http://evolve.elsevier.com/McCuistion/pharmacology
A nurse who is providing care to children must make certain adaptations in assessment, treatment, and evaluation of nursing care because of the physiologic, psychological, and developmental differences inherent in the pediatric population. This is especially true in the science of pharmacology, in both the administration of drugs to children and evaluation of the therapeutic and adverse effects of a drug. This chapter addresses pediatric nursing adaptations and discusses the impact of a child’s growth and development on many aspects of pharmacology: pharmacokinetics, pharmacodynamics, dosing and monitoring, methods of drug administration, and nursing implications.
Pediatric pharmacology is limited to available research in the provision of dosing protocols, safe practices, key assessments, and important nursing implications. Most available information about drugs is derived from studies that use adult samples, small sample sizes, or samples with healthy children. Few studies have been conducted to determine the effectiveness of drugs in the pediatric population. Generalizing the results of studies using adult patients to pediatric populations may result in serious errors and ignores the impact of growth and development on pharmacology.
Research related to pediatric patients is limited because of several factors. Research risks and obtaining informed consent make it difficult to recruit a pediatric sample. Parents and guardians are reluctant to provide permission for children to participate in research studies because of the risk involved and the potentially invasive nature of data gathering. Pharmaceutical companies invest fewer resources in pediatric drug research because of the smaller market share afforded to pediatric drugs. However, many contend that lack of pediatric data reflects lack of due diligence, especially when drugs are administered to pediatric patients without supporting research data on which to base safe practices. As a result, less is known about the effects, uses, and dosages of pediatric drugs, and nurses must investigate pediatric drugs carefully to provide knowledgeable nursing care for children.
Closely aligned with the conflicts that affect pediatric pharmacologic research are those associated with drug labeling and dosing instructions. Because many drugs have not undergone the clinical trials required for federal approval, they have not been approved for pediatric use. Safe use for children may be guided by small studies or the judgment of the clinician and may be based on anecdotal evidence rather than scientific study. These conflicts have generated new legislation designed to protect pediatric patients and provide health care professionals with better information and resources.
Despite the permanent reauthorization of the Pediatric Research Equity Act (PREA) in 2012, which requires drug manufacturers to study pediatric drug use and offers incentives for pediatric pharmacology research, only half of all drugs carry federally approved indications for use in children. This means many drugs prescribed for children are being prescribed off label, which means the drug is being used for some purpose for which it has not been approved. Current research agendas reinforce the need for pediatric drug research and establishment of safe guidelines for pediatric drug dosing, administration, and evaluation.
Pharmacokinetics
Significant differences exist in drug pharmacokinetics for pediatric patients versus adults. These distinctions stem from differences in body composition and organ maturity and appear to be more pronounced in neonates and infants but less significant in school-age and adolescent children. Pharmacokinetics may be defined as the study of the time course of drug absorption, distribution, metabolism, and excretion.
Absorption
The degree and rate of drug absorption are based on factors such as age (Table 5.1), health status, weight, and route of administration. As children grow and develop, the absorption of drugs generally becomes more effective; therefore less developed absorption in neonates and infants must be considered in dosage and administration. In contrast, poor nutritional habits, changes in physical maturity, and hormonal differences during the adolescent years may cause slowing of drug absorption. Hydration status, presence of underlying disease, and gastrointestinal (GI) disorders in the child may be significant factors in the absorption of drugs.
