CHAPTER 31 Menstruation and menstrual disorders
Introduction
Menstrual abnormality is a common reason why women present at gynaecological outpatient clinics, and heavy menstrual bleeding (HMB) is one of the most common causes of iron-deficiency anaemia in Western women (Cohen and Gibor 1980). The average woman now experiences approximately 400 cycles, in contrast to the 40 or so she would have experienced 50 years ago. This is mainly due to smaller family size, availability of effective contraception, occurrence of an earlier menarche and later onset of menopause (Higham 1994). In addition to having an increased number of cycles, a significant number of women now work outside home, where disruption of work due to episodes of flooding is even less tolerable. Consequently, disorders of menstruation have become a significant public health issue.
Menstruation
At the end of the ovarian cycle, a major portion of the endometrium in primates undergoes periodic necrosis and sloughing associated with blood loss. Hence, the endometrium is a site of recurrent physiological injury and repair (Critchley et al 2001). During menstruation, the gonadal steroids reach their lowest levels. The nature of the supportive effect on the endometrium is unknown, although possible mechanisms will be discussed later.
Anatomy of the uterus
The wall of the uterus consists of three layers: the serous coat, the myometrium and the endometrium. The serous coat is firmly adherent to the myometrium, which consists of smooth muscle fibres, the main branches of the blood vessels, and the nerves of the uterus and connective tissue. The endometrium consists principally of glandular and stromal cells, although its structure does vary spatially within the uterus and temporally with the stage of the menstrual cycle. The human endometrium is a dynamic tissue that, in response to the prevailing steroid environment of sequential oestrogen and progesterone exposure, undergoes well-characterized cycles of proliferation, differentiation and tissue breakdown on a monthly basis (Jabbour et al 2006).
Blood supply of the uterus
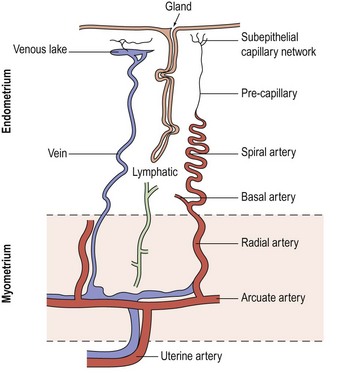
The blood supply of the uterus (Figure 31.1) is via the uterine artery, a branch of the anterior division of the internal iliac artery. It passes medially across the pelvic floor above the ureter, reaching the uterus at the supravaginal part of the cervix. Giving a branch to the cervix and vagina, the vessel turns upwards between the leaves of the broad ligament to run alongside the uterus as far as the entrance of the fallopian tube, where it anastomoses with the tubal branch of the ovarian artery. In its course, it gives off branches that penetrate the walls of the uterus. Within the myometrium, the uterine and ovarian arteries form the arcuate arteries. These, in turn, give rise to the radial arteries which, after passing through the endometrial–myometrial junction, branch into the basal arterioles, supplying the basal endometrium, and the spiral arterioles, supplying the superficial layer of the endometrium. Spiral arterioles are end arterioles and are only present in species that menstruate. Each supplies an area of 4–9 mm2. Branching of the spiral arterioles occurs throughout the superficial layer of the endometrium. Just below the surface epithelium, they break up into a prominent subepithelial plexus that drains into venous sinuses.
The endometrial vasculature is unlike any other vascular bed owing to its cyclic remodelling and regression during the menstrual cycle. These vessels are sensitive to changes in gonadal steroid levels, and at menstruation, the capillaries are shed with the glands and stroma (Rogers et al 1998). A recent study confirmed that crucial structural components in the endometrium during the menstrual process are the component blood vessels and the dynamic population of leukocytes that influx at this time (Jabbour et al 2006).
Histology of the uterus
The endometrium
In the uterus, the superficial endometrial layer is characterized by rapid proliferation during the follicular phase of the cycle, followed by secretory transformation of the glandular epithelium, predecidualization of the stromal compartment and influx of uterine natural killer cells in the luteal phase of the cycle (King et al 1998).
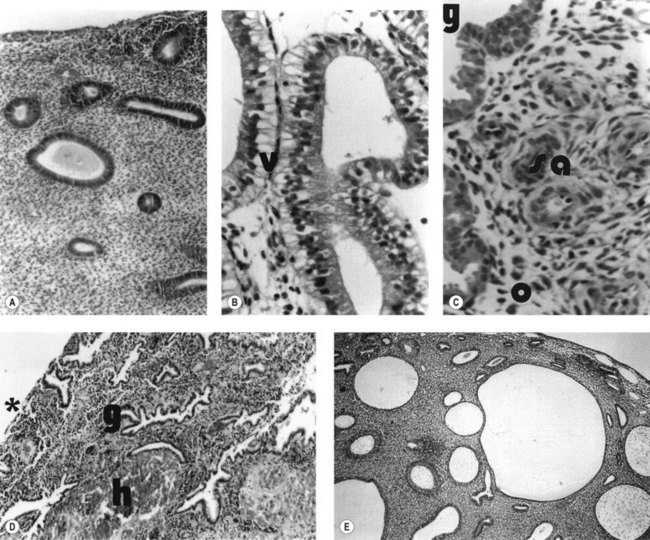
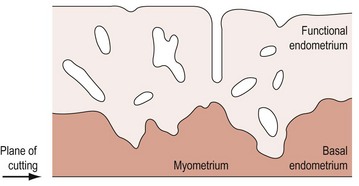
During the proliferative phase, the short, straight epithelial glands elongate and become tortuous (Figure 31.2A). Changes occur in the position of the nuclei and the number of mitoses. During the secretory phase, the glands increase in diameter and tortuosity, and vacuoles appear in the cellular cytoplasm (Figure 31.2B). The tissue also becomes markedly oedematous.
These morphological events are controlled by highly coordinated activation of certain gene sets essential for the regulation of uterine function. For instance, after ovulation, the sequential expression of progesterone-dependent genes defines a limited period of uterine receptivity or, in the absence of pregnancy, maintains vascular integrity prior to menstruation (Tabibzadeh and Babaknia 1995, Lockwood et al 1998). In addition, recent application of knowledge from the human genome, utilizing microarray technologies, has allowed several groups to contribute to a rapidly expanding literature on gene profiles during the ‘putative window’ of implantation (Carson et al 2002, Kao et al 2002, Riesewijk et al 2003).
The microvascular blood supply of the endometrium undergoes the unique process of benign angiogenesis, and is under the control of ovarian steroids during reproductive life. Following cessation of menstruation, they are simple in form, extending just into the endometrium. The secretory phase is characterized by the growth of arterioles. In the late secretory phase, coiling occurs due to proliferation and extension of the arterioles (Figure 31.2C). With the fall in steroid concentrations, menstrual shedding of the functional layer of the endometrium occurs (Figure 31.2D). Dramatic changes occur in the spiral arterioles at menstruation. These changes were described by Markee (1948) after experiments involving the transplantation of endometrium into the anterior chamber of the eye of the Rhesus monkey. This work was the cornerstone of current concepts of menstruation. On observing the bleeding process, Markee suggested that the arteriolar coiling caused constriction of the vessel lumen with vascular stasis and leukocytic infiltration. Approximately 24 h premenstrually, intense vasoconstriction led to ischaemic damage, followed by vasodilatation with haemorrhage from both arterial and venous vessels: 75% of the loss was arteriolar, 15% was venous and 10% was diapedesis of erythrocytes (Markee 1940).
The myometrium
The human myometrium is structurally and functionally polarized during the reproductive years. Compared with the outer myometrium, the subendometrial layer or junctional zone (JZ) is characterized by higher cell density, lower cellular nuclear:cytoplasmic ratios and the expression of different extracellular matrix (ECM) components (Brosens et al 1998, Campbell et al 1998). Additionally, evidence from 31P nuclear magnetic resonance spectroscopic studies has demonstrated biochemical heterogeneity between both myometrial layers (Xu et al 1997).
The differentiation of the myometrium into two distinct layers is strictly under ovarian hormonal control. In premenarchal girls and postmenopausal women, the zonal anatomy is often indistinct. Ovarian suppression with gonadotrophin-releasing hormone (GnRH) analogues leads to a magnetic resonance imaging (MRI) appearance of the uterus mimicking that of postmenopausal women, whilst hormone replacement therapy in postmenopausal women results in the reappearance of myometrial zonal anatomy (Demas et al 1985).
Further evidence for hormone responsiveness of the JZ is provided by the work of Wiczyk et al (1988), who demonstrated changes in JZ thickness throughout the menstrual cycle in conjunction with endometrial thickness.
Mechanisms of Normal Menstruation and Control of Blood Loss
Withdrawal of progesterone from an oestrogen-primed endometrium results in menstrual shedding. Shedding arises because of the induction of matrix metalloproteinases (MMPs) from the endometrium, in particular MMPs 1, 2 and 9 (Jeziorska et al 1996, Marbaix et al 1996). This arises through a mechanism of upregulation of transforming growth factor-beta (TGF-β) (Bruner et al 1995). The blood vessels are thus denuded of their support. Spiral arterioles and venules are cleaved at the level of the JZ between the endometrial functionalis and basalis, with subsequent bleeding.
Factors involved in the control of menstrual blood loss are:
Derangement of any of these mechanisms is likely to lead to excessive menstrual loss.
Haemostasis
Coagulation occurs in the distal endometrium, and platelets found within the endometrial cavity are deactivated and do not respond to collagen, as they would elsewhere. Once clinical bleeding and tissue shedding have started, haemostatic plug formation occurs, but less rapidly and less completely than is observed in human skin wounds (Christiaens et al 1982). Certain haemorrhagic conditions (e.g. thrombocytopenic purpura) are associated with increased incidence of HMB, suggesting that abnormalities of platelet structure may be important.
The coagulation cascade operates in the uterus and endometrium as in other tissues. Platelet accumulation, platelet degranulation and fibrin deposition occur within hours of the onset of menstruation, sealing endometrial vessels. The platelet count in menstrual discharge is only one-tenth of that seen in peripheral blood. This is probably due to the consumption of platelet aggregates in endometrial blood vessels early in the haemostatic processes of menstruation (de Merre et al 1967). Additionally, these platelets have been found to be devoid of granules, and they fail to aggregate when challenged with aggregating stimuli such as adenosine diphosphate and collagen. Compared with peripheral blood platelets, those in menstrual fluid do not produce appreciable cyclo-oxygenase products from arachidonic acid (Christiaens et al 1981, Sheppard et al 1983, Rees et al 1984).
Since prevention of clot formation is necessary to deter scarring and obliteration of the endometrial cavity, there appears to be an active fibrinolytic system in the endometrium mediated by plasmin through proteolytic cleavage of plasminogen by the activators urokinase-type plasminogen activator (uPA) and tissue-type plasminogen activator (tPA). These activators are regulated by plasminogen activator inhibitor 1 (PAI-1) and PAI-2, both of which are expressed in the endometrium. The presence of these fibrinolytic agents suggests that coagulation occurs but is rapidly reversed. Oestradiol stimulates uPA, whilst progesterone inhibits uPA (Casslen et al 1986). Furthermore, progesterone inhibits production of tPA, and this effect is amplified by production of PAI. Blood coagulation products have also been found to be severely depleted in menstrual fluid, suggesting consumption during menstruation (Hahn 1980).
