CHAPTER 13 Methods for Cardiac Magnetic Resonance Imaging
CLINICAL APPLICATIONS
TEMPORAL RESOLUTION AND SPATIAL RESOLUTION
Because the heart is in constant motion, the duration of image acquisition (temporal resolution) must be sufficiently brief (<100 ms) to avoid motion blurring artifacts. Single-shot MRI techniques that acquire all necessary k-space data after a single radiofrequency (RF) excitation, such as echo planar imaging (EPI),1–3 single-shot fast spin-echo (FSE),4 or spiral imaging methods,5,6 yield images with insufficient spatial resolution, insufficient signal-to-noise ratio (SNR), or too long an image acquisition window to image the beating heart properly. The necessary MRI (i.e., k-space) data required to reconstruct the image must be acquired in segmented fashion across multiple cardiac cycles.7 These segmented k-space acquisition techniques incorporate the repeated acquisition of MRI k-space data over a small temporal window in the cardiac R–R interval, after ECG triggering. The acquisition is repeated over multiple cardiac cycles until sufficient k-space data are collected to reconstruct an image.
APPLICATION-SPECIFIC CARDIAC MRI METHODS
Ventricular Function: Cine Imaging
For the assessment of ventricular function (e.g., assessment of left ventricular wall motion), images of the heart at multiple time points or phases in the cardiac cycle are acquired. An optimal MRI pulse sequence provides good contrast between the ventricular blood pool and the myocardium, and high spatial and high temporal resolution. Gradient-recalled-echo (GRE) techniques are typically used. A variant of steady-state GRE acquisition, balanced steady-state free precession (SSFP), has gained acceptance as the primary method for cardiac cine imaging at 1.5 T. Gradient-echo imaging is still used at 3.0 T, however, because of the sensitivity of balanced SSFP to off-resonance effects that can cause significant black banding and ghosting image artifacts. The balanced SSFP pulse sequence (depending on MRI scanner manufacturers known as trueFISP, FIESTA, or balanced-FFE) yields the highest image SNR by refocusing all available magnetization at the end of each sequence repetition period (TR).8,9
Images acquired at different cardiac time points are played back in a movie loop to enable the assessment of the contractile function of the left ventricle—hence the name cine (referring to the motion picture technique). There are several techniques for generating a cine series. Because the imaging pulse sequence is unable to complete image acquisition within the 25- to 100-ms time frame of a phase in the cardiac cycle, the acquisition is segmented over several cardiac cycles. As shown in Figure 13-1A, the number of views, or k-space lines, acquired in a cardiac cycle, or R–R interval, is known as the views-per-segment (vps). Segments are acquired repeatedly throughout the cardiac cycle, with each segment at a different temporal phase, starting from the R wave trigger. This segment acquisition continues until the next cardiac R wave trigger is encountered, at which time the next set of k-space encoding views are updated, and the process is repeated until all k-space encoding views have been acquired. An acquisition that is prescribed for a phase encoding matrix dimension of 128 requires a total of 128 views and 16 heartbeats to complete, using 8 vps (128 views ÷ 8 vps = 16 segments; 1 segment per heartbeat for a total of 16 heart beats).
where TR is repetition time. The number of acquired cardiac phases that can fit into an R–R interval is limited by the temporal resolution of the acquisition and the patient’s heart rate. The number of acquired phases is:
where avail_RRtime is the cardiac R–R interval time and is given (in ms) as:
where the heart rate is given in beats per min and trig_window is the fraction of the cardiac R–R interval where data acquisition is disabled to wait for the next cardiac R wave trigger. The number of heart beats that are required to reconstruct an image is given by:
where yres is the image resolution in the phase-encoding direction and represents the number of k-space lines in the image.
For a heart rate of 80 beats/min, TR = 5 ms, and vps = 12, with a 10% trigger window (trig_window = 0.10), 11 phases or segments can be acquired per cardiac interval. For fast heart rates and low temporal resolution, the number of acquired phases is small, and does not allow a smooth depiction of cardiac motion. The effective temporal resolution can be substantially increased, however, using view sharing.10
View sharing effectively doubles the number of reconstructed cardiac phases by sharing k-space lines between adjacent acquired data segments to generate an intermediate-phase image. As shown in Figure 13-1B, where a view-sharing factor of 2 is used for a 4-vps acquisition, k-space encoding lines k1, … , k4 are acquired in each segment. To generate an intermediate phase image, k-space encoding lines k3 and k4 from segment n are combined with k-space encoding lines k1 and k2 lines from segment n + 1 to form an intermediate time point or phase image between acquired phases n and n + 1.
This nearest neighbor interpolation reconstructs an image at a time point midway between two adjacent acquired phases. Although the acquired temporal resolution is still given by Equation 1, the effective temporal resolution is now doubled to:
where vvs is the view sharing factor, and typically, vvs = 2.
The number of acquired segments for each cardiac R–R interval can change owing to possible variations in the patient’s heart rate. These variations in the number of segments and the R–R interval are tracked over time, however, by the MRI scanner’s computer or real-time data processor. After data acquisition is complete for each cardiac cycle, the acquired views for each cardiac R–R interval are sorted retrospectively and binned or interpolated into the appropriate cardiac phase.11 As shown in Figure 13-2, with retrospective gating or sorting, we can choose to reconstruct an arbitrary number of phases for any given number of actual acquired segments. With retrospective interpolation, each cardiac R–R interval is divided into equal time points. The acquired views for each cycle are interpolated to each reconstructed time point using a linear interpolation scheme.
Black Blood Imaging
Blood suppression is achieved by using a double inversion recovery (IR) technique.12 As shown in Figure 13-3, a nonselective IR RF pulse inverts all spins in the RF coil volume. This inversion is followed immediately by a slice-selective IR RF pulse to restore the longitudinal magnetization only within the imaged slice. The aim of the reinversion RF pulse is to ensure that the spins within the imaged slice are unperturbed. At an appropriate inversion time (TI), selected so that TI = TInull,blood, the point in time where the inverted longitudinal magnetization of blood is zero (TInull,blood = T1,blood loge 2), the FSE image acquisition is applied. In this TI interval, blood within the imaged slice is replaced with blood from outside the slice (with inverted longitudinal magnetization). At the moment of data acquisition (just after application of the 90-degree RF excitation pulse), the longitudinal magnetization of blood passes through zero resulting in a suppression of blood signal in the imaged slice. Image acquisition usually spans two R–R intervals to allow for a more complete recovery of the longitudinal magnetization (in the second R–R interval), for increased image SNR.
Typical image acquisition parameters for a black blood FSE acquisition are a 256 × 192 acquisition matrix with an ETL = 20 and two R–R intervals per acquisition segment; this translates to an approximate breath-hold time per slice of about 20 seconds (assuming 60 beats/min heart rate). Complete coverage of the heart using black blood FSE requires about 8 to 12 slice locations with separate breath-holds at each location. To further improve the efficiency of the black blood FSE acquisition, the inversion and image acquisition can be compacted into a single R–R interval (e.g., FSEuno). By removing the time for recovery of the longitudinal magnetization in the second R–R interval, increased T1-weighting is also achieved. Additionally, the acquisition of four slices in the same time as a single slice in a conventional two R–R interval gated FSE pulse sequence (e.g., FSEquatro) can be realized by grouping adjacent slice locations together.13 As shown in Figure 13-4, two slice locations are acquired in each R–R interval (for four slices in total for a two R–R interval acquisition). Because the slices are adjacent to each other, the slice-selective reinversion RF pulse now spans two slice locations providing blood suppression for both slices. The number of breath-holds for whole heart coverage is reduced by a factor of 4 (Fig. 13-5), significantly speeding up the cardiac examination and reducing patient fatigue from frequent and repeated breath-holds.
Myocardial Perfusion
To achieve robust image contrast, a 90-degree saturation recovery magnetization preparation segment, followed by a fixed time TI, is used for myocardial perfusion studies. A 90-degree rather than a 180-degree preparation RF pulse is used because the longitudinal magnetization recovers from the same level (Mz = 0) during the TI interval for each ECG-gated acquisition during the contrast uptake. With saturation recovery magnetization preparation, the tissue signal intensity is independent of the cardiac R–R interval, eliminating signal variations caused by arrhythmia (Fig. 13-6). The relatively short TI time (100 to 200 ms) of the saturation recovery preparation pulse suppresses signals from long T1-weighted tissues, such as the unenhanced myocardium and blood, while emphasizing tissues with markedly reduced T1 times from contrast medium uptake. With the correct selection of acquisition parameters and contrast medium dose, saturation recovery magnetization preparation can yield signal intensity changes that are linear with contrast medium concentration, providing an opportunity for quantitative assessment of myocardial perfusion.
