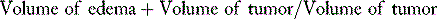CHAPTER 9 Meningiomas and Brain Edema
INTRODUCTION
According to the Central Brain Tumor Registry of the United States, meningiomas account for more than 30% of all primary brain tumors.1 It is well recognized that there is a significant difference in the incidence of meningiomas between females and males. Among females, meningiomas represent about 38% of all intracranial tumors compared to 20% of all tumors in males, a difference that is much higher for spinal extramedullary tumors.2 Age represents another relevant factor in their epidemiology, with the incidence of meningiomas increasing from 0.3/100,000 in childhood to more than 8.4/100,000 in the elderly population.3
Despite their prevalence, the vast majority of meningiomas are extra-axial, benign slow-growing tumors arising from arachnoid cap cells. Hence, many meningiomas, including those that are relatively large, are asymptomatic and do not require active intervention, especially in the elderly or in those with medical comorbidities. However, meningiomas can cause neurologic symptoms due to local growth and compression of adjacent brain structures and cranial nerves; irritation of the adjacent cortex leading to seizures; and pain syndromes due to involvement of cranial nerves, adjacent dura, and skull base structures. The majority of these symptoms evolve slowly and are related to the insidious growth of the benign meningioma. Elective surgery, supplemented with adjuvant radiation when indicated, usually provides long-term cure or tumor control with minimal morbidity, as discussed elsewhere. However, certain locations and biological subtypes of meningiomas still pose significant management challenges requiring a multidisciplinary approach and ongoing research into the molecular biology of these tumors.2
MENINGIOMA AND PERITUMORAL BRAIN EDEMA
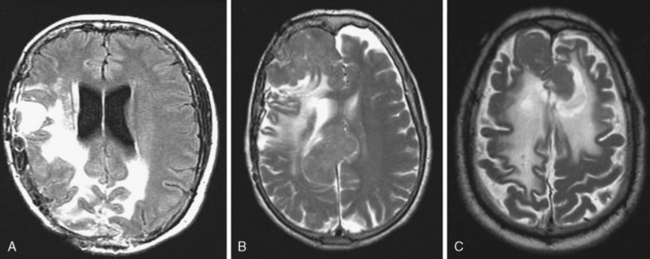
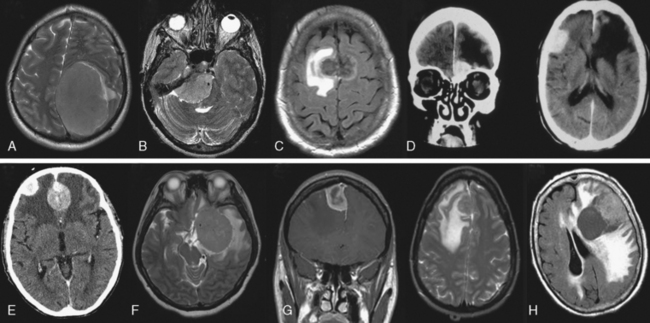
The presence of peritumoral brain edema (PTBE) associated with meningiomas is relatively common and has been quoted in different series to be present between 40% and 92% of the time.4–6 The incidence of PTBE resulting in significant neurologic deficit, thereby reducing the patient’s performance status and negatively affecting the surgical outcome, is unknown. Although this number is likely much lower, PTBE still represents a significant pre- and postoperative clinical issue in a sizable number of patients.7,8 As discussed in the text that follows, several structural, pathologic, and molecular correlates of meningiomas and associated PTBE have been proposed, with likely no one single etiology that adequately explains all cases. For example, although the incidence of significant PTBE is more with malignant meningiomas, the vast majority occur in the much more common totally benign meningioma (Figs. 9-1 and 9-2). Location around major venous sinuses or draining veins has been implicated as another association, but meningiomas with significant PTBE are found in the convexity, skull base, as well as those in the parasagittal region (see Fig. 9-2). Size of the meningioma has also been linked to PTBE, but large series have not clearly documented this to be true, as demonstrated by the significant PTBE in the smaller of two meningiomas in the same patient in (Fig. 9-2D).
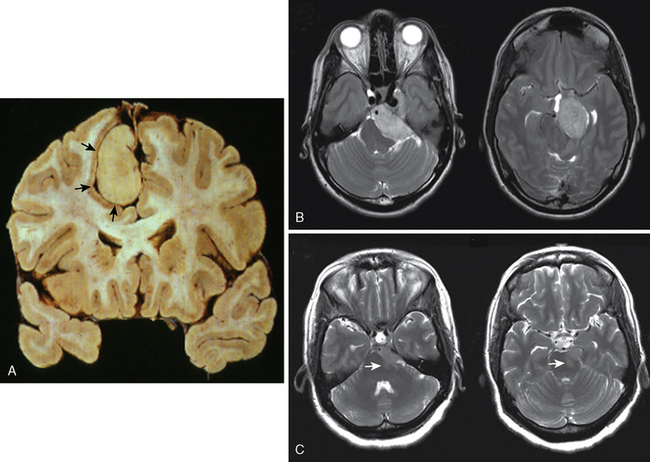
There is agreement that at a histologic level, ablation of the usually present intact arachnoid plane separating the tumor from adjacent brain (Fig. 9-3A) or subpial extension of the tumor, such as in malignant meningioma, is associated with increased incidence and severity of PTBE. The presence of T2 signal change in the parenchyma at the tumor–brain interface correlates well to the loss of a patent arachnoid plane (Fig. 9-3B, C). These subtle but important preoperative radiologic features should be recognized to properly plan the extent of resection with minimal exacerbation of neurologic deficits, especially in meningiomas that grow against eloquent regions of the brain, such as the brain stem (Fig. 9-3C).
