Meningitis, Encephalitis, and Other Infections of the Central Nervous System
1. Describe the anatomy of the central nervous system (CNS), and list the anatomic structures that compose it.
2. Define meninges; name the three separate layers and describe their function.
3. Define the cerebrospinal fluid, and list the functions of the cerebrospinal fluid (CSF).
4. Describe the routes of infection for the central nervous system.
5. Explain the host defense mechanisms that protect the central nervous system from infection.
6. Define meningitis, and describe the two major types of meningitis including the etiologic agents.
7. When discussing the etiology of acute meningitis, explain the host predisposing factors for the neonate and identify the most commonly associated bacterial pathogens.
8. Discuss how the advent of the Hib vaccine in the United States has helped to prevent pediatric cases of meningitis.
9. Compare acute and chronic meningitis; outline the distinguishing symptoms and CSF findings for each, including cell counts and chemistry laboratory results.
10. Explain the disease processes for encephalitis and meningoencephalitis.
11. Discuss two ways that meningoencephalitis infections, brain abscesses, or other CNS infections are caused by parasites; identify the associated infecting organisms and the population of patients at increased risk for developing these conditions.
12. Explain the collection, transport, and specimen storage requirements for CSF; include specimen processing and the appropriate distribution of specimen throughout the laboratory.
13. List the culture media used to identify the causative agent of meningitis in bacterial, mycobacterial, and fungal infections; what incubation conditions are required for each type of organism?
General Considerations
Anatomy
Coverings and Spaces of the CNS
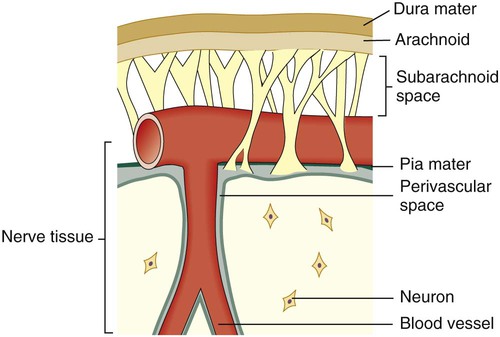
Between and around the meninges are spaces that include the epidural, subdural, and subarachnoid spaces. The relative location of the meninges and spaces to one another in the brain are depicted in Figure 71-1. The location and nature of the meninges and spaces are summarized in Table 71-1.
TABLE 71-1
Inner Coverings (Meninges) of the Brain, Spinal Cord, and Surrounding Spaces
| Anatomic Structure | Relative Location | Key Features |
| Epidural space | Outside the dura mater yet inside the skull | Cushion of fat and connective tissues |
| Dura mater | Outermost membrane | Membrane that adheres to the skull; white fibrous tissue |
| Subdural space | Between the dura mater and the arachnoid membrane | Cushion of lubricating serous fluid |
| Arachnoid membrane | Between the dura mater and pia mater | Delicate, cobweb-like membrane covering the brain and spinal cord |
| Subarachnoid space | Beneath the arachnoid membrane | Contains a significant amount of CSF in an adult (~125-150 mL) |
| Pia mater | Beneath the subarachnoid space | Adheres to the outer surface of the brain and spinal cord; contains blood vessels |
Cerebrospinal Fluid
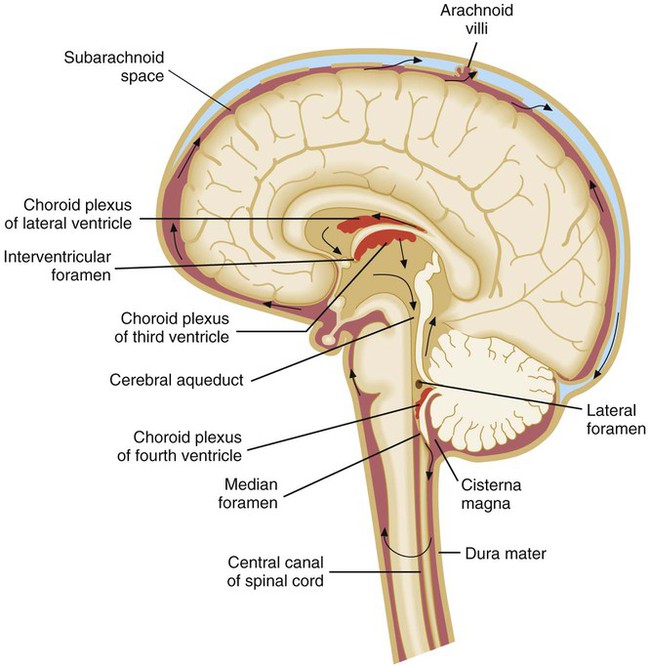
CSF is found in the subarachnoid space (see Table 71-1) and within cavities and canals of the brain and spinal cord. There are four large, fluid-filled spaces within the brain referred to as ventricles. Specialized secretory cells, called the choroid plexus, produce CSF. The choroid plexus is located centrally within the brain in the third and fourth ventricles. Approximately 23 mL of CSF are contained within these ventricles in an adult. The fluid travels around the outside areas of the brain within the subarachnoid space, driven primarily by the pressure produced initially at the choroid plexus (Figure 71-2). By virtue of its circulation, chemical and cellular changes in the CSF may provide valuable information about infections within the subarachnoid space.