To achieve full coverage of the left ventricle during the first pass of the contrast medium, it must be possible to fit an optimal number of acquisition segments (with each segment consisting of the magnetization preparation pulse, TI interval, and data acquisition section) into either one or two R–R intervals. The TI interval section and the data acquisition segment can be optimized to provide the shortest possible time with best possible image quality. Although single-shot EPI (all k-space lines acquired after a single RF excitation pulse) has poor image quality for cardiac perfusion studies, a segmented and interleaved approach accomplishes short scan time and good image quality.14–16 In interleaved EPI, only a few echoes per RF excitation pulse (ETL = 4) are used, resulting in short (6 ms) TR times; this yields an acquisition approach with high efficiency (time per echo). With improvements in gradient hardware, however, fast gradient echoes (and balanced SSFP) with an ETL = 1 and TR = 2 ms or less have also yielded images with comparable image quality and with equal compactness in time.17–19
Effective assessment of myocardial perfusion defects requires maximal tissue contrast, which is obtained from an optimal TI time at typical contrast medium doses of 0.05 to 0.10 mmol/kg.20 To maximize the number of slice locations that can be acquired per R–R interval, however, the time for each acquisition segment needs to be as compact as possible. By minimizing the TI time, more slice locations can be acquired per R–R interval; however, this leads to compromises in image contrast because there is an inherent tradeoff between TI and image contrast-to-noise ratio (CNR). Several approaches allow for significantly reduced TI times, while maintaining sufficient tissue contrast. By interleaving the saturation recovery RF pulse and image acquisition using a slice-selective notched saturation, longer TI times can be achieved with minimal dead times.21 Alternatively, the center of k-space in the image acquisition section can be reordered to the end of the acquisition segment, yielding a longer “effective” TI time. Both approaches produce satisfactory image contrast with as compact an acquisition segment as possible.
Sections of the myocardium where there is a regional perfusion deficit are hypointense relative to the normal myocardium because of reduced uptake of contrast medium. By a qualitative assessment of signal intensity changes, myocardial perfusion deficits can be easily identified (Fig. 13-7). A troubling artifact with myocardial perfusion using MRI is the so-called “dark rim” artifact, which is characterized by a transient and distinct hypointense thin region in the endocardium that mimics a perfusion defect (Fig. 13-8).22 This phenomenon usually occurs immediately after the contrast medium bolus first arrives in the left ventricle, but does not persist over time. Improvements in spatial resolution and substantial reduction in image acquisition times reduce the occurrence of these artifacts, as does requiring positive identification of a defect that spans several slices and persists through multiple frames of the scan.
Myocardial Viability
Myocardial delayed enhancement (MDE) imaging provides an accurate method for determination of myocardial viability.23,24 On first-pass perfusion imaging, infarcted myocardial tissue does not enhance after gadolinium-chelate contrast agent administration because of its restricted blood flow. Over time, the gadolinium-chelate contrast agent diffuses into the infarcted region. The poor vascular supply to the infarcted region is associated with diminished washout of contrast agent. Infarcted tissue exhibits delayed gadolinium uptake and delayed washout. Normally perfused myocardium has much faster early uptake and brisk washout. Delayed imaging of the left ventricle (beginning 5 to 10 minutes after contrast agent administration) enables optimal differentiation of infarcted from noninfarcted (i.e., normal) myocardium. On delayed imaging, infarcted myocardium appears more enhanced (i.e., “hyperenhanced”) relative to that of normal enhancing myocardium (T1 shortening).
The best technique for visualizing delayed hyperenhancement is an IR GRE pulse sequence that suppresses signal of the normally enhancing myocardial tissue, while maximizing the signal difference with that of the hyperenhancing infarcted tissue.25 To maximize the image contrast between normal myocardium and hyperenhancing infarcted tissue, the TI time is selected to null normal myocardial signal during the acquisition of the central k-space view. Because hyperenhancing infarcted tissue has a higher concentration of gadolinium contrast agent, it has a substantially shorter T1 time (approximately 75 ms) relative to normal myocardium (approximately 290 ms). Nulling the signal from normal myocardium yields a high contrast image differentiating the bright infarcted regions from the dark background. The primary image acquisition technique is an ECG-gated, segmented k-space, two-dimensional acquisition where each slice is acquired in a single breath-hold (Fig. 13-9), requiring multiple breath-holds for complete coverage of the left ventricle.
Although a temporal resolution of about 40 to 50 ms is usually targeted in cine imaging to best visualize motion over the whole cardiac cycle, MDE imaging is able to tolerate temporal resolutions of 150 ms without significant cardiac motion degradation. The number of vps can be substantially increased over a cine scan (see Equation 1), shortening the overall scan time. Typical image acquisition occurs in mid-to-end systole or at a delay of 300 to 400 ms from the cardiac R wave.
To improve image CNR, there are two approaches. With a two R–R interval approach, image acquisition occurs in the first R–R interval, and the second R–R interval is used for more complete magnetization recovery.24 An alternative approach is a single R–R acquisition with double the number of excitations. In the latter approach, there is less time for longitudinal magnetization recovery, but by doubling the number of signal averages, some reduction in motion-related artifacts is realized. In both schemes, the overall scan time is the same, where one slice location is acquired in a single breath-hold.
Maximizing image CNR between normal and infarcted myocardial tissue requires a judicious choice of TI times. As noted in Figure 13-10, the contrast at different TI times varies according to whether phase-sensitive inversion recovery (PSIR) or magnitude IR is used. With PSIR, the signal phase is preserved, allowing for negative values of signal intensity (i.e., to account for negative signal intensities when the longitudinal magnetization is <0).26,27 In magnitude IR, phase information is lost, and all signal intensities are positive (>0). PSIR and magnitude IR provide identical tissue contrast for TI > tnull,myo, the null point for normal myocardium. The advantage in PSIR occurs when TI is significantly shorter than tnull,myo, where contrast is maintained between normal and infarcted myocardium, whereas in magnitude IR, contrast diminishes and inverts if the TI time is decreased further. Although PSIR is less sensitive to TI times when TI < tnull,myo, there is no benefit if TI ≥ tnull,myo. The optimum TI must still be determined for maximum contrast to avoid setting too long a TI time.
The optimal TI time can be chosen by performing repeated test scans at several different TI times; this can be time-consuming because each acquisition requires a separate breath-hold. As contrast medium concentrations diminish over time, the optimal TI time also changes as a function of time after contrast injection. Alternatively, the appropriate TI time can be determined using a scout acquisition that acquires images at different TI times in a single acquisition. By selecting the best image that has complete suppression of normal myocardium, the corresponding TI time can be used in the MDE acquisition. One such TI scout technique uses a GRE cine pulse sequence with an IR pulse applied immediately at the cardiac R wave trigger.28,29 This cine IR approach reconstructs images at different times from the IR pulse where each cardiac phase represents a different TI time. Images at multiple TI times can be obtained in a single breath-hold acquisition to choose the appropriate TI time (Fig. 13-11).
As with a two-dimensional cine scan, an MDE study can be challenging for a patient because of repeated breath-holding. Single-shot methods using shorter TR gradient-echo or balanced SSFP have longer acquisition windows, but allow multiple slice locations to be acquired in a single breath-hold or in a series of much shorter breath-holds.30,31 Compared with a conventional MDE study where the acquisition window of 120-150 ms per cardiac cycle is used, single-shot techniques use acquisition windows in the range of 220 to 250 ms. In addition, a much shorter TR with higher receiver bandwidth leads to a reduction in image CNR. By using approximately the same acquisition window per R–R interval, the MDE study can be extended to a three-dimensional acquisition where a 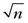 SNR advantage is realized (where n is the number of slice partitions in the volume scan).32–34 By trading off some increased motion blurring (that does not compromise diagnosis) against higher SNR, the efficiency of the myocardial viability study can be improved. In addition, variations that include fat suppression allow for assessment of specific patterns of hyperenhancement as may be seen in arrhythmogenic right ventricular dysplasia.35
SNR advantage is realized (where n is the number of slice partitions in the volume scan).32–34 By trading off some increased motion blurring (that does not compromise diagnosis) against higher SNR, the efficiency of the myocardial viability study can be improved. In addition, variations that include fat suppression allow for assessment of specific patterns of hyperenhancement as may be seen in arrhythmogenic right ventricular dysplasia.35
Tagging and Wall Motion Assessment
Although cine MRI allows the assessment of wall motion by visualizing global myocardial contractility, MRI also has the ability to measure regional myocardial wall function directly. The basic approach is to set up a series of grid lines at the start of systole using a magnetization preparation segment consisting of nonselective RF pulses and gradient pulses, and to follow the evolution of these tagged lines through systole.36,37 Tag lines in the image are applied using a tagging sequence as shown in Figure 13-12. In a simple view with a two-RF pulse tagging sequence, the first RF-excitation pulse tips into the transverse plane all spins within the coil volume. The gradient pulse causes the spins to acquire a relative phase as a function of position. At some time τ, a second RF pulse restores the longitudinal magnetization except for the spins that are aligned along the x′-axis in the rotating frame. Spatial locations that have acquired a relative phase where φ = π/2, 3π/2, … , (2n + 1)π/2 will have minimal signal (φ = 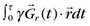 , where
, where 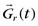 is the magnetic field gradient,
is the magnetic field gradient, 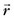 is the position, τ is the gradient waveform pulse width, and γ is the gyromagnetic ratio).
is the position, τ is the gradient waveform pulse width, and γ is the gyromagnetic ratio).