MECHANISM(S) AND QUANTIFICATION OF BRAIN EDEMA
Vasogenic edema is the most relevant type of edema associated with intra- or extra-axial tumor-associated PTBE, including meningiomas.9 Vasogenic edema is characterized by an increase in extracellular fluid volume due to breakdown of the blood–brain barrier (BBB), with increased permeability of brain capillary endothelial cells to macromolecular serum proteins such as albumin. The main ultrastructural basis of the intact BBB is the presence of endothelial cell tight junctions or zona occludens, a key differentiating factor of CNS versus systemic vasculature. In addition to tight junctions, the fully mature BBB is also dependent on the glial foot processes that wrap around the vessels and lack of pinocytic transcellular vesicular transport in the CNS endothelial cells. The onset of vasogenic edema is more subacute to chronic compared to cytotoxic edema, as it requires disruption of the substrates of the BBB described in the preceding text. However, acute cytotoxic edema is usually followed within the next few hours to days by the development of vasogenic edema, as endothelial and glial cell death leads to disruption of the BBB. Breakdown of the ultrastructural substrates of the BBB mentioned earlier in the peritumoral brain is usually not secondary to a primary cytotoxic injury. It likely is a result of combination of potentially reversible mechanical, physiologic, and molecular alterations triggered by the tumor, as discussed in detail later. To better understand and potentially treat PTBE it is essential to be able to quantify it both in preclinical and clinical settings. Accordingly, the simple edema index defined by magnetic resonance imaging (MRI) volumetric measurements as:
has proven to be reliable measurement of communication between investigators.10–13 Accordingly, an edema index of 1 by definition implies lack of any PTBE.
POTENTIAL ETIOLOGIES OF MENINGIOMA-ASSOCIATED PTBE
Gender, Age, Size, and Location
It is well recognized that meningiomas express estrogen and progesterone receptors,14–16 perhaps linked to the underlying prevalence of these tumors in females and occasional increased growth noted with pregnancy. A correlation between number of sex hormone receptors and PTBE in meningiomas has been proposed.17 In this study, estrogen and progesterone receptors were measured in 22 meningiomas and correlated to the amount of PTBE estimated on computed tomography (CT) scans. Of the 22 cases, 19 were positive for progesterone receptors and all of these meningiomas exhibited significant PTBE. None of these 22 meningiomas was estrogen receptor positive. If indeed true, such a link between progesterone receptors and PTBE may have therapeutic implications, using antiprogesterone therapy in the treatment of meningioma-related PTBE. Although encouraging, several systematic studies have failed to consistently demonstrate such a link with sex hormones and gender on PTBE,18,19 in keeping with a clear lack of efficacy of antiprogesterone therapy to check the growth of meningiomas.
Similar to gender, increasing age has been suggested to confer a higher prevalence of significant PTBE in meningiomas and thereby contribute to a higher surgical morbidity.18 However, most authors have not been able to verify this observation, although increasing age does put the patient at much higher surgical risk, but due to systemic comorbidities and not increased PTBE.19 Tumor size has also failed to correlate unambiguously with the amount of PTBE. In a cohort of 175 patients, larger size meningiomas correlated with increased PTBE.20,21 However, in two separate series of 55 and 25 meningiomas, tumor size did not correlate with PTBE.18 Our own experience is similar, as demonstrated in Figure 9-2D, where significant PTBE was present in the smaller anterior skull base meningioma, necessitating surgery, versus the much larger but asymptomatic contralateral meningioma.
Location of meningioma and associated PTBE also remains controversial. Several authors have reported increased incidence of PTBE in frontal sphenoidal ridge meningiomas in comparison to meningiomas in other areas of brain.22,23 It is postulated that this resulted from impeded venous drainage through the middle cerebral vein. However, as discussed later, the degree of obstruction to venous drainage did not clearly show a relationship to PTBE. Similarly, tumors of the falx, convexity, and anterior fossa have also been linked with greater PTBE.19 However, other studies examining meningiomas originating from the convexity, falx, sphenoid ridge, tentorium, suprasellar region, cerebellopontine angle, and posterior fossa, did not find any correlation with PTBE.18 There does seem some consensus that posterior fossa meningiomas generally do not have significant PTBE on presentation.18,19,24,25 A plausible explanation for this observation may lie in the relative paucity of white matter, which is preferentially affected by vasogenic edema, in the posterior fossa compared to the supratentorial cortex.25 However, perhaps a more likely explanation is that meningioma and other tumors in the posterior fossa are diagnosed earlier, before PTBE, due to early onset of symptoms.
