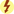http://evolve.elsevier.com/McCuistion/pharmacology/
Reproductive health requires the production of adequate quantities of various hypothalamic, pituitary, and gonadal hormones as well as the appropriate hormone receptors. It requires normal development and patency of the reproductive tract. In addition, reproductive health implies that men and women at developmentally appropriate life stages are fertile (i.e., able to produce gametes [sperm or eggs]). Finally, reproductive health entails the ability to engage in sexual intercourse with ejaculation by the male.
Alterations in male reproductive health reflect a wide range of developmental, endocrine, infectious, inflammatory, hypertrophic, malignant, and psychoemotional processes. Review the introduction to this unit to gain a better understanding of ways in which reproductive health is affected, including anatomy and physiology, sperm production, regulation of male sexual functioning, and sexual intercourse.
The drug family most clearly associated with male reproductive processes is the androgens. Because synthetic anabolic steroids and antiandrogens affect male reproduction, they are also discussed.
Drugs Related to Male Reproductive Disorders
Androgens
Androgens, or male sex hormones, control the development and maintenance of sexual processes, accessory sexual organs, cellular metabolism, and bone and muscle growth. Testosterone, an anabolic steroid, is the principal male sex hormone. It is the prototype of the androgen hormones, synthesized primarily in the testes and, to a lesser extent, in the adrenal cortex. In women, the ovaries synthesize small amounts of testosterone. In adult males, normal plasma concentrations of testosterone are 270 to 1070 ng/dL, with a slow decline of 1% per year after the age of 30 (Table 53.1). Prototype Drug Chart 53.1 lists the natural and synthetic androgens and their dosages, uses, and considerations.
TABLE 53.1
| Age | T Level (ng/dL) |
| 0-5 months | 75-400 |
| 6 mos.-9 yrs. | <7-20 |
| 10-11 yrs. | <7-130 |
| 12-13 yrs. | <7-800 |
| 14 yrs. | <7-1,200 |
| 15-16 yrs. | 100-1,200 |
| 17-18 yrs. | 300-1,200 |
| 19+ yrs. | 240-950 |
| Avg. Adult Male | 270-1,070 |
| 30+ yrs. | -1% per year |
From: http://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/83686
Pharmacokinetics
Testosterone secretion is greater in men than in women in most stages of life. About 98% of circulating testosterone is bound to both sex hormone–binding globulin (SHBG) and albumin protein, leaving about 2% unbound, or circulating free in the plasma; this unbound portion is biologically active. Estrogen elevates the production of SHBG, resulting in more protein-bound testosterone in women than in men. The half-life of endogenous, or naturally occurring, free testosterone in the blood is 10 to 20 minutes.
Because as much as 50% of testosterone is metabolized on its first pass through the hepatic circulation when taken orally, oral testosterone is not available for prescription in the United States. Testosterone can be combined with esters to form esterified testosterone in an oil base for intramuscular (IM) injection.
Ninety percent of testosterone is excreted in the urine as glucuronic and sulfuric acid conjugates and its metabolites. About 6% of the hormone is excreted unconjugated in the feces. Synthetic androgens may be excreted as unaltered hormone or as metabolites. In some tissues the action of testosterone depends on its reduction to 5-alpha-dihydrotestosterone (DHT), whereas in other tissues testosterone itself is the active hormone. In the central nervous system, the metabolite estradiol affects hormonal action.
Pharmacodynamics
Testosterone is responsible for the development of male sex characteristics. The biologic effects of testosterone may be mediated directly by testosterone or by its metabolites. Testosterone and dihydrotestosterone act as androgens by way of a single androgen receptor officially designated NR3A. The hormones bind to sites on certain responsive genes, causing a change to take place in the target cell. The effects of the testosterone depend on which receptor it activates and the tissues in which these effects occur. The manufacture of protein within the target cells results in the buildup of cellular tissue (anabolism), especially in muscles. This leads to development of secondary sex characteristics such as pubic hair growth, beard and body hair growth, baldness, deepening of the male voice, thickening of the skin, sebaceous gland activity, increased musculature, bone development, and red blood cell formation.
Fetal testes begin to produce testosterone during the first 3 months in utero. After birth until just before puberty, production is negligible. During puberty, production increases rapidly and continues until later adulthood. As men age, the number of Leydig cells decreases, sperm production declines, and luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels rise. Levels of unbound testosterone are reduced in older men to one third to one fifth the peak value. If men experience osteoporosis and anemia, and if their testosterone levels are 300 ng/dL or less, testosterone replacement therapy should be considered. Testosterone boosters are also an option.
