CHAPTER 274 Metabolic and Other Nondegenerative Causes of Low Back Pain
About two thirds of adults suffer from low back pain at some point in their lives.1–3 Low back complaints are the primary reason for lost productivity and the second most common reason to seek medical attention.4 Table 274-1 shows a broad differential diagnosis for low back pain and the estimated prevalence of various disorders observed in a primary care setting. Degenerative and mechanical disorders of the lower back account for the vast majority of patients who present with low back pain.4,5 In a significantly small proportion of patients, nondegenerative disease is the cause of low back pain; this includes metabolic, inflammatory, infectious, neoplastic, hematologic, and vascular causes, as well as visceral referred pain syndromes. An understanding of nondegenerative causes of low back pain is necessary for the appropriate evaluation and triage of patients. Management often requires a collaborative effort among medical specialists, pain specialists, and surgeons. Many of these topics are covered in depth elsewhere; the following serves as a concise review of these conditions as they relate to low back pain.
TABLE 274-1 Differential Diagnosis of Low Back Pain
| MECHANICAL LOW BACK PAIN (97%) | NONMECHANICAL SPINAL CONDITIONS (1%) | VISCERAL DISEASE (2%) |
|---|---|---|
| Lumbar strain, sprain | Neoplasia | Disease of pelvic organs |
| Degenerative processes | Multiple myeloma | Prostatitis |
| Herniated disk | Metastatic carcinoma | Endometriosis |
| Spinal stenosis | Lymphoma and leukemia | Chronic pelvic inflammatory disease |
| Osteoporotic compression fracture | Spinal cord tumors | Renal disease |
| Spondylolisthesis | Retroperitoneal tumors | Nephrolithiasis |
| Congenital disease | Primary vertebral tumors | Pyelonephritis |
| Severe kyphosis | Infection | Perinephric abscess |
| Severe scoliosis | Osteomyelitis | Aortic aneurysm |
| Transitional vertebrae | Septic diskitis | Gastrointestinal disease |
| Spondylolysis | Epidural abscess | Pancreatitis |
| Internal disk disruption or diskogenic low back pain | Shingles | Cholecystitis |
| Presumed instability | Inflammatory arthritis | Penetrating ulcer |
| Ankylosing spondylitis | ||
| Psoriatic spondylitis | ||
| Reactive arthritis | ||
| Inflammatory bowel disease | ||
| Scheuermann’s disease | ||
| Paget’s disease of bone | ||
| Osteoporosis/osteopetrosis |
Data from Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001;344:363-370.
Metabolic Bone Disease
Metabolic bone disease, which includes osteomalacia, Paget’s disease, and osteoporosis, is considered a major health threat in the United States because more than 18 million individuals have compromised bone density. Many of these patients are at increased risk for vertebral body fractures. These fractures are one of the main causes of severe debilitating back pain that results in a reduced quality of life, physical function, and survival.6,7 The occurrence of a single vertebral fracture substantially increases the likelihood of future fractures and progressive kyphotic deformity.8 Open surgical intervention is usually reserved for patients with neurological deficits or spinal instability. However, minimally invasive procedures, such as vertebroplasty and kyphoplasty, have become integral to the management of acute vertebral fractures.6,8
Osteomalacia
Osteomalacia is the failure of the bone matrix to mineralize normally.9,10 The most frequent causes of osteomalacia are a lack of extracellular calcium and phosphate, abnormal osteoblast function, defective collagen production, and low pH at the sites of mineralization. Osteomalacia less commonly results from vitamin D deficiency, which can be caused by inadequate exposure to sunlight or by gastrointestinal disease that interrupts normal vitamin D absorption. Abnormal intestinal absorption can be caused by biliary fistula, chronic steatorrhea, sprue, or surgical resection of a large portion of the distal jejunum and ileum.11,12 Osteomalacia can also result from chronic renal disease, with impaired synthesis of 1,25-dihydroxycholecalciferol and the loss of calcium resorption. Phosphate-deficient diets also cause osteomalacia. Vegetarians who eliminate dairy products from their diet are susceptible to phosphate deficiency, as are patients who take large doses of aluminum hydroxide,13 which can block the intestinal absorption of phosphate. Other causes include systemic acidosis,14 drug side effects,15,16 tumor toxins,13 and primary mineralization defects. Severe phosphate wasting is associated with certain tumors such as sclerosing hemangioma, angiosarcoma, hemangiopericytoma, and nonossifying fibroma.13
There is an initial decrease in bone density that is indistinguishable from osteoporosis. The pathognomonic radiologic finding in osteomalacia is the pseudofracture of long bones, characterized by a radiolucent band running perpendicular to the bone surface.17 These fractures are sometimes referred to as Fraser’s zones or milkman’s fractures. As the disease progresses, compression fractures of the vertebrae may occur with little or no trauma.
Osteoporosis
Osteoporosis is defined as a skeletal disorder characterized by loss of bone mass that causes fragility, predisposing an individual to an increased risk of fracture. It can be categorized as primary or secondary. Primary osteoporosis is most often seen in postmenopausal women and older men.8 An estimated 20% of women suffer an osteoporotic fracture by age 65 years, and 40% of women have an osteoporotic fracture at some point during their lives.18 Vertebral, hip, and wrist fractures are the most common. The major predisposing factors are sex hormone deficiency and reduced calcium intake in the elderly.18–22 Other risk factors include alcohol abuse, smoking, immobilization, and lack of exercise.23 Paradoxically, women who exercise excessively and experience weight loss and amenorrhea are at risk for osteoporosis.24,25 Secondary osteoporosis is seen with the use of corticosteroids, thyroid hormone, and anticonvulsant drugs.15,16,26–29 It is also associated with genetic disorders such as Turner’s syndrome and Klinefelter’s syndrome and can occur in children and adolescents.18
Vertebral compression fractures are one of the most common manifestations of osteoporosis, and more than 700,000 compression fractures are seen each year.30 Localized back pain is often the presenting complaint, sometimes associated with pain in a radicular distribution. Progressive loss of stature results in shortening of the paraspinal musculature; therefore, prolonged active contraction of these muscles is required to maintain posture.31 This is a major cause of back pain in spinal osteoporosis. Weight bearing aggravates the pain, whereas offloading the spine with bed rest improves the pain. Healing of vertebral body fractures may take 4 to 8 weeks; however, subsequent vertebral fractures may occur, producing chronic pain complaints, dorsal kyphosis (i.e., widow’s hump or dowager’s hump), and loss of height.
Plain radiographs and dual-energy x-ray absorptiometry (DXA), which is used to assess bone density, are the diagnostic modalities of choice. A biconcave central compression fracture or burst fracture is usually seen in the lumbar spine, and an anterior wedge fracture is seen in the thoracic spine.31
Optimal treatment for osteoporosis is prevention by maximizing bone mineral density. Medical management includes supplemental calcium, vitamin D, and bisphosphonate therapy. Calcitonin and parathyroid hormone therapy have also been used. Estrogen replacement therapy is helpful but has been associated with a higher incidence of breast cancer. The risks and benefits of hormone replacement therapy are complex and are discussed in the medical literature.32
For symptomatic fractures, conservative treatment includes pain management and physical therapy. Thoracolumbosacral orthoses and Jewett braces may help prevent further vertebral compression. Open surgical management of osteoporotic vertebral fractures is rarely employed except in cases of neurological deterioration or significant instability. The compromised bone density makes stabilization and fusion of the osteoporotic spine a difficult endeavor. Vertebral body augmentation techniques, such as kyphoplasty and vertebroplasty, are safe and effective at reducing pain.8 These techniques consist of injecting polymethyl methacrylate (PMMA) bone cement into the compression fracture. These interventions are often preferred because they are less invasive and can be done in an outpatient setting.