Fibrin formation occurs during the process of menstruation (Beller 1971, Sheppard et al 1983). The high levels of fibrin degradation products in menstrual fluid are not solely due to direct digestion. Although menstrual blood contains high levels of fibrin and fibrin degradation products, it contains no fibrinogen. Similar levels of protein C1 inactivator, α2-macroglobulin and α2-antitrypsin have been found in peripheral and menstrual blood (Daly et al 1990). However, compared with peripheral plasma, lower levels of activated prothrombin, antithrombin, antiplasmin, plasminogen, protein C and factors V, VII, VIII and X have been reported in menstrual fluid (Rees et al 1985, Daly et al 1990).
Compared with peripheral plasma, menstrual blood has a marked increase in the level of tPA antigen with reduced levels of PAI. Levels of fibrinolytic enzymes in menstrual fluid vary according to cycle stage. Throughout the cycle, higher levels of tPA are found in the myometrium compared with the endometrium in the normal menstrual cycle (Sheppard 1992), whilst during the late secretory phase, several studies have demonstrated a significant increase in endometrial tPA levels.
The above mechanisms ensure that progesterone falls and oestradiol rises during menstruation. There is increased fibrinolytic activity in the endometrium of women with increased menstrual blood loss. The endometrium also generates factors that inhibit platelet aggregation and platelet adhesion. Such factors include prostacyclin, nitric oxide and platelet-activating factor (PAF) (Kelly et al 1984, Alecozay et al 1991).
Vasoconstriction
In the menstruating uterus, haemostatic events are strikingly different from the rest of the body (Christiaens et al 1980). At the onset of menstruation, damaged blood vessels are sealed by intravascular thrombi of platelets and fibrin. However, as menstruation progresses, the functional endometrium is shed and so these haemostatic thrombi are lost. Subsequently, during the first 20 h of menstruation, intense vasoconstriction of the spiral arterioles occurs; this assures haemostasis until regeneration of the endometrial surface is complete.
The role of prostaglandin F2α (PGF2α) in vasoconstriction is well established. The effects of the vasoconstricting PGF2α are balanced by those of the vasodilating prostaglandin E2 (PGE2). Concentrations of both these prostaglandins are increased in the luteal phase. Overproduction of the vasodilatory prostaglandins or reduced production of the vasoconstrictors is likely to lead to excessive blood loss at the time of menstruation. This is confirmed by work showing an elevated PGE2/PGF2α ratio, increased endometrial PGE2 or increased PGE2 receptor concentrations in women with HMB (Smith et al 1981, Adalantado et al 1988). Steroid hormones influence endometrial prostaglandin synthesis, and the highest levels of the latter are found during menstruation. This is particularly true for PGF2α, the synthesis of which rises significantly during the secretory phase of the menstrual cycle under the influence of progesterone. It is also likely that the upregulation of the cyclo-oxygenase pathway impacts on angiogenesis in the endometrium.
Other vasoconstrictors, such as endothelins and PAF, may also contribute. Endothelins are powerful vasoconstrictors, and are found in human endometrium (Cameron et al 1992). They are thought to be involved in paracrine regulation of a number of endometrial functions, such as the induction of vascular and myometrial smooth muscle contractions, fibroblast mitogenesis and the release of other paracrine agents (Ohbuchi et al 1995). Endothelins can also induce PGF2α. It has been proposed that as endometrial glandular epithelium breaks down during menstruation, stored endothelin gains access to the spiral arterioles, causing long-lasting vasoconstriction (Cameron and Davenport 1992).
PAF is present in the endometrium in the luteal phase and has an ambiguous effect on spiral arteriolar tone. PAF itself is a vasoconstrictor but it stimulates production of the vasodilator PGE2 (Björk and Smedegård 1983, Smith and Kelly 1988).
Endometrial repair
Vasculature
Seventy percent of menstrual loss arises from the spiral arterioles, and most of this bleeding occurs within the first 3 days of menses (Haynes et al 1977). Concordantly, vessel repair begins within the first 2–3 days of the onset of bleeding. As might be expected, the endometrium is a rich source of angiogenic growth factors (Smith 1998). The fibroblast growth factor (FGF) and the vascular endothelial growth factor (VEGF) families are the best known.
Vascular endothelial growth factor
VEGF is a heterodimeric angiogenic growth factor which is expressed and secreted by a variety of endometrial cells, including macrophages and large decidualized cells. They cluster around the spiral arterioles during the late luteal phase (Gospodarowicz et al 1989, Charnock-Jones et al 1993). The variants of VEGF differ in their cellular localization.
Steroids and hypoxia regulate VEGF, with steroid regulation being around two orders of magnitude lower than that of hypoxia. VEGF mRNA is abundant in the endometrium at the time of menstruation (Charnock-Jones et al 1993). This upregulation probably follows the hypoxia induced by spiral arteriole vasoconstriction (Sharkey et al 2000). Its expression is followed by rapid angiogenesis when the functional endometrium is lost.
Vascular smooth muscle cells (VSMCs) in endometrium also express certain VEGF receptors. This provides a direct link between the development of spiral arterioles and VEGF-A expression. The finding of reduced proliferation of VSMCs in patients with HMB could be explained by altered VEGF-A expression. A novel angiogenic factor, endocrine gland VFGF, and its receptors have recently been reported to be expressed in the uterus (Battersby et al 2004).
Fibroblast growth factor
The FGF family consists of at least eight members, some of which are expressed in the endometrium and are hormone dependent with expression during the proliferative phase and reduction during the secretory phase (Basilico and Moscatelli 1992, Ferriani et al 1993). FGF synergizes with VEGFs in inducing angiogenesis (Pepper et al 1992). Inhibition of FGF action does not fully inhibit angiogenesis, but this is possible when anti-VEGF agents are used. VEGFs promote the release of FGF from the ECM. FGF and platelet-derived growth factor are also known to stimulate angiogenesis, and have been demonstrated in the endometrium of a number of a species (Weston and Rogers 2000).
Angiopoietins
The angiopoietins are another family of molecules which influence vascular development and maintenance by stabilizing the endothelial cell and vascular smooth muscle structure (Suri et al 1998). Recently, expression patterns of the angiopoetin family in the human endometrium have been examined, although results have been conflicting, perhaps reflecting relatively low expression of these factors (Rogers and Abberton 2003). It is possible that this will alter the function of the VSMCs that are essential for controlling the blood loss at menstruation, and that HMB is due to aberrant build-up of VSMCs. Angiopoietin expression and signalling are regulated by the cyclo-oxygenase enzymes.
Epithelium
Once the functional layer of the endometrium is shed, the regeneration of all cell types (epithelial, endothelial and stromal) occurs rapidly. This regeneration occurs from the cells of the remaining basal layer, which acts as the germinal compartment of the functional endometrium (Chan et al 2004).
Given its dynamic nature, growth factors and cytokines are especially important in the development of the endometrium. In addition, tissue remodelling is dependent on the integrity of the ECM. Profound hormone-dependent tissue remodelling is seen in the endometrium, although currently there is no direct evidence linking disorders of tissue regeneration with HMB. Proliferation of the endometrium is most active during menstruation. The proliferation starts on day 2 in the basal glands and is complete by day 5, leaving a completely re-epithelialized endometrial surface. Various agents regulate this process, the best-known being epidermal growth factor (EGF). EGF is expressed in human epithelial cells throughout the cycle and stimulates endometrial epithelial proliferation (Haining et al 1991, Zhang et al 1995). Oestradiol induces the expression of EGF and its receptor. However, during menstruation, when the concentration of oestradiol is at its lowest, there are sufficient amounts of residual EGF remaining in the epithelial cells as growth continues before oestradiol levels increase during the proliferative phase. Additionally, EGF has been shown to mimic the proliferative effect of oestradiol in the endometrium of transgenic mice lacking the oestradiol receptor (Ignar Trowbridge et al 1993). Numerous other growth factors (insulin-like growth factor, FGF) and their binding proteins are expressed in the endometrium, and all promote cell proliferation. The complexities of tissue remodelling suggest that altered wound healing may be a factor in the aetiology of HMB, and its role warrants further investigation.
Matrix
Integrins are the agents which link the ECM with epithelial cells of the endometrium (Hynes 1987, Lessey et al 1992). The MMPs are a highly regulated family of calcium and zinc endopeptidases, which are able to degrade most components of the ECM in the activated state. These enzymes are active in normal and pathological processes involving tissue remodelling. MMP activity is greatest during days 1–4 of the cycle. Progesterone withdrawal and migratory leukocytes activate some MMPs, and this may be a significant step in the initiation of menstruation. Local release of agents such as MMP-1 and MMP-3 from stromal cells may activate other proteases released from the invading neutrophils (Schatz et al 1999). This is supported by the presence of substantially more latent MMP-9 than active MMP-9 before menstruation (Rigot et al 2001). Abnormalities in the dynamic turnover of collagen production are likely to be important in menstrual upset given their importance in other vascular and fibrotic processes.
Normal Menstrual Cycle
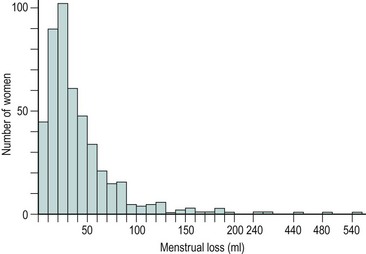
The majority of cycles lie between 24 and 32 days, and a normal cycle is considered to last for 28 days. Menstrual cycle length varies during reproductive life, being most regular between the ages of 20 and 40 years. It tends to be longer after the menarche and shorter as the menopause approaches. The mean menstrual blood loss per menstruation in a healthy Western European population ranges between 37 and 43 ml; 70% of the loss occurs in the first 48 h. Despite large interpatient variability, loss between consecutive menses in the same woman does not vary largely (Figure 31.3). Only 9–14% of women lose more than 80 ml per menses, and 60% of these women are actually anaemic. The upper limit of normal menstruation is thus taken as 80 ml per menses (Rybo 1966). However, total fluid loss (mucus, tissue, etc.) may be considerably more than the blood loss alone and amounts vary. These parameters have been reviewed recently (Fraser and Inceboz 2000).
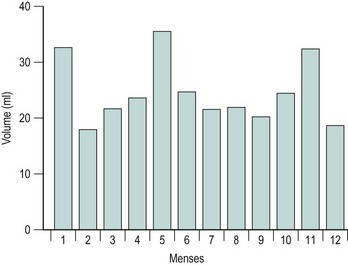
Figure 31.3 The variability in menstrual blood loss in a single individual: values for 12 consecutive periods.
From Hallberg L, Nilsson L 1964 Consistency of individual menstrual blood loss. Acta Obstetrica et Gynecologica Scandinavica 43:352–359, Blackwell Science.
The pattern of spontaneous myometrial contractions varies during the menstrual cycle (Lumsden et al 1983). Time-lapse ultrasound studies have shown that propagated myometrial contractions in the non-pregnant uterus emanate from the JZ. The frequency and direction of these contractions depend on the phase of the cycle. In the follicular and periovulatory phases, cervicofundal subendometrial contractions can be seen, the amplitude and frequency of which increase notably towards ovulation. Short, asymmetrical myometrial waves are present during the luteal phase, but propagated fundocervical subendometrial contraction waves are noted during menstruation (de Vries et al 1990, Chalubinski et al 1993).
