CHAPTER 130 Metastatic Brain Tumors
Brain metastases represent a significant source of morbidity and mortality in patients with systemic cancer, as first reported by Bucholz1 in 1898. They are neoplasms that originate in tissues outside the central nervous system (CNS) and spread secondarily to the brain. In adults, cerebral metastases are by far the most common intracranial tumors, and their incidence seems to be rising as systemic cancer therapies have improved, thereby extending patients’ lives. This chapter describes current thought on the epidemiology of brain (parenchymal) metastases and strategies for their treatment by surgery, radiation therapy, radiosurgery, and chemotherapy.
Epidemiology
The incidence of brain metastasis is difficult to determine with precision. The bulk of older estimates originated from historical neurosurgical series, and because neurosurgeons were reluctant to operate on patients with known systemic cancer, these series grossly underestimated the actual incidence of brain metastasis. Similarly, major ascertainment and underreporting problems are limiting factors in obtaining accurate epidemiologic data from large patient populations. In the national survey for intracranial neoplasms reported by Walker and associates,2 only 20% of the metastatic cases diagnosed during 1973 and 1974 were verified by tissue examination. Estimates of incidence from earlier studies of large populations in the United States, Iceland, and central Finland ranged from 2.8 to 11.1 per 100,000 individuals.2–5 More recent series and autopsy studies indicate a much higher incidence of brain metastasis. These studies place brain metastases first in frequency among all intracranial tumors.2,6,7 It is currently estimated that between 100,000 and 200,000 patients will develop brain metastases each year in the United States (the wide range reflects the uncertainty of the estimates). It is also estimated that between one fourth and one fifth of patients with cancer will have brain metastases at autopsy.8–10 This prevalence at autopsy translates into 112,620 to 140,775 cancer patients per year who will die with brain metastases, based on the American Cancer Society’s 1999 estimate of 563,100 cancer deaths11—an increase over previous figures. How much of this increase is real is unclear. An increased incidence of lung cancer and melanoma, longer survival times of patients with cancer, and an aging patient population may have resulted in a true increase. However, a more adequate representation of brain metastasis in more recent neurosurgical series, advances in neuroimaging techniques, and routine staging that assesses the CNS may have artificially inflated the figures.
The incidence of brain metastasis and the spectrum of metastasizing primary cancers vary with patient age.9,12–14 Brain metastases occur more frequently in adults than in children.6,8,15–19 Among adults, the highest incidence is observed in the fifth to seventh decades.9,15 The most common sources of brain metastases in this patient group are cancers of the lung, breast, and skin, in descending order. In children, the most common cause of brain metastasis is leukemia, followed by lymphoma.9 Osteogenic sarcoma and rhabdomyosarcoma are the most frequent causes of solid brain metastases among children younger than 15 years, whereas germ cell tumors are the most frequent producers of brain metastases in patients 15 to 21 years old.15
The overall incidence of brain metastasis is not affected by the patient’s gender, nor is the incidence of brain metastasis from a given primary tumor. The only apparent exception is melanoma, which is more likely to spread to the brain in male patients.9,20,21 The fact that melanoma primaries in males develop in locations that are more likely to spread to the brain, namely the head, neck, or trunk, could explain this observation.22,23 Overall, differences in the incidence of primary cancers between the two sexes result in differences in the sources of brain metastasis in male and female patients. For example, lung cancer is the most common source of brain metastasis in men, whereas breast cancer is the most common source in women.2,9
The primary tumor’s histologic type appears to be the most important dictator of the frequency and pattern of intracranial extension. Lung cancer, breast cancer, melanoma, renal cancer, and colon cancer account for most brain metastases and are listed in order of decreasing relative frequency. Primary lung tumors account for 30% to 60% of all brain metastasis cases.9,24–32 Breast cancer ranks second, contributing 10% to 30% of all brain metastases among women.9,24,25,27,30,32–34 Melanoma ranks third; of patients with brain metastases, approximately 5% to 21% have melanoma as the primary tumor.16,27,28,30–32,35 Renal and colon cancers infrequently metastasize to the brain. Metastases to the brain are even rarer from other types of cancers, such as sarcoma and genitourinary primaries.36–44 Virtually any malignancy can metastasize to the brain, however, and patients with no known history of cancer frequently present with symptoms caused by a brain metastasis from an undiagnosed primary malignancy. The frequency of such presentation varies.45
The picture is different when one considers the ability of a primary tumor to spread to the brain. Interestingly, malignant melanoma, which represents only 4% of all cancers,46 has the highest propensity of all systemic malignant tumors to metastasize to the brain.23,35,47 The incidence of brain metastases among patients with malignant melanoma varies from 6% to 43% in clinical series48–50 and from 12% to 90% in autopsy series23,35,47,51; it was 6.9% in a recent large, population-based study of the incidence of brain metastasis from single primary cancers.52 Lung cancer ranks second in overall number of brain metastases produced. Of patients with lung cancer, 18% to 65% will develop brain metastasis,9,53–55 and the primary tumor histology is very important in determining metastatic frequency. Indeed, more than 40% of patients with small cell lung cancer (SCLC) and lung adenocarcinoma have brain metastases at autopsy, a prevalence of more than twice that found in the other types of lung carcinoma such as squamous cell carcinoma.9,56,57 Breast cancer ranks third in overall contribution to brain metastases. Historically, it has been suggested that approximately 20% to 30% of patients with breast cancer will develop brain metastasis.6,12,33,35,53,58 However, a large population-based study by Barnholtz-Sloan and colleagues52 showed that only 5.1% of breast cancer patients with a single primary tumor developed brain metastasis and that renal cancer had a slightly higher cumulative incidence of 6.5%.
Treatment Modalities
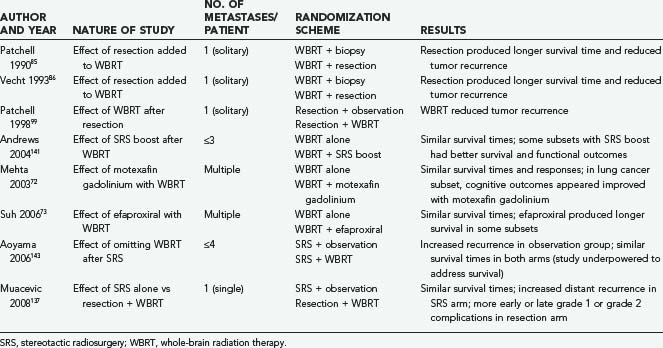
High-dose corticosteroids constitute the initial treatment of patients with symptomatic brain metastases, with the objective of decreasing the edema that typically surrounds these tumors and helping to restore neurological function. Systemic chemotherapy is not very effective against the most common types of primary tumors metastasizing to the brain, which tend to be chemoresistant; however, it appears to be a useful adjunct to other therapies against metastases from SCLC and germ cell tumors. The major weapons in the clinician’s arsenal against brain metastases include whole-brain radiation therapy (WBRT), surgical resection by open craniotomy, and stereotactic radiosurgery (SRS). Table 130-1 lists some key randomized clinical trials relevant to the management of brain metastases.
Radiation Therapy
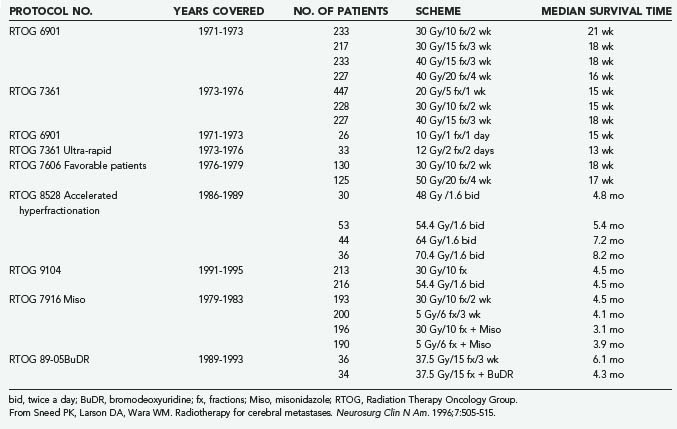
For the past 50 years, radiation therapy has played a major role in the palliation of metastatic brain disease. In 1954 Chao and coworkers59 were the first to report the use of WBRT for the treatment of brain metastases. Subsequently, numerous publications (reviewed by Sawaya and associates60) have considered the role of WBRT in treating brain metastases. WBRT (20 to 40 Gy delivered over 1 to 4 weeks) results in a median survival time of 4 to 6 months, as established by trials conducted by the Radiation Therapy Oncology Group (RTOG)61 (Table 130-2). In terms of improved symptoms, the published response rate ranges from 70% to 90%.61,62 Headaches, seizures, or symptoms of increased intracranial pressure show a complete response to WBRT in more than 50% of cases, but the durability of that response at 1 year is 65%. Cranial nerve deficits also improve in more than 40% of patients.62
Patient Parameters and Prognostic Factors
Although WBRT can provide effective palliation of brain metastases and can reduce the likelihood of death due to neurological causes, which translates into improved quality of life, patient-related factors such as age, performance status, presence of extracranial metastases, and status of the primary tumor remain the primary determinants of patient outcome.63,64 Patient parameters are an important database for the evaluation of WBRT treatment response and for the prediction of patient outcome when similar patient groups are compared. To identify favorable subgroups of patients for future protocols, Diener-West and colleagues63 used multivariate analysis in a large RTOG study (RTOG 7916) and identified four factors associated with improved survival: Karnofsky Performance Scale (KPS) score of 70 or greater, an unknown or controlled primary tumor, age younger than 60 years, and metastatic spread limited to the brain. Patients with all four favorable characteristics had a predicted 200-day survival of 52%. Patients with none of the favorable factors had a predicted survival time of 1.8 months.63 These prognostic factors were identified again when a database from three consecutive RTOG trials examining dose escalation and radiosensitizers was subjected to recursive partitioning analysis.64 Patients were sorted into three classes. Class 1 included patients with a KPS score of 70 or greater who were younger than 65 years and had a controlled primary tumor and no extracranial metastases; these patients experienced a median survival time of 7.1 months. Class 3 included patients with a KPS score less than 70; they survived for a median of only 2.3 months. Class 2 included all remaining patients.64
Dose-Fractionation Schemes for Whole-Brain Radiation Therapy
The optimal dose-fractionation schedule was studied by the RTOG using cobalt 60 and linear accelerator equipment (4 to 6 MV). Two studies using five different fractionation schemes were reported together: RTOG 6901 and RTOG 7361 (see Table 130-2).61 All treatment schedules were comparable with regard to frequency and duration of improvement, time to progression, survival, and palliative index.61 The median survival times in these two studies were 18 weeks and 15 weeks, respectively. WBRT improved neurological function in approximately 50% of patients.61 An optional “ultra-rapid high-dose irradiation schedule” was used to randomize patients to treatment with either 10 Gy in 1 fraction or 12 Gy in 2 fractions.65 These patients were compared with control patients receiving 20 to 40 Gy during a period of 1 to 4 weeks. The improvement in neurological function in patients receiving the ultra-rapid treatment was comparable to that of patients receiving more protracted schedules. Promptness of response, morbidity, and median survival time were also comparable. However, duration of improvement, time of progression to improved neurological status, and rate of complete disappearance of neurological symptoms were generally less favorable for patients receiving 10 to 12 Gy, leading the authors to conclude that ultra-rapid schedules may not be as effective as higher-dose schedules in palliating brain metastases.65
A follow-up study of patients from the first two studies who had a favorable prognosis was performed by the RTOG.66 Its purpose was to test the hypothesis that for selected patients with a favorable prognosis, the duration of palliative effect is greater with higher total doses of radiation. The study found no advantage in treating patients with more than 20 Gy in 1 week; thus, it was concluded that this schedule could be used for effective palliation with less inconvenience and cost to the patient, although late effects of radiation were not addressed. Gelber and coworkers66 classified ambulatory breast cancer patients with no soft tissue metastases, ambulatory lung cancer patients with the primary not found or with no extracerebral metastases, and ambulatory patients with other primaries and no extracerebral metastases as favorable subgroups who had a median survival of 28 weeks, in contrast to 11 weeks for the remaining patients. Many radiation oncologists commonly prescribe 30 Gy of WBRT in 10 fractions and adjust fractionation based on expected prognosis. A hypofractionated course of WBRT should be reserved for patients with severely limited life expectancies, and more protracted courses can be given to patients with more favorable prognoses.
