Menopause
The formal definition of menopause is the permanent cessation of menses, clinically defined as 12 months after a woman’s last menstrual period. It marks the end of a woman’s normal ovarian function.
2. When does menopause usually occur?
The median age for the last menses is defined at 51.4 years. There is large variability in the exact age at which menopause occurs. A genetic component appears to be involved because women often experience menopause around the same time as their mother or sister, but there are numerous examples of circumstances when this does not hold true. It also appears that women in Asia may experience menopause at an earlier age (42–49 years old).
3. How is menopause diagnosed clinically?
In a woman who is more than 45 years old, 12 months of secondary amenorrhea is sufficient to diagnose menopause. Although a pelvic examination may reflect some atrophy of the vaginal mucosa, this is not always remarkable. Generally, there are elevations in both follicle-stimulating hormone (FSH), which rises significantly (approximately 10- to 20-fold), and luteinizing hormone (LH), which has a more modest rise (approximately 3-fold). It is generally considered that an FSH level higher than 40 IU/L indicates ovarian failure, but these levels are not reliable for diagnosis because in certain circumstances these hormones may be elevated before menopause.
Menopause should not be thought of as occurring suddenly, but rather as a transitional process over time. Perimenopause (also called menopause transition) describes the transition toward menopause, in which women’s cycles can vary in frequency and severity. Menopause symptoms often begin to appear during this time.
5. Physiologically, what determines the timing of menopause?
The absence of ovarian oocytes signals the cessation of menstruation in women. Oocyte numbers decline throughout a woman’s life; they actually peak in number in utero and decline quite rapidly before birth, by which time approximately 80% of oocytes have been lost. Exhaustion of oocytes causes cessation of menses.
6. What is premature ovarian failure?
Ovarian failure is considered premature when it occurs in women who are less than 35 years old. Symptoms are very similar to those experienced by women entering menopause. There can be several causes of this condition, including autoimmune disorders, chromosomal defects, chemotherapy treatment, and some unknown causes. The incidence of this condition is estimated at 0.3% within the United States.
7. What are the symptoms of menopause?
The hallmark symptom of menopause, which often occurs during the perimenopause transition, consists of hot flashes. During these episodes, which last seconds to minutes, the woman experiences a tremendous warming of her body, often with accompanying redness of the skin and sweat production. Menopausal women often describe this variable temperature control as occurring at night, a condition termed “night sweats.” Other symptoms that are less prevalent include the following: insomnia; short-term memory loss or “mental fogginess”; a loss of “youthfulness” to the skin, hair, and nail cells; and skin and vaginal dryness.
8. Will all women experience menopause symptoms?
It is estimated that most women (approximately 85%) will experience some types of vasomotor symptoms with menopause. These can vary greatly in severity; the most severe symptoms usually occur in women who have had their ovaries surgically removed, thereby transitioning to what has been called “instant menopause.”
9. Do menopausal symptoms last indefinitely?
Generally, menopausal symptoms are worst during perimenopause or the first few years after menopause. Most women are able to tolerate these symptoms after approximately 3 to 5 years. Some women, however, continue to suffer from hot flashes and other menopause symptoms 10 years or longer after menopause occurs, at almost the same severity as when the symptoms first appeared.
10. What physiologic changes accompany menopause?
Loss of bone calcium, increased rates of coronary artery disease, skin and vaginal atrophy, hot flashes, and alterations in the lipid profile (mainly increased triglycerides and low-density lipoprotein cholesterol, and decreased high-density lipoprotein cholesterol) all occur through and after menopause.
11. What estrogens are present in a woman’s body?
Women naturally have three different estrogens present in varying amounts: estrone (E1), estradiol (E2), and estriol (E3). Estradiol is the most potent of the three estrogens, binds equally to both the α- and β-estrogen receptors, and converts freely back and forth with estrone in the body. Some evidence suggests that estrone has a higher carcinogenic risk compared with the other estrogens, possibly because of its higher affinity to the α-estrogen receptor (approximately 5:1 α:β). Estriol is a metabolite of estradiol and estrone and will not convert back to E1 or E2 once formed, but rather is excreted out through the urine. It has a higher selective binding to the β-estrogen receptor (3:1 β:α).
12. What is the predominant circulating estrogen during and after menopause?
Estradiol, or E2, is the most abundant estrogen in a woman’s body during reproductive years, and it is produced by the ovaries. During menopause, this production wanes and then stops, and estrone, or E1, becomes the dominant estrogen, converted in adipose tissue from androstenedione, which is secreted from the adrenal glands.
13. There was a major shift in managing women at menopause after the Women’s Health Initiative (WHI) trial. Why did this occur?
