Chapter 36 Meningiomas
• Meningiomas are believed to derive from the arachnoid cap cells around arachnoid granulations near venous sinuses, cisterns, ventricles, and brain. They can be found anywhere there is known pia, arachnoid, or dura. These tumors exhibit a wide variety of behaviors from benign to extremely aggressive. The etiology of meningiomas is unclear but some are associated with genetic aberrations such as partial loss of chromosome 22, prior trauma, and radiation therapy.
• The latest World Health Organization (WHO) grading system of meningiomas evaluates these neoplasms from grades I to III. Grade I meningiomas have nine subtypes ranging from fibroblastic to psammomatous. Despite the different histological patterns there is no prognostic significance among the subtypes of grade I meningioma. Grade II represents an atypical meningioma and implies the presence of mitosis, or three or more of such features as increased cellularity, brain invasion, or necrosis. Grade III anaplastic malignant meningiomas are characterized by highly active mitosis, and their tumor cells resemble carcinoma or sarcoma. Metastases are rare in meningioma but can occur in the lungs, liver, bone, and heart.
• The treatment of meningiomas depends on a variety of factors such as their growth rate, radiological characteristics, location, and patient clinical status and age. The advent of magnetic resonance imaging (MRI) has brought the age of more incidentally diagnosed lesions. The natural history of meningiomas varies, as do the growth rates. Some incidentally discovered meningiomas remain stable and can be observed, especially in elderly patients with few symptoms or signs. Meningiomas are symptomatic in a wide range of patient ages and locations and thus warrant excision. Total surgical excision of meningiomas is the treatment of choice.
• A wide spectrum of surgical approaches can be employed to radically excise a meningioma. Preoperative embolization can decrease intraoperative blood loss in selected patients. Postoperative radiation therapy, radiosurgery, and hormonal therapy is required for incompletely resected lesions or those with malignant characteristics.
• Simpson classification of meningioma provides a general estimate of recurrence after resection. The resection ranges from grade I resection, which is complete removal, to grade IV subtotal resection, and grade V, which is decompression of the tumor. The recurrence rates are as low as 9% for grade I resections and as high as 40% for grade IV resections.
In 1922, Harvey Cushing presented a series of 85 meningeal tumors in his Cavendish lecture and coined the term meningioma to describe these lesions.1 Years later with Louise Eisenhardt, he created a definitive monograph on these tumors.2 He believed that all meningeal tumors arose from the arachnoidal cap cells that are particularly abundant in the arachnoid granulations.
Meningiomas are the most common brain tumor and have a wide variety of clinical behaviors. Although most of them behave rather nicely, there are some that are extremely aggressive. Little is known about the reasons for this difference in natural history. The etiology of meningiomas remains unknown, but previous radiation therapy and monosomy or partial loss of chromosome 22 are important factors. Radiation therapy, at a high or low dosage, can cause meningiomas even after several years of treatment.3
Epidemiology
Meningiomas constitute 15% to 20% of all primary intracranial tumors in surgical series, but their incidence in routine screening is 1 in 100 population. Their incidence increases with advancing age.4 They predominantly affect women with an overall male-female ratio of 1:2.5. This difference is increased for intraspinal meningiomas with a ratio of 1:10 in comparison with an intracranial ratio of 2:3. Meningeal tumors are rare in children but tend to be more aggressive when they occur in children. They represent 0.4% to 4.1% of all childhood brain tumors and constitute 1.5% to 1.8% of all meningiomas.5,6 Pediatric meningiomas tend to be more frequent in males with a male-female ratio of 1.2 to 1.9:1 and have a higher incidence of ventricular location.7,8
In one study, symptomatic meningiomas were encountered in 2.0 per 100,000 of the population and asymptomatic ones in 5.7 per 100,000, with an overall incidence of 7.7 per 100,000.9
Imaging
Computed Tomography
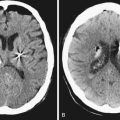
Meningiomas on nonenhanced CT usually appear as well-circumscribed extra-axial lesions, hyperdense (70-75%), isodense (25%), or hypointense (1-5%) to adjacent parenchyma.8 Calcifications ranging from microscopic psammoma bodies to dense sclerosis are found in 25% of patients. Necrosis, cysts, and hemorrhage are seen occasionally (8-23%).8,10 With contrast agent, they usually enhance brightly.
Magnetic Resonance Imaging
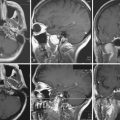
T1-weighted imaging shows meningiomas as isointense or moderately hypointense to gray matter lesions. Calcifications and highly fibrous areas are hypointense. FLAIR (fluid-attenuated inversion recovery) is helpful to demonstrate edema, seen as a hyperintense signal in the adjacent parenchyma. T2-weighted imaging may present a wide range of possible signal intensities. Usually isointense or mildly hyperintense, it can show hypointensity if the meningioma is calcified or highly fibrous. Massive surrounding edema is seen as a hyperintense signal. Arterial feeders to tumor are seen as arborizing flow voids (hypointense). Pial blood vessels present as surface flow voids between tumor and parenchyma.11 T2-weighted gradient echo (GRE) may “bloom” as parenchymal low signal, suggesting calcifications or intratumoral microhemorrhages.
T1 contrast enhancement shows meningiomas as heterogeneous clearly defined hyperintensive images. The “dural tail” may enhance in 35% to 80%, but is not specific.11,12 Magnetic resonance (MR) angiography and venography are noninvasive options to demonstrate tumor blood supply, vascularization, drainage veins, and sinus compromise.
Functional MRI is based on increased brain hemodynamics in response to cortical neuronal activity due to certain stimulus performed during imaging. It can be helpful in surgical planning for localization of motor, sensory, and language regions. Diffusion may differentiate benign from atypical or malignant meningiomas.13,14 Perfusion reveals differentials in relative cerebral blood volume, allows us to distinguish meningiomas from dural metastases,15 and according to some authors also discerns typical from atypical histological grades.8,16 Spectroscopy shows high choline peak, low or absent N-acetylaspartate (NAA) and creatinine levels, and variable amounts of lactate. Some of them also present high alanine and glutamate/glutamine levels on MR spectroscopy.17
Positron Emission Tomography
The role of positron emission tomography (PET) in patients with meningiomas is still not clear. The benign variants of these tumors usually show isometabolism with [18F]-fluorodeoxyglucose (FDG) markers while atypical or anaplastic meningeal tumors may exhibit hypermetabolism.18,19 FDG uptake in meningioma could be a predictive factor in tumor recurrence.20 In one study FDG PET had 80% sensitivity but only 57% specificity for detecting meningiomas.21
Single-Photon Emission Computed Tomography/Scintigraphy
Meningiomas have many somatostatin receptors (SSr) and this is the base of the scintigraphy in which SSr-positive tumors can be imaged in vivo through single-photon emission computed tomography (SPECT). Octreotide is a somatostatin analog with high binding affinity for SSr subtype 2 and a longer plasma half-life than native somatostatin. Therefore, octreotide is a better SSr imaging agent than somatostatin.22 Octreotide SPECT had 83% sensitivity and 27% specificity for identifying meningiomas.21
Pathology
Hormone Receptors
Meningioma growth may be related to hormonal status due to the presence of estrogen and progesterone receptors. The tumor may become clinically evident during pregnancy or in the luteal phase of the menstrual cycle.23 The expression of progesterone receptors alone in a meningioma could be related to a favorable behavior. Absence of both progesterone and estrogen receptors or the presence of estrogen receptors alone correlates with aggressive clinical behavior, progression, and recurrence after complete surgical resection.24 Despite the presence of these receptors, drug therapies targeting hormonal status have not been particularly successful.
Classification
Histological subtypes are classified according to the most recent WHO grading system published in 200725 (Box 36.1). In the 2000 WHO classification of meningiomas, brain invasion was associated with aggressive behavior and increased probability of recurrence, but was not included as a diagnostic criterion for grade II or grade III tumor.3 Perry and associates demonstrated that brain invasion indicated a greater likelihood of recurrence and felt that it should be considered one of the diagnostic features of grade II meningioma.26 Following these findings, brain invasion by a meningioma is now an independent criterion for WHO grade II. Subtype classification does not appear to influence prognosis unless atypia or malignancy is evident.
