition.
5. A true spore is a highly refractile body formed within the vegetative bacterial cell.
6. They function as a protective coat around nucleic material that may remain inside the cell (endospore) or extend beyond the width of the cell (exospore).
7. They are metabolically active and contain essential enzymes of Krebs’ cycle.
IV Bacterial Growth Requirements
A Growth medium: Needs vary with specific bacteria.
1. Simple nutrients: Water, carbon, hydrogen, nitrogen, oxygen, sulfur, phosphorus, calcium, potassium, and magnesium
2. Complex nutrients: Sugar, amino acids, and blood products
B Atmospheric gas requirements
1. Obligate anaerobes reproduce only in an oxygen-free environment. Oxygen is toxic to these organisms.
2. Aerotolerant anaerobes are organisms unaffected by exposure to oxygen.
3. Facultative anaerobes reproduce under aerobic or anaerobic conditions.
4. Microaerophilic anaerobes reproduce best at low oxygen tensions. High oxygen tensions are inhibitory.
5. Obligate aerobes require oxygen for reproduction.
6. Chemolithotrophic and photolithotrophic bacteria use carbon dioxide as their principal source of carbon.
C Temperature requirements, optimal growth ranges:
1. Psychrophilic: −5° C to 30° C (optimum 10° C to 20° C)
2. Mesophilic (pathogenic): 10° C to 45° C (optimum 20° C to 40° C)
D The osmotic pressure requirement varies with each bacterial species. Most require a 0.9% saline environment.
E Hydrogen ion (pH variations)
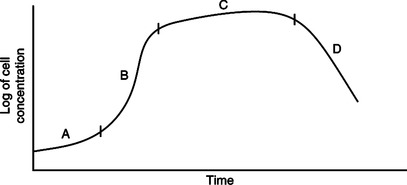
VI Growth Pattern: A new culture of bacteria will develop similar to the growth curve seen in Figure 3-3.
A Lag phase: Adaptation to new environment; little reproduction.
B Exponential phase: Stage of rapid cell growth.
C Stationary phase: Equal death and growth rates.
D Death phase: Depletion of culture nutrients and buildup of toxic waste; death rate exceeds growth rate.
A Autotroph: An organism capable of using simple inorganic matter as nutrients (nonpathogenic).
B Heterotroph: An organism that requires organic matter for growth and survival (pathogenic).
C Symbiosis: Two dissimilar organisms existing together.
1. Amensalism: One organism is inhibited, and the other is not affected.
2. Commensalism: One organism benefits, and the other is not affected.
3. Mutualism: Each organism benefits and is unable to survive without the other.
4. Parasitism: One organism benefits, and the other is harmed.
5. Synnecrosis: Both organisms are harmed by the relationship.
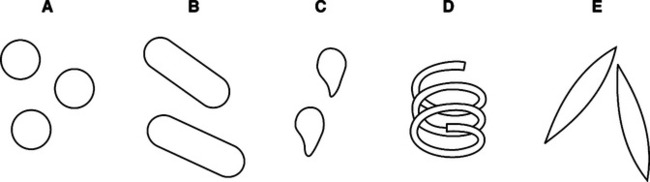
IX Microbial Shapes (Figure 3-4)
A Purpose: Used to identify and categorize bacteria based on cell components.
1. Bacteria can be separated into two general categories by virtue of their staining properties: gram-positive or gram-negative.
2. Variation in staining is determined by cell wall construction.
a. Basic dye is crystal violet (all organisms take up this dye).
b. The specimen is then covered with Gram’s iodine.
c. A water rinse is then performed.
d. The specimen is then decolorized with acetone.
e. A water wash is then performed.
f. The specimen is counterstained with red dye (usually safranin).
XI Definitions Related to Microorganisms
A Contamination: Presence of a microorganism in an otherwise sterile environment.
B Pathogen: Any disease-producing microorganism.
C Virulence: Heightened ability of an organism to produce infection in its host.
D Aerobic: Growth only in the presence of oxygen.
E Anaerobic: Growth in the absence of oxygen.
F Toxins: Poisonous substances produced by bacteria.
G Vegetative cell: Metabolically active form of a bacterium in which reproduction can occur.
H Vector: An insect, animal, or other carrier that transfers an infecting agent or pathogen from one host to another.
I Host: An organism that harbors or furnishes nutrition to a dissimilar organism.
J Bacteremia: The presence of bacteria in the blood.
K Septicemia: A condition in which pathogens and their associated toxins are present in the blood.
L Toxemia: The presence of bacterial toxins in the blood.
N Pyemia: Condition in which pus-forming bacteria have entered the bloodstream.
XII Definitions Related to Immunologic Response
A Infection: An inflammatory process resulting from the presence and growth of a pathogenic organism.
B Inflammation: A tissue response to injury or stress that can cause local vascular dilation, fluid exudation, and/or leukocyte accumulation at the site caused by a microorganism or some other stress.
C Superinfection: Infection developed primarily in the debilitated or immunosuppressed patient previously treated with antibiotics.
D Nosocomial infection: Hospital-acquired infection.
E Immunity: The ability of the body to resist or overcome infection or disease.
F Plasma cell: Cells that specialize in the production of antibodies.
G Eosinophils: White blood cells with two- or three-lobed nucleus and large cytoplasmic granules.
1. They are able to phagocytize antigen-antibody complexes.
2. Increase in number occurs with hypersensitivity and parasitic infections.
H Lymphocyte: White blood cells formed in the lymphatic system and by the thymus gland.
1. Have a single nucleus with no granules.
2. Can become plasma cells that may form antibodies.
3. Can attract and localize macrophages to an area of infection.
I T lymphocytes: Specialized white blood cells formed by the thymus gland.