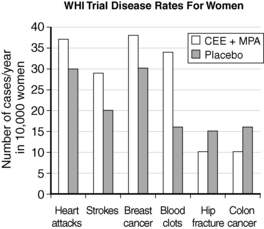
The WHI trial was a landmark trial from the National Institutes of Health (NIH) that enrolled more than 27,000 women 50 to 79 years old. Those women with no previous hysterectomy received combined conjugated equine estrogen (CEE) plus medroxyprogesterone acetate (MPA) or placebo (16,608 women), and those women who had previously had a hysterectomy received either CEE or placebo (10,739 women). The primary outcomes for this trial were coronary heart disease events. Much of the media attention toward this trial came when the combined arm of CEE and MPA was stopped early in July 2002 after investigators found that the associated health risks outweighed potential benefits of therapy. Data showed an increased risk of invasive breast cancer, along with an increased incidence of coronary heart disease, pulmonary embolism, and stroke. Positive outcomes included a reduction in colon cancer and fewer hip fractures. The estrogen-alone arm of the trial also showed an increased risk of stroke and blood clots without preventing heart disease (Fig. 49-1).
14. What are some of the limitations of the WHI trial data?
One of the largest recognized limitations to these data is the age of women at enrollment; it is widely believed that a woman’s greatest benefit derived from hormone replacement therapy (HRT) occurs in the years just following menopause, or in the age range of 50 to 60 years. Enrolling women 50 to 79 years old included a large number of women more than 60 years old, and this may have affected the results shown from the trial. The investigators identified that only one hormone regimen (CEE 0.625 mg/day and MPA 2.5 mg/day) was used in the arm of the trial in which women had an intact uterus, so the rationale of extrapolating these results to state that all hormone products carry the same risk has been questioned.
15. Should women be taking HRT at menopause?
The American Association of Clinical Endocrinologists (AACE) clinical guidelines recommend against long-term HRT and recommend that estrogen therapy not be used for cardiovascular disease prevention. Estrogen is indicated for the prevention of osteoporosis, but the risks of long-term use are thought to outweigh the benefits. Therefore, this indication is generally reserved for women less than 60 years old, and it should be carefully evaluated based on the individual woman’s risk and possible use of other treatments, including bisphosphonates. Similarly, the American Congress (formerly College) of Obstetrics and Gynecologists (ACOG) and the North American Menopause Society (NAMS) recommend estrogen and possible progestin supplementation only for symptomatic relief. They further recommend that women should take the lowest dose possible for the shortest amount of time.
The decision regarding whether a woman should be treated for severe hormone symptoms should be an individual choice made in collaboration between the patient and provider. Perceived risks based on the WHI trial and accompanying literature should be presented and weighed against the severity of symptoms and the woman’s health-related quality of life. Because the greatest benefits of HRT are seen in women less than 60 years old, it is generally thought that any HRT use should occur between the ages of 50 and 60 years, and attempts should be made to taper the therapy to discontinuation every 3 to 5 years.
16. What is a bio-identical hormone?
The term bio-identical was coined to represent hormones that are identical to what the body naturally produces. Although bio-identical hormones are often promoted from compounding pharmacy sources, many of the prescription hormone products that are approved by the Food and Drug Administration (FDA), such as estradiol and progesterone, are also bio-identical. This term has been used to contrast these hormones from other hormone products, most notably CEE, which originates from the urine of pregnant horses, or MPA, a synthetic progestin compound that binds to the progesterone receptor.
17. Are bio-identical hormones safer for supplementation?
There was a tremendous amount of publicity and promotion for bio-identical hormones following the WHI trial, as women sought other options for controlling severe menopause symptoms. Currently, most of the evidence suggests that all estrogen products are similar in nature and effect, regardless of source. Proponents of bio-identical hormones believe that the physiologic similarity of these molecules yields benefits to the body that are not seen with other estrogen and progestin hormones. Some compounded hormones include estriol, or E3, which is not FDA approved for use in the United States, although it is used in Europe and other countries. Estriol has limited data to support its efficacy, and the FDA took a strong stance in 2008 in response to a petition from Wyeth Ayerst to prohibit the use of estriol, by citing a lack of safety and efficacy with its use. With regard to progesterone, some data support different physiologic activities when compared with MPA, and evidence from observational trials and controlled primate trials suggests a better safety profile than MPA, but large randomized placebo-controlled trials are still lacking to verify this potential benefit.