Indications for Androgen Therapy
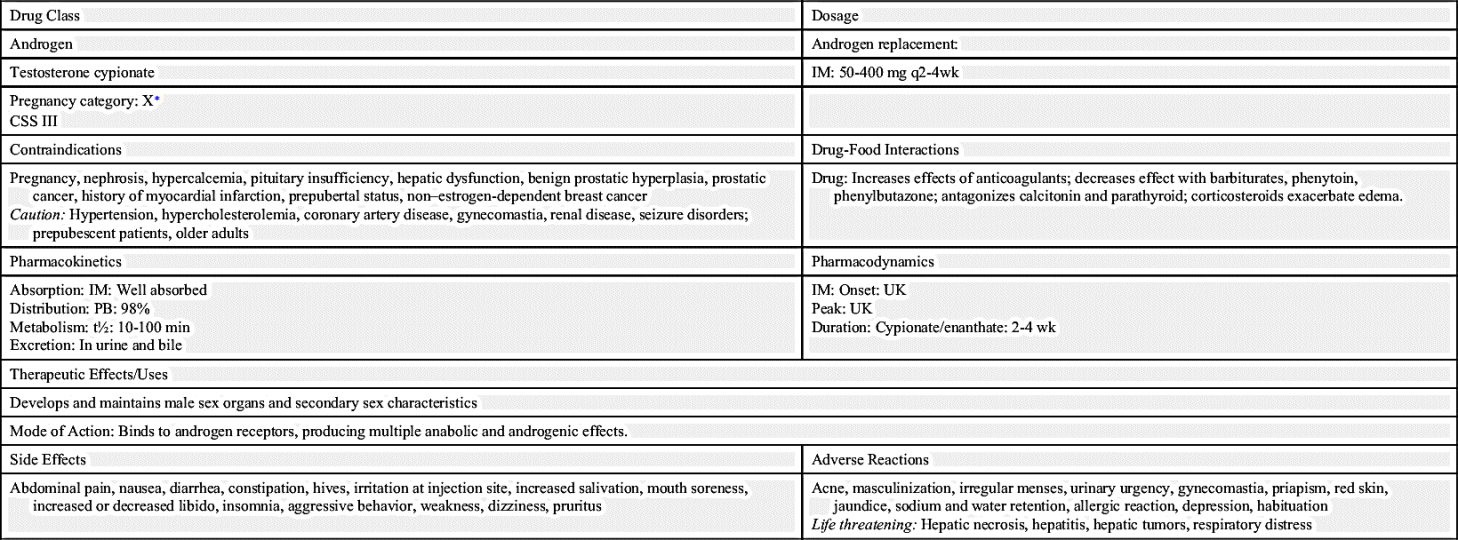
Table 53.2 lists the natural and synthetic androgens with their dosages, uses, and considerations. Androgen therapy is approved for use in androgen deficiency in males, specifically hypogonadism; replacement therapy for testicular failure in adult males; and delayed puberty in adolescents.
Hypogonadism
The clearest indication for exogenous androgen therapy is hypogonadism. Male hypogonadism is a defect of the reproductive system that results in failure of the testes to produce testosterone, sperm, or both. Deficiency of sex hormones can result in defective primary or secondary sexual development, and defective sperm development can result in infertility. Hypogonadism is either primary, reflecting testicular abnormality, or secondary, reflecting hypothalamic or pituitary failure. A combination of disorders can also occur. Inadequate pituitary function will severely affect young boys and results in infertility and a lack of secondary sex characteristics. Adult men may experience testicular atrophy, impotence, decreased libido, decreased bone density, loss of muscle mass, hair loss, gynecomastia, fatigue, difficulty concentrating, or vasomotor flushing.
The timing and extent of treatment depend on the clinical manifestations. Because accelerated bone maturation can lead to premature closure of bone epiphyses and short stature, androgen therapy should be used cautiously in children and only by specialists aware of the adverse effects on bone maturation. Skeletal maturation must be monitored every 6 months by radiography of the hand and wrist. Artificial induction of puberty is undertaken only after boys reach age 15 to 17 years and after hypothalamic and pituitary function has been assessed. A 4- to 6-month trial of androgen therapy is implemented, followed by a similar period of rest for reevaluation. If prolonged therapy is required, testosterone cypionate is used, 50 to 400 mg IM every 2 to 4 weeks. It should be given deep in the IM. Inspect vials visually for particulate matter and discoloration before administration, and warm and shake the vial to dissolve any crystals that may have formed during storage. It takes 3 or 4 years for sexual development to occur, and plasma testosterone levels should be monitored and dosages adjusted as needed to maintain normal levels; if the serum testosterone level is below the normal range, the provider will adjust the dose upward. Therapy may be lifelong.
Testosterone may be administered buccally, nasally, transdermally, or parenterally. Drug selection depends on the balance of growth and sexual maturation desired and on the preferred route of administration. A buccal mucoadhesive system is available that provides a 30-mg dose every 12 hours. Advise the patient to place the rounded surface of the system against the gum above an incisor tooth and hold it firmly in place with finger over the lip and against the product for 30 seconds. To remove, slide gently downward toward the tooth to avoid scratching the gums. Sites must be rotated with each application. If the product falls off within the 12-hour dosing interval, or if it falls out of position within 4 hours before next dose, remove it and apply a new system. The patient should not chew or swallow the system. Advise the patient to regularly inspect gums where the system has been applied. Testosterones are considered Schedule III controlled substances.