J Macrophage: Large mononuclear phagocytic cell.
K Monocyte: White blood cell (leukocyte) with a single nucleus (mononuclear) that is capable of phagocytosis.
L Polymorphonucleated leukocyte (neutrophil): White blood cell with a multilobed nucleus capable of phagocytosis.
M Antibody: Developed in response to antigen.
1. Produced by lymphoid tissue in response to an antigen.
2. Antibodies can cause invading antigens or microbes to clump, rendering them easier for ingestion by macrophages and granulocytes.
N Antigen: A substance, often a protein, that gains access to the bloodstream or a body tissue.
A Infection occurs when a pathogen is able to overcome the barriers of a host.
B Three elements must be present for an infection to develop:
C Factors that increase host susceptibility to infection include:
D The high incidence of nosocomial gram-negative bacterial pneumonias is associated with factors that promote colonization of the pharynx (Box 3-1).
E Patients with artificial airways are at highest risk of nosocomial pneumonia.
F Pathogens can be transmitted to a host by five major routes (Table 3-1).
TABLE 3-1
Routes of Infectious Disease Transmission
| Mode | Type | Examples |
| Contact | Direct | Hepatitis A |
| Venereal disease | ||
| HIV | ||
| Staphylococcus | ||
| Enteric bacteria | ||
| Indirect | Pseudomonas aeruginosa | |
| Enteric bacteria | ||
| Hepatitis B and C | ||
| HIV | ||
| Droplet | Haemophilus influenzae (type B) pneumonia and epiglottitis | |
| Neisseria meningitidis pneumonia | ||
| Diphtheria | ||
| Pertussis | ||
| Streptococcal pneumonia | ||
| Influenza | ||
| Mumps | ||
| Rubella | ||
| Adenovirus | ||
| Vehicle | Waterborne | Shigellosis |
| Cholera | ||
| Foodborne | Salmonellosis | |
| Hepatitis A | ||
| Airborne | Aerosols | Legionellosis |
| Droplet nuclei | Tuberculosis | |
| Varicella | ||
| Measles | ||
| Dust | Histoplasmosis | |
| Vectorborne | Ticks and mites | Rickettsia, Lyme disease |
| Mosquitoes | Malaria | |
| Fleas | Bubonic plague |
From Wilkins RL, et al: Egan’s Fundamentals of Respiratory Care, ed 8. St. Louis, Mosby, 2003,
2. Droplet: Contact with large droplets produced by an infected or contaminated individual.
4. Common vehicle: Pathogen transmitted to host in contaminated food or water.
5. Vectorborne: Transmission by an animal from infected individual to host, usually by insects.
XIV Notable Gram-Positive Pathogenic Bacteria
A Bacillus anthracis: Causes skin infections, septicemia, enteritis, meningitis, anthrax, and pneumonia (woolsorter’s disease).
XV Notable Gram-Negative Bacteria (see Box 3-1)
1. Gram-negative aerobe and facultative anaerobe.
2. Rod shaped and found as a single organism.
3. Causes up to 10% of all hospital-acquired infections.
4. Causes pneumonia with characteristic green odoriferous sputum.
5. Causes wound infection, urinary tract infection, empyema, meningitis, and septicemia.
1. Gram-negative aerobe and facultative anaerobe.
2. Rod shaped and found as a single organism.
3. Responsible for approximately 45% of all nosocomial infections.
4. Causes necrotizing pneumonia, septicemia, endocarditis, meningitis, wound infections, and urinary tract infections.
1. Gram-negative aerobe and facultative anaerobe.
2. Short rod and found as a single organism.
4. Causes necrotizing pneumonia with characteristic “red currant jelly” sputum.
1. Gram-negative aerobe and facultative anaerobe.
2. Minute rod found as a single organism.
3. Most common cause of epiglottitis in children.
4. Causes meningitis, laryngitis, croup, and subacute bacterial endocarditis.
1. Gram-negative aerobe and facultative anaerobe.
2. Rod shaped and found as single organism.
5. Transmitted through contaminated water and, less frequently, contaminated food.
1. Found in animals, particularly shellfish, swine, and fowl.
2. Causes enteritis that may progress to meningitis, encephalitis, or nephritis.
3. Transmitted orally via contaminated milk, turtles, eggs, undercooked chicken, fish, clams, and pork.
2. Coccus is found as diplococci with their adjacent sides flattened.
3. Causes meningococcal meningitis, bacteremia, and pneumonia.
J Proteus mirabilis and Proteus vulgaris
1. Gram-negative aerobe and facultative anaerobe.
2. Rod shaped and found as a single organism.
3. Causes chronic urinary tract infections, pneumonia, gastroenteritis, and bacteremia.
K Normal human flora by body site (Table 3-2)
TABLE 3-2
Normal Human Flora by Body Site
| Site | Normal Flora |
| Pharynx and upper gastrointestinal tract | Moraxella catarrhalis; Staphylococcus epidermidis and Staphylococcus aureus; α-hemolytic streptococci; viridans-group streptococci; Streptococcus pneumoniae; Peptostreptococcus, Lactobacillus, and Fusobacterium species; Actinomyces israelii; Haemophilus influenzae and Haemophilus parainfluenzae; Corynebacterium species; Neisseria meningitidis, Bacteroides species and other anaerobes; and Candida (yeast) species. |
| Colon | Enterococcus species; Escherichia coli; Pseudomonas, Bacteroides, and Clostridium species and other gram-negatives and anaerobes; also Candida (yeast) species; organisms known as coliforms or enteric bacteria because of their location in the colon. |
| Skin | Staphylococcus epidermidis and Staphylococcus aureus; streptococci; Corynebacterium species; Clostridium perfringens; Propionibacterium acnes; and Candida (yeast) species. |
| Lower respiratory tract | Essentially sterile; possibility of colonization when illness or structural lung disease compromises immune function. |
From Hess D, et al: Respiratory Care: Principles and Practice. Philadelphia, WB Saunders, 2002.