BOX 36.1 World Health Organization (WHO) Grading of Meningiomas, 2007
Grade II
Clear cell type is distinguished by lobulated or sheet-like proliferations of polygonal cells with clear, abundant glycogen cytoplasm (periodic acid–Schiff positive [PAS+]). This type is associated with a higher incidence of recurrence, frequently affects young patients, and commonly arises in spinal or cerebellopontine locations.27
Chordoid type is marked by the presence of cords of eosinophilic, epithelial-like, and vacuolated cells in a prominent myxoid background, similar to the appearance of chordomas. It is associated with chronic inflammation cell pattern, dysgammaglobulinemia, and microcytic anemia (features observed in Castelman’s disease).28 This variant also presents a high rate of recurrence after subtotal resection.29
Grade III
Rhabdoid variant is characterized by rhabdoid-like cells with prominent eosinophilic cytoplasm, prominent nucleolus, and eccentric nuclei. This histological presentation has been associated with increased risk of recurrence and distant metastases.
Decision Making
In order to integrate these variables some treatment algorithms have been developed. Dr. Takeshi Kawase and his group (Adachi and associates30) in 2006 presented a set of rules for treating cranial base meningiomas. They give a score to each tumor based on predetermined risk characteristics:
A higher score number (risk factor) implies a lower chance of complete resection.
Dr. Joung Lee31 and his group at the Cleveland Clinic designed the “CLASS” algorithm for the treatment of all meningiomas. This algorithm compares negative features (comorbidity, location, and age) against benefits (size and symptoms) and assigns a score:
Conservative Treatment
Around two thirds of asymptomatic meningiomas do not continue to grow and may be observed at appropriate time intervals. Absolute growth rates of meningiomas vary between 0.03 and 2.62 cm3 per year. Several studies following the behavior of asymptomatic meningiomas showed minimal growth during the follow-up period. In a retrospective study of tumor growth rate in 37 patients, 9 of the 37 (24.3%) showed tumor growth during a mean follow-up period of 4.2 years. Annual growth rates were calculated as the difference in tumor volume between the initial and latest imaging, divided by the time interval (years) between these determinations, with tumor growth defined as an annual increase in tumor volume more 1 cm3 per year. In this study they associate the age of patients and the volume of the tumor at its initial diagnosis with growth rate increased. They concluded that young patients and those with large tumors should be carefully observed.32 Nakamura and colleagues33 studied 41 patients with asymptomatic meningiomas, reporting a majority (66%) of growth rates less than 1 cm3 per year. They also correlated growth rate with patient age but did not consider initial tumor size as a predictive factor for tumor growth. Yano and Kuratsu in their study of surgical indications for asymptomatic meningiomas reported 37% of tumor growth during a period of observation of 3.9 years and only 6.4% becoming symptomatic.34 Some authors recommend the surgical resection of meningiomas when the tumor growth rate is greater than 1 cm3 per year.32,33 Radiological features such as partial or complete calcification is related to slow growth rate or absence of it, so these tumors may be kept only under observation. Meningiomas that remain asymptomatic but show displacement and compression of delicate structures as spinal cord, optic nerve, chiasm, and brainstem, or with considerable surrounding edema, should be considered for early treatment. Observation alone, with periodic neurological and MRI evaluation follow-up, first at 3 months, second at 6 months, and then every year, is reasonable for asymptomatic or minimal symptomatic elderly patients with fewer than 10 to 15 years of remaining life expectancy.
Surgical Treatment
General Surgical Planning
Preoperative Embolization
The main goal of this procedure is to decrease intraoperative blood loss in meningiomas with high vascular supply. The superselective catheterization makes this procedure safer and allows for controlling the aggressiveness of embolization. The proximal occlusion of the tumor-feeding arteries only reduces the blood supply temporarily and collateral flow quickly develops. The time between embolization and surgery is controversial. The possibility of necrosis induced by vascular occlusion and therefore the softening of tumoral tissue should be compared to the increase of collateral supply development on time. The optimal interval could be between 3 and 9 days. Complications such as painful trismus, facial pain, scalp necrosis, ischemic stroke, and intratumoral hemorrhage could occur but are infrequent.
General Recurrence Rate
In 1957 Simpson35 classified meningioma resection as follows: grade I, complete removal, including resection of dura and bone; grade II, complete tumor removal with coagulation of dural attachment; grade III, complete tumor removal without resection or coagulation of dural attachments; grade IV, subtotal removal; and grade V, decompression. This classification remains useful for evaluating recurrences. In Simpson’s series, grade I through grade IV tumors had recurrence rates of 9%, 19%, 29%, and 40%, respectively, at a follow-up period of 10 years.
Considerations by Location
Convexity Meningiomas
Surgical Technique
Approach
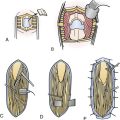
Depending on the location and size of the lesion a linear or horseshoe incision is done with preservation of scalp vascularity. The scalp incision should extend at least 2 cm away from the craniotomy; this should allow extending the dura resection around 2 cm beyond the meningioma border. The pericranium is dissected from the skull and prepared for later grafting. Bur holes are placed around the tumor, and the dura is separated from the overlying bone with a blunt dissector. The bone flap is cut with a high-speed craniotome. Bleeding from bone edges is controlled with bone wax. The infiltrated dura or identified tumor-feeding vessels are coagulated.
Operative Results
In a series of convexity meningiomas Black and co-workers36 reported no surgical fatality and no significant difference in morbidity between age groups younger and older than 65 years. The overall morbidity rate was 5.5%. The 5- and 10-year survival rate was 90% with overall recurrence rate of 4.3%. The 5-year recurrence rate for WHO grade I tumors was zero, for grade II 27.2%, and for grade III was 50%. In their series 15 patients (9%) underwent radiation therapy.
Parasagittal Meningiomas
Type I: Attachment to outer surface of the sinus wall
Type II: Fragment inside the lateral recess
Type III: Invasion of the ipsilateral wall
Type IV: Invasion of the lateral wall and roof
Types V and VI: Complete sinus occlusion, with or without one wall free, respectively37
To achieve a Simpson grade I or II radical resection, the infiltrated SSS should be removed with the tumor. They represent about 16.8% to 25.6% of all intracranial meningiomas.38
Pathology
The histological pattern in a reported series of 106 was 79.6% for WHO grade I tumors, 14.8% for grade II, and 3.7% for grade III meningiomas.39
Treatment
Observation is acceptable in asymptomatic elderly patients or with tumor less than 3 cm in diameter. The SSS total or partial invasion can be treated by radical resection of the sinus with or without venous reconstruction. Resecting the SSS is associated with an increased risk of intraoperative and postoperative hemorrhage, sinus occlusion, corticovenous thrombosis, and venous infarction leading to brain edema. A less aggressive surgical approach, with satisfactory long-term effect and fewer complications, is to resect the tumor up to the sinus wall and leave the sinus intact.36 Residual tumor can be followed up and treated with radiosurgery at recurrence. Radiosurgery as first-line treatment can offer good results for tumors smaller than 3 cm.
Surgical Technique
Operative Results
In Black and co-workers’36 series the anterior third of the SSS was involved in 12.8% of tumors, the middle third in 69.2%, and the posterior third in 17.9%. In 63.2% of patients there was total tumor resection, Simpson grades I and II. In 14 patients (36.8%) residual tumor was found on postoperative imaging, and 13.2% of those had tumor progression. Recurrence-free survival rate was 94.7% at 5 years.
Falx Meningiomas
These meningiomas arise from the falx cerebri and tend to grow and compress the medial surface of the cerebral hemispheres. They can be classified according to involvement of the falx in longitudeal dimension. Like parasagittal meningiomas, they can be divided into anterior, middle, or posterior types. The classification proposed by Yasargil40 separated them into outer falx meningiomas, which arise from the body of the falx, and inner falx meningiomas that arise adjacent to the inferior sagittal sinus (ISS). Falcine meningiomas represent 8.5% of all intracranial meningiomas.41
Evaluation
About 60% of falx meningiomas present the dural tail sign.41 MRVA or angiographies are useful to determine the displacement or involvement of the anterior cerebral artery (ACA). The venous phase shows the SSS of ISS invasion and the localization of venous drainage.
Surgical Technique
Operative Results
In the series of 68 patients presented by Chung and colleagues,41 85.2% had total resection with no evident recurrence and 92.6% achieved a good outcome (no neurological deficit or complications). SRS was performed as a postoperative adjunctive treatment in six patients.
Olfactory Groove Meningiomas
Surgical Technique
Positioning
An external lumbar cerebrospinal fluid (CSF) drainage is placed to prevent or reduce brain retraction. For a frontotemporal approach the patient is placed in the supine position. The patient’s trunk and head are elevated 20 degrees.The head is turned to the contralateral shoulder, 30 degrees for more anterior lesions and 20 degrees for posterior tumors. The head is flexed, taking the chin to the ipsilateral clavicle and then slightly hyperextended so that the maxillary eminence reaches the highest point in the surgical field. For the bifrontal approach the patient is also placed in a supine position but with the head in a neutral position and minimally extended inferiorly.