Kunz et al (1996) demonstrated the role of preovulatory cervicofundal contractions in assisting the rapid transport of sperm through the female reproductive tract. Others have postulated that the asymmetrical myometrial peristalsis during the luteal phase serves to maintain the developing blastocyst within the uterine fundus. The role of fundocervical contractions during menstruation is likely to be important in controlling menstrual flow and limiting retrograde menstruation (Chalubinski et al 1993).
Abnormal Menstruation
Heavy menstrual bleeding (HMB)
The causes of HMB fall into four categories:
Dysfunctional uterine bleeding
Anovulatory dysfunctional uterine bleeding
Occasionally, anovulatory cycles occur in all women. Chronic anovulation, however, is associated with an irregular and unpredictable pattern of bleeding ranging from short cycles with scanty bleeding to prolonged periods of irregular heavy loss. Normal bleeding occurs in response to withdrawal of both progesterone and oestradiol. If ovulation does not occur, the absence of progesterone results in an absence of secretory changes in the endometrium, accompanied by abnormalities in the production of steroid receptors, prostaglandins and other locally active endometrial products. Unopposed oestrogen gives rise to persistent proliferative or hyperplastic endometrium, and oestrogen withdrawal bleeding is characteristically painless and irregular. It tends to occur at the extremes of reproductive life but is rare at other times. Only 20% of those cycles with excessive menstrual blood loss are anovulatory (Haynes et al 1979); this same study failed to demonstrate any abnormalities in gonadotrophin or circulating steroid concentrations. In anovulatory cycles, the endometrium is unable to produce factors whose synthesis is controlled by progesterone, such as PGF2α (Smith et al 1982). This may account for the painless nature of the bleeds. Ovulation occurs in response to the mid-cycle surge of luteinizing hormone (LH). If this fails to occur due to insufficient oestradiol secretion or impaired positive feedback, ovulation will not occur.
Failure of follicular development
Follicular development which is insufficient to produce an oestrogen signal strong enough to induce an LH surge is one of the common reasons for the irregularities in menstrual cycle pattern in premenopausal women. This occurs perimenopausally and in PCOS (see Chapter 18 for information regarding the aetiology and treatment of PCOS).
Anovulatory bleeding may be associated with cystic glandular hyperplasia of the endometrium (see Figure 31.2E). This occurs in some older women and also in peripubertal girls where unopposed oestrogen secretion occurs. The first few cycles after the menarche are commonly anovulatory. However, if anovulation persists, a long period of amenorrhoea is accompanied by endometrial hyperplasia. This is probably a result of multiple follicular development (multicystic ovaries) with failure of antral follicle formation. Endometrial hyperplasia may cause excessive bleeding, anaemia, infertility and even cancer of the endometrium.
Ovulatory dysfunctional uterine bleeding: idiopathic bleeding
As described above, an important factor in the control of menstrual blood loss is vasoconstriction. It appears that there are a number of endometrial products which alter the degree of vasoconstriction, and thus may affect the volume of menstrual blood lost. In the mid-1970s, a relationship between prostaglandin production and HMB was suggested by work showing that total endometrial prostaglandin content was proportional to menstrual loss. It appears that a shift in endometrial conversion of endoperoxide from the vasoconstrictor prostaglandins (PGF2α) to the vasodilator prostaglandins (PGE2 or prostacyclin) occurs. However, it is likely that it is not only prostaglandins that are of importance, and work is now being performed on the role of endothelin in heavy menstrual loss. Endothelins are very potent vasoconstrictors that are produced within the endometrial vessels; their receptors are also present, although it is not yet clear whether either of these two factors differ in women with heavy menstrual loss. Although studies to date are limited, Marsh (1996) showed reduced immunostaining for endothelin in the endometrium of women with HMB, implicating this peptide in the pathophysiology of increased menstrual blood loss. This is a rapidly changing area of knowledge, and it is likely that other elements will come to light which will be significant. According to Jabour et al (2006): ‘Further challenge in the future will be the development of experimental strategies that will allow us to assess the exact role of the various factors deduced from gene mining studies in menstrual function/dysfunction and which of these gene pathways constitute a sensible target for novel therapeutic application in the clinic.’
It is still uncertain why there is a difference in production of local factors in those with heavy menstrual loss compared with those with normal loss. Interest has centered on the role of steroid hormones, but it has been impossible to demonstrate either a difference in the circulating levels of oestradiol and progesterone or in the receptor concentration within the endometrium (Critchley et al 1994). It is possible that there is a genetic difference altering the production of local hormones and growth factors, or that there is a multifactorial aetiology. Reference has been made above, in discussion of the control of menstrual blood loss, to areas of research which may throw new light on the mechanism of HMB.
Coagulation disorders
Although certain haemorrhagic conditions, e.g. thrombocytopenic purpura and von Willebrand’s disease, are associated with an increased incidence of HMB, coagulation disorders have a variable effect overall. There is no impairment of systemic coagulation in those with excess menstrual loss, nor are fibrin degradation products elevated in the menstrual fluid of those with heavy menstrual loss (Bonnar et al 1983). In women with thrombocytopenia, menstrual blood loss correlates broadly with platelet count at the time of the menses. Splenectomy has been known to reduce menstrual blood loss dramatically in these patients.
Women with factor VII deficiency exhibit a spectrum of bleeding symptoms, with HMB being one of the most common (Kulkarni et al 2006). There is no need to test for these routinely unless HMB has been present from adolescence or the woman encountered bleeding problems with dental extraction.
Dysmenorrhoea
Idiopathic (primary) dysmenorrhoea
Uterine hyperactivity
The significance of uterine hyperactivity in women with dysmenorrhoea was first proposed in 1932. Since then, there has been much research which suggests that women with dysmenorrhoea have increased uterine activity during menstruation (Novak and Reynolds 1932, Filler and Hall 1970). Patients often describe the pain as ‘labour-like’, and an increase in uterine contractility can be demonstrated by measuring intrauterine pressure in those with dysmenorrhoea compared with women without dysmenorrhoea (Lumsden and Baird 1985). The increased uterine contractility also appears to be related to uterine blood flow and the presence of pain (Åkerlund and Anderssen 1976).
During the reproductive years, the myometrium is structurally and functionally polarized (see above). Using transvaginal ultrasound scanning or MRI, it is possible to delineate between the myometrial zones (Brosens et al 1998, Lesny et al 1999).
Patients with endometriosis and adenomyosis have been found to have structural and functional abnormalities of the JZ (Brosens et al 2000). Leyendecker et al (1996) demonstrated marked hyperperistalsis of the JZ during the early- and mid-follicular phase in women with endometriosis, associated with a marked increase in the transport of inert particles from the vaginal depot to the peritoneal cavity.
Dysmenorrhoeic patients have been found to exhibit profound structural changes in the JZ, including irregular thickening, and smooth muscle hyperplasia characterized by closely packed smooth muscle fibres which are poorly oriented and less vascular than the smooth muscle of normal inner myometrium (Togashi et al 1989, Brosens et al 1998). Consequently, the term ‘junctional zone hyperplasia’ was coined for this disorder (Brosens et al 1998).
Dysperistalsis and hyperactivity of the uterine JZ are important mechanisms of primary dysmenorrhoea, and possibly menstrual pain associated with adenomyosis and endometriosis (Brosens et al 2000).
Endothelins
Endothelins are potent uterotonins in the non-pregnant uterus. They are thought to be involved in the induction of myometrial smooth muscle contraction in a juxtacrine fashion. The greatest density of endothelin-binding sites is found on glandular epithelium in the endomyometrial junction, and recent evidence suggests that endothelins in the endometrium can induce PGF2α and further endothelin release in a paracrine and autocrine fashion (Bacon et al 1995). Local ischaemia could further increase the expression of endothelins and prostaglandins, which could further aggravate uterine dysperistalsis.
Prostaglandins
It has been shown that dysmenorrhoeic women have increased endometrial synthesis of PGF2α and enhanced concentrations of PGF2α and PGE2 in menstrual blood compared with asymptomatic women (Lundstrom and Green 1978, Lumsden et al 1983). PGF2α is a potent oxytocic and vasoconstrictor. When administered into the uterus, it will give rise to dysmenorrhoea-like pain and occasionally menstrual bleeding. Menstrual fluid PGF2α concentrations also correlate with uterine work during the menses in women with dysmenorrhoea (Lumsden et al 1983). These properties of PGF2α could thus lead to ‘angina’ of the myometrium. The role of PGE2 is less clear, although its administration may increase the sensitivity of nerve endings.
Vasopressin
The treatment of HMB with desmopressin is efficacious and safe if patients are instructed to self-administer the agent only during the first 2 or 3 days of heavy menstrual blood loss, for a maximum of three to four doses and with no more than two consecutive administrations during a 12-h interval (Rodeghiero 2008).
There are other stimulants of the non-pregnant uterus, such as vasopressin, a vasoconstrictor that also stimulates uterine contractility. On the first day of menstruation, circulating vasopressin levels are higher in those with dysmenorrhea than in those without dysmenorrhea (Åkerlund et al 1979). Infusion of hypertonic saline results in increased uterine contractility and pain in women with dysmenorrhoea as a result of stimulation of endogenous vasopressin release, as well as a reduction in concentrations of PGF2α metabolites. Preliminary studies also indicate that vasopressin analogues may have a place in the treatment of dysmenorrhoea.
Secondary dysmenorrhoea
Those women presenting with dysmenorrhoea who have no other complaints or abnormalities on examination can be safely treated without further investigation. Pierzynski et al (2006) have shown that tamoxifen, a selective oestrogen-receptor modulator, directly inhibits uterine contractions, causing improvement in uterine blood flow and a decrease in menstrual pain and cramps. This could be considered for application in selected groups of dysmenorrheic women; for instance, carriers of breast-cancer-associated antigen genes, breast cancer survivors or women with advanced endometriosis (Pierzynski et al 2006).
Letzel et al (2006) reported that aceclofenac (100 mg) and naproxen (500 mg) effectively reduced the pain associated with primary dysmenorrhoea, and both were more effective than placebo at easing menstrual pain assessed by various pain relief criteria. Laparoscopy is indicated for those with a provisional diagnosis of endometriosis or pelvic inflammatory disease. Hysteroscopy, endometrial sampling and examination under anaesthetic are only required if uterine abnormality is suspected. The standard treatment for dysmenorrhoea (prostaglandin synthetase inhibitors and the oral contraceptive pill) is so effective that laparoscopy in treatment failures will often demonstrate previously unsuspected abnormalities such as mild endometriosis, even in teenage girls.
Epidemiology of Menstrual Abnormality
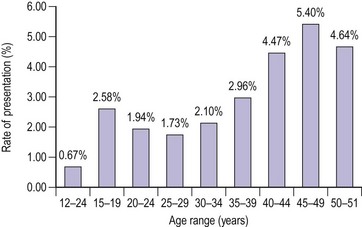
The distribution of blood loss for a normal population shows a positively skewed distribution, as mentioned above (Figure 31.4) (Cole et al 1971). Age per se does not influence menstrual blood loss until the sixth decade (Figure 31.5, Table 31.1). This may be due to an increased incidence of pathology (e.g. fibroids or perimenopausal endocrine abnormalities). An hereditary influence has been demonstrated following twin studies, and parity is thought to be an important factor; parous women have greater menstrual blood loss than nulliparous women. Uterine pathology, particularly fibroids, is a well-documented cause for HMB, although endometrial pathology is rather uncommon in these women; it is found as a reasonable cause for HMB in approximately 6% of cases.
| Recommendations with significant resource impact | Annual cost (£millions) |
|---|---|
| Full blood count investigations | 0.9 |
| Transvaginal ultrasound investigations | 10.4 |
| Endometrial biopsy for suspected endometrial cancer | −0.9 |
| Pharmaceutical treatments levonorgestrel-releasing intrauterine system | −0.6 |
| Substitution of hysterectomy with endometrial ablation | −1.2 |
| Total net cost of implementing the HMB guideline | 8.7 |
Source: National Institute for Health and Clinical Excellence 2007 NICE Clinical Guideline No. 44. HMB, heavy menstrual bleeding.