TABLE 5.1
| Classification | Age |
| Term neonate | Birth at 38 or more weeks’ gestation to 27 days |
| Infant/toddler | 28 days to 23 months |
| Children | 24 months to 11 years |
| Adolescent | 12 years to 16 or 18 years (regional difference) |
From U.S. Food and Drug Administration. (2014). Pediatric exclusivity study age group (C-DRG-00909). Retrieved from http://www.fda.gov/Drugs/DevelopmentApprovalProcess/FormsSubmissionRequirements/ElectronicSubmissions/DataStandardsManualmonographs/ucm071754.htm
Drug absorption is initially influenced by the route of administration. For oral drugs, conditions in the stomach and intestine such as gastric acidity, gastric emptying, gastric motility, GI surface area, enzyme levels, and intestinal flora all mediate drug absorption. Lack of maturation of the GI tract is most pronounced in infancy, making the neonatal and infancy periods those most affected by changes in absorption physiology. Gastric pH is alkaline at birth; acid production begins in the neonatal period, and gastric acid secretion reaches adult levels around 2 to 3 years of age. A low pH, or acidic environment, favors acidic drug absorption, whereas a high pH, or alkaline environment, favors basic drug formulations; therefore differences in pH may hinder or enhance drug absorption. Gastric emptying and GI motility are unpredictable in neonates and infants; however, it approaches that of adults between 6 and 8 months of age. Gastric emptying is affected by feeding, and breast-fed infants have faster gastric emptying than formula-fed infants. Unpredictable GI motility may hinder or enhance absorption of oral drugs, depending on the usual site of chemical absorption.
Intestinal surface area in neonates does not reach that of adults until 20 weeks; prior to this, the reduced surface area leads to reduced drug absorption. Immature enzyme function may also affect drug absorption; neonates have inadequate production of bile salts and pancreatic enzymes, which leads to reduced absorption of lipid-soluble drugs. Intestinal microbial colonization begins in the first few hours after birth and is influenced by gestational age and whether the neonate is breast or formula fed; GI microbial colonization reaches adult levels in adolescence. All of these factors must be considered when assessing the effectiveness of drugs administered by the oral route.
For drugs administered via the subcutaneous (subcut) or intramuscular (IM) routes, absorption occurs at the tissue level. The level of peripheral perfusion and effectiveness of circulation affects drug absorption. Conditions that alter perfusion—dehydration, cold temperatures, and alterations in cardiac status—may impede absorption of drugs in the tissues. Intravenous (IV) drugs are administered directly into the bloodstream and are immediately absorbed and distributed.
Distribution
Drug distribution is affected by factors such as body fluid composition, body tissue composition, protein-binding capability, and effectiveness of various barriers to drug transport. In neonates and infants, the body is about 75% water, compared with 60% in adults. This increased body fluid proportion allows for a greater volume of fluid in which to distribute drugs, which results in a lower drug concentration. Until about age 2 years, the pediatric patient requires higher doses of water-soluble drugs to achieve therapeutic levels. Younger patients also have higher levels of extracellular fluids, which increases the tendency for children to become dehydrated and changes the distribution of water-soluble drugs. Compared with older children, neonates and infants have fat stores with an increased ratio of water to lipids, which alters the distribution of some lipid-soluble drugs. Close monitoring of drug levels (e.g., anti-epileptic drugs) can help ensure drug safety.
To varying degrees, drugs become bound to circulating plasma proteins in the body. Only drugs that are free, or unbound, are available to cross the cell membrane and exert their effect. Neonates and infants have decreased protein concentrations compared with adults, and they have fewer protein receptor sites with an affinity for drug binding in the first 12 months after birth; this results in higher levels of unbound drug and an increased risk of drug toxicity.
In neonates, high bilirubin levels may pose a health risk related to drug administration. Bilirubin molecules may bind with protein receptor sites, which makes the sites unavailable to drugs or displaces drugs from binding sites, allowing large amounts of drug to remain free and available for effect. When drugs are prescribed to neonates, dosages must be decreased and closely monitored to both avoid adverse effects and ensure therapeutic effectiveness.
Anatomic barriers to drug distribution, such as the blood-brain barrier (BBB), must be considered when drugs are administered to pediatric patients. This barrier in neonates is relatively immature and allows drugs to pass easily into central nervous system (CNS) tissue, thereby increasing the likelihood for toxicity. As a child matures, the BBB becomes more impervious to drugs, and drug dosages must be titrated accordingly.