Operative Results
In Obeid and Al-Mefty’s42 series of 13 benign OGMs gross total resection was achieved in 93.3% of patients. Vision remained stable in six patients and improved in eight with no recurrence in a median follow-up period of 3.7 years. They reviewed the recurrence rate for OGMs reported in the literature and found that it ranged from 5% to 41%, and concluded that radical tumor resection, including the dural attachment and any involved bone during the initial surgery, is the best way to reduce the chances of recurrence.42
Tuberculum Sellae Meningiomas
Tuberculum sellae meningiomas (TSMs) arise from the dura of the tuberculum sellae, diaphragma sellae, chiasmatic sulcus, and limbus sphenoidale. Usually bilateral, they grow from the midline over one side. They can invade the suprasellar region as other meningiomas with different dural origins. TSM can be distinguished from OGM by the displacement of the optic nerves and chiasm. TSMs elevate the chiasm and optic nerves superolaterally, but OGMs displace the chiasm downward and posteriorly as they grow. TSMs represent 5% to 10% of all intracranial meningiomas.43
Clinical Presentation
The most frequent presentation of a TSM is optic atrophy with bitemporal hemianopia. The asymmetrical visual loss usually begins in an insidious way and progresses slowly. Other occasional symptoms include headache, mental status deterioration, seizures, anosmia, hyperprolactinemia due to pituitary stalk posterior displacement and compression, and hydrocephalus in cases of third ventricle compression.
Expanded Endonasal Approach
Provide access to the anterior skull base extending from the crista galli to the foramen magnum;44 all 12 cranial nerves and the carotid and vertebrobasilar arteries can be seen through the nose. This approach should be considered only for small TSMs measuring less than 4 cm owing to the limited lateral explosion. Tumors arising lateral to the optic nerve or beyond the midline of the superior orbit are best approached via craniotomy if the objective of surgery is total removal.44 Neuronavigation is commonly used.
Postoperative Care
ATB therapy is used until the endonasal tamponades are removed 48 hours after the surgery.
Operative Results
In Nakamura and Samii’s43 series of 72 TSMs, total tumor removal could be achieved in 91.7% of patients (Simpson grades I and II). They found a visual improvement rate of 71% in small tumors (maximum diameter <3 cm) and 64% in larger tumors (diameter ≥3 cm) but the difference was not statistically significant. Recovery is thought to be related to tumor size, duration of visual symptoms, and patient edge. Gardner and associates’45 series of anterior cranial base meningiomas resected endoscopically and endonasally reported that 85% of 13 patients underwent complete resection (Simpson grade I), and one patient underwent 95% resection. The remaining tumor had a 78% resection, based on volumetric analysis. The postoperative CSF leak rate of their entire series was 40%, mostly in TSMs.
Complications
To minimize brain retraction the sphenoid ridge must be drilled and flattened as far as possible. When the dural base and hyperostotic bone are drilled care should be taken to avoid opening the sphenoid sinus. In case of eventual opening a pericranial flap or fascia of the temporal muscle with addition of fibrin glue is used for covering the defect. The surgeon who performs endoscopic surgery for TSM removal must have significant technical experience to deal with critical encased vessels or tumor inside the optic canal. The dural opening cannot be closed by primary means; a vascularized nasoseptal flap should be used in every case to avoid CSF leakage. In the event a leak does occur immediate and early reexploration is recommended rather than diversion with a lumbar drain.45
Optic Nerve Sheath Meningiomas
Optic nerve sheath meningiomas (ONSMs) involve the optic nerve and the anterior visual pathways. They usually arise from the arachnoidal membrane of the intraorbital nerve and extend through the optic canal to the anterior fossa. Without treatment, slowly but progressive growth often results in unremitting visual loss. Schick and colleagues46 classify the ONSM as three types:
Type I: Intraorbital lesions (Ia, flat extension around the optic nerve; Ib, bulbiform mass around the optic nerve; Ic, exophytic tumor around the optic nerve)
Type II: Intraorbital tumors with intracranial extension through the optic canal or superior orbital fissure (IIa, intraorbital growth through the optic canal; IIb, growth through the superior orbital fissure or cavernous sinus)
Type III: Intraorbital tumors with widespread intracranial tumor extension (IIIa, extension to chiasm; IIIb, extension to chiasm, contralateral optic nerve, and planum sphenoidale)
Pathology
The most common histological presentations are meningothelial and transitional.47 Aggressive tumors tend to infiltrate rather than compress of the globe or optic nerve.
Treatment
Schick and colleagues46 recommended radiotherapy without biopsy for type Ia meningiomas; type Ib should be treated with surgery only if it is causing painful eye discomfort without useful vision. Otherwise, these tumors can be observed and treated with radiation once visual decline begins. Type Ic tumors with large exophytic portions should be treated surgically. Type IIa and IIb tumors causing visual impairment should be explored intradurally achieving decompression of the optic canal and superior orbital fissure (SOF). Subtotal resection must be followed by RT. Cavernous sinus involvement should be treated with RT. Type III tumors are operated on to prevent affecting the optic chiasm and contralateral optic nerve. The intraorbital portion should be treated with radiation once visual symptoms or signs occur.
Surgical Technique
Microsurgical Resection
The sylvian fissure aperture and the ipsilateral optic nerve and carotid cisterns are identified. The tumor capsule is coagulated and dissected around the dura of the optic canal. The falciform ligament of the ipsilateral optic canal is identified and opened to release the nerve. The optic nerve sheath is opened until the annulus of Zinn is reached; this maneuver expands the operative field mainly in the opticocarotid triangle, facilitating access to meningiomas in the suprasellar and subchiasmatic regions. The tumor among the infiltrated dura and the optic nerve is removed. Complete neurectomy and tumor resection are performed in blind patients with disfiguring painful proptosis. The intracranial tumor removal follows the classical steps of microsurgical resection.
Operative Results
Visual improvement after surgical treatment is unusual. In a large reported series of 79 patient with OSNM treated with surgery approximately 7.5% had visual improvement after surgery, 78.5% maintained their vision, and 14% suffered visual deterioration postoperatively.46 Delfini and co-workers48 reported that 11 (84%) of 13 patients treated with surgery for ONSM developed postoperative amaurosis. In a large review of meningiomas involving the orbit, Dutton49 reported a mortality rate of 0%, the rate of operative complication was 30%, and the recurrence rate was 25%. Postoperative visual improvement was shown in only 5% of cases, in contrast with approximately 78% of patients experiencing no light perception. Recurrence rates for ONSMs have been reported to be 6.9% for WHO grade I, 34.6% for WHO grade II, and 72.7% for WHO grade III.
Radiotherapy
RT demonstrates stabilization and even improvement in vision. In early studies Dutton reported outcomes for ONSM treated with RT: in 75% of cases visual acuity improved, in 8% vision remained stable, and in 17% vision declined. Turbin and Pokorny47 reported 64 patients with ONSM managed with observation, surgery, surgery and adjuvant RT, or RT alone and concluded that treatment with RT alone resulted in the best long-term visual outcomes. They recommended fractionated external beam radiation between 50 and 55 Gy. However, 33% of these patients developed complications related to radiation. Complication rates improved with the introduction of precisely targeted radiation in the form of SRS or SCRT (stereotactic conformal radiotherapy). Andrews and associates,50 in a series of 24 eyes treated with SCRT, used doses of 54 Gy and demonstrated visual improvement in 41.6%, stabilization in 50%, and complications in only 4%. Finally, Baumert and colleagues51 reported on the fractionated SCRT treatment of 23 eyes with a mean follow-up period of 20 months and found 70% showed visual improvement and 22% had stable vision, and they reported the same complication rate of 4%. Fractionated SCRT has proved to be an important noninvasive treatment alternative for ONSM with preservation and improvement of visual function in approximately 80% of the patients.