This results in a sinusoidal variation of signal intensity according to position. Two-dimensional grid tags can easily be applied by first applying the tagging sequence in one direction, followed by the same tagging sequence but with a gradient field in an orthogonal direction. The tag lines can also have better definition (sharpness) by increasing the number of RF pulses and corresponding gradient pulses, and varying the relative amplitudes of the component RF pulses.38 The tag separation can be controlled better with an increased number of RF and gradient pulses in the tagging sequence.
As the tagged regions evolve over the cardiac cycle, normal myocardial wall motion is seen as a contraction of the pattern of grid lines in systole, followed by separation or relaxation in diastole (Fig. 13-13). Nonfunctioning sections of the myocardium exhibit no such contractility. Quantitative assessment of myocardial strain is possible from analysis of the deforming tag lines. More efficient techniques have been introduced that allow faster computation of myocardial strain by analyzing the phase evolution of the tag lines in k-space.39,40
Coronary Artery Imaging
Developments in navigator technology and faster acquisition pulse sequences have resulted in substantial progress in using MRI for coronary artery stenosis assessment. Coronary artery imaging methods can be divided into whole heart imaging41 and targeted oblique acquisitions.42,43 In the latter technique, the volume (three-dimensional) acquisition is targeted to specific vessels. Typically, to image the left main coronary artery, left anterior descending coronary artery, right coronary artery, and left circumflex coronary artery, three separate acquisitions are required. For whole heart imaging approaches, the entire heart is imaged in a single acquisition, followed by segmentation of the coronary arteries using postprocessing algorithms available on image analysis workstations.
Short breath-hold three-dimensional techniques42,44 are most suitable for the targeted approach. With breath-hold times of 20 to 28 seconds, an oblique three-dimensional volume encompassing the coronary artery can be easily and rapidly acquired. Three targeted, breath-hold coronary three-dimensional MR angiography acquisitions that include the left main and proximal segments of the left anterior descending coronary artery, right coronary artery, and left circumflex coronary artery are typically imaged within 5 minutes, assuming the patient is able to tolerate repeated breath-holding. The short scan times allow the coronary artery assessment to be completed immediately after the first-pass perfusion study and before performing the myocardial viability assessment.
Because most patients with cardiac disease are unable to tolerate long breath-holds, free-breathing or respiratory-navigator techniques are employed.45 In navigator imaging of the coronary arteries, the position of the diaphragm is monitored before data acquisition in each R–R interval. The navigator acquisition is a two-dimensional slice selective column that is prescribed typically over the right hemidiaphragm in the cranial-caudal direction. The right hemidiaphragm is chosen for tracking diaphragmatic excursion because the lung-liver interface provides an excellent tissue-contrast interface. The placement of the tracker in the right hemithorax places the dark saturation bands related to navigator gating away from the heart. In each R–R interval, the profile along the navigator column is measured and analyzed to track the displacement of the lung-liver interface. A training phase is usually performed to determine automatically the end-expiration position; this is used as a reference profile (Fig. 13-14). During the navigator scan, a least-squares determination of the displacement of the measured profile is made relative to the reference profile (end-expiration). If the position falls within an acceptance window around the end-expiration position, the data are accepted. If not, the same k-space encoding views are repeated until the diaphragm position is noted to fall within the acceptance criteria.46
where xd is the displacement of the profile from its measured position. The value of xd is the displacement of the subject navigator profile from the reference profile. This value is plotted as a function of time (as shown in Fig. 13-14) in real time either to accept or to reject that acquisition view.
Specifically for coronary artery imaging, the navigator segment must be integrated with the data acquisition segment and a fat suppression segment (Fig. 13-15). Fat suppression is required because the coronary arteries are embedded in epicardial fat that yields high signal intensities similar to that of blood. The navigator gating sequence synchronizes image acquisition for end-expiration, and enables the extension of the imaging window over many respiratory cycles for improved high spatial resolution, whole heart imaging of the coronary arteries. From the three-dimensional volume, planar reformations or surface rendering can better depict long lengths of the coronary artery.47
Free-breathing techniques have disadvantages. In practice, the shorter the scan time, the greater the chances that the scan is successful. There is a normal drift in the location of the diaphragm over time48 that changes the end-expiration position, resulting in poor performance of the navigator compensation. If the scan time can be shortened (e.g., using parallel imaging), the probability of diaphragm drift can be reduced, improving the image quality. The ongoing development of coronary artery imaging techniques has focused on minimizing the effects of cardiac and respiratory motion, primarily by reducing the overall acquisition time, but also by determining the optimal quiescent period of the heart when there is minimal motion.
Phase Contrast Imaging
In addition to excellent soft tissue visualization, MRI affords the ability to quantify flow in a manner similar to Doppler ultrasonography, but without the restrictions of limited acoustic windows. Phase contrast angiography49–51 discriminates between flowing spins and stationary tissue by measuring differences in accumulated phase in the direction of a flow-sensitizing gradient. Phase contrast data can provide the velocity of flowing spins (i.e., flowing blood) and the direction of blood flow.
where 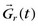 describes the time-varying gradient (direction and amplitude), γ describes the gyromagnetic ratio, and r0 describes the initial position of the spins. The terms M0 and M1 represent the zero and first moments of motion of the spins in the gradient field. If we follow this first gradient waveform with a second waveform that is identical but of opposite polarity, the accumulated phase is then:
describes the time-varying gradient (direction and amplitude), γ describes the gyromagnetic ratio, and r0 describes the initial position of the spins. The terms M0 and M1 represent the zero and first moments of motion of the spins in the gradient field. If we follow this first gradient waveform with a second waveform that is identical but of opposite polarity, the accumulated phase is then:
where 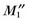 represents the first gradient moment from the combined bipolar gradient waveform. The phase accumulation from this bipolar gradient waveform (Fig. 13-16) arises only from flowing spins, with the contribution from stationary spins (the r0 component) canceled out by the gradient lobes of equal amplitude but opposite polarity. Only flowing spins contribute to nonzero phase accumulation, the magnitude and sign of which are directly related to the velocity and direction of flow.
represents the first gradient moment from the combined bipolar gradient waveform. The phase accumulation from this bipolar gradient waveform (Fig. 13-16) arises only from flowing spins, with the contribution from stationary spins (the r0 component) canceled out by the gradient lobes of equal amplitude but opposite polarity. Only flowing spins contribute to nonzero phase accumulation, the magnitude and sign of which are directly related to the velocity and direction of flow.
A single acquisition with a bipolar flow-sensitizing gradient ideally provides an image whose phase represents flow in the direction of the applied gradient as given by Equation 9. Residual eddy currents, magnetic field inhomogeneity, and miscentering of the MRI echo in the acquisition window contribute to a spatially varying nonzero phase unrelated to flow or motion (Fig. 13-17A and B). To overcome this problem, two images with bipolar gradients of opposite sign (toggled bipolar gradients) are subtracted. Any nonzero phase in stationary tissue is canceled out, leaving an image with the phase difference accumulated in the two acquisitions owing only to flow (Fig. 13-17C). The phase difference (ΔØ) from the toggled bipolar flow gradient is then:
with:
where G(t) is the bipolar gradient waveform.