Tumor Margin, Subtype, and Grade
The gross appearance of tumor–brain margin has been postulated to be an important determinant of PTBE. Meningiomas with irregular margins are grouped as “lobulated,” whereas meningiomas with smooth margin can be considered “nonlobulated.” One report links meningiomas with tumor lobulation to have increased PTBE26 however, this was not verified in another study.18 Several studies have implicated histologic subtypes or the WHO grading of meningiomas with the occurrence of PTBE.10,12,27,28 In particular, “meningothelial” meningiomas, which potentially are more invasive and able to damage the leptomeninges and cortex, thereby allowing the transmission of edema into the white matter, have been associated with increased PTBE compared to the “fibroblastic” subtype.29 However, other studies do not corroborate these observations and do not find any correlation between PTBE and subtype of benign meningiomas.18,19,30 In contrast to subtypes of benign meningiomas, there is general agreement that atypical or malignant meningiomas induce a greater degree of PTBE. This may be related to their rapidity of growth with breach of the arachnoid plane and subpial extension, with and without frank brain invasion. In addition, these higher grades of meningiomas also secrete a larger number and greater quantities of edemogenic cytokines, as discussed later.
Tumor Vascularization
The vascular supply of meningiomas has been implicated in PTBE, especially in tumors which also derive some of their blood supply from the cerebral-pial circulation.10 MRI correlated to cerebral angiograms revealed that PTBE is directly related to the degree of cerebral–pial blood supply of the tumor and to the location of pial blush. In contrast, meningiomas supplied only by dural meningeal arteries had minimal associated PTBE. The link to pial supply and PTBE is to be expected, as the presence of pial supply to the meningioma implies a discontinuation of the critical arachnoid plane between the tumor and brain, a critical histopathologic correlate involved in development of PTBE.12 Interestingly VEGF vascular endothelial growth factor (VEGF) expression, a strong molecular correlate of vasogenic edema as discussed later, was increased in tumors with cerebral–pial blood supply and not with meningiomas that derived their blood supply strictly from dural meningeal arteries. Although the link between cerebral–pial blood supply and PTBE is logical and supported by several studies, it is not universally accepted as per the reports from other groups of investigators.10,12
Venous Obstruction
Venous stasis due to poor efferent venous drainage of the tumor has been hypothesized to be one of the major causes of PTBE. In a study on 25 patients with supratentorial meningiomas, MRI and superselective angiography were performed to assess the arterial supply and venous drainage.31 One result of this study was that tumors with poor efferent venous drainage have an increased incidence of PTBE, related to increased intratumoral venous pressure. The intratumoral venous congestion could increase concentrations of vasogenic cytokines, including VEGF, and collectively lead to an increase in the permeability of the cerebral–pial arteries, causing alterations in the BBB and PTBE. Another mechanism of meningiomas inducing venous hypertension, and thereby PTBE, is by direct cortical vein compression. The ability to preserve critical cortical veins wrapping around the meningiomas and draining into the major sinuses is a vital component of meningioma surgery. Failure to do so can result in significant morbidity with postsurgical edema and venous infarcts. Although cortical venous obstruction can provide an explanation why some small meningiomas are associated with significant PTBE (see Fig. 9-2), this is not a universal finding as authors have reported no direct correlation of cortical vein compression and PTBE.31
Sequelae of Treatment
PTBE can be induced or worsened after surgery or radiation therapy of meningiomas. In a study to assess the pattern of resolution of the edema after surgical resection of meningiomas, serial CT and MRI were done postoperatively. It was found that 50% of the PTBE, as denoted by hypodensity on CT, resolved within 4 days, with 90% resolution in 14 days.32 Beyond this time, persistent hypodensity likely represents regions of permanent postoperative encephalomalacia. Delayed worsening of PTBE after conventional, fractionated, or single-fraction radiosurgery is well recognized.33,34 In one series after radiosurgery the mean time of significant PTBE postradiation was approximately 5.5 months, with an average duration of 16 months.34 Of interest, postradiosurgery PTBE was more common in meningiomas located in the parasagittal regions. Although unproven, a plausible mechanism maybe radiation related tumor necrosis, inflammation, and hyalinization of the blood vessels with subacute expansion of the tumor and occlusion of cortical draining veins or the sagittal sinus itself.33,34 In support, hypoxic regulated and edemogenic genes such as VEGF and HIF-1, as discussed in the text that follows, were increased in meningiomas that required surgical removal due to clinically significant PTBE that could not be managed with systemic corticosteroids.34
MOLECULAR BIOLOGY OF MENINGIOMA-RELATED PTBE
As per the preceding discussion, there does not seem to be one unifying epidemiologic, pathologic, clinical, or structural cause for all meningioma-associated PTBE. However, general agreement exists that disruption of the BBB is mandatory for PTBE, and this requires a breach of the arachnoid plane at the tumor–brain interface.29,35 However, breach of the arachnoid plain likely does not suffice to induce PTBE by itself, but facilitates transgress of various edemogenic cytokines being released by the tumor to disrupt the integrity of the BBB in the adjacent parenchymal vessels.36 Loss of the arachnoid plane prevents removal and dilution of these cytokines by the CSF to facilitate penetration of these cytokines into the brain by disruption of the pial border. In addition, cytokines such as VEGF are not only edemogenic but also often angiogenic, thereby promoting neo-angiogenesis and establishment of cerebral–pial tumor vessels. As discussed, these cerebral–pial vessels usually lack a mature BBB and thereby further exacerbate PTBE.