Routes of Infection
Organisms may gain access to the CNS through several primary routes:
• Hematogenous spread: followed by entry into the subarachnoid space through the choroid plexus or through other blood vessels of the brain. This is the most common route of infection for the CNS.
• Direct spread from an infected site: the extension of an infection close to or contiguous with the CNS can occasionally occur; examples of such infections include otitis media (infection of middle ear), sinusitis, and mastoiditis.
• Anatomic defects in CNS structures: anatomic defects as a result of surgery, trauma, or congenital abnormalities can allow microorganisms easy and ready access to the CNS.
• Travel along nerves leading to the brain (direct intraneural): the least common route of CNS infection caused by organisms such as rabies virus, which travels along peripheral sensory nerves, and herpes simplex virus.
Diseases of the Central Nervous System
Meningitis
Purulent Meningitis.
Acute.
Chronic meningitis can often occur in patients who are immunocompromised, although this is not always the case. Patients experience an insidious onset of disease, with some or all of the following symptoms: fever, headache, stiff neck, nausea and vomiting, lethargy, confusion, and mental deterioration. Symptoms may persist for a month or longer before treatment is sought. The CSF usually manifests an abnormal number of white blood cells (usually lymphocytic), elevated protein, and decrease in glucose content (Table 71-2). The pathogenesis of chronic meningitis is similar to that of acute disease.
TABLE 71-2
| Clinical Setting | Leukocytes/mm3 | Predominant Cell Type | Protein | Glucose* |
| Normal | 0-5 | None | 15-50 mg/dL | 45-100 mg/dL |
| Viral infection | 2-2000 (mean of 80) | Mononuclear† | Slightly elevated (50-100 mg/dL) or normal | Normal |
| Purulent infection | 5-20,000 (mean of 800) | PMN | Elevated (>100 mg/dL) | Low (<45 mg/dL), but may be normal early in the course of disease |
| Tuberculosis and fungi | 5-2000 (mean of 100) | Mononuclear | Elevated (>50 mg/dL) | Normal or often low (>45 mg/dL) |
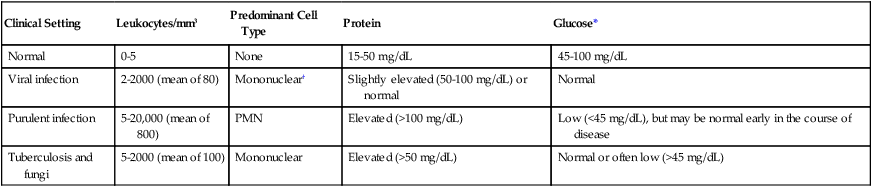
*Must consider CSF glucose level in relation to blood glucose level. Normally, the CSF glucose serum ratio is 0.6, or 50% to 70% of the blood glucose normal value.
†About 20% to 75% of cases may have PMN leukocytosis early in the course of infection.
Epidemiology/Etiologic Agents-Acute Meningitis.
Important causes of meningitis in the adult, in addition to the meningococcus in young adults, include pneumococci, Listeria monocytogenes, and, less commonly, Staphylococcus aureus and various gram-negative bacilli. Meningitis caused by the latter organisms results from hematogenous seeding from various sources, including urinary tract infections. The percentage of adults with nosocomial bacterial meningitis at large urban hospitals has been increasing. The various etiologic agents of chronic meningitis are listed in Box 71-1.
Encephalitis/Meningoencephalitis
Viral.