The phase contrast approach to velocity imaging can be applied to cine pulse sequences (see Figs. 13-1 and 13-2) to generate ECG-gated images that depict flow velocities in the heart and blood vessels as a function of the cardiac cycle. With phase contrast cine images, the magnitude and direction of intracardiac shunts and valvular dysfunction (Fig. 13-18) can be easily observed. It is also possible to compute net flow (integrated over the entire cardiac cycle) through vessel cross sections (Fig. 13-19), and the forward and reverse components of flow. Phase contrast cine is an essential tool for flow quantification in patients with atrial septal defects or similar congenital cardiac disease where the measurement of regurgitant volumes or shunt flow is required.52,53
ACCELERATED CARDIAC MRI
Fast Pulse Sequences
A primary method for speeding up imaging of the heart has been the adoption of faster pulse sequences. The first of these to be developed, the echo-planar imaging (EPI) sequence,1 executes a raster pattern back and forth across all of raw-data space, or “k-space,”5 after a single RF excitation. This is in contrast to conventional acquisition approaches that acquire a single line of k-space data per excitation (Fig. 13-20). Although this sequence dates from the earliest days of MRI, it did not become practical for cardiac imaging until the advent of improved gradient hardware.3,54 This improved hardware has also enabled real-time54 EPI movie loops and high-quality phase contrast interleaved EPI images15 of the heart. Interleaved EPI pulse sequences,14,15 which employ multiple excitation pulses with interleaved k-space trajectories, were developed later to reduce the ETL, and minimize some of the artifacts associated with EPI sequences.
Improvements in gradient hardware also benefited another class of fast imaging sequence, balanced SSFP.8,55 This method relies on balanced gradient waveforms and short TR to rewind coherently the magnetization after each readout, and avoid signal saturation effects that degrade other short TR sequences. Balanced SSFP images are characterized by relatively high SNR and bright blood signal, but require TR on the order of 3 ms or less (at 1.5 T) to avoid dark banding artifacts arising from off-resonance effects. The greater the inhomogeneity present, the shorter the TR times required.
Another class of imaging sequence replaces rectilinear k-space trajectories with curved paths that push the gradient slew rate (which can be viewed as the rate of change of motion along the trajectory) more evenly over the entire sequence. The most successful of these arguably has been the spiral trajectory5,6 and its variants. The spiral trajectory can be traversed at a nonuniform angular rate to produce constant gradient amplitudes or slew rates, and maximize bandwidth.56 The advantages of reduced echo times gained by interleaving in EPI also hold for interleaved spirals. Spirals have the further benefits of good flow characteristics57 and relative insensitivity to motion,58 making them especially useful for cardiac imaging. Off-resonance and susceptibility effects can affect image quality, however, and measures such as automated field-map calculation and correction in the image reconstruction are generally necessary to prevent regional image blurring.59
Parallel Imaging
The conventional approach to faster MRI pulse sequences, through reductions in the sequence TR time, is inherently limited by gradient amplitude and slew-rate constraints. These constraints arise partly from hardware limitations, but more fundamentally from the potential for peripheral nerve stimulation. A newer class of methods for speeding up MRI acquisitions, parallel imaging,60,61 overcomes this limitation by shifting some of the responsibility for spatial encoding from the gradients to an array of RF receiver coils.62 This shift removes the dependence for shortening scan times on reducing TR by instead reducing the amount of data to be collected.
This method of undersampling k-space and using spatially arrayed RF coils to reconstruct artifact-free images is termed parallel imaging. Parallel imaging techniques fall into two classes: k-space-based techniques such as SMASH (simultaneous acquisition of spatial harmonics)60 and GRAPPA (generalized autocalibrating partially parallel acquisitions),63 and image-space–based methods such as SENSE (sensitivity encoding).61 These techniques typically use either a separate acquisition first to generate spatial sensitivity maps for each coil or autocalibration, in which the center of k-space is fully sampled within an undersampled acquisition, and low-resolution sensitivity maps are generated from that portion of the data.
Early application of parallel imaging to the heart used the SMASH method in coronary MRI, to bring a factor of 2 improvement to breath-hold times, spatial resolution, or time resolution.64 Soon after this, SENSE imaging with a half-Fourier segmented EPI sequence was applied to breath-hold and real-time imaging to accelerate scanning by factors up to 3.2, resulting in low spatial resolution images in times as short as 13 ms using six-element cardiac phased-array coils.65,66 Acceleration factors are constrained by the number of spatially arrayed coil elements. The higher the acceleration factor, the greater the undersampling of k-space data, and the greater the reliance on more coil elements for spatial encoding. Exceeding the threshold acceleration factor for the number of coil elements present leads to decreased ability to unwrap aliased pixels.
As channel counts increased, 32-coil torso67 and cardiac68 arrays were used for cardiac MRI, enabling acceleration factors of 16 for whole heart imaging. This development allows all three main branches of the coronary tree to be captured in a single breath-hold (Fig. 13-21). Arrays with higher channel counts have also brought improved image quality for accelerated real-time balanced-SSFP imaging at frame rates of 50 frames per second, potentially enabling improved stress testing.69 An example of real-time cardiac imaging with a 32-channel cardiac array is shown in Figure 13-22. Channel counts have since continued to increase beyond 32 to 128,70,71 resulting in improved quality for highly accelerated breath-hold cardiac MRI. Cardiac imaging with 128 channels has been shown more recently to yield acceleration rates greater than 12 to 20.72 Although coil losses and electrodynamics considerations seem to indicate that we may be nearing the useful limits of channel count (at least for field strengths of 1.5 to 3 T), more work remains to be done to explore the best use of high-channel cardiac MRI.
k-t Methods
In some cases, redundancies in the MRI signal acquired at different times in the cardiac cycle can be exploited to accelerate cardiac MRI effectively without the need for stronger gradients or multiple RF coils. In the earliest example of this, a sliding-window reconstruction was used to improve the effective frame rate of real-time fluoroscopic MRI.73 In this method, as each new line (or group of lines) of k-space is acquired, it is combined with other, most recently acquired lines to compose a complete data set that is reconstructed to form an image for that point in time. In this way, the image reconstruction window is “slid” along in time, updating different parts of k-space at each new time point. Essentially, only a small but different portion of k-space is acquired at each time point, yielding a sparsely sampled k-t space (a data matrix with k-space along one dimension and time in the other). This method has also been applied to real-time cardiac MRI using interleaved spirals.58
In a more recent development called UNFOLD (unaliasing by Fourier-encoding the overlaps using the temporal dimension), data from the dynamic object (the beating heart) are made to populate k-t space in a denser, more efficient manner.74 Specifically, the data are undersampled by a factor of 2 and acquired in a time series where every odd image in the series uses a sampling function shifted in k-space by half of a line spacing compared with that used for the even images. After reconstructing each undersampled k-space data set at each time point (Fourier transformation in the k-dimension), this results in the signal intensity of aliased pixels alternating in position (shifted by half an image field of view) in each time frame, while unaliased pixels remain constant. When the time frames are played out as in a movie loop, the aliased pixels appear to flicker (i.e., change with high temporal frequency), whereas the unaliased pixels are steady (i.e., they exhibit only low temporal frequencies). After reconstructing the k-t data set to a series of images in time, this yields a pixel (x)-time (t) data set (or x-t data set).
A more generalized k-t approach does not rely on such separation of temporal frequency data. This technique, called k-t BLAST (broad-use linear acquisition speed-up technique),75 enables much higher acceleration factors than UNFOLD, while similarly not being constrained by the number of coil elements. The principle of k-t BLAST is simple. The time-series MRI data are uniformly undersampled in k-t space (i.e., different parts of k-space are collected at different times to form a sheared grid pattern—“quasi” diamond pattern—in k-t space). On Fourier transformation of this undersampled k-t data set, a replication of objects in the corresponding x-f space is obtained (i.e., pixels are aliased). Fully sampled data would yield x-f space that has fairly localized signal with noise everywhere else (i.e., pixels are not aliased). In the undersampled case, the object in x-f space is replicated throughout, and may overlap. This is in contrast to the case of UNFOLD, where the aliased or replicated objects do not overlap with the primary object. Because the replicated objects may overlap, the use of a low-pass filter as in UNFOLD would also remove data from the unaliased pixels. A much better method to remove the aliased pixels is needed.
An inverse Fourier transformation back to k-t space yields an approximation of a fully sampled set that can be reconstructed normally to obtain alias-free images. In this manner, eightfold acceleration for cardiac imaging with reduced artifacts relative to a sliding-window reconstruction has been achieved without the use of multiple element phased-array coils.75,76
Real-Time MRI
Rapid imaging pulse sequences are most useful when employed in the context of a system capable of high-speed reconstruction, real-time image display, and interactive scan-plane control. Such a setup was first developed and demonstrated by Wright et al.,77 who used a workstation and array processor connected to a conventional MRI scanner. Other systems were developed later to enable real-time graphic control of the scan plane, or control by the use of three-dimensional hardware controllers.78,79 Real-time interactive MRI systems have been applied to coronary MRI,80 evaluation of left-ventricular function, MRI color flow,81 and interventional guidance,82 among other applications in the heart, and commercial real-time MRI systems are now widely available.
