Chapter 151
Mesenteric Vascular Disease
General Considerations
Ruby C. Lo, Marc L. Schermerhorn
Based on a chapter in the seventh edition by Juan Carlos Jimenez and William J. Quinones-Baldrich
Mesenteric ischemia occurs when perfusion of the visceral organs fails to meet normal metabolic requirements. This disorder is categorized as either acute or chronic on the basis of the duration of symptoms. Acute mesenteric ischemia (AMI) occurs rapidly during hours to days and frequently leads to acute intestinal infarction requiring resection (see Chapter 153). The most common causes are embolization to the mesenteric arteries and acute thrombosis related to a preexisting plaque. Chronic mesenteric ischemia (CMI) is a more insidious process and progresses during weeks to several months (see Chapter 152). The most common cause is progressive occlusive disease of the visceral arteries, usually related to atherosclerosis. Because CMI is relatively uncommon, it is frequently misdiagnosed as a gastrointestinal disorder. Patients typically have undergone an extensive workup for other potential causes before being diagnosed with CMI.
The first case of AMI was diagnosed and treated successfully with intestinal resection and reanastomosis by Elliott in 1895. Goodman first described chronic intestinal angina as a clinical disorder in 1918.1 Dunphy, a surgical resident at the Peter Bent Brigham Hospital, in 1936 reported a case of a patient with weight loss and pain out of proportion to abdominal findings who subsequently died and was found to have mesenteric occlusive disease on autopsy. After reviewing the medical records of 12 patients who died of intestinal angina, he found that 7 of the 12 (58%) had a history of chronic abdominal pain, thus introducing the potential for early intervention to prevent disease progression and death.2 Warren and Eberhard were the first to describe mesenteric venous thrombosis (MVT) as a distinct cause of intestinal infarction in 1935, differentiating it from occlusion of the mesenteric arteries.3
In the modern era, Klass4 performed the first superior mesenteric artery (SMA) embolectomy in 1950, avoiding intestinal resection. Although the patient died several days later of a heart-related condition, autopsy revealed the bowel to be normal. In 1958, Shaw and Maynard5 performed the first successful thromboendarterectomy of the SMA at the Massachusetts General Hospital. Morris et al6 performed the first successful retrograde bypass graft from the infrarenal aorta to the SMA in 1962. Stoney and Wylie7 at the University of California, San Francisco, first described antegrade aortovisceral bypass and transaortic visceral thromboendarterectomy in 1966. Ushering in the current era of percutaneous treatment of visceral arterial occlusive disease, Furrer8 and Novelline9 separately published the initial reports of endovascular dilatation of the SMA in 1980, and Finch10 first reported the treatment of a celiac artery stenosis with a Palmaz stent.
Anatomy of The Visceral Arteries
The primitive dorsal aorta gives rise to the abdominal aorta during fetal development. Ventral segmental arteries emerge from the primitive ventral aorta, which disappears around the fourth week of gestation. Multiple segmental branches from the primitive ventral aorta—the 10th, 13th, and 21st—persist and develop into the celiac artery, SMA, and inferior mesenteric artery (IMA), respectively. Disparity in the regression of the primitive ventral aorta and its segmental branches infrequently causes deviations in the visceral arterial anatomy.
The celiac artery arises from the abdominal aorta just caudal to the diaphragm at the level of L1 and is bordered by the median arcuate ligament at the aortic hiatus superiorly and the superior border of the pancreas inferiorly. Traditionally, the three branches from this common trunk include the left gastric, splenic, and common hepatic arteries. However, multiple variations of the true “trifurcation” can exist. Most frequently, the common hepatic artery and its branches arise from the SMA or directly from the abdominal aorta.
Exposure of the celiac trunk is best achieved through a midline transabdominal incision, which also allows visual assessment of bowel viability during surgical revascularization. The celiac trunk and its branches are surrounded by the celiac plexus of nerves, which must be divided for proximal exposure. A midline laparotomy is performed, and the triangular ligament is divided. The gastrohepatic ligament is then divided longitudinally to the level of the posterior parietal peritoneum. The liver is carefully retracted to the right of midline with a self-retaining retractor. Placement of a nasogastric tube facilitates identification of the esophagus. The posterior peritoneum overlying the diaphragmatic crus is divided sharply to expose the celiac trunk. The first vessel encountered is usually the common hepatic artery traversing to the right of midline toward the liver. The hepatic artery can be exposed back to the origin of the celiac trunk, which is covered by lymphatic tissue and the celiac nerve plexus. The diaphragmatic crus is divided to expose the celiac origin and the supraceliac aorta.
The SMA arises a few centimeters caudal to the celiac trunk, and its origin is crossed by the neck of the pancreas and the splenic vein. It arises at a less acute and downward-sloped angle than the celiac trunk and lies superior to both the uncinate process of the pancreas and the third portion of the duodenum. The superior mesenteric vein runs parallel adjacent to the artery, usually along its right border. The first important branch of the SMA is usually the inferior pancreaticoduodenal artery, which supplies collateral circulation with the celiac artery through the gastroduodenal and superior pancreaticoduodenal arteries. The second major branch of the SMA is frequently the middle colic artery, which arises at the inferior border of the pancreas. The right colic, ileocolic, and third-order mesenteric branches arise distally, supplying the small bowel within the mesentery.
During surgical exposure, the SMA can be approached either anteriorly at the base of the transverse colon mesentery or lateral to the fourth portion of the duodenum. Anterior SMA exposure involves lifting the transverse colon superiorly to clearly expose the base of its mesentery. The small intestine is covered in a moist towel or a bowel bag and retracted to the right. A horizontal incision is made through the posterior peritoneum at the base of the mesentery at the level of the proximal jejunum and extended to the right of midline. The middle colic artery can be used as a landmark within the transverse colon mesentery and to localize the main SMA trunk. Palpation often aids in localizing the SMA. The superior mesenteric vein is often visualized first, and the SMA can be palpated adjacent and to the left of it. This approach provides excellent exposure of the SMA. Exposure of the more proximal SMA to the left and lateral to the fourth portion of the duodenum can also be achieved. The ligament of Treitz is divided, and the lateral wall of the duodenum is mobilized off the anterior surface of the aorta. The SMA can be identified just distal to its origin from the aorta.
The IMA is usually located 3 to 4 cm cephalad to the aortic bifurcation, just to the left of midline, and usually arises at the level of the third lumbar vertebra. The main trunk frequently divides into sigmoidal branches and the left colic artery. The ascending left colic artery forms the inferior marginal artery of Drummond, which is the major collateral arcade between the SMA and IMA. The SMA and IMA are also linked by the meandering mesenteric artery (of Moskowitz), known historically as the arc of Riolan. This vessel runs more centrally, medial to the mesenteric border of the colon and through the middle of the mesenteric arcade near the inferior mesenteric vein. The meandering mesenteric artery is likely to be produced by the dilation of a normal collateral vessel in response to significant stenosis or occlusion of the SMA or IMA.11 Sigmoidal branches lead to the left and right superior rectal arteries, which collateralize with branches of the hypogastric arteries in the pelvis.
Physiology of Splanchnic Blood Flow
Normal intestinal function and nutrient absorption rely on adequate perfusion and oxygenation to the microvascular splanchnic circulation. Various autoregulatory mechanisms ensure adequate gut circulation through both vasoconstriction and relaxation of arterial smooth muscle. The degree of visceral artery dilatation and constriction determines the relatively large fluctuations in splanchnic blood flow during fasting, postprandial states, and periods of extreme stress. Visceral blood flow can vary dramatically, ranging from 10% of cardiac output in the setting of shock or hypovolemia, to 20% to 25% at rest or while fasting, and up to 35% after a large carbohydrate meal.12,13 Seventy percent to 80% of mesenteric blood flow supplies the mucosal and submucosal layers. The severely diminished blood flow observed in patients with nonocclusive mesenteric ischemia results from severe vasospasm related to this process. Duplex studies demonstrate moderate to high arterial resistance in the SMA circulation, with low diastolic flow and slight flow reversal during fasting states. In the postprandial period, low-resistance signals are noted throughout both systole and diastole, indicative of dilated splanchnic arteriolar beds; flow reversal does not occur. In contrast, low arterial resistance signals are noted in the celiac artery circulation regardless of feeding, probably because of the influence of the low-resistance hepatic vascular bed. Perko et al14 also noted that in fasting subjects performing submaximal exercise, splanchnic vascular resistance doubled and exhibited a 50% reduction in hepatosplenic blood flow and a 25% reduction in mesenteric blood flow.
Multiple mechanisms are responsible for regulating mesenteric arteriolar smooth muscle tone, and they are often interdependent. Extrinsic factors include sympathetic efferent nerves in the prevertebral celiac and mesenteric ganglia, which initiate stimuli for arterial vasoconstriction. Hormonal pathways also contribute to extrinsic regulation of splanchnic blood flow. The renin-angiotensin feedback mechanism causes mesenteric vasoconstriction through the direct action of angiotensin II during hypovolemic states. Low-volume states and hyperosmolarity stimulate the neurohypophysis, which releases vasopressin,15 a hormone that causes splanchnic vasoconstriction, reduction in portal venous pressure, and venodilatation.15 In addition, when subjected to shear stress, activation of the nitric oxide synthase enzymes on the surface of red blood cell membranes occurs, leading to significant dilation of mesenteric arteries under hypoxic conditions.16
Intrinsic regulation also occurs through metabolic and myogenic feedback mechanisms.15 In the metabolic pathway, mucosal ischemia prompts the release of metabolic byproducts, causing vasodilatation in arteriolar smooth muscle and preferentially shunting increased blood flow to the intestinal mucosa. In the myogenic pathway, which dominates the regulation of blood flow in the small intestines,17 abrupt decreases in perfusion pressure are sensed by arteriolar baroreceptors, which respond by decreasing arteriolar wall tension to maintain blood flow.18 Together, these mechanisms maintain mucosal perfusion and integrity during periods of relative ischemia.