Paget’s Disease
Paget’s disease (osteitis deformans) is a focal disorder of bone metabolism characterized by uncontrolled bone reabsorption and formation caused by excessive numbers of osteoblasts and osteoclasts, leading to the development of thick but soft bone. Paget’s disease has a predilection for the lumbosacral spine. It has a heterogeneous geographic distribution, with a much higher incidence in England and Germany; it is rarely encountered in Scandinavia, Africa, the Middle East, or Asia. Nuclear inclusions, resembling virus particles, have been seen in osteoclasts, suggesting a slow virus as a possible cause of Paget’s disease.33,34
Back or radicular pain results from compression fractures or foraminal compromise. Severe focal bone pain may also occur with sarcomatous degeneration. Radiologic abnormalities range from purely osteolytic lesions to combined osteolytic and sclerotic lesions. Thickening of the vertebrae, compression fractures, and disappearing vertebrae can be encountered.35 A bone scan is the most sensitive diagnostic modality to screen for Paget’s disease.
The majority of patients are asymptomatic. Serum calcium, phosphate, magnesium, and parathyroid hormone levels are normal. There are marked increases in the concentrations of alkaline phosphatase and acid phosphatase. Treatment includes calcitonin and bisphosphonate therapy.36,37 Spine surgery may be indicated for severe spinal stenosis.
Inflammatory Disorders
Ankylosing Spondylitis
Human leukocyte antigen B27 (HLA-B27), the B27 haplotype of the major histocompatibility complex, confers a strong genetic predisposition for AS. HLA-B27 is seen in up to 90% of white western European patients with AS, compared with 8% of the general population38; the prevalence of HLA-B27 among blacks in the United States is about 2%. An unknown environmental factor in genetically predisposed patients is the likely trigger for this disease.39 AS is 3 to 9 times more prevalent in men than in women. AS is milder and less rapidly progressive in women. Typically, the disease begins in people 15 to 40 years of age.38,40
An insidious onset of low back pain and stiffness is the usual manifestation. This pain may extend from the thoracic spine to the buttocks, radiating into the legs above the knees. It is usually worse in the morning but improves with exercise. Peripheral joint complaints and nonspecific systemic manifestations of fatigue, anemia, low-grade fevers, and weight loss are often present. Neurological symptoms can occur with spinal cord or nerve root compression, and vertebral fractures are commonly seen.41
Traditional treatment of AS begins with physical therapy. Analgesics and nonsteroidal anti-inflammatory drugs are very effective means of pain relief. Recent evidence has shown that disease-modifying antirheumatic drugs, including sulfasalazine and methotrexate, may be helpful, but so far the data are inconclusive.39 Additionally, corticosteroid injections and bisphosphonates can improve pain control. Recent evidence from randomized controlled studies indicates that inhibitors of tumor necrosis factor, such as etanercept, infliximab, and adalimumab, have had a profound impact on the treatment of spine pain, function, and peripheral joint disease in those with AS.39
Reactive Arthritis
The term reactive arthritis generally refers to rheumatic disorders seen after an infection, as long as the pathogen was not found in the affected joint. This term also refers to the triad of urethritis, conjunctivitis, and arthritis, which is the classic description of what was formerly called Reiter’s syndrome. In 2003 leaders in the field of rheumatology agreed to expunge the term Reiter’s syndrome from the literature and replace it with reactive arthritis.42 This syndrome is associated with nongonococcal urethritis and enteral infections with Shigella, Salmonella, Campylobacter, and Yersinia, and it usually occurs during or shortly after these infections. The HLA-B27 antigen is present in 80% of these patients.38,40 The arthritic component of reactive arthritis typically includes a periarterial arthropathy that is acute, involves a few joints (often asymmetrically), and is commonly localized within the lower extremities. Axial involvement, which occurs in more than one third of patients, includes sacroiliitis and arthropathy of the lumbosacral spine. The sacroiliitis in reactive arthritis may be unilateral and asymmetric, unlike AS and inflammatory bowel disease, but it is otherwise indistinguishable. Spinal involvement, including ossification and syndesmophyte formation, occurs less often and in a more random fashion than in AS.
Genitourinary symptoms occur in 93% of patients. Iritis and conjunctivitis with pain and photophobia occur in 20%.38 One third experience fever, anorexia, and fatigue. Mucocutaneous lesions are also common. The arthritis occurs 1 to 3 weeks after the initial infection, most commonly with asymmetric pain in the knees, ankles, feet, and sacroiliac joint. The sacroiliac joint is involved in 30% to 90% of these patients and accounts for most back pain.38,40 Nonsteroidal anti-inflammatory drugs and steroid injections are the main agents for treatment. Antibiotic therapy is often used to treat the underlying infection.43
Psoriatic Arthropathy
The uric acid concentration is elevated in 20% of patients, who may develop gout. Thirty-five percent to 60% of those with axial skeletal involvement have elevated expression of HLA-B27.38,40
Psoriasis occurs equally in men and women, with most cases occurring in temperate climates. Among all patients with psoriasis, 5% develop psoriatic arthritis, usually after the onset of skin changes. Severe skin and nail changes increase the risk of developing psoriatic arthritis but do not correlate with arthritis symptoms. Spine involvement occurs in 20% of psoriatic arthritic patients, tends to predominate in males, and has an onset later in life.38,40 Additionally, it more often affects the cervical spine than the lumbar spine.
Enteropathic Arthritis
Enteropathic arthritis is associated with the inflammatory bowel diseases Crohn’s disease and ulcerative colitis. Joint involvement may also occur with certain gastrointestinal disorders such as celiac disease or Whipple’s disease and even following intestinal bypass surgery for obesity.44
Peripheral arthritis occurs concurrently with exacerbations of bowel disease, whereas involvement of the spine progresses independently of the bowel disease. Of all patients with enteropathic arthropathy, 5% are HLA-B27 positive, but among patients with axial skeletal involvement, this percentage increases to 50% to 75%.38
Spondyloarthropathy occurs in about 2% to 12% of patients with ulcerative colitis and Crohn’s disease.38 Pain and stiffness of peripheral and axial joints that improve with ambulation are common, along with the symptoms and signs associated with the bowel disease. Symptoms usually manifest as inflammatory spine pain and alternating buttock pain or chest pain. The bony ankylosis generally moves from the lumbar spine toward the cervical spine. Ulcerations of the gastrointestinal tract, iritis, erythema nodosum, and pyoderma gangrenosum are common.
Myofascial Pain Syndrome
Myofascial pain syndrome (MPS) may be one of the most common causes of persistent musculoskeletal pain, particularly in the cervical and lumbar region. MPS is defined as a musculoskeletal pain disorder caused by one or more myofascial trigger points and their associated reflexes. Trigger points are small nodules of hypersensitivity located in rope-like bands of skeletal muscle. They are detected through palpation on examination.45 This syndrome frequently affects the lower back and neck; it usually occurs in middle-aged patients, with a marked female preponderance. It is often misdiagnosed as fibromyalgia. Although similar, the characteristic difference is that MPS is associated with regional pain and trigger points, whereas fibromyalgia is associated with generalized pain and tender points. MPS is frequently initiated by injury or overuse. The treatment consists of injecting the trigger points with local analgesics, followed by stretching exercises of the involved muscle groups. Strengthening exercises should be avoided. Heat and massage may be beneficial.45–47 Although MPS may be prevalent, there is no modality that can render a definitive diagnosis. The diagnosis is made based on the presence of major and minor clinical criteria.45
Infection
Pyogenic Infection
Pyogenic infections of the spine almost exclusively involve the vertebral body and only rarely the posterior elements of the spine. The most frequent site of infection is the lumbar spine, followed by the thoracic, cervical, and sacral bones. The usual source is hematogenous spread of an infectious process elsewhere in the body. The routes of dissemination are thought to be the arterioles within the vertebral metaphyseal region or the valveless paraspinous venous plexus (Batson’s plexus veins).48 These infections may manifest acutely, subacutely, or chronically, depending on the virulence of the organism and the host response to the infection.