KEY POINTS
 Methods for imaging the heart using MRI are described. Descriptions on how cine images of the heart can be obtained over several cardiac cycles, and the basics for acquiring images that provide information on myocardial viability, perfusion, and cardiac wall motion are provided.
Methods for imaging the heart using MRI are described. Descriptions on how cine images of the heart can be obtained over several cardiac cycles, and the basics for acquiring images that provide information on myocardial viability, perfusion, and cardiac wall motion are provided. In addition to soft tissue contrast, functional information can be obtained with MRI. Specifically, MRI is able to acquire images with information on the direction and velocity of flow. This information allows measurements of flow volumes and other physiologic indicators of cardiovascular disease.
In addition to soft tissue contrast, functional information can be obtained with MRI. Specifically, MRI is able to acquire images with information on the direction and velocity of flow. This information allows measurements of flow volumes and other physiologic indicators of cardiovascular disease. Image acquisition times are currently longer than the periods of cardiac motion. Several cardiac cycles are required to acquire sufficient data to reconstruct images depicting the heart at different cardiac phases. Fast imaging techniques using parallel acceleration that reduce the k-space data requirements for image reconstruction have substantially sped up image acquisition. With a larger number of receiver coils, high acceleration factors permit real-time image acquisition with acceptable spatial resolution; this allows imaging with the patient breathing freely throughout image acquisition, rather than requiring repeated breath-holding.
Image acquisition times are currently longer than the periods of cardiac motion. Several cardiac cycles are required to acquire sufficient data to reconstruct images depicting the heart at different cardiac phases. Fast imaging techniques using parallel acceleration that reduce the k-space data requirements for image reconstruction have substantially sped up image acquisition. With a larger number of receiver coils, high acceleration factors permit real-time image acquisition with acceptable spatial resolution; this allows imaging with the patient breathing freely throughout image acquisition, rather than requiring repeated breath-holding.Earls JP, Ho VB, Foo TK, et al. Cardiac MRI: recent progress and continued challenges. J Magn Reson Imag. 2002;16:111-127.
Lima JAC, Milind DY. Cardiovascular magnetic resonance imaging: current and emerging applications. J Am Coll Cardiol. 2004;44:1164-1171.
Pruessmann KP. Encoding and reconstruction in parallel MRI. NMR Biomed. 2006;19:288-299.
1 Mansfield P, Pykett I. Biological and medical imaging by NMR. J Magn Reson. 1978;29:355-373.
2 Young I. Nuclear magnetic resonance systems. US patent 4,355,282. 1979.
3 Rzedzian RR, Pykett IL. Instant images of the human heart using a new, whole-body MR imaging system. AJR Am J Roentgenol. 1987;149:245-250.
4 Semelka RC, Kelekis NL, Thomasson D, et al. HASTE MR imaging: description of technique and preliminary results in the abdomen. J Magn Reson Imaging. 1996;6:698-699.
5 Likes R. Moving gradient zeugmatography. US patent 4,307,343 August 1979.
6 Meyer CH, Hu BS, Nishimura DG, et al. Fast spiral coronary artery imaging. Magn Reson Med. 1992;28:202-213.
7 Atkinson DJ, Edelman RR. Cineangiography of the heart in a single breath hold with a segmented turboFLASH sequence. Radiology. 1991;178:357-360.
8 Oppelt A, Graumann R, Barfuss H, et al. FISP—a new fast MRI sequence. Electromedica. 1986;54:15-18.
9 Barkhausen J, Ruehm SG, Goyen M, et al. MR evaluation of ventricular function: true fast imaging with steady-state precession versus fast low-angle shot cine MR imaging: feasibility study. Radiology. 2001;219:264-269.
10 Foo TKF, Bernstein MA, Aisen AM, et al. Improved ejection fraction and flow velocity estimates with use of view sharing and uniform repetition time excitation with fast cardiac techniques. Radiology. 1995;195:471-478.
11 Feinstein JA, Epstein FH, Arai AE, et al. Using cardiac phase to order reconstruction (CAPTOR): a method to improve diastolic images. J Magn Reson Imaging. 1997;7:794-798.
12 Simonetti OP, Finn JP, White RD, et al. “Black blood” T2-weighted inversion-recovery MR imaging of the heart. Radiology. 1996;199:49-57.
13 Saranathan M, Slavin GS. Quadrupled efficiency for black blood imaging using slice-pair interleaving (abstract). Proceedings of the 11th meeting of the International Society of Magnetic Resonance in Medicine, Toronto, Canada, July 2003.
14 McKinnon GC. Ultrafast interleaved gradient-echo-planar imaging on a standard scanner. Magn Reson Med. 1993;30:609-616.
15 McKinnon GC, Debatin JF, Wetter DR, et al. Interleaved echo planar flow quantitation. Magn Reson Med. 1994;32:263-267.
16 Ding S, Wolff SD, Epstein FH. Improved coverage in dynamic contrast-enhanced cardiac MRI using interleaved gradient-echo EPI. Magn Reson Med. 1998;39:514-519.
17 Klocke FJ, Simonetti OP, Judd RM, et al. Limits of detection of regional differences in vasodilated flow in viable myocardium by first-pass magnetic resonance perfusion imaging. Circulation. 2001;104:2412-2416.
18 Schreiber WG, Schmitt M, Kalden P, et al. Dynamic contrast-enhanced myocardial perfusion imaging using saturation-prepared TrueFISP. J Magn Reson Imaging. 2002;16:641-652.
19 Foo TK, Wu KC, Azevedo CF, et al. Partial Fourier steady-state free precession (FIESTA) first-pass perfusion with improved image quality and efficient spatial coverage (abstract). Proceedings of the 12th Annual Meeting International Society of Magnetic Resonance in Medicine, Kyoto, Japan, May 2004.
20 Bertschinger KM, Nanz D, Buechi M, et al. Magnetic resonance myocardial first-pass perfusion imaging: parameter optimization for signal response and cardiac coverage. J Magn Reson Imaging. 2001;14:556-562.
21 Slavin GS, Wolff SD, Gupta SN, et al. First-pass myocardial perfusion MR imaging with interleaved notched saturation: feasibility study. Radiology. 2001;219:258-263.
22 Di Bella EV, Parker DL, Sinusas AJ. On the dark rim artifact in dynamic contrast-enhanced MRI myocardial perfusion studies. Magn Reson Med. 2005;54:1295-1299.
23 Lima JA, Judd RM, Bazille A, et al. Regional heterogeneity of human myocardial infarcts demonstrated by contrast-enhanced MRI: potential mechanisms. Circulation. 1995;92:1117-1125.
24 Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445-1453.
25 Simonetti OP, Kim RJ, Fieno DS, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215-223.
26 Kellman P, Arau AE, McVeigh ER, et al. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med. 2002;47:272-283.
27 Setser RM, Chung YC, Weaver JA, et al. Effect of inversion time on delayed-enhancement magnetic resonance imaging with and without phase-sensitive reconstruction. J Magn Reson Imaging. 2005;21:650-655.
28 Ho VB, Hood MN, Montequin M, et al. cine inversion recovery (IR): rapid tool for optimized myocardial delayed enhancement imaging (abstract). Proceedings of the 13th Annual Meeting International Society of Magnetic Resonance in Medicine, Miami, May 2005.
29 Setser RM, Kim JK, Chung YC, et al. Cine delayed-enhancement MR imaging of the heart: initial experience. Radiology. 2006;239:856-862.
30 Huber A, Schoenberg SO, Spannagl B, et al. Single-shot inversion recovery TrueFISP for assessment of myocardial infarction. AJR Am J Roentgenol. 2006;186:627-633.
31 Sievers B, Elliott MD, Hurwitz LM, et al. Rapid detection of myocardial infarction by subsecond, free-breathing delayed contrast-enhancement cardiovascular magnetic resonance. Circulation. 2007;115:236-244.
32 Foo TK, Stanley DW, Castillo E, et al. Myocardial viability: breath-hold 3D MR imaging of delayed hyperenhancement with variable sampling in time. Radiology. 2004;230:845-851.