Transdermal testosterone (TT) patches achieve adequate serum concentrations when applied to the arm, back, or upper buttocks. TT patches can be applied to any healthy skin site other than the scrotum or bony areas. Daily application of one to two TT 2 mg/24 h or 4 mg/24 h skin patches at 10 PM results in serum testosterone concentrations approaching those of healthy young men. The first day of dosing results in morning serum testosterone concentrations within the normal range. There is no testosterone accumulation with continued use. After removal of TT patches, hypogonadal status returns within 24 hours. Keep testosterone gel out of reach of children.
Testosterone gel is applied to clean dry skin of shoulders or upper arms. It should not be applied to the genitals. Hands should be thoroughly washed with soap and water following application. Testosterone gel carries a boxed warning, as it can be transferred to others through personal contact with skin or clothing. Children should avoid contact with unwashed application sites or application sites not covered by clothing. Children can experience virilization from secondary exposure. Caution is advised. 
Side Effects
Hypogonadal men on androgen therapy may experience frequent erections or priapism (painful, continuous erection), gynecomastia (mammary gland enlargement in men), or urinary urgency. Continued use of androgens by normal men can halt spermatogenesis (formation of spermatozoa). The sperm count may be low (oligospermia) for 3 or more months after therapy is stopped.
Other side effects of androgen therapy include abdominal pain, nausea, insomnia, diarrhea or constipation, hives or redness at the injection site, increased salivation, mouth soreness, and increased or decreased sexual desire. Advise the patient to notify the health care provider if side effects persist, worsen, or are bothersome.
Adverse Reactions
Androgen therapy may cause hypercalcemia by stimulating bone resorption in immobilized patients and those with breast cancer. The drug should be discontinued and appropriate measures instituted if signs of hypercalcemia occur; signs include nausea and vomiting, lethargy, decreased muscle tone, polyuria, and increased urine and serum calcium.
Virilization refers to the development of male secondary sex characteristics in women or hypogonadal males. Such characteristics include growth of facial hair, acne and skin oiliness, and vocal huskiness. Menstrual irregularities or amenorrhea, suppressed ovulation or lactation, baldness or increased hair growth (hirsutism), and hypertrophy of the clitoris may develop in women undergoing androgen therapy. Although most adverse effects slowly reverse themselves after short-term therapy is completed, vocal changes may be permanent. With long-term therapy, as in the treatment of breast cancer, adverse effects may be irreversible.
Children may experience profound virilization as well as impaired bone growth. During pregnancy, androgens can cross the placenta and cause masculinization of the fetus. Virilization can occur in those secondarily exposed to testosterone gel and may cause teratogenic effects in fetuses. Women and children should not handle the gel and should avoid contact with application sites in men using testosterone gel.
Less frequent adverse effects include dizziness, weakness, changes in skin color, frequent headaches, confusion, respiratory distress, depression, pruritus, allergic skin rash, edema of the lower extremities, jaundice, bleeding, paresthesias, chills, polycythemia, muscle cramps, and sodium and water retention. Hepatocellular carcinoma can occur in patients who have received selected androgens for long-term therapy and in cases of abuse of androgenic hormones by athletes.
Serum cholesterol may become elevated during androgen therapy. Other alterations in laboratory tests include increased hematocrit, altered thyroid and liver function tests, and elevated urine 17-ketosteroids (a by-product of the breakdown of androgens). Rare complications of long-term therapy include hepatic necrosis, hepatic peliosis (blood-filled cysts), hepatic tumors, and leukopenia.
Contraindications
Androgen therapy is contraindicated during pregnancy and in individuals with nephrosis or those in the nephrotic phase of nephritis; it is also contraindicated in patients with hypercalcemia, pituitary insufficiency, hepatic dysfunction, benign prostatic hyperplasia (BPH), prostate cancer, or history of myocardial infarction. Men with breast cancer are not treated with androgens, nor are women whose breast cancer is not estrogen dependent.
Caution must be exercised when using androgen therapy in individuals with hypertension, hypercholesterolemia, coronary artery disease, renal disease, or seizure disorder. It is used with caution in infants and prepubertal children because of the potential for growth disturbances and in older men because of their increased risk for BPH and prostate cancer.
Drug Interactions
Androgens potentiate the effects of oral anticoagulants, necessitating a decrease in anticoagulant dosage. Androgens antagonize calcitonin and parathyroid hormones. Because androgens can decrease blood glucose in patients with diabetes, dosages of insulin or other antidiabetic agents may need to be reduced. Concurrent use of corticosteroids exacerbates the edema that can occur with androgen therapy. Barbiturates, phenytoin, and phenylbutazone decrease the effects of androgens.