Metabolism
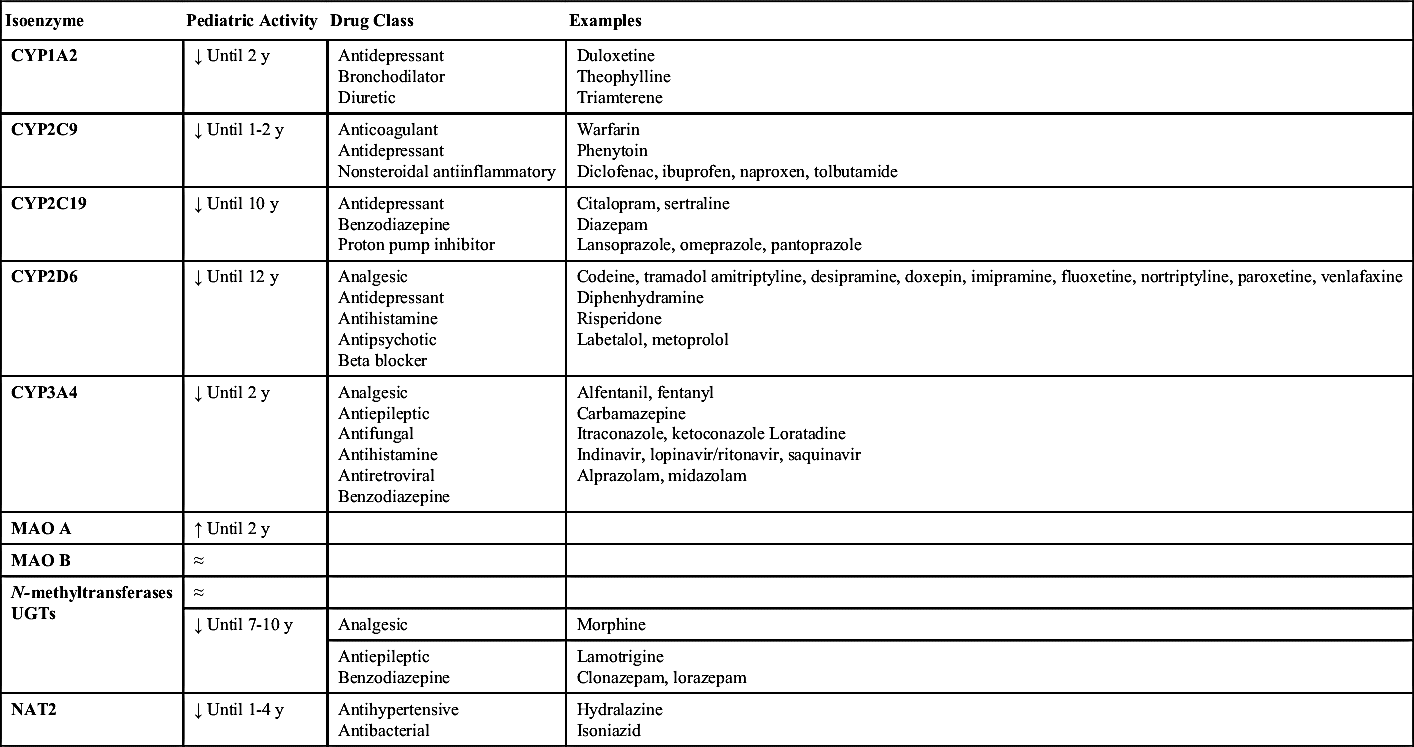
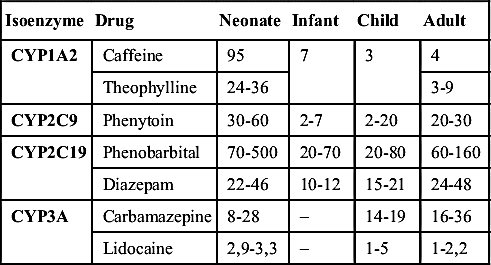
The metabolism of drugs depends greatly on the maturation level of the pediatric patient and varies from child to child. Metabolism is carried out primarily in the liver, with the kidneys and lungs playing a small part in metabolism. Infants have reduced hepatic blood flow and drug-metabolizing enzymes; however, by the time they reach 1 year of age, hepatic blood flow has reached that of an adult. Whereas drug-metabolizing enzymes reach an adult level at around age 11, it is important to understand that the isoenzymes involved in the cytochrome P450 system—CYP1, CYP2, and CYP3 (Table 5.2)—develop at different rates and demonstrate individual variation. Drug prescribing should be based on therapeutic effect and drug concentration. Such differences in drug metabolism, as with other pharmacokinetic factors, reinforce the importance of the nurse evaluating therapeutic effects and monitoring the adverse effects of drugs.