33 Saranathan M, Rochitte CE, Foo TK. Fast, three-dimensional free-breathing MR imaging of myocardial infarction: a feasibility study. Magn Reson Med. 2004;51:1055-1060.
34 Tatli S, Zou KH, Fruitman M, et al. Three-dimensional magnetic resonance imaging technique for myocardial-delayed hyperenhancement: a comparison with the two-dimensional technique. J Magn Reson Imaging. 2004;20:378-382.
35 Tandri H, Saranathan M, Rodriguez ER, et al. Noninvasive detection of myocardial fibrosis in arrhythmogenic right ventricular cardiomyopathy using delayed-enhancement magnetic resonance imaging. J Am Coll Cardiol. 2005;45:98-103.
36 Zerhouni EA, Parish DM, Rogers WJ, et al. Human heart: tagging with MR imaging—a method for noninvasive assessment of myocardial motion. Radiology. 1988;169:59-63.
37 Axel L, Dougherty L. MR imaging of motion with spatial modulation of magnetization. Radiology. 1989;171:841-845.
38 Axel L, Dougherty L. Heart wall motion: improved method of spatial modulation of magnetization for MR imaging. Radiology. 1989;172:349-350.
39 Osman NF, Kerwin WS, McVeigh ER, et al. Cardiac motion tracking using cine harmonic phase (HARP) magnetic resonance imaging. Magn Reson Med. 1999;42:1048-1060.
40 Aletras AH, Ding S, Balaban RS, et al. DENSE: displacement encoding with stimulated echoes in cardiac functional MRI. J Magn Reson. 1999;137:247-252.
41 Weben OM, Martin AJ, Higgins CB. Whole-heart steady-state free precession coronary artery magnetic resonance angiography. Magn Reson Med. 2003;50:1223-1228.
42 Wielopolski PA, van Geuns RJ, de Feyter PJ, et al. Breath-hold coronary MR angiography with volume-targeted imaging. Radiology. 1998;209:209-219.
43 Stuber M, Botnar RM, Danias PG, et al. Double-oblique free-breathing high resolution three-dimensional coronary magnetic resonance angiography. J Am Coll Cardiol. 1999;34:524-531.
44 Foo TKF, Ho VB, Saranathan M, et al. High spatial resolution 3D breath-held coronary artery imaging: feasibility of integration with myocardial perfusion and viability studies. Radiology. 2005;235:1025-1030.
45 Ehman RL, Felmlee JP. Adaptive technique for high-definition MR imaging of moving structures. Radiology. 1989;173:255-263.
46 Stuber M, Botnar RM, Danias PG, et al. Submillimeter three-dimensional coronary MR angiography with real-time navigator correction: comparison of navigator locations. Radiology. 1999;212:579-587.
47 Etienne A, Botnar RM, Van Muiswinkel AM, et al. “Soap-Bubble” visualization and quantitative analysis of 3D coronary magnetic resonance angiograms. Magn Reson Med. 2002;48:658-666.
48 Taylor AM, Jhooti P, Wiesmann F, et al. MR navigator-echo monitoring of temporal changes in diaphragm position: implications for MR coronary angiography. J Magn Reson Imaging. 1997;7:629-636.
49 Moran PR. A flow velocity zeugmatographic interlace for NMR imaging in humans. Magn Reson Imaging. 1982;1:197-203.
50 Nayler GL, Firmin DN, Longmore DB. Blood flow imaging by cine magnetic resonance. J Comp Assist Tomogr. 1986;10:715-722.
51 Dumoulin CL, Souza SP, Walker MF. Three-dimensional phase contrast angiography. Magn Reson Med. 1989;9:139-149.
52 Sechtem U, Pflugfelder P, Cassidy MC, et al. Ventricular septal defect: visualization of shunt flow and determination of shunt size by cine MR imaging. AJR Am J Roentgenol. 1987;149:689-692.
53 Beerbaum P, Korperich H, Barth P, et al. Noninvasive quantification of left-to-right shunt in pediatric patients: phase-contrast cine magnetic resonance imaging compared with invasive oximetry. Circulation. 2001;103:2476-2482.
54 Chapman B, Turner R, Ordidge RJ, et al. Real-time movie imaging from a single cardiac cycle by NMR. Magn Reson Med. 1987;5:246-254.
55 Patz S, Hawkes RC. The application of steady-state free precession to the study of very slow fluid flow. Magn Reson Med. 1986;3:140-145.
56 Hardy C, Cline H. Broadband nuclear magnetic resonance pulses with two-dimensional spatial selectivity. J Appl Phys. 1989;66:1513-1516.
57 Nishimura DG, Irarrazabal P, Meyer CH. A velocity k-space analysis of flow effects in echo-planar and spiral imaging. Magn Reson Med. 1995;33:549-556.
58 Kerr AB, Pauly JM, Hu BS, et al. Real-time interactive MRI on a conventional scanner. Magn Reson Med. 1997;38:355-367.
59 Irarrazabal P, Hu BS, Pauly JM, et al. Spatially resolved and localized real-time velocity distribution. Magn Reson Med. 1993;30:207-212.
60 Sodickson DK, Manning WJ. Simultaneous acquisition of spatial harmonics (SMASH): fast imaging with radiofrequency coil arrays. Magn Reson Med. 1997;38:591-603.
61 Pruessmann KP, Weiger M, Scheidegger MB, et al. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42:952-962.
62 Roemer PB, Edelstein WA, Hayes CE, et al. The NMR phased array. Magn Reson Med. 1990;16:192-225.
63 Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med. 2002;47:1202-1210.
64 Jakob PM, Griswold MA, Edelman RR, et al. Accelerated cardiac imaging using the SMASH technique. J Cardiovasc Magn Reson. 1999;1:153-157.
65 Weiger M, Pruessmann KP, Boesiger P. Cardiac real-time imaging using SENSE. SENSitivity Encoding scheme. Magn Reson Med. 2000;43:177-184.
66 Pruessmann KP, Weiger M, Boesiger P. Sensitivity encoded cardiac MRI. J Cardiovasc Magn Reson. 2001;3:1-9.
67 Niendorf T, Hardy CJ, Giaquinto RO, et al. Toward single breath-hold whole-heart coverage coronary MRA using highly accelerated parallel imaging with a 32-channel MR system. Magn Reson Med. 2006;56:167-176.
68 Hardy CJ, Cline HE, Giaquinto RO, et al. 32-element receiver-coil array for cardiac imaging. Magn Reson Med. 2006;55:1142-1149.
69 Hardy CJ, Darrow RD, Marinelli L, et al. 32-channel real-time MRI at ~50 frames per sec for irregular heart motion (abstract). Proceedings of the 14th Annual Meeting International Society of Magnetic Resonance in Medicine, Seattle, May 2006.
70 Schmitt M, Potthast A, Sosnovik DE, et al. A 128-channel receive-only cardiac coil for highly accelerated cardiac MRI at 3 Tesla. Magn Reson Med. 2008;59:1431-1439.
71 Hardy CJ, Giaquinto RO, Piel JE, et al. 128-channel body MRI with a flexible high-density receiver-coil array. J Magn Reson Imaging. 2008;28:1219-1225.
72 Shankaranarayanan A, Fung M, Beatty P, et al. 128-channel highly-accelerated breath-held 3D coronary MR imaging. Proceedings of the 16th annual meeting of ISMRM, Toronto, 2008.
73 Riederer SJ, Tasciyan T, Farzaneh F, et al. MR fluoroscopy: technical feasibility. Magn Reson Med. 1988;8:1-15.
74 Madore B, Glover GH, Pelc NJ. Unaliasing by Fourier-encoding the overlaps using the temporal dimension (UNFOLD), applied to cardiac imaging and fMRI. Magn Reson Med. 1999;42:813-828.
75 Tsao J, Boesiger P, Pruessmann KP. k-t BLAST and k-t SENSE: dynamic MRI with high frame rate exploiting spatiotemporal correlations. Magn Reson Med. 2003;50:1031-1042.
76 Tsao J, Kozerke S, Boesiger P, et al. Optimizing spatiotemporal sampling for k-t BLAST and k-t SENSE: application to high-resolution real-time cardiac steady-state free precession. Magn Reson Med. 2005;53:1372-1382.
77 Wright RC, Riederer SJ, Farzaneh F, et al. Real-time MR fluoroscopic data acquisition and image reconstruction. Magn Reson Med. 1989;12:407-415.
78 Hardy CJ, Darrow RD, Nieters EJ, et al. Real-time acquisition, display, and interactive graphic control of NMR cardiac profiles and images. Magn Reson Med. 1993;29:667-673.