Anabolic Steroids
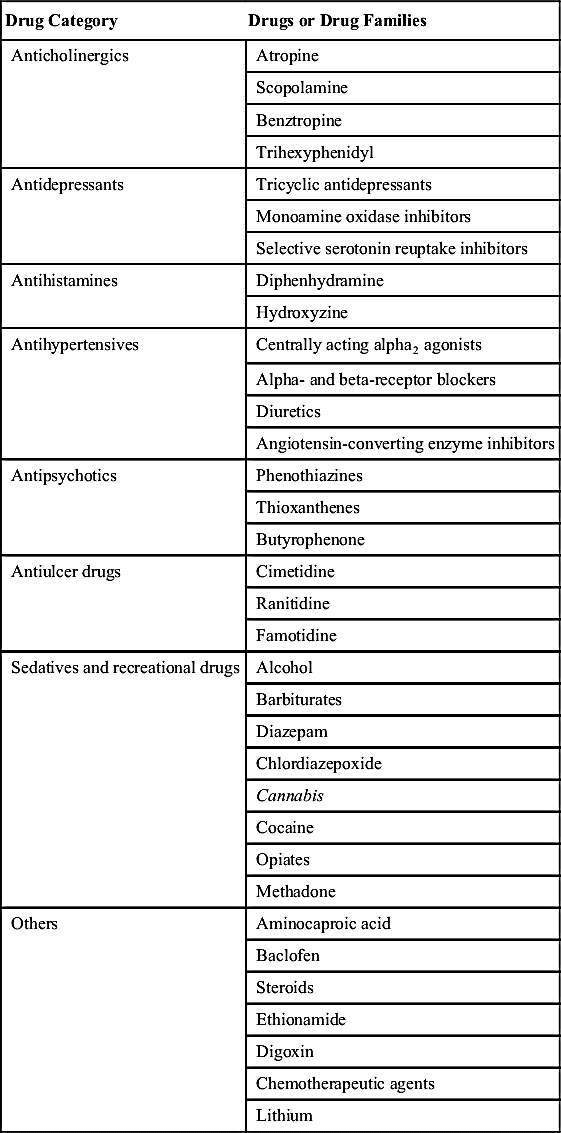
Anabolic steroids, or anabolic-androgenic steroids (AASs), are a class of steroid hormones related to the hormone testosterone. They increase protein synthesis within cells, which results in the buildup of cellular tissue (anabolism), especially in muscles. Anabolic steroids also have androgenic and virilizing properties, including the development and maintenance of masculine characteristics such as the growth of the vocal cords and body hair. While the American College of Sports Medicine notes that AASs, combined with sufficient diet and exercise, can contribute to increased lean body mass in some individuals, they also note it is in the best interest of all sports to eradicate the use of AASs by athletes, due to the risk of serious harm or death in those who use AASs.  See Chapter 7, Drugs in Substance Use Disorder, for further information.
See Chapter 7, Drugs in Substance Use Disorder, for further information.
 See Chapter 7, Drugs in Substance Use Disorder, for further information.
See Chapter 7, Drugs in Substance Use Disorder, for further information.Testosterone precursors available as nutritional supplements include androstenediol, androstenedione, and dehydroepiandrosterone (DHEA). Older teens are the heaviest users, but more than one-half million junior high school students also use them. Marketed as “sport supplements” or “teen formulas,” they can be purchased without a prescription in stores and on the Internet. A sudden dramatic increase in weight and body size, increased acne, and changes in mood and behavior can be signs of exogenous anabolic steroid use. Individuals using anabolic steroids may become more aggressive and physical, and health risks can result from long-term use or excessive intake of anabolic steroids; these effects include increased low-density lipoprotein (bad) cholesterol and decreased high-density lipoprotein (good) cholesterol, acne, high blood pressure, liver damage, and dangerous changes in the structure of the left ventricle of the heart. Adverse effects may not be recognized until years later. See Chapter 7, Drugs in Substance Use Disorder, for further information.
Two other steroids that have gained popularity, especially with athletes, are human chorionic gonadotropin (hCG) and tetrahydrogestrinone (THG); hCG is a hormone used to treat infertility and stimulate testosterone production, and THG is a potent androgen developed to escape detection on urine drug screens, although tests have since been developed for rapid screening. It is not approved by the U.S. Food and Drug Administration (FDA) and is not legally marketed. All major athletic organizations prohibit the use of anabolic steroids, but their continued use despite bans has led to “antidoping” investigations and punitive action.
 Cultural Considerations
Cultural Considerations• Assess how the patient’s cultural group regards expressions of sexuality.
• Ascertain any culturally defined expectations about male-female or male-male relationships, including health care relationships.
• Ask if the patient has restrictions related to sexuality, exposure of body parts, or discussion of sexual functioning.
Evaluation
• Monitor the patient’s ability to adhere to a treatment regimen and response to prescribed drugs. The ability to adhere to a treatment plan and discuss treatment and effects knowledgeably suggests that teaching has been effective and that the patient accepts the treatment.
• Ask the patient about therapeutic and adverse drug effects on follow-up visits. Monitoring of weight, blood pressure, and laboratory tests will continue throughout therapy with alterations in the plan of care as needed.
• Periodically assess children and women who experience virilizing effects or acne for the ability to cope with these changes and to maintain a positive self-concept.
• Assess sexual function when appropriate.