Excretion
Renal excretion is the predominant means of drug elimination. The glomerular filtration rate (GFR) in term neonates is roughly 30% that of adults. During infancy, the GFR rises, and by 12 months, it reaches adult levels. Nurses must carefully monitor renal function, urine flow, and drug effectiveness to evaluate the impact of drug administration on patient status.
Pharmacodynamics
Pharmacodynamics refers to the mechanisms of action and effects of a drug on the body and includes the onset, peak, and duration of effect of a drug. It can also be described as the intensity and time course of therapeutic and adverse effects of drugs. The variables of pharmacokinetics—absorption, distribution, metabolism, and excretion—all affect the parameters of pharmacodynamics. These processes determine the time a drug begins to function, reaches its peak, and sustains its length of action. Variables such as organ function, developmental factors, and administration issues affect drug pharmacodynamics and drug half-life in pediatric patients (Table 5.3), and these have an impact on drug dosing.
Nursing Implications
Pediatric Drug Dosing and Monitoring
Because of the changes in pharmacokinetics and pharmacodynamics inherent in pediatric patients, a key nursing role is to monitor the patient for therapeutic effect and adverse reactions. The processes described earlier in the chapter may be measured using plasma or serum drug levels, which indicate the amount of drug in a patient’s body. The therapeutic ranges established for many drug levels are based on adult studies; therefore close monitoring of serum drug levels can assist in establishing appropriate dosages, schedules, and routes of administration. Monitoring can also assist in indicating when the dose is subtherapeutic or becomes toxic. Serum blood levels are not available for all drugs, so patient clinical responses to drugs are especially important when monitoring drug effects.
The calculation of pediatric dosages is based in part on U.S. Food and Drug Administration (FDA) recommendations; as a result of the Best Pharmaceuticals for Children Act (BPCA) and PREA, pediatric dosing is now available for over 450 drugs. For those drugs without pediatric dosing schedules, dosing is based on approved protocols, research studies, and provider experience. Drugs for pediatric patients are ordered based on either the child’s weight in kilograms (mg/kg) or body surface area (BSA; or mg/m2). Body surface is based on a percentage of adult surface area (1.73 m2). Dosing must also consider the individual child’s status, including age, organ function, health, and route of administration.
Pediatric Drug Administration
Developmental and cognitive differences must always be considered in pediatric drug administration. It is important for the nurse to differentiate the child’s developmental age from chronologic age, because this difference has an impact on the child’s response to drug administration. The pediatric patient’s ability to understand the process, the reason for drug administration, and the need to cooperate with the procedure must always figure prominently in the nurse’s plan of care. The child’s temperament may influence understanding and level of cooperation. The concept of family-centered care is essential to ensuring safety during and after health care interventions, especially drug administration. Teaching is directed toward both family members or caregivers and patients, commensurate with the cognitive level of the child. When possible, family members or caregivers should be solicited to assist in drug administration. These significant persons in the child’s life, individuals who see the child on a day-to-day basis, are usually in the best position to evaluate the effectiveness of a drug and observe for adverse reactions. Some adverse drug reactions in children, such as ringing in the ears and nausea, may be difficult to evaluate; those closest to the child may be in the best position to assess for these reactions. However, family members or caregivers may request not to participate in invasive procedures such as injections. This request should be respected, and family members or caregivers should be encouraged to provide comfort to the child after drugs are administered. Family members or caregivers should always be supported in their caring function so that the child feels safe and secure.
Pediatric patients must be assessed for the ability to understand the reason for the drug, the need for the drug despite unpleasant taste or method of administration, and the need to complete all doses and courses of the drug. When the family is taught about pediatric drug administration, education for the child at a developmentally appropriate level must also be included. Communication with the child and family members or caregivers must always consider level of knowledge, developmental age, cultural factors, and anxiety levels. The nurse should use optimal interpersonal skills to ensure the best outcome in drug administration to pediatric patients.