L Common causes of community, hospital, and nosocomial pneumonias are listed in Box 3-2.
XVI Mycobacterium: Genus Characteristics
A Consists of acid-fast, gram-positive, aerobic rods.
B Inert forms are found singly; virulent strains are found in “cords”; two chains in a side-by-side parallel arrangement.
C Identified by acid-fast staining and immunofluorescence staining.
1. Very slow growing organisms, requiring 3 to 6 weeks for culturing.
2. Reaction time in newly infected individuals 3 to 6 weeks for a positive skin test.
3. Causes pulmonary tuberculosis, spinal tuberculosis, and miliary tuberculosis.
4. Transmitted through inhalation of droplet nuclei.
5. Causes a necrotizing lesion with caseating center.
6. Treatment is long-term therapy (6 to 12 months) with a combination of various agents.
F Mycobacterium avium complex (MAC) and Mycobacterium avium intracellulare (MAI)
1. Gram-negative motile rods (difficult to stain).
2. Can survive as long as 139 days at room temperature in distilled water.
3. Can survive ≥1 year in tap water.
4. Growth can occur in tap water.
5. Found in air-conditioning cooling towers and evaporative condensers.
a. Causes 10% to 30% of hospital-acquired pneumonias.
b. Causes epidemic pneumonia, sporadic pneumonia, and a mild upper respiratory illness called “Pontiac fever.”
c. Legionnaire’s disease varies from mild pneumonia to adult respiratory distress syndrome.
d. Incubation is 2 to 10 days.
e. There is rapid onset of high fever, nonproductive cough, chills, headache, myalgias, and diarrhea.
f. A productive cough follows in 3 to 4 days, with patchy or segmental alveolar infiltrates usually in one lobe.
g. May be accompanied by pleural effusion, empyema, pneumothorax, hyponatremia, and respiratory failure.
A A true bacterium but is an obligate intracellular organism.
B Gram-positive but requires special staining techniques for identification.
C It is a pleomorphic rod or coccus.
D Occurs singly, paired, chained, or in filaments.
E Diseases have clinical findings of fever, headaches, malaise, and rash.
C An obligate intracellular parasite.
D Chlamydia pneumoniae is responsible for some atypical pneumonias.
a. Simple virus: Protein coat.
b. Complex virus: Protein coat with some polysaccharides and lipids present (lipoprotein membrane).
2. Nucleus: No nucleus; single strand of DNA or RNA present.
1. Do not stain by conventional means.
2. Come in a variety of shapes and forms, all of which are small (maximum diameters, 0.02 to 0.3 μm).
3. Obligate intracellular parasite.
4. Replication by diverting host metabolism to produce new viruses.
C Table 3-3 lists viruses important in human respiratory disease.
TABLE 3-3
Viruses Important in Human Respiratory Disease
| Virus | Resulting Disease |
| Rhinoviruses, adenoviruses, coronaviruses | URI; “common cold” |
| Herpesviruses | Diverse important diseases |
| Herpes simplex virus (HSV) | Herpetic skin lesions; infection of the lungs and brain, causing pneumonia and encephalitis |
| Varicella zoster virus (VZV) | Chickenpox and shingles, both of which may involve the lung and central nervous system |
| Cytomegalovirus (CMV) | Systemic infection, including pneumonia, usually in immunocompromised individuals |
| Epstein-Barr virus (EBV) | Infectious mononucleosis (“mono”) |
| Retroviruses (include HIV) | Diverse respiratory manifestations resulting from HIV |
| Flaviviruses (include yellow fever and dengue viruses) | Yellow fever and dengue, diseases common in Central and South America |
| Orthomyxoviruses | Influenza |
| Paramyxoviruses | Measles, mumps, parainfluenza |
| Respiratory syncytial virus (RSV) | Bronchiolitis in infants; milder disease in children and adults |
| Togaviruses | Diverse illnesses, including rubella |
| Coronavirus | Severe acute respiratory syndrome (SARS) |
From Hess D, et al: Respiratory Care: Principles and Practice. Philadelphia, WB Saunders, 2002.
A These are primarily decomposers of dead and decaying matter (saprophytes).
1. Occasionally they result in a pulmonary infection or pneumonia.
2. Usually they are acquired through inhalation of the spore form.
3. Some parasitic fungi can derive their food directly from living plants or animals.
4. Diseases in humans are usually restricted to the skin or mucous membranes (e.g., lung, pneumonia).
2. Tubular strands of single cells: More complex forms; hyphae, or series of branching filaments, that form mycelium.
C Table 3-4 lists important fungal respiratory pathogens in normal hosts.