Altered Fractionation Schemes
An RTOG phase I-II trial of accelerated fractionation for brain metastases suggested that dose escalation may improve survival.67 An incremental nonstatistically significant improvement in survival was noted with escalating doses. A follow-up randomized phase III study was performed that assigned 445 patients who had not undergone resection and whose KPS scores were 60 or greater to undergo either accelerated hyperfractionation (at a dose of 1.6 Gy twice a day to an end point of 54.4 Gy) or accelerated fractionation (30 Gy in 10 fractions). The phase III trial failed to demonstrate any improvement in survival in the group receiving 54.4 Gy.68
Radiosensitizers
The RTOG evaluated the radiation sensitizer misonidazole by randomly assigning patients to four treatment arms: 3 Gy in 10 fractions with or without a 1 g/m2 dose of misonidazole versus 5 Gy in 6 fractions with or without a 2 g/m2 dose of misonidazole. Patient survival times did not vary significantly among the four treatment arms.69
Bromodeoxyuridine, a halogenated pyrimidine that has been studied in the treatment of malignant gliomas, was evaluated for use against brain metastases in an RTOG trial in which 72 patients were randomly assigned to treatment with WBRT (37.5 Gy in 15 fractions) either with or without bromodeoxyuridine at a dose of 0.8 g/m2 per day for 4 days on each of 3 consecutive weekends.70 Although the drug caused significant grade 4 and grade 5 hematologic and skin toxicity in five patients, there was no significant difference in patient survival between the two treatment arms.
Further investigation of radiosensitizers has continued with the evaluation of gadolinium texaphyrins.71 Motexafin gadolinium (MGd) was used in a randomized controlled trial that evaluated survival as well as neurological and neurocognitive function in 401 patients with multiple brain metastases (including 251 with non–small cell lung cancer [NSCLC]) who underwent WBRT (see Table 130-1).72 Patients were randomly assigned to receive 30 Gy with or without 5 mg/kg per day of MGd. Although overall survival times and responses were similar in the two trial arms, in the subset of patients with lung cancer, MGd administration appeared to produce better cognitive outcomes.
Subsequently, Suh and associates73 conducted a phase III study of the use of efaproxiral, a noncytotoxic radiosensitizer, as an adjuvant to WBRT in 515 patients with multiple brain metastases (see Table 130-1). Again, overall survival times were not significantly different between the groups receiving and not receiving the radiosensitizer, but the subset of patients with breast cancer who received efaproxiral had better survival (although not significantly so).
Prophylactic Cranial Irradiation for Small Cell Lung Cancer
Patients with SCLC may be considered for prophylactic cranial irradiation (PCI) because of their high likelihood of developing brain metastases and consequent neurological deficits. About 10% of SCLC patients have metastasis to the CNS at diagnosis, and another 20% to 25% will develop CNS metastasis later on.74 There is debate regarding the use of PCI because of concerns that it may contribute to neurological deficits. The pertinent issues are quality of life and survival. The Prophylactic Cranial Irradiation Overview Collaboration Group reported a meta-analysis in which data on 987 patients with SCLC in complete remission were collected in seven trials that randomized patients to receive or not receive PCI.75 The main end point of the study was survival. The risk of death in the treatment group relative to the control group was 0.84 (P = .01), corresponding to a 5.4 percentage point increase in the rate of survival at 3 years (15.3% in the control group versus 20.7% in the treatment group). PCI also decreased the relative risk (RR) of recurrence or death to 0.75 (P < .001) and decreased the cumulative RR of brain metastasis to 0.46 (P < .001). Larger doses of radiation led to greater decreases in the risk of brain metastasis, according to an analysis of four total doses (8 Gy, 24 to 25 Gy, 30 Gy, and 36 to 40 Gy) (P for trend = .02), but the effect on survival did not differ significantly by dose. Critics point to the neurocognitive impairment seen in patients who have undergone PCI. However, the two largest trials in this meta-analysis used neuropsychological tests to evaluate most patients before, during, and after treatment; neurocognitive impairment was often detected at initial diagnosis, but no deterioration was found after PCI.76,77 This meta-analysis makes a strong case for using PCI as standard treatment in all patients with SCLC in complete remission. To minimize neurological toxicity, PCI should not be given concurrently with chemotherapy.78 Determining the optimal sequencing and dose of PCI to maximally reduce the incidence of brain metastasis while minimizing toxicity will be the goal of future trials.
Complications of Whole-Brain Radiation Therapy
Acute effects of WBRT include mild fatigue, reversible hair loss, mild scalp erythema, and hyperpigmentation. Somnolence syndrome, described as persistent fatigue, anorexia, and irritability (especially in children), may occur 3 to 10 weeks after WBRT and resolve within 6 weeks.79,80 In long-term survivors with metastatic brain disease, long-term toxicities associated with WBRT can become apparent. DeAngelis and colleagues81 reported a series of 12 patients who developed progressive dementia, ataxia, and urinary incontinence within 5 to 36 months of treatment with WBRT, causing severe disability and leading to death in 7 patients; computed tomography (CT) scans showed cortical atrophy and hypodense white matter in all 12 patients. Nevertheless, Patchell and Regine82 have suggested that the frequency of long-term neuropsychological side effects of WBRT in adult patients with brain metastases may be overestimated. Although 5 of 47 patients (11%) in DeAngelis’s study experienced dementia 1 year after WBRT for nonrecurrent brain metastases, all 5 received either abnormally high daily radiation fractions (3 to 6 Gy—a dose not currently given) or radiation-sensitizing agents, potentially increasing damage to normal tissue.81 Yet none of the 15 patients who were treated with more modern fractionation schemes (<3 Gy/fraction) had dementia at 1 year. Moreover, Langer and Mehta83 recently showed that the risk of neurological decline induced by recurrent disease outweighs the potential loss of neurocognitive function after WBRT. Although the untoward long-term effects of WBRT are probably less significant than previously thought, neurocognitive decline remains a possible complication, and it may be reasonable to consider administering WBRT in daily fractions of 1.8 to 2 Gy to a total of 40 to 45 Gy to reduce long-term sequelae in patients with more favorable prognoses.
Surgical Resection
Surgical resection is an important component in the therapeutic arsenal for cerebral metastases. Although initial reports from the early 20th century concluded that surgery was not warranted because of high morbidity and poor postoperative survival,84 advances in surgical technique since the 1970s have dramatically decreased the operative complication rates and increased survival times. Most important, two prospective randomized trials from the early 1990s (see Table 130-1) demonstrated that surgery followed by WBRT is superior to WBRT alone for patients with single brain metastases and good neurological performance scores.85,86 Moreover, recent reports have suggested that surgery may benefit patients with multiple or recurrent metastases, who have traditionally been excluded from surgical intervention.87,88 Thus, in the modern era, surgery is often considered the primary and optimal treatment of brain metastases.
Surgery has certain advantages over other treatments. First, complete excision of a metastatic lesion provides palliation by immediately eliminating the effects of increased intracranial pressure and the direct irritation of surrounding brain tissue. This effect may be greater for metastases than for primary intraparenchymal tumors because metastases grow by expansion and compression rather than by infiltration and often produce a large amount of edema. Although corticosteroids may provide immediate palliation of symptoms, their effects are not long lasting. Second, surgery provides tissue to confirm the diagnosis of metastasis. This is important because as many as 10% to 15% of patients with a clinical diagnosis of metastasis may actually have nonmetastatic lesions such as abscesses or primary tumors.85 Last, surgery may provide local cure if all the tumor cells are removed. These advantages must be weighed against the requisite invasiveness of surgery, which subjects patients to potential intraoperative and postoperative problems, including bleeding, wound infection, pulmonary emboli, myocardial infarction, and sepsis.
Patient Selection and Prognostic Factors
Radiographic Features
Tumor Number
Patients with single brain metastases are the most appropriate surgical candidates. Oldberg89 was the first to recognize that surgery for single brain metastases could result in longer survival times than other treatments. Multiple retrospective surgical series consistently verified this finding, but it was not until Patchell and colleagues85 and Vecht and associates86 reported the results of their randomized prospective trials that surgical resection became the standard treatment for single brain metastases. These studies demonstrated that patients with single metastases who were treated with surgery and radiation lived statistically longer, had fewer recurrences, and had a better quality of life than patients treated with WBRT alone. One caveat about these studies is that they included patients with limited systemic disease and those with a KPS score greater than 70.90 This is important because a randomized study by Mintz and coworkers91 that failed to demonstrate any advantage of surgery over WBRT included many patients with extensive systemic disease and poor performance status. Thus, the value of surgery for single brain metastases may apply only to patients with the potential for long-term survival (see later).
For patients with multiple metastases, the role of surgery is more controversial. The historical bias against resecting multiple brain metastases was questioned by Bindal and coworkers,87 who retrospectively reviewed 56 patients who underwent resection of two or three brain metastases at The University of Texas M. D. Anderson Cancer Center. Among these patients, 30 had one or more lesions left unresected (group A), and 26 underwent resection of all lesions (group B). These patients were compared with 26 matched controls with single surgically resected metastases (group C). There was no difference in surgical mortality (3%, 4%, and 0% for groups A, B, and C, respectively) or morbidity (8%, 9%, and 8%), regardless of treatment group. Patients with multiple metastases that were all resected (group B) had a significantly longer survival time (median, 14 months) than patients with multiple metastases in whom some lesions were not resected (group A; median survival, 6 months). The survival of patients in group B was similar to that of patients with resected single metastases (group C; median survival, 14 months). Thus, the authors concluded that resecting multiple brain metastases (typically two to four) is as effective as resecting a single brain metastasis as long as all the lesions are resected. Although intriguing, these results have not been confirmed by a prospective randomized trial. Moreover, it is important to note that no more than three metastases (per patient) were treated in Bindal’s study, and most patients received WBRT after surgery.
Tumor Size
The size of a metastasis also influences surgical decision making. Although tumor size has never been shown to influence survival after surgery, it has become an increasingly important factor in decision making because of the possibility of treating metastases with SRS (discussed later). Tumors can be divided into three groups, according to size. First are tumors greater than 3 cm in maximal diameter. For such large tumors, surgical resection is the primary and best option because these lesions are too large for SRS. Second are very small tumors measuring less than 5 mm in maximal diameter. For these lesions, SRS is probably most appropriate (see later), particularly if they are located deep within the brain. In our experience, the trend toward screening cancer patients for brain metastases using contrast-enhanced MRI has resulted in the early detection of small, asymptomatic lesions that would not have been detected in the past, even with CT. It can be argued that these tumors are most suitable for SRS, considering the potential difficulties associated with locating such small lesions during surgery.92 Last are intermediately sized metastases that typically range from 1 to 3 cm in greatest diameter. Decision making is particularly challenging for these lesions because in many cases surgery and SRS may be considered equally appropriate. This is the group for which a randomized prospective study is required to determine which approach is more efficacious (see later). However, until such a trial is completed, the decision for surgical intervention must rely on an assessment of other variables such as the potential for surgical morbidity, the need to reverse neurological deficits, the extent of systemic disease, and the presence of medical comorbidities.
Clinical Assessment
The most significant determinant of a patient’s ultimate outcome is the status of the systemic disease, which is defined as the activity and extent of the primary tumor and systemic (noncerebral) metastases. The importance of systemic disease status in determining outcome has been emphasized in nearly all the studies examining factors that predict survival. Moreover, in the prospective randomized trial of Patchell and colleagues,85 as many as 70% of patients undergoing surgery for single brain metastases died from progression of systemic disease rather than from neurological causes. The importance of the extent of systemic disease was further illustrated in the prospective study of Mintz and coworkers,91 who failed to find any benefit of surgery compared with standard fractionated WBRT for patients with single brain metastases. In this study, more than 45% of patients had external metastases, and 41% had KPS scores of 50 or less. In contrast, only 38% of patients in Patchell’s study had external metastases, and all patients had KPS scores of 70 or greater.85 Thus, these studies suggest that extensive external disease may make it more difficult to detect a survival advantage for surgery. It has been suggested that to reap the benefits of surgical resection, patients should have a life expectancy of more than 3 to 4 months, based on the extent and activity of their systemic disease. To state it another way, surgery is most beneficial to patients with absent, controlled, or limited systemic disease.