The variation between menses for an individual (intramenses) is between 20% and 40%. Approximately 90% of blood is lost during the first 3 days of the menses in women with normal blood loss and women with HMB. These studies are based on objective measurement of menstrual blood loss, which is rarely done except for research purposes (Hallberg and Nilsson 1964). New methods of measuring menstrual symptoms are being designed in order to allow for better objective assessment, with consideration given to the variety of symptoms that contribute to the overall complaint such as irregularity, HMB, pain and premenstrual syndrome.
The results of epidemiological studies performed over the last 50 years give a variable incidence for dysmenorrhoea. This is because pain is a subjective symptom and cannot be assessed accurately by an outsider. Different women will react to the same pain in different ways. How each woman perceives the pain will vary according to her individual circumstances. In addition, the definition and diagnosis allow different interpretations by different workers. Severe dysmenorrhoea, which causes disruption of daily routine with time off work or study, occurs in 3–10% of 19-year-olds (Andersch and Milsom 1982), while mild discomfort occurs in the majority of women. The relative risk of dysmenorrhoea in those who have smoked for 10–20 years is up to six-fold higher than the risk in non-smokers (Parazzini et al 1994). The incidence of dysmenorrhoea is inversely correlated with age, and parous women are less likely to report the condition. There is no correlation between the incidence of emotional stress factors and dysmenorrhoea.
Presentation
Clinical history
Risk factors for endometrial carcinoma should be noted carefully in the history (Box 31.1, Figure 31.6).
Examination
General examination may reveal signs of hypothyroidism, anaemia or blood clotting disorders. Abdominal and pelvic examination is only mandatory in women complaining of HMB if structural abnormality is expected (National Institute for Health and Clinical Excellence 2007). Clues to diagnosis may be revealed on pelvic examination. A tender fixed uterus may suggest endometriosis or pelvic inflammatory disease, whilst fibroids can often be palpated. A symmetrically enlarged uterus is more typical of adenomyosis or endometrial carcinoma. Atrophic and inflammatory vulvar and vaginal lesions can be visualized, and cervical polyps and invasive lesions of cervical carcinoma that may present with irregular bleeding can be seen. Anaemia may also be present since HMB is the most common cause of iron-deficiency anaemia in the Western world. HMB may arise because of disorders of the haemopoietic system, as described above.
Investigation
Haemoglobin concentration should be determined in all women with HMB, and iron supplementation should be given if required. Although thyroid disease is a cause of menstrual derangement in some women, screening thyroid-stimulating hormone and thyroxine measurements are not justified unless there are other features in the history suggestive of thyroid disease (Fraser et al 1986, National Institute for Health and Clinical Excellence 2007). Similarly, routine screening for bleeding diatheses is not appropriate.
Assessment of the uterine cavity
A wide array of methods are available for endometrial assessment including:
Endometrial sampling
The risk of endometrial carcinoma in women with perimenopausal HMB is approximately 1%; hence, all women with irregular bleeding that is unresponsive to treatment need endometrial assessment. Although women under 40 years of age have a very low risk of developing endometrial carcinoma, further investigation may be needed in high-risk symptomatic women (Royal College of Obstetricians and Gynaecologists 1994).
Hysteroscopy
Hysteroscopy facilitates intrauterine surgical procedures such as endometrial ablation. Hence, it has long been accepted into UK gynaecological practice for diagnostic and therapeutic purposes. Hysteroscopy and directed biopsy appear to be superior in identifying benign endometrial pathology (Gimpleson and Rappold 1988, Loffer 1989). More pertinently, in a significant minority of women with endometrial carcinoma, the diagnosis is missed when conventional curettage is used (Stovall et al 1989), and it appears that hysteroscopy and directed biopsy is more sensitive in the diagnosis of this condition. Ultrasound is better at identifying fibroids unless it is intracavity, and hysteroscopy should only be used when ultrasound is unsatisfactory.
The development of a narrow diameter (4 mm) scope allows this investigation to be performed in the outpatient setting in a carefully selected patient group following counselling (Taylor and Hamou 1983). Patient selection is important to maximize the success of the procedure; for example, women with a previous cervical cone biopsy or Manchester repair may have cervical stenosis, making passage of the hysteroscope through the internal os difficult.
These studies provide increasing evidence that hysteroscopy and endometrial sampling in combination is a superior diagnostic tool. This has therefore replaced D&C as the investigation of choice in menstrual disorders (Lewis 1990, 1994).
Quantification of Menstrual Blood Loss
The objective assessment of menstrual blood loss is rarely made, except for research purposes. However, menstrual blood loss can be assessed either in a semiquantitative manner, relying on the patient’s assessment of the bleeding, or by the use of pictorial aids (Higham et al 1990). The pictorial method has been shown to correlate well with the gold standard alkaline haematin method (Higham et al 1990, Wyatt et al 2001). However, this correlation has also been challenged (Reid et al 2000). Attention is being given to the development of aids, including the use of hand-held computers, to assess the menstrual complaint as a whole, which consists of more than heavy bleeding. Many women also complain of pain and premenstrual syndrome. Again, these are not yet available for general use.
Treatment
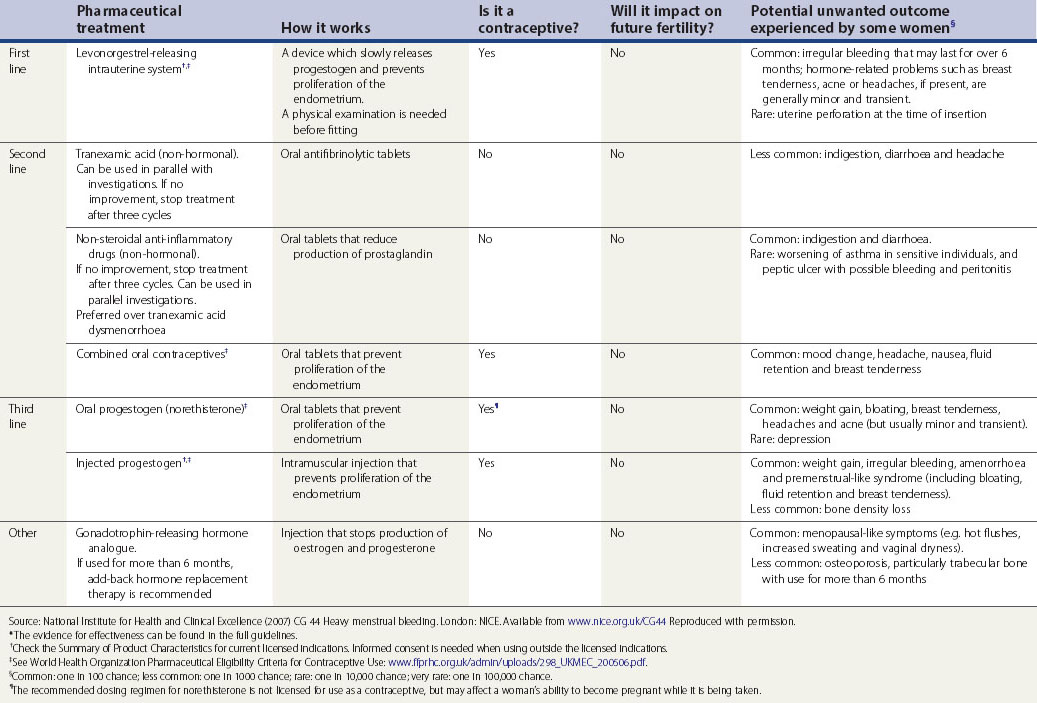
Medical treatment
Current medical therapy falls into two broad groups: non-hormonal (prostaglandin synthetase inhibitors, antifibrinolytics and ethamsylate) and hormonal (progestogens, oral contraceptives, hormone replacement therapy, danazol, gestrinone, GnRH analogues) (see Table 31.2). Non-hormonal treatment may be taken during menstruation, which avoids teratogenicity and is therefore suitable for women who wish to conceive. Some women, once reassured that there is no major pathology, will require no further treatment.
Table 31.2 Pharmaceutical treatments proven to reduce menstrual bleeding*
Discuss hormonal and non-hormonal options, and provide time and support to help the woman to decide which option is best for her.
Non-hormonal treatment
Prostaglandin synthetase inhibitors
NSAIDs remain a popular choice for the treatment of HMB (Coulter et al 1995). Their principal mechanism of action is to decrease endometrial prostaglandin concentrations. The endometrium is a rich source of PGE2 and PGF2α, and a number of studies have shown that prostaglandin concentrations are greater in the endometrium of women with HMB than they are in the endometrium of women with normal blood loss (Cameron and Norman 1995).
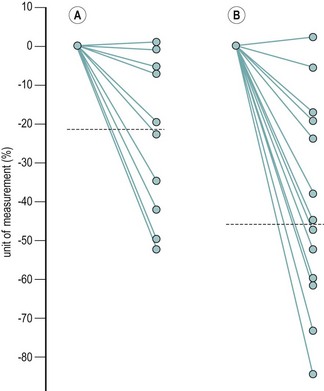
There are four groups of prostaglandin synthetase inhibitors, of which the fenamates are the most widely used. These are unique amongst the prostaglandin synthetase inhibitors in that, in addition to inhibition of prostaglandin synthesis, they bind and block the prostaglandin receptor (Rees et al 1988). Menstrual blood loss is reduced by a median of 25–40% in three-quarters of women with HMB (Figure 31.7). The beneficial effect of mefenamic acid on menstrual blood loss (and other symptoms including dysmenorrhoea, headache, nausea, diarrhoea and depression) can be long term, but if it is not effective within 3 months, treatment should be stopped. Side-effects, mainly gastrointestinal (dyspepsia, nausea and diarrhoea), are mild and not frequently reported.
Antifibrinolytics
As discussed above, the endometrium possesses an active fibrinolytic system which is more active in the endometrium of women with HMB than in those without HMB. Antifibrinolytic agents such as tranexamic acid reduce menstrual loss by approximately 50%. This is greater than the reduction following administration of prostaglandin synthetase inhibitors (Ylikorkala and Viinikka 1983, Milsom et al 1991).
The incidence of adverse effects is dose dependent. One-third of women experience gastrointestinal side-effects following treatment with tranexamic acid 3–6 g/day. As 90% of menstrual blood is lost in the first 3 days of full flow, dose-related side-effects can be reduced by limiting the number of days on which the drug is taken to the first 3 or 4 days of the menses. Serious side-effects are uncommon. No increase in the incidence of thromboembolic disease has been seen in women of reproductive age in Scandinavia, where tranexamic acid has been used since the early 1970s as a first-line treatment for HMB (Rybo 1991). Antifibrinolytic agents therefore represent a relatively effective first-line treatment to reduce the degree of menstrual bleeding.