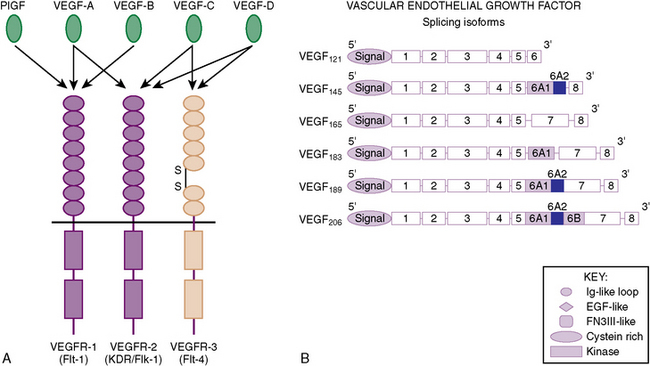
What are some of these edemogenic and angiogenic cytokines released by meningiomas that may underlie PTBE? One of the most important and best studied growth factors in cancers, including meningiomas, is VEGF. VEGF was originally described as a potent edemogenic factor and coined to be vascular permeability factor (VPF).37 The subsequent independent isolation and sequencing of VPF and VEGF demonstrated absolute homology and realization they were the same protein. VEGF is a 34- to 45-kDa highly soluble dimeric glycosylated protein, with structural homology to platelet-derived growth factor (PDGF). Several VEGF family members have been identified (VEGF-A, VEGF-B, VEGF-C, VEGF-D, PIGF), with different but overlying function in regulating not only the vascular but also related lymphatic system (Fig. 9-4A). VEGF-A is synonymous to VEGF and plays a pivotal role in normal developmental and physiological angiogenesis, as well as pathological neoangiogenesis such as in cancer. There are several isoforms of VEGF in humans, with the most abundant, diffusible, and active being the smallest two isoforms, VEGF121 and VEGF165 (Fig. 9-4B). VEGF is a potent mitogen and chemotactic factor for endothelial cells, as well as inducing the production of proteases (plasminogen activators, collagenases, etc.), to degrade the extracellular matrix.38–40 The important tumor angiogenic role of VEGF is not detailed in this chapter, but its importance is demonstrated by promising clinical trials in neurooncology utilizing a variety of strategies to inhibit VEGF with the potential of inhibiting brain tumor angiogenesis and thereby growth.
VEGF is able to increase vascular permeability of normal blood vessels to plasma proteins, without inducing inflammation or endothelial cell injury.41 It is approximately 1000 times more potent as histamine in inducing vascular edema, by acting directly on endothelial cells of venules and arterioles, but not on smooth muscle cells or fibroblasts. The specificity toward endothelial cells is a result of the two cognate VEGF receptors, VEGFR-1 (or Flt-1) and VEGFR-2 (or Flk-1/KDR), being specifically expressed on endothelial cells (see Fig. 9-4A). VEGF, especially the most abundant VEGF165 isoform, has the highest affinity to bind VEGFR-2 and plays a more dominant role in tumor angiogenesis and growth.42–44 VEGF expression at the mRNA level and PTBE in meningiomas has been linked.45 We extended these observations to VEGF proteins expression by immunohistochemical analysis and found it to correlate to meningioma vascularization.36 We then correlated the degree of meningioma vascularization to degree of PTBE, as measured by the previously described edema index. Although all meningiomas with a significant edema index (>2, where the amount of maximal PTBE was twice the maximal size of the meningioma) had a high degree of tumor angiogenesis and VEGF expression, not all highly vascular meningiomas had significant PTBE. We postulate that for PTBE to occur, both breach of the arachnoid plane and high levels of VEGF secretion by the tumor cells were required. In support of this postulate another group demonstrated that expression of both VEGFR-1 and VEGFR-2 in meningioma endothelium cells is related to degree of VEGF expression and associated PTBE in these tumors.46
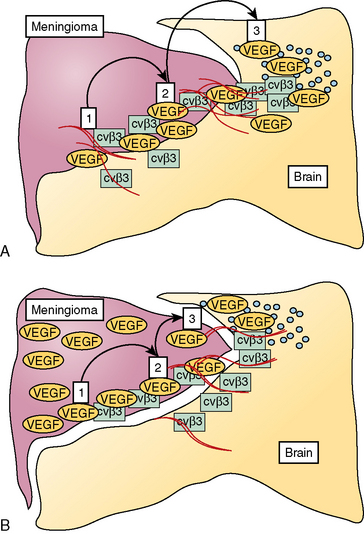
Although implicating VEGF, the sequence of events how VEGF induces PTBE development in meningiomas is still matter of debate. From the literature two potential mechanisms, which are not mutually exclusive but could act independently or in synergism, have been proposed, as schematically depicted in Figure 9-5. One mechanism suggests that VEGF acted as a potent edemogenic factor only when growth of the meningioma physically leads to disruption of the arachnoid (see Figs. 9-2 and 9-3).47 After breakdown of the arachnoid plane, VEGF, which is highly soluble, can induce vascular permeability as well as proliferation of subpial microvessels leading to cerebral-pial vascular supply to the tumor. Another potential but not exclusive mechanism takes into account the noted observation that meningiomas with significant PTBE express high levels of VEGF.36 VEGF regulates expression of several proteases, which in addition to the physical growth of the tumor can facilitate breakdown of the arachnoid plane and extracellular matrix (see Figs. 9-2 and 9-3).48 In support, PTBE