79 Debbins JP, Riederer SJ, Rossman PJ, et al. Cardiac magnetic resonance fluoroscopy. Magn Reson Med. 1996;36:588-595.
80 Hardy CJ, Darrow RD, Pauly JM, et al. Interactive coronary MRI. Magn Reson Med. 1998;40:105-111.
81 Nayak KS, Pauly JM, Kerr AB, et al. Real-time color flow MRI. Magn Reson Med. 2000;43:251-258.
82 Guttman MA, Lederman RJ, Sorger JM, et al. Real-time volume rendered MRI for interventional guidance. J Cardiovasc Magn Reson. 2002;4:431-442.

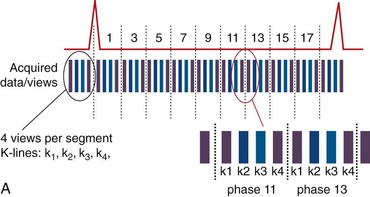
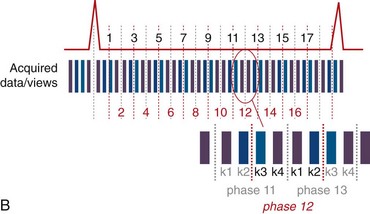
 FIGURE 13-1
FIGURE 13-1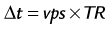
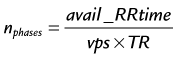
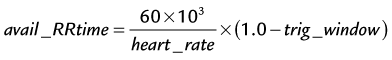
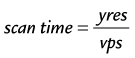
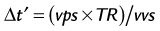
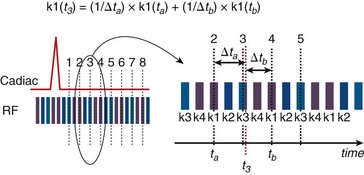
 FIGURE 13-2
FIGURE 13-2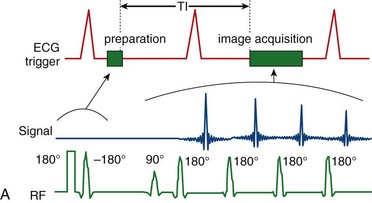
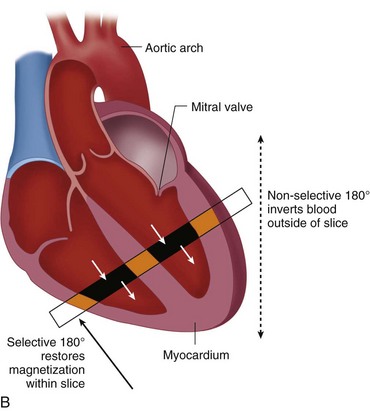
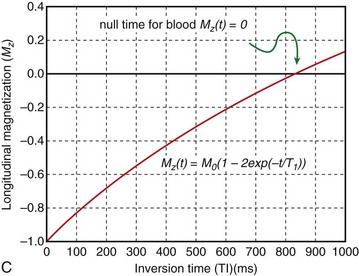
 FIGURE 13-3
FIGURE 13-3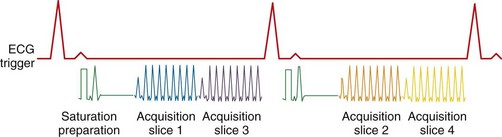
 FIGURE 13-4
FIGURE 13-4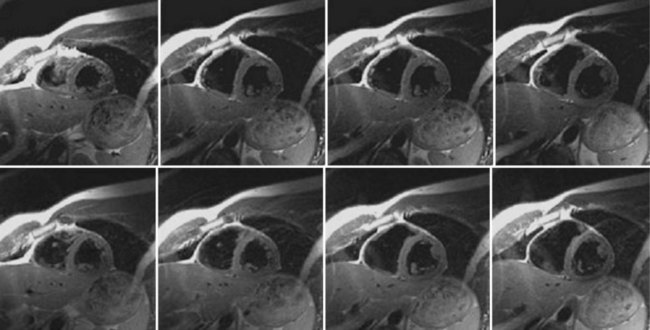
 FIGURE 13-5
FIGURE 13-5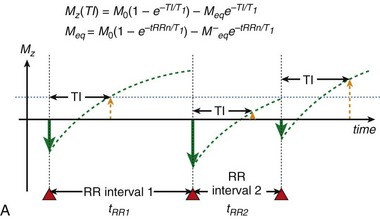
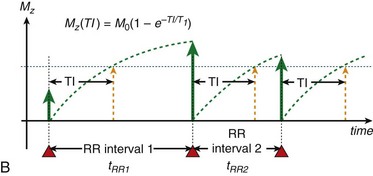
 FIGURE 13-6
FIGURE 13-6 is defined as the longitudinal magnetization just prior to the application of the IR pulse in RR interval n (i.e., the longitudinal magnetization at the end of RR interval (n − 1).