Viral encephalitis, which cannot always be distinguished clinically from meningitis, is common in the warmer months. The primary agents are enteroviruses (coxsackie viruses A and B, echoviruses), mumps virus, herpes simplex virus, and arboviruses (West Nile virus, togavirus, bunyavirus, equine encephalitis, St. Louis encephalitis, and other encephalitis viruses). Other viruses—such as measles, cytomegalovirus, lymphocytic choriomeningitis, Epstein-Barr virus, hepatitis, varicella-zoster virus, rabies virus, myxoviruses, and paramyxoviruses—are less commonly encountered. Any preceding viral illness and exposure history are important considerations in establishing a cause by clinical means. Since 1999, with the first debut of West Nile in the United States, the West Nile virus has been an important consideration in the diagnosis of viral encephalitis. The Centers for Disease Control and Prevention (CDC) reports that the incidence of West Nile infection peaked in 2003 with 9862 cases of West Nile infection; 2860 were reported cases of meningitis and encephalitis, resulting in 264 deaths. Since then the rates of infection have dropped: human cases reported to the CDC in 2010 were significantly lower with 1021 total reported cases of West Nile; 629 were neuroinvasive cases resulting in 57 deaths; a state-by-state breakdown of the disease incidence is outlined in Table 71-3. In 2012, a deadly resurgence of West Nile virus occurred, including neuroinvasive and non-neuroinvasive, for a total of 4531 cases through mid-October, according to the CDC.
TABLE 71-3
Final 2010 West Nile Virus Human Infections in the United States*
| State | Neuroinvasive Disease Cases | Non-neuroinvasive Disease Cases | Total Cases | Deaths | Presumptive Viremic Donors* |
| Alabama | 1 | 2 | 3 | 0 | 0 |
| Arizona | 107 | 60 | 167 | 15 | 31 |
| Arkansas | 6 | 1 | 7 | 1 | 0 |
| California | 72 | 39 | 111 | 6 | 24 |
| Colorado | 26 | 55 | 81 | 4 | 1 |
| Connecticut | 7 | 4 | 11 | 0 | 5 |
| District of Columbia | 3 | 3 | 6 | 0 | 0 |
| Florida | 9 | 3 | 12 | 2 | 1 |
| Georgia | 4 | 9 | 13 | 0 | 1 |
| Idaho | 0 | 1 | 1 | 0 | 0 |
| Illinois | 45 | 16 | 61 | 4 | 5 |
| Indiana | 6 | 7 | 13 | 1 | 0 |
| Iowa | 5 | 4 | 9 | 2 | 1 |
| Kansas | 4 | 15 | 19 | 0 | 1 |
| Kentucky | 2 | 1 | 3 | 1 | 7 |
| Louisiana | 20 | 7 | 27 | 0 | 7 |
| Maryland | 17 | 6 | 23 | 2 | 0 |
| Massachusetts | 6 | 1 | 7 | 0 | 1 |
| Michigan | 25 | 4 | 29 | 3 | 2 |
| Minnesota | 4 | 4 | 8 | 0 | 1 |
| Mississippi | 3 | 5 | 8 | 0 | 2 |
| Missouri | 3 | 0 | 3 | 0 | 0 |
| Nebraska | 10 | 29 | 39 | 2 | 10 |
| Nevada | 0 | 2 | 2 | 0 | 0 |
| New Hampshire | 1 | 0 | 1 | 0 | 0 |
| New Jersey | 15 | 15 | 30 | 2 | 0 |
| New Mexico | 21 | 4 | 25 | 1 | 6 |
| New York | 89 | 39 | 128 | 4 | 16 |
| North Dakota | 2 | 7 | 9 | 0 | 0 |
| Ohio | 4 | 1 | 5 | 0 | 0 |
| Oklahoma | 1 | 0 | 1 | 0 | 1 |
| Pennsylvania | 19 | 9 | 28 | 0 | 0 |