Hormonal treatment
Hormonal treatments for HMB are summarized in Box 31.2.
Progestogens
Cyclical progestogens
It has been shown that low-dose, short-duration therapy of oral progestogens during the luteal phase is ineffective for the reduction of menstrual loss (Figure 31.8) (Cameron et al 1990). However, they may be successful if given at a dose of 5 mg three times daily from days 5 to 26 of the cycle. On this regime, menstrual loss can be reduced by up to 30% (Irvine et al 1998). Cyclical progestogens can be used to regulate the onset of bleeding and are useful for those with an irregular, unpredictable cycle.
Intrauterine progestogens
The most recently described medicated device is the levonorgestrel intrauterine system (LNG-IUS; Mirena). This delivers LNG 20 µg to the endometrium every 24 h in a sustained-release formulation that can last for up to 5 years (Luukkainen et al 1986). Direct administration of the progestogen to the uterus results in minimal systemic absorption (Andersson and Rybo 1990), giving a better side-effect profile (Cheng Chi 1991).
The efficacy of the LNG-IUS for the treatment of HMB compares well with transcervical resection of the endometrium (TCRE) at 12 months post treatment (Crosignani et al 1997, Rauramo et al 2004). It has also been suggested that it might be an acceptable alternative to hysterectomy (Lahteenmaki et al 1998). Moreover, it also appears to be cost-effective when compared with thermal balloon ablation for treatment of HMB (Brown et al 2006).
The LNG-IUS has a local effect on the endometrium causing endometrial glandular atrophy, stromal decidualization and epithelial cell inactivation. This is seen within 1 month of insertion and occurs independently of ovarian activity (Silverberg et al 1986). When removed, the endometrium recovers quickly and biopsies taken at 1–3 months show no sign of progesterone administration, regardless of duration of treatment. The LNG-IUS has a weak effect on ovarian function, with ovulation continuing in approximately 75% of women. The most significant side-effect is irregular bleeding that may present for more than 6 months; women require careful counselling prior to insertion if this is not to lead to removal of the device.
Oestrogen/progestogen regimes
The combined oral contraceptive pill is widely used to treat HMB. It causes endometrial atrophy and, in this way, reduces endometrial prostaglandin synthesis and fibrinolysis. Menstrual loss can be reduced by as much as 50% (Fraser and McCarron 1991). Although the use of the low-dose oestrogen pill in the management of HMB has been affected by adverse publicity associated with its use in older women, it is now felt to be quite safe in women of any age who are not obese, do not smoke and are not hypertensive. It has the added advantage of providing a regular menstrual cycle and being effective in the treatment of dysmenorrhoea (Ekstrom et al 1989, Davis 2005). The limiting factor is that many of the women will have been sterilized and are therefore reluctant to continue with something they see as a contraceptive agent. Hormone replacement therapy can be used in perimenopausal women with excessive menstrual bleeding (Rees and Barlow 1991).
Danazol
Danazol, a derivative of testosterone, causes endometrial atrophy by inhibiting release of the pituitary gonadotrophins and can reduce menstrual loss by as much as 50% (Chimbira et al 1980, Dockeray et al 1989). The use of danazol is limited by its androgenic side-effects, which include weight gain, acne, depression and other long-term metabolic sequelae, all of which reduce compliance. This drug is no longer recommended.
Gonadotropin-releasing hormone analogues
GnRH analogues act by causing pituitary downregulation and subsequent inhibition of cyclical ovarian activity, resulting in amenorrhoea. Their use is limited to less than 6 months because of problems related to the hypo-oestrogenic state, including hot flushes, dry vagina and decreased bone mineral density unless ‘add-back’ oestrogen/progestogen therapy is used concurrently. They are useful in a minority of women (Shaw and Fraser 1984) and also in those with fibroids before surgery.
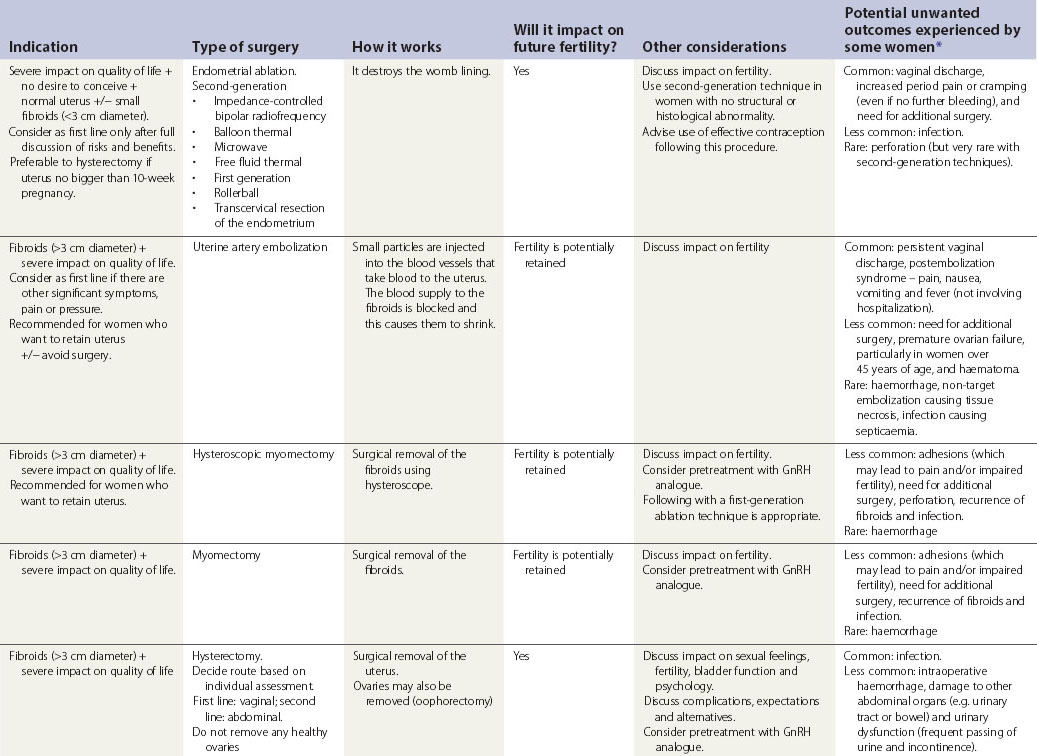
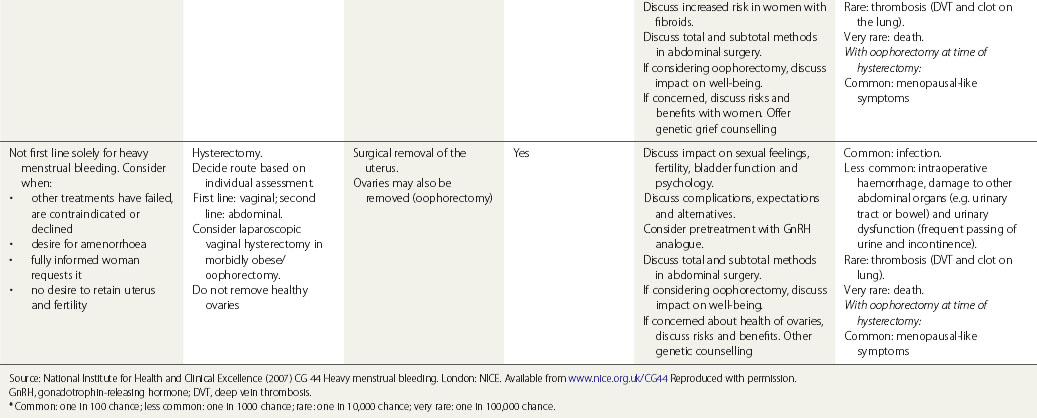
Surgical treatment
Surgical treatments range from minor conservative procedures to hysterectomy (see Table 31.3). First- and second-generation ablation methods are a less invasive alternative to hysterectomy and are associated with high levels of satisfaction, but in a proportion, surgery may be required repeatedly, and there is some risk of perioperative morbidity. Hysterectomy is the definitive treatment to stop HMB, with satisfaction rates consistently greater than 90%. However, hysterectomy is a major operation with the potential for serious morbidity and, rarely, mortality. The inconvenience to the patient and the cost to both the patient and the health services need to be balanced against the high levels of satisfaction that are reported after hysterectomy (Lethaby and Farquhar 2003).
Table 31.3 Surgical and radiological treatment options for women whose quality of life is severely impacted
Provide information to the woman before her outpatient appointment.
Endometrial ablation techniques
Hysteroscopic techniques (first generation) allow visualization of the uterine cavity, and enable the endometrium to be ablated under direct vision. Since regeneration of the endometrium occurs from the basal layer, it is essential to destroy all the endometrium. As the endometrial–myometrial border is irregular, the superficial layer of myometrium must also be included (Figure 31.8). There are a number of methods of achieving this, including removal of the tissue using a cutting loop, or coagulation or vaporization of the tissue using laser.
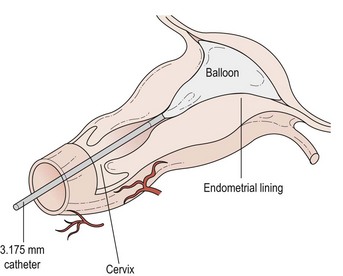
Thermal balloon ablation (Figure 31.9)
This involves the blind insertion of a balloon through the cervix, which is then filled with fluid until a pressure of 160–180 mmHg is reached. The fluid is then heated to 75–90°C (average 87°C). Endometrial destruction occurs over the next 8 min. The diameter of the insertion tube is very small and no cervical dilatation is required. This means that the procedure is particularly appropriate for women who are not suitable for or who do not wish to have a general anaesthetic. It is uncomfortable but pain relief can be improved by prior administration of a preparation such as diclofenac. Some sedation may be useful in certain instances. The success rate of this procedure appears to be similar to that for TCRE. One of the main advantages appears to be that pretreatment with a GnRH agonist is not required. Since the procedure is done in the outpatient setting in a number of units and has even been performed in general practice, it is likely to be a cheap and popular modality in the future. Outpatient thermal balloon ablation (Thermachoice III) can be performed in the majority of women, and is associated with similar overall pain scores but significantly less nausea, vomiting, need for antiemetics and less time spent in hospital compared with day-case Thermachoice III (Marsh et al 2007)
The procedure is effective and patient satisfaction is rated between good and excellent in more than 90% of cases. Complications of thermal balloon ablation include endometritis, urinary tract infection and haemorrhage, but all are uncommon (National Institute for Health and Clinical Excellence 2003).
Microwave endometrial ablation
MEA requires the insertion of a probe that emits microwaves into the uterus. The probe is then slowly moved across the fundus and down through the uterine cavity, destroying the endometrium. Provided that the probe is in the cavity of the uterus, this is a very safe procedure as the temperature rises as the endometrium is treated and the probe moved to another area. Randomized comparisons of this technique with TCRE suggest that it is equally effective; like TCRE, MEA also requires pretreatment with a GnRH agonist. In addition, since it requires dilatation of the cervix to 9 mm, it is unusual to perform this procedure under local anaesthetic. However, it is likely that smaller probes will be developed in order to achieve this result. The curative effect of MEA is similar to that of total hysterectomy, especially when preservation of the uterus and postoperative recovery is considered (Lin 2006).