Radiographic changes usually do not manifest for 2 to 3 weeks, with a paravertebral shadow or loss of disk space height often being the initial sign. Vertebral end plates show an increased density at approximately 12 weeks, and lytic changes in these end plates develop later, leading to vertebral destruction and collapse. Computed tomography (CT) reveals bone erosion and vertebral destruction quite early and delineates the soft tissue abnormalities at the infection site. Magnetic resonance imaging (MRI) is becoming the most sensitive and specific method of diagnosing pyogenic infections of the spine, with a sensitivity of 96%, specificity of 92%, and accuracy of 94%.49 The administration of gadolinium demonstrates disk space enhancement early in the course of the infection. A bone scan may localize the area before radiographic evidence exists and is extremely sensitive to early inflammatory changes.
Image-guided needle biopsy (either CT or fluoroscopy) can be used in the diagnosis and treatment of spine infections and has low morbidity and mortality. Treatment includes rest, immobilization if pain is severe, and appropriate antibiotics after cultures are obtained. A 6-week course of intravenous antibiotics is a general guideline, followed by repeat imaging to assess the efficacy of treatment. Often patients can be discharged home or to a nursing facility during the treatment period. Surgical débridement should be considered for the following situations: no clinical response is seen within the first 6 weeks, neurological compromise exists or progresses, radiographic imaging demonstrates severe neural element compression or deformity, or evidence of chronic infection exists (e.g., draining sinus tract). If there is concern about instability, spinal fixation and fusion are warranted. Reports have shown that the use of spinal instrumentation and autologous bone graft in the presence of infection does not lead to a higher risk of persistent or recurrent infection.50
Granulomatous Disease
Laboratory evaluation and medical treatment are specific for each disease. In general, the goals of management are to eradicate the infection and prevent or treat neurological deficits and spine deformity. Tissue biopsy is necessary for diagnosis; biopsies must be evaluated for appropriate cultures and also for pathologic study. Uncommon infections such as tuberculosis and fungal infections are often first identified by microscopic evaluation. Treatment is guided by the results of the cultures. However, surgery is frequently necessary. An operation may be performed to drain abscesses, débride sequestered bone and disk, decompress the spinal cord, or stabilize the spine to prevent or correct deformity.51
The most common granulomatous infection is tuberculosis. Fungal infections caused by Blastomyces dermatitidis, Cryptococcus neoformans, Coccidioides immitis, Aspergillus, and Histoplasma capsulatum, as well as bacteria of the genus Brucella, Actinomyces bovis, and Actinomyces israelii, can also lead to granulomatous disease of the spine.51
Neoplastic Disease
Both primary bone tumors and metastatic lesions can be found in all regions of the spine and in all age groups. The vertebral column is the most common bone site for secondary malignancy, and up to 90,000 new cases of metastatic disease to the spine are diagnosed each year in the United States alone. By contrast, primary bone tumors are rare and account for a very small percentage of spine tumors, with a prevalence of 2.5 to 8.5 cases per 100,000 persons per year.52 A variety of extradural and intradural soft tissue tumors may also give rise to low back pain.
Focal pain is the most common symptom in tumors of the spine, although radicular symptoms may also be present. The pain is progressive, unremitting, and usually worse at night. Compression fractures are often associated with osteolytic lesions, which may lead to instability and deformity of the spine. This may also progress to weakness in the lower limbs, sensory loss, and loss of sphincter control. Although bowel and bladder dysfunction may be seen at the initial presentation, they are almost never isolated findings.53–55
In addition to the history and physical examination, the diagnostic work-up includes imaging studies, such as CT and MRI, to evaluate the bone and soft tissue involvement (Table 274-2).56 Additionally, spinal angiography, positron emission tomography, and bone scans may be indicated to aid in diagnosis.
Benign Primary Tumors
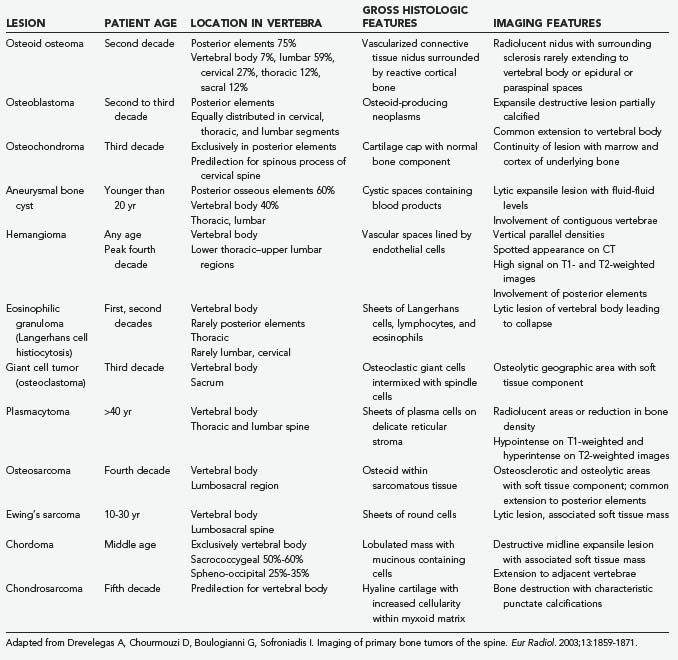
Osteoid Osteoma and Osteoblastoma
Osteoid osteoma and osteoblastoma are pathologically similar diseases differentiated by size. Osteoid osteomas are less than 1.5 cm in diameter, and osteoblastomas are 1.5 cm or larger.57 These lesions are solitary and painful and cause an osteoblastic reaction in the involved bone, encountered primarily in the long bones of the lower extremities.58,59 In reported series of osteoma and osteoblastoma, 10% to 41% of patients have spine involvement.
The lumbar spine is most often involved, with the posterior elements acting as the predominant site of origin; the vertebral body is rarely involved. These lesions are more common in males and occur most frequently in the second and third decades of life. Nocturnal pain is the characteristic complaint, and 14% to 19% of patients exhibit dramatic improvement with aspirin and nonsteroidal anti-inflammatory drugs.58–60 Some patients have associated radicular involvement. Neurological deficits are more common with the larger osteoblastoma; approximately 25% to 75% of these patients present with some form of neurological deficit, compared with 0% to 30% of patients with osteoid osteoma. Osteoblastomas tends to be more aggressive and have the capacity for malignant transformation to osteosarcoma.61
Excision of the lesion is the treatment of choice, but curettage is effective when excision is not feasible. Fusion is recommended only when stability is compromised.62 With complete excision, pain is eliminated in 92% of patients.59 Persistence of pain postoperatively is suggestive of residual tumor.
Osteochondroma
Osteochondromas are common benign bone lesions, with vertebral involvement occurring in roughly 7% of all cases. Hyaline cartilage–capped bony growths extend from the growth plate as solitary or multiple lesions that usually grow quite slowly. They typically involve the posterior elements, and the majority are identified in the cervical spine, particularly at C2. These lesions are also seen in patients with hereditary multiple exostoses. This condition is associated with a higher rate of malignant transformation (3% to 5%), as opposed to a 1% transformation incidence observed with solitary lesions.63
These lesions are usually asymptomatic, but neurological deficits can occur if there is compression of the spinal cord or roots. Radiographic examination reveals the bony portion of the lesion, but the cartilaginous cap is rarely visualized on plain radiography. CT, MRI, and myelography may be required for complete visualization. The pathologic and radiologic hallmark of osteochondroma is continuity of the lesion with the marrow and cortex of the underlying bone.56
Aneurysmal Bone Cyst
Aneurysmal bone cysts are benign neoplastic lesions of bone consisting of anastomosis of cavernous spaces; they constitute approximately 15% of all primary spine tumors.64 The lumbar spine is the most frequent site, followed by the thoracic spine, with cysts typically involving the posterior elements. Most lesions appear in children and young adults and are more common in women.