Postoperative Whole-Brain Radiation Therapy
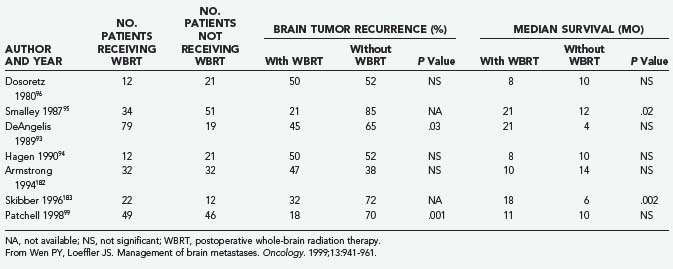
The role of postoperative WBRT has not been clearly defined. WBRT theoretically destroys residual cancer cells at the site of resection, as well as microscopic deposits at other sites. However, it is unclear whether postoperative adjuvant WBRT should be given to all patients after surgical resection. Although several retrospective studies examining WBRT after resection of single brain metastases have shown a beneficial effect,93–95 others have not shown any benefit (Table 130-3).31,96,97 Moreover, WBRT is associated with a significant risk of dementia and other long-term neurotoxicities. Looper and associates98 noted a high incidence of severe neurological problems after combined modality therapy (chemotherapy and WBRT) in long-term survivors with SCLC. Sundaresan and Galicich31 noted that after surgical resection, 50% of 2-year survivors developed hydrocephalus ex vacuo or evidence of leukoencephalopathy on CT scans; almost 17% developed evidence of radiation necrosis.
Patchell and colleagues99 reported the results of a randomized prospective trial examining the benefits of adjunctive WBRT in the surgical treatment of single brain metastases (see Table 130-1). After surgery, patients were randomly assigned to either treatment with 50.4 Gy over 5.5 weeks or observation (median follow-up, 43 weeks), and the patients were classified according to extent of disease and primary tumor type. The patients who received WBRT showed a striking reduction in tumor recurrence (distant and local) relative to the observation group (18% versus 70%; P < .001). The local recurrence rate was 20% in the surgery only group and 3% in the surgery plus WBRT group, and patients in the radiotherapy group were less likely than those in the observation group to die of neurological causes (14% versus 44%; P = .003). Nevertheless, overall patient survival was not improved by adjunctive WBRT. Moreover, the KPS scores for patients undergoing WBRT declined at the same rate as the scores of those in the surgery only (observation) group, raising the possibility that the toxicity of WBRT offsets its beneficial effect. An unexplained result was that among patients who died from systemic disease, those not receiving WBRT survived longer than those in the observation group. Although the authors concluded that WBRT is a valuable adjunct to surgical resection (partly on the basis of preventing death from neurological causes), the lack of overall survival improvement, the use of a higher than standard radiation dose (50 Gy rather than 30 Gy), and the potential for radiation toxicity leave some unresolved questions regarding the best treatment for patients with single brain metastases. Confirmation of these findings with more careful assessments of cognitive function would be helpful.
For patients with tumors having so-called radioresistant histologies, including metastatic melanoma and RCC, postoperative WBRT is controversial. Based on the study by Patchell and colleagues,99 it is difficult to draw conclusions for patients with RCC or melanoma because each arm of the study contained only one melanoma patient and an unspecified number of RCC patients. A randomized trial of postoperative WBRT exclusively for RCC or melanoma patients is needed to resolve the controversy.
Surgical Techniques
Metastasis Anatomy
As viewed microscopically, brain metastases are composed of a solid tumor mass without intervening brain tissue. There may be some degree of infiltration, but this typically does not extend beyond a radius of 5 mm from the solid tumor.31,60,100 There may be a central area of necrosis in larger lesions. At the macroscopic level, typical cerebral metastases are round and well demarcated from the surrounding edematous brain. Tumor cysts may also be present, particularly in metastases from bronchogenic carcinoma. At surgery, a gliotic pseudocapsule is often identifiable surrounding the metastasis. Dissection in this gliotic plane generally ensures gross total resection because there are typically no tumor cells in this zone. The tumor mass corresponds to the region of contrast enhancement seen on CT or MRI.101
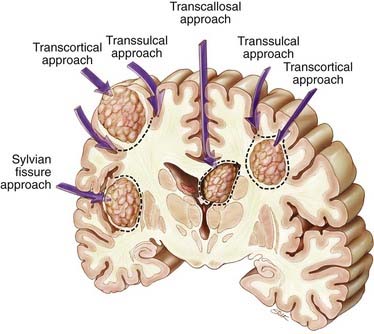
Supratentorial metastases can be surgically defined by their relationship to adjacent sulci and gyri (Fig. 130-1).101–103 Metastases may occur superficially just below the cortex, filling a gyrus (subcortical); deep within a sulcus, either at the side of the sulcus (subgyral) or at its base (subsulcal); or deep within the white matter, independent of a single sulcus or gyrus (lobar). These same patterns may arise near the cerebral fissures. For example, tumors in the subinsular cortex are deeply located relative to the sylvian fissure. Midline metastases, such as those in the cingulate gyrus, should be viewed in relation to the interhemispheric fissure. Metastatic tumors occasionally arise within the ventricles (see Fig. 130-1).
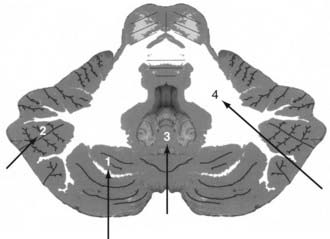
The less common cerebellar metastases can be divided into deep and hemispheric locations. Hemispheric lesions can be categorized as lateral or medial; a subset arises directly within the vermis. Cerebellar tumors can be subdivided into those occurring in superior and inferior locations (Fig. 130-2).
Surgical Approaches
Surgical approaches are based on the anatomic location of the brain metastasis.101,102 Supratentorial subcortical lesions are best resected by an incision in the apex of the sulcus and circumferential dissection of the tumor (transcortical approach; see Fig. 130-1). Removal of a cortical plug above the lesion improves exposure. This may be problematic when the lesion arises within eloquent cortex. In these situations, a longitudinal incision dictated by local mapping with direct brain stimulation and, for large metastases, an “inside-out” piecemeal (rather than en bloc) resection may minimize injury to the surrounding brain.
Lesions in the subgyral or subsulcal location are best approached by splitting the sulcus leading to the lesion. Subgyral tumors are removed by making an incision in the side of the split sulcus, whereas subsulcal lesions are entered at the sulcal base (transsulcal approach; see Fig. 130-1).
Metastases located deep within the white matter, independent of a single sulcus or gyrus (lobar), can be approached either transcortically or transsulcally (see Fig. 130-1). Tumors in the subinsular cortex can be approached by splitting the sylvian fissure. Midline metastases are best approached by splitting the interhemispheric fissure. Tumors can then be resected by further splitting or entering a deep gyrus (see Fig. 130-1). Intraventricular lesions can be approached transcallosally or transcortically (Fig. 130-3; see also Fig. 130-1).
Cerebellar tumors are best approached along the shortest transparenchymal route to the lesion (Fig. 130-4; see also Fig. 130-2). Superior hemispheric lesions are approached via the supracerebellar cistern and by incising the cerebellum at the closest point to the tumor. This requires a high suboccipital craniotomy with exposure of the transverse sinus. Lateral hemispheric lesions are approached directly from a posterior trajectory. Entering the paracerebellar cisterns is generally not necessary, thus avoiding exposure of the cranial nerves. Inferior cerebellar tumors require opening of the foramen magnum. Midline tumors can be resected after splitting the vermis.
Reoperation for Recurrent Metastases
Brain metastases may recur locally if all the neoplastic cells are not removed from the tumor bed at surgery. New metastases arising at sites other than the original location are termed distant recurrences. Recurrent tumors are found in up to 40% of patients.31,87,88 At M. D. Anderson, Bindal and coworkers88 studied outcomes retrospectively in 48 patients who underwent surgery for recurrent brain metastases. No operative morbidity or mortality was reported, and 75% of patients improved neurologically after surgery. These patients survived for a median of 11.5 months, with 26% surviving for 2 years and 17% for 5 years. These results are consistent with those of a prior study of the same type104 and indicate that in appropriate patients, reoperation for recurrent brain metastases can improve the quality of life and increase survival time.
Outcome and Prognosis
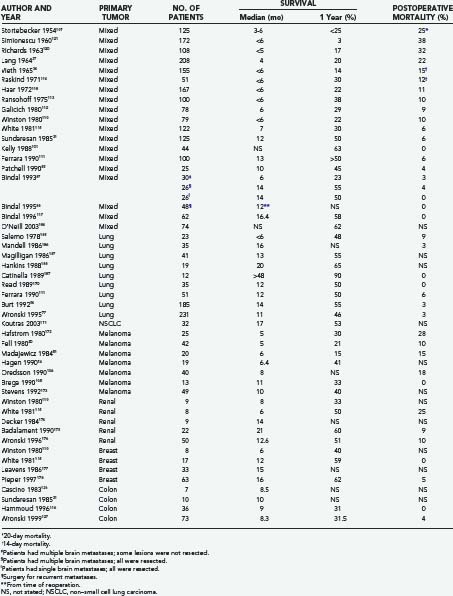
Table 130-4 lists the results of major series of patients treated surgically for brain metastases. These studies include primary tumors with different histologies. Most of the series are retrospective and report on selected patients with single brain metastases, limited systemic disease, and good neurological function. Although some series include small numbers of patients with multiple metastases,20,31,97,105,106 Bindal and colleagues87 reported the only series dedicated to surgery for these patients. In many of the earlier series, WBRT was not routinely used,84,107–110 whereas in most of the later series, a high proportion of patients received WBRT.31,85,96,101,111–115 Corticosteroids were used perioperatively in most series published after 1960.
Surgical Mortality
Most studies define surgical mortality as death that occurs within 30 days of operation, although some of the earlier surgeons used shorter periods.34,109,114 Other series include death occurring after 30 days if the patient did not leave the hospital.107,112 Surgical mortality has decreased dramatically since the earliest reports. For example, Cushing107 found that the mortality after resection of brain metastases (38%) was quite high compared with that for other tumor types such as astrocytoma (15%) and gliobastoma multiforme (23%). In contrast, in the 1990s, using modern techniques, surgical mortalities of 3% or less have often been reported. In fact, some of the more recent series report no mortality after surgery for brain metastases (see Table 130-4).88,105,116,117 In the randomized trial of Patchell and associates,85 the 30-day operative mortality and the 30-day postradiotherapy mortality were both 4%. Thus, surgery was no riskier than WBRT in the short term. Bindal and colleagues87 found no difference in mortality after a craniotomy for single lesions (0%) compared with multiple craniotomies for multiple lesions (0%).
Postoperative Morbidity
Postoperative morbidity after surgery for brain metastases includes worsening of neurological status and nonneurological complications such as postoperative hematoma, wound infection, deep venous thrombosis, pneumonia, and pulmonary embolism. Some studies separate these two aspects of morbidity,31,87,88,102 others consider them together,85,86,118 and a few report only neurological morbidity27,110,111,114,119; many do not report morbidity at all.34,84,109,112,113,115,120,121 In the modern era, neurological worsening can be expected to occur in 5% or fewer of patients undergoing surgery for brain metastases.31,85,87,105 Nonneurological complications typically occur in 8% to 10% of patients.87,122,123 Patchell and associates85 reported a favorable operative morbidity of 8%, compared with a 30-day postradiotherapy morbidity of 17%.
Leptomeningeal disease (LMD) is a relatively rare but serious complication of metastatic brain disease. A recent study at M. D. Anderson considered whether 260 patients with brain metastases in the posterior fossa who underwent conventional resection were at greater risk for LMD than 119 patients undergoing SRS.124 Although there was no significant difference in the risk for LMD in patients undergoing en bloc tumor resection or SRS, piecemeal tumor resection (137 cases) was associated with a significantly higher risk of LMD than en bloc resection (123 cases; P = .006) or SRS (P = .006). A similar study at M. D. Anderson that focused on the impact of surgery on the leptomeningeal dissemination of supratentorial metastases also found a significantly increased risk of LMD with piecemeal resection relative to en bloc resection (P = .009) or SRS (P < .001).125 These studies are the first to indicate that the way a brain tumor is resected can affect its dissemination, and further assessment of this aspect of resection is warranted in a controlled prospective setting.