Antiandrogens
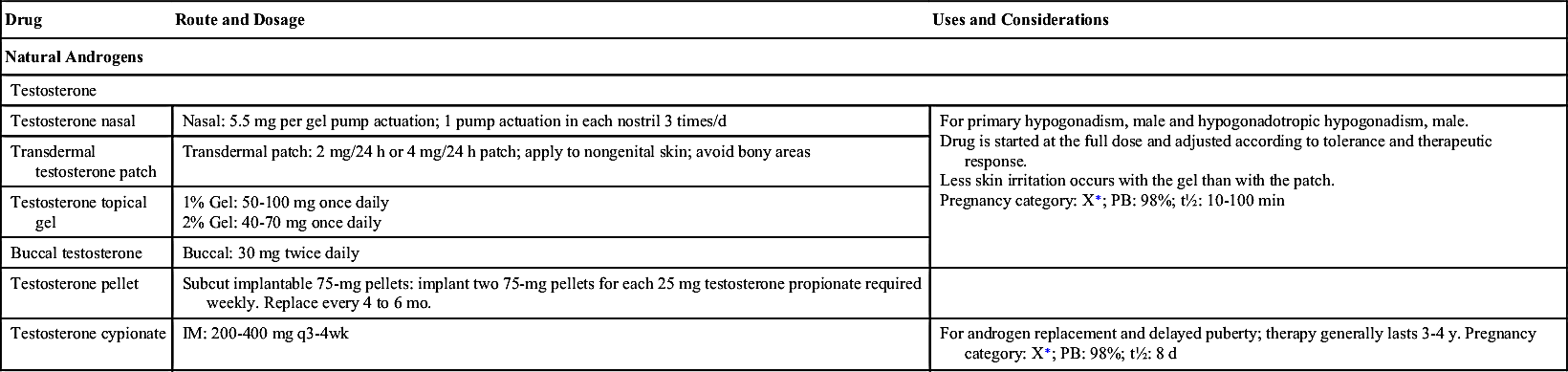
Antiandrogens, or androgen antagonists, block the synthesis or action of androgens (Table 53.3). These drugs are used in the treatment of BPH, advanced prostatic cancer, and as hormonal therapy in the treatment of endometriosis. They may also be used to treat male-pattern baldness (MPB), acne, hirsutism, virilization syndrome in women, and precocious puberty in boys, although their effectiveness is not well established.
Gonadotropin-releasing hormone (GnRH), or a synthetic analogue such as leuprolide, is the most effective inhibitor of testosterone synthesis. When such agents are given over time, LH and testosterone levels fall. Normally, GnRH is released in a pulsatile fashion, but the sustained activity of leuprolide leads to decreased production of FSH and LH. In the male, this activity stops testosterone production in the testis. In the female, it stops estrogen production in the ovaries. This drug should not be used in combination with herbal or dietary supplements such as black cohosh, chasteberry, or DHEA.
Ketoconazole, an antifungal drug, has testosterone-suppressing effects similar to those of GnRH analogues when given at doses higher than those required for antifungal activity (400 mg orally every 8 hours as opposed to 200 to 400 mg daily for the treatment of fungal infections). The mechanism of action of this non–GnRH drug is unclear. GnRH analogues act directly through their impact on testosterone production for the treatment of prostate cancer. Ketoconazole as treatment of prostate cancer is an off-label use.
Flutamide is an oral nonsteroidal antiandrogen drug used as an antihormonal agent in the treatment of metastatic prostate cancer. It is most effective when used simultaneously with luteinizing hormone–releasing hormone (LHRH) analogues such as leuprolide acetate. When used alone, the best response is seen in untreated patients. Flutamide is not effective in treating other hormonally dependent diseases such as breast cancer or BPH. Flutamide competes with androgens at androgen receptor sites in the prostate gland, blocking the conversion of testosterone to dihydrotestosterone; by doing so, it prevents the androgens from stimulating the prostate cancer cells to grow. Men receiving flutamide show elevations in plasma LH and testosterone levels. Bicalutamide is structurally related to flutamide but has a long plasma half-life that allows once-daily dosing, compared with thrice-daily dosing for flutamide. In addition, bicalutamide is more selective for the peripheral androgen receptor and has less activity at the central androgen receptor on the hypothalamic-pituitary axis.
Spironolactone is a weak potassium-sparing diuretic used primarily to treat high blood pressure, heart failure, and ascites in patients with liver disease. Although not FDA approved for these indications, spironolactone has also been used to treat acne vulgaris and idiopathic hirsutism. Dosage ranges from 25 to 200 mg/day in divided doses with a maximum adult dose of 400 mg/day.
Finasteride, a synthetic compound, inhibits conversion of testosterone to DHT. This orally active agent decreases the concentration of dihydrotestosterone in plasma and in the prostate without elevated plasma concentrations of LH or testosterone. Finasteride is used to treat BPH and MPB. The recommended 5-mg daily dose of finasteride for BPH needs to be reevaluated at 6 months and periodically thereafter. The 1-mg dose of finasteride for MPB is taken once a day for 12 months, and continued treatment is recommended for sustained results. Following discontinuation of therapy, reversal of effect is typically seen within 1 year. Adverse reactions include impotence, decreased libido, and decreased ejaculate. Women of childbearing age must not use finasteride and should not handle crushed or broken tablets because the active ingredient may cause abnormalities of a male fetus’s sex organs.