Radiographically, these lesions often extend between multiple adjacent vertebrae. The expansive osteolytic capita with their thin cortices have a bubbly appearance that gives the lesion its name. In addition to CT and MRI, a preoperative angiogram may identify feeding vessels that can be embolized. Selective arterial embolization can reduce the vascularity of the lesion and possibly decrease intraoperative blood loss. If no feeding vessel is identified, direct percutanous embolization may be a viable option.64,65
The treatment of choice is en bloc excision when feasible, but curettage seems to be similarly efficacious.64 Cysts recur in approximately 10% to 15% of cases, but a second resection or curettage is quite effective. Depending on the destructive nature of the lesion, a postoperative stabilization procedure may be indicated.
Hemangioma
These radiosensitive tumors can sometimes be cured by low-dose irradiation, around 30 to 40 Gy.66 Surgical resection should be considered in symptomatic cases when irradiation is not feasible or in the presence of neurological deterioration. These tumors are often very vascular, and surgery can be complicated by significant blood loss. Endovascular intervention can facilitate surgical resection by eliminating the arterial tumor supply and dramatically reduce intraoperative bleeding.67,68 Other modalities, including vertebroplasty and ethanol injection, have been reported for pain relief but are usually secondary to selective angiography, surgery, and radiation.69
Eosinophilic Granuloma
Eosinophilic granuloma is a benign lesion that is one of the reticuloendothelial proliferative diseases. Called Langerhans histiocytosis or histiocytosis X, eosinophilic granuloma has an uncertain origin. When the spine is affected, these lesions are generally found in the vertebral body, typically in a single cervical vertebra; lumbar involvement occurs much less often. Eosinophilic granulomas can occur in all age groups, but it predominantly affects children, adolescents, and young adults.70
Eosinophilic granuloma is usually self-limited, and many patients do not require treatment. A percutaneous biopsy can be performed, particularly when it is necessary to differentiate the lesion from other neoplasms. These lesions show evidence of spontaneous resolution by fibrosis within 1 to 2 years. When neurological compromise occurs, the combination of steroids and low-dose radiation therapy with immobilization is a widely accepted treatment. Curettage and excision may be another curative option.71
Giant Cell Tumor
Giant cell tumors of bone (osteoclastoma) are benign lesions made up of mononuclear cells interposed with multinucleated osteoclast-like giant cells. Although histologically benign, these lesions tend to be locally aggressive. Spine involvement occurs in 2% to 3% of patients with these lesions and occurs predominantly in the sacrum.72,73 Almost all lesions involve the vertebral body, but the posterior elements can also be involved. Women are affected slightly more frequently than men, with a peak incidence in the third decade. Metastasis to the lungs is possible and often portends a worse prognosis.74
Paraspinous muscle spasm is the most common presenting symptom. Pain and radicular changes are less common. If there is sacral involvement, bowel or bladder symptoms may be clinically evident.74
A lytic lesion that is well circumscribed or expansive, depending on the aggressiveness of the lesion, can be seen on plain radiography. A well-defined pseudocapsular margin is visible on CT, which allows complete tumor delineation. MRI reveals a heterogeneously enhancing lesion with paraspinal and epidural disease. Hemorrhage and compression fractures may be associated. Diagnosis is usually achieved with CT-guided core biopsy. Although this core needle biopsy technique may be susceptible to sampling error, a recent study recommends fine-needle aspiration biopsy and reports a diagnostic accuracy of greater than 94%.74,75
Because of the locally aggressive nature of these lesions, complete en bloc excision is the treatment of choice; however, local recurrence is commonly observed at 3 years after surgery.76 Phenol injections, cement, cryosurgery, curettage, and radiotherapy may all play a role as alternatives to en bloc resection.
Malignant Primary Tumors
Multiple Myeloma and Plasmacytoma
Multiple myeloma is a monoclonal proliferation of malignant plasma cells of bone marrow that can spread throughout the body. The marrow of the spine is affected in about two thirds of patients, and the thoracic and lumbar regions are most commonly involved.56 Multiple myeloma is rare, occurring in 4.7 of 100,000 white men and 3.2 of 100,000 white women; the frequency is twice that among blacks. In most cases the disease appears after the sixth decade, with a male preponderance in both races.77 Plasmacytoma represents the solitary form of the disease. Plasmacytoma may occur as a lesion outside the bone marrow (extramedullary plasmacytoma) or as a solitary lesion of bone (solitary plasmacytoma). The prognosis for patients with extramedullary plasmacytoma appears to be better than that for patients presenting with multiple myeloma or solitary bone plasmacytoma.78 Overt multiple myeloma ultimately develops in 50% to 60% of patients with solitary plasmacytoma of the bone. In one series, two thirds of patients who progressed did so within 3 years or less. In a study done at the Mayo Clinic in a cohort of 46 patients, 77% of those who progressed did so within 4 years, although progression occurred as late as 13 years.79–81 The spine is the initial presenting site in 25% to 50% of cases of solitary plasmacytoma. These tumors usually appear after the fifth decade of life and are 3 times more common in men than women. The thoracic spine is the most common site of involvement, but the lumbar spine is frequently involved.
Plasmacytoma and multiple myeloma are considered very radiosensitive and chemosensitive tumors, and these modalities are the primary treatment methods. Stereotactic radiosurgery has also been used in select patients.82 Surgery may be necessary to address progressive neurological deficits as well as mechanical instability as a result of osteolytic bone destruction and diffuse osteoporosis.83 Kyphoplasty and vertebroplasty can be used to treat pathologic fractures and can aid in pain management.84 Medical management has also been used effectively to reduce skeletal-related events. Bisphosphonates, corticosteroids, and antineoplastic agents can be used.
The prognosis is considerably worse in multiple myeloma than in solitary plasmacytoma. Disease-free survival is possible for patients with solitary lesions, but for those with systemic multiple myeloma, median survival is 28 months, with a 25% to 29.3% 5-year survival rate.77 With spinal cord involvement, the prognosis is worse in both diseases.