| South Carolina | 1 | 0 | 1 | 0 | 0 |
| South Dakota | 4 | 16 | 20 | 0 | 0 |
| Tennessee | 2 | 2 | 4 | 0 | 1 |
| Texas | 77 | 12 | 89 | 6 | 14 |
| Utah | 1 | 1 | 2 | 0 | 3 |
| Virginia | 4 | 1 | 5 | 1 | 2 |
| Washington | 1 | 1 | 2 | 0 | 0 |
| Wisconsin | 0 | 2 | 2 | 0 | 1 |
| Wyoming | 2 | 4 | 6 | 0 | 0 |
| Totals | 629 | 392 | 1021 | 57 | 144 |
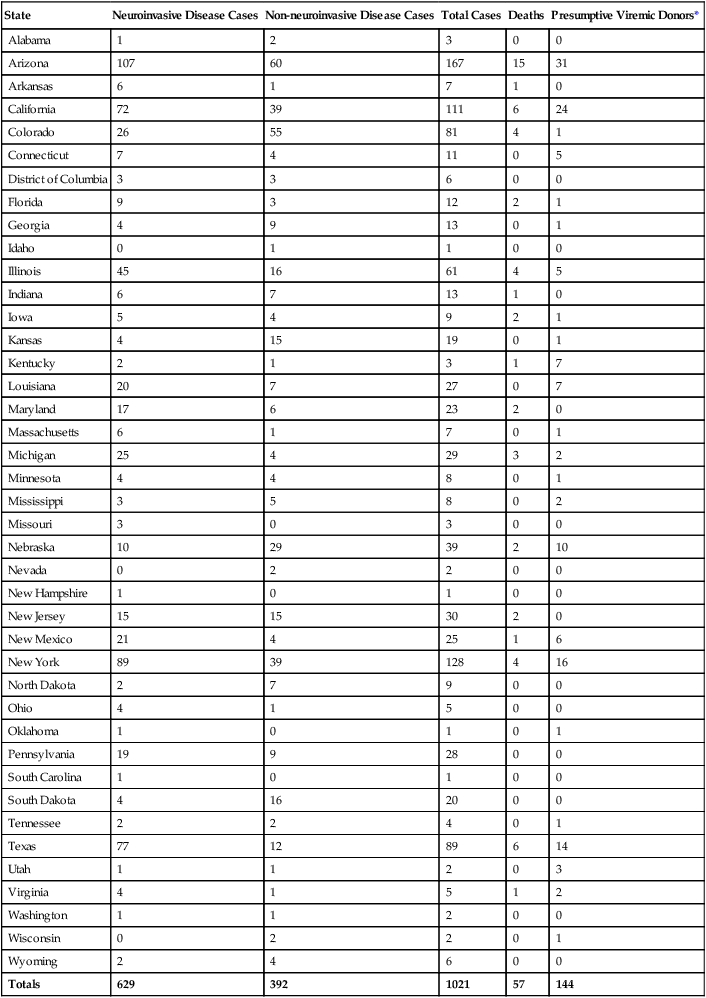
Neuroinvasive disease refers to severe cases of disease that affect a person’s nervous system. These include encephalitis, meningitis, and acute flaccid paralysis that is an inflammation of the spinal cord that can cause a sudden onset of weakness in the limbs or breathing muscles.
Laboratory Diagnosis of Central Nervous System Infections
Meningitis
Except in unusual circumstances, a lumbar puncture (spinal tap) is one of the first steps in the diagnosis of a patient with suspected CNS infection, in particular, meningitis. Refer to Table 5-1 to review the procedure for collecting, transporting, and processing specimens obtained from the central nervous system.
Initial Processing
Initial processing of CSF for bacterial, fungal, or parasitic studies includes centrifugation of all specimens with a volume greater than 1 mL for at least 15 minutes at 1500× g. Specimens in which cryptococci or mycobacteria are suspected require special handling. (Discussions of techniques for culturing CSF for mycobacteria and fungi are found in Chapters 43 and 59, respectively.) If fewer than 1 mL of CSF is available, the specimens should be gram stained and plated directly to blood and chocolate agar plates. The supernatant is removed to a sterile tube, leaving approximately 0.5 mL of fluid. The remaining fluid is used to suspend the sediment for visual examination or culture. Mixing of the sediment after the supernatant has been removed is critical. Forcefully aspirating the sediment up and down into a sterile pipette several times will adequately disperse the organisms that remained adherent to the bottom of the tube after centrifugation. Laboratories that use a sterile pipette to remove portions of the sediment from underneath the supernatant will miss a significant number of positive specimens. The supernatant can be used to test for the presence of antigens, rapid diagnostic test (vertical flow immunochromatography), for N. meningitidis, or for chemistry evaluations (e.g., protein, glucose, lactate, C-reactive protein). As a safeguard, keep the supernatant even if it has no immediate use.
CSF Laboratory Results
As previously mentioned, CSF is also removed for analysis of cells, protein, and glucose. Ideally, the glucose content of the peripheral blood is determined simultaneously for comparison to CSF levels. General guidelines for the interpretation of results are shown in Table 71-2.
Visual Detection of Etiologic Agents
Stained Smear of Sediment.