Success rates
There is little variation in success rates between the different methods. Overall, approximately 30% of women will be amenorrhoeic and a further 45–50% will have significantly decreased loss, giving a satisfaction rate of approximately 75% (Magos et al 1991, Scottish Hysteroscopy Audit Group 1995, Cooper et al 1999). Careful counselling is essential in that if a woman is keen to have amenorrhoea, this will not be the treatment of choice for her. Some women also experience relief from symptoms of dysmenorrhoea and premenstrual tension.
Comparison of endometrial resection with hysterectomy
Endometrial resection was developed as an alternative to hysterectomy. However, it appears that the number of hysterectomies being performed is continuing to rise, suggesting that it is not serving this purpose. There have been a number of randomized comparisons between endometrial ablation and hysterectomy which show that satisfaction with both procedures is high (Pinion et al 1994). In addition, the operation can be performed on a day-case basis and recovery time is short, making it a cheap alternative to hysterectomy. However, it does appear that 20% of patients will require further surgical treatment, and there is still uncertainty regarding whether or not the number of failures will increase with time.
Endometrial resection is most likely to be successful in women over 40 years of age who have a normal-sized uterus and no intrauterine pathology. Its use in dysmenorrhoea is equivocal since it appears to make women worse in approximately 12% of cases, which may be due to the formation of haematometra. However, approximately 40% will improve so the presence of dysmenorrhoea is not an absolute contraindication. The menstrual cycle length will not be changed by the procedure, and hysterectomy may be an appropriate alternative in women with very irregular bleeding (Lewis 1994).
Novasure
Without the side-effects of hormones or the risks of hysterectomy, NovaSure has a quick recovery time. Most women experience no pain after the procedure and can return to work the next day. With more than 500,000 patients treated to date, NovaSure is proven to be safe and successful. A clinical trial has shown that more than nine out of 10 women return to normal or lower than normal bleeding levels following treatment with NovaSure (Cooper et al 2002). For some women, their menses stopped completely. In a small percentage of patients, side-effects include cramping, nausea/vomiting, discharge and spotting.
Hysterectomy
Abdominal hysterectomy involves a laparotomy incision that is transverse or longitudinal. A vaginal hysterectomy involves an incision through the vaginal wall. For many surgeons, vaginal hysterectomy is the preferred method for women with some degree of uterine descent and where the uterus is not excessively large. Abdominal hysterectomy allows better visualization of the pelvic cavity should there be pathology or where difficulty is anticipated. Non-randomized studies indicate that the complication rate for abdominal hysterectomy is greater than that for vaginal hysterectomy, and women who have vaginal procedures are able to go home sooner than women who have abdominal operations (Dicker et al 1982, Clinch 1994).
Recently, it has become possible to convert an abdominal hysterectomy to a vaginal hysterectomy using endoscopic techniques. The laparoscope allows visualization of the pelvic cavity, and all or part of the hysterectomy may be performed using specially designed laparoscopic instruments. A laparoscopic-assisted vaginal hysterectomy allows tissues accessible through the vagina to be ligated and the uterus to be removed by the vaginal route. This method facilitates the removal of ovaries and aids the operation when there is no uterine descent. It allows for dissection of adhesions and the treatment of pelvic pathology, such as endometriosis, prior to the hysterectomy. Published complication rates are low but recovery does not appear to be quicker than that following abdominal hysterectomy (Lumsden et al 2000), although the length of hospital stay is reduced (Boike et al 1993). Compared with abdominal hysterectomy, laparoscopic hysterectomy is associated with a higher rate of major complications, less postoperative pain and shorter hospital stay, but takes longer to perform (Boike et al 1993). The ovarian pedicles were only secured with laparoscopic sutures in 7% of cases, but this was associated with 25% of the complications. Abdominal laparoscopic hysterectomy is associated with a significantly higher risk of major complications and takes longer to perform than abdominal hysterectomy. Abdominal laparoscopic hysterectomy is, however, associated with less pain, quicker recovery and better short-term quality of life after surgery than abdominal hysterectomy. The cost-effectiveness of laparoscopic hysterectomy is finely balanced and is also influenced by the choice of reusable vs disposable equipment (Garry et al 2004).
Dysmenorrhoea
In young women, dysmenorrhoea is frequently the only menstrual abnormality. Prostaglandin synthetase inhibitors and the oral contraceptive pill are very effective (Anderson 1981), but in a minority of women, even in the absence of disease, these measures are insufficient. Calcium channel blockers (e.g. nifedipine) have been used in Scandinavia and are effective, although their use is limited by cardiovascular side-effects. Oxytocin/vasopressin receptor antagonists may become available when effective oral agents are developed.
Conclusions
KEY POINTS
Adalantado JM, Rees MCP, Bernal AL, Turnbull AC. Increased intrauterine prostaglandin E receptors in menorrhagic women. British Journal of Obstetrics and Gynaecology. 1988;95:162.
Åkerlund M, Anderssen KE. Vasopressin response and turbutaline inhibition of the uterus. Obstetrics and Gynecology. 1976;48:528-536.
Åkerlund M, Stromberg P, Forsling ML. Primary dysmenorrhoea and vasopressin. British Journal of Obstetrics and Gynaecology. 1979;86:484-487.
Alecozay AA, Harper MJK, Schenken RS, Hanahan DJ. Paracrine interactions between platelet-activating factor and prostaglandins in hormonally-treated human luteal phase endometrium in vitro. Journal of Reproduction and Fertility. 1991;91:301-312.
Andersch B, Milsom I. An epidemiologic study of young women with dysmenorrhoea. American Journal of Obstetrics and Gynecology. 1982;144:655-660.
Anderson A. The role of prostaglandin synthetase inhibitors in gynaecology. Practitioner. 1981;225:1460-1470.
Andersson K, Rybo G. Levonorgestrel-releasing intrauterine device in the treatment of menorrhagia. British Journal of Obstetrics and Gynaecology. 1990;97:690-694.
Bacon CR, Morrison JJ, O’Reilly G, Cameron IT, Davenport AP. ETA and ETB endothelin receptors in human myometrium characterised by the subtype selective ligands BQ123, BQ3020, FR139317 and PD15142. Journal of Endocrinology. 1995;144:127-134.
Basilico C, Moscatelli D. The FGF family of growth factors and oncogenes. Advances in Cancer Research. 1992;59:115-165.
Battersby S, Critchley HOD, Morgan K, Millar RP, Jabbour HN. Expression and regulation of prokineticins (endocrine-gland-derived vascular endothelial growth factor and Bv8) and their receptors in the human endometrium across the menstrual cycle. Journal of Clinical Endocrinology and Metabolism. 2004;89:2463-2469.
Beller FK. Observations on the clotting of menstrual blood and clot formation. American Journal of Obstetrics and Gynecology. 1971;11:535-546.
Björk J, Smedegård G. Acute microvascular effects of PAF-acether, as studied by intravital microscopy. European Journal of Pharmacology. 1983;96:87.
Boike GM, Elfstrand EP, Del Priore G, et al. Laparoscopically assisted vaginal hysterectomy in a university hospital: report of 82 cases and comparison with abdominal and vaginal hysterectomy. American Journal of Obstetrics and Gynecology. 1993;168:1690-1701.
Bonnar J, Sheppard BL, Dockeray CJ. The haemostatic system and dysfunctional uterine bleeding. Research and Clinical Forums. 1983;5:27-36.
Brosens JJ, Barker FG, deSouza NM. Myometrial zonal differentiation and uterine junctional zone hyperplasia in the non-pregnant uterus. Human Reproduction Update. 1998;4:496-502.
Brosens JJ, Mak I, Brosens I. Mechanisms of dysmenorrhoea. In: O’Brien S, Cameron IT, MacLean A, editors. Disorders of the Menstrual Cycle. London: RCOG Press; 2000:113-132.
Brown PM, Farquhar CM, Lethaby A, Sadler LC, Johnson NP. Cost-effectiveness analysis of levonorgestral intrauterine system and thermal balloon ablation for heavy menstrual bleeding. BJOG: an International Journal of Obstetrics and Gynaecology. 2006;113:797-803.
Bruner KL, Rodgers WH, Old LI et al 1995 Transforming growth factor beta mediates progesterone suppression of an epithelial metalloproteinase by adjacent stroma in the human endometrium. Proceedings of the National Academy of Sciences USA 92: 7362–7366.
Cameron IT, Haining R, Lumsden MA, Reid-Thomas V, Smith SK. The effects of mefenamic acid and norethisterone on measured menstrual blood loss. Obstetrics and Gynecology. 1990;76:85-88.
Cameron IT, Davenport AP. Endothelins in reproduction. Reproductive Medicine Review. 1992;1:99-113.
Cameron IT, Davenport AP, van Papendorp CL, et al. Endothelin-like immunoreactivity in human endometrium. Journal of Reproduction and Fertility. 1992;95:623-628.
Cameron IT, Norman JE. Endometrial biochemistry in menorrhagia. In: Studd J, Asch R, editors. Progress in Reproductive Medicine, Volume II. London: Parthenon Publishing Group; 1995:267-279.
Campbell S, Young A, Stewart CJR et al 1998 Laminin beta 2 distinguishes inner and outer myometrial layers of the human myometrium. Journal of Reproduction and Fertility 22: 12.
Carson DD, Lagow E, Thathiah A, et al. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high density microarray screening. Molecular Human Reproduction. 2002;8:871-879.
Casslen B, Andersson A, Nilsson IM, Astedt B. Hormonal regulation of the release of plasminogen activators and of a specific activator inhibitor from endometrial tissue in culture (42360). Proceedings of the Society for Experimental Biology and Medicine. 1986;182:419-424.
Chalubinski K, Deutinger J, Bernaschek G. Vaginosonography for recording of cycle-related myometrial contractions. Fertility and Sterility. 1993;59:225-228.
Chamberlain G, Freeman R, Price F, Kennedy A, Green D, Eve L. A comparative study of ethamsylate and mefenamic acid in dysfunctional uterine bleeding. British Journal of Obstetrics and Gynaecology. 1991;98:707-711.
Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biology of Reproduction. 2004;70:1738-1750.
Charnock-Jones DS, Sharkey AM, Rajput-Williams J, et al. Identification and localization of alternately spliced mRNAs for vascular endothelial growth factor in human uterus and estrogen regulation in endometrial carcinoma cell lines. Biology of Reproduction. 1993;48:1120-1128.
Cheng Chi I. An evaluation of the levonorgestrel-releasing IUD: its advantages and disadvantages when compared to the copper-releasing IUDs. Contraception. 1991;44:573-587.
Chimbira TH, Anderson ABM, Turnbull AC. Relation between measured menstrual blood loss and patients’ subjective assessment of loss, duration of bleeding, number of sanitary towels used, uterine weight and endometrial surface area. British Journal of Obstetrics and Gynaecology. 1980;87:603-609.
Christiaens G, Sixma JJ, Haspels AA. Morphology of haemostasis in menstrual endometrium. British Journal of Obstetrics and Gynaecology. 1980;87:425-439.
Christiaens G, Sixma JJ, Haspels AA. Fibrin and platelets in menstrual discharge before and after the insertion of an intrauterine contraceptive device. American Journal of Obstetrics and Gynecology. 1981;140:793-798.