Osteosarcoma
Osteosarcoma is the most common primary malignant tumor of bone after myeloma, accounting for nearly 20% of osseous tumors. These osteoblastic tumors develop most commonly in the rapidly growing bones of the extremities as primary osteosarcoma; they can also occur in a variety of other conditions, such as Paget’s disease or fibrous dysplasia, or result from previous radiotherapy. Spine involvement occurs in 1% to 3% of patients, particularly in the thoracic spine and sacrum. Osteogenic sarcoma is most common between the ages of 10 and 20 years, but involvement of the spine is more common in adults. Secondary lesions occur later in life. Men are affected slightly more often than women.85
Osteosarcomas are aggressive and radioresistant. Current treatment protocols include surgery, radiation, and chemotherapy. The prognosis for patients with this disease is poor, probably owing to the difficulty of en bloc resection and the inability to achieve adequate surgical margins. Additionally, close proximity of the spinal cord limits the radiation dose to the tumor bed.86 Osteosarcoma of the spine has an especially poor prognosis.55
Ewing’s Sarcoma
Ewing’s sarcoma accounts for 0.5% of primary malignant bone tumors of the spine, and only 3% to 15% of Ewing’s sarcoma occurs in the spine. These small, round-cell tumors of unknown origin arise predominantly in the sacrum and lumbar spine and demonstrate a male predominance. They usually appear during the second decade of life, with the average age of onset being 16.5 years.86,87
Localized pain is the most common presenting symptom. Neurological deficits have been reported in up to 40% to 60% of patients. Bowel and bladder problems are less frequent.86
Plain radiographic examination reveals lytic, moth-eaten destruction of bone with a sclerotic rim. Vertebral collapse may develop in severe disease. However, these changes do not occur until late in the disease; CT and MRI with and without contrast are the diagnostic modalities of choice. CT demonstrates aggressive patterns of bone destruction and the soft tissue extension. MRI is superior for the delineation of the soft tissue mass, which shows homogeneous enhancement.88
Diagnosis can be confirmed with image-guided needle aspiration. Treatment includes chemotherapy, radiation, and surgery. Radiation is particularly helpful because these lesions are radiosensitive. Surgical excision is the treatment of choice, and en bloc spondylectomy allows wide or marginal resection with an acceptable morbidity in the hands of experienced surgeons.89 The reported 5-year survival rate is 48% to 58% and has been improving with more recent therapies.86
Retroperitoneal Sarcoma
Connective tissue tumors located in the retroperitoneal space are known as retroperitoneal sarcomas. These tumors rarely involve the spine and thus usually do not come to the attention of neurosurgeons. However, pelvic retroperitoneal sarcomas, such as liposarcoma and leiomyosarcoma, can involve the spinal and paraspinal muscles, resulting in pain, fractures, and neurological deficit. These tumors tend to be very aggressive. Management is similar to that for the other sarcomatous lesions previously discussed and includes a combination of surgical resection, radiation, and chemotherapy.86
Chordoma
Chordomas are rare primary malignant tumors that arise from remnants of the primitive notochord at the sacrococcygeal and basioccipital regions and occasionally from notochordal rests in the vertebrae. These lesions account for about 20% of malignant tumors of the axial skeleton, 50% in the sacrococcygeal region, and 15% in the vertebrae above the sacrum.90 Men develop sacral chordomas 3 times more often than women, and most of these tumors appear in the fifth through seventh decades of life. There is no sex difference in the incidence of clival or vertebral chordomas.90–94
Pain in the lower back and sacrum is the most common presenting symptom, and a palpable mass is frequently encountered. Radiculopathy, with or without bladder and bowel dysfunction, is suggestive of foraminal extension of the tumor.95
The most frequent radiographic appearance is that of a destructive expansile lesion of the vertebral body centered in the midline, with a large associated soft tissue mass. There can also be calcifications in the sacrum and lytic destruction of the vertebrae, with a sclerotic rim in the lumbar spine. The intervertebral disk space is often involved as the tumor spreads locally. CT and MRI are useful for determining the extent of the tumor. Images on CT reveal destruction of bone without any associated sclerosis and low attenuation within the soft tissue mass, which is due to the gelatinous material and cystic degeneration within the tumor. On MRI there is enhancement of the lesion. Notably, chordomas have a very high signal intensity on T2-weighted images, similar to the nucleus pulposus.56,88
Complete surgical resection with wide margins is the treatment of choice. However, these are often technically demanding cases and may require a multidisciplinary approach. Surgical considerations include a detailed knowledge of the anatomy, an understanding of multiple surgical exposures, and acceptance of the possibility of intentional sacrifice of the sacral roots, resulting in loss of bladder, bowel, and sexual function. Additionally, sacral reconstruction and spinopelvic stabilization are often necessary. For chordomas of the spine, either spondylectomy or intralesional extracapsular excision can be performed.96 Inadequate tumor margin is the main factor negatively affecting the prognosis and is the cause of seeding of the tumor. The rate of local recurrence after intralesional extracapsular excision, even if followed by conventional radiation therapy, is consistently higher and earlier compared to complete resection followed by radiotherapy.97 Although chordomas are considered radioresistant, high-dose radiation therapy may serve a palliating role in the control of pain and neurological deficits.98 Newer therapies include intensity-modulated radiotherapy and proton therapy.95 Chemotherapy is generally ineffective. The median 5-year survival is 66% for sacral chordomas and 50% for vertebral chordomas.94
Chondrosarcoma
Chondrosarcoma, a rare malignant tumor of cartilaginous origin, may arise as a primary malignancy or develop from benign cartilaginous tumors after irradiation. Approximately 10% of these tumors arise in the spine, and up to 60% arise in the thoracic spine; the remainder occur in the cervical and lumbar spine. Men are affected more commonly than women, and the mean age at presentation is in the fourth decade.99
Radiographically, these lesions appear as lytic and destructive, and an associated soft tissue mass is frequently calcified. CT and MRI can delineate the tumor’s extent. MRI may show a peripheral ring of enhancement or heterogeneous enhancement of the entire tumor.99
Complete surgical excision is the treatment of choice. The extent of surgical resection has a significant impact on the recurrence rate.100 These lesions are not sensitive to radiotherapy or chemotherapy. The median survival rate is poor, with 50% mortality between 2 and 5 years.99
Malignant Secondary Tumors
The axial skeleton is the third most common site of metastases, after the lungs and liver. Metastases are by far the most common skeletal tumors, and the spine is the most common site of involvement. Metastases account for 70% of all tumors of the spine, and the thoracic spine is most frequently involved, followed by the lumbar and then cervical regions.2 The incidence of bone metastasis has risen because of a worldwide increase in the incidence of cancer, which is largely attributed to a longer life expectancy in cancer patients.101 The most common metastatic tumor types are breast carcinoma, lung carcinoma, lymphoma, and prostate carcinoma.
Important considerations before deciding on treatment include the patient’s age, preoperative functional status, medical comorbidities, life expectancy, and need for tissue diagnosis. Typically, surgical intervention should be offered to patients with malignant spinal cord compression.102 Surgery is often reserved for patients with a life expectancy of at least 12 months, but it should not be withheld if it will significantly improve the patient’s quality of life. Radiotherapy is often the treatment of choice for certain spinal metastases. Tumors of the prostate and lymphoreticular system are very radiosensitive. Most breast carcinomas are sensitive, but gastrointestinal and renal tumors are resistant. The radiosensitivity of the lesions often determines management.101 In some cases, a histologic diagnosis may be necessary, and a biopsy of a peripheral lesion or of the spine may be indicated. As a general guideline, radiation therapy is usually indicated if pain is the presenting syndrome with no neurological deficit, or if there is a stable neurological deficit.103 If the patient’s life expectancy is very short (<3 months) or the patient is a poor surgical candidate, radiation therapy should be considered the initial treatment alternative. Chemotherapy is also used, and its effectiveness greatly depends on the relative sensitivity of the tumor. In the acute phase of management, corticosteroids are often given to patients with new or progressive neurological deficits.
Nonvertebral Spinal Tumors
A variety of intradural and extradural soft tissue tumors arising in proximity to the spinal column can produce low back pain. Spinal cord tumors are an uncommon cause of low back pain. The heterogeneous cell composition of the spine results in a histologically variable group of neoplasms.104
Intramedullary Tumors of the Spine
Twenty percent to 30% of intradural tumors are intramedullary spinal cord neoplasms. Eighty percent of these tumors are glial in origin, represented primarily by astrocytomas and ependymomas. Oligodendrogliomas, mixed gliomas, subependymomas, hemangioblastomas, lipomas, angiomatous lesions, intramedullary metastases, and developmental tumors such as teratomas are uncommon.91,104 Spine tumors occur predominantly in young or middle-aged adults and are less common in childhood and old age. Although tumors are more common in the thoracic region, when the actual lengths of the various portions of the spinal cord are taken into consideration, the distribution is relatively equal.105 There is no sex preference.