TABLE 3-4
Important Fungal Respiratory Pathogens in Normal Hosts
| Organism | Disease | Comments |
| Coccidioides immitis | Coccidioidomycosis | The organism is commonly found in the arid regions of the southwest United States (e.g., Arizona and central California), and the disease causes valley fever, with high fever and bilateral pneumonia and may later form thin-walled pulmonary cavities. |
| Histoplasma capsulatum | Histoplasmosis | The organism is commonly found along the Mississippi and Ohio river valleys, and inhaled Histoplasma inoculum may cause acute fever and pneumonia. Some patients develop disseminated infection, often causing skin lesions; in rare cases, fibrosis of the mediastinum may result. Most residents of endemic areas have had asymptomatic infection, causing elevated antibody titers, and often one or more calcified granulomas visible on chest x-ray film. |
| Blastomyces dermatitidis | Blastomycosis | The organism is common in the southern United States, and the disease varies from mild fever and pulmonary infiltrates to severe illness, nodular pulmonary infiltrates, and dissemination. |
| Paracoccidioides brasiliensis | Paracoccidioidomycosis | Occurring in Central America, this clinical disease is similar to mild coccidioidomycosis. |
From Hess D, et al: Respiratory Care: Principles and Practice. Philadelphia, WB Saunders, 2002.
D Table 3-5 lists opportunistic fungal respiratory pathogens.
TABLE 3-5
Opportunistic Fungal Respiratory Pathogens
| Organism | Disease | Comments |
| Candida albicans | Candidiasis (e.g., thrush, esophagitis, and intertrigo | Organism is commonly found in infants and the elderly and also in HIV-infected and critically ill patients. Thrush also may be precipitated by inhaled steroid deposition in the mouth. True candidal pneumonia is rare. |
| Aspergillus species | Aspergillosis | Disease causes otitis externa in normal hosts; may infect skin, sinuses, or lung of immunocompromised individuals; and can disseminate, with extremely high mortality. Preexisting lung cavities from tuberculosis or emphysema are particularly prone to infection, with formation of a “fungus ball” inside. |
| Cryptococcus neoformans | Cryptococcosis | Organism is found in kitten feces and causes pneumonia and meningitis, a feared complication of AIDS. |
| Mucor and Rhizopus species | Mucormycosis | Organism can infect the sinuses, lungs, or gut, forming a black eschar. Treatment is difficult, often requiring surgical debridement. |
HIV, Human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome.
From Hess D, et al: Respiratory Care: Principles and Practice. Philadelphia, WB Saunders, 2002.
1. Widely distributed in animals in nature but is normally not pathogenic.
2. Rarely problematic in healthy people.
a. In most individuals it may be dormant, with no host damage.
b. Seventy percent of healthy subjects have humoral antibody to the organism.
3. An extracellular opportunist that can cause a diffuse pneumonia.
4. Assumed mode of transmission is by inhalation.
5. In an immunocompromised host, the organism occurs in massive numbers; a common infection in patients with HIV.
6. Usually there is panlobular involvement in the lungs.
7. Clinical onset is usually abrupt, with fever, tachypnea, hypoxia, cyanosis, and asphyxia in the acute stage.
XXIII Definitions Related to Disinfection and Sterilization
1. –cide: When a killing action is applied to a microorganism.
2. –statis or –static: When the organism is only inhibited in growth or prevented from reproducing.
B Bactericide (bactericidal): Kills or destroys bacteria.
C Fungicide (fungicidal): Kills or destroys fungi.
D Virucide (virucidal): Kills or destroys viruses.
E Tuberculocide (tuberculocidal): Kills or destroys Mycobacterium tuberculosis and related mycobacterium.
F Sporicidal: Killing of bacterial spores.
G Germicide: Chemical agent that kills vegetative cells of microorganisms.
H Bacteriostatic: Inhibits or retards growth of bacteria.
I Antiseptic: Opposes sepsis or putrefaction either by killing microorganisms or by preventing their growth; free from living organisms.
J Antisepsis: Preventing the growth of bacteria or stopping bacterial activity.
K Medical asepsis: Killing or inhibiting the growth of pathogenic microorganisms to prevent their transmission from one person to another.
L Surgical asepsis: Sterilization or decontamination of items used in the operating room.
M Antiseptic: Free from living microorganisms; also an agent that destroys or inhibits the growth of microorganisms.
N Cleaning: The removal of all foreign matter such as sputum, blood, dirt, or organic matter from an item that may provide a favorable environment for bacterial growth; precedes sterilization.
O Disinfectant: Germicidal agent used on inanimate objects.
P Sanitizer: An agent that reduces the number of bacteria to a safer level for handling of material.
Q Sterilization: Complete destruction or inactivation of all forms of microorganisms.
R Disinfection: A process that eliminates vegetative, pathogenic microorganisms on inanimate objects.
S High-level disinfectants: Germicidal agents capable of killing all microorganisms except their spores.
T Intermediate-level disinfectants: Germicidal agents capable of killing all gram-negative bacteria and fungi but have variable activity against spores and certain viruses.
U Low-level disinfectants: Germicidal agents capable of killing some, but not all, vegetative bacteria, fungi, and lipophilic viruses.
V Decontamination: The process of removing a contaminant by chemical or physical means.
W Sanitization: Any process that reduces total bacterial contamination to a level consistent with safety in handling.
X Semicritical items: Objects that come in contact with mucous membranes but that do not enter tissue or the vascular system.
Y Noncritical items: Objects that do not come in contact with mucous membranes or skin that is not intact.
Z Critical items: Devices introduced in the bloodstream or other parts of the body.
XXIV Dynamics of Disinfection and Sterilization
A Selection of the procedure is determined by the situation.
1. Items used for surgery, intravascularly, or within tissues must be sterile.
2. Media and glassware for the microbiology laboratory must be sterile.
3. Items not in contact with mucous membranes or that touch only intact skin may only need to be disinfected.
1. Criterion of death is the irreversible loss of the ability to reproduce.
2. Exposure of a bacterial population to a lethal agent results in a time interval in which there is a progressive reduction in the number of survivors.
a. This reduction may be logarithmic with time when the sterilizing agent is strong.
b. This reduction may be sigmoidal, the rate being slower at the beginning, if the agent is less potent.