Survival
Table 130-4 reveals that both the median and 1-year survival times for patients with brain metastases have improved in the modern era (post-CT era). This is a reflection of improvements in both neurosurgical management and the control of noncerebral systemic disease. Overall, modern neurosurgery (from about 1975 to the present) for these lesions is associated with a median survival time of 11 months (range, 6 to 16 months) and a 1-year survival of 42% of patients (range, 22% to 63%). Kelly and coworkers101 reported a 1-year survival of 63% using computer-assisted stereotactic craniotomy. Studies from M. D. Anderson report a median survival time of 14 months and a 1-year survival of 50% for patients with solitary brain metastases.87,88 Similar median (14 months) and 1-year (55%) survivals were observed in patients with multiple metastases in whom all the lesions were removed.87
The effects of surgery on brain metastases may be better estimated by using death from neurological causes (alone) as the outcome statistic. Patchell and associates85 reported a median “neurological survival” of 16 months after surgery and WBRT, compared with only 6 months after WBRT alone. In the same study, there was no difference in survival between the surgery and irradiation groups when death due to systemic causes alone was used as the end point. As one would anticipate, resection of brain metastases may treat the cerebral disease, but it does not alter the progression of disease outside the nervous system.
Table 130-4 also shows the survival data for patients with brain metastases originating from different tumor types. In the case of lung cancer, SCLC is particularly radiosensitive and represents only a minority of cases in most surgical series. NSCLC is best treated by surgical resection and radiation therapy. Almost all the studies from the post-CT era included in the table represent outcomes for patients having single brain metastases. Based on the combined results of these studies, the median survival time after surgical resection is 14 months, with a 1-year survival of 57% of patients.
Representative series of patients treated in the post-CT era for melanoma metastatic to the brain are also shown in Table 130-4. The combined results of these series indicate a median survival time of 7 months (range, 5 to 11 months) and a 1-year survival of 30% of patients after surgical resection.
Although patients with brain metastases from melanoma have poorer survival after surgery than patients with other types of cancer, those who undergo surgery show better results than those who do not. In 1980 Fell and colleagues20 retrospectively reviewed 80 patients treated at M. D. Anderson and found a significant difference in median survival between the group that underwent operation (5 months, n = 42) and the group that did not (6 weeks, n = 38). These authors attempted to correct for selection bias by comparing the survival figures for all patients resected (n = 23) and unresected (n = 29) who were known to have widespread melanoma (stage 4) when their brain metastases were diagnosed; surgery was still beneficial in this subset of patients. In addition, surgery was associated with neurological improvement in 88% of patients, whereas radiotherapy and chemotherapy led to neurological improvement in only 39% of patients. They also noted that patients with multiple brain metastases fared better with craniotomy than without.20
Based on the post-CT era surgical series included in Table 130-4 that report breast cancer metastases to the brain, a median survival time of approximately 12 months and a 1-year survival of 54% of patients can be expected. As with other tumor types, these series include primarily patients with single metastases and limited extracranial disease.
There are few reports on the surgical treatment of brain metastases from RCC. The compiled results of the post-CT era series included in Table 130-4 suggest a median survival time of 12 months and a 1-year survival of 48% of patients after surgical resection.
Similarly, brain metastases from colon carcinoma are rare.8,60 They occur late in the course of the disease126 and do not respond well to radiation therapy or chemotherapy, making resection the best treatment for patients whose medical condition permits it. The median survival time for such patients averages 9 months, with more recent studies showing a 1-year survival of about 31% of patients.116,127
Stereotactic Radiosurgery
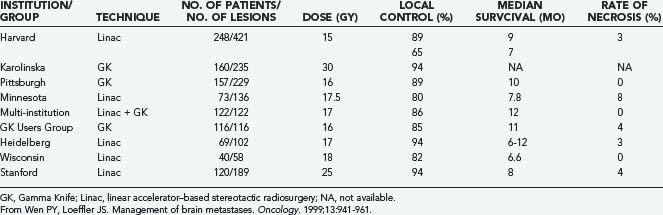
A growing body of experience from different institutions supports the use and effectiveness of SRS for brain metastases (Table 130-5). SRS is a technique of external beam radiation that uses multiple noncoplanar beams to deliver a single concentrated dose of radiation to a small target volume.71 Stereotactic radiation techniques exhibit rapid dose falloff at the target edges, permitting significant sparing of normal brain tissue.71,128
Stereotactic Radiosurgery versus Conventional Surgery
Clearly, there are benefits to both conventional surgery and SRS. The advantages of surgery include immediate resolution of mass effect, procurement of tissue for pathologic diagnosis, and no risk of radiation necrosis.129 The advantages of SRS include decreased risk of hemorrhage and infection and no risk of tumor seeding. SRS is also less invasive, is potentially less costly, and requires shorter hospital stays than standard craniotomy. SRS has even been shown to ameliorate symptoms from tumors resistant to conventional radiation, such as melanoma.60 Median survival times of 6 to 10 months have been reported.130–132 Disadvantages of SRS include the potential exacerbation of peritumoral edema, the requirement for long-term steroid administration, and radiation necrosis.100,132,133
Auchter and coworkers134 reported a multi-institutional retrospective outcome and prognostic factor analysis of patients with single cerebral metastases who were treated with SRS plus WBRT. The goal of the study was to “examine the results of radiosurgery in a population of patients that would be considered eligible for surgical resection.” From their database of 533 patients with brain metastases treated with SRS and WBRT, they selected 122 patients who fulfilled the criteria for surgical resection established in the prospective randomized trial of Patchell and colleagues85: at least 18 years old and with a single brain metastasis that was surgically resectable, no prior radiotherapy or surgical treatment, independent functional status (KPS score ≥70), a nonradiosensitive tumor, and no urgent need for surgery.
Auchter’s group134 compared the outcome of these 122 patients with that of the patients treated with surgery and WBRT reported in the randomized trials of Patchell and colleagues85 and Noordijk and associates.135 The actuarial median survival was 56 weeks after SRS plus WBRT,134 compared with 40 weeks85 and 43 weeks135 after surgery plus WBRT. Death was attributed to progressive CNS disease in 25% of patients undergoing SRS plus WBRT,134 compared with 29%85 and 35%135 of patients who underwent surgery plus WBRT. The median duration of functional independence was 38 weeks after SRS and WBRT,134 compared with 38 weeks85 and 33 weeks135 after surgery and WBRT. There was local recurrence in 14% of patients undergoing SRS plus WBRT,134 compared with 20%85 of patients treated with surgery and WBRT. The authors reported “no treatment-related deaths or major acute toxicity” after SRS.134 These comparisons suggested to Auchter and coworkers that SRS combined with WBRT produces better outcomes than surgery combined with WBRT, and they favor the use of SRS rather than surgery for patients with single brain metastases.
Bindal and colleagues117 also reported a retrospective comparison of SRS and conventional surgery in patients with brain metastases. This analysis matched 31 patients who underwent SRS with 62 patients who underwent conventional surgery for these lesions. Patients were matched on the basis of age, sex, primary tumor histology, extent of systemic disease, pretreatment KPS score, time to appearance of brain metastases, and number of brain metastases. Patient eligibility criteria for SRS were the same as those for surgery. Additional criteria for SRS included the presence of small lesions (<3 cm in maximal diameter) and patient preference for that treatment. Retrospective analysis of the tumors treated by SRS revealed that 81% were surgically resectable lesions.
The authors reported a median survival time of 7.5 months for the SRS group and 16.4 months for the surgery group.117 The 1-year survival rate was 27% for the SRS group and 58% for the surgery group. Fifty percent of patients treated with SRS died from neurological causes, compared with only 19% of patients treated surgically. The 1-year neurological survival (i.e., freedom from neurological death) for the surgery group was 83%, compared with 40% for the SRS group. Similarly, 13% of surgically treated patients suffered a local recurrence, whereas 39% of SRS patients suffered local progression of disease. The complication rate was higher in the SRS group (23%) than in the surgery group (5%). Three patients in the SRS group eventually underwent surgery for tumor resection because SRS failed to control their lesions. In contrast to the conclusions of Auchter’s group,134 Bindal’s117 concluded that surgery was superior to SRS in clinically similar patients in terms of survival, local recurrence, and morbidity. They favor surgery rather than SRS in the treatment of single brain metastases.
Cho and associates136 evaluated their experience with 225 single brain metastases in patients treated with WBRT alone, surgery plus WBRT, or SRS plus WBRT. Patients in all three groups had a similar distribution of prognostic factors such as age, sex, KPS score, and location of metastasis; however, extracranial disease was more prevalent in the group treated with SRS plus WBRT than it was in the surgery group. The actuarial survival was the same for the surgery group and the SRS group, and both these groups fared better than patients receiving WBRT alone. The authors concluded that “given that SRS is minimally invasive, is able to treat lesions in surgically inaccessible locations, and is potentially more cost-effective than surgery, it is a reasonable and potentially more attractive alternative than surgery in the management of single brain metastases.”136
Recently, Muacevic and associates137 compared surgery plus WBRT with SRS alone in a randomized prospective study (see Table 130-1) that treated patients with single small brain metastases (≤3 cm in maximal diameter). Owing to poor patient accrual, there were only 33 patients in the surgery group and 31 patients in the SRS group. No significant differences were found with respect to patient survival interval, neurological death rate, or freedom from local tumor recurrence; however, patients undergoing SRS experienced significantly more distant tumor recurrences than those in the surgery group. Patients in the surgery plus WBRT group experienced significantly more early or late grade 1 or 2 complications than those in the SRS group. The authors concluded that SRS alone is as effective as surgery plus WBRT in controlling local tumor recurrence, but SRS salvage treatment may be necessary to curb distant recurrence in the absence of adjuvant WBRT.
An additional prospective study with both randomized and nonrandomized arms that compared patients with single brain metastases treated with either conventional surgery or SRS has just been completed at M. D. Anderson.138 In the randomized arm, 30 patients received surgical resection and 29 received SRS. In the nonrandomized arm, 89 patients chose surgery and 66 chose SRS. In the nonrandomized cohort, follow-up of patients who were eligible for randomization was identical to that in the randomized arm. It was possible to compare tumor recurrence rates (but not overall survival times) using multivariate analyses that took into account both randomized and nonrandomized groups (and compensated for confounding covariates such as age, sex, WBRT treatment, primary tumor type, extent of disease, tumor volume and location, KPS score, and recursive partitioning analysis [RPA] class). Contrary to the findings of Muacevic’s group,137 these analyses showed that patients receiving SRS experienced significantly more local recurrences than those undergoing conventional surgery, and they found no difference in distant recurrence between the two groups.
Stereotactic Radiosurgery Plus Whole-Brain Radiation Therapy
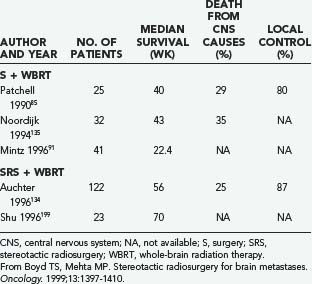
The retrospective study of Auchter and coworkers134 showed that SRS in conjunction with WBRT for single brain metastases can produce substantial functional survival 56 weeks from the date of SRS, especially in patients with good performance status and without extracranial metastasis. Moreover, it appears that in patients with single brain metastases, the results of treatment with SRS plus WBRT are comparable to those in selected randomized trials that include resection and WBRT (Table 130-6).