Epidemiology
Asymptomatic occlusive disease of the visceral arteries is a common finding in elderly patients. Wilson et al15 demonstrated that 17.5% of 553 consecutive patients older than 65 years examined with duplex ultrasonography (DUS) had a critical stenosis of at least one visceral vessel. In addition, autopsy studies have estimated the prevalence of atherosclerosis involving the mesenteric arteries to be between 6% and 10%.19
Despite an aging population, hospitalizations for AMI in the United States have declined from 9.6 to 6.7 per 100,000 from 1998 to 2010.20 There is some evidence that this decline may be related to the increasing and widespread use of statins and the efficacy of warfarin in the prevention of thromboembolic events in patients with atrial fibrillation.21 Probably in part because of differences in longevity, AMI and CMI disproportionately affect women, with a female-to-male ratio of approximately 3 : 1.22
Because the majority of patients with mesenteric occlusive disease manifest no symptoms, the exact incidence of CMI is not known. However, admissions for CMI account for less than 1 per 100,000 admissions23 and have been increasing steadily in recent years in the United States.22 Whether these figures represent an actual increasing incidence or simply increased re-intervention due to restenosis from endovascular therapy remains to be determined. Despite the high prevalence of individuals with asymptomatic mesenteric arterial occlusive disease, patients usually demonstrate involvement of two or more mesenteric vessels before symptoms arise owing to the development of extensive collateralization over time. In fact, in a study by Thomas et al,24 who observed 980 consecutive patients with asymptomatic significant (50%) stenosis of at least one mesenteric artery, only four patients developed mesenteric ischemia and all of them had significant three-vessel disease after follow-up of 1 to 6 years. The variability of symptoms in patients with chronic abdominal pain often makes the diagnosis challenging, resulting in treatment delays and increased morbidity.
Pathophysiology
Chronic Mesenteric Ischemia
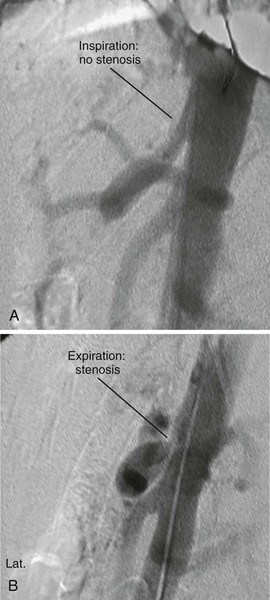
Atherosclerosis is the most common cause of CMI, and patients frequently have a history of smoking, hypertension, and hyperlipidemia. They may also have evidence of atherosclerotic disease in other vascular beds, particularly coronary, cerebrovascular, renal, aortoiliac, and other peripheral arteries. Although it is much more uncommon, CMI may also be seen in association with vasculitis and other inflammatory conditions, such as lupus, Buerger’s disease, and radiation arteritis. Median arcuate ligament syndrome is a separate entity that may lead to symptoms of CMI and is caused by the compression of the celiac artery by the median arcuate ligament. Symptoms are classically exacerbated by full expiration.
Acute Mesenteric Ischemia
Embolism
Arterial emboli are the most common cause of AMI, representing 40% to 50% of cases.25 The proximal source of the embolus is frequently intracardiac mural thrombus that develops in patients with atrial tachyarrhythmias, myocardial infarction, cardiomyopathy, structural heart defects, and cardiac tumors. Endocarditis can result in septic emboli from affected valve leaflets. Mural thrombus in proximal aneurysms in the thoracic or proximal abdominal aorta can also serve as an embolic source, as can atheromatous plaque even in the absence of aneurysm. The SMA is the most common final destination for mesenteric emboli, perhaps because of its relative size and the decreased angle of takeoff from the abdominal aorta compared with the other mesenteric vessels. In addition, such emboli tend to lodge several centimeters from the vessel’s origin, usually distal to the middle colic artery (Fig. 151-1A). The angiographic hallmark of an embolic occlusion seen on computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) is an abrupt cutoff just beyond the origin of the middle colic artery.26
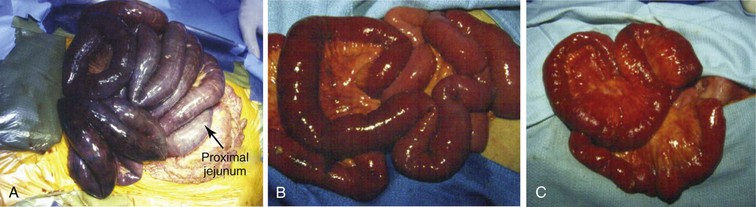
Figure 151-1 A, Operative findings typical of acute mesenteric ischemia secondary to an embolus. Note that the first portion of the jejunum is spared because the embolus lodged distal to the middle colic artery. B, Operative findings typical of acute mesenteric ischemia secondary to arterial thrombosis. Note that the entire bowel is affected. C, Appearance of the small bowel at second-look surgery after revascularization. Note the hemorrhagic changes in the mesentery.
Arterial Thrombosis
Arterial thrombosis constitutes the next most common cause of AMI and occurs in 20% to 35% of cases.27,28 Preexisting atherosclerotic plaque affecting all visceral vessels is the most common finding. Hypercoagulability syndromes can also predispose to acute visceral artery thrombosis. The affected segment of artery is usually its origin at the level of the aorta. Patients with acute arterial thrombosis frequently have preexisting symptoms of CMI. Schoots et al29 reviewed 45 observational studies encompassing 3692 patients with AMI and found that mortality from acute thrombosis of a mesenteric artery was 77.4%, compared with 54.1% for patients with acute arterial embolism to visceral vascular beds. This increased mortality is likely due to the involvement of larger segments of bowel because occlusions are typically within the first 2 cm of the SMA origin (Fig. 151-1B). This theory was corroborated in a Swedish study, in which 213 patients with acute thromboembolic occlusion of the SMA and intestinal infarction were examined post mortem.30 The cause of occlusion was embolic in 57.3% of patients and thrombotic in 41.3% (indeterminate in 1.4%). The extent of intestinal infarction was significantly greater in patients with SMA thrombosis compared with embolus. Acute extension of an aortic dissection can also serve as a mechanism for abrupt mesenteric vessel occlusion and thrombosis.
Nonocclusive Mesenteric Ischemia
Impaired intestinal perfusion in the absence of thromboembolic occlusion is termed nonocclusive mesenteric ischemia (NOMI) and makes up approximately 5% to 15% of cases.31 Visceral ischemia can occur from a low-flow state, which is exacerbated by the presence of any intestinal atherosclerotic disease. It is theorized that in such circumstances, in an effort to maintain cardiac and cerebral perfusion, excessive sympathetic output results in mesenteric vasospasm. NOMI most commonly occurs secondary to cardiac disease, particularly severe congestive heart failure, and in patients who have undergone cardiac surgery. Atrial fibrillation, commonly a cause of cardiac thrombi and visceral embolization, can also induce NOMI by reducing left ventricular function and causing low cardiac output. Other risk factors for NOMI include age, hypovolemia, systemic vasoconstrictors, vasoactive drugs (e.g., digoxin, α-adrenergic agents, β-receptor blocking agents, cocaine), aortic insufficiency, cardiopulmonary bypass, abdominal and cardiovascular surgery, abdominal compartment syndrome, and liver failure.25,32
In recent years, NOMI has been increasingly observed in patients on hemodialysis. Quiroga et al33 reported incidence rates more than 40 times greater among patients on hemodialysis compared with the general population. Using the Taiwan National Health Insurance Research Database, Li et al34 similarly reported a 44-fold higher risk of mesenteric ischemia in patients on hemodialysis or peritoneal dialysis compared with the general population. Older age and a heavier burden of cardiovascular risk factors undoubtedly contribute to these disparities. In addition, patients with end-stage renal disease are frequently taking erythropoietin, which elevates red cell mass and can lead to relative hyperviscosity. However, hypotension during dialysis has been implicated as the most important and immediate precipitating risk factor for development of NOMI.35 Therefore, special care should be made to avoid dialysis-related hypotension in patients at particularly high risk. High-risk patients include those who are older, are taking erythropoietin, have a longer history of dialysis, and are diabetics.33,35
Mesenteric Venous Thrombosis
MVT constitutes 5% to 15% of all cases of mesenteric ischemia.36,37 Involvement is usually limited to the superior mesenteric vein, but the inferior mesenteric, splenic, and portal veins can also be involved. MVT is classified as either primary (idiopathic) or secondary. Secondary MVT occurs when an underlying disease process is present; this type accounts for 90% of all patients with this disorder. Conditions associated with MVT can be categorized into three major categories: direct injury, local venous stasis or congestion, and thrombophilia. Causes of direct injury include abdominal surgery, trauma, and local inflammation, such as that seen with inflammatory bowel disease, pancreatitis, and diverticulitis.38,39 Splanchnic venous stasis may occur in the case of increased intra-abdominal pressure, obesity, and intraoperative manipulation of the mesenteric vessels. Inherited or acquired hypercoagulable diseases, including protein C and protein S deficiency, myeloproliferative disorders, antithrombin III deficiency, antiphospholipid antibody syndrome, and factor V Leiden mutation, are frequent causes of thrombophilia and thus also predispose to MVT.40
The extent of bowel ischemia depends largely on the degree of venous involvement and the presence of collateral vessels. The transition from normal to ischemic intestine is slower with MVT than with arterial occlusive disease.25 Edema and hemorrhage of the intestinal wall are frequently seen, followed by focal sloughing of the mucosa.25 The origin of thrombosis varies, depending on the etiologic process. When an intra-abdominal process is the cause, thrombosis begins in the larger mesenteric veins and progresses to involve the smaller venous arcades and arcuate channels.41 MVT caused by hypercoagulable conditions usually begins in the smaller mesenteric veins. Symptomatic acute MVT is associated with a 20% to 50% mortality rate.41 A Swedish population-based autopsy study showed that MVT-related death is more likely in patients with portal vein thrombosis, systemic venous thromboembolism, and obesity.42
Clinical Presentation
Acute Mesenteric Ischemia
The most common symptom of AMI associated with arterial thromboembolic disease is the sudden onset of abdominal pain. Because of a lack of collateral flow to the visceral organs, the presentation of AMI is more dramatic and severe, often with rapid clinical deterioration. Nausea, vomiting, diarrhea, emptying symptoms, and distention can also occur. Classically, the pain is out of proportion to the findings on physical examination. Initially, bowel sounds are hyperactive as the failure to relax the bowel smooth muscle leads to emptying symptoms. Bowel sounds are typically diminished in the later stages. Abdominal guarding and rebound tenderness are absent in the early stages of AMI; however, as intestinal ischemia and infarction progress, these signs become more pronounced. They are typically late findings, so their absence should not delay the diagnosis and treatment of AMI. Other late findings include fever, oliguria, dehydration, confusion, tachycardia, and shock.25 Metabolic abnormalities can include leukocytosis, metabolic acidosis, hyperamylasemia, elevated liver function values, and lactic acidemia.