Drugs Used in Other Male Reproductive Disorders
Delayed Puberty
Puberty is considered to be delayed when testicle enlargement, followed by penile growth and pubic hair development, has not begun by age 14. Delay in growth may be a normal part of the maturation process, but the cause could also be androgen deficiency or a deficiency of growth hormone. Secretion of GnRH, LH, or FSH is insufficient in up to 5% of cases of delayed puberty. Treatment is only begun after 14 years of age, following a full evaluation, including serum LH, FSH, thyroid-stimulating hormone (TSH), and testosterone levels. Therapy with testosterone cyprionate 50 mg IM every month, for 3 to 6 months or less before epiphyseal closure, may result in linear growth without adverse permanent effects on hypothalamic, pituitary, or gonadal maturation.
Pituitary, Thyroid, and Adrenal Disorders
Inadequate pituitary function can be another cause of hypogonadism. Menotropins are purified combination preparations of the human pituitary gonadotropins FSH and LH. These drugs are indicated when both LH and FSH levels are low. When given in combination with hCG, the drug stimulates testosterone production in men to encourage spermatogenesis when injected three times a week for 4 to 9 months to ensure adequate spermatozoa production. One ampule of a menotropin contains 75 international units each of LH and FSH. The initial dose is 75 to 150 international units subcutaneously (subcut) or 3 times per week. Subsequent doses are adjusted according to individual response but not exceeding 75 to 150 international units per adjustment; the maximum dose is 300 units 3 times per week. Use the lower abdomen (alternating sides) for subcut administration. Menotropin must be used immediately after reconstitution and must be protected from light. Advise patients to discard unused drug. Adverse effects include nausea, vomiting, diarrhea, gynecomastia, and fever.
Inadequate thyroid and adrenal gland function can also affect sexuality. Hypothyroidism, a deficiency in thyroid hormone, can be the result of insufficient thyroid hormone production or resistance to its effects at the target organs. The problem can be congenital or acquired. It can cause inhibited sexual desire, a lack of or decreased interest in sexual activity, and ED. In Addison disease, there is a deficit of both cortisol and the mineralocorticoid aldosterone. Men with Addison disease may experience inhibited sexual desire, ED, or diminished fertility. Both hypothyroidism and Addison disease are highly responsive to replacement therapy with the appropriate hormones. (Refer to Chapter 46 for a discussion of Addison disease.)
Sexual Dysfunction
Sexual dysfunction is the inability to experience sexual desire, erection, ejaculation, and/or detumescence—the four phases of the sexual response cycle in men. Inhibited sexual desire can result from androgen deficiency, an affective disorder, or discord in the sexual relationship. Many drugs can also cause sexual dysfunction (Table 53.4).
Ejaculatory dysfunction, impaired ejection of seminal fluid from the male urethra, can be psychogenic or a result of drug therapy, androgen deficiency, or sympathetic degeneration. Failure of detumescence (reduction of penile engorgement) is most commonly caused by penile disease, systemic disease, trauma, or adverse effects of drugs. Individuals who experience premature ejaculation related to excessive anxiety about sexual intercourse may be helped by treatment with one of the many antidepressants alone or in conjunction with psychotherapy.
Erectile dysfunction (ED) is the inability to achieve or maintain an erection satisfactory for sexual performance. ED happens when not enough blood flows to the penis during sexual stimulation. This may be caused by psychoemotional problems, diabetes, hypertension, lower urinary tract symptoms, pelvic surgery, vascular insufficiency, neurologic disorders, androgen deficiency or resistance, or diseases of the penis. ED caused by vascular insufficiency is occasionally treated on a short-term basis by local vasoactive drugs, including papaverine, phentolamine, prostaglandin E, and nitroglycerin. Other drug options for the treatment of ED include oral drugs, drugs injected into the penis, intraurethral alprostadil, or testosterone.
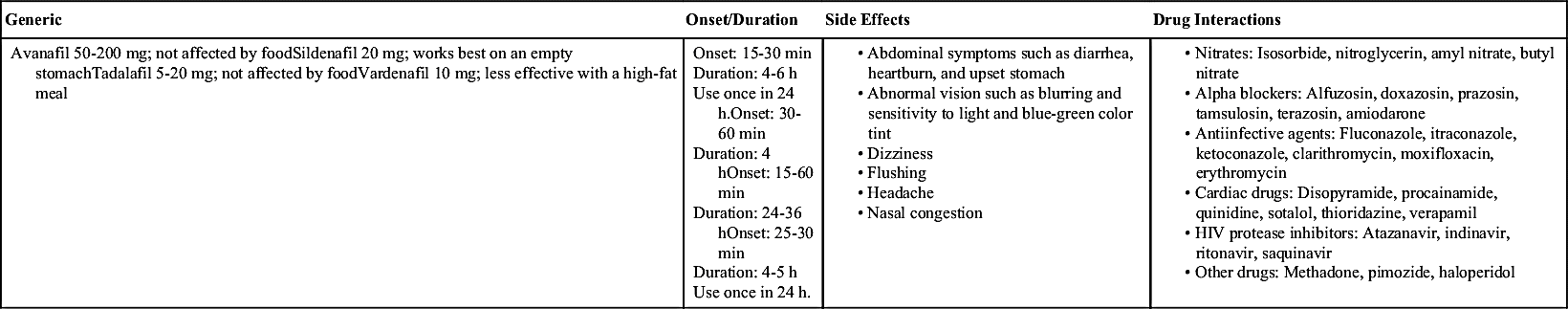
A class of drugs called phosphodiesterase inhibitors facilitate erection by enhancing blood flow to the penis. Phosphodiesterase-5 (PDE-5) inhibitors are currently the most commonly used medical treatment for ED. These drugs have a high success rate, are easy to use, and often result in erection during sexual stimulation. The PDE-5 inhibitors currently approved by the FDA are sildenafil, tadalafil, vardenafil, and avanafil. PDE-5 inhibitors are taken before sexual activity with variations in effective start time and duration of benefit (Table 53.5).