TABLE 130-6 Resection and Whole-Brain Radiation Therapy versus Stereotactic Radiosurgery and Whole-Brain Radiation Therapy
A relevant question raised by Auchter’s multi-institutional SRS series134 is whether combining SRS with WBRT can improve survival in patients with multiple brain metastases. A study from the University of Pittsburgh addressed this question by randomly assigning 27 patients who had two to four brain metastases (<25 mm in diameter) to initial management with WBRT alone or to treatment with WBRT and SRS.139 The failure rate of local tumor control at 1 year was 100% in patients receiving WBRT alone but only 8% in patients who received SRS. The study was stopped prematurely after interim analysis at the 60% accrual point because of these differences. The median time to failure of local control was 6 months after WBRT alone, compared with 36 months after WBRT plus SRS (P = .0005). The median time to any brain tumor local control failure was improved in the SRS group (34 months) relative to the WBRT only group (5 months; P = .002). Patients who received WBRT alone lived for a median of 7.5 months thereafter, whereas those who received WBRT plus SRS lived for 11 months (P = .22). It is possible that the inclusion of patients with three or four metastases prevented a statistically significant survival benefit from being detected, in light of data from a Stanford University study. The Stanford group reported outcomes for 120 patients whose brain metastases were treated with SRS and most of whom also received WBRT. The survival duration was 37 weeks and was equivalent for patients who had either one or two metastases; this was notably similar to results reported for patients with solitary metastases managed by surgical resection and WBRT. For patients with three or more metastases, survival after SRS was similar to that reported for patients receiving only WBRT (14 weeks).140
A phase III trial (RTOG 95-08; see Table 130-1) randomized patients with no more than three unresected brain metastases to treatment with WBRT alone (n = 164) or WBRT with an SRS boost (n = 167).141 This trial stratified patients by number of brain metastases and status of extracranial disease. The overall survival times were similar in both treatment arms. Univariate analysis showed a survival advantage for patients with single brain metastases who received the SRS boost (6.5 months) relative to those who did not (4.9 months; P = .039). Patients in the SRS arm of the study had better KPS scores at the 6-month follow-up visit than those not receiving SRS. Multivariate analysis of the two study arms showed that survival improved in patients receiving the SRS boost who had RPA class 1 status (P < .0001) or a favorable histologic tumor type (P = .0121).
Investigators at the University of California at San Francisco reported 106 patients with single or multiple brain metastases who were managed initially with SRS or SRS plus WBRT; median survival times were 11.3 and 11.1 months, respectively, and the percentages of patients experiencing 1-year local freedom from progression were 71% and 79%, respectively.142 The percentage of patients exhibiting freedom from progression within the brain was significantly less for patients treated with SRS alone than for those receiving SRS plus WBRT (28% versus 69% at 1 year); however, analysis of local tumor control within the brain, allowing for successful salvage of a first failure (time to second, rather than first, local control failure in the brain was scored), was not significantly different for patients treated with SRS alone versus SRS plus WBRT (62% versus 73% at 1 year; P = .56).
In 2006 Aoyama and coworkers143 performed a prospective randomized study of 132 patients with one to four brain metastases (<3 cm in maximal diameter) who were treated with either SRS alone (n = 67) or SRS plus WBRT (n = 65) (see Table 130-1). They reported that although adding WBRT to SRS did not increase survival time, it significantly reduced the number of recurrent brain metastases. Omission of WBRT did not produce any difference in either gross neurological or neurocognitive functioning. Nevertheless, the authors concluded that WBRT is not necessary and can safely be omitted, provided frequent monitoring of brain tumor status is conducted.
Subsequently, Patchell and colleagues144,145 pointed out that exactly the opposite conclusion could be drawn from this study. Although Aoyama’s group143 stated that the main reason for omitting WBRT was to avoid long-term neurotoxic effects, they found no difference between the groups treated with or without WBRT in terms of neurological or neurocognitive functioning, radiation-induced adverse effects, or survival times. Because WBRT seemed to significantly reduce the number of recurrent brain metastases without demonstrable neurotoxic effects, the trial actually offers strong support for the use of WBRT as an up-front treatment for brain metastases. Patchell and colleagues145 also demonstrated that because Aoyama’s study143 included patients with up to four brain metastases—whereas only patients with single brain metastases had been shown to live longer with aggressive treatment in prior randomized trials85,86,141—the study was statistically underpowered to demonstrate a meaningful survival benefit of WBRT plus SRS over SRS alone, or even whether these treatments were equivalent.
Another randomized trial comparing SRS with SRS plus WBRT to better assess survival, freedom from progression in the brain, and quality of life is currently under way (see http://www.cancer.gov/clinicaltrials/NCCTG-N0574).
Metastases from Renal Cell Carcinoma and Melanoma
A review of M. D. Anderson’s experience with patients having RCC metastatic to the brain showed that 119 patients had been treated with WBRT alone.146 For this group, the overall median survival time from diagnosis of brain metastases was 4.4 months. The cause of death was neurological in 76% of patients, whereas 16% died from systemic cancer. Because of these unsatisfactory results, more aggressive approaches, including surgery or SRS, were suggested by the authors. The group at the University of Pittsburgh reported results of the radiosurgical treatment of 52 brain metastases from RCC in 35 patients during a 9-year period; 28 of 35 patients also received WBRT.147 The median survival interval for these patients was 11 months after treatment with SRS. Local control was achieved in 90% of tumors (21% disappeared, 44% regressed, 26% were stable), and the addition of WBRT did not improve survival. Failure to control distant tumor recurrence within the brain was similar in both groups (46% for SRS treatment alone; 50% for SRS plus WBRT). Local control failure was observed only in the SRS only group, leading the authors to postulate that WBRT might contribute to local control. The number of local control failures was small in this study (approximately 5), making it difficult to draw any firm conclusions about the ability of WBRT to increase local control. A randomized trial is needed to better assess the role of post-SRS WBRT, specifically with respect to patients with RCC brain metastases. The use of WBRT in treating these patients remains controversial and requires one to weigh the possible theoretical benefits of improved local control and decreased distant control failure against the long-term morbidity associated with treatment.
A study from Harvard University has shown that radioresistant tumors such as melanoma and RCC can be controlled with SRS just as effectively as radiosensitive tumor types.148 Investigators from the University of California at San Francisco also reported their Gamma Knife SRS series for 55 melanoma patients with brain metastases, including 16 patients with recurrences, 11 patients receiving SRS boosts, and 28 who underwent SRS alone as the initial treatment.149 The median survival time for patients in this study was 35 weeks overall. According to multivariate analysis, total target volume was the only factor significantly affecting survival. Log-rank analysis showed no significant differences in actuarial freedom from development of intracranial progression (P = .85) among patients treated with SRS alone, SRS boost plus WBRT, or SRS for recurrence.
Just as the role of post-SRS radiation therapy is not clear in the case of RCC, the role of postoperative WBRT in treating melanoma must be better defined. Hagen and associates94 from Memorial Sloan-Kettering Cancer Center reported their experience with 35 patients who underwent resection of a single brain metastasis from melanoma. Although the difference was not significant, the median survival time was 6.4 months in patients receiving WBRT and 8.3 months in those not receiving it; however, 24% of patients receiving WBRT and 85% of those not receiving it died of neurological causes. The authors concluded that although control of CNS disease was improved with postoperative WBRT, survival ultimately depended on the control of systemic disease. In contrast, the Pittsburgh group150 concluded, based on a series of patients with solitary brain metastases from melanoma, that SRS alone is an appropriate management strategy because WBRT combined with SRS did not improve survival or local tumor control. Although new brain metastases developed less frequently in patients receiving WBRT in addition to SRS, this was not statistically significant. Evidence that SRS can be effective in treating radioresistant solitary brain metastases comes from a multi-institutional study that found tumor control to be significantly improved (using multivariate analysis) for patients with malignant melanoma or RCC (P = .0006) compared with other tumor histologies.151
Reirradiation with Stereotactic Radiosurgery or Whole-Brain Radiation Therapy
SRS can be used in the treatment of recurrent brain metastases or metastases that persist despite treatment with WBRT. Loeffler and colleagues152 used SRS to treat 18 patients who had 21 recurrent or persistent brain metastases. To be eligible for this therapy, patients were required to have a KPS score greater than 70 and no evidence of stable systemic disease. There was a median follow-up of 9 months, and all tumors within the SRS field were controlled. No cases of symptomatic radiation necrosis occurred, despite these patients’ previous exposure to radiotherapy.
The RTOG has attempted to determine the maximal acutely tolerable dose of single-fraction SRS in patients with recurrent, previously irradiated primary brain tumors or brain metastases.153 Thirty-eight patients (median prior dose, 60 Gy) had recurrent primary brain tumors, and 64 patients (median prior dose, 30 Gy) had recurrent brain metastases that were 40 mm or less in maximal diameter. Initially, patients were entered into arms of the study based on the diameter of the recurrent lesion: lesions less than 20 mm received 18 Gy, those ranging from 21 to 30 mm received 15 Gy, and those ranging from 31 to 40 mm received 12 Gy. Dose escalation was later carried out such that lesions with a diameter less than 20 mm received 21 Gy, those ranging from 21 to 30 mm received 18 Gy, and those ranging from 31 to 40 mm received 15 Gy. Unacceptable acute toxicity secondary to cerebral edema was observed in 0%, 7%, and 5%, respectively, in the three arms of the first group. In the dose escalation group, no unacceptable acute toxicity was seen. Multivariate analysis of the data showed that a tumor volume greater than 8200 mm3 and a ratio of maximal dose to prescription dose that was greater than 2 were significantly associated with unacceptable toxicity. Symptomatic necrosis requiring operation was necessary in 6% of patients. The results of this RTOG study can be used as guidelines for dose selection to minimize the occurrence of unacceptable toxicities after treatment with SRS.
The largest published series on external beam reirradiation of brain metastases was reported for 86 patients by the Mayo Clinic.154 The median dose of the first course of radiation was 30 Gy, followed by 20 Gy. The median survival time after reirradiation was 4 months, and 27% of patients experienced resolution of their symptoms, 43% had partial improvement, and 29% had either no change or worse neurological symptoms. The majority of patients had no significant toxicity secondary to reirradiation. Absence of extracranial disease was the only significant factor in multivariate analysis that was associated with improved survival. A series from New York University reported 52 adults patients selected for reirradiation of recurrent cerebral metastases.155 Selected patients were required to be in relatively good general condition for at least 4 months after the initial course of radiation therapy and to have renewed deterioration of their neurological condition. Initial treatment administered to the whole brain was 30 Gy in 10 fractions over 2 weeks. Reirradiation consisted of 25 Gy delivered in 10 fractions. Forty-two percent of patients responded to reirradiation. Patient survival time after the second treatment averaged 5 months. SRS should be considered as the radiotherapy treatment of choice for patients with recurrent brain metastases, if they are eligible.152 If SRS is not feasible, reirradiation with WBRT can be carefully considered, if appropriate.
Chemotherapy
In theory, there are obvious advantages to using chemotherapy to treat patients with brain metastases. Unlike surgery and SRS, which provide only localized treatment, systemic chemotherapy permits treatment of the entire brain and allows concurrent treatment of extracranial sites of cancer. Nevertheless, chemotherapy has historically been considered ineffective in the management of these patients.156 Explanations offered for the limited effectiveness of chemotherapy for brain metastases include the presence of the blood-brain barrier, the relatively drug-resistant nature of cancers that metastasize to the brain, the frequent observation of brain metastases in patients who fail to respond to chemotherapy, and the use of suboptimal chemotherapeutic agents in past trials.156–158
In the past it was assumed that the blood-brain barrier largely restricted the entry of many chemotherapeutic drugs into the CNS, especially large polar or hydrophobic compounds. In the case of patients who have brain metastases, it is now generally recognized that the blood-brain barrier is variably disrupted, as demonstrated by the fact that nearly all tumors metastasizing to the brain show contrast enhancement on CT or MRI. This enhancement indicates leakage of contrast material from the tumor vasculature to the interstitium. Several studies have shown that cytotoxic drugs (cisplatin, etoposide, nimustine, aziquinone) administered systemically before surgery reach pharmacologically relevant levels in brain tumor tissue samples removed at surgery, which is consistent with their entering tumor tissue via a disrupted blood-brain barrier.159–163 Nevertheless, in animal studies, concentrations of these drugs in brain tumors were lower than those observed for subcutaneous tumors after systemically administered chemotherapy.164 Drug delivery into CNS tumors may be further limited by the routine use of corticosteroids for symptomatic brain metastases.165 These steroids may facilitate the reestablishment of the disrupted blood-brain barrier function, thereby providing a protective effect against cytotoxic agents.166,167
Tumors that frequently metastasize to the brain, such as NSCLC and malignant melanoma, are relatively insensitive to systemic chemotherapy. The results of chemotherapy in patients with these tumors have been mixed.168–172 Of all primary tumors known to metastasize to the brain in significant numbers, SCLC, breast cancer, germ cell tumor, and lymphoma are the only ones considered relatively chemosensitive. It has been shown that chemotherapy does indeed have significant effects on brain metastases from these cancers. Chemotherapy for brain metastases from germ cell tumors such as choriocarcinoma173 and germinoma174 is considered standard therapy. Whether administered along with surgery and WBRT or as the sole treatment, chemotherapy’s effectiveness against these types of brain metastases has been well documented.175 For brain metastases from SCLC and breast cancer, the effectiveness of chemotherapy is less well defined. In patients with these metastases, chemotherapy produces response rates similar to those observed for their systemic tumors.170,176–178 This may mean that the complicating effect of the blood-brain barrier is minimal. Some have even proposed that chemotherapy be standard treatment for patients who develop brain metastases from SCLC.179 To date, it has not been conclusively determined whether chemotherapy provides better results than WBRT alone or whether chemotherapy could be a useful adjunct to WBRT. However, a phase III study of teniposide administration in SCLC patients treated with or without WBRT showed a higher response rate of brain metastases and a longer time to progression in the WBRT cohort (although survival was similarly poor in both groups).180
Defining the role of chemotherapy in the management of patients with brain metastases awaits the performance of a well-designed randomized, controlled clinical trial.181 Until evidence of its effectiveness becomes available from such a study, the use of chemotherapy in patients with brain metastases from cancers other than SCLC and germ cell tumors will remain experimental.
Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665-1672.
Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483-2491.
Auchter RM, Lamond JP, Alexander E, et al. A multiinstitutional outcome and prognostic factor analysis of radiosurgery for resectable single brain metastasis. Int J Radiat Oncol Biol Phys. 1996;35:27-35.
Auperin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341:476-484.
Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865-2872.
Bindal AK, Bindal RK, Hess KR, et al. Surgery versus radiosurgery in the treatment of brain metastasis. J Neurosurg. 1996;84:748-754.
Bindal RK, Sawaya R, Leavens ME, et al. Reoperation for recurrent metastatic brain tumors. J Neurosurg. 1995;83:600-604.
Bindal RK, Sawaya R, Leavens ME, et al. Surgical treatment of multiple brain metastases. J Neurosurg. 1993;79:210-216.
Borgelt B, Gelber R, Larson M, et al. Ultra-rapid high dose irradiation schedules for the palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1981;7:1633-1638.
Chang EL, Hassenbusch SJ3rd, Shiu AS, et al. The role of tumor size in the radiosurgical management of patients with ambiguous brain metastases. Neurosurgery. 2003;53:272-280.
DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789-796.
Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745-751.
Lang FF, Sawaya R. Surgical management of cerebral metastases. Neurosurg Clin N Am. 1996;7:459-484.
Mehta MP, Rodrigus P, Terhaard CH, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol. 2003;21:2529-2536.
Muacevic A, Wowra B, Siefert A, et al. Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. J Neurooncol. 2008;87:299-307.
Noordijk EM, Vecht CJ, Haaxma-Reiche H, et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys. 1994;29:711-717.
Patchell RA, Regine WF. The rationale for adjuvant whole brain radiation therapy with radiosurgery in the treatment of single brain metastases. Technol Cancer Res Treat. 2003;2:111-115.
Patchell RA, Regine WF, Loeffler JS, et al. Radiosurgery plus whole-brain radiation therapy for brain metastases. JAMA. 2006;296:2089-2090.
Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485-1489.
Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494-500.
Posner JB, Chernik NL. Intracranial metastases from systemic cancer. Adv Neurol. 1978;19:579-592.
Suh JH, Stea B, Nabid A, et al. Phase III study of efaproxiral as an adjunct to whole-brain radiation therapy for brain metastases. J Clin Oncol. 2006;24:106-114.
Suki D, Abouassi H, Patel AJ, et al. Comparative risk of leptomeningeal disease after resection or stereotactic radiosurgery for solid tumor metastasis to the posterior fossa. J Neurosurg. 2008;108:248-257.
Takakura K, Sano K, Hojo S, et al. Metastatic Tumors of the Central Nervous System. New York: Igaku-Shoin; 1982.
Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993;33:583-590.
1 Bucholz A. Kasuistischer Beitrag zur Kenntis der Karzinome des Zentralnervensystems. Monatsschr Psychiatr Neurol. 1898;4:183-210.
2 Walker AE, Robins M, Weinfeld FD. Epidemiology of brain tumors: the national survey of intracranial neoplasms. Neurology. 1985;35:219-226.
3 Guomundsson KR. A survey of tumors of the central nervous system in Iceland during the 10-year period 1954-1963. Acta Neurol Scand. 1970;46:538-552.
4 Percy AK, Elveback LR, Okazaki H, et al. Neoplasms of the central nervous system. Epidemiologic considerations. Neurology. 1972;22:40-48.
5 Fogelholm R, Uutela T, Murros K. Epidemiology of central nervous system neoplasms: a regional survey in central Finland. Acta Neurol Scand. 1984;69:129-136.
6 Posner JB. Neurologic Complications of Cancer. Philadelphia: F. A. Davis, 1995.
7 Wingo PA, Tong T, Bolden S. Cancer statistics, 1995. CA Cancer J Clin. 1995;45:8-30.
8 Posner JB, Chernik NL. Intracranial metastases from systemic cancer. Adv Neurol. 1978;19:579-592.
9 Takakura K, Sano K, Hojo S, et al. Metastatic Tumors of the Central Nervous System. New York: Igaku-Shoin; 1982.
10 Cairncross JG, Posner JB. The management of brain metastases. In: Walker MD, editor. Oncology of the Nervous System. Boston: Martinus Nijhof; 1983:341-377.
11 Landis SH, Murray T, Bolden S, et al. Cancer statistics, 1999. CA Cancer J Clin. 1999;49:9-31.
12 Aronson SM, Garcia JH, Aronson BE. Metastatic neoplasms of the brain: their frequency in relation to age. Cancer. 1964;17:558-563.
13 de la Monte SM, Hutchins GM, Moore GW. Influence of age on the metastatic behavior of breast carcinoma. Hum Pathol. 1988;19:529-534.
14 Sorensen JB, Hansen HH, Hansen M, et al. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol. 1988;6:1474-1480.
15 Graus F, Walker RW, Allen JC. Brain metastases in children. J Pediatr. 1983;103:558-561.
16 Posner JB. Brain metastases: a clinician’s view. In: Weiss L, Gilbert HA, Posner JB, editors. Brain Metastasis, Vol 2. Boston: G. K. Hall; 1980:2-29.
17 Posner JB. Management of brain metastases. Rev Neurol (Paris). 1992;148:477-487.
18 Vannucci RC, Baten M. Cerebral metastatic disease in childhood. Neurology. 1974;24:981-985.
19 Tasdemiroglu E, Patchell RA. Cerebral metastases in childhood malignancies. Acta Neurochir (Wien). 1997;139:182-187.
20 Fell DA, Leavens ME, McBride CM. Surgical versus nonsurgical management of metastatic melanoma of the brain. Neurosurgery. 1980;7:238-242.
21 Sampson JH, Carter JHJr, Friedman AH, et al. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11-20.
22 Robinson W, Jobe K, Stevens R. Central nervous system metastases in malignant melanoma. In: Nathanson L, editor. Basic and Clinical Aspects of Malignant Melanoma. Boston: Nijhoff; 1987:155-163.
23 Amer MH, Al-Sarraf M, Baker LH, et al. Malignant melanoma and central nervous system metastases: incidence, diagnosis, treatment and survival. Cancer. 1978;42:660-668.
24 Baker AB. Metastatic tumors of the nervous system. Arch Pathol Lab Med. 1942;34:495-537.
25 Baker GS, Kernohan JW, Kiefer EJ. Metastatic tumors of the brain. Surg Clin North Am. 1951;31:1143-1145.
26 Chang DB, Yang PC, Luh KT, et al. Late survival of non-small cell lung cancer patients with brain metastases. Influence of treatment. Chest. 1992;101:1293-1297.
27 Lang EF, Slater J. Metastatic brain tumors: results of surgical and nonsurgical treatment. Surg Clin North Am. 1964;44:865-872.
28 Le Chevalier T, Smith FP, Caille P, et al. Sites of primary malignancies in patients presenting with cerebral metastases. A review of 120 cases. Cancer. 1985;56:880-882.
29 MacGee EE. Surgical treatment of cerebral metastases from lung cancer. The effect on quality and duration of survival. J Neurosurg. 1971;35:416-420.
30 Markesbery WR, Brooks WH, Gupta GD, et al. Treatment for patients with cerebral metastases. Arch Neurol. 1978;35:754-756.
31 Sundaresan N, Galicich JH. Surgical treatment of brain metastases. Clinical and computerized tomography evaluation of the results of treatment. Cancer. 1985;55:1382-1388.
32 Zimm S, Wampler GL, Stablein D, et al. Intracerebral metastases in solid-tumor patients: natural history and results of treatment. Cancer. 1981;48:384-394.
33 Tsukada Y, Fouad A, Pickren JW, et al. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer. 1983;52:2349-2354.
34 Vieth RG, Odom GL. Intracranial metastases and their neurosurgical treatment. J Neurosurg. 1965;23:375-383.
35 Chason J, Walker F, Landers J. Metastatic carcinoma in the central nervous system and dorsal root ganglia. Cancer. 1963;16:781-787.
36 Castaldo JE, Bernat JL, Meier FA, et al. Intracranial metastases due to prostatic carcinoma. Cancer. 1983;52:1739-1747.
37 Taylor HG, Lefkowitz M, Skoog SJ, et al. Intracranial metastases in prostate cancer. Cancer. 1984;53:2728-2730.
38 Stein M, Steiner M, Klein B, et al. Involvement of the central nervous system by ovarian carcinoma. Cancer. 1986;58:2066-2069.
39 Bloch JL, Nieh PT, Walzak MP. Brain metastases from transitional cell carcinoma. J Urol. 1987;137:97-99.
40 Dauplat J, Nieberg RK, Hacker NF. Central nervous system metastases in epithelial ovarian carcinoma. Cancer. 1987;60:2559-2562.
41 Steinfeld AD, Zelefsky M. Brain metastases from carcinoma of bladder. Urology. 1987;29:375-376.
42 Lewis AJ. Sarcoma metastatic to the brain. Cancer. 1988;61:593-601.
43 Anderson RS, el-Mahdi AM, Kuban DA, et al. Brain metastases from transitional cell carcinoma of urinary bladder. Urology. 1992;39:17-20.
44 Martinez-Manas RM, Brell M, Rumia J, et al. Brain metastases in endometrial carcinoma. Gynecol Oncol. 1998;70:282-284.
45 Khansur T, Routh A, Hickman B. Brain metastases from unknown primary site. J Miss State Med Assoc. 1997;38:238-242.
46 Landis SH, Murray T, Bolden S, et al. Cancer statistics, 1998. CA Cancer J Clin. 1998;48:6-29.
47 Pickren JW, Lopez G, Tsukada Y, et al. Brain metastases: an autopsy study. Cancer Treat Sympos. 1983;2:295-313.
48 Amer MH, Al-Sarraf M, Vaitkevicius VK. Clinical presentation, natural history and prognostic factors in advanced malignant melanoma. Surg Gynecol Obstet. 1979;149:687-692.
49 Atkinson L. Melanoma of the central nervous system. Aust N Z J Surg. 1978;48:14-16.
50 McNeer G, das Gupta T. Problem of recurrence in the management of melanoma. CA Cancer J Clin. 1965;15:270-274.
51 Madajewicz S, Karakousis C, West CR, et al. Malignant melanoma brain metastases. Review of Roswell Park Memorial Institute experience. Cancer. 1984;53:2550-2552.
52 Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865-2872.
53 Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma: analysis of 1000 autopsied cases. Cancer. 1950;3:74-85.
54 Burt M, Wronski M, Arbit E, et al. Resection of brain metastases from non-small-cell lung carcinoma. Results of therapy. Memorial Sloan-Kettering Cancer Center Thoracic Surgical Staff. J Thorac Cardiovasc Surg. 1992;103:399-410.
55 Nugent JL, Bunn PAJr, Matthews MJ, et al. CNS metastases in small cell bronchogenic carcinoma: increasing frequency and changing pattern with lengthening survival. Cancer. 1979;44:1885-1893.
56 Cox JD, Komaki R. Prophylactic cranial irradiation for squamous cell carcinoma, large cell carcinoma, and adenocarcinoma of the lung: indications and techniques. In: Mountain CF, Carr DT, editors. Lung cancer: Current Status and Prospects for the Future, Vol 28. Chicago: Year Book Medical Publishers; 1986:233-237.