Patients with NOMI or MVT typically present with a slower, more insidious clinical course. Frequently, patients with NOMI are critically ill, hospitalized, intubated patients who experience a sudden deterioration in their clinical condition. These patients are often administered intravenous pressors, worsening mesenteric vasoconstriction and thus decreasing splanchnic perfusion. In patients with MVT, fever, abdominal pain and distention, nausea and vomiting, and bloody stools are the most common findings. Dehydration and profound fluid shifts lead to bloody ascites and a hypovolemic state, causing further propagation of venous thrombosis.25 Although nonspecific, normal D-dimer levels may help rule out MVT.43
To develop better diagnostic criteria to reduce delays in diagnosis, Mitsuyoshi et al44 reviewed their 13-year experience treating 22 patients with NOMI. Multidetector row computed tomography (MDCT) was unavailable at the time the first 13 patients were treated, and 9 subsequently died of intestinal necrosis. These first 13 cases were used to devise four criteria for determining which patients warranted MDCT evaluation once this technology became available: (1) ileus or abdominal pain, (2) catecholamine requirement, (3) episode of hypotension, and (4) gradual rise in serum transaminase level. If three of the four criteria were present, patients received MDCT and underwent treatment with high-dose intravenous prostaglandin E1. Of the nine patients treated with this algorithm, only one died.
Chronic Mesenteric Ischemia
Postprandial abdominal pain and progressive weight loss are the most common symptoms in patients with CMI. Pain is often described as dull and crampy and located in the midepigastric region. Pain often occurs 15 to 45 minutes after a meal, and the severity varies according to the size and type of meal. Patients typically develop “food fear” and decrease their oral intake in anticipation of severe pain after meals. Consequently, weight loss is a common finding and in fact, when present, can help distinguish CMI from other functional bowel disorders when the diagnosis is in question.45 Changes in bowel habits, nausea, and vomiting are less common findings. CMI is seen more frequently in elderly women, who represent 70% of patients. The variable nature of symptoms often makes the diagnosis confusing and can result in delayed treatment. The traditional risk factors for atherosclerosis are usually present. A heavy smoking history is frequently obtained, and the majority of patients also have a history of symptomatic manifestations in other vascular beds, most commonly cerebrovascular, coronary, and peripheral arteries.46
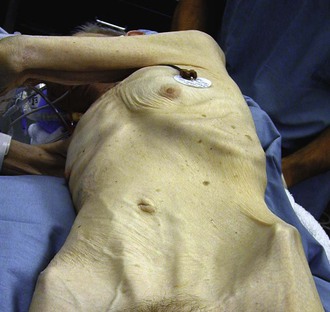
Physical examination findings are usually nonspecific. Patients are commonly undernourished and cachectic (Fig. 151-2). An abdominal bruit can sometimes be auscultated but is not always present. Bowel sounds are frequently hyperactive. Guarding and rebound tenderness are usually absent. Low prealbumin and albumin levels are often seen owing to the patient’s chronic malnourished state.
Diagnostic Evaluation
Noninvasive Evaluation
DUS is a useful tool for the early, noninvasive diagnosis of visceral ischemic syndromes. Color Doppler scanning can be used to assess the flow velocities and resistance index in the splanchnic arteries and their arterial beds as well as to evaluate end-organ vascularity. The intestinal wall can also be assessed with a high degree of accuracy by high-resolution transabdominal ultrasound.47 Transmural hemorrhage, inflammation, and necrotic thickening in the bowel wall can be imaged sonographically. Asymmetrical wall thickening with associated ileus as well as ascites and free peritoneal air can be seen in patients with AMI.48 DUS combined with expiratory maneuvers is also an excellent screening examination for median arcuate ligament syndrome.
Moneta et al49 performed blinded DUS studies in 100 patients who previously underwent arteriography of the celiac trunk and SMA. They hypothesized that lack of flow or a peak systolic velocity (PSV) in the SMA of greater than 275 cm/s, or no flow or a PSV of greater than 200 cm/s in the celiac trunk, was a reliable indicator of 70% or greater angiographic stenosis.50 With use of these criteria, duplex sensitivity for detection of lesions in the SMA and celiac artery was 92% and 87%, respectively. Overall accuracy for detection of a 70% lesion in the SMA and celiac artery was 96% and 82%, respectively. The IMA is not generally assessed because it is generally difficult to visualize and is of lower clinical importance. However, patency of the IMA can usually be determined.
Limitations of transabdominal duplex scanning include the wide variation in examination quality, which is operator dependent. Patient-related factors, such as obesity, excessive intraluminal bowel gas, variation in local anatomy, and effects of respiration, can affect image quality.51 Care must be taken to clearly define the origin of each vessel to avoid inaccurate measurements. DUS is generally not recommended in the workup of AMI.
DUS is also the primary imaging modality used for surveillance after both bypass and stenting. However, no specific criteria exist for determining restenosis. Baker et al52 evaluated the accuracy and utility of DUS for detection of in-stent stenosis in 23 patients who underwent successful (<20% residual stenosis on completion angiography) mesenteric stenting for CMI (20 SMA alone, 3 both SMA and celiac artery). Preprocedure DUS was performed in 13 patients with a mean PSV of 464 cm/s. Initial surveillance DUS was performed in 21 patients at a mean of 0.9 month after revascularization. Mean PSV at this time was 335 cm/s, and for 12 of these patients, the first postoperative PSV in successfully stented vessels was higher than the 275 cm/s threshold used to diagnose high-grade native SMA stenosis. In addition, there was no correlation between surveillance PSV and the degree of angiographic stenosis seen at the time of re-intervention.
In their review of 107 patients who underwent endovascular therapy for CMI, Schoch et al53 similarly reported that although 83% of patients had recurrent stenosis on surveillance DUS, 53% of patients remained asymptomatic and required no further intervention. These findings therefore mandate obtaining an early baseline DUS against which future surveillance scans can be compared. They also stress the importance of considering the clinical context and symptom recurrence in making the decision to re-intervene.
The predictive value of DUS for detection of disease recurrence after mesenteric bypass has also been studied. Liem et al54 evaluated 167 duplex examinations used in the surveillance of 43 bypass grafts in 38 patients. On comparison of antegrade and retrograde bypass configurations, differences in mean PSV were demonstrated only at the inflow artery. There were no differences in PSV at the proximal or distal anastomosis, in the midgraft, or in the outflow artery. Between the first postoperative DUS examination and the latest follow-up scan (on average 38 months), PSV measurements did not change significantly. Graft failure occurred in two patients, both of whom had no findings to suggest impending occlusion on the DUS examinations immediately preceding failure. No predictors of graft thrombosis were identified on multivariable analysis.
Computed tomography (CT) is an accurate, noninvasive imaging modality for diagnosis of mesenteric ischemia. Modern MDCT enables imaging with excellent spatial and temporal resolution. A meta-analysis of six studies published between 1996 and 2009 on the diagnostic accuracy of MDCT in AMI showed a pooled sensitivity of 93% and specificity of 96%.55 Advantages over conventional angiography include the relative ease and speed of performance; the rapid infusion of contrast agent through peripheral intravenous lines; and the ability to simultaneously image the mesenteric arteries, veins, and visceral organs. CTA is also useful for evaluating patency of previously placed grafts and stents. Common radiographic findings in the bowel wall related to AMI include increased thickening, dilatation, and attenuation, which can be easily detected with CT. Pneumatosis intestinalis, portal venous air, mesenteric edema, and ascites can also be detected. During the arterial phase of contrast infusion, the mesenteric vessels can be evaluated for thrombosis, embolus, dissection, and aneurysm. Venous engorgement and subtle intestinal findings may be identified in cases of mesenteric venous occlusion. A “target sign” may be seen in the superior mesenteric vein, with thrombus in the center of the lumen and surrounding contrast-enhanced blood flow peripherally. The portal venous phase is more accurate for diagnosis of MVT, and images during the venous phase may be acquired if the diagnosis is still in question or for surveillance of clot burden or extension in patients with MVT. In many centers, a biphasic scan, which includes a delayed venous phase in addition to an arterial phase, has been used in the diagnosis of AMI because it enables detection not only of arterial occlusion but also of MVT and improves the ability to detect changes in the bowel wall as well.
Disadvantages of CT include radiation, risk of contrast nephropathy, and hypersensitivity reactions to iodinated contrast agents. Inaccurate timing of contrast infusion during the arterial phase may provide indeterminate images and delay diagnosis. Because calcification at the vessel origins enhances in a similar fashion to intravenous contrast material, it is possible to underestimate the degree of stenosis. Therefore, the non–contrast-enhanced images should be reviewed. In addition, with careful adjustment of the window and level, calcification can usually be distinguished from intravascular contrast material. Last, CT serves strictly as a diagnostic modality; treatment must be performed through a separate angiographic procedure or laparotomy.
MRA is useful for diagnosis of mesenteric occlusive disease. Although MRA takes significantly longer to perform than CTA, it avoids the radiation exposure associated with CTA. Patients with hypersensitivity to iodinated contrast agents may also benefit from MRA. However, CTA has superior spatial resolution and allows better visualization of the IMA, peripheral splanchnic vessels, calcified plaque, and previously placed stents, giving it a distinct advantage.26 For these same reasons, CTA is also preferred to MRA for surveillance after revascularization. Our standard workup includes DUS to screen those thought to have CMI. CTA is used routinely before intervention for AMI, CMI, and MVT.
Invasive Evaluation
Conventional angiography remains the “gold standard” in the diagnosis of mesenteric ischemia. Anteroposterior and lateral views of the visceral aorta (Fig. 151-3) as well as selective catheterization of the celiac trunk, SMA, and IMA provide the most accurate and specific localization of stenotic and occlusive lesions. Therapeutic alternatives such as balloon angioplasty, stenting, and thrombolysis and percutaneous thrombus extraction can all be used to restore luminal visceral blood flow. These options are discussed in more detail in the next section.
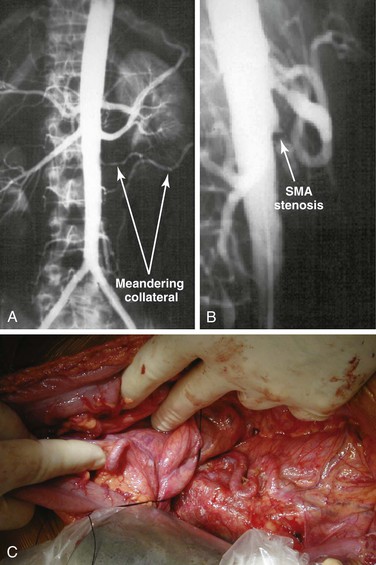
Figure 151-3 A, Anteroposterior angiogram of a patient with chronic mesenteric ischemia. Note the meandering mesenteric collateral vessels. B, Lateral aortogram of a patient with chronic mesenteric ischemia. Lesions are typically located at the origin of the vessel and often protrude into the aortic lumen. SMA, Superior mesenteric artery. C, Meandering mesenteric collateral vessels in a patient with chronic mesenteric ischemia at the time of exploration. In patients undergoing surgery for other reasons, this finding should raise the suspicion of significant mesenteric occlusive disease.