Biopsy, often with the goal of gross total resection, is the primary treatment of choice. Certain tumors, such as ependymomas and hemangioblastomas, can be cured with complete excision. Astrocytomas are usually not amenable to complete excision and have a high recurrence rate; adjuvant therapy with chemotherapy and radiation is advocated, but it has a minimal effect on disease progression.104
Extramedullary Tumors of the Spine
Two thirds of intradural tumors are extramedullary and are usually benign. Nerve sheath tumors and meningiomas account for nearly 70% of extramedullary tumors and occur with equal frequency. Less common are myxopapillary ependymomas, which arise from the conus and filum. Rare extramedullary tumors include hemangiopericytoma, lipoma, paraganglioma, epidermoid cyst, and dermoid cyst.104 For the most common tumors (i.e., nerve sheath tumors, meningiomas), the frequency increases with increasing age. Neurofibromas affect men and women equally, but 80% of meningiomas occur in women.91
Retroperitoneal and Pelvic Disorders
Disease processes in the retroperitoneal space or pelvis may manifest as back pain. Periodic low back pain may occur with endometriosis and can be associated with leg pain if the lumbosacral plexus is involved. Back pain usually begins just before the onset of menses, and the usual treatment is hormone manipulation. Retroperitoneal inflammatory processes, such as perforation of a retroperitoneal appendix, sigmoid diverticulitis, and posterior wall duodenal ulcer, may manifest as low back pain. Tumors in the retroperitoneal space involving the pancreas, kidney, or rectum are associated with back pain. Aortic aneurysms with dissection produce severe, acute back pain. All these processes are characterized by nonfocal pain, unlike the very focal pain that is characteristic of vertebral involvement. Early diagnosis and referral to the appropriate subspecialist are essential for the appropriate management of these disorders.54
Anderson;. 2006; Anderson. Tumors of the Spine. In: Herkowitz HN, Garfin SR, Eismont FJ, et al, editors. The Spine, II. Philadelphia: Saunders; 2006:1235-1264.
Bartels RH, van der Linden YM, van der Graaf WT. Spinal extradural metastasis: review of current treatment options. CA Cancer J Clin. 2008;58(4):245-259.
Bilsky MH, Azeem S. Multiple myeloma: primary bone tumor with systemic manifestations. Neurosurg Clin N Am. 2008;19(1):31-40.
Boriani S, Bandiera S, Biagini R, et al. Chordoma of the mobile spine: fifty years of experience. Spine. 2006;31(4):493-503.
Burch S, Hu S, Berven S. Aneurysmal bone cysts of the spine. Neurosurg Clin N Am. 2008;19(1):41-47.
Chi JH, Bydon A, Hsieh P, et al. Epidemiology and demographics for primary vertebral tumors. Neurosurg Clin N Am. 2008;19(1):1-4.
Currier BL, Kim C, Eismont F. Infections of the Spine. In: Herkowitz HN, Garfin SR, Eismont FJ, Bell GR, Balderston RA, editors. Rothman-Simeone. The Spine, Vol II. Philadelphia: Saunders; 2006:1265-1316.
Dakwar E, Reddy J, Vale FL, Uribe JS. A review of the pathogenesis of ankylosing spondylitis. Neurosurg Focus. 2008;24(1):E2.
Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001;344(5):363-370.
Drevelegas A, Chourmouzi D, Boulogianni G, Sofroniadis I. Imaging of primary bone tumors of the spine. Eur Radiol. 2003;13(8):1859-1871.
Erlemann R. Imaging and differential diagnosis of primary bone tumors and tumor-like lesions of the spine. Eur J Radiol. 2006;58(1):48-67.
Hoffmann RT, Jakobs TF, Trumm C, et al. Vertebroplasty in the treatment of osteoporotic vertebral body fracture. Eur Radiol. 2007;17(10):2656-2662.
Junming M, Cheng Y, Dong C, et al. Giant cell tumor of the cervical spine: a series of 22 cases and outcomes. Spine. 2008;33(3):280-288.
Kan P, Schmidt MH. Osteoid osteoma and osteoblastoma of the spine. Neurosurg Clin N Am. 2008;19(1):65-70.
Kanter AS, Jagannathan J, Shaffrey CI, et al. Inflammatory and dysplastic lesions involving the spine. Neurosurg Clin N Am. 2008;19(1):93-109.
Luther N, Bilsky MH, Hartl R. Giant cell tumor of the spine. Neurosurg Clin N Am. 2008;19(1):49-55.
McLoughlin GS, Sciubba DM, Wolinsky JP. Chondroma/Chondrosarcoma of the spine. Neurosurg Clin N Am. 2008;19(1):57-63.
McVeigh CM, Cairns AP. Diagnosis and management of ankylosing spondylitis. BMJ. 2006;333(7568):581-585.
Mendoza S, Urrutia J, Fuentes D. Surgical treatment of solitary plasmocytoma of the spine: case series. Iowa Orthop J. 2004;24:86-94.
, 2001 Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285(6):785-795.
Pateder DB, Brems J, Lieberman I, et al. Masquerade: nonspinal musculoskeletal disorders that mimic spinal conditions. Cleve Clin J Med. 2008;75(1):50-56.
Prasad D, Schiff D. Malignant spinal-cord compression. Lancet Oncol. 2005;6(1):15-24.
Quinones-Hinojosa A, Jun P, Jacobs R, et al. General principles in the medical and surgical management of spinal infections: a multidisciplinary approach. Neurosurg Focus. 2004;17(6):E1.
Raj PP, Paradise LA. Myofascial pain syndrome. Semin Pain Med. 2004;2:167-174.
Sansur CA, Pouratian N, Dumont AS, et al. Part II: spinal-cord neoplasms—primary tumours of the bony spine and adjacent soft tissues. Lancet Oncol. 2007;8(2):137-147.
Sciubba DM, Chi JH, Rhines LD, Gokaslan ZL. Chordoma of the spinal column. Neurosurg Clin N Am. 2008;19(1):5-15.
Sciubba DM, Hsieh P, McLoughlin GS, Jallo GI. Pediatric tumors involving the spinal column. Neurosurg Clin N Am. 2008;19(1):81-92.
Traul DE, Shaffrey ME, Schiff D. Part I: spinal-cord neoplasms—intradural neoplasms. Lancet Oncol. 2007;8(1):35-45.
Wang VY, Potts M, Chou D. Sarcoma and the spinal column. Neurosurg Clin N Am. 2008;19(1):71-80.
1 Martinelli TA, Wiesel SW. Low back pain: the algorithmic approach. Compr Ther. 1991;17:22-27.
2 McCowin PR, Borenstein D, Wiesel SW. The current approach to the medical diagnosis of low back pain. Orthop Clin North Am. 1991;22:315-325.
3 Posner JB. Back pain and epidural spinal cord compression. Med Clin North Am. 1987;71:185-205.
4 Graw BP, Wiesel SW. Low back pain in the aging athlete. Sports Med Arthrosc. 2008;16:39-46.
5 Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001;344:363-370.
6 Hoffmann RT, Jakobs TF, Trumm C, et al. Vertebroplasty in the treatment of osteoporotic vertebral body fracture. Eur Radiol. 2007;17:2656-2662.
7 Stallmeyer MJ, Zoarski GH, Obuchowski AM. Optimizing patient selection in percutaneous vertebroplasty. J Vasc Interv Radiol. 2003;14:683-696.
8 Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-795.
9 Frame B, Parfitt AM. Osteomalacia: current concepts. Ann Intern Med. 1978;89:966-982.
10 Goldring SR, Krane SM. Disorders of calcification: Osteomalacia and rickets. In: DeGroot LJ, Besser GM, Cahill GFJr, editors. Endocrinology. Philadelphia: WB Saunders; 1989:1165-1187.
11 Fraser D, Kooh SW, Kind HP, et al. Pathogenesis of hereditary vitamin-D-dependent rickets. An inborn error of vitamin D metabolism involving defective conversion of 25-hydroxyvitamin D to 1 alpha,25-dihydroxyvitamin D. N Engl J Med. 1973;289:817-822.
12 Morgan DB, Hunt G, Paterson CR. The osteomalacia syndrome after stomach operations. QJM. 1970;39:395-410.
13 Ryan EA, Reiss E. Oncogenous osteomalacia. Review of the world literature of 42 cases and report of two new cases. Am J Med. 1984;77:501-512.
14 Morris RCJr, McSherry E. Symposium on acid-base homeostasis. Renal acidosis. Kidney Int. 1972;1:322-340.
15 Hahn TJ, Hendin BA, Scharp CR, Haddad JGJr. Effect of chronic anticonvulsant therapy on serum 25-hydroxycalciferol levels in adults. N Engl J Med. 1972;287:900-904.
16 Maclaren N, Lifshitz F. Vitamin D-dependency rickets in institutionalized, mentally retarded children on long term anticonvulsant therapy. II. The response to 25-hydroxycholecalciferol and to vitamin D2. Pediatr Res. 1973;7:914-922.