in meningiomas correlated significantly with expression of matrix metalloproteinase-9 (MMP-9) by the tumor cells, one of the most common and potent proteolytic enzymes capable of breaking down the basal membrane (collagen IV) and the connective tissue.48 In addition, integrins such as αvβ3 and αvβ5 play a fundamental role in angiogenesis and invasion and are expressed in meningiomas, particularly at the brain–tumor interface where cerebral–pial vascularization and PTBE occur.49 In contrast to normal brain, the peritumoral brain around meningiomas express high levels of these integrins, especially α v β 5, thereby representing a state of activated, immature and edemogenic vasculature.49 In support of the role of integrins, tenascin, which is an ECM glycoprotein and a ligand of α v β5, correlates to the extent of PTBE and VEGF expression in meningiomas.50
Why some meningiomas express high levels of VEGF and thereby potentially lead to significant PTBE, while others do not, is not known. Primary mutations, amplifications, and translocations leading to increased VEGF expression have not been identified in meningiomas, or in fact other human cancers. Differences in intratumoral hypoxia are suspect, as hypoxia is the most potent physiologic inducer of VEGF.51 In meningiomas, different degrees of ischemia have been demonstrated at the meningioma–brain interface, suggesting that this hypoxic environment might be able to induce VEGF and thus PTBE development.52 Hypoxia leads to VEGF induction by increasing its transcription by stabilizing the transcription factor hypoxia-inducible factor-1α (HIF-1α), which acts on the hypoxia-responsive element (HRE) promoter region of VEGF. Furthermore, hypoxia stabilizes VEGF at the mRNA level53–55 and induces the expression of growth factors such as PDGF and epidermal growth factor (EGF), which in turn induces VEGF expression.
However, a variation in tumor hypoxia likely is not the main underlying etiology for noted differences in the VEGF expression profile among meningiomas. If it was, one would predict that larger meningiomas with greater hypoxic regions may have higher levels of VEGF and thereby demonstrate more PTBE, a correlation that has not been verified, as previously discussed. This brings forth the thesis that primary growth-promoting molecular aberrations in certain meningiomas may also increase VEGF expression by these tumors and make them more susceptible to PTBE if there is breakdown of the arachnoid–pial plane. These include cytokines such as transforming growth factor (TGF-β) fibroblast growth factor (FGF), interleukin-6 (IL-6), platelet-activating factor (PAF), and prostaglandins.11,56 Further, downstream signaling pathways to these cytokine receptors, such as p21-Ras and PI3 kinase, also can induce VEGF.36 In summary, meningiomas and other cancers lack a primary genetic basis for increased VEGF expression. However, VEGF can be secondarily induced by tumor hypoxia and primary genetic alterations in the tumors, such as aberrant growth factor-receptor–signaling pathways, that may underlie the molecular mechanisms of PTBE in meningiomas.
PRINCIPLES OF TREATMENT OF PTBE IN MENINGIOMAS
The long-term permanent therapy of PTBE associated with meningiomas is microneurosurgical removal of the tumor, with preservation of the tumor–arachnoid plane. In some instances, the surgical volume of the symptomatic meningioma that requires removal is actually small, with most of the clinical symptoms due to PTBE (see Fig. 9-2). Preservation of the arachnoid plane at the tumor–brain interface, avoiding damage to cortical veins and not attempting tumor removal from the posterior two thirds of the sagittal sinus, are important to minimize parenchymal dysfunction and postoperative neurologic worsening due to increased PTBE or venous engorgement and subsequent venous infarction. The presence of preoperative edema as detected on MRI would indicate those regions of the tumor–brain interface to have a breached arachnoid plane (see Fig. 9-3). Attempted total removal of the tumor in these interfaces has a high probability of increasing PTBE, leading to significant mortality, especially in highly eloquent regions such as the brain stem. In these cases, it is more prudent to leave a “carpet” of tumor over these interfaces. In less eloquent regions, total tumor removal can be achieved without added postoperative deficits, with meticulous dissection of the tumor–brain interface.