is defined as the longitudinal magnetization just prior to the application of the IR pulse in RR interval n (i.e., the longitudinal magnetization at the end of RR interval (n − 1).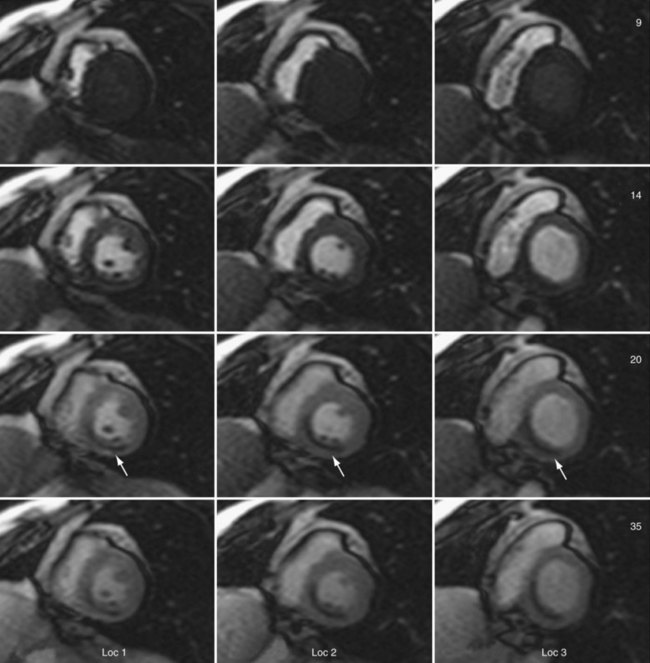
 FIGURE 13-7
FIGURE 13-7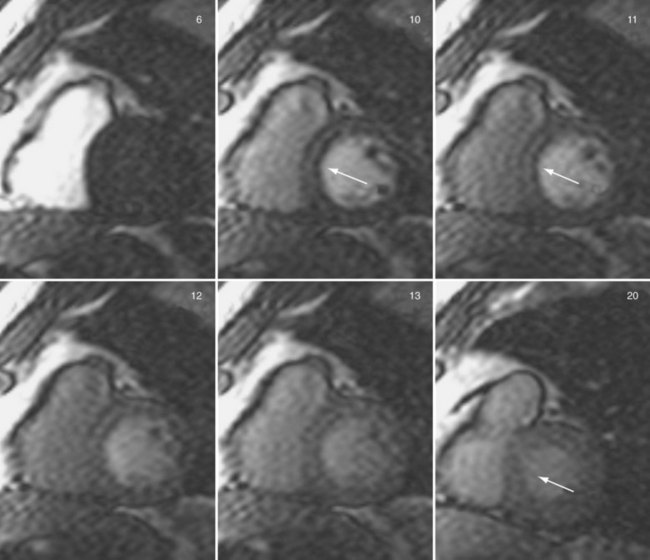
 FIGURE 13-8
FIGURE 13-8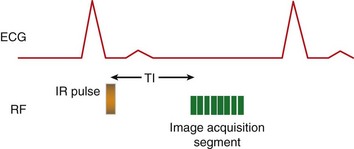
 FIGURE 13-9
FIGURE 13-9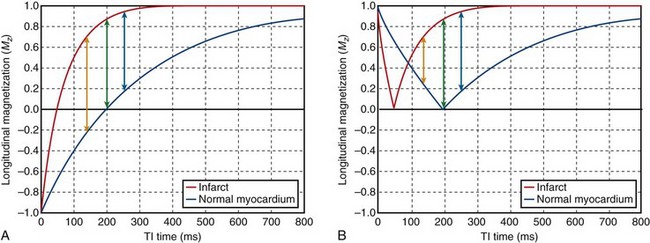
 FIGURE 13-10
FIGURE 13-10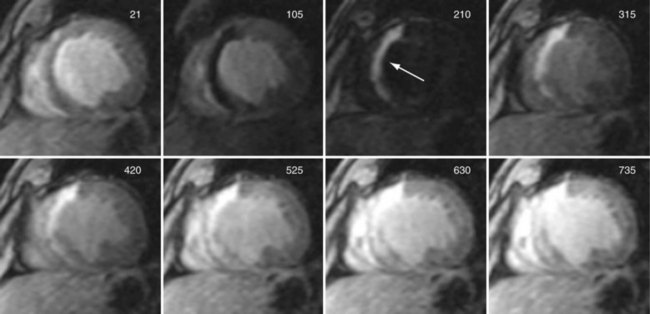
 FIGURE 13-11
FIGURE 13-11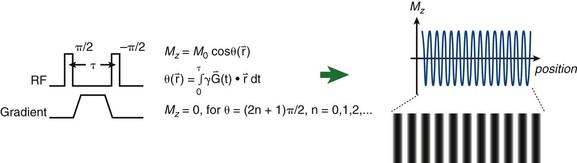
 FIGURE 13-12
FIGURE 13-12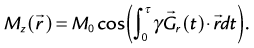
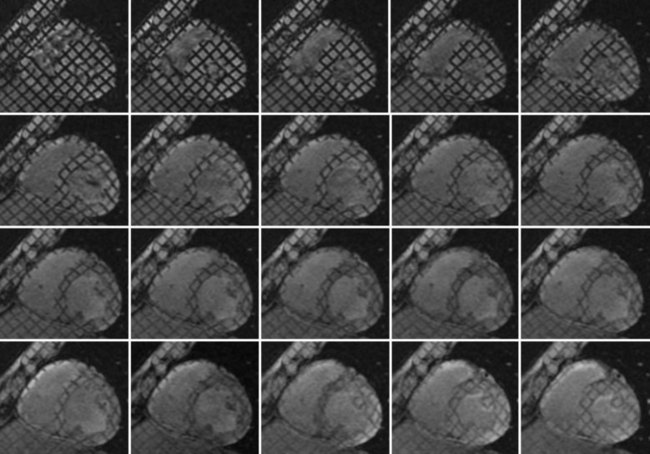
 FIGURE 13-13
FIGURE 13-13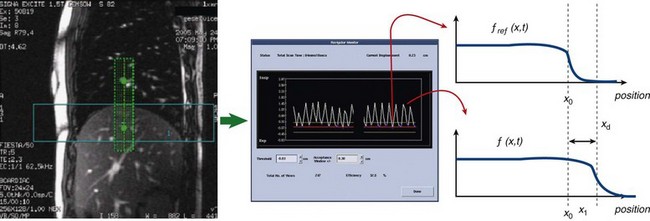
 FIGURE 13-14
FIGURE 13-14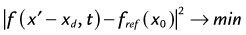
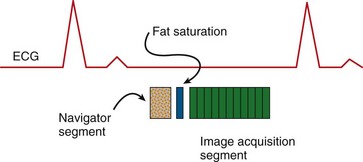
 FIGURE 13-15
FIGURE 13-15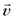 , in that direction is:
, in that direction is: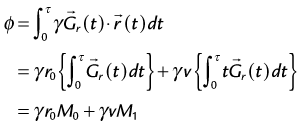
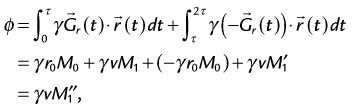
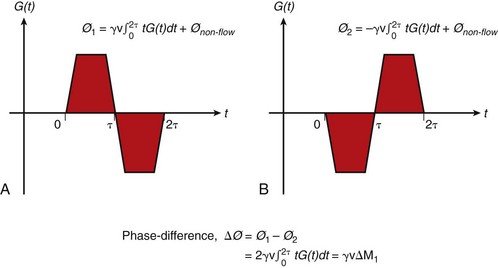
 FIGURE 13-16
FIGURE 13-16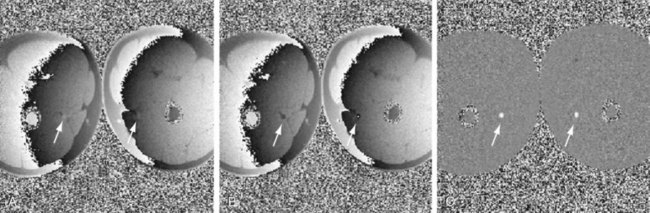
 FIGURE 13-17
FIGURE 13-17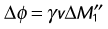
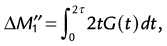
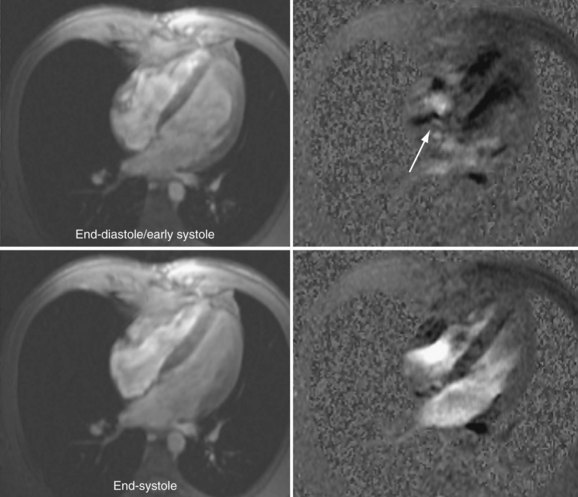
 FIGURE 13-18
FIGURE 13-18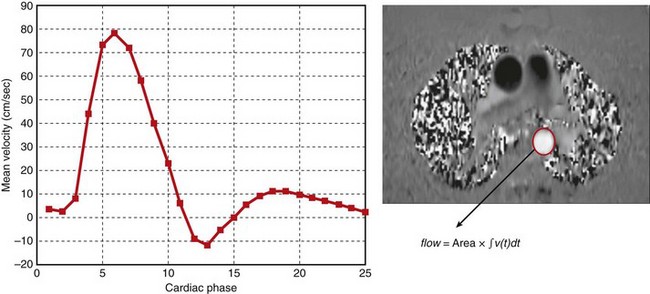
 FIGURE 13-19
FIGURE 13-19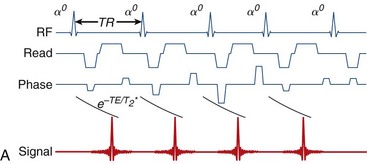
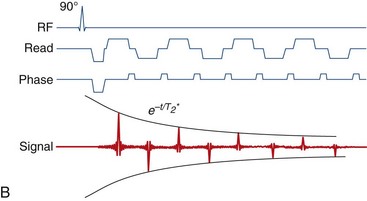
 FIGURE 13-20
FIGURE 13-20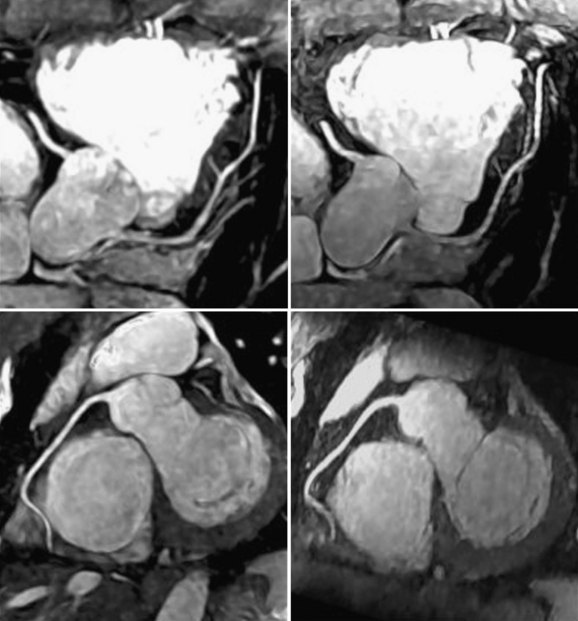
 FIGURE 13-21
FIGURE 13-21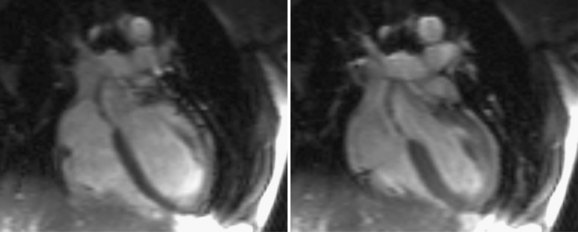
 FIGURE 13-22
FIGURE 13-22