Anterior Clinoidal Meningiomas
Anterior clinoidal meningiomas (ACMs) arise from the meningeal covering of the anterior clinoid process (ACP). Also called medial sphenoid wing meningiomas, they are considered one of the most challenging to treat because of failure of total removal, high surgical mortality and morbidity rates, and a high rate of recurrence. Al-Mefty52 classified clinoidal meningiomas based on their origin in three groups. In group I, the tumor origin is proximal to the end of the carotid cistern, and in its growth enwraps the carotid artery without intervening arachnoid. In group II tumors originate from the superior or lateral aspect of the anterior clinoid above the segment of the carotid invested in the carotid cistern and enwrap the carotid with intervening arachnoid. Finally in group III, tumors originate at the optic foramen, extending into the optic canal and the tip of the anterior clinoid process. Pamir and co-workers53 combined the coronal diameter of the clinoidal meningiomas (suprasellar extension) with the classical Al-Mefty classification. They graded each tumor numerically to correspond to the classification of Al-Mefty and added a capital letter to represent the tumor size on coronal section. The letter A corresponds to a tumor measuring less than 2 cm, B applies to tumors between 2 and 4 cm, and C designates a tumor larger than 4 cm. Factors such as arachnoidal membrane covering of the tumor, size, and neurovascular relationship are important in determining the surgical resectability. ACMs represent 6.5% of all meningiomas and 24.5% of all meningiomas in the anterior fossa.
Clinical Presentation
Visual disturbances were present in 84% of patients. Visual loss preceded diagnosis by an average of 25 months.52 Other common findings are optic atrophy, papilledema, seizures, headache, and oculomotor or trigeminal nerve impairment.
Surgical Technique
The frontotemporal approach is used.
Extradural Considerations
The sphenoid ridge is drilled out and a limited posterior orbitotomy is performed with the removal of the posterolateral orbital wall to completely decompress the superior orbital fissure. The optic canal is unroofed to avoid entering into the ethmoid or sphenoid sinus. The optic nerve is exposed and the dura is dissected from the ACP. The ACP and optic strut are drilled and gently fractured. The dura is opened in a C-shape fashion with an anterior base. Under microscopic guidance the dural incision is continued from the falciform ligament along the length of the optic nerve sheath extending to the annulus of Zinn. The intradural ICA is identified and with gentle maneuvers the tumor around the optic nerve is removed.
Operative Results
Al-Mefty52 in his analysis of 24 patients achieved total resection in the 75%, with a low recurrence rate of 4% in a median follow-up at 57 months. Lee and associates54 reported total resection rates of 72% in 42 patients with cavernous meningioma; 22 of them presented with visual deficits and 11 had visual improvement after surgery. No patient in their series showed loss of vision postoperatively.
According to the classification proposed by Pamir and co-workers53 for a series of 43 cavernous meningiomas, 2 tumors were type IB (4.7%), 8 were type IIA (18.6%), 14 were type IIB (32.5%), 16 were type IIC (37.2%), and 3 (6.9%) were type IIIA. They achieved total surgical removal in 39 cases (90.7%). Vision improvement was found in 22 of the 26 patients who had visual problems, and none of the 43 patients presented with vision deterioration after operation. However, they reported a postoperative complication rate of 18% and a recurrence rate of 11% over a median follow-up period of 32 months.
Spheno-Orbital Meningiomas
Clinical Presentation
The most common presentation of SOM is progressive exophthalmos (55-88%), visual impairment (32-78%), and ocular paresis (15-20%).55–57
Pathology
Bone infiltration shows a histological periosteal pattern of hyperostosis. These tumors can have extensive intraosseous involvement without dural infiltration. In Shivastava and colleagues’56 series all treated SOMs were low grade (WHO I), and the most frequently found variety was meningothelial.
Surgical Technique
Approach
A bicoronal incision is planned from the ipsilateral zygoma extending to the contralateral superior temporal line just behind the hairline. The skin flap is elevated and retracted anteriorly. An interfascial dissection is performed to protect the frontal branch of the facial nerve. The temporalis muscle is divided and reflected inferiorly. The anterior edge of the orbit, the foramen, and the supraorbital nerve are identified. The periorbita is dissected from the orbit ridge superiorly and laterally, and the supraorbital nerve is gently released from its notch or foramen. A keyhole is drilled behind the suture between the frontal bone and the frontal process of the zygomatic bone. Additional bur holes are made in the temporal bone and above the superior temporal line. The first cut is performed with a craniotome from the temporal squama bur hole and extended superiorly to the bur hole above the superior temporal line and anteriorly to the orbital edge, just lateral to the supraorbital notch. The next cut extends from the temporal squama bur hole anteriorly and parallel to the zygomatic arch and then turns superiorly toward the sphenoid ridge until stopped by the bony ridge. A cut then is made from the keyhole to the sphenoid ridge. The final cuts are made using the craniotome without the footplate. A cut is made through the orbital ridge and roof connecting with the first cut performed. Then a cut is made from the lateral orbital and frontal process of the zygoma to the keyhole, and finally, a superficial cut is made in the sphenoid spine allowing its fracture when the bone flap is elevated. Osteotomes are used to fracture the orbital roof and lateral wall. An anterior clinoidectomy with unroofing of the optic canal is performed in patients with visual deficits or intracanalicular tumor. The bone infiltration is drilled away, and during this procedure the dura is left intact as long as possible.
Operative Results
In an early report, Carrizo and Basso58 in a series of 25 patients presented postoperative improvement of exophthalmos without sequelae in 80% of the patients. Ringel and associates57 presented a large series of 63 patients, with a median follow-up of 4.5 years, achieving proptosis improvement in 77%. Tumor residuals were found in 66%, of which 61% were stable and 39% were progressive. Scarone and colleagues59 achieved a subtotal resection (Simpson grade II) in 90% of their patients. Radiological evaluation at a median follow-up of 61 months showed no contrast enhancement in 14 patients (47%), residual contrast enhancement without evolution in 13 (43%), and recurrence (new contrast enhancement) in 3 (10%). The exophthalmos improved at a median follow-up period of 61 months in 28 patients (93%).
Cavernous Sinus Meningiomas
Meningiomas involving the cavernous sinus (CS) are one of the most challenging tumors in regard to achieving radical surgical resection. They can arise and remain within the sinus, extend outside the sinus and infiltrate its lateral wall, or growth inside and outside the sinus. The true cavernous sinus meningiomas (CSMs) are seen infrequently; almost all the meningiomas that involve the CS arise in surrounding parasellar dura. Advances in skull base techniques allow surgical tumor resection in this area, previously considered inaccessible, although with significant morbidity, including hemorrhage, cranial nerve deficits, and ICA injury. RT in combination with microsurgical resection or alone has led to new treatment strategies for these tumors. In a large series of meningiomas involving the CS only 8% truly arise from the dural covering of the sinus.60
Pathology
Pathological classification of these tumors shows that 92.6% are WHO grade I (transitional 41%, meningothelial 23%, and angiomatous 22%), 5.4% are WHO grade II, and 2% are WHO grade III.60
Treatment
Primary management options range from observation to conservative surgical resection (without opening the sinus), aggressive surgical resection, RT, SRS, SCRT, and a combination of these therapies. Observation can be offered to asymptomatic elderly patients or those with minimal symptoms such as mild facial tingling or numbness. In young and asymptomatic patients with an extracavernous component, the natural growth rate of the disease and the pros and cons of the treatment options should be explained. Yano and Kuratsu34 found a growth rate of 0.19 cm per year in 37.3% of patients, with a median follow-up greater than 5 years in their study of asymptomatic CSMs. Close observation followed by conservative surgical treatment if the patient becomes symptomatic should be considered in these patients. Conservative surgical resection as first-line treatment may be offered for CSM with visual or brainstem compression symptoms or with radiological evidence of progressive tumor growth. Adjuvant therapy with RT or SRS is used in selected cases.60 For intracavernous meningiomas infiltrating the ICA or cranial nerves, SRS can be considered as the first-line treatment because of its long-lasting progression-free survival.54,61
Surgical Technique
A standard frontopterional approach is preferred.
Microsurgical Resection
The sylvian fissure is opened initially in order to avoid injuring the ICA. The tumor is debulked in order to expose the MCA branches surrounding the tumor capsule posteriorly. This maneuver will allow identification of the main trunk of the ICA. After identifying the ICA and the optic nerve, the tumor capsule is dissected from extracavernous structures. If it is applicable, decompression of the oculomotor and cranial nerves at their entry into the CS is performed, leaving the cavernous sinus unopened.
Operative Results
Pichierri and co-workers60 in a series of 147 patients compared a group treated with open sinus surgery with a second group treated with closed sinus surgery. The mean follow-up time was 9.7 years. They found a statistical difference in postoperative morbidity rate between the two groups. Early postoperative morbidity rate was 62.5% for the first group and 31.7% for the second; permanent postoperative morbidity rates were 45.8% and 20.3%, respectively. They didn’t find statistical differences in recurrence rates and progression between groups. Lee and associates54 presented 159 patients, 52% of whom had SRS as their primary treatment. For this group, in 83 patients the control growth rate was 96.9% at 5 years. They concluded that SRS should be considered as the first-line treatment for tumors with a diameter less than 3 cm or volume less than 15 cm3. Similar findings were reported by Nicolato and colleagues61 showing an overall progression-free survival rate at 5 years of 96.5% in CSMs treated with SRS as the primary therapeutic option.