Gastric tonometry, or the measurement of PCO2 levels in the gastric, jejunal, or colonic mucosa through a nasogastric catheter, has been shown to be a useful adjunct for the diagnosis of mesenteric ischemia56,57 but is not currently widely used in the United States.
Treatment of Acute and Chronic Mesenteric Ischemia
The goal of therapy for patients with mesenteric ischemia is the prompt restoration of blood flow to the visceral organs. Specifics of the treatment of AMI and CMI are also discussed in Chapters 152 and 153.
Medical Treatment
Preventive risk factor modification helps control the progression of atherosclerosis in the mesenteric circulation, but medical treatment alone is not effective in patients with symptomatic mesenteric ischemia. Patients with known risks for inheritable hypercoagulable disorders should undergo screening and should be treated with systemic anticoagulation if indicated.
Before operation, aggressive fluid resuscitation with restoration of adequate urine output is required owing to the frequent finding of severe dehydration on presentation. Electrolyte abnormalities and metabolic acidosis should also be corrected. Patients with CMI are frequently malnourished, so albumin, prealbumin, transferrin, and C-reactive protein levels should be checked before revascularization. Preoperative total parenteral nutrition or enteral nutrition should be considered in severely malnourished patients. Finally, because the intestinal mucosa is damaged during periods of prolonged ischemia, bacterial translocation can occur, contributing to systemic sepsis. Broad-spectrum intravenous antibiotics with aggressive fluid resuscitation can lead to decreased mortality in these patients.58 Treatment against gram-negative and anaerobic organisms is especially important.
A recent Cochrane review of 22 randomized controlled trials investigating the use of different antiplatelet regimens after endovascular treatment of peripheral vascular disease concluded that there is limited evidence to support the use of antiplatelet drugs in the reduction of restenosis and reocclusion.59 No comparable studies for mesenteric angioplasty and stenting have been published of which we are aware. Unless it is contraindicated, our practice is to administer an initial 300-mg clopidogrel load after the procedure, followed by 75 mg daily for 1 to 3 months of treatment. Patients are also prescribed aspirin indefinitely.
Endovascular Treatment
General Principles
Advances in endovascular techniques have greatly expanded the role of percutaneous interventions for patients with mesenteric ischemia in recent years. Balloon angioplasty with stenting has surpassed open surgery as the dominant method of revascularization for CMI,22 and an endovascular approach is now generally accepted as primary therapy.60–62 In contrast, the adoption of endovascular modalities for treating AMI has been slower, and a decline in the number of open revascularizations has not been observed.22 This is likely because most patients with AMI have some degree of bowel ischemia and many require laparotomy for adequate evaluation and potential resection. In those with short-segment stenoses, cardiac and pulmonary comorbidities, prior abdominal surgery, coagulopathy, or malnutrition, endovascular therapy is often favored.
Efficacy
Acute Mesenteric Ischemia.
Because AMI occurs so infrequently and most revascularizations for AMI until recently have been performed open, there are relatively few retrospective reviews describing outcomes after endovascular treatment of AMI. Arthurs et al63 at the Cleveland Clinic reported their experience with revascularization in 70 consecutive patients with AMI from 1998 to 2008. Over time, their institution adopted an endovascular-first approach, such that 81% of the patients in their series were treated with endovascular therapy. The etiology was more often thrombotic (endovascular 72% vs open 36%) than embolic (endovascular 28% vs open 64%), and age was older in patients undergoing endovascular-first therapy (65 years vs 60 years), but there were no other significant differences in comorbidities or clinical presentation between the treatment groups. Technical success was 87%, and 31% of patients were able to avoid laparotomy. Compared with patients undergoing open revascularization, those who underwent endovascular revascularization had shorter lengths of bowel resected, developed fewer complications, and had lower perioperative mortality (39% vs 50%; P < .05).
Ryer et al64 also published their institution’s 2-decade (1990-2010) experience with revascularization for 93 patients with AMI. In addition, they performed a subgroup analysis between the 45 patients undergoing revascularization in the first decade compared with the 48 treated in the second decade. More patients underwent endovascular revascularization in the latter decade (7% vs 17%), but this difference was not significant, and the majority (88%) of all patients still underwent open revascularization. Improvements in mortality between the first and second decade were modest and nonsignificant (30-day mortality: 27% vs 17%, P = .28; 1-year mortality: 51% vs 31%, P = .11).
Chronic Mesenteric Ischemia.
A large body of literature now exists documenting early success and favorable outcomes for patients with CMI undergoing percutaneous endovascular therapy,53,65–71 and several institutions have recently published their midterm outcomes. Between 1995 and 2007, Dias et al72 successfully stented 47 of 49 mesenteric vessels in 43 patients with CMI. No technical failures occurred after a 3-year learning curve. Intraoperative complications occurred in seven (15%) patients, but all were successfully managed with endovascular techniques. No patient died within 30 days, and 87% of patients experienced symptom relief. Three-year survival was 76%, and re-intervention–free survival was 60%.
Peck et al62 also published their intermediate outcomes with similar results. From 2002 to 2008, 66 mesenteric arteries were treated in 49 patients. All patients had at least one mesenteric vessel successfully treated, but five technical failures occurred (four mesenteric occlusions could not be crossed and one IMA could not be stented). There was one in-hospital death due to a myocardial infarction in a patient who underwent a peripheral bypass during the same admission. Symptom relief was achieved in 90%, and major complications occurred in 16%. Three-year primary patency was 64%, and freedom from symptomatic recurrence was 63%.
Compared with traditional open approaches, endovascular revascularization results in lower or similar perioperative mortality, fewer complications, and shorter hospital stays but is associated with higher rates of recurrence of symptoms, restenosis, and re-intervention73–75 Oderich et al73 performed a risk-stratified comparison of 146 patients who underwent open and 83 patients who underwent endovascular revascularization for CMI. Operative risk was assessed by the Society for Vascular Surgery scores, and patients were classified as high risk by the presence of at least one high-risk criterion. Patients undergoing endovascular revascularization were significantly older (71 years vs 65 years; P < .5) and higher risk (58% vs 31%; P < .001). Angiographic features and extent of disease were similar between the endovascular and open treatment groups. For both low- and high-risk patients, there were no differences in procedure-related mortality, but patients in the endovascular group had fewer complications and shorter intensive care unit and hospital stays. At 5-year follow-up, patients who had undergone open revascularization had superior recurrence-free survival (89% vs 51%) and primary patency (88% vs 41%). Patients undergoing endovascular revascularization had 5.1-fold higher odds of restenosis and 4.3-fold higher odds of re-intervention than did patients undergoing open revascularization.
In a study by Atkins et al,74 42 mesenteric vessels treated with angioplasty (with stenting in 87%) were compared with 88 vessels treated with open revascularization. Mean follow-up was 15 months in the percutaneous transluminal angioplasty (PTA) group and 42 months in the open surgery group. No difference was noted in major morbidity or mortality. Radiographic primary patency and primary-assisted patency in the PTA group at 1 year were significantly lower (58% and 65%, respectively) compared with the open surgery group (90% and 96%, respectively). PTA with stenting was also associated with the need for earlier re-intervention. Surprisingly, the rates of symptomatic recurrence requiring re-intervention were high in both groups, with no significant difference noted.
No randomized studies comparing open and endovascular repair have been published to date. However, van Petersen et al76 performed a systematic review of the literature between 1988 and 2009. In a pooled comparison of 412 open repairs and 227 endovascular repairs, they found similar technical success, immediate symptom relief, and early mortality (in-hospital and 30-day) between the two treatment groups. Patients undergoing open revascularization experienced higher in-hospital and 30-day morbidity (32% vs 11%; P < .0001) and were more likely to receive intensive care unit care (88% vs 6%; P < .0001) but had lower rates of recurrent stenosis (15% vs 37%; P < .0001), recurrent symptoms (13% vs 30%; P < .0001), and re-intervention (9% vs 20%; P = .0004) and higher primary patency (86% vs 51%; P < .0001). These differences were seen at a mean follow-up of 35 months for the open group and 20 months for the endovascular group.
Using a large national administrative database with 22,413 patients undergoing mesenteric revascularization, we found that mortality was lower after angioplasty and stenting compared with bypass for both CMI (3.7% vs 13%; P < .01) and AMI (16% vs 28%; P < .01).22 Bowel resection was shown to be a significant risk factor for perioperative mortality (bypass: 45% vs 17%, P < .001; angioplasty and stenting: 29% vs 11%, P < .001). More patients undergoing bypass underwent bowel resection, indicating greater disease severity among this subgroup.
Mesenteric angioplasty and stenting have been associated with high restenosis and re-intervention rates. Fortunately, outcomes after re-intervention have been associated with low mortality and excellent symptom improvement. Tallarita et al77 studied re-interventions among 157 patients with CMI who initially underwent mesenteric artery angioplasty and stenting. In-stent restenosis (defined as PSV >330 cm/s or angiographic stenosis >60%) developed in 36% of patients, and 30 patients underwent re-intervention (87% by repeated endovascular and 13% by open revascularization). Among patients undergoing repeated endovascular revascularization, 13 bare metal stents and 4 covered stents were used. One patient undergoing repeated stenting died, and 27% experienced perioperative complications. Of the 24 patients who had developed recurrent symptoms, 92% had symptom improvement after re-intervention. At a mean follow-up of 29 months, 50% of patients developed a second restenosis and 23% required another re-intervention. Of note, however, none of the patients in whom covered stents were used experienced recurrence of symptoms, restenosis, or re-intervention.
Lesions most amenable to endovascular treatment are short, focal stenosis/occlusions with less calcification and thrombus. More complex lesions and complete arterial occlusions traditionally are better treated with open revascularization. However, feasibility of endovascular recanalization of complete occlusions has been reported. Sharafuddin et al78 published the largest series to date on the endovascular management of totally occluded mesenteric arteries and reported a technical success rate of 85% for the recanalization of 27 nonembolic total occlusions of the SMA and celiac arteries. All four failures were in SMA occlusions. Interestingly, technical success was not related to presence of stump (an appropriate probing point used to initiate the recanalization sequence and to establish a stable catheter position), ostial plaque, intraluminal versus subintimal route, occlusion length, or vessel diameter. Instead, success often depended on the ability to identify an appropriate probing point, for which preoperative cross-sectional imaging was crucial, and a stable catheter position, which frequently necessitated a brachial approach. As a result, the access site complication rate was relatively high (22%).