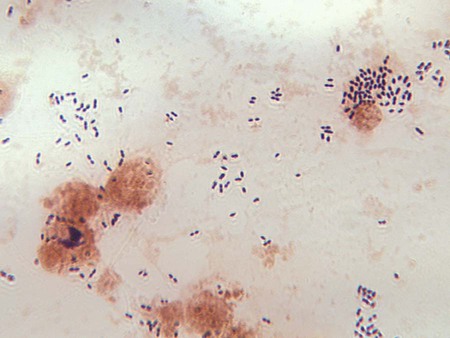
Gram stain must be performed on all CSF sediments. False-positive smears have resulted from inadvertent use of contaminated slides. Therefore, use of alcohol-dipped and flamed or autoclaved slides is recommended. After thoroughly mixing the sediment, a heaped drop is placed on the surface of a sterile or alcohol-cleaned slide. The sediment should never be spread out on the slide surface, because this increases the difficulty of finding small numbers of microorganisms. The drop of sediment is allowed to air dry, is heat or methanol fixed, and is stained by either Gram (Figure 71-3) or acridine orange. The acridine orange fluorochrome stain may allow faster examination of the slide under high-power magnification (400×) and thus a more thorough examination. The brightly fluorescing bacteria will be easily visible. All suspicious smears can be stained using the Gram stain (directly over the acridine orange) to confirm the presence and morphology of organisms.
Using a cytospin centrifuge to prepare slides for staining has also been found to be an excellent alternative procedure. This method for preparing smears for staining concentrates cellular material and bacterial cells up to a 1000-fold. By centrifugation, a small amount of CSF (or other body fluid) is concentrated onto a circular area of a microscopic slide (Figure 71-4), fixed, stained, and then examined.
Wet Preparation.
Amoebas are best observed by examining thoroughly mixed sediment as a wet preparation under phase-contrast microscopy. If a phase-contrast microscope is not available, observing under light microscopy with the condenser closed slightly can be used as an alternative. Amoebas are identifiable by their typical slow, methodical movement in one direction via pseudopodia. (The organisms may require a little time under the warm light of the microscope before they begin to move.) Organisms must be distinguished from motile macrophages, which occasionally occur in CSF. Following a suspicious wet preparation, a trichrome stain can assist in the differentiation of amoebas from somatic cells. The pathogenic amoebas can be cultured on a lawn of Klebsiella pneumoniae or Escherichia coli (see Chapter 47).
Direct Detection of Etiologic Agents
Antigen.
Commercial reagents and kits are available for the rapid detection of antigen in the CSF; a review of the methodologies used will be discussed in the following sections; for more detailed specifics, please refer back to Chapter 9.
Bacteria.
Rapid antigen detection from CSF has been largely accomplished by the techniques of latex agglutination (see Chapter 9). All commercial agglutination systems use the principle of an antibody-coated particle capable of binding to specific antigen, resulting in macroscopically visible agglutination. The soluble capsular polysaccharide found in the common etiologic agents of meningitis, including the group B streptococcal polysaccharide, are well suited to serve as bridging antigens. The agglutination assays may contain either a polyclonal or monoclonal antibody or an antigen from an infectious agent.
In general, the commercial systems have been developed for use with CSF, urine, or serum, although results with serum have not been as diagnostically useful as those with CSF. Soluble antigens from Streptococcus agalactiae and Haemophilus influenzae may concentrate in the urine. Urine, however, seems to produce a higher incidence of nonspecific reactions than either serum or CSF. The manufacturers’ directions must be followed for performance of antigen detection test systems for different specimen types. Although some of the systems require pretreatment of samples (usually heating for 5 minutes), not all manufacturers recommend such a step. The reagents, however, may yield false positive or cross-reactions unless the specimen is pretreated. Interference by rheumatoid factor and other substances, more often present in body fluids other than CSF, has also been reported. The method of Smith and colleagues has been shown to effectively reduce a substantial portion of nonspecific and false-positive reactions, at least for tests performed with latex particle reagents. This pretreatment, called rapid extraction of antigen procedure (REAP; see Procedure 71-1 on the Evolve site), is recommended for laboratories that use commercial body fluid antigen detection kits. Certain commercial systems have an extraction procedure included in the protocol.
Culture
Bacteria and Fungi.
The symptoms of chronic meningitis that prompt a physician to request fungal cultures are the same as those for tuberculous meningitis. Cultures for mycobacteria are addressed in Chapter 43. For CSF fungal cultures, two drops of the well-mixed sediment should be inoculated onto Sabouraud dextrose agar or other non-blood-containing medium and brain-heart infusion with 5% sheep blood. Fungal media should be incubated in air at 30° C for 4 weeks. If possible, two sets of media should be inoculated, with one set incubated at 30° C and the other at 35° C.