The main medical therapy for PTBE in patients who cannot undergo surgery for medical reasons or in the perioperative or postoperative period is corticosteroids.57,58 Steroids reduce VEGF expression by the tumor cell and inhibit the edemogenic response of the brain microvascular bed to VEGF and other cytokines, by stabilization of endothelial cell permeability and inhibiting vasogenic edema.59 In support, VEGF applied to cultured endothelial cells increases intracellular influx of calcium associated with cytoskeletal rearrangement, a response antagonized by steroids.60 The effect of dexamethasone on PTBE and associated clinical sequelae can be quite abrupt and gratifying to the patient and family. However, the long-term use of steroids is significant, including weight gain, diabetes, immunosuppression, osteoporosis, gastrointestinal problems, myopathy, increased risk of deep vein thrombosis, or pulmonary embolism. These side effects need close monitoring and preclude using steroids on a prolonged basis for the management of PTBE in meningiomas.
Therapeutic strategies that decrease PTBE other than steroids include agents that selectively target VEGF or VEGF receptors. VEGF antagonists currently under preclinical and clinical study include neutralizing or blocking antibodies to VEGF and small-molecule VEGF-receptor inhibitors.61 In addition to PTBE, VEGF antagonists may also inhibit overall tumorigenic growth by their antiangiogenic effects. Another group of compounds that also deserve attention in the management of PTBE are the cycloxygenase-2 (COX-2) inhibitors, as ubiquitous expression of COX-2 in meningiomas has been demonstrated.62 COX-2 is an enzyme responsible for the production of relevant mediators of inflammation and is the rate-limiting enzyme for the synthesis of prostaglandins from arachidonic acid. Prostaglandins possess tumorigenic properties via their angiogenic, antiapoptotic, and cell proliferative effects. Nonsteroidal anti-inflammatory drugs (NSAIDs) and selective COX-2 inhibitors are effective antagonists of prostaglandin synthesis and potentially also possess antitumorigenic effects. In addition, COX-2 inhibitors also reduce expression of the transcription factor Sp1, which inhibits generation of VEGF transcripts and thereby expression.63 In support, the COX-2 inhibitor SC-236 demonstrated promising results in a preclinical gliosarcoma model.64 Rofecoxib, another specific COX-2 inhibitor, was as effective in reducing PTBE as dexamethasone also in animal models of PTBE. Celecoxib, a specific COX-2 inhibitor, was found to be effective in management of PTBE, although the effect was not immediate and varied with duration of use.62 Although promising, none of these nonsteroidal agents has been conclusively demonstrated to be equivalent or superior to conventional steroids, which remains the prime medical therapy of PTBE when indicated.
[1] CBTRUS (2005). Statistical Report: Primary Brain Tumors in the United States, 1998–2002. Hinsdale: Central Brain Tumor Registry of the United States, 2006.
[2] Bernstein M., Berger M.S. Neuro-Oncology: The Essentials. New York: Thieme, 2008.
[3] Youmans J.R. Neurological Surgery. Philadelphia: WB Saunders, 1996.
[4] Abe T., Black P.M., Ojemann R.G., Hedley-White E.T. Cerebral edema in intracranial meningiomas: evidence for local and diffuse patterns and factors associated with its occurrence. Surg Neurol. 1994;42(6):471-475.
[5] Al-Mefty O. Meningiomas. New York: Raven Press, 1991.
[6] Schmidek H.H. Meningiomas and Their Surgical Management. Philadelphia: WB Saunders, 1991.
[7] Sawaya R., Hammoud M., Schoppa D., et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42(5):1044-1055. discussion 1055–1046
[8] Sacko O., Sesay M., Roux F.E., et al. Intracranial meningioma surgery in the ninth decade of life. Neurosurgery. 2007;61(5):950-954. discussion 955
[9] Stummer W. Mechanisms of tumor-related brain edema. Neurosurg Focus. 2007;22(5):E8.
[10] Bitzer M., Wockel L., Luft A.R., et al. The importance of pial blood supply to the development of peritumoral brain edema in meningiomas. J Neurosurg. 1997;87(3):368-373.
[11] Constantini S., Tamir J., Gomori M.J., Shohami E. Tumor prostaglandin levels correlate with edema around supratentorial meningiomas. Neurosurgery. 1993;33(2):204-210. discussion 211
[12] Inamura T., Nishio S., Takeshita I., Fujiwara S., Fukui M. Peritumoral brain edema in meningiomas: influence of vascular supply on its development. Neurosurgery. 1992;31(2):179-185.
[13] Bitzer M., Opitz H., Popp J., et al. Angiogenesis and brain oedema in intracranial meningiomas: influence of vascular endothelial growth factor. Acta Neurochirurg. 1998;140(4):333-340.
[14] Carroll R.S., Glowacka D., Dashner K., Black P.M. Progesterone receptor expression in meningiomas. Cancer Res. 1993;53(6):1312-1316.
[15] Carroll R.S., Zhang J., Black P.M. Expression of estrogen receptors alpha and beta in human meningiomas. J Neuro-oncol. 1999;42(2):109-116.