Christiaens G, Sixma JJ, Haspels AA. Haemostasis in menstrual endometrium: a review. Obstetrical and Gynecological Survey. 1982;37:281-303.
Clinch J. Length of hospital stay after vaginal hysterectomy. British Journal of Obstetrics and Gynaecology. 1994;101:253-254.
Cohen BJB, Gibor Y. Anaemia and menstrual blood loss. Obstetrical and Gynecological Survey. 1980;35:597-618.
Cole SK, Billewicz WZ, Thomson AM. Sources of variation in menstrual blood loss. Journal of Obstetrics and Gynaecology of the British Commonwealth. 1971;78:933-939.
Cooper KG, Parkin DE, Garrett AM, Grant AM. Two year follow-up of women randomised to medical management or transcervical resection of the endometrium for heavy menstrual loss; clinical and quality of life outcomes. British Journal of Obstetrics and Gynaecology. 1999;106:258-265.
Cooper J, Gimpleson R, Laberge P, et al. A randomized, multicenter trial of safety and efficacy of the NovaSure® system in the treatment of menorrhagia. Journal of the American Association of Gynecologic Laparoscopists. 2002;9:418-428.
Coulter A, Kelland J, Peto V, Rees M. Treating menorrhagia in primary care: an overview of drug trials and a survey of prescribing practice. International Journal of Health Technology Assessment in Health Care. 1995;11:456-471.
Critchley H, Abberton KM, Taylor NH, Healy DL, Rogers AW. Endometrial sex steroid receptor expression in women with menorrhagia. British Journal of Obstetrics and Gynaecology. 1994;101:428-434.
Critchley HO, Kelly RW, Brenner RM, Baird DT. The endocrinology of menstruation — a role for the immune system. Clinical Endocrinology. 2001;55:701-710.
Crosignani PG, Vercellini P, Mosconi P, Oldani S, Cortesi I, de Giorgi O. Levonorgestrel-releasing intrauterine device versus hysteroscopic endometrial resection in the treatment of dysfunctional uterine bleeding. Obstetrics and Gynecology. 1997;90:257-263.
Daly L, Sheppard BL, Carroll E, Hennelly B, Bonnar J. Coagulation and fibrinolysis in menstrual and peripheral blood in dysfunctional uterine bleeding. Irish Journal of Medical Science. 1990;159:24-25.
de Merre LJ, Moss JD, Pattison OS. The haematological study of menstrual discharge. Obstetrics and Gynecology. 1967;30:830-833.
de Vries K, Lyons EA, Ballard G, Levi CS, Lindsay DJ. Contractions of the inner third of the myometrium. American Journal of Obstetrics and Gynecology. 1990;162:679-682.
Demas BE, Hricak H, Jaffe RB. Uterine MR imaging: effects of hormonal stimulation. Radiology. 1985;159:123-126.
Davis AR. Oral contraceptives for dysmenorrhoea in adolescent girls: a randomized trial. Obstetrics and Gynecology. 2005;106:97-104.
Dicker RC, Greenspan JR, Strauss LT, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. American Journal of Obstetrics and Gynecology. 1982;144:841-848.
Dockeray CJ, Sheppard BL, Bonnar J. Comparison between mefenamic acid and danazol in the treatment of established menorrhagia. British Journal of Obstetrics and Gynaecology. 1989;96:840-844.
Ekstrom P, Juchnicka E, Laudanski T, Åkerlund M. Effect of an oral contraceptive in primary dysmenorrhoea — changes in uterine activity and reactivity to agonists. Contraception. 1989;40:39-47.
Ferriani RA, Charnock-Jones DS, Prentice A, Thomas EJ, Smith SK. Immunohistochemical localization of acidic and basic fibroblast growth factors in normal human endometrium and endometriosis and the detection of their mRNA by polymerase chain reaction. Human Reproduction. 1993;8:11-16.
Filler WW, Hall WC. Dysmenorrhoea and its therapy: a uterine contractility study. American Journal of Obstetrics and Gynecology. 1970;106:104-109.
Fraser IS, McCarron G, Markham R, Resta T, Watts A. Measured menstrual blood loss in women with menorrhagia associated with pelvic disease or coagulation disorder. Obstetrics and Gynecology. 1986;68:630-633.
Fraser IS, McCarron G. Randomised trial of two hormonal and two prostaglandin-inhibiting agents in women with a complaint of menorrhagia. Australian and New Zealand Journal of Obstetrics and Gynaecology. 1991;31:66-70.
Fraser IS, Inceboz US. Defining disturbances of the menstrual cycle. In: O, Brien PMS, Cameron I, Maclean A, editors. Disorders of the Menstrual Cycle. London: RCOG Press; 2000:141-152.
Garry R, Fountain J, Brown J, et al. EVALUATE hysterectomy trial: a multicentre randomized trial comparing abdominal, vaginal and laparoscopic methods of hysterectomy. Health Technology Assessment. 2004;8:1-154.
Gimpleson RJ, Rappold HO. A comparative study between panoramic hysteroscopy with directed biopsies and dilatation and curettage. American Journal of Obstetrics and Gynecology. 1988;158:489-492.
Gospodarowicz D, Abraham JA, Schilling J. Isolation and characterization of a vascular endothelial cell mitogen produced by pituitary-derived follicular stellate cells. Proceedings of the National Academy of Sciences USA. 1989;86:7311-7315.
Hahn L. Composition of menstrual blood. In: Diczfalusy E, Fraser IS, Webb FTG, editors. Endometrial Bleeding and Steroidal Contraception. Bath: Pitman Press; 1980:107-137.
Haining REB, Cameron IT, van Papendorp CL, et al. Epidermal growth factor in human endometrium: proliferative effects in culture and immunocytochemical localisation in normal and endometriotic tissues. Human Reproduction. 1991;6:1200.
Hallberg L, Nilsson L. Consistency of individual menstrual blood loss. Acta Obstetrica et Gynecologica Scandinavica. 1964;43:352-359.
Haynes P, Hodgson H, Anderson A, Turnbull A. Measurement of menstrual blood loss in patients complaining of menorrhagia. British Journal of Obstetrics and Gynaecology. 1977;84:763-768.
Haynes P, Anderson ABM, Turnbull AC. Patterns of menstrual blood loss in menorrhagia. Research and Clinical Forums. 1979;1:73-78.
Higham JM. Medical treatment of menorrhagia. Progress in Obstetrics and Gynaecology. 1994;68:335-337.
Higham JM, O’Brien PMS, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. British Journal of Obstetrics and Gynaecology. 1990;97:734-739.
Hynes RO. Integrins: a family of cell surface receptors. Cell. 1987;48:549-554.
Ignar Trowbridge DM, Teng CT, Ross KA, Parker MG, Korach KS, McLachlan JA. Peptide growth factors elicit estrogen receptor-dependent transcriptional activation of an estrogen-responsive element. Molecular Endocrinology. 1993;7:992-998.
Irvine GA, Campbell-Brown MB, Lumsden MA, Heikkila A, Walker JJ, Cameron IT. Randomised comparative trial of the levonorgestrel intrauterine system and norethisterone for treatment of idiopathic menorrhagia. British Journal of Obstetrics and Gynaecology. 1998;105:592-598.
Jabbour HN, Kelly RW, Fraser HM, Critchley HO. Endocrine regulation of menstruation. Endocrine Reviews. 2006;27:17-46.
Jeziorska M, Nagase H, Salamonsen LA, Woolley DE. Immunolocalisation of the matrix metalloproteinase gelatinase B and stromelysin 1 in human endometrium throughout the menstrual cycle. Journal of Reproductive Fertility. 1996;107:43-51.
Kao LC, Tulac S, Lobo S, et al. Global gene profiling in human endometrium during the window of implantation. Endocrinology. 2002;143:2119-2138.
Kelly RW, Lumdsen MA, Abel MH, Baird DT. The relationship between menstrual blood loss and prostaglandin production in the human: evidence for increased availability of arachidonic acid in women suffering from menorrhagia. Prostaglandins, Leukotrienes and Medicine. 1984;16:69-78.
King A, Burrows T, Vernas S, Hiby S, Loke YW. Human uterine lymphocytes. Human Reproduction Update. 1998;5:480-485.
Kulkarni A, Lee CA, Griffeon A, Kadir RA. Disorders of menstruation and their effect on the quality of life in women with congenital factor VII deficiency. Haemophilia. 2006;12:248-252.
Kunz G, Beil D, Deininger H, Wildt L, Leyendecker G. The dynamics of rapid sperm transport through the female genital tract: evidence from vaginal sonography of uterine peristalsis and hysterosalpingoscintigraphy. Human Reproduction. 1996;11:627-632.
Lahteenmaki P, Haukkamaa M, Puolakka J, et al. Open randomised study of use of levonorgestrel releasing intrauterine system as an alternative to hysterectomy. BMJ (Clinical Research Ed.). 1998;316:1122-1126.
Lesny P, Killick SR, Tetlow RL, et al. Ultrasound evaluation of the uterine zonal anatomy during in-vitro fertilization and embryo transfer. Human Reproduction. 1999;14:1593-1598.
Lessey BA, Castelbaum AJ, Buck CA, Lei Y, Yowell CW, Sun J. Integrin adhesion molecules in the human endometrium. Correlation with the normal and abnormal menstrual cycle. Journal of Clinical Investigation. 1992;90:188-195.
Lethaby S, Farquhar C. Treatments for heavy menstrual bleeding. BMJ (Clinical Research Ed.). 2003;327:1243-1244.
Letzel H, Megard Y, Lamarca R, Raber A, Fortea J. The efficacy and safety of aceclofenac versus placebo and naproxen in women with primary dysmenorrhoea. European Journal of Obstetrics, Gynaecology and Reproductive Biology. 2006;129:162-168.
Lewis BV. Hysteroscopy for the investigation of abnormal uterine bleeding. British Journal of Obstetrics and Gynaecology. 1990;97:283-284.
Lewis BV. Guidelines for endometrial ablation. British Journal of Obstetrics and Gynaecology. 1994;101:470.
Leyendecker G, Kunz G, Wildt L, Beil D, Deiniger H. Uterine hyperperistalsis and dysperistalsis as dysfunctions of the mechanism of rapid sperm transport in patients with endometriosis and infertility. Human Reproduction. 1996;11:1542-1551.
Lin H. Comparison between microwave endometrial ablation and total hysterectomy. Chinese Medical Journal. 2006;119:1195-1197.
Lockwood CJ, Krikun G, Hausknecht VA, Papp C, Schatz F. Matrix metalloproteinase and matrix metalloproteinase inhibitor expression in endometrial stromal cells during progestin-initiated decidualisation and menstruation. Endocrinology. 1998;139:4607-4613.
Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstetrics and Gynecology. 1989;73:16-20.
Lumsden MA, Kelly RW, Baird DT. Is prostaglandin F2 involved in the increased myometrial contractility of primary dysmenorrhoea? Prostaglandins. 1983;25:683-692.
Lumsden MA, Baird DT. Intrauterine pressure in dysmenorrhoea. Acta Obstetrica et Gynecologica Scandinavica. 1985;64:183-186.
Lumsden MA, Twaddle S, Hawthorn R, et al. A randomised comparison and economic evaluation of laparoscopic-assisted hysterectomy and abdominal hysterectomy. British Journal of Obstetrics and Gynaecology. 2000;107:1386-1391.