1. Which of the following are specialized structures of the meninges that function to absorb the spinal fluid and allow it to pass into the blood?
2. What bacteria are responsible for outbreaks of meningitis among neonates in hospital nurseries?
3. All of the following are pathogenic sources capable of causing brain abscesses except:
4. When processing CSF specimens for laboratory diagnosis, the specimen appears red in some of the tubes, a sign of red blood cells and bleeding; to determine whether the blood is due to a bloody tap or a subarachnoid hemorrhage, cell counts are done on which of the following tubes:
5. Refer to the previous question. Which tube is used for chemistry and immunologic studies?
6. Culture for the determination of the etiologic agent causing meningitis is set up from which tube of CSF?
7. Which of the following organisms is a parasite that grows intracellularly, destroys brain parenchyma, and is a common CNS affliction in HIV-infected patients with AIDS?
8. Which of the following causes of pediatric meningitis was significantly reduced as the result of an effective vaccination program?
9. When a physician suspects Cryptococcus neoformans as the etiologic agent of a CNS infection, what is the best way to test for it?
10. The cerebrospinal fluid that surrounds the brain and spinal fluid functions to:
a. carry essential metabolites into the neural tissue
b. protect the central nervous system against microbial invasion by phagocytosis
c. provide a means by which the brain monitors changes in the internal environment
_____ In cushioning and providing buoyancy for the bulk of the brain, the effective weight of the brain is reduced by a factor of 30.
_____ The three layers of protective membranes, which surround the brain and spinal column, are called the meninges.
_____ The elderly population of patients have the highest prevalence of meningitis.
_____ Encephalitis is an inflammation of the brain parenchyma and is normally caused by bacteria.
_____ The first Hib vaccine was not efficacious in children younger than 18 months of age.
_____ The normal CSF glucose serum ratio is 0.6, or 50% to 70% of the blood glucose normal value.
_____ A component of syphilis infections is aseptic meningitis.
_____ CSF cultures from patients suffering from brain abscesses are typically positive for anaerobes or viridians streptococci.
_____ CSF is found in the subdural spaces of the brain.
_____ The entire volume of CSF is exchanged every 5 to 6 hours.
_____ When collecting CSF for culture studies, it is imperative to collect the correct volume of CSF.
_____ When transporting CSF to the laboratory for bacterial studies, the CSF must be refrigerated and kept at a temperature of 2° to 8°C.
_____ If a physician orders viral studies on a CSF and the transport to the laboratory will be longer than 2 to 3 hours after collection, the CSF specimen must be frozen at −20°C.
_____ The most sensitive method for detecting encephalitis-causing viruses in the CSF is PCR.
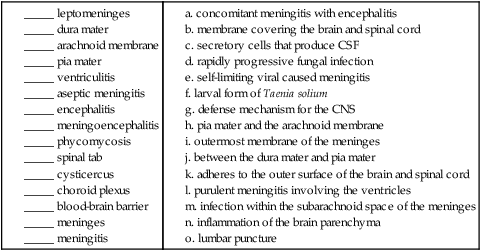
12. Matching: Match the term with the appropriate definition.
|
|
a. concomitant meningitis with encephalitis
b. membrane covering the brain and spinal cord
c. secretory cells that produce CSF
d. rapidly progressive fungal infection
e. self-limiting viral caused meningitis
f. larval form of Taenia solium
g. defense mechanism for the CNS
h. pia mater and the arachnoid membrane
i. outermost membrane of the meninges
j. between the dura mater and pia mater
k. adheres to the outer surface of the brain and spinal cord
l. purulent meningitis involving the ventricles
m. infection within the subarachnoid space of the meninges
1. Name the least common route of CNS infection caused by an organism?
2. Name a unique property of the HIV virus that predisposes infected individuals to viral encephalitis?
3. What is a cytospin centrifuge, and how does it assist in processing CSF specimens?
4. What is diagnostic in the smear when using the India ink stain for the presence of Cryptococcus neoformans?
5. What is the purpose of the pretreatment REAP procedure? What does REAP stand for?
6. What population of patients has the highest prevalence of meningitis? Describe the associated predisposing factors.
7. What is one of the most critical steps in processing CSF for culture?
8. Explain the reasoning behind using tubes 3 and 4 in CSF collection for cell count and differential.