[16] Black P.M. Meningiomas. Neurosurgery. 1993;32(4):643-657.
[17] Benzel E.C., Gelder F.B. Correlation between sex hormone binding and peritumoral edema in intracranial meningiomas. Neurosurgery. 1988;23(2):169-174.
[18] Gurkanlar D., Er U., Sanli M., Ozkan M., Sekerci Z. Peritumoral brain edema in intracranial meningiomas. J Clin Neurosci. 2005;12(7):750-753.
[19] Brandis A., Mirzai S., Tatagiba M., et al. Immunohistochemical detection of female sex hormone receptors in meningiomas: correlation with clinical and histological features. Neurosurgery. 1993;33(2):212-217. discussion 217–218
[20] Bitzer M., Wockel L., Morgalla M., et al. Peritumoural brain oedema in intracranial meningiomas: influence of tumour size, location and histology. Acta Neurochirurg. 1997;139(12):1136-1142.
[21] Bitzer M., Topka H., Morgalla M., et al. Tumor-related venous obstruction and development of peritumoral brain edema in meningiomas. Neurosurgery. 1998;42(4):730-737.
[22] Go K.G., Kamman R.L., Wilmink J.T., Mooyaart E.L. A study on peritumoral brain edema around meningiomas by MRI and contrast CT. Acta Neurochir Suppl (Wien). 1994;60:365-368.
[23] Stevens J.M., Ruiz J.S., Kendall B.E. Observations on peritumoral oedema in meningioma. Part II: Mechanisms of oedema production. Neuroradiology. 1983;25(3):125-131.
[24] Maiuri F., Gangemi M., Cirillo S., et al. Cerebral edema associated with meningiomas. Surg Neurol. 1987;27(1):64-68.
[25] Tamiya T., Ono Y., Matsumoto K., Ohmoto T. Peritumoral brain edema in intracranial meningiomas: effects of radiological and histological factors. Neurosurgery. 2001;49(5):1046-1051. discussion 1051–1042
[26] Nakano T., Asano K., Miura H., Itoh S., Suzuki S. Meningiomas with brain edema: radiological characteristics on MRI and review of the literature. Clin Imaging. 2002;26(4):243-249.
[27] Go K.G., Wilmink J.T., Molenaar W.M. Peritumoral brain edema associated with meningiomas. Neurosurgery. 1988;23(2):175-179.
[28] Lobato R.D., Alday R., Gomez P.A., et al. Brain oedema in patients with intracranial meningioma. Correlation between clinical, radiological, and histological factors and the presence and intensity of oedema. Acta Neurochirurg. 1996;138(5):485-493. discussion 493–484
[29] Ide M., Jimbo M., Kubo O., et al. Peritumoral brain edema and cortical damage by meningioma. Acta Neurochir Suppl (Wien). 1994;60:369-372.
[30] Gilbert J.J., Paulseth J.E., Coates R.K., Malott D. Cerebral edema associated with meningiomas. Neurosurgery. 1983;12(6):599-605.
[31] Tanaka M., Imhof H.G., Schucknecht B., et al. Correlation between the efferent venous drainage of the tumor and peritumoral edema in intracranial meningiomas: superselective angiographic analysis of 25 cases. J Neurosurg. 2006;104(3):382-388.
[32] Shirotani T., Shima K., Chigasaki H. Resolution of peritumoral brain edema following excision of meningioma. Acta Neurochir Suppl (Wien). 1994;60:416-418.
[33] Chen C.H., Shen C.C., Sun M.H., et al. Histopathology of radiation necrosis with severe peritumoral edema after gamma knife radiosurgery for parasagittal meningioma. A report of two cases. Stereotact Funct Neurosurg. 2007;85(6):292-295.
[34] Kan P., Liu J.K., Wendland M.M., Shrieve D., Jensen R.L. Peritumoral edema after stereotactic radiosurgery for intracranial meningiomas and molecular factors that predict its development. J Neuro-oncol. 2007;83(1):33-38.
[35] Ide M., Jimbo M., Yamamoto M., et al. MIB-1 staining index and peritumoral brain edema of meningiomas. Cancer. 1996;78(1):133-143.
[36] Provias J., Claffey K., delAguila L., et al. Meningiomas: role of vascular endothelial growth factor/vascular permeability factor in angiogenesis and peritumoral edema. Neurosurgery. 1997;40(5):1016-1026.
[37] Senger D.R., Galli S.J., Dvorak A.M., et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science (NY). 1983;219(4587):983-985.
[38] Leung D.W., Cachianes G., Kuang W.J., Goeddel D.V., Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science (NY). 1989;246(4935):1306-1309.
[39] Unemori E.N., Ferrara N., Bauer E.A., Amento E.P. Vascular endothelial growth factor induces interstitial collagenase expression in human endothelial cells. J Cell Physiol. 1992;153(3):557-562.
[40] Pepper M.S., Ferrara N., Orci L., Montesano R. Vascular endothelial growth factor (VEGF) induces plasminogen activators and plasminogen activator inhibitor-1 in microvascular endothelial cells. Biochem Biophys Res Commun. 1991;181(2):902-906.