57 Sen M, Demiral AS, Cetingoz R, et al. Prognostic factors in lung cancer with brain metastasis. Radiother Oncol. 1998;46:33-38.
58 Cifuentes N, Pickren JW. Metastases from carcinoma of mammary gland: an autopsy study. J Surg Oncol. 1979;11:193-205.
59 Chao J, Phillips R, Nickson J. Roentgen-ray therapy of cerebral metastases. Cancer. 1954;7:682-689.
60 Sawaya R, Bindal RK, Lang FF, et al. Metastatic brain tumors. In: Kaye AH, Laws ER, editors. Brain Tumors: An Encyclopedic Approach. 2nd ed. Edinburgh: Churchill Livingstone; 2001:999-1026.
61 Borgelt B, Gelber R, Kramer S, et al. The palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1980;6:1-9.
62 Coia LR. The role of radiation therapy in the treatment of brain metastases. Int J Radiat Oncol Biol Phys. 1992;23:229-238.
63 Diener-West M, Dobbins TW, Phillips TL, et al. Identification of an optimal subgroup for treatment evaluation of patients with brain metastases using RTOG study 7916. Int J Radiat Oncol Biol Phys. 1989;16:669-673.
64 Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745-751.
65 Borgelt B, Gelber R, Larson M, et al. Ultra-rapid high dose irradiation schedules for the palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1981;7:1633-1638.
66 Gelber RD, Larson M, Borgelt BB, et al. Equivalence of radiation schedules for the palliative treatment of brain metastases in patients with favorable prognosis. Cancer. 1981;48:1749-1753.
67 Sause WT, Scott C, Krisch R, et al. Phase I/II trial of accelerated fractionation in brain metastases RTOG 85-28. Int J Radiat Oncol Biol Phys. 1993;26:653-657.
68 Murray KJ, Scott C, Greenberg HM, et al. A randomized phase III study of accelerated hyperfractionation versus standard in patients with unresected brain metastases: a report of the Radiation Therapy Oncology Group (RTOG) 9104. Int J Radiat Oncol Biol Phys. 1997;39:571-574.
69 Komarnicky LT, Phillips TL, Martz K, et al. A randomized phase III protocol for the evaluation of misonidazole combined with radiation in the treatment of patients with brain metastases (RTOG-7916). Int J Radiat Oncol Biol Phys. 1991;20:53-58.
70 Phillips TL, Scott CB, Leibel SA, et al. Results of a randomized comparison of radiotherapy and bromodeoxyuridine with radiotherapy alone for brain metastases: report of RTOG trial 89-05. Int J Radiat Oncol Biol Phys. 1995;33:339-348.
71 Wen PY, Loeffler JS. Management of brain metastases. Oncology (Williston Park). 1999;13:941-961.
72 Mehta MP, Rodrigus P, Terhaard CH, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol. 2003;21:2529-2536.
73 Suh JH, Stea B, Nabid A, et al. Phase III study of efaproxiral as an adjunct to whole-brain radiation therapy for brain metastases. J Clin Oncol. 2006;24:106-114.
74 Ihde DC, Pass HI, Glatstein E. Small cell lung cancer. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 5th ed. Philadelphia: Lippincott-Raven; 1997:911-949.
75 Auperin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341:476-484.
76 Arriagada R, Le Chevalier T, Borie F, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst. 1995;87:183-190.
77 Gregor A, Cull A, Stephens RJ, et al. Prophylactic cranial irradiation is indicated following complete response to induction therapy in small cell lung cancer: results of a multicentre randomised trial. United Kingdom Coordinating Committee for Cancer Research (UKCCCR) and the European Organization for Research and Treatment of Cancer (EORTC). Eur J Cancer. 1997;33:1752-1758.
78 Carney DN. Prophylactic cranial irradiation and small-cell lung cancer. N Engl J Med. 1999;341:524-526.
79 Boldrey E, Sheline G. Delayed transitory clinical manifestations after radiation treatment of intracranial tumors. Acta Radiol. 1966;5:5-10.
80 Littman P, Rosenstock J, Gale G, et al. The somnolence syndrome in leukemic children following reduced daily dose fractions of cranial radiation. Int J Radiat Oncol Biol Phys. 1984;10:1851-1853.
81 DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789-796.
82 Patchell RA, Regine WF. The rationale for adjuvant whole brain radiation therapy with radiosurgery in the treatment of single brain metastases. Technol Cancer Res Treat. 2003;2:111-115.
83 Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol. 2005;23:6207-6219.
84 Grant FC. Concerning intracranial malignant metastases: their frequency and the value of surgery in their treatment. Ann Surg. 1926;84:635-646.
85 Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494-500.
86 Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993;33:583-590.
87 Bindal RK, Sawaya R, Leavens ME, et al. Surgical treatment of multiple brain metastases. J Neurosurg. 1993;79:210-216.
88 Bindal RK, Sawaya R, Leavens ME, et al. Reoperation for recurrent metastatic brain tumors. J Neurosurg. 1995;83:600-604.
89 Oldberg E. Surgical considerations of carcinomatous metastases to the brain. JAMA. 1933;101:1458-1461.
90 Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM, editor. Evaluation of Chemotherapeutic Agents. New York: Columbia University Press; 1949:191-205.
91 Mintz AH, Kestle J, Rathbone MP, et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer. 1996;78:1470-1476.
92 Chang EL, Hassenbusch SJ3rd, Shiu AS, et al. The role of tumor size in the radiosurgical management of patients with ambiguous brain metastases. Neurosurgery. 2003;53:272-280.
93 DeAngelis LM, Mandell LR, Thaler HT, et al. The role of postoperative radiotherapy after resection of single brain metastases. Neurosurgery. 1989;24:798-805.
94 Hagen NA, Cirrincione C, Thaler HT, et al. The role of radiation therapy following resection of single brain metastasis from melanoma. Neurology. 1990;40:158-160.
95 Smalley SR, Schray MF, Laws ERJr, et al. Adjuvant radiation therapy after surgical resection of solitary brain metastasis: association with pattern of failure and survival. Int J Radiat Oncol Biol Phys. 1987;13:1611-1616.
96 Dosoretz DE, Blitzer PH, Russell AH, et al. Management of solitary metastasis to the brain: the role of elective brain irradiation following complete surgical resection. Int J Radiat Oncol Biol Phys. 1980;6:1727-1730.
97 Wronski M, Arbit E, Burt M, et al. Survival after surgical treatment of brain metastases from lung cancer: a follow-up study of 231 patients treated between 1976 and 1991. J Neurosurg. 1995;83:605-616.
98 Looper JD, Einhorn LH, Garcia SA, et al. Severe neurological problems following successful therapy for small cell lung cancer. Proc Am Soc Clin Oncol. 1984:231.
99 Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485-1489.
100 Kondziolka D, Lunsford LD. Brain metastases. In: Apuzzo MLJ, editor. Brain Surgery: Complication Avoidance and Management, Vol 1. New York: Churchill Livingstone; 1993:615-641.
101 Kelly PJ, Kall BA, Goerss SJ. Results of computed tomography-based computer-assisted stereotactic resection of metastatic intracranial tumors. Neurosurgery. 1988;22:7-17.
102 Kelly PJ. Nonglial mass lesions. In: Tumor Stereotaxis. Saunders; 1991:358-369.
103 Yasargil MG. Topographic anatomy for microsurgical approaches to intrinsic brain tumors. In: Microneurosurgery. New York: Thieme Medical Publishing; 1994:2-114.
104 Sundaresan N, Sachdev VP, DiGiacinto GV, et al. Reoperation for brain metastases. J Clin Oncol. 1988;6:1625-1629.
105 Brega K, Robinson WA, Winston K, et al. Surgical treatment of brain metastases in malignant melanoma. Cancer. 1990;66:2105-2110.
106 Oredsson S, Ingvar C, Stromblad LG, et al. Palliative surgery for brain metastases of malignant melanoma. Eur J Surg Oncol. 1990;16:451-456.
107 Cushing H. Notes upon a series of two thousand verified cases with surgical-mortality percentages pertaining thereto. Springfield, IL: Charles C Thomas; 1932.
108 Elvidge AR, Baldwin M. Clinical analysis of eighty-eight cases of metastatic carcinoma involving the central nervous system. With an outline of therapeutic principles. J Neurosurg. 1949;6:495-502.
109 Stortebecker TP. Metastatic tumors of the brain from a neurosurgical point of view. A follow-up study of 158 cases. J Neurosurg. 1954;11:84-111.
110 Winston KR, Walsh JW, Fischer EG. Results of operative treatment of intracranial metastatic tumors. Cancer. 1980;45:2639-2645.
111 Ferrara M, Bizzozzero L, Talamonti G, et al. Surgical treatment of 100 single brain metastases. Analysis of the results. J Neurosurg Sci. 1990;34:303-308.
112 Galicich JH, Sundaresan N, Arbit E, et al. Surgical treatment of single brain metastasis: factors associated with survival. Cancer. 1980;45:381-386.
113 Ransohoff J. Surgical management of metastatic tumors. Semin Oncol. 1975;2:21-27.
114 Raskind R, Weiss SR, Manning JJ, et al. Survival after surgical excision of single metastatic brain tumors. AJR Am J Roentgenol. 1971;111:323-328.
115 White KT, Fleming TR, Laws ERJr. Single metastasis to the brain. Surgical treatment in 122 consecutive patients. Mayo Clin Proc. 1981;56:424-428.
116 Hammoud MA, McCutcheon IE, Elsouki R, et al. Colorectal carcinoma and brain metastasis: distribution, treatment, and survival. Ann Surg Oncol. 1996;3:453-463.
117 Bindal AK, Bindal RK, Hess KR, et al. Surgery versus radiosurgery in the treatment of brain metastasis. J Neurosurg. 1996;84:748-754.
118 Haar F, Patterson RHJ. Surgery for metastatic intracranial neoplasm. Cancer. 1972;30:1241-1245.
119 Sause WT, Crowley JJ, Morantz R, et al. Solitary brain metastasis: results of an RTOG/SWOG protocol evaluation surgery + RT versus RT alone. Am J Clin Oncol. 1990;13:427-432.
120 Richards P, McKissock W. Intracranial metastases. BMJ. 1963;1:15-18.
121 Simionescu MD. Metastatic tumors of the brain: a follow-up study of 195 patients with neurosurgical considerations. J Neurosurg. 1960;17:361-373.
122 Constantini S, Kornowski R, Pomeranz S, et al. Thromboembolic phenomena in neurosurgical patients operated upon for primary and metastatic brain tumors. Acta Neurochir (Wien). 1991;109:93-97.
123 Sawaya R, Zuccarello M, Elkalliny M, et al. Postoperative venous thromboembolism and brain tumors: part I. Clinical profile. J Neurooncol. 1992;14:119-125.
124 Suki D, Abouassi H, Patel AJ, et al. Comparative risk of leptomeningeal disease after resection or stereotactic radiosurgery for solid tumor metastasis to the posterior fossa. J Neurosurg. 2008;108:248-257.
125 Suki D, Hatiboglu MA, Patel AJ, et al. Comparative risk of leptomeningeal dissemination of cancer after surgery or stereotactic radiosurgery for a single supratentorial solid tumor metastasis. Neurosurgery. 2009;64:664-674.
126 Cascino TL, Leavengood JM, Kemeny N, et al. Brain metastases from colon cancer. J Neurooncol. 1983;1:203-209.
127 Wronski M, Arbit E. Resection of brain metastases from colorectal carcinoma in 73 patients. Cancer. 1999;85:1677-1685.
128 Phillips MH, Stelzer KJ, Griffin TW, et al. Stereotactic radiosurgery: a review and comparison of methods. J Clin Oncol. 1994;12:1085-1099.
129 Loeffler JS, Alexander E3rd. Radiosurgery for the treatment of intracranial metastases. In: Alexander, Loeffler JS, Lunsford LD. Stereotactic Radiosurgery. New York: McGraw-Hill; 1993:197-206.
130 Alexander, Loeffler JS. Radiosurgery using a modified linear accelerator. Neurosurg Clin N Am. 1992;3:167-190.
131 Coffey RJ, Flickinger JC, Lunsford LD, et al. Solitary brain metastasis: radiosurgery in lieu of microsurgery in 32 patients. Acta Neurochir Suppl (Wien). 1991;52:90-92.