17 Steinbach HL, Noetzli M. Roentgen appearance of the skeleton in osteomalacia and rickets. Am J Roentgenol Radium Ther Nucl Med. 1964;91:955-972.
18 Riggs BL. Osteoporosis. In: DeGroot LJ, Besser GM, Cahill GFJr, editors. Endocrinology. Philadelphia: Saunders; 1989:1188-1207.
19 Cann CE, Martin MC, Genant HK, Jaffe RB. Decreased spinal mineral content in amenorrheic women. JAMA. 1984;251:626-629.
20 Ettinger B, Genant HK, Cann CE. Long-term estrogen replacement therapy prevents bone loss and fractures. Ann Intern Med. 1985;102:319-324.
21 Lindsay R, Hart DM, Aitken JM, et al. Long-term prevention of postmenopausal osteoporosis by oestrogen. Evidence for an increased bone mass after delayed onset of oestrogen treatment. Lancet. 1976;1:1038-1041.
22 Riggs BL, Melton LJ3rd. Involutional osteoporosis. N Engl J Med. 1986;314:1676-1686.
23 Aloia JF, Cohn SH, Ostuni JA, et al. Prevention of involutional bone loss by exercise. Ann Intern Med. 1978;89:356-358.
24 Drinkwater BL, Nilson K, Chesnut CH3rd, et al. Bone mineral content of amenorrheic and eumenorrheic athletes. N Engl J Med. 1984;311:277-281.
25 Marcus R, Cann C, Madvig P, et al. Menstrual function and bone mass in elite women distance runners. Endocrine and metabolic features. Ann Intern Med. 1985;102:158-163.
26 Paul TL, Kerrigan J, Kelly AM, et al. Long-term L-thyroxine therapy is associated with decreased hip bone density in premenopausal women. JAMA. 1988;259:3137-3141.
27 Nissen-Meyer LS, Svalheim S, Tauboll E, et al. How can antiepileptic drugs affect bone mass, structure and metabolism? Lessons from animal studies. Seizure. 2008;17:187-191.
28 Pack A. Bone health in people with epilepsy: is it impaired and what are the risk factors? Seizure. 2008;17:181-186.
29 Petty SJ, O’Brien TJ, Wark JD. Anti-epileptic medication and bone health. Osteoporos Int. 2007;18:129-142.
30 Disorders, Diseases, & Injuries of the Spine. In Skinner HB, editor: Current Diagnosis & Treatment in Orthopedics, 4th ed, New York: McGraw-Hill Medical, 2006.
31 Wheeless CRIII. Wheeless’ Textbook of Orthopaedics. In: Wheeless ICR, Nunley IJA, Urbaniak JR, editors. Wheeless’ Textbook of Orthopaedics. Data Trace Internet Publishing, 2008.
32 Morgan SL, Kitchin B. Osteoporosis: handy tools for detection, helpful tips for treatment. J Fam Pract. 2008;57:311-320.
33 Arnold A. Paget’s disease of bone: Pathophysiology and diagnosis. In: DeGroot LJ, Besser GM, Cahill GFJr, editors. Endocrinology. Philadelphia: Saunders; 1989:1208-1244.
34 Singer FR, Mills BG. Evidence for a viral etiology of Paget’s disease of bone. Clin Orthop Relat Res. 1983:245-251.
35 Freeman DA. Paget’s disease of bone. Am J Med Sci. 1988;295:144-158.
36 Strewler GJ. Paget’s disease of bone. West J Med. 1984;140:763-768.
37 Wallach S. Treatment of Paget’s disease. In: Gtollerman GH, editor. Advances in Internal Medicine. Chicago: Year Book Medical Publishers; 1982:1-43.
38 Khan MA, van der Linden SM. Ankylosing spondylitis and other spondyloarthropathies. Rheum Dis Clin North Am. 1990;16:551-579.
39 McVeigh CM, Cairns AP. Diagnosis and management of ankylosing spondylitis. BMJ. 2006;333:581-585.
40 Wollheim FA. Ankylosing spondylitis. In: Kelly WM, Harris JED, Ruddy S, editors. Textbook of Rheumatology. 4th ed. Philadelphia: Saunders; 1993:943-960.
41 Dakwar E, Reddy J, Vale FL, Uribe JS. A review of the pathogenesis of ankylosing spondylitis. Neurosurg Focus. 2008;24:E2.
42 Panush RS, Wallace DJ, Dorff RE, Engleman EP. Retraction of the suggestion to use the term “Reiter’s syndrome” sixty-five years later: the legacy of Reiter, a war criminal, should not be eponymic honor but rather condemnation. Arthritis Rheum. 2007;56:693-694.
43 Rihl M, Klos A, Kohler L, Kuipers JG. Infection and musculoskeletal conditions: reactive arthritis. Best Pract Res Clin Rheumatol. 2006;20:1119-1137.
44 Holden W, Orchard T, Wordsworth P. Enteropathic arthritis. Rheum Dis Clin North Am. 2003;29:513-530.
45 Raj PP, Paradise LA. Myofascial pain syndrome. Semin Pain Med. 2004;2:167-174.
46 Buskila D. Fibromyalgia, chronic fatigue syndrome, and myofascial pain syndrome. Curr Opin Rheumatol. 2001;13:117-127.
47 Yunus M, Masi AT, Calabro JJ, et al. Primary fibromyalgia (fibrositis): clinical study of 50 patients with matched normal controls. Semin Arthritis Rheum. 1981;11:151-171.
48 Lee KC, Tsai YT, Lin CY, et al. Vertebral osteomyelitis combined streptococcal viridans endocarditis. Eur J Cardiothoracic Surg. 2003;23:125-127.
49 Modic MT, Feiglin DH, Piraino DW, et al. Vertebral osteomyelitis: assessment using MR. Radiology. 1985;157:157-166.
50 Quinones-Hinojosa A, Jun P, Jacobs R, et al. General principles in the medical and surgical management of spinal infections: a multidisciplinary approach. Neurosurg Focus. 2004;17:E1.
51 Currier BL, Kim C, Eismont F. Infections of the spine. In: Herkowitz HN, Garfin SR, Eismont FJ, et al, editors. Rothman-Simeone The Spine. 5th ed. Philadelphia: Saunders; 2006:1265-1316.
52 Spinal Tumors. eMedicine.com, 2008.
53 Anderson ME, McLain RF. Tumors of the Spine. In: Herkowitz HN, Garfin SR, Eismont FJ, et al, editors. Rothman-Simeone The Spine. 5th ed. Philadelphia: Saunders-Elsevier; 2006:1235-1264.
54 Chi JH, Bydon A, Hsieh P, et al. Epidemiology and demographics for primary vertebral tumors. Neurosurg Clin N Am. 2008;19:1-4.
55 Sansur CA, Pouratian N, Dumont AS, et al. Part II: spinal-cord neoplasms—primary tumours of the bony spine and adjacent soft tissues. Lancet Oncol. 2007;8:137-147.
56 Drevelegas A, Chourmouzi D, Boulogianni G, Sofroniadis I. Imaging of primary bone tumors of the spine. Eur Radiol. 2003;13:1859-1871.
57 Nemoto O, Moser RPJr, Van Dam BE, et al. Osteoblastoma of the spine. A review of 75 cases. Spine. 1990;15:1272-1280.
58 Byers PD. Solitary benign osteoblastic lesions of bone. Osteoid osteoma and benign osteoblastoma. Cancer. 1968;22:43-57.
59 Kirwan EO, Hutton PA, Pozo JL, Ransford AO. Osteoid osteoma and benign osteoblastoma of the spine. Clinical presentation and treatment. J Bone Joint Surg Br. 1984;66:21-26.