3. The larger the initial number of cells to be killed, the longer and more intense the required treatment for sterilization.
4. The rate of disinfection is related to the concentration of the disinfecting agent.
C Factors affecting the potency of disinfectants
1. Concentration and type of agent and organism involved.
3. Hydrogen ion concentration, which affects bactericidal action.
1. Killing action increases with temperature.
2. At low temperatures, a 10° C increase doubles the death rate.
1. Species and presence of special structures such as capsules or spores.
F Presence of extraneous material such as organic or foreign matter
XXV Processing of Equipment for Disinfection or Sterilization
A Cleaning the equipment: Removal of dirt and organic material is the first step in processing equipment.
1. Should take place in a designated facility.
2. Clean and dirty equipment should always be separated.
3. Before cleaning, all equipment should be dissembled and parts examined for function.
4. Detergents should be used to clean equipment.
5. Equipment that cannot be immersed in water should have its surface disinfected with 70% ethyl alcohol or equivalent solution.
B After cleaning, equipment should be completely dried.
1. Water dilutes and alters the pH of chemical disinfectants.
2. Water combines with ethylene oxide to form ethylene glycol, which is toxic to tissue.
C After drying, equipment should be reassembled and packed appropriately when indicated.
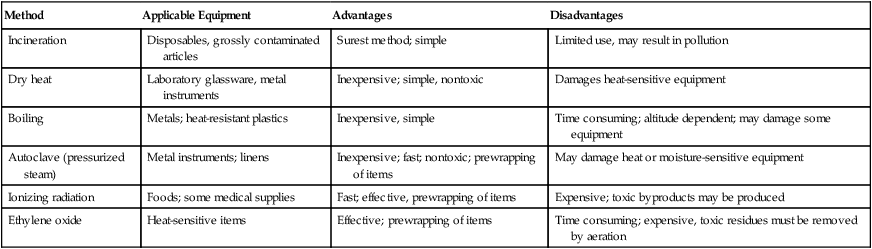
XXVI Sterilization and Disinfection by Temperature Change (Table 3-6)
TABLE 3-6
Comparison of Sterilization Methods
| Method | Applicable Equipment | Advantages | Disadvantages |
| Incineration | Disposables, grossly contaminated articles | Surest method; simple | Limited use, may result in pollution |
| Dry heat | Laboratory glassware, metal instruments | Inexpensive; simple, nontoxic | Damages heat-sensitive equipment |
| Boiling | Metals; heat-resistant plastics | Inexpensive, simple | Time consuming; altitude dependent; may damage some equipment |
| Autoclave (pressurized steam) | Metal instruments; linens | Inexpensive; fast; nontoxic; prewrapping of items | May damage heat or moisture-sensitive equipment |
| Ionizing radiation | Foods; some medical supplies | Fast; effective, prewrapping of items | Expensive; toxic byproducts may be produced |
| Ethylene oxide | Heat-sensitive items | Effective; prewrapping of items | Time consuming; expensive, toxic residues must be removed by aeration |
From Wilkins RL, et al: Egan’s Fundamentals of Respiratory Care, ed 8. St. Louis, Mosby, 2003.
A Heat is the most reliable and universally applied method of sterilization.
1. The time required is inversely related to temperature.
2. Heat causes denaturation of proteins and coagulation.
3. The efficiency of heat sterilization is determined by the heat capacity of the gas involved in the sterilization process.
4. The heat capacity of water at any temperature significantly exceeds the heat capacity of air.
5. Steam has a heat capacity many times greater than that of water at the same temperature because of the latent heat of vaporization of water molecules.
6. The heat capacity of steam increases logarithmically with increasing pressure.
7. Order of efficiency of sterilization and disinfection by heat
1. Steam and pressure are used to produce the most efficient method of sterilization.
2. Sterilization occurs as a result of heat transfer from the condensation and evaporation of steam on the surface of the substance being sterilized.
3. Equipment is packaged in material that allows steam to enter but prevents microorganisms from entering.
4. Variables involved in proper autoclaving
5. Holding time is the minimum amount of time necessary to kill spores at a specific pressure.
6. The actual sterilization time is 1.5 times the holding time.
7. Examples of autoclaving cycles
a. 121° C at 15 pounds per square inch (psi) pressure for 15 minutes
8. Heat-sensitive indicators are applied to all equipment to be autoclaved.
9. Biologic indicators are used to ensure that actual sterilization has been accomplished.
a. Bacillus stearothermophilus spores are normally used because of their high heat resistance.
b. Capsules containing 106 spores should be placed in at least one load each day and inspected for viable cells.
10. Oil and grease must be removed from items before processing.
11. Autoclaving is not effective for substances that cannot be penetrated by steam, such as paraffin or oil.
12. It may melt some plastics and rubbers.
13. It may be corrosive to some metals.
14. Many types of respiratory care equipment cannot be autoclaved.
1. Efficiency is considerably lower than steam heat.
2. In general, dry heat should be used only on materials in which moist heat would be deleterious or unable to permeate the product being sterilized.
3. Temperature-time relationships
4. Dry heat is limited primarily to the sterilization of glassware and materials such as oils, powders, jellies, glass, dressings, and cutting instruments that cannot be penetrated by steam or may be damaged by the moisture.
5. The lethal action is the result of heat conveyed from the material with which the organisms are in contact.
6. It is important that heating is uniform.
7. The most widely used type is the hot air oven.
8. Another form includes incineration of disposable objects.
1. This is a fractional method of sterilization that consists of three separate heatings on three consecutive days of a liquid or semisolid to be sterilized.