Sphenoid Wing Meningiomas
An early classification of sphenoid wing meningiomas (SWMs) designated them as (1) inner or clinoidal; (2) middle or alar; and (3) outer or pterional. The classification proposed by Pirotte and Brotchi62 in 2008 distinguished them as (A) deep or clinoidal or sphenocavernous; (B) invading en plaque of the sphenoid wings; (C) invading en masse of the sphenoid wings, which combines the features of groups A and B; (D) middle ridge meningiomas; and (E) pterional or sylvian point meningiomas. Clinoidal meningiomas, also named anterior clinoidal meningiomas or medial sphenoid wing meningiomas, were already described. Middle and lateral SWMs are more surgically accessible and resectable than clinoidal meningiomas. They represent up to 20% of intracranial meningiomas.63
Petroclival and Upper Clival Meningiomas
Clinical Presentation
The most frequent cranial nerves involved are the fifth, causing facial paresthesia, and the eighth nerve, leading to hearing loss. Facial nerve disturbance can occur in 30% to 50% of patients, and low cranial nerves are affected in approximately 30% of the cases.64,65 Compression of the brainstem and cerebellum may present as gait disturbances and somatomotor and sensitive deficits.
Evaluation
CT scan may reveal bone erosion, hyperostosis, or both. Displacement and compression of normal structures are best demonstrated by MRI. Edema in the brainstem may be shown on T2-weighted imaging scans and implies disruption of the arachnoidal plane between the tumor and brainstem; thus, aggressive resection of the brainstem must be avoided. MRVA and conventional angiography shows tumor vascularization degree, feeder branches, and displacement or encasement of the basilar artery and the principal branches. Angiography allows embolization of the tumor vascular supply, usually from the external carotid artery. As a distinctive sign the meningohypophyseal trunk (Bernasconi-Cassinari artery) may be enlarged.
Posterior Petrosal Approach
Approach
The transverse-sigmoid sinus junction is exposed with the craniotomy. The cortical bone of the mastoid is drilled out and a mastoidectomy is performed, keeping the bony labyrinth intact. The presigmoid dura is exposed and the sigmoid sinus is skeletonized down to the jugular bulb. The dura is opened anterior to the sigmoid sinus, along the floor of the temporal fossa. The vein of Labbé must be identified at its insertion into the transverse sinus and preserved. The superior petrosal sinus is coagulated and ligated between two stitches, and is incised, allowing the connection with the anterior dura to be open. The tentorium is sectioned parallel to the transverse sinus and perpendicular to the superior petrosal sinus after the identification and preservation of the fourth cranial nerve insertion. The posterior temporal lobe is elevated and the sigmoid sinus is retracted posteriorly, allowing a wide exposure of the cerebellum.
Total Petrosectomy
Operative Results
In a series of 97 patients with PCM presented by Al-Mefty and co-workers,64 28 patients were treated using the anterior petrosal approach, 27 with the posterior petrosal approach, 34 with the combined petrosal approach, and 8 underwent to a total petrosectomy. Eight patients presented with complications related to the approach. There were no cases of trigeminal neuralgia after the zygomatic anterior petrosal approach. Only 8% of the patients who underwent the posterior petrosal or combined petrosal approaches experienced hearing loss.
Petrosal Meningiomas
The cerebellopontine angle (CPA) meningiomas describe a group of tumors that compromise a common anatomical region, the CPA. These lesions may have their true origins in different locations, even outside the CPA. Because of their different clinical presentations and outcomes in relation to their site of origin,66 they have been classified in reference to the internal auditory meatus (IAC) as anterior petrous meningiomas (group 1), tumors involving the IAC (group 2), superior petrous meningiomas (group 3), inferior petrous meningiomas (group 4), and posterior petrous meningiomas (group 5).67 They are the second most frequent tumor in the CPA after vestibular schwannomas (10-15%), representing approximately 8% to 23% of all intracranial meningiomas.
Clinical Presentation
The main form of presentation is the hearing loss (73%), followed by vertigo, tinnitus, trigeminal dysfunction, and facial nerve dysfunction. The brainstem compression can cause gait disturbance and obstructive hydrocephalus in 10% to 20% of these patients.
Pathology
The most frequent histological presentation is WHO I type meningioma. The pattern may differ in relation to its localization, presenting 68% of meningothelial subtype in anterior petrosal meningioma, whereas 69% of posterior petrosal meningiomas and 62% of superior petrosal meningiomas had fibrous histological appearance.68
Surgical Technique
Operative Results
Nakamura and associates67 present a series of 347 CPA meningiomas. Total tumor removal (Simpson grades 1 and 2) was achieved in 85.9% and subtotal removal in 14.1% of the patients, with best initial postoperative seventh and eighth nerve function in tumors located posterior and superior to the IAC. A good postoperative facial nerve function (House-Brackmann grade 1 or 2) was observed in 88.9% of patients with a mean follow-up time of 62.3 months. Hearing preservation among patients with preoperative functional hearing was documented in 90.8%. Similar findings were reported by Sade and Lee68 in a group or 58 patients, with an informed gross total resection in 84% of the patients. New-onset hearing loss was present in 11% of patients. The best surgical results were also obtained with superior and posterior petrosal meningiomas.
Foramen Magnum Meningiomas
These tumors arise anteriorly from the inferior third of the clivus to the superior edge of the C2 body, laterally from the jugular tubercle to the C2 laminae, and posteriorly from the anterior border of the occipital squama to the spinal process of C2. The dentate ligament divided the foramen into anterior and posterior compartments. According to the insertion on the dura, foramen magnum meningiomas (FMMs) can be defined in the anteroposterior plane as anterior, if insertion is on both sides of the anterior midline; lateral, if insertion is between the midline and the dentate ligament; and posterior, if insertion is posterior to the dentate ligament.69 The FMMs are located in the anterior face of the foramen in 70%, anterolateral in 21%, and posterolateral in 9% of the patients.70 They represent 1.5% to 3.2% of all intracranial meningiomas.
Surgical Technique
Postoperative Care
The patient is sent to the intensive care unit intubated, and if the cough and gag reflex is present on the next day the patient is extubated. If new lower cranial deficits are present, tracheotomy and nasogastric feeding must be considered.70 Postoperative cranial nerve deficits require aggressive and early rehabilitation.
Operative Results
Wu and colleagues70 presented a series of 114 patients with FMM. Gross total resection was achieved in 86.0% of patients and subtotal resection in 14.0%. Surgical mortality rate was 1.8%. Ninety-three patients were followed for a median period of 90.3 months: 59 (63.4%) had a normal life (Karnofsky performance scale [KPS] score 80-100), 28 (30.1%) had moderate disabilities (KPS score 50-80), and 6 (6.5%) presented with severe disabilities.
Jugular Foramen Meningiomas
Clinical Presentation
The most common symptoms are hearing loss (52.3%), dysphagia (23.2%), and tinnitus (17.4%), followed by dysphonia, dizziness, ataxia, and cervical pain.71
Evaluation
On CT scans JFMs may show an irregular enlargement of the JF margins with a mixed permeative-sclerotic appearance, whereas GJTs present a permeative-destructive pattern. Schwannomas gradually enlarge the JF by pressure erosion conferring an expanded and scalloped but well-defined corticated margin.72 The presence of dural tails, even when they are not pathognomonic of meningiomas, can be useful in the differential diagnosis. Another important differentiating characteristic seen in MRI scans is the absence of flow voids in the meningioma mass—this feature is often present in GJTs owing to their rich vascularization.72
Pathology
The frequency of histological subtypes is as follows: 88.5% WHO grade I, 6.5% WHO grade II, and 5% WHO grade III.71
Surgical Technique
Positioning
The patient is placed in the supine position with the head turned to the contralateral side.
Operative Results
In a recent series of 13 patients presented by Sanna and co-workers73 their findings showed gross total tumor removal (Simpson grades I and II) in 11 (84.6%) cases without evidence of tumor recurrence at a mean follow-up of 47 months. Good facial nerve function (grades I and II) was achieved in 46.1% of cases. A new deficit of one or more of the lower cranial nerves was recorded in eight (61.5%) patients. Ramina and associates74 achieved gross total resection in 50% of their cases. Two patients died in the immediate postoperative period and four patients died because of disease progression, with a mean survival time of 35 months. They concluded that the incidence of postoperative deficit of cranial nerves is higher than in other benign tumors of the JF.