Nitroglycerin and other nitrate drugs used to treat heart disease can cause a significant decrease in blood pressure when taken with PDE-5 inhibitors, therefore this class of drugs is contraindicated for use by any patient using organic nitrates in any form. Patients with a prescription for as-needed (PRN) sublingual nitroglycerin can take PDE-5 inhibitors but should still be cautioned about taking them concomitantly. Organic nitrates include nitroglycerin, isosorbide mononitrate, isosorbide nitrate, isosorbide dinitrate/phenobarbital, pentaerythritol tetranitrate, erythritol tetranitrate, and illicit substances (amyl nitrate/nitrite, butyl nitrate). Sildenafil is contraindicated in patients with significant cardiovascular disease and in individuals who have anatomic deformities or conditions that predispose them to priapism.
Studies have shown that vardenafil and tadalafil are safer for patients with heart failure or a history of myocardial infarctions. Like sildenafil, they are contraindicated if the patient is taking nitrate-containing drugs. Common side effects of these drugs are headache (most common), dyspepsia, nasal congestion, and nasopharyngitis. Other rare side effects can also occur, and the patient should be taught about them; these effects include blurred vision, photosensitivity, changes in color perception (especially blue and green), hearing loss, seizures, priapism and urinary tract symptoms such as frequent or painful urination and cloudy or bloody urine. Patients are instructed to notify their health care provider of any side effects they experience.
The PDE-5 inhibitors interact with grapefruit juice and grapefruit products by increasing the amount of the PDE-5 inhibitor absorbed, thereby increasing the risk of side effects. Many drugs interact with PDE-5 inhibitors, so be sure to obtain a complete list of drugs taken by the patient.
Certain drugs are often abused by individuals seeking a heightened sexual experience. Amyl nitrate is commonly believed to be an aphrodisiac. Sudden death, myocardial infarction, and methemoglobinemia have been reported with its use. Cantharides (Spanish fly) causes bladder and urethral irritation, accounting for its use as a sexual stimulant. Permanent penile damage has been reported with its use.
Complementary and Alternative Medicine
To self-treat sexual problems or to enhance sexual performance, consumers use a wide variety of herbs and plant-derived compounds (phytochemicals). Despite new science-based therapies, men are attracted to phytochemicals because they are easy to obtain and may be cheaper than prescription drugs and therapies, procedures, or surgeries not covered by insurance. Consumers may mistakenly perceive natural products as providing health benefits beyond sexual performance because they can be purchased at nutrition centers or health food stores. Common herbs used for sexual health and performance include Pausinystalia yohimbine, Panax quinquefolius, ginkgo biloba, saw palmetto, muira puama, Lepidium meyenii, Mandia whitei, and Tribulus terrestris. Limited studies have been conducted on these products; all studies have yielded mixed results. As such, there is insufficient evidence to recommend any complementary or alternative medicine (CAM). Its benefits appear to reflect popular or cultural beliefs. Reports in health magazines and advertising are anecdotal and based on a small number of users. However, it is important for the nurse to be aware of CAM, as ED affects 50% of men between the ages of 50 and 70, but only 10% seek medical attention. These statistics indicate men may be choosing to use CAM to avoid the discomfort of discussing this sensitive topic with their health care provider.
Non–Sexually Transmitted Infections
Drugs used to treat acute or chronic prostatitis, orchitis, or epididymitis are the same as those used to treat urinary tract infections (see Chapter 48).
Benign Prostatic Hyperplasia
As a man ages, the glandular units in the prostate gland begin to undergo tissue hyperplasia, an abnormal increase in the number of cells, which results in prostatic hypertrophy, or enlargement of the gland. The exact cause of BPH is unknown, but its development is almost universal in older men. The enlarged prostate gland contributes to overall lower urinary tract symptoms either through direct bladder outlet obstruction or from resistance within the enlarged gland itself. The man experiences storage and/or voiding disturbances, such as a sensation of bladder fullness, frequency, nocturia, hesitation when trying to begin urinating, dribbling of urine, and erectile dysfunction. Although BPH is not a life-threatening condition, the impact of symptoms on quality of life can be significant. Because these are the same symptoms that prostate cancer may cause, the patient should have a prostate-specific antigen (PSA) blood test and may undergo a prostate biopsy to make sure no cancer is present. Traditionally, the primary goal of treatment has been to alleviate bothersome symptoms. More recently, treatment has also been focused on the alteration of disease progression and prevention of complications. Drugs from several pharmacologic classes are used; they include alpha-adrenergic antagonists (alpha blockers), 5-alpha-reductase inhibitors (5-ARIs), anticholinergics (only used in men without large post-void residuals), and the PDE-5 inhibitor tadalafil. Choosing the correct medical treatment for BPH is complex (Table 53.6). The American Urological Association 2010 Guidelines are a useful reference on the effective evidence-based management of lower urinary tract symptoms caused by BPH. The guidelines advise a discussion of benefits and risks of each recommended treatment alternative (e.g., watchful waiting; medical, surgical, or minimally invasive surgical treatments). The choice of treatment is reached in a shared decision-making process between provider and patient.