18. If a woman is taking HRT, should dosing be based on serum or saliva levels?
According to AACE guidelines, HRT should be based on a woman’s symptoms, not on specific drug levels. There is some debate about whether serum or saliva levels are optimal for measuring hormone levels. Saliva test proponents cite the findings that hormones are generally quite lipophilic and are not widely found in the blood, and that saliva levels more closely represent intracellular levels of hormone. Saliva levels, however, are documented to vary substantially depending on the time of day when they are collected, and they have not been established as a reliable indicator of therapeutic response. They are not FDA approved and are generally not considered to correlate with serum hormone levels. It is also often difficult for women to obtain saliva level tests through their medical benefits, and the cost of measuring saliva levels can be significant. There is no clearly established therapeutic range for estrogen or progestin in the body, so current recommendations are to base therapy on the lowest possible dose that will adequately control menopausal symptoms.
19. What is the role of progestin supplementation?
Progestins, most notably MPA, are used in conjunction with estrogen treatment to protect women from developing endometrial cancer. They are given to women who have not received a hysterectomy. There is a suggestion based on smaller studies that supplementing progesterone may yield additional benefits, even in women who have received a hysterectomy, but it is not routinely recommended for use beyond endometrial protection.
20. Do women need androgen supplementation?
This is a controversial area. The Endocrine Society’s consensus statement recommends against a diagnosis of androgen deficiency in women because of a lack of data documenting “normal” androgen levels in women across their life span. Evidence indicates that, in some women, supplementing low levels of testosterone (often starting with 1% testosterone creams) may help treat symptoms of fatigue or low energy and decreased libido. When androgen is supplemented, dosing is often limited by unwanted side effects (oily skin and acne, hirsutism, voice lowering).
21. Are compounded hormones superior to other hormone treatment?
Currently, there is no evidence that compounded hormones outperform other HRTs. Compounded medications depend on a reliable source, so women who seek compounded hormones need to identify a pharmacy that performs its own quality assurance on products, follows proper protocols, and has reliable equipment to provide consistent, accurate products. Some data suggest that compounded progesterone creams may not provide high enough systemic levels to provide adequate protection to the endometrium.
22. Does male menopause exist?
There is literature to suggest that androgen levels decline in men, and indications exist for supplementation in some circumstances. Generally, this is not considered “male menopause,” most likely because it does not appear to be a physiologically programmed event.
23. What are some other options for managing menopausal symptoms?
Based on the severity of the woman’s symptoms and the specific symptoms she is experiencing, there are numerous other approaches for managing these without HRT. Antidepressant drugs such as fluoxetine, paroxetine, and venlafaxine have documented evidence for helping to manage hot flash symptoms, and clonidine has been used for this indication, particularly at night, although side effects (hypotension and dry mouth) limit clonidine’s use. There are herbal products that have some documented success, some of which have weaker phytoestrogen activity, such as soy products and black cohosh. These herbal products typically do not have the potency to control more severe menopausal symptoms, but they may manage mild to moderate symptoms.
24. What are some good menopause and HRT references?
General menopause reference: Endotext.com: The post-menopausal woman chapter by Dr. McAvey and Dr. Santoro.
Good review of the literature regarding bio-identical hormones: Cirigliano M: Bioidentical hormone therapy: a review of the evidence. J Womens Health (Larchmt) 16:600–631, 2007.
Holtorf K: The bioidentical hormone debate: are bioidentical hormones (estradiol, estriol, and progesterone) safer or more efficacious than commonly used synthetic versions in hormone replacement therapy? Postgrad Med 121:73–85, 2009.
Anderson, GL, Limacher, M, Assaf, AR, et al, Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. the Women’s Health Initiative randomized controlled trial. JAMA 2004;291:1701–1712.
Cirigliano, M, Bioidentical hormone therapy. a review of the evidence. J Womens Health (Larchmt) 2007;16:600–631.
Goodwin, TM. Management of common problems in obstetrics and gynecology, ed 5. Hoboken, NJ: Wiley-Blackwell; 2010.
Grady, D, Clinical practice. management of menopausal symptoms. N Engl J Med 2006;355:2338–2347.
Holtorf, K, The bioidentical hormone debate. are bioidentical hormones (estradiol, estriol, and progesterone) safer or more efficacious than commonly used synthetic versions in hormone replacement therapy. Postgrad Med 2009;121:73–85.
Nelson, HD, Commonly used types of postmenopausal estrogen for treatment of hot flashes. scientific review. JAMA 2004;291:1610–1620.
Nelson, HD, Postmenopausal estrogen for treatment of hot flashes. clinical applications. JAMA 2004;291:1621–1625.
Rossouw, JE, Anderson, GL, Prentice, RL, et al, Risks and benefits of estrogen plus progestin in healthy postmenopausal women. principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321–333.
Weinstein, M, O’Connor, K, New York Academy of Sciences. Reproductive aging. Boston: Blackwell Publishing on behalf of the New York Academy of Sciences;2009.