Retrograde Mesenteric Stenting
Techniques for retrograde mesenteric stenting during laparotomy for AMI were developed by Milner et al and Wyers et al, and success with this method has since been duplicated and adapted (Fig. 151-4).79–83 During laparotomy, the SMA is dissected at the base of the transverse mesocolon, and a sheath is inserted in the vessel either percutaneously or by arteriotomy. Wyers et al80 described placing a longitudinal arteriotomy in the SMA, performing a local thromboendarterectomy if required, and performing patch angioplasty. The sheath is then placed through the distal end of the patch for retrograde access to the SMA. Hand-injected lateral aortography is performed, and a wire is placed retrograde across the lesion into the aorta. A balloon-expandable stent is positioned and deployed to allow protrusion of the stent 1 to 2 mm into the aorta. In the review by Wyers et al,80 they compared patients treated in this manner with patients who underwent antegrade stenting and traditional surgical bypass for AMI. Mortality was 17% for the patients who underwent retrograde stenting compared with 100% for the antegrade stenting group and 80% for those who underwent surgical bypass. The technical success rate for the retrograde stenting group was 100%.
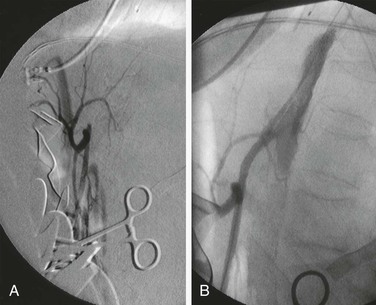
Figure 151-4 A, Retrograde superior mesenteric arteriogram during exploration in a patient with acute mesenteric ischemia secondary to arterial thromboses. Note the proximal occlusion of the superior mesenteric artery. B, Intraoperative placement of a proximal superior mesenteric artery stent for acute revascularization of the small bowel.
In a study by Moyes et al,81 four patients were treated similarly with retrograde stenting of the SMA for AMI. Two of the patients were alive at 2 years. One patient experienced stent thrombosis on day 14 and required surgical bypass. The remaining patient died on postoperative day 6 due to multiorgan failure. Stout et al83 also used the technique in three female patients, one of whom required bowel resection. After a mean follow-up of 8.4 months, the primary patency rate was 100%. Pisimisis et al82 described adapting the procedure by performing endarterectomy and patch angioplasty after stenting rather than before. Advantages to this alternative include improved ability to flush and to inspect the lumen for residual thrombus and avoidance of a stenosis with primary closure. In addition, the authors reported that inflating the angioplasty balloon within the stent allowed excellent proximal control during the endarterectomy and anastomosis.
Although early results demonstrate good technical success, the long-term efficacy of retrograde mesenteric stenting has not been established. As with antegrade stenting, the main advantage of this technique is reduced invasiveness and morbidity. However, retrograde stenting also provides the ability to inspect the bowel before and after reperfusion and can potentially reduce operative times because the exposure of proximal inflow vessels (aorta, iliac) and the harvest of autogenous conduit for surgical bypass are avoided. Retrograde SMA stenting may also be useful in patients if a good source of inflow is unavailable for bypass or as a temporizing measure until definitive bypass can be accomplished (e.g., in the case of peritoneal contamination). Potential disadvantages include inadvertent injury to the vessel or aortic dissection during wire manipulation and restenosis due to intimal hyperplasia.
Limitations
Endovascular therapy should be the treatment of choice in high-risk patients with CMI. High technical success rates and decreased patient morbidity and mortality rates have been reasonably well established in such individuals. However, in patients who are good surgical candidates, the advantage is not so clear. Restenosis and symptomatic recurrence rates remain relatively high, as documented in the current literature, and re-intervention is often required earlier and more frequently than with open surgery. Placement of stents in the mesenteric arteries may also complicate future surgical intervention, especially in the celiac trunk, which is relatively short before branching. However, anastomosis to the hepatic artery is usually not compromised by prior celiac stent placement. Because the origins of the visceral arteries are angulated downward, especially the SMA, percutaneous access from the femoral arteries may be difficult and result in suboptimal stent placement. Thus, a brachial approach is often preferred. One study found a significant improvement in primary patency in patients who underwent treatment through the brachial artery compared with a femoral approach.84 In contrast, Turba et al85 did not find a significant association of femoral versus brachial access to primary patency of the celiac, SMA, or IMA when reviewing their 28-year experience treating 166 patients with CMI. Furthermore, as noted before, brachial access is associated with higher rates of access site–related complications.78 Technologic advances, such as deflecting tip sheaths, may improve results with femoral access.
The risk of distal embolization is reported at approximately 8% without embolic protection devices.86 With increasingly aggressive treatment of more complex stenotic lesions and total occlusions, the risk of distal embolization theoretically increases. However, Sharafuddin et al78 detected no angiographically significant embolic events in their experience with endovascular recanalization of 27 nonembolic total occlusions, but the possibility of microembolization could not be excluded. Therefore, distal protection, such as that used during carotid stenting, can be considered, especially for patients with complete occlusions, long lesions, or severe calcification. Other complications described in the literature include access site problems, renal insufficiency, dissection, stent thrombosis, dislodgement or embolization, and vessel perforation. Oderich et al.87 studied complications that developed in 156 patients who underwent angioplasty and stenting for CMI. On univariate analysis, antiplatelet therapy was associated with lower rates of distal embolization or vessel thrombosis. There were no significant multivariable predictors. Stent fracture producing restenosis and recurrent ischemia has been documented.88,89 Reperfusion hemorrhage, traditionally associated with open surgical revascularization, has also been described after balloon angioplasty.90 Despite these limitations, the role of endovascular techniques will likely continue to expand for patients with mesenteric ischemia. Early restenosis rates are likely to improve with the development of new balloons, drug-eluting stents, and stent-grafts.
Recommendations
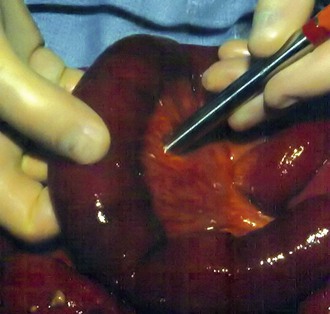
At our institution, we perform mesenteric angiography for all patients with CMI and attempt primary stenting as first-line therapy. The brachial approach has been preferred for selective catheterization of the SMA owing to its acute downward angle at its origin. However, recently we have started using a deflecting tip sheath from a femoral approach with good results in selected patients. Patients with isolated stenosis of the celiac artery due to extrinsic compression by the median arcuate ligament and appropriate symptoms undergo laparoscopic lysis of the median arcuate ligament first (Fig. 151-5). Balloon angioplasty and stenting are avoided initially in these patients but may be used after median arcuate ligament release in patients with persistent abdominal symptoms and duplex- or CTA-demonstrated persistent stenosis. We conduct duplex surveillance 1 month after the initial procedure and then at 6-month intervals to assess for continued vessel patency.
Surgical Treatment
General Considerations
Laparotomy with visceral revascularization can be used to treat patients with both AMI and CMI. Patients presenting with signs and symptoms of AMI require urgent abdominal exploration, assessment of bowel viability, and revascularization. Several techniques for the restoration of intestinal perfusion are available to the vascular surgeon, and familiarity with a variety of options is crucial. Before revascularization, large segments of both small and large intestine may appear dusky, ischemic, or necrotic. Because SMA emboli typically lodge distal to its origin, the middle colic artery and ileocolic branches may remain patent. Thus, the pattern of bowel necrosis after embolus may be less extensive and spare the proximal jejunum and transverse colon distribution from ischemia (see Fig. 151-1A). Acute thrombosis usually occurs at the orifice of the SMA, and a more extensive pattern of hypoperfusion and necrosis is present owing to the involvement of proximal SMA branches (see Fig. 151-1B).
After vascular reconstruction and reperfusion, the bowel should be reassessed. Segments of bowel that otherwise would have been resected may be spared owing to improvement after revascularization. A wide range of techniques to assess bowel viability have been described, including pulse oximetry, measurement of oxygen tension, spectrophotometry, Doppler ultrasound, fluorescein dye, and examination with Wood’s lamp, laser Doppler flowmetry, and infrared imaging.91 At our institution, we favor a combination of clinical assessment (using criteria such as serosal color, bowel peristalsis, and vessel palpation) and Doppler ultrasound (Fig. 151-6). Intraoperative angiography after vascular reconstruction may be performed to accurately assess the restoration of mesenteric flow. Most patients should undergo “second-look” laparotomy in 24 hours to reassess bowel viability and the need for further resection (see Fig. 151-1C). The decision to perform a second-look laparotomy should be made during the initial procedure and should not be changed on the basis of subsequent clinical improvement. For those in whom bowel resection is performed at initial exploration, the ends are stapled and no anastomosis is performed until second-look laparotomy.
Acute Mesenteric Ischemia
Detailed descriptions of the techniques of surgical visceral revascularization for AMI are highlighted in Chapter 153.
Chronic Mesenteric Ischemia
Detailed descriptions of the techniques of surgical visceral revascularization for CMI are highlighted in Chapter 152.
Transaortic Endarterectomy.
Stoney and Wylie first described the “trap-door” approach for transaortic endarterectomy (Fig. 151-7) in 1966 at the University of California, San Francisco.7 The procedure involves medial visceral rotation, dissection of the SMA, and longitudinal aortotomy. It has been performed during the past 4 decades with acceptable morbidity and mortality rates and proven durability.92 Although it is rarely indicated anymore, this approach may be useful in patients who have failed to respond to endovascular therapy or in those with hostile abdomens. Advantages of this operation include removal of atheroma from the aorta and both visceral arteries simultaneously. Limitations include the need for extended exposure of the upper abdominal aorta and incomplete plaque removal if the atheroma extends to the distal artery or if transmural calcification is present.
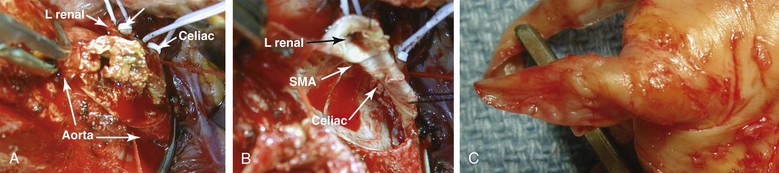
Figure 151-7 A, Transaortic endarterectomy using a trap-door incision in a patient with severe aortic, mesenteric, and renal occlusive disease. B, Appearance of the visceral vessel orifice after transaortic endarterectomy. The aortotomy is usually closed with pledgeted sutures because the arterial wall is very thin after endarterectomy. C, Typical specimen after transaortic endarterectomy of an orificial lesion. Note the tapered endpoint. Patients selected for this technique should have lesions limited to the proximal segment of the artery. Failure to obtain an endpoint can be corrected by intraoperative placement of a stent under direct vision. L, Left; SMA, superior mesenteric artery.