Lundstrom V, Green K. Endogenous levels of prostaglandin F2α and its main metabolites in plasma and the endometrium of normal and dysmenorrhoeic women. American Journal of Obstetrics and Gynecology. 1978;130:640-646.
Luukkainen T, Allonen H, Haukkamaa M, Lahteenmaki P, Nilsson CG, Toivonen J. Five years experience with levonorgestrel-releasing IUCDs. Contraception. 1986;33:139-148.
Magos AL, Baumann R, Lockwood GM, Turnbull AC. Experience with the first 250 endometrial resections for menorrhagia. The Lancet. 1991;337:1074-1078.
Marbaix E, Kokorine I, Moulin P, Donnez J, Eeckhout Y, Courtoy PJ 1996 Menstrual breakdown of human endometrium can be mimicked in vitro and is selectively and reversibly blocked by inhibitors of matrix metalloproteinases. Proceedings of the National Academy of Sciences USA 93: 9120–9125.
Markee JE. Menstruation in intraocular endometrial transplants in the rhesus monkey. Contributions to Embryology, Carnegie Institute. 1940;28:219-308.
Markee JE. Morphological basis for menstrual bleeding. Relation of regression to the initiation of bleeding. Bulletin of the New York Academy of Medicine. 1948;24:253-270.
Marsh M. Endothelin and menstruation. Human Reproduction. 1996;11:83-89.
Marsh F, Thewlis J, Duffy S. Randomised controlled trial comparing Thermachoice III in the outpatient versus day-case setting. Fertility and Sterility. 2007;87:642-650.
Migram S, Benedetto C, Zonka M, Leo Rosscerg I, Lubbert H, Hammerstein J. Increased concentrations of eicosanoids and platelet-activating factor in menstrual fluid in women with primary dysmenorrhoea. Eicosanoids. 1991;4:137-141.
Milsom I, Andersson K, Andersch B, Rybo G. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. American Journal of Obstetrics and Gynecology. 1991;164:879-883.
National Institute for Health and Clinical Excellence. Balloon thermal endometrial ablation. Interventional Procedure Guidance 6. NICE. Available at www.nice.org, 2003.
National Institute for Health and Clinical Excellence. Heavy Menstrual Bleeding. Clinical Guideline 44. NICE. Available at www.nice.org, 2007.
Novak E, Reynolds SRM. The cause of primary dysmenorrhoea with special reference to hormonal factors. JAMA: the Journal of the American Medical Association. 1932;99:1466-1472.
Ohbuchi H, Nagai K, Yamaguchi M, et al. Endothelin-1 and big endothelin-1 increase in human endometrium during menstruation. American Journal of Obstetrics and Gynecology. 1995;173:1483-1490.
Parazzini F, Tozzi L, Mezzopane R, Luchini L, Marchini M, Fedele L. Cigarette smoking, alcohol consumption, and risk of primary dysmenorrhoea. Epidemiology. 1994;5:469-472.
Pepper MS, Ferrara N, Orci L, Monteano R. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochemical and Biophysical Research Communications. 1992;189:824-831.
Pierzynski P, Swiatecka J, Oczeretko E, Laudanski P, Batra S, Laudanski T. Effect of short-term, low-dose treatment with tamoxifen in patients with primary dysmenorrhoea. Gynecological Endocrinology. 2006;22:698-703.
Pinion SB, Parkin DE, Abramovich DR, et al. Randomised trial of hysterectomy, endometrial laser ablation and transcervical endometrial resection for dysfunctional uterine bleeding. BMJ (Clinical Research Ed.). 1994;309:979-983.
Rauramo I, Elo I, Isre O. Long-term treatment of menorrhagia with levonorgestral intrauterine system versus endometrial resection. Obstetrics and Gynecology. 2004;104:1314.
Rees MCP, Demers LM, Anderson ABM, Turnbull AC. A functional study of platelets in menstrual fluid. British Journal of Obstetrics and Gynaecology. 1984;91:667-693.
Rees MCP, Cederholm-Williams SA, Turnbull AC. Coagulation factors and fibrinolytic proteins in menstrual fluid collected from normal and menorrhagic women. British Journal of Obstetrics and Gynaecology. 1985;92:1164-1168.
Rees MCP, Canete-Soler R, Lopez-Bernal A, Turnbull A. Effect of fenamates on prostaglandin E receptor binding. The Lancet. 1988;ii:541-542.
Rees MCP, Barlow DH. Quantitation of hormone replacement induced withdrawal bleeds. British Journal of Obstetrics and Gynaecology. 1991;98:106-107.
Reid PC, Coker A, Coltart R. Assessment of menstrual blood loss using pictorial chart: a validation study. British Journal of Obstetrics and Gynaecology. 2000;107:320-322.
Riesewijk A, Martin J, van Os R, et al. Gene expression profiling of human endometrial receptivity on days LH+2 versus LH+7 by microarray technology. Molecular Human Reproduction. 2003;9:253-264.
Rigot V, Marbaix E, Lemoine P, Courtoy PJ, Eechout Y. In vivo perimenstrual activation of progelatinase B (ProMMP-9) in the human endometrium and its dependence on stromelysin 1(MMP-3) ex vivo. Biochemical Journal. 2001;358:275-280.
Rodeghiero F. Management of menorrhagia in women with inherited bleeding disorders: general principles and use of desmopressin. Haemophilia. 2008;14(Suppl 1):21-30.
Rogers PAW, Lederman F, Taylor N. Endometrial microvascular growth in normal and dysfunctional states. Human Reproduction Update. 1998;4:503-508.
Rogers PA, Abberton KM. Endometrial arteriogenesis: vascular smooth muscle cell proliferation and differentiation during the menstrual cycle and changes associated with endometrial bleeding disorders. Microscopy Research and Technique. 2003;60:412-419.
Royal College of Obstetricians and Gynaecologists. Inpatient Treatment 1-1 D&C in Women Aged 40 or Less. Guideline No. 3. London: RCOG; 1994.
Rybo G. Plasminogen activator in the endometrium. II. Clinical aspects. Acta Obstetrica et Gynecologica Scandinavica. 1966;45:97-118.
Rybo G. Tranexamic acid therapy: effective treatment in heavy menstrual bleeding. Clinical update on safety. Therapeutic Advances. 1991;4:1-8.
Schatz F, Krikun G, Runic R, Wang EY, Hausknecht V, Lockwood CJ. Implications of decidualization-associated protease expression in implantation and menstruation. Seminars in Reproductive Endocrinology. 1999;17:3-12.
Scottish Hysteroscopy Audit Group. A Scottish audit of hysteroscopic surgery for menorrhagia — complications and follow up. British Journal of Obstetrics and Gynaecology. 1995;102:249-254.
Sharkey AM, Day K, McPherson A, et al. Vascular endothelial growth factor expression in human endometrium is regulated by hypoxia. Journal of Clinical Endocrinology and Metabolism. 2000;85:402-409.
Shaw RW, Fraser HM. Use of a superactive luteinizing hormone-releasing hormone (LHRH) agonist in the treatment of menorrhagia. British Journal of Obstetrics and Gynaecology. 1984;91:913-916.
Sheppard BL. Physiology of dysfunctional uterine bleeding. In: Lowe D, Fox H, editors. Advances in Gynaecological Pathology. Edinburgh: Churchill Livingstone; 1992:191-204.
Sheppard BL, Dockeray CJ, Bonnar J. An ultrastructural study of menstrual blood in normal menstruation and dysfunctional uterine bleeding. British Journal of Obstetrics and Gynaecology. 1983;90:259-265.
Silverberg SG, Haukkamaa M, Arko H, Nilsson CG, Luukkainen T. Endometrial morphology during long-term use of levonorgestrel-releasing intrauterine devices. International Journal of Gynecological Pathology. 1986;5:235-241.
Smith SK. Angiogenesis, vascular endothelial growth factor and the endometrium. Human Reproduction. 1998;4:519.
Smith SK, Abel MH, Kelly RW, Baird DT. Prostaglandin synthesis in the endometrium of women with ovular dysfunctional uterine bleeding. British Journal of Obstetrics and Gynaecology. 1981;88:434-442.
Smith SK, Abel MH, Kelly RW, Baird DT. The synthesis of prostaglandins from persistent proliferative endometrium. Journal of Clinical Endocrinology and Metabolism. 1982;55:284-289.
Smith SK, Kelly RW. Effect of platelet-activating factor on the release of PGF2α and PGE2 by separated cells of human endometrium. Journal of Reproduction and Fertility. 1988;82:271-276.
Stovall TG, Solomon SK, Ling FW. Endometrial sampling prior to hysterectomy. Obstetrics and Gynecology. 1989;73:405-408.
Suri C, McClain J, Thurston DM, et al. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998;282:468-471.
Tabibzadeh S, Babaknia A. The signals and molecular pathways involved in implantation, a symbiotic interaction between blastocyst and endometrium involving adhesion and tissue invasion. Molecular Human Reproduction. 1995;1:1579-1602.
Taylor PJ, Hamou J. Hysteroscopy. Journal of Reproductive Medicine. 1983;28:359-389.
Togashi K, Ozasa H, Konishi I, et al. Enlarged uterus: differentiation between adenomyosis and leiomyoma with MR imaging. Radiology. 1989;171:531-534.
Weston G, Rogers PA. Endometrial angiogenesis. Baillières Best Practice and Research Clinical Obstetrics and Gynaecology. 2000;14:919-936.
Wiczyk HP, Janus CL, Richards CJ, et al. Comparison of magnetic resonance imaging and ultrasound in evaluating follicular and endometrial development throughout the normal cycle. Fertility and Sterility. 1988;49:969-972.
Wyatt KM, Dimmok PW, Walker TJ, O’Brien PMS. Determination of total menstrual blood loss. Fertility and Sterility. 2001;76:125-131.
Xu S, Yang Y, Gregory CD, et al. Biochemical heterogeneity in hysterectomised uterus measured by 31P NMR using SLIM localisation. Magnetic Resonance in Medicine. 1997;37:736-743.
Ylikorkala O, Viinikka L. Comparison between anti-fibrinolytic and anti-prostaglandin treatment in the reduction of increased menstrual blood loss in women with intrauterine contraceptive devices. British Journal of Obstetrics and Gynaecology. 1983;90:78-83.
Zhang L, Rees MCP, Bicknell R. The isolation and long term culture of normal human endometrial epithelium and stroma. Journal of Cell Science. 1995;108:323-331.
Cameron IT. Medical treatment of menorrhagia. In: Yearbook of the RCOG. London: RCOG Press; 1993:55-64.
Cameron IT, Fraser IS, Smith SK. Clinical Disorders of the Endometrium and Menstrual Cycle. Oxford: Oxford University Press; 1998.
Fraser IS, Jansen RPS, Lobo RA, Whitehead M, editors. Estrogens and Progestogens in Clinical Practice. London: Churchill Livingstone, 1998.
O’Brien S, Cameron I, Maclhean A, editors. Disorders of the Menstrual Cycle. London: RCOG Press, 2000.
Sheth S, Sutton C. Menorrhagia. Oxford: Isis Medical Media; 1999.
Swiet MD, Chamberlein G, Bennet P. Basic Science in Obstetrics and Gynaecology, 3rd edn. London: Churchill Livingstone; 2002.
Vollenhoven B (ed) 1998 Uterine fibroids. Baillière’s Clinical Obstetrics and Gynaecology 12: 177–195.