2. For the sterilization of certain liquids or semisolids that are easily destroyed by heat, this provides a fractional method of sterilization.
3. The material is heated at 80° C to 100° C for 30 minutes on three consecutive days.
4. The rationale is that vegetative cells and some spores are killed during the first heating. More resistant spores subsequently germinate and are killed on either the second or third heating.
5. It is used for sterilizing heat-sensitive culture media such as serum or egg.
1. Pasteurization is the submergence of equipment in medium hot water for a specified period.
2. It is effective only in the destruction of vegetative cells and most viruses, including HIV.
3. Pasteurization is useful for respiratory care equipment because spore-forming bacteria are not associated with nosocomial pneumonia.
4. Equipment is immersed for 30 minutes in water that is 70° C.
5. When the equipment is removed from the pasteurization unit, it must be dried and packaged in a sterile manner to avoid contamination.
XXVII High-Energy Waves for Sterilization and Disinfection
A Sunlight possesses some bactericidal activity.
1. UV rays cause the disinfectant action.
2. Most rays are screened by glass or ozone in the atmosphere.
3. UV light is absorbed by bacteria and damages DNA.
4. This effect is reversible if cells are immediately irradiated with visible light (photoreactivation).
5. Mercury vapor lamps produce UV radiation.
6. It is used primarily in control of airborne infection for disinfection of enclosed areas such as operating rooms.
B Ionizing radiation (see Table 3-6)
1. Ionizing rays collide with orbiting electrons, causing electron ejection. As the ejected electrons move through a medium, the electrons ionize, exciting other atoms.
2. This causes ionization of water molecules, which form free hydroxl and hydrogen ions. These then react with DNA molecules, causing lysis.
3. Spores are the most radiation-resistant microorganism known but can be destroyed by radiation.
1. Gamma waves are very short wavelength light waves possessing extremely high energy and having the ability to ionize a substance.
2. Ionization of water molecules inactivates DNA molecules by increasing the rate of reaction of DNA with hydrogen and hydroxyl ions.
A These are widely used disinfectant and sterilizing agents for surgical instruments and respiratory therapy equipment.
B They are a form of cold, liquid disinfectant that kills by the binding to SH or amino groups of proteins in microorganisms, interrupting metabolism and reproduction.
C Protein-containing materials do not decrease effectiveness.
D They are most commonly used in respiratory care for disinfection of noncritical items.
E Material must be washed and all foreign matter removed before soaking in this liquid to expose all surfaces.
F All surfaces must be in contact with the agent for it to be effective.
G A variety of glutaraldehydes are available on the market.
H In general, they are bactericidal, tuberculocidal, fungicidal, and virucidal in 10 to 30 minutes and sporicidal in approximately 10 hours.
I Shelf life varies from approximately 2 to 4 weeks.
J Glutaraldehydes in general are irritating to skin, mucous membranes, and eyes; are damaging to some rubbers and plastics; and are corrosive to carbon steel instruments.
K Items must be rinsed thoroughly after use, dried, and packaged in a sterile or clean manner.
A An alkylating agent extensively used in gas sterilization.
B Characteristics of ethylene oxide (ETO).
1. It is a gas at room temperature but liquefies readily under moderate pressure.
2. It has a pleasant ethereal odor.
3. It causes irritation to tissues, especially the mucous membranes.
4. It is flammable and explosive at certain concentrations and temperatures.
5. Normally it is used in 10% to 12% mixtures with carbon dioxide or halogenated hydrocarbons (e.g., dichlorodifluoromethane [Freon 12], which acts as a damping agent), making up the remainder of the gas mixture. These mixtures are nonflammable at temperatures up to 55° C.
1. Alkylation occurs at specific enzyme sites and interrupts normal metabolism and reproduction.
2. Coordination of the following factors is necessary for proper sterilization:
3. The addition of H2O vapor increases sensitivity of vegetative cells and spores to ETO.
4. Sterilization proceeds most rapidly at relative humidity of 30% and slows progressively below or above that level.
5. Other factors being equal, the effectiveness of ETO doubles for each 10° C increase in temperature up to 60° C.
6. System pressure is between 20 and 30 psi for carbon dioxide mixtures and between 5 and 7 psi for hydrocarbon mixtures.
a. Temperature between 50° C and 60° C, relative humidity of 30% to 60%, and pressure of 5 to 7 psi with 450 mg of ETO/L of air for 1.5 to 6 hours results in sterilization.
b. If the concentration of ETO is increased to 850 mg/L, sterilization time is decreased.
8. The packaging material used should be permeable to humidity and ETO but not to microorganisms.
9. The use of indicator tape identifies exposure to ETO but does not guarantee sterility of equipment.
10. A biologic indicator is used to ensure that conditions necessary for sterility have been achieved. Cultures of Bacillus subtilis var. globigi should be used daily.
a. Most materials require at least a 24-hour aeration time in a well-ventilated area (aeration chambers can significantly decrease this time) at room temperature.
b. At a temperature of 50° C aeration time is decreased to 12 hours and at 60° C to 8 hours.
a. Substances that have been previously gamma irradiated, especially PVC, react with ETO to form ethylene chlorhydrin, which is irritating to tissue.
b. If the material to be sterilized is not dry, the water on it reacts with ETO to form ethylene glycol. (This usually results in a sticky residue on the material.)