60 Janin Y, Epstein JA, Carras R, Khan A. Osteoid osteomas and osteoblastomas of the spine. Neurosurgery. 1981;8:31-38.
61 Kan P, Schmidt MH. Osteoid osteoma and osteoblastoma of the spine. Neurosurg Clin N Am. 2008;19:65-70.
62 Crist BD, Lenke LG, Lewis S. Osteoid osteoma of the lumbar spine. A case report highlighting a novel reconstruction technique. J Bone Joint Surg Am. 2005;87:414-418.
63 Sciubba DM, Hsieh P, McLoughlin GS, Jallo GI. Pediatric tumors involving the spinal column. Neurosurg Clin N Am. 2008;19:81-92.
64 Codd PJ, Riesenburger RI, Klimo PJr, et al. Vertebra plana due to an aneurysmal bone cyst of the lumbar spine. Case report and review of the literature. J Neurosurg. 2006;105:490-495.
65 Burch S, Hu S, Berven S. Aneurysmal bone cysts of the spine. Neurosurg Clin N Am. 2008;19:41-47.
66 Yang ZY, Zhang LJ, Chen ZX, Hu HY. Hemangioma of the vertebral column. A report on twenty-three patients with special reference to functional recovery after radiation therapy. Acta Radiol Oncol. 1985;24:129-132.
67 Graham JJ, Yang WC. Vertebral hemangioma with compression fracture and paraparesis treated with preoperative embolization and vertebral resection. Spine. 1984;9:97-101.
68 Hemmy DC, McGee DM, Armbrust FH, Larson SJ. Resection of a vertebral hemangioma after preoperative embolization. Case report. J Neurosurg. 1977;47:282-285.
69 Acosta FLJr, Sanai N, Chi JH, et al. Comprehensive management of symptomatic and aggressive vertebral hemangiomas. Neurosurg Clin N Am. 2008;19:17-29.
70 Berry DH, Becton DL. Natural history of histiocytosis-X. Hematol Oncol Clin North Am. 1987;1:23-34.
71 Kanter AS, Jagannathan J, Shaffrey CI, et al. Inflammatory and dysplastic lesions involving the spine. Neurosurg Clin N Am. 2008;19:93-109.
72 Dahlin DC. Giant-cell tumor of vertebrae above the sacrum: a review of 31 cases. Cancer. 1977;39:1350-1356.
73 Savini R, Gherlinzoni F, Morandi M, et al. Surgical treatment of giant-cell tumor of the spine. The experience at the Istituto Ortopedico Rizzoli. J Bone Joint Surg Am. 1983;65:1283-1289.
74 Luther N, Bilsky MH, Hartl R. Giant cell tumor of the spine. Neurosurg Clin N Am. 2008;19:49-55.
75 Mehrotra R, Singh M, Singh PA, et al. Should fine needle aspiration biopsy be the first pathological investigation in the diagnosis of a bone lesion? An algorithmic approach with review of literature. Cytojournal. 2007;4:9.
76 Shimada Y, Hongo M, Miyakoshi N, et al. Giant cell tumor of fifth lumbar vertebrae: two case reports and review of the literature. Spine J. 2007;7:499-505.
77 Riedel DA, Pottern LM. The epidemiology of multiple myeloma. Hematol Oncol Clin North Am. 1992;6:225-247.
78 Knowling MA, Harwood AR, Bergsagel DE. Comparison of extramedullary plasmacytomas with solitary and multiple plasma cell tumors of bone. J Clin Oncol. 1983;1:255-262.
79 Dimopoulos MA, Goldstein J, Fuller L, et al. Curability of solitary bone plasmacytoma. J Clin Oncol. 1992;10:587-590.
80 Frassica DA, Frassica FJ, Schray MF, et al. Solitary plasmacytoma of bone: Mayo Clinic experience. Int J Radiat Oncol Biol Phys. 1989;16:43-48.
81 Vincent S, Rajkumar M. Diagnosis and management of solitary plasmacytoma. In UpToDate Online 162. June 2008 ed www.uptodateonline.com, 2008.
82 Gibbs IC, Chang SD. Radiosurgery and radiotherapy for sacral tumors. Neurosurg Focus. 2003;15:E8.
83 Mendoza S, Urrutia J, Fuentes D. Surgical treatment of solitary plasmocytoma of the spine: case series. Iowa Orthop J. 2004;24:86-94.
84 Bilsky MH, Azeem S. Multiple myeloma: primary bone tumor with systemic manifestations. Neurosurg Clin N Am. 2008;19:31-40.
85 Sundaresan N, Schiller AL, Rosenthal DI. Osteosarcoma of the spine. In: Sunaresan N, Schmidek HH, Schiller AL, editors. Tumors of the Spine. Philadelphia: Saunders; 1990:128-145.
86 Wang VY, Potts M, Chou D. Sarcoma and the spinal column. Neurosurg Clin N Am. 2008;19:71-80.
87 Bradway JK, Pritchard DJ. Ewing’s tumor of the spine. In: Sunaresan N, Schmidek HH, Schiller AL, editors. Tumors of the Spine. Philadelphia: WB Saunders; 1990:235-239.
88 Erlemann R. Imaging and differential diagnosis of primary bone tumors and tumor-like lesions of the spine. Eur J Radiol. 2006;58:48-67.
89 Liljenqvist U, Lerner T, Halm H, et al. En bloc spondylectomy in malignant tumors of the spine. Eur Spine J. 2008;17:600-609.
90 Healey JH, Lane JM. Chordoma: a critical review of diagnosis and treatment. Orthop Clin North Am. 1989;20:417-426.
91 Connolly E. Spinal cord tumors in adults. In: Youmans JR, editor. Neurological Surgery. 2nd ed. Philadelphia: Saunders; 1982:3196-3215.
92 O’Neill P, Bell BA, Miller JD, et al. Fifty years of experience with chordomas in southeast Scotland. Neurosurgery. 1985;16:166-170.
93 Paavolainen P, Teppo L. Chordoma in Finland. Acta Orthop Scand. 1976;47:46-51.
94 Sundaresan N, Galicich JH, Chu FC, Huvos AG. Spinal chordomas. J Neurosurg. 1979;50:312-319.
95 Sciubba DM, Chi JH, Rhines LD, Gokaslan ZL. Chordoma of the spinal column. Neurosurg Clin N Am. 2008;19:5-15.
96 Gallia GL, Haque R, Garonzik I, et al. Spinal pelvic reconstruction after total sacrectomy for en bloc resection of a giant sacral chordoma. Technical note. J Neurosurg Spine. 2005;3:501-506.
97 Boriani S, Bandiera S, Biagini R, et al. Chordoma of the mobile spine: fifty years of experience. Spine. 2006;31:493-503.
98 Roux CH, Chami H, Breuil V, et al. Lumbosacral pain: chordoma—a diagnosis not to forget. Clin Rheumatol. 2007;26:781-783.
99 McLoughlin GS, Sciubba DM, Wolinsky JP. Chondroma/chondrosarcoma of the spine. Neurosurg Clin N Am. 2008;19:57-63.
100 Lloret I, Server A, Bjerkehagen B. Primary spinal chondrosarcoma: radiologic findings with pathologic correlation. Acta Radiol. 2006;47:77-84.
101 Bartels RH, van der Linden YM, van der Graaf WT. Spinal extradural metastasis: review of current treatment options. CA Cancer J Clin. 2008;58:245-259.
102 Prasad D, Schiff D. Malignant spinal-cord compression. Lancet Oncol. 2005;6:15-24.
103 Lin A, Ray ME. Targeted and systemic radiotherapy in the treatment of bone metastasis. Cancer Metastasis Rev. 2006;25:669-675.
104 Traul DE, Shaffrey ME, Schiff D. Part I: spinal-cord neoplasms—intradural neoplasms. Lancet Oncol. 2007;8:35-45.
105 Parsa AT, Chi JH, Acosta FLJr, et al. Intramedullary spinal cord tumors: molecular insights and surgical innovation. Clin Neurosurg. 2005;52:76-84.