[41] Senger D.R., Van de Water L., Brown L.F., et al. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastas Rev. 1993;12(3–4):303-324.
[42] Millauer B., Shawver L.K., Plate K.H., Risau W., Ullrich A. Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature. 1994;367(6463):576-579.
[43] Millauer B., Longhi M.P., Plate K.H., et al. Dominant-negative inhibition of Flk-1 suppresses the growth of many tumor types in vivo. Cancer Res. 1996;56(7):1615-1620.
[44] Millauer B., Wizigmann-Voos S., Schnurch H., et al. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72(6):835-846.
[45] Kalkanis S.N., Carroll R.S., Zhang J., Zamani A.A., Black P.M. Correlation of vascular endothelial growth factor messenger RNA expression with peritumoral vasogenic cerebral edema in meningiomas. J Neurosurg. 1996;85(6):1095-1101.
[46] Otsuka S., Tamiya T., Ono Y., et al. The relationship between peritumoral brain edema and the expression of vascular endothelial growth factor and its receptors in intracranial meningiomas. J Neuro-oncol. 2004;70(3):349-357.
[47] Yoshioka H., Hama S., Taniguchi E., et al. Peritumoral brain edema associated with meningioma: influence of vascular endothelial growth factor expression and vascular blood supply. Cancer. 1999;85(4):936-944.
[48] Paek S.H., Kim C.Y., Kim Y.Y., et al. Correlation of clinical and biological parameters with peritumoral edema in meningioma. J Neuro-oncol. 2002;60(3):235-245.
[49] Bello L., Zhang J., Nikas D.C., et al. Alpha(v)beta3 and alpha(v)beta5 integrin expression in meningiomas. Neurosurgery. 2000;47(5):1185-1195.
[50] Kilic T., Bayri Y., Ozduman K., et al. Tenascin in meningioma: expression is correlated with anaplasia, vascular endothelial growth factor expression, and peritumoral edema but not with tumor border shape. Neurosurgery. 2002;51(1):183-192. discussion 192–193
[51] Shweiki D., Itin A., Soffer D., Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359(6398):843-845.
[52] Tatagiba M., Mirzai S., Samii M. Peritumoral blood flow in intracranial meningiomas. Neurosurgery. 1991;28(3):400-404.
[53] Levy A.P., Levy N.S., Wegner S., Goldberg M.A. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995;270(22):13333-13340.
[54] Stein I., Neeman M., Shweiki D., Itin A., Keshet E. Stabilization of vascular endothelial growth factor mRNA by hypoxia and hypoglycemia and coregulation with other ischemia-induced genes. Mol Cell Biol. 1995;15(10):5363-5368.
[55] Levy A.P., Levy N.S., Goldberg M.A. Post-transcriptional regulation of vascular endothelial growth factor by hypoxia. J Biol Chem. 1996;271(5):2746-2753.
[56] Hirashima Y., Hayashi N., Fukuda O., et al. Platelet-activating factor and edema surrounding meningiomas. J Neurosurg. 1998;88(2):304-307.
[57] Ruderman N.B., Hall T.C. Use of glucocorticoids in the palliative treatment of metastatic brain tumors. Cancer. 1965;18:298-306.
[58] Jelsma R., Bucy P.C. The treatment of glioblastoma multiforme of the brain. J Neurosurg. 1967;27(5):388-400.
[59] Merrill M.J., Oldfield E.H. A reassessment of vascular endothelial growth factor in central nervous system pathology. J Neurosurg. 2005;103(5):853-868.
[60] Criscuolo G.R., Lelkes P.I., Rotrosen D., Oldfield E.H. Cytosolic calcium changes in endothelial cells induced by a protein product of human gliomas containing vascular permeability factor activity. J Neurosurg. 1989;71(6):884-891.
[61] Takamoto T., Sasaki M., Kuno T., Tamaki N. Flk-1 specific kinase inhibitor (SU5416) inhibited the growth of GS-9L glioma in rat brain and prolonged the survival. Kobe J Med Sci. 2001;47(4):181-191.
[62] Ragel B.T., Jensen R.L., Gillespie D.L., Prescott S.M., Couldwell W.T. Ubiquitous expression of cyclooxygenase-2 in meningiomas and decrease in cell growth following in vitro treatment with the inhibitor celecoxib: potential therapeutic application. J Neurosurg. 2005;103(3):508-517.
[63] Wei D., Wang L., He Y., et al. Celecoxib inhibits vascular endothelial growth factor expression in and reduces angiogenesis and metastasis of human pancreatic cancer via suppression of Sp1 transcription factor activity. Cancer Res. 2004;64(6):2030-2038.
[64] Portnow J., Suleman S., Grossman S.A., Eller S., Carson K. A cyclooxygenase-2 (COX-2) inhibitor compared with dexamethasone in a survival study of rats with intracerebral 9L gliosarcomas. Neuro-oncol. 2002;4(1):22-25.