13. The ETO residues are mutagenic for bacteria.
14. Toxicity to humans includes mutagenicity and carcinogenicity.
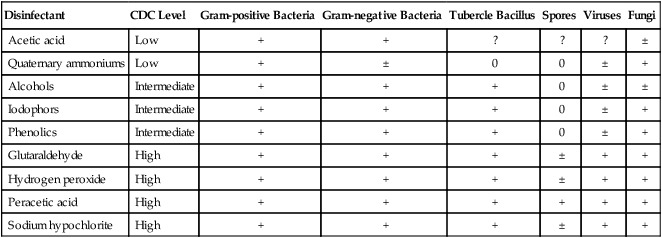
XXX Liquid Disinfectants (Table 3-7)
TABLE 3-7
| Disinfectant | CDC Level | Gram-positive Bacteria | Gram-negative Bacteria | Tubercle Bacillus | Spores | Viruses | Fungi |
| Acetic acid | Low | + | + | ? | ? | ? | ± |
| Quaternary ammoniums | Low | + | ± | 0 | 0 | ± | + |
| Alcohols | Intermediate | + | + | + | 0 | ± | ± |
| Iodophors | Intermediate | + | + | + | 0 | ± | + |
| Phenolics | Intermediate | + | + | + | 0 | ± | + |
| Glutaraldehyde | High | + | + | + | ± | + | + |
| Hydrogen peroxide | High | + | + | + | ± | + | + |
| Peracetic acid | High | + | + | + | + | + | + |
| Sodium hypochlorite | High | + | + | + | ± | + | + |
From Wilkins RL, et al: Egan’s Fundamentals of Respiratory Care, ed 8. St. Louis, Mosby, 2003.
1. Alcohol disorganizes the lipid structure of membranes and also denatures cellular proteins.
2. It is used extensively for skin cleaning before cutaneous injections and is also used for disinfection of thermometers.
3. It is active against gram-positive, gram-negative, and acid-fast bacteria.
4. It is only slightly irritating, leaves no residue, removes fats and lipids from skin surfaces, and is inexpensive.
5. Alcohols are not sporicidal or virucidal to all viruses.
a. Ethanol has been shown to be effective against HIV.
b. Neither ethanol nor isopropanol is effective against tubercle bacillus in sputum.
6. Alcohol is a volatile and a powerful organic solvent that may damage rubber and plastic materials.
7. Ethanol (ethyl alcohol) is used at 70% concentration.
8. Isopropyl alcohol is used at 90% concentration.
9. Toxic effects of isopropanol are greater and longer lasting than ethanol if ingested or inhaled in large quantities.
10. Sometimes alcohol is used as part of the composition of other disinfecting agents such as Lysol spray (ethanol).
B Quaternary ammonium compounds
1. These are surface-acting, cationic agents containing the ammonium ion that cause a loss of membrane semipermeability and leakage of nitrogen- and phosphorus-containing compounds from the cell.
a. Lysis of the cell is followed by the action of the cell’s own autolytic enzymes.
b. The agent may then enter the cell and denature its proteins.
2. Activity is greatest at an alkaline pH.
3. These compounds are bactericidal for a wide range of organisms, but gram-positive species are more susceptible.
4. Antibacterial activity is reduced by the presence of organic matter.
6. Ineffective against tuberculosis bacillus spores, enteroviruses, hepatitis B, bacterial spores, or some fungi and notably are not very pseudomonocidal.
1. Acetic acid has long been known as a preservative.
2. It inhibits the growth of many bacteria and fungi.
3. Antimicrobial activity is related to its acidity.
a. Acidotic pH prevents organisms from maintaining a normal pH balance as excessive hydrogen ions enter the cell and cannot be expelled rapidly enough.
b. It can result in denaturation of cellular proteins.
c. It has an indirect effect because of ionization of organic compounds in the medium that permits them to penetrate the cell more rapidly and disrupt cell metabolism.
4. A 1.25% acetic acid solution seems to be sufficiently effective to disinfect equipment; one part vinegar (5% acetic acid) and three parts water.
5. Used extensively in the cleaning of respiratory care equipment, such as hand-held nebulizers, in the home.
6. Effectiveness is significantly reduced if a solution is reused.
1. They cause leakage of cell contents and irreversible inactivation of membrane-bound enzymes.
2. Cresols are simple alkyl phenols obtained industrially from the distillation of coal tar.
3. The three primary types are orthocresol, metacresol, and paracresol (usually used as a mixture of tricresol).
4. Examples are Lysol and Creolin, which are emulsified mixtures of cresols and green soap.
E Chlorine and related compounds
1. Like iodine, they are strong oxidizing agents that inactivate enzymes.
2. In addition to chlorine compounds, the hypochlorites and the inorganic and organic chloramines are also oxidizing disinfectants.
3. They are effective against most bacteria, viruses, and fungi.
4. They are not sporicidal at room temperatures.
5. They have no effect in the presence of organic matter.
6. They are highly corrosive to metals and cannot be used with rubber.
7. Chlorine is used to purify the water supply and is widely used in swimming pools (0.6 to 1.0 ppm).
8. Hypochlorites are widely used in the food and dairy industries for sanitization.
a. They are also used as sanitizers in hospitals, households, and public buildings.
b. A 0.2% solution of sodium hypochlorite has been recommended by the Pasteur Institute for use on inanimate objects for inactivation of HIV with a 10-minute contact time.
9. Commercial household bleach is usually bottled as a 5.25% hypochloride solution (storage reduces percentage and effectiveness).
10. A 1:50 dilution of household bleach is able to kill gram-negative bacteria, bacterial spores, and M. tuberculosis in 10 minutes.
11. The Centers for Disease Control and Prevention recommends a 1:10 dilution of bleach to clean blood spills after the area is cleaned of gross organic matter.
1. This is a strong oxidizing agent similar to iodine and chlorine in bacterial activity.
2. The reaction of peroxide with iron may produce additional free hydroxide radicals that may account for part of its germicidal activity.