Antegrade Mesenteric Bypass.
The majority of open mesenteric revascularizations consist of bypass with a bifurcated synthetic graft from the supraceliac aorta to the celiac artery and SMA. Historically, antegrade bypass has demonstrated efficacy and durability.93–95 Jimenez et al95 reviewed the results of 47 patients with CMI who underwent antegrade synthetic aortoceliac and aortomesenteric bypass during a 14-year period. The in-hospital mortality rate was 11%, and the mean length of hospital stay was 32 ± 30 days. At a mean follow-up of 37 months, 100% of surviving patients were symptom free, and 86% had significant weight gain. Primary, primary-assisted, and secondary graft patency rates at 5 years were 69%, 94%, and 100%, respectively. Actuarial survival was 74%. Although antegrade bypass was associated with significant perioperative morbidity and mortality, it was also associated with excellent durability and symptom-free survival.95 Cunningham and Reilly found similar results in their review of 26 antegrade bypasses for CMI.93,96 They reported a slightly lower perioperative mortality rate (7.7%). The percentage of patients who were symptom free at 1 and 5 years was 96% and 86%, respectively. English et al97 performed antegrade revascularization for CMI in 80 mesenteric vessels. Their in-hospital mortality rate was 29%. The presence of intestinal gangrene correlated significantly with perioperative death and escalation of symptoms before bypass (acute-on-chronic). Symptom-free survival at 70 months was 57%.
Retrograde Mesenteric Bypass.
In retrograde mesenteric bypass, the inflow is typically from the infrarenal aorta or either of the iliac arteries, although case reports have documented the success of bypasses originating from the splenic artery.98 Wylie et al99 first described retrograde mesenteric bypass (Fig. 151-8), and the debate continues about its efficacy compared with antegrade bypass. Retrograde bypass is favored in patients who are elderly, are cachectic, or have severe cardiac, pulmonary, and renal comorbidity or those who have extensive plaque and calcification in the supraceliac aorta.100 Despite this controversy, no convincing evidence favors one approach over the other, and no randomized controlled trials have been conducted. Foley et al19 from Oregon Health Sciences University examined their results with 50 bypass grafts to the SMA alone for both AMI and CMI. All grafts originated from either the infrarenal aorta or the iliac artery. Perioperative mortality was 3% in patients with CMI and 24% in patients with AMI. All survivors had relief of symptoms in the postoperative period. There were two late deaths related to graft occlusion. Nine-year primary-assisted graft patency was 79%, and the 5-year survival was 61%.
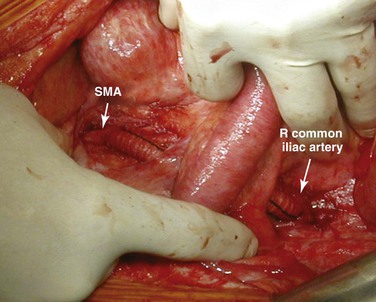
Figure 151-8 Right iliomesenteric bypass for retrograde revascularization of the superior mesenteric artery (SMA). The graft is passed between the leaves of the base of the mesentery to avoid contact with the intestines.
In a combined study from Beth Israel Deaconess Medical Center and University of California, Los Angeles, the results of 39 mesenteric bypass procedures were reviewed during a 9-year period.101 Symptom-free survival after antegrade (n = 21) and retrograde (n = 18) bypass was compared by the Kaplan-Meier life-table method. No significant difference was found between the two techniques. Although the overall incidence of postoperative complications was higher in the antegrade group, the number of major complications in both groups was similar. The 30-day mortality was higher in the antegrade group (14.3%) than in the retrograde group (0%), and none of the patients who died were revascularized for acute ischemia.
Outcomes of Open Repair
Acute Mesenteric Ischemia.
Although AMI-related mortality after open revascularization has declined from 50% in the 1990s to 30% in the 2000s,22 mortality remains high in patients with AMI even after successful surgical revascularization. One of the contributing factors is ischemia-reperfusion intestinal injury. Although the goal of therapy is prompt restoration of mesenteric blood flow, revascularization may lead to a paradoxical exacerbation of tissue damage. Although the exact mechanism is not completely understood, the production of toxic oxygen radicals is thought to play an important role.102 Ischemia-reperfusion injury has been linked to postoperative myocardial depression,103,104 sepsis,102 acute lung injury,105 and multiorgan failure.106 Immediately after reperfusion, direct injury to the intestinal wall occurs and triggers a series of events, including the release of various inflammatory mediators, activation and aggregation of neutrophils, and bacterial translocation.103 The intestine may take several weeks to months to recover, and patients may experience prolonged diarrhea and periods of malabsorption.106
Several translational studies have reported promising strategies to attenuate the effects of ischemia-reperfusion injury. In murine models, when revascularization after a prolonged period of SMA occlusion is preceded by brief SMA clamping (termed preconditioning), epithelial narrowing and hyperpermeability, leukocyte rolling and adherence, and bacterial translocation are all reduced.107–109 Similarly, postconditioning, or brief periods of reocclusion in the first few minutes after revascularization, has also been shown to decrease the extent of damage caused by oxidative stress and cytokine release.110 The protective effects of conditioning are thought to be mediated through the upregulation of heat shock proteins and antioxidant enzymes and the downregulation of apoptosis and the inflammatory response.18
Aside from ischemia-reperfusion effects, other factors can contribute to worse outcomes after mesenteric revascularization. Kougias et al111 reviewed the records for 72 patients who underwent surgical visceral revascularization for AMI. The overall 30-day mortality was 31%. Factors associated with increased mortality were renal insufficiency, age older than 70 years, metabolic acidosis, symptom duration, and need for bowel resection during second-look operations. Increased age and prolonged symptom duration were independent predictors of mortality, with relative risks of 3.6 and 4.6, respectively.
In their 20-year experience with revascularization for AMI encompassing 93 patients, the group at the Mayo Clinic found that Society for Vascular Surgery comorbidity score, congestive heart failure, and chronic kidney disease predicted higher early mortality, whereas advanced age and connective tissue disease predicted long-term mortality.64 Thirty-day mortality was 22%, and the primary causes of death were ongoing mesenteric ischemia, multiorgan failure, adverse cardiac events, and withdrawal of care by the health care proxy.
Chronic Mesenteric Ischemia.
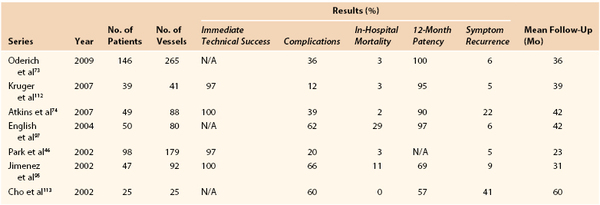
Oderich et al73 reviewed 265 vessels (143 SMA, 113 celiac, and 9 IMA) in 146 patients with CMI who underwent open revascularization (93% bypass, 7% transaortic endarterectomy). Symptoms were improved in 97%, and procedure-related morbidity and mortality were 36% and 3%, respectively. At 5 years, overall survival was 72% and symptom-free survival was 89%. All patients with recurrent symptoms underwent re-intervention. Half of these (n = 4) required emergency operation for acute thrombosis. The other half underwent angioplasty and stenting. Table 151-1 presents the results of revascularization for CMI from selected series.
Treatment of Nonocclusive Mesenteric Ischemia
NOMI represents an insidious disease process that is distinct from thromboembolic AMI but has a similarly high mortality. Cardiac surgery and low-flow states are the most common causes of this disorder, and treatment is directed toward improving circulatory support and increasing cardiac output. Selective mesenteric angiography remains the best invasive diagnostic modality, which can be followed by catheter-based interventions. These include direct infusion of intra-arterial vasodilators, such as papaverine and prostaglandin E1, as well as angioplasty and stenting if necessary.28 Intravenous rather than intra-arterial prostaglandin E1 has also demonstrated efficacy in treatment of vasospasm associated with NOMI.44 In a model of cardiac tamponade–induced acute NOMI, Kang et al114 demonstrated that low doses of intra-arterial iloprost (prostacyclin), a potent inhibitor of platelet aggregation with fibrinolytic activity, exhibited a significant vasodilatory effect on mesenteric blood flow.
Treatment of Mesenteric Venous Thrombosis
A detailed discussion of the diagnosis and treatment of MVT is contained in Chapter 154. The mainstay for treatment of acute and subacute MVT is the prompt initiation of systemic anticoagulation, which improves survival and reduces the risk of recurrence.41 Heparin should be initiated with a bolus followed by a continuous infusion to keep the partial thromboplastin time between 50 and 70 seconds. Intravenous antibiotics should be administered to decrease bacterial translocation from the intestinal mucosa. Aggressive fluid resuscitation and circulatory support should be performed because of severe bowel edema and shifting of fluid into the peritoneal cavity. Nasogastric decompression, bowel rest, and administration of total parenteral nutrition are also indicated.
Patients with suspected peritonitis, severe gastrointestinal bleeding, or intestinal stricture require abdominal exploration and resection of nonviable bowel. Bowel resection should be conservative in patients with MVT. We frequently perform a second-look laparotomy 24 hours after the initial operation, which helps avoid resection of viable bowel during the initial operation. Several case reports have been published describing the successful use of direct percutaneous thrombolytic infusion into the mesenteric veins through the transjugular115 or transhepatic route116 or indirectly through the SMA.117 However, Grisham et al118 reviewed the outcomes of 24 patients with MVT and noted a significantly higher mortality rate in those treated with thrombolytic therapy compared with those receiving systemic anticoagulation alone. There was no significant difference in length of hospital stay between the two groups. In contrast, Semiz-Oysu et al119 performed multiple percutaneous interventions for prehepatic MVT, including venous recanalization (n = 19), thrombolysis (n = 1), and mechanical thrombectomy (n = 5). They noted an improvement in symptoms in 83% of patients treated, with relatively low mortality (13.6%). Other endovascular treatment options include mechanical or aspiration thrombectomy, angioplasty, and portosystemic shunt.
Dentali et al120 performed the first large, multicenter, retrospective review to determine the efficacy of oral anticoagulant therapy for prevention of MVT recurrence. After a median follow-up of 36 months, reported recurrence rates were 45.9 events/1000 patient-years for patients who had ceased anticoagulation therapy and 10.5 events/1000 patient-years for those with ongoing treatment. Major bleeding events occurred in 2.6% while receiving anticoagulation. In light of such findings, lifelong anticoagulation with vitamin K antagonists is recommended for patients with MVT because recurrence is common (up to 36% without anticoagulation) and potentially catastrophic. Patients may be transitioned from heparin to a vitamin K antagonist once bowel function has normalized.