The primary concerns in drug administration to infants are maintaining safety and providing care while ensuring as much comfort as possible. Family members or caregivers must be able to practice and repeat the psychomotor skills associated with drug administration. The following are tips to enhance safe drug administration and facilitate comfort:
• Toddlers may react violently and negatively to drug administration. Simple explanations, a firm approach, and enlisting the imagination of a toddler through play may enhance success.
• Preschoolers are fairly cooperative and respond well to age-appropriate explanations. Allowing some level of choice and control may facilitate success with preschool children.
• School-age children, although often cooperative, may fear bodily injury and should be permitted even more control, involvement in the process, and information.
• Age-appropriate fears related to pain, changes in body image, and injury are prevalent among older school-age and adolescent patients. The nurse should establish a positive rapport with the patient, develop the plan of care in collaboration with the patient, and ensure privacy in all aspects of drug administration.
Atraumatic care principles should be used when possible. Donna Wong’s Principle of Atraumatic Care is “the philosophy of providing therapeutic care through the use of interventions that eliminate or minimize the psychologic and physical distress experienced by children and families.” Atraumatic care is achieved by decreasing the separation of children from their family members or caregivers, identifying family and patient stressors, decreasing pain, and providing care within the framework of a collaborative partnership.
TABLE 5.4
Dosage Form Variability for Pediatric Age Groups
| Neonates: 0-4 weeks | ??? |
| Infants: 1 month-2 years | Liquids—small volumes (syrups, solutions) |
| Children: 2-5 years | Liquids; effervescent tablets dispersed in liquids; sprinkles on food |
| Children: 6-11 years | Solids (chewable tablets, orally disintegrating tablets, oral films) |
| Adolescents: 12-18 years | Solids (typical adult dosage forms—tablets, capsules) |
From Pinto, J. C. (n.d.). Pediatric dosage development: Where are we? [PowerPoint slides]. Retrieved from http://www.fda.gov/downloads/NewsEvents/MeetingsConferencesWorkshops/UCM415217.pdf
Most pediatric drugs are administered via the oral route (Table 5.4). This route is the least invasive and easiest to use and can be used by family members or caregivers. Topical, rectal, and parenteral routes are also used to deliver drugs to pediatric patients for whom the oral route is contraindicated. Because of tissue differences among children, the IV route is more predictable than other routes.
Most oral drugs administered to children under the age of 6 are given using an oral syringe. Oral syringes ensure more exact dosing and are relatively easy to use. Syringes may be marked to ensure correct dosages. The syringe is inserted into either side of the mouth and is pointed toward the buccal mucosa. Depositing the drug too close to the front of the mouth increases the likelihood that it will be spit out. Pointing the syringe directly toward the back of the mouth may increase the risk for gagging or choking. Infants may suck drugs from a bottle nipple into which the measured drug has been squirted from an oral syringe. Preschool and school-age children are usually able to inject oral drugs into their own mouths, enhancing their sense of control over what can be an anxiety-provoking situation.
Nurses may need to crush pills or dissolve the contents of capsules in fluid for administration to pediatric patients. The nurse should work closely with the pharmacist and in compliance with hospital policies to determine the advisability of crushing or dissolving a drug before administration; some drugs, particularly timed-release and enteric-coated drugs, should not be crushed or dissolved. Some drugs may be made more palatable by adding jam, yogurt, or honey (although infants younger than 1 year should not be given honey because of the risk of botulism). Small volumes (10 mL) should be used to dilute drugs so the patient is ensured the full dose. For children who require tube feeding, oral drugs can be administered via nasogastric, orogastric, or gastrostomy tubes, if the drugs can be crushed or dissolved prior to administration.
When drug injection or venipuncture is necessary, topical anesthetic protocols may be followed to reduce the pain associated with the procedure. Agents such as eutectic mixture of local anesthetics (EMLA), topical liposomal 4% lidocaine cream (LMX4), or a vapocoolant spray may be effective in reducing the pain and fear associated with invasive procedures, such as injection or venipuncture, in children.