3. A 3% solution is used as a mild antiseptic for wound cleaning.
4. At room temperature a 6% solution is bactericidal, fungicidal, and virucidal in 10 minutes.
5. A 6% solution is sporicidal in 6 hours.
6. Used increasingly in recent years as a disinfectant of medical-surgical devices and soft plastic contact lenses.
7. It is also used for cleaning tracheostomy tubes and the incision site.
A To avoid contamination of yourself and cross-contamination of patients, the Centers for Disease Control and Prevention has outlined specific precautions that should be taken with all patient contact and specific precautions that should be exercised when certain infections are present.
B Standard precautions: Should be used wherever there is a probability that a caregiver will be exposed to blood, body fluids, or other excretions from any patient or contaminated medical equipment.
1. The most important aspect of disease transmission prevention is handwashing.
a. Hands should be disinfected with an alcohol-based hand rinse.
(1) Before entering a patient’s room.
(2) After interaction with a patient.
(3) After removal of any protective equipment, gloves, gowns, or mask.
b. If hands are visibly soiled they should be vigorously washed with soap and water before applying the alcohol-based disinfectant.
a. Clean, nonsterile gloves must be worn when touching blood, body fluids, secretions, mucous membranes, and contaminated medical equipment.
b. Remove gloves after use and before touching any noncontaminated object.
a. Clean, nonsterile gowns must be worn to protect skin and to prevent soiling of clothing during activities that may generate splashes or spray of blood, body fluids, secretions, or excretions.
4. Mask and eye protection or face shield should be worn to protect mucous membranes of the eyes, nose, and mouth during activities that could result in splashes of blood, body fluids, secretions, or excretions.
1. Required in treatment of patients where transmission of pathogen occurs by body-to-body contact.
2. See Box 3-3 for a listing of diseases requiring contact precautions.
4. Gowns for direct contact with patient, environmental surfaces, or equipment in room.
5. Private room required or patients need to be cohorted by infection.
6. Equipment should be dedicated to the single patient for length of stay.
7. If equipment is removed from room it must be properly disinfected.
1. Required in treatment of patients where transmission of pathogen occurs by droplet nuclei or dust particles.
2. Respiratory protection is critical; an N-95/HEPA-filtered mask must be worn when entering the room.
3. See Box 3-4 for a list of diseases requiring airborne precautions.
4. If immune to chickenpox and measles, an N-95 mask is unnecessary.
5. Patient should be placed in a negative pressure isolation room. Door must remain closed.
1. Surgical mask when within 3 ft of patient.
2. See Box 3-5 for a list of diseases requiring droplet precautions.
3. Private room required or cohort patients by disease.
XXXII Equipment-Related Infection Control Issues (Table 3-8)
TABLE 3-8
Frequency of Respiratory Care Equipment Change for Infection Control Purposes
| Equipment | Change Frequency |
| Ventilator circuits and humidifiers | Most frequent every 7 days |
| Least frequent when equipment malfunctions | |
| Inline suction catheter | When ventilator circuit changed |
| When system malfunctions | |
| Heat and moisture exchangers | Every 48-96 hours |
| Nebulizers | Every 24 hours |
| Oxygen therapy equipment | Between patients or if malfunctioning |
| Ventilator | Surface disinfection between patients |
| Pulmonary function equipment | Circuits, mouthpieces, etc. between patients |
| External surfaces no recommended cleaning frequency | |
| Manual ventilators (Ambu Bags) | Weekly |
From Infection Control Manual, Massachusetts General Hospital, Boston, MA, 2004. Massachusetts General Hospital
A Ventilator circuits and humidifiers
1. Ventilator circuits and their condensate have been implicated for decades in the development of ventilator-associated pneumonia (VAP).
2. However, recent data clearly indicate that VAP is primarily associated with aspiration of oral secretions and gastric contents.
a. Steps should always be taken to ensure that cuffs on airways are properly inflated, but this cannot prevent the small silent aspiration that continually occurs via folds in inflated airway cuffs during mechanical ventilation.
b. Ideally, the head of the bed should be elevated 30 degrees to prevent regurgitation of gastric fluid.
c. Some also recommend the use of pharmacologic agents to decontaminate the upper digestive tract of gram-negative bacteria and Candida species, but this is still controversial.
d. Good oral hygiene and the removal of secretions above the cuff assist in minimizing aspiration.
3. American Association for Respiratory Care practice guidelines recommend that ventilator circuits be changed no more frequently than every 7 days.
4. Good evidence indicates that circuits need not be changed at all provided they are functional.
5. Ideally, the circuit should not be disconnected from the patient once attached.
6. Inline suction catheters eliminate contamination of the circuit by repeated removal for suctioning.
7. Properly maintained inline catheters need only be changed when ventilator circuits are changed.
B Ventilator circuit condensate
1. Normally, within hours the condensate of ventilator circuits is contaminated.
2. However, this contaminate is from the patient’s airway provided appropriate precautions are taken while handling the circuit.
3. Condensate is an infectious hazard to caregivers, but it should not be a hazard to the patient.
4. Careful regular drainage of condensate away from the patient should occur.
1. Current data indicate that they can be changed as infrequently as every 48 to 96 hours without an increased risk of nosocomial pneumonia.
1. Because no aerosol is produced during humidification of delivered oxygen if appropriate precautions are taken, equipment only needs to be changed between patients or if equipment fails.
1. The outside surface of the ventilator should be cleaned periodically while in use.
2. Disinfection should always occur between patients.
3. The use of a filter at the ventilator on the exhalation side can prevent internal contamination of the ventilator. No data indicate that the ventilator itself is a cause of nosocomial pneumonia.