Selected Key References
Acosta S. Epidemiology of mesenteric vascular disease: clinical implications. Semin Vasc Surg. 2010;23:4–8.
Arthurs ZM, Titus J, Bannazadeh M, Eagleton MJ, Srivastava S, Sarac TP, Clair DG. A comparison of endovascular revascularization with traditional therapy for the treatment of acute mesenteric ischemia. J Vasc Surg. 2011;53:698–704.
Baker AC, Chew V, Li CS, Lin TC, Dawson DL, Pevec WC, Hedayati N. Application of duplex ultrasound imaging in determining in-stent stenosis during surveillance after mesenteric artery revascularization. J Vasc Surg. 2012;56:1364–1371.
Foley MI, Moneta GL, Abou-Zamzam AM Jr, Edwards JM, Taylor LM Jr, Yeager RA, Porter JM. Revascularization of the superior mesenteric artery alone for treatment of intestinal ischemia. J Vasc Surg. 2000;32:37–47.
Jimenez JG, Huber TS, Ozaki CK, Flynn TC, Berceli SA, Lee WA, Seeger JM. Durability of antegrade synthetic aortomesenteric bypass for chronic mesenteric ischemia. J Vasc Surg. 2002;35:1078–1084.
Oderich GS, Bower TC, Sullivan TM, Bjarnason H, Cha S, Gloviczki P. Open versus endovascular revascularization for chronic mesenteric ischemia: risk-stratified outcomes. J Vasc Surg. 2009;49:1472–1479.e3.
Wyers MC, Powell RJ, Nolan BW, Cronenwett JL. Retrograde mesenteric stenting during laparotomy for acute occlusive mesenteric ischemia. J Vasc Surg. 2007;45:269–275.
Description of technique and outcomes of retrograde mesenteric stenting..
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Bacelli F. Angina abdominis. [cited by Goodman EH] Am J Med Sci. 1918;155:524–528.
2. Dunphy JE. Abdominal pain of vascular origin. Am J Med Sci. 1936;192:109–113.
3. Warren S, et al. Mesenteric venous thrombosis. Surg Gynecol Obstet. 1935;61:102–121.
4. Klass AA. Embolectomy in acute mesenteric occlusion. Ann Surg. 1951;134:913–917.
5. Shaw RS, et al. Acute and chronic thrombosis of the mesenteric arteries associated with malabsorption; a report of two cases successfully treated by thromboendarterectomy. N Engl J Med. 1958;258:874–878.
6. Morris GC Jr, et al. Revascularization of the celiac and superior mesenteric arteries. Arch Surg. 1962;84:95–107.
7. Stoney RJ, et al. Recognition and surgical management of visceral ischemic syndromes. Ann Surg. 1966;164:714–722.
8. Furrer J, et al. Treatment of abdominal angina with percutaneous dilatation of an arteria mesenterica superior stenosis. Preliminary communication. Cardiovasc Intervent Radiol. 1980;3:43–44.
9. Novelline RA. Percutaneous transluminal angioplasty: newer applications. AJR Am J Roentgenol. 1980;135:983–988.
10. Finch IJ. Use of the Palmaz stent in ostial celiac artery stenosis. J Vasc Interv Radiol. 1992;3:633–635.
11. Lin PH, et al. Embryology, anatomy, and surgical exposure of the great abdominal vessels. Surg Clin North Am. 2000;80:417–433.
12. Gallavan RH, et al. Pathophysiology of the gastrointestinal system. American Physiological Society: Bethesda, Md; 1989.
13. Rosenblum JD, et al. The mesenteric circulation. Anatomy and physiology. Surg Clin North Am. 1997;77:289–306.
14. Perko MJ, et al. Mesenteric, coeliac and splanchnic blood flow in humans during exercise. J Physiol. 1998;513(Pt 3):907–913.
15. Wilson DB, et al. Clinical course of mesenteric artery stenosis in elderly Americans. Arch Intern Med. 2006;166:2095–2100.
16. Ulker P, et al. Nitric oxide generated by red blood cells following exposure to shear stress dilates isolated small mesenteric arteries under hypoxic conditions. Clin Hemorheol Microcirc. 2012.
17. Hansen MB, et al. Profile of neurohumoral agents on mesenteric and intestinal blood flow in health and disease. Physiol Res. 1998;47:307–327.
18. Vollmar B, et al. Intestinal ischemia/reperfusion: microcirculatory pathology and functional consequences. Langenbecks Arch Surg. 2011;396:13–29.
19. Foley MI, et al. Revascularization of the superior mesenteric artery alone for treatment of intestinal ischemia. J Vasc Surg. 2000;32:37–47.
20. Lo RC, et al. The decline of mesenteric ischemia-related mortality in the last decade. Abstract presented at the 41st Annual Symposium of the Society for Clinical Vascular Surgery, Miami, Fla.. 2013.
21. Go AS, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA. 2003;290:2685–2692.
22. Schermerhorn ML, et al. Mesenteric revascularization: management and outcomes in the United States, 1988-2006. J Vasc Surg. 2009;50:341–348.e1.
23. Mitchell EL, et al. Mesenteric duplex scanning. Perspect Vasc Surg Endovasc Ther. 2006;18:175–183.
24. Thomas JH, et al. The clinical course of asymptomatic mesenteric arterial stenosis. J Vasc Surg. 1998;27:840–844.
25. Bradbury AW, et al. Mesenteric ischaemia: a multidisciplinary approach. Br J Surg. 1995;82:1446–1459.
26. Shih MC, et al. CTA and MRA in mesenteric ischemia: part 1, role in diagnosis and differential diagnosis. AJR Am J Roentgenol. 2007;188:452–461.
27. Mansour MA. Management of acute mesenteric ischemia. Arch Surg. 1999;134:328–330.
28. Oldenburg WA, et al. Acute mesenteric ischemia: a clinical review. Arch Intern Med. 2004;164:1054–1062.
29. Schoots IG, et al. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br J Surg. 2004;91:17–27.
30. Acosta S, et al. Clinical implications for the management of acute thromboembolic occlusion of the superior mesenteric artery: autopsy findings in 213 patients. Ann Surg. 2005;241:516–522.
31. Acosta S. Epidemiology of mesenteric vascular disease: clinical implications. Semin Vasc Surg. 2010;23:4–8.
32. Bjorck M, et al. Nonocclusive mesenteric hypoperfusion syndromes: recognition and treatment. Semin Vasc Surg. 2010;23:54–64.
33. Quiroga B, et al. Detection of patients at high risk for non-occlusive mesenteric ischemia in hemodialysis. J Surg Res. 2013;180:51–55.
34. Li SY, et al. Mesenteric ischemia in patients with end-stage renal disease: a nationwide longitudinal study. Am J Nephrol. 2012;35:491–497.
35. Archodovassilis F, et al. Nonocclusive mesenteric ischemia: a lethal complication in peritoneal dialysis patients. Perit Dial Int. 2007;27:136–141.
36. Grendell JH, et al. Mesenteric venous thrombosis. Gastroenterology. 1982;82:358–372.
37. Kumar S, et al. Mesenteric venous thrombosis. N Engl J Med. 2001;345:1683–1688.
38. Jackson CS, et al. Mesenteric vascular thromboembolism in inflammatory bowel disease: a single center experience. J Gastrointest Surg. 2011;15:97–100.
39. Gonzelez HJ, et al. Splanchnic vein thrombosis in severe acute pancreatitis: a 2-year, single-institution experience. HPB (Oxford). 2011;13:860–864.
40. Boley SJ, et al. History of mesenteric ischemia. The evolution of a diagnosis and management. Surg Clin North Am. 1997;77:275.
41. Deitch EA, et al. Effect of hemorrhagic shock on bacterial translocation, intestinal morphology, and intestinal permeability in conventional and antibiotic-decontaminated rats. Crit Care Med. 1990;18:529–536.
42. Acosta S, et al. Mesenteric venous thrombosis with transmural intestinal infarction: a population-based study. J Vasc Surg. 2005;41:59–63.
43. Acosta S, et al. D-dimer testing in patients with suspected acute thromboembolic occlusion of the superior mesenteric artery. Br J Surg. 2004;91:991–994.
44. Mitsuyoshi A, et al. Survival in nonocclusive mesenteric ischemia: early diagnosis by multidetector row computed tomography and early treatment with continuous intravenous high-dose prostaglandin E1. Ann Surg. 2007;246:229–235.
45. White CJ. Chronic mesenteric ischemia: diagnosis and management. Prog Cardiovasc Dis. 2011;54:36–40.
46. Park WM, et al. Current results of open revascularization for chronic mesenteric ischemia: a standard for comparison. J Vasc Surg. 2002;35:853–859.
47. Dietrich CF, et al. Sonographic assessment of splanchnic arteries and the bowel wall. Eur J Radiol. 2007;64:202–212.
48. Rhee RY, et al. Mesenteric venous thrombosis: still a lethal disease in the 1990’s. J Vasc Surg. 1994;20:688–697.
49. Moneta GL, et al. Mesenteric duplex scanning: a blinded prospective study. J Vasc Surg. 1993;17:79–84.
50. Moneta GL, et al. Duplex ultrasound criteria for diagnosis of splanchnic artery stenosis or occlusion. J Vasc Surg. 1991;14:511–518.
51. Geelkerken RH, et al. Pitfalls in the diagnosis of origin stenosis of the coeliac and superior mesenteric arteries with transabdominal color duplex examination. Ultrasound Med Biol. 1996;22:695–700.
52. Baker AC, et al. Application of duplex ultrasound imaging in determining in-stent stenosis during surveillance after mesenteric artery revascularization. J Vasc Surg. 2012;56:1364–1371.
53. Schoch DM, et al. Management of chronic mesenteric vascular insufficiency: an endovascular approach. J Am Coll Surg. 2011;212:668–675.
54. Liem TK, et al. Duplex scan characteristics of bypass grafts to mesenteric arteries. J Vasc Surg. 2007;45:922–927.
56. Otte JA, et al. Jejunal tonometry for the diagnosis of gastrointestinal ischemia. Feasibility, normal values and comparison of jejunal with gastric tonometry exercise testing. Eur J Gastroenterol Hepatol. 2008;20:62–67.
57. Sana A, et al. Radiological imaging and gastrointestinal tonometry add value in diagnosis of chronic gastrointestinal ischemia. Clin Gastroenterol Hepatol. 2011;9:234–241.