TABLE 53.6
Drugs Used for Treatment of Benign Prostatic Hyperplasia

| Safety in Administration |
| Women of childbearing age must not handle crushed or broken tablets due to possible absorption and potential risk to male fetuses; immediately wash contact area with soap and water if contact occurs. Inform patients of an increase in high-grade prostate cancer in men treated with 5-alpha-reductase inhibitors indicated for BPH treatment. Inform patients that the volume of ejaculate may be decreased and that impotence or decreased libido may occur. Instruct patients to promptly report to their physician any changes in breasts (lumps, pain, or nipple discharge). |

| Safety in Administration |
|
Alpha-adrenergic blocking agents are also used to control blood pressure. To avoid hypotensive episodes, advise using at bedtime, and reconcile all drugs taken before adding another antihypertensive drug.
Instruct patients to take these drugs with food. Counsel about possible symptoms of postural hypotension (dizziness); caution patients about driving, operating machinery, or performing hazardous tasks while using these drugs. Inform patients that orgasm with reduced or no semen does not pose a safety concern and is reversible when the drug is stopped. Advise patients to notify their ophthalmologist about use before cataract surgery or other eye procedures.
|

| Safety in Administration |
| Tadalafil should not be used by patients taking nitrates such as nitroglycerin because the combination may trigger an unsafe drop in blood pressure. Tadalafil should not be used with alpha blockers for the treatment of BPH because the combo therapy has not been adequately studied and may increase the risk of lowering blood pressure. |
With initiation of drug therapy, the patient should be followed every 1 to 3 months, depending on the class of drug chosen, to assess success and inquire about side effects. The patient with an enlarged prostate should avoid drugs that can cause urinary retention, such as anticholinergics, antihistamines, and decongestants.
Malignant Tumors
Prostatic cancer accounts for about 10% of all cancer deaths among American men. Most prostatic cancers are adenocarcinomas. Metastasis to lymph nodes, bone, lungs, liver, and adrenal glands is common. Prostatic cancer is often asymptomatic, but urinary obstruction is commonly the first sign. Treatment may include a combination of surgical resection, cryotherapy, antiandrogen administration, radiation therapy, chemotherapy, and pain management.
Testicular tumors peak in early adulthood. They include malignant germinal cell tumors and benign Leydig or Sertoli cell tumors. Treatment depends on the type and stage of the tumor. Surgical excision, radiation therapy, and chemotherapy are used alone or in combination.
Approximately 1% of breast cancer cases occur in men, most commonly after the age of 60 years. Treatment is similar for men and women and entails surgery, radiation therapy, chemotherapy, and endocrine therapy. Carcinoma of the penis represents less than 1% of all malignancies among men. In situ, treatment entails local excision, radiation therapy, and local application of 5-fluorouracil cream or solution. Invasive carcinoma is treated by surgical resection of the penis and the involved nodes. Radiation and chemotherapy follow as needed.
Critical Thinking Case Study
MT, age 16 years, is a high school junior who is 59.3 inches tall and weighs 126 pounds. He is having increased feelings of discomfort about not fitting in with the other students at school because he has not yet begun sexual maturation. He is a good student and an accomplished violinist in the school orchestra. His father states that he also was a “late bloomer,” but both parents are concerned about MT’s increasing social withdrawal and seem determined to seek medical intervention for him. The nurse at the clinic assesses the needs and status of MT and his parents.
1. What is the patient’s primary complaint?
2. What is concerning MT’s parents?
3. What information must be included in the history and physical examination?
4. What education should the nurse prepare before the parents decide whether to start their son on androgen therapy?The decision is made to prescribe testosterone 30 mg every 12 hours by buccal tablet (held inside the cheek until it dissolves). MT will be on this regimen for 4 months, during which time he is to come to the clinic at monthly intervals.
5. MT asks why he will be treated for 4 months. What will the nurse reply?
6. About what adverse effects do MT and his parents need to be educated?
7. What physical and psychosocial parameters will be assessed at MT’s monthly visits?
8. What special hygiene needs does MT have while on this regimen?
9. When should MT have x-rays taken? Explain your answer.
10. During a clinical visit, MT mentions that he heard that the use of anabolic steroids might improve his chances of making the wrestling team. What should he be told about the safety and efficacy of anabolic steroid use?