Tentorial Meningiomas
Group I: Anteromedial, arising from the apex of the tentorial margin
Group II: Anterolateral, arising from the lateral aspect of the tentorial incisural margin
Group III: Intermediate, arising from the intermediate aspect of the tentorium remote from the incisura and the dural sinuses
Group IV: Posteromedial, arising from posteromedial aspect of the tentorium close to straight sinus or venous confluence at the torcula; this group also includes the falcotentorial and torcular meningiomas
Group V: Posterolateral, arising from the posterolateral aspect of the tentorium close to the sigmoid sinus75–77
TM represents 3% to 6% of all intracranial meningiomas.
Clinical Presentation
The most frequent symptoms are headache (75%), dizziness (49%), gait disturbance (45%), mental changes (12%), visual disturbance (11%), and hearing loss (9%).75
Pathology
Grade I tumors represent approximately 95% of the histological pattern, grade II tumors account for 3.75%, and grade III accounts for 1.2%. The most frequent benign meningomas in these locations are fibroblastic and meningothelial.75
Surgical Technique: Occipital Interhemispheric Approach
Approach
The head is slightly turned to the side of the lesion and fixed with a three-point headrest. A supraoccipital craniotomy is made exposing at its inferior margin the edge of the superior sagittal and transverse sinuses. For supra- and infratentorial tumors the exposure is completed by transection of the tentorium as far as the incisura, at least 1 cm parallel to the straight sinus.76
Microsurgical Resection
The occipital lobe is gently retracted laterally, allowing a wide exposure followed by coagulation of the surrounding falx and tentorium in order to reduce tumor vascularity. The meningioma is detached from its tentorial origin and resected. If the meningioma extends to the contralateral side it can be reached through a fenestration in the falx cerebri. In selected cases the tentorium opening will provide access to the pineal region and infratentorial portion of the tumor. Excessive occipital lobe retraction or venous injuries may result in postoperative hemiparesis or visual field deficits.76
Subtemporal Approach
The subtemporal approach can be used for group II supratentotrial meningiomas.
Paramedian Supracerebellar Infratentorial Retrosigmoid Approach
Operative Results
Bassiouni and colleagues75 in a series of 81 TMs treated with surgery reported Simpson grades I and II resection in 91% of patients with permanent surgical morbidity and mortality rates of 19.8% and 2.5%, respectively. The recurrence rate was 8.6% in a mean follow-up of 5.9 years. Recently Shukla and co-workers77 reported similar morbidity results with only 46% of a gross total resection of Simpson grades I and II tumors.
Intraventicular Meningiomas
Pathology
In a recent series all IVMs were WHO grade I; in the lateral ventricle five were angiomatous, three were meningothelial, and one was psammomatous. The sole third ventricle tumor was angiomatous. In the fourth ventricle one was fibroblastic and one was meningothelial.78
Posterior Transcallosal Approach
Approach
After parieto-occipital craniotomy, the dura is reflected toward the superior sagittal sinus.
Parieto-Occipital Transcortical Approach
Positioning
The patient is placed in the park-bench or prone position. The head is elevated 20 degrees.
Midline Suboccipital Infratentorial Approach
The position and approach have already been described.
Operative Results
Liu and associates79 reported a series of 24 IVMs with total tumor removal in 87.5% of patients. No deaths or postoperative hydrocephalus occurred in their group although the morbidity rate was approximately 25%. Tumor recurrence rate was 8.3% in a follow-up period from 6 months to 15 years.
Cerebellar Convexity Meningiomas
Group A: Pure convexity meningiomas arising from the dura over the posterior convexity of the cerebellum
Group B: Inferior peritorcular meningiomas arising from or invading the inferior wall of the torcular herophili or the medial transverse sinus
Group C: Parasinus meningiomas arising in the angle between petrous and convexity dura, including the wall of the sigmoid and transverse sinuses
Group D: Meningiomas with secondary invasion of cerebellar convexity/fossa
CCMs represent approximately 1.5% of all intracranial meningiomas.80
Surgical Technique
Operative Results
In Delfini80 and colleagues’ series of 37 CCMs, total surgical resection was achieved in approximately 84% of patients. No postoperative morbidity or death was reported in the entire group. Tumor recurrence was 5.4% for the resection group and in one case treated with radiosurgery.
Spinal Meningiomas
Spinal meningiomas (SMs) are among the most frequently encountered primary spinal tumors. SMs arise at the junction of the spinal arachnoids and the dura of the nerve root sheath. They usually present as an intradural extramedullary tumor that grows from intradural attachments and tends to spread laterally in the subarachnoid space. SMs generally respect the pial layer of the spinal cord. SMs are mostly located dorsally to the spinal cord with a component that extends laterally. SMs account for 7.5% to 12.7% of all meningiomas. They represent 20% to 46% of all intradural extramedullary primary intraspinal tumors. Approximately 83% to 94% have an intradural component, 5% to 14% are extradural, and 10% may grow in both compartments. About 73% of spinal meningiomas occur in the thoracic spine, 16% are in the cervical location, and 5% arise in the lumbar region.81
Pathology
In a recent series of SMs presented by Sandalcioglu and co-workers,81 98.5% of the encountered tumors correspond to WHO grade I and 1.5% to WHO grade II. Of the benign meningiomas the psammomatous subtype appears to be most frequent.82
Surgical Technique
Approach
Through a posterior approach a hemilaminectomy, laminectomy, or laminoplasty is performed. For lateral tumors, a hemilaminectomy is preferred. If the tumor has an intraforaminal extension a laminectomy with partial or complete facetectomy may be considered. Laminectomy provides a wide exposure for safer resection, but a multilevel laminectomy may cause instability, and in this case laminoplasty should be considered to avoid postoperative kyphosis and subluxation. For ventrally located tumor a more lateral approach is preferred, and the patient can be rotated away from the surgeon and additional bony resection by drilling the pedicles may aid anterior visualization.
Operative Results
Sandalcioglu and co-workers,81 in their series of 137 SMs, reported complete surgical resection in 97% of patients and improved or unchanged neurological state in 96.2% of patients in a mean follow-up time of 61 months. Permanent operative morbidity and mortality rates were 3% and 0.8%, respectively. Recurrence rate was observed in 3% of patients after a mean follow-up period of 76.5 months. Schaller82 demonstrated that the resection of psammomatous meningiomas of the spine is associated with a less favorable neurological outcome postoperatively than resection of spinal meningiomas of other pathological subtypes.
Radiation Therapy
Fractionated Radiotherapy
External beam radiotherapy has proved to be effective in the treatment of primary, unresectable, aggressive, residual, and recurrent meningiomas. Conventional fractionated radiation may be offered instead of surgery to patients with poor clinical status or unresectable meningiomas that involve critical central nervous system and vascular structures. Conventional fraction radiation doses between 50 and 55 Gy provide improvement of symptoms and tumor control up to 80% to 86% at 5 years.83,84 In patients with aggressive meningiomas (grade II or III), even with a gross total resection, radiotherapy must be considered to reduce the high risk of recurrence.84
Radiation therapy may be offered as a standard adjuvant treatment following subtotal resection of selected meningiomas. External beam RT allows an 89% progression-free survival rate at 5 years for benign meningiomas with subtotal resection as compared with the 43% for those followed by observation only.85,86 Several studies support the use of salvage RT in patients with (not previously irradiated) recurrent meningiomas and the treatment outcomes appear to be superior to those achieved with repeat resection alone.84,86,87 Complications such as worsening of neurological symptoms, radionecrosis, memory and cognitive deficit, and chronic otitis apparently decreased since the use of novel high-precision RT techniques such as stereotactic radiosurgery (SRS), fractionated stereotactic conformal radiotherapy (SCRT), proton beam radiotherapy (PBT), and intensity-modulated radiotherapy (IMRT).