Based on the cognitive level of the child, other nonpharmacologic methods of pain and anxiety control such as distraction, diversion, relaxation, and creative imagery can also be used to decrease the perception of pain. Injections should never be given to a sleeping child with the intent to surprise the child with a quick procedure. The child may subsequently experience a lack of trust and may be reluctant to sleep in the future.
IV infusion sites must be protected, especially in infants and toddlers, who do not understand the rationale or importance of maintaining the IV site. Commercial products are available to protect the IV site and maintain an intact IV infusion set. Stocking-like covers may hide the IV site from infants before they master the concept of object permanence. The patency of an IV site should be checked prior to each drug administration to avoid infiltration and extravasation. Any injection site on a preschooler should be covered with a bandage, preferably a decorated one, so that the child does not fear “leakage” from the area. Selection of injection and IV sites is made based on developmental variables, site of preference, and access to administration sites. The ventrogluteal or vastus lateralis are preferred sites for pediatric IM injections. The length of the needle depends on the child’s muscle mass, subcutaneous tissue, and the site of injection. Children may prefer subcutaneous injections in the leg or upper arm rather than in the abdomen. IV sites may be difficult to find in children. The amount of fatty tissue, hydration status of the child, and ability to isolate and immobilize veins are all mitigating factors.
When administering drugs to children, follow these basic principles: honesty, respect, age-appropriate teaching and explanations, attention to safety, atraumatic care, use of the least amount of restraint necessary (e.g., swaddling a neonate), providing positive reinforcement for age-appropriate cooperation, refraining from use of negative messages or behaviors, and upholding family-centered principles. These standards may be used throughout the pediatric life span and highlight the need for nursing interventions that are sensitive, individualized, and caring.
Considerations for the Adolescent Patient
Adolescent patients need individualized nursing care specific to their developmental stage. Age-oriented developmental considerations include physical changes, cognitive level and abilities, emotional factors, and impact of chronic illness.
Physically, adolescence is a highly diverse period of growth and development. Growth rates during these years may be affected by nutrition, factors within the environment, genetics and heredity, and gender. A group of adolescents of similar ages may manifest very different sizes, height-to-weight proportions, timing of secondary sex characteristics, and other indicators of physical maturity. These differences may warrant individualization of drug dosage based on weight or body surface area, even when the adolescent meets or exceeds the size of standard adults. For example, an adjustment may be required in the dosage of a lipid-soluble drug because of the changes in lean-to-fat body mass, especially in young adolescent males, that coincide with physical maturation. Hormonal changes and growth spurts may necessitate changes in drug dosages; many children with chronic illnesses require dosage adjustments in the early teen years as a result of these transitions. Sleep requirements and metabolic rates may greatly increase during the teen years, along with appetite and food consumption, which may affect the scheduling of and response to drugs. Although adolescents’ physical appearance and organ structure and function resemble those of adults, their bodies continue to grow and change; this requires increased vigilance in monitoring therapeutic and toxic drug levels.
The cognitive level and abilities of adolescents may pose additional considerations. Cognitive theorists have posited that adolescents progress from concrete to abstract reasoning. Individuals who are still in the concrete operational stage may have difficulty comprehending how a drug exerts its effects on the body and the importance of meticulous dosing and administration. Adolescents may also have difficulty understanding such concepts as drug interactions, side effects, adverse reactions, and therapeutic levels. For example, the patient taking birth control pills may or may not be able to comprehend the reduced action of birth control pills caused by antibiotics taken during an acute infection and may fail to take extra precautions to prevent pregnancy.
An understanding of the adolescent brain and the ongoing development of social, reasoning, and decision-making skills can be used to guide nursing assessment and interventions with the pediatric patient. As adolescents learn to reason in an abstract manner, teaching may be based on more complex information. Potentially, adolescent perception of invulnerability and difficulty relating future consequences to current actions may dictate that the nurse adapt teaching to address specific adolescent thought processes. An adolescent who is told that an insulin injection schedule must be adhered to in order to avoid long-term complications may not understand the rationale for treatment if it is only substantiated by abstract, future-oriented risks. The same patient may find the relationship between using insulin to maintain normoglycemia and the ability to participate in sports more immediate and relevant. Allowing the adolescent to verbalize concerns about the drug and its regimen may offer opportunities for clarifying misconceptions and teaching new concepts.