58. Jamieson WG, et al. Effect of antibiotic and fluid resuscitation upon survival time in experimental intestinal ischemia. Surg Gynecol Obstet. 1988;167:103–108.
59. Robertson L, et al. Antiplatelet and anticoagulant drugs for prevention of restenosis/reocclusion following peripheral endovascular treatment. Cochrane Database Syst Rev. 2012;(8).
60. Fioole B, et al. Percutaneous transluminal angioplasty and stenting as first-choice treatment in patients with chronic mesenteric ischemia. J Vasc Surg. 2010;51:386–391.
61. Pecoraro F, et al. Chronic mesenteric ischemia: critical review and guidelines for management. Ann Vasc Surg. 2013;27:113–122.
62. Peck MA, et al. Intermediate-term outcomes of endovascular treatment for symptomatic chronic mesenteric ischemia. J Vasc Surg. 2010;51.
63. Arthurs ZM, et al. A comparison of endovascular revascularization with traditional therapy for the treatment of acute mesenteric ischemia. J Vasc Surg. 2011;53:698–704.
64. Ryer EJ, et al. Revascularization for acute mesenteric ischemia. J Vasc Surg. 2012;55:1682–1689.
65. Matsumoto AH, et al. Percutaneous transluminal angioplasty and stenting in the treatment of chronic mesenteric ischemia: results and longterm followup. J Am Coll Surg. 2002;194(Suppl):S22–S31.
66. Sharafuddin MJ, et al. Endovascular treatment of celiac and mesenteric arteries stenoses: applications and results. J Vasc Surg. 2003;38:692–698.
67. Landis MS, et al. Percutaneous management of chronic mesenteric ischemia: outcomes after intervention. J Vasc Interv Radiol. 2005;16:1319–1325.
68. Silva JA, et al. Endovascular therapy for chronic mesenteric ischemia. J Am Coll Cardiol. 2006;47:944–950.
69. Sarac TP, et al. Endovascular treatment of stenotic and occluded visceral arteries for chronic mesenteric ischemia. J Vasc Surg. 2008;47:485–491.
70. Brown DJ, et al. Mesenteric stenting for chronic mesenteric ischemia. J Vasc Surg. 2005;42:268–274.
71. van Wanroij JL, et al. Endovascular treatment of chronic splanchnic syndrome. Eur J Vasc Endovasc Surg. 2004;28:193–200.
72. Dias NV, et al. Mid-term outcome of endovascular revascularization for chronic mesenteric ischaemia. Br J Surg. 2010;97:195–201.
73. Oderich GS, et al. Open versus endovascular revascularization for chronic mesenteric ischemia: risk-stratified outcomes. J Vasc Surg. 2009;49:1472–1479.e3.
74. Atkins MD, et al. Surgical revascularization versus endovascular therapy for chronic mesenteric ischemia: a comparative experience. J Vasc Surg. 2007;45:1162–1171.
75. Davies RS, et al. Surgical versus endovascular reconstruction for chronic mesenteric ischemia: a contemporary UK series. Vasc Endovascular Surg. 2009;43:157–164.
76. van Petersen AS, et al. Open or percutaneous revascularization for chronic splanchnic syndrome. J Vasc Surg. 2010;51:1309–1316.
77. Tallarita T, et al. Reinterventions for stent restenosis in patients treated for atherosclerotic mesenteric artery disease. J Vasc Surg. 2011;54:1422–1429.e1.
78. Sharafuddin MJ, et al. Endovascular recanalization of total occlusions of the mesenteric and celiac arteries. J Vasc Surg. 2012;55:1674–1681.
79. Milner R, et al. Superior mesenteric artery angioplasty and stenting via a retrograde approach in a patient with bowel ischemia—a case report. Vasc Endovascular Surg. 2004;38:89–91.
80. Wyers MC, et al. Retrograde mesenteric stenting during laparotomy for acute occlusive mesenteric ischemia. J Vasc Surg. 2007;45:269–275.
81. Moyes LH, et al. Intraoperative retrograde mesenteric angioplasty for acute occlusive mesenteric ischaemia: a case series. Eur J Vasc Endovasc Surg. 2008;36:203–206.
82. Pisimisis GT, et al. Technique of hybrid retrograde superior mesenteric artery stent placement for acute-on-chronic mesenteric ischemia. Ann Vasc Surg. 2011;25:132.e7–132.e11.
83. Stout CL, et al. Retrograde open mesenteric stenting for acute mesenteric ischemia is a viable alternative for emergent revascularization. Vasc Endovascular Surg. 2010;44:368–371.
84. Sivamurthy N, et al. Endovascular versus open mesenteric revascularization: immediate benefits do not equate with short-term functional outcomes. J Am Coll Surg. 2006;202:859–867.
85. Turba UC, et al. Chronic mesenteric ischaemia: 28-year experience of endovascular treatment. Eur Radiol. 2012;22:1372–1384.
86. Oderich GS, et al: Anatomic measurements and factors associated with embolic events during superior mesenteric artery stenting: implications for use of embolic protection devices. Abstract book of the 37th Society for Clinical Vascular Surgery Annual Symposium, 2009.
87. Oderich GS, et al. Mesenteric artery complications during angioplasty and stent placement for atherosclerotic chronic mesenteric ischemia. J Vasc Surg. 2012;55:1063–1071.
88. Klepczyk L, et al. Superior mesenteric artery stent fracture producing stenosis and recurrent chronic mesenteric ischemia: case report. Vasc Endovascular Surg. 2008;42:79–81.
89. Schellekens JF, et al. Superior mesenteric artery stent fracture leading to recurrent mesenteric ischemia. Vasc Endovascular Surg. 2011;45:654–659.
90. Moore M, et al. Reperfusion hemorrhage following superior mesenteric artery stenting. Cardiovasc Intervent Radiol. 2008;31(Suppl 2):S57–S61.
91. Urbanavicius L, et al. How to assess intestinal viability during surgery: a review of techniques. World J Gastrointest Surg. 2011;3:59–69.
92. Mell MW, et al. Outcomes after endarterectomy for chronic mesenteric ischemia. J Vasc Surg. 2008;48:1132–1138.
93. Bergman RT, et al. The role of intravenous fluorescein in the detection of colon ischemia during aortic reconstruction. Ann Vasc Surg. 1992;6:74–79.
94. Rapp JH, et al. Durability of endarterectomy and antegrade grafts in the treatment of chronic visceral ischemia. J Vasc Surg. 1986;3:799–806.
95. Jimenez JG, et al. Durability of antegrade synthetic aortomesenteric bypass for chronic mesenteric ischemia. J Vasc Surg. 2002;35:1078–1084.
96. Cunningham CG, et al. Chronic visceral ischemia. Three decades of progress. Ann Surg. 1991;214:276–287.
97. English WP, et al. Chronic visceral ischemia: symptom-free survival after open surgical repair. Vasc Endovascular Surg. 2004;38:493–503.
98. Mukherjee D, et al. Splenic artery–to–superior mesenteric artery bypass for chronic mesenteric ischemia—a case report. Vasc Endovascular Surg. 2004;38:465–468.
99. Wylie EJ, et al. Manual of vascular surgery. Springer-Verlag: New York; 1980.
100. Oderich GS, et al. Open surgical treatment for chronic mesenteric ischemia in the endovascular era: when it is necessary and what is the preferred technique? Semin Vasc Surg. 2010;23:36–46.
101. Kansal N, et al. A comparison of antegrade and retrograde mesenteric bypass. Ann Vasc Surg. 2002;16:591–596.
102. Horton JW, et al. Oxygen radicals, lipid peroxidation, and permeability changes after intestinal ischemia and reperfusion. J Appl Physiol. 1993;74:1515–1520.
103. Horton JW, et al. Free radical scavengers prevent intestinal ischemia-reperfusion–mediated cardiac dysfunction. J Surg Res. 1993;55:282–289.
104. Horton JW, et al. Cardiac contractile injury after intestinal ischemia-reperfusion. Am J Physiol. 1991;261(Pt 2):H1164–H1170.
105. Gerkin TM, et al. Intestinal ischemia-reperfusion injury causes pulmonary endothelial cell ATP depletion. Ann Surg. 1993;217:48–56.
107. McCallion K, et al. Ischemic preconditioning ameliorates ischemia- and reperfusion-induced intestinal epithelial hyperpermeability in rats. Shock. 2000;14:429–434.
108. Aksoyek S, et al. Intestinal ischemic preconditioning protects the intestine and reduces bacterial translocation. Shock. 2002;18:476–480.
109. Moore-Olufemi SD, et al. Intestinal ischemic preconditioning after ischemia/reperfusion injury in rat intestine: profiling global gene expression patterns. Dig Dis Sci. 2010;55:1866–1877.
110. Liu KX, et al. Immediate postconditioning during reperfusion attenuates intestinal injury. Intensive Care Med. 2009;35:933–942.
111. Kougias P, et al. Determinants of mortality and treatment outcome following surgical interventions for acute mesenteric ischemia. J Vasc Surg. 2007;46:467–474.
112. Kruger AJ, et al. Open surgery for atherosclerotic chronic mesenteric ischemia. J Vasc Surg. 2007;46:941–945.
113. Cho JS, et al. Long-term outcome after mesenteric artery reconstruction: a 37-year experience. J Vasc Surg. 2002;35:453–460.
114. Kang H, et al. Intravenous iloprost increases mesenteric blood flow in experimental acute nonocclusive mesenteric ischemia. Crit Care Med. 2002;30:2528–2534.
115. Wang MQ, et al. Acute symptomatic mesenteric venous thrombosis: treatment by catheter-directed thrombolysis with transjugular intrahepatic route. Abdom Imaging. 2011;36:390–398.
116. Goldberg MF, et al. Treatment of acute superior mesenteric vein thrombosis with percutaneous techniques. AJR Am J Roentgenol. 2003;181:1305–1307.
117. da Motta Leal Filho JM, et al. Infusion of recombinant human tissue plasminogen activator through the superior mesenteric artery in the treatment of acute mesenteric venous thrombosis. Ann Vasc Surg. 2011;25:840.e1–840.e4.
118. Grisham A, et al. Deciphering mesenteric venous thrombosis: imaging and treatment. Vasc Endovascular Surg. 2005;39:473–479.
119. Semiz-Oysu A, et al. Interventional radiological management of prehepatic obstruction of [corrected] the splanchnic venous system. Cardiovasc Intervent Radiol. 2007;30:688–695.
120. Dentali F, et al. Natural history of mesenteric venous thrombosis in patients treated with vitamin K antagonists: a multi-centre, retrospective cohort study. Thromb Haemost. 2009;102(3):501–504.