Stereotactic Radiosurgery
This procedure delivers a single high dose of precisely targeted radiation; modalities include gamma knife, linear accelerator, proton beam, and the CyberKnife, a robotic radiosurgery system. Doses of approximately 15 Gy are equally efficacious to conventionally fractionated treatment and reduce the complication rate. The use of SRS in meningiomas smaller than 3 to 4 cm was associated with better local control.88
Fractionated Stereotactic Conformal Radiotherapy
This treatment modality advantage is the possibility of delivering higher doses but maintaining stereotactic accuracy. Numerous reports show optimal tumor control rate in short-term follow-up with doses ranging from 50 to 54 Gy.89,90
Proton Beam Therapy
PBT delivers protons instead of radiotherapy photons. Protons are more conformal and homogeneous than photons and their use decreases the dose in surrounding tissue compared to photon beam therapy.91 However, the treatment results of PBT appear to be similar to those for IMRT. A group from Harvard University reported the outcome of 46 patients treated with combined photon and proton beam radiation therapy for biopsied, resected, or recurrent meningiomas. The 5- and 10-year overall survival rates were 93% and 77%, respectively.92
Chemotherapy
Adjuvant chemotherapy treatment is generally ineffective against meningiomas. Many modalities have been tested including cytotoxic drugs, immunomodulation, molecular agents, and hormonal therapy. None of them has shown significant success. Conventional combined chemotherapy—cyclophosphamide, adriamycin, and vincristine—showed a modest activity against malignant meningiomas and may improve the median survival time.93 Treatment with interferon alpha-2b has presented some success in preventing meningioma growth.94 Hydroxyurea has also been suggested for treatment of unresectable and recurrent meningiomas.95 This agent arrests meningioma cell growth in the S phase of the cell cycle as a result of DNA synthesis inhibition, therefore inducing apoptosis. Its early promise appears not to have held out over time, however.
Igaki H., Maruyama K., Koga T., et al. Stereotactic radiosurgery for skull base meningioma. Neurol Med Chir (Tokyo). 2009;49(10):456-461.
Nakamura M., Roser F., Michel J., et al. The natural history of incidental meningiomas. Neurosurgery. 2003;53:62-71.
Pamir N., Black P., Fahlbusch R. Meningiomas: A Comprehensive Text. New York: Elsevier; 2010.
Perry A., et al. Meningiomas. In: Louis D.N., Ohgaki H., Wiestler O.D., et al, editors. World Health Organization Classification of Tumours of the Central Nervous System. 4th ed. Lyon: IARC Press; 2007:164-172.
Yano S, Kuratsu J. Indications for surgery in patients with asymptomatic meningiomas based on an extensive experience. J Neurosurg. 200;105:538-543.
Please go to expertconsult.com to view the complete list of references.
1. Cushing H. The meningiomas (dural endotheliomas): their source and favored seats of origin (Cavendish Lecture). Brain. 1922;45:282-316.
2. Cushing H., Eisenhardt L. Meningiomas: Their Classification, Regional Behaviour, Life History, and Surgical End Results. Springfield, IL: Charles C Thomas; 1938.
3. Louis D.N., Scheithauer B.W., Budka H., et al. Meningiomas. In: Kleihues P., Cavenee W.K., editors. Tumours of the Nervous System. Lyon: IARC Press; 2000:176-184.
4. Kuratsu J., Ushio Y. Epidemiological study of primary intracranial tumors in elderly people. J Neurol Neurosurg Psychiatry. 1997;63:116-118.
5. Perry A., Dehner L.P. Meningeal tumours of childhood and infancy. An update and literature review. Brain Pathol. 2003;13:386-408.
6. Menon G., Nair S., Sudhir J., et al. Childhood and adolescent meningiomas: a report of 38 cases and review of literature. Acta Neurochir. 2009;151:239-244.
7. Kuratsu J., Ushio Y. Epidemiological study of primary intracranial tumors in childhood: a population-based survey in Kumamoto prefecture. Japan. Pediatr Neurosurg. 1996;25:240-247.
8. Baumgartner J.E., Sorenson J.M. Meningioma in the pediatric population. J Neurooncol. 1996;29:223-228.
9. Park B.J., Kim H.K., Sade B., Epidemiology In: Lee J.H. Meningiomas, Diagnosis, Treatment and Outcomes. New York: Springer-Verlag; 2008. :111-114, Chap
10. Weber J., Gassel A.M., Hoch A., et al. Intraoperative management of cystic meningiomas. Neurosurg Rev. 2003;26(1):62-66.
11. Osborn A. Diagnostic Imaging: Brain. Salt Lake City, UT: Amirsys; 2004. :56-59
12. Guermazi A., Lafitte F., Miaux Y., et al. The dural tail sign—beyond meningioma. Clin Radiol. 2005;60:171-188.
13. Filippi C.G., Edgar M.A., Ulug A.M., et al. Appearance of meningiomas on diffusion-weighted images: correlating diffusion constants with histopathologic findings. AJNR Am J Neuroradiol. 2001;22:65-72.
14. Toh C.H., Castillo M., Wong A.M.C., et al. Differentiation between classic and atypical meningiomas with use of diffusion tensor imaging. AJNR J Neuroradiol. 2008;29:1630-1635.
15. Kremer S., Grand S., Remy C., et al. Contribution of dynamic contrast MR imaging to the differentiation between dural metastasis and meningioma. Neuroradiology. 2004;46:642-648.
16. Yang S., Law M., Zagzag D., et al. Dynamic contrast-enhanced perfusion MR imaging measurements of endothelial permeability: differentiation between atypical and typical meningiomas. AJNR Am J Neuroradiol. 2003;24:1554-1559.
17. Yue Q., Isobe T., Shibata Y., et al. New observations concerning the interpretation of magnetic resonance spectroscopy of meningioma. Eur Radiol. 2008;18:2901-2911.
18. Lippitz B., Cremerius U., Mayfrank L., et al. PET-study of intracranial meningiomas: correlation with histopathology, cellularity and proliferation rate. Acta Neurochir Suppl. 1996;65:108-111.
19. Park Y.S., Jeon B.C., Oh H.S., et al. FDG PET/CT assessment of the biological behavior of meningiomas. J Korean Neurosurg Soc. 2006;40:428-433.
20. Lee J.W., Kang K.W., Lee S.M., et al. 18F-FDG PET in the assessment of tumor grade and prediction of tumor recurrence in intracranial meningioma. Eur J Nucl Med Mol Imaging. 2009;36:1574-1582.
21. Chang A.S., Ross J.S. Diagnostic Neuroradiology: CT, MRI, fMRI, MRS, PET, and Octreotide SPECT. Meningiomas, Diagnosis, Treatment and Outcomes. New York: Springer-Verlag; 2008. :255-266
22. Krenning E.P., Kwekkeboom D.J., Bakker W.H., et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med. 1993;20:716-731.
23. Bickerstaff E.R., Small J.M., Guest I.A. The relapsing course of certain meningiomas in relation to pregnancy and menstruation. J Neurol Neurosurg Psychiatry. 1958;21:89-91.
24. Pravdenkova S., Al-Mefty O., Sawyer J., Husain M. Progesterone and estrogen receptors: opposing prognostic indicators in meningiomas. J Neurosurg. 2006;105(2):163-173.
25. Perry A., et al. Meningiomas. In: Louis D.N., Ohgaki H., Wiestler O.D., et al, editors. World Health Organization Classification of Tumours of the Central Nervous System. 4th ed. Lyon: IARC Press; 2007:164-172.
26. Perry A., Scheithauer B.W., Stafford S.L., et al. “Malignancy” in meningiomas: a clinicopathologic study of 116 patients, with grading implications. Cancer. 1999;85:2046-2056.
27. Dhall S.S., Tumialan L.M., Brat D.J., Barrow D.L. Spinal intradural clear cell meningioma following resection of a suprasellar clear cell meningioma. Case report and recommendations for management. J Neurosurg. 2005;103:559-563.
28. Lee D.K., Kim D.G., Choe G., et al. Chordoid meningioma with polyclonal gammopathy. Case report. J Neurosurg. 2001;94:122-126.
29. Couce M.E., Aker F.V., Scheithauer B.W. Chordoid meningioma. A clinicopathologic study of 42 cases. Am J Surg Pathol. 2000;24:899-905.
30. Adachi K., Kawase T., Yoshida K., et al. A proposed ABC Surgical Risk Score for skull base meningioma based on preoperative findings. Proceedings of the Fifth International Conference on Meningiomas and Cerebral Veins, Mount Fuji, Japan, 2006 (abstr). Later published in J Neurosurg. 2009;111:1053-1069.
31. Sade B., Lee J. A novel “CLASS” algorithmic scale for patient selection in meningioma surgery. Mount Fuji, Japan: Proceedings of the Fifth International Conference on Meningiomas and Cerebral Veins; 2006.
32. Yoneoka Y., Fujii Y., Tanaka R. Growth of incidental meningiomas. Acta Neurochir (Wien). 2000;142:507-511.
33. Nakamura M., Roser F., Michel J., et al. The natural history of incidental meningiomas. Neurosurgery. 2003;53:62-71.
34. Yano S., Kuratsu J. Indications for surgery in patients with asymptomatic meningiomas based on an extensive experience. J Neurosurg. 2006;105:538-543.