Emotional development of the adolescent also occurs on an individual basis. The adolescent years are characterized by sensation seeking, risk taking, questioning, formation of identity, and increasing influences exerted by peer groups. To avoid potential drug interactions, the nurse should assess for high-risk behaviors that include use of alcohol, tobacco, and recreational drugs. Other issues, including sexual practices and stressful family and social situations, may affect the patient’s response to drugs. Nurses must be respectful of the emotional needs of adolescence while attending to the mental health issues that may surface during these years. A comprehensive history must be solicited from adolescent patients to ensure appropriate drug administration. The nurse must also be conscious of the need to exercise care in offering confidentiality in the event that information needs to be divulged to other health care providers, family members, or caregivers to ensure patient safety.
As adolescents attain greater levels of independence from their parents, self-care behaviors increase. The nurse should assess the patient’s abilities to self-administer drugs and monitor therapeutic and adverse reactions. Adolescents spend less time with family members and caregivers and may need increased instruction about their drug regimen and the key observations that are needed. Although adolescents frequently display “breaking away” behaviors in response to parental bonds, they often continue to use family members or caregiver drug habits as models for their own drug behaviors.
For the pediatric patient with a chronic illness, issues may change during adolescence. Engaging peers in the plan of care for drug administration, allowing the adolescent to make safe choices and have flexibility within that plan, setting up mutual drug contracts, and permitting the patient to design their own adult-monitored drug regimen may facilitate adherence. The nurse can facilitate required adaptations and support both the patient and family members during these times.
Nursing Process: Family-Centered Collaborative Care
In working with pediatric patients, key developmental differences must be considered when administering and monitoring drugs. The nursing process provides the framework to guide nursing practice in administering drugs, planning and evaluating nursing care, providing patient and family teaching, and incorporating the family into all aspects of treatment.
Family and patient teaching is a key role for the nurse. Issues such as indications for the drug, the side effects, the dose, how to measure the dose, how to administer the dose, the therapeutic effect, adverse effects to monitor for, the duration, and the frequency are all important information needed by the family or caregiver. Specifics such as the need for refrigeration, the need to shake the medicine, the difference between household and prescriptive measurements, and other issues should be addressed to ensure patient safety. Adherence to the drug regimen is of paramount importance with children and families; providing written instructions or a drug calendar may facilitate this through concrete reminders.
Nurses should also be aware of the tendency for parents to treat infants and children with over-the-counter (OTC) analgesics. Parents may provide frequent analgesia to their children and may be largely unaware of the potential for misuse and overuse in the pediatric population. Additional concern has arisen regarding the inappropriate use of OTC cough and cold remedies with children. Deaths and significant illness have been attributed to lack of label recommendations, misuse of adult drugs, poor drug instructions, and overdose, which warrants rigid restrictions on the use of these drugs in the pediatric population.
Evaluation
• Evaluate the child’s physiologic and psychological response to the drug regimen.
• Evaluate the family member’s knowledge about the drug, the dosage, the schedule for administration, and the side effects.
• Evaluate the therapeutic and adverse effects of the drug(s).
Critical Thinking Case Study
A 9-month-old infant weighing 20 pounds comes to the emergency department with a 3-day history of vomiting, fever greater than 102.5°F, and significant pain. Physical assessment reveals acute otitis media, for which the doctor prescribed amoxicillin 500 mg three times a day for 5 days and ibuprofen 2.5 mL every 6 hours.
1. Prior to administration of amoxicillin, what must the nurse assess for?
2. If the safe dose of amoxicillin for a child under the age of 2 is 80 mg/kg/day in divided doses, is the prescribed dose safe? How do you know?
3. How will you instruct the family member to safely administer the drugs?