132 Sturm V, Kimmig B, Engenhardt R, et al. Radiosurgical treatment of cerebral metastases. Method, indications and results. Stereotact Funct Neurosurg. 1991;57:7-10.
133 Vecil GG, Suki D, Maldaun MV, et al. Resection of brain metastases previously treated with stereotactic radiosurgery. J Neurosurg. 2005;102:209-215.
134 Auchter RM, Lamond JP, Alexander E, et al. A multiinstitutional outcome and prognostic factor analysis of radiosurgery for resectable single brain metastasis. Int J Radiat Oncol Biol Phys. 1996;35:27-35.
135 Noordijk EM, Vecht CJ, Haaxma-Reiche H, et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys. 1994;29:711-717.
136 Cho KH, Hall WA, Lee AK, et al. Stereotactic radiosurgery for patients with single brain metastasis. J Radiosurg. 1998;1:79-85.
137 Muacevic A, Wowra B, Siefert A, et al. Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: a randomized controlled multicentre phase III trial. J Neurooncol. 2008;87:299-307.
138 Lang FF, Suki D, Maor M, et al. Conventional surgery versus stereotactic radiosurgery in the treatment of single brain metastases: a prospective study with both randomized and nonrandomized arms. American Association of Neurological Surgeons Meeting [abstract]. Article ID: 48938. 2008.
139 Kondziolka D, Patel A, Lunsford LD, et al. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45:427-434.
140 Joseph J, Adler JR, Cox RS, et al. Linear accelerator-based stereotaxic radiosurgery for brain metastases: the influence of number of lesions on survival. J Clin Oncol. 1996;14:1085-1092.
141 Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665-1672.
142 Sneed PK, Lamborn KR, Forstner JM, et al. Radiosurgery for brain metastases: is whole brain radiotherapy necessary? Int J Radiat Oncol Biol Phys. 1999;43:549-558.
143 Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483-2491.
144 Patchell RA, Regine WF, Loeffler JS, et al. Radiosurgery plus whole-brain radiation therapy for brain metastases. JAMA. 2006;296:2089-2090.
145 Patchell RA, Regine WF, Renschler M, et al. Comments about the prospective randomized trial by Aoyama et al. Surg Neurol. 2006;66:459-460.
146 Wronski M, Maor MH, Davis BJ, et al. External radiation of brain metastases from renal carcinoma: a retrospective study of 119 patients from the M. D. Anderson Cancer Center. Int J Radiat Oncol Biol Phys. 1997;37:753-759.
147 Mori Y, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for brain metastasis from renal cell carcinoma. Cancer. 1998;83:344-353.
148 Alexander, Moriarty TM, Davis RB, et al. Stereotactic radiosurgery for the definitive, noninvasive treatment of brain metastases. J Natl Cancer Inst. 1995;87:34-40.
149 Seung SK, Sneed PK, McDermott MW, et al. Gamma Knife radiosurgery for malignant melanoma brain metastases. Cancer J Sci Am. 1998;4:103-109.
150 Mori Y, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for cerebral metastatic melanoma: factors affecting local disease control and survival. Int J Radiat Oncol Biol Phys. 1998;42:581-589.
151 Flickinger JC, Kondziolka D, Lunsford LD, et al. A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. Int J Radiat Oncol Biol Phys. 1994;28:797-802.
152 Loeffler JS, Kooy HM, Wen PY, et al. The treatment of recurrent brain metastases with stereotactic radiosurgery. J Clin Oncol. 1990;8:576-582.
153 Shaw E, Scott C, Souhami L, et al. Radiosurgery for the treatment of previously irradiated recurrent primary brain tumors and brain metastases: initial report of Radiation Therapy Oncology Group protocol 90-05. Int J Radiat Oncol Biol Phys. 1996;34:647-654.
154 Wong WW, Schild SE, Sawyer TE, et al. Analysis of outcome in patients reirradiated for brain metastases. Int J Radiat Oncol Biol Phys. 1996;34:585-590.
155 Cooper JS, Steinfeld AD, Lerch IA. Cerebral metastases: value of reirradiation in selected patients. Radiology. 1990;174:883-885.
156 Buckner JC. The role of chemotherapy in the treatment of patients with brain metastases from solid tumors. Cancer Metastasis Rev. 1991;10:335-341.
157 Greig NH. Chemotherapy of brain metastases: current status. Cancer Treat Rev. 1984;11:157-186.
158 Siegers HP. Chemotherapy for brain metastases: recent developments and clinical considerations. Cancer Treat Rev. 1990;17:63-76.
159 Savaraj N, Lu K, Feun LG, et al. Intracerebral penetration and tissue distribution of 2,5-diaziridinyl 3,6-bis(carboethoxyamino) 1,4-benzoquinone (AZQ, NSC-182986). J Neurooncol. 1983;1:15-19.
160 Stewart DJ, Benvenuto JA, Leavens M, et al. Penetration of 3-deazauridine into human brain, intracerebral tumor, and cerebrospinal fluid. Cancer Res. 1979;39:4119-4122.
161 Stewart DJ, Leavens M, Maor M, et al. Human central nervous system distribution of cis-diamminedichloroplatinum and use as a radiosensitizer in malignant brain tumors. Cancer Res. 1982;42:2474-2479.
162 Stewart DJ, Benvenuto JA, Leavens M, et al. Human central nervous system pharmacology of pentamethylmelamine and its metabolites. J Neurooncol. 1983;1:357-364.
163 Stewart DJ, Richard MT, Hugenholtz H, et al. Penetration of teniposide (VM-26) into human intracerebral tumors. Preliminary observations on the effect of tumor type, rate of drug infusion and prior treatment with amphotericin B or oral glycerol. J Neurooncol. 1984;2:315-324.
164 Stewart DJ. A critique of the role of the blood-brain barrier in the chemotherapy of human brain tumors. J Neurooncol. 1994;20:121-139.
165 Nakagawa H, Groothuis DR, Owens ES, et al. Dexamethasone effects on [125I]albumin distribution in experimental RG-2 gliomas and adjacent brain. J Cereb Blood Flow Metab. 1987;7:687-701.
166 Weller M, Schmidt C, Roth W, et al. Chemotherapy of human malignant glioma: prevention of efficacy by dexamethasone? Neurology. 1997;48:1704-1709.
167 Mariotta M, Perewusnyk G, Koechli OR, et al. Dexamethasone-induced enhancement of resistance to ionizing radiation and chemotherapeutic agents in human tumor cells. Strahlenther Onkol. 1999;175:392-396.
168 Cascino TL, Byrne TN, Deck MD, et al. Intra-arterial BCNU in the treatment of metastatic brain tumors. J Neurooncol. 1983;1:211-218.
169 Ushio Y, Arita N, Hayakawa T, et al. Chemotherapy of brain metastases from lung carcinoma: a controlled randomized study. Neurosurgery. 1991;28:201-205.
170 Lange OF, Scheef W, Haase KD. Palliative radio-chemotherapy with ifosfamide and BCNU for breast cancer patients with cerebral metastases. A 5-year experience. Cancer Chemother Pharmacol. 1990;26:S78-S80.
171 Dvorak J, Melichar B, Zizka J, et al. Complete response of multiple melanoma brain metastases after treatment with temozolomide. Onkologie. 2004;27:171-174.
172 Seute T, Leffers P, Wilmink JT, et al. Response of asymptomatic brain metastases from small-cell lung cancer to systemic first-line chemotherapy. J Clin Oncol. 2006;24:2079-2083.
173 Rustin GJ, Newlands ES, Begent RH, et al. Weekly alternating etoposide, methotrexate, and actinomycin/vincristine and cyclophosphamide chemotherapy for the treatment of CNS metastases of choriocarcinoma. J Clin Oncol. 1989;7:900-903.
174 Rustin GJ, Newlands ES, Bagshawe KD, et al. Successful management of metastatic and primary germ cell tumors in the brain. Cancer. 1986;57:2108-2113.
175 Spears WT, Morphis, Lester SG, et al. Brain metastases and testicular tumors: long-term survival. Int J Radiat Oncol Biol Phys. 1992;22:17-22.
176 Rosner D, Nemoto T, Pickren J, et al. Management of brain metastases from breast cancer by combination chemotherapy. J Neurooncol. 1983;1:131-137.
177 Boogerd W, Dalesio O, Bais EM, et al. Response of brain metastases from breast cancer to systemic chemotherapy. Cancer. 1992;69:972-980.
178 Twelves CJ, Souhami RL, Harper PG, et al. The response of cerebral metastases in small cell lung cancer to systemic chemotherapy. Br J Cancer. 1990;61:147-150.
179 Twelves CJ, Souhami RL. Should cerebral metastases be treated by chemotherapy alone? Ann Oncol. 1991;2:15-17.
180 Postmus PE, Haaxma-Reiche H, Smit EF, et al. Treatment of brain metastases of small-cell lung cancer: comparing teniposide and teniposide with whole-brain radiotherapy—a phase III study of the European Organization for the Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol. 2000;18:3400-3408.
181 Rock JP, Haines S, Recht L, et al. Practice parameters for the management of single brain metastasis. Neurosurg Focus. 9, 2000. Clinical Pearl 2, 9 pp
182 Armstrong JG, Wronski M, Galicich J, et al. Postoperative radiation for lung cancer metastatic to the brain. J Clin Oncol. 1994;12:2340-2344.
183 Skibber JM, Soong SJ, Austin L, et al. Cranial irradiation after surgical excision of brain metastases in melanoma patients. Ann Surg Oncol. 1996;3:118-123.
184 O’Neill BP, Iturria NJ, Link MJ, et al. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys. 2003;55:1169-1176.
185 Salerno TA, Munro DD, Little JR. Surgical treatment of bronchogenic carcinoma with a brain metastasis. J Neurosurg. 1978;48:350-354.
186 Mandell L, Hilaris B, Sullivan M, et al. The treatment of single brain metastasis from non-oat cell lung carcinoma. Surgery and radiation versus radiation therapy alone. Cancer. 1986;58:641-649.
187 Magilligan, Duvernoy C, Malik G, et al. Surgical approach to lung cancer with solitary cerebral metastasis: twenty-five years’ experience. Ann Thorac Surg. 1986;42:360-364.
188 Hankins JR, Miller JE, Salcman M, et al. Surgical management of lung cancer with solitary cerebral metastasis. Ann Thorac Surg. 1988;46:24-28.
189 Catinella FP, Kittle CF, Faber LP, et al. Surgical treatment of primary lung cancer and solitary intracranial metastasis. Chest. 1989;95:972-975.
190 Read RC, Boop WC, Yoder G, et al. Management of nonsmall cell lung carcinoma with solitary brain metastasis. J Thorac Cardiovasc Surg. 1989;98:884-890.
191 Koutras AK, Marangos M, Kourelis T, et al. Surgical management of cerebral metastases from non-small cell lung cancer. Tumori. 2003;89:292-297.
192 Hafstrom L, Jonsson PE, Stromblad LG. Intracranial metastases of malignant melanoma treated by surgery. Cancer. 1980;46:2088-2090.
193 Stevens G, Firth I, Coates A. Cerebral metastases from malignant melanoma. Radiother Oncol. 1992;23:185-191.
194 Decker DA, Decker VL, Herskovic A, et al. Brain metastases in patients with renal cell carcinoma: prognosis and treatment. J Clin Oncol. 1984;2:169-173.
195 Badalament RA, Gluck RW, Wong GY, et al. Surgical treatment of brain metastases from renal cell carcinoma. Urology. 1990;36:112-117.
196 Wronski M, Arbit E, Russo P, et al. Surgical resection of brain metastases from renal cell carcinoma in 50 patients. Urology. 1996;47:187-193.
197 Leavens ME, Moser RP, Obbens EAMT, et al. Surgical treatment of metastatic brain tumors. Cancer Bull. 1986;38:39-44.
198 Pieper DR, Hess KR, Sawaya RE. Role of surgery in the treatment of brain metastases in patients with breast cancer. Ann Surg Oncol. 1997;4:481-490.
199 Shu HKG, Sneed PK, Shiau CY, et al. Factors influencing survival after Gamma Knife radiosurgery for patients with single and multiple brain metastases. Cancer J Sci Am. 1996;2:335.