35. Simpson D. Recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22-39.
36. Black P.M., Morokoff A.P., Zauberman J. Surgery for extra-axial tumors of the cerebral convexity and midline. Neurosurgery. 2008;62(6 Suppl 3):1115-1121.
37. Sindou M.P., Alvernia J.E. Results of attempted radical tumor removal and venous repair in 100 consecutive meningiomas involving the major dural sinuses. J Neurosurg. 2006;105:514-525.
38. Wilkins R. Parasagittal meningiomas. In: Al-Mefty O., editor. Meningiomas. New York: Raven Press; 1991:329-343.
39. Di Meco F., Li K.W., Casali C., et al. Meningiomas invading the superior sagittal sinus: surgical experience in 108 cases. Neurosurgery. 2004;55(6):1263-1274.
40. Yasargil M.G. Microneurosurgery of CNS Tumors, IV-B. Stuttgart: Georg Thieme; 1996. :134–165
41. Chung S.B., ChY Kim, ChK Park, et al. Falx meningiomas: surgical results and lessons learned from 68 cases. J Korean Neurosurg Soc. 2007;42:276-280.
42. Obeid F., Al-Mefty O. Recurrence of olfactory groove meningiomas. Neurosurgery. 2003;53:534-542.
43. Nakamura N., Samii M. Surgical management of tuberculum sellae meningiomas. In: Ramina R., Pires Aguiar P., Tatagiba M., editors. Samii’s Essentials in Neurosurgery. New York: Springer-Verlag; 2008:91-98., Chap. 10
44. Dehdashti A.R., Ganna A., Witterick I., Gentili F. Expanded endoscopic endonasal approach for anterior cranial base and suprasellar lesions: indications and limitations. Neurosurgery. 2009;64(4):677-687.
45. Gardner P.A., Kassam A.B., Thomas A., et al. Endoscopic endonasal resection of anterior cranial base meningiomas. Neurosurgery. 2008;63(1):36-52.
46. Schick U., Dott U., Hassler W. Surgical management of meningiomas involving the optic nerve sheath. J Neurosurg. 2004;101:951-959.
47. Turbin R.E., Pokorny K. Diagnosis and treatment of orbital optic nerve sheath meningioma. Cancer Control. 2004;11:334-341.
48. Delfini R., Missori P., Tarantino R. Primary benign tumors of the orbital cavity: comparative data in a series of patients with optic nerve glioma, sheath meningioma, or neurinoma. Surg Neurol. 1996;45:147-153.
49. Dutton J.J. Optic nerve sheath meningiomas. Surv Ophthalmol. 1992;37:167-183.
50. Andrews D.W., Faroozan R., Yang B.P., et al. Fractionated stereotactic radiotherapy for the treatment of optic nerve sheath meningiomas: preliminary observations of 33 optic nerves in 30 patients with historical comparison to observation with or without prior surgery. Neurosurgery. 2002;51:890-904.
51. Baumert B.G., Villa S., Studer G., et al. Early improvements in vision after fractionated stereotactic radiotherapy for primary optic nerve sheath meningioma. Radiother Oncol. 2004;72:169-174.
52. Al-Mefty O. Clinoidal meningiomas. J Neurosurg. 1990;73:840-849.
53. Pamir M.N., Belirgen M., Özduman K., et al. Anterior clinoidal meningiomas: analysis of 43 consecutive surgically treated cases. Acta Neurochir (Wien). 2008;150:625-636.
54. Lee J.Y., Niranjan A., McInerney J., et al. Stereotactic radiosurgery providing longterm tumor control of cavernous sinus meningiomas. J Neurosurg. 2002;97:65-72.
55. Honig S., Trantakis C., Frerich B., et al. Spheno-orbital meningiomas: outcome after microsurgical treatment: a clinical review of 30 cases. Neurol Res. 2010;32(3):314-325.
56. Shrivastava R.K., Chandranath S., Costantino P., Della Rocca R. Sphenoorbital meningiomas: surgical limitations and lessons learned in their long-term management. J Neurosurg. 2005;103:491-497.
57. Ringel F., Cedzich C., Schramm J. Microsurgical technique and results of a series of 63 spheno-orbital meningiomas. Neurosurgery. 2007;60(Suppl 4):214-222.
58. Carrizo A., Basso A. Current surgical treatment for sphenoorbital meningiomas. Surg Neurol. 1998;50(6):574-578.
59. Scarone P., Leclerq D., Héran F., Robert G. Long-term results with exophthalmos in a surgical series of 30 sphenoorbital meningiomas. J Neurosurg. 2009;111:1069-1077.
60. Pichierri A., Santoro A., Raco A., et al. Cavernous sinus meningiomas: retrospective analysis and proposal of a treatment algorithm. Neurosurgery. 2009;64:1090-1101.
61. Nicolato A., Foroni R., Alessandrini F., et al. Radiosurgical treatment of cavernous sinus meningiomas: experience with 122 treated patients. Neurosurgery. 2002;51:1153-1159.
62. Pirotte B., Brotchi J. Lateral and middle sphenoid wing meningiomas. In: Lee J.H., editor. Meningiomas, Diagnosis, Treatment and Outcomes. New York: Springer-Verlag; 2008:371-378., Chap. 39
63. Roser F., Nakamura M., Jacobs C., et al. Sphenoid wing meningiomas with osseous involvement. Surg Neurol. 2005;64:37-43.
64. Al-Mefty O., Fox J.L., Smith R.R. Petrosal approach for petroclival meningiomas. Neurosurgery. 1988;22:510-517.
65. Hakuba A., Nishimura S., Tanaka K., et al. Clivus meningioma: six cases of total removal. Neurol Med Chir (Tokyo). 1977;17:63-77.
66. Schaller B., Merlo A., Gratzl O., Probst R. Premeatal and retromeatal cerebellopontine angle meningioma: two distinct clinical entities. Acta Neurochir (Wien). 1999;141:465-471.
67. Nakamura M., Roser F., Dormiani M., et al. Facial and cochlear nerve function after surgery of cerebellopontine angle meningiomas. Neurosurgery. 2005;57:77-90.
68. Sade B., Lee J.H. Petrous meningiomas. II: Ventral, posterior and superior subtypes. In: Lee J.H., editor. Meningiomas, Diagnosis, Treatment and Outcomes. New York: Springer-Verlag; 2008:443-447., Chap. 48
69. Bruneau M., George B. Foramen magnum meningiomas: detailed surgical approaches and technical aspects at Lariboisière Hospital and review of the literature. Neurosurg Rev. 2008;31:19-33.
70. Wu Z., Hao S., Zhang J., et al. Foramen magnum meningiomas: experiences in 114 patients at a single institute over 15 years. Surg Neurol. 2009;72(4):376-382.
71. Bakar B. Jugular foramen meningiomas: review of the major surgical series. Neurol Med Chir (Tokyo). 2010;50:89-97.
72. Sasaki T., Kawahara N. Jugular foraman meningiomas I. In: Lee J.H., editor. Meningiomas, Diagnosis, Treatment and Outcomes. New York: Springer-Verlag; 2008:515-520., Chap. 55
73. Sanna M., Bacciu A., Falconi M., et al. Surgical management of jugular foramen meningiomas: a series of 13 cases and review of the literature. Laryngoscope. 2007;117:1710-1719.
74. Ramina R., Neto M.C., Fernandes Y.B., et al. Meningiomas of the jugular foramen. Neurosurg Rev. 2006;29:55-60.
75. Bassiouni H., Hunold A., Asgari S., Stolke D. Tentorial meningiomas: clinical results in 81 patients treated microsurgically. Neurosurgery. 2004;55:108-118.
76. Bret P., Guyotat J., Madarassy G., et al. Tentorial meningiomas. Report on twenty-seven cases. Acta Neurochir (Wien). 2000;142(5):513-526.
77. Shukla D., Behari S., Jaiswal A.K., et al. Tentorial meningiomas: operative nuances and perioperative management dilemmas. Acta Neurochir (Wien). 2009;151:1037-1051.
78. Bhatoe H.S., Singh P., Dutta V. Intraventricular meningiomas: a clinicopathological study and review. Neurosurg Focus. 2006;20:E9.
79. Liu M., Wei Y., Liu Y., et al. Intraventricular meningiomas: a report of 25 cases. Neurosurgery. 2006;29:36-40.
80. Delfini R., Santoro A., Pichierri A. Cerebellar convexity meningiomas. In: Lee J.H., editor. Meningiomas, Diagnosis, Treatment and Outcomes. New York: Springer-Verlag; 2008:457-463., Chap. 50
81. Sandalcioglu I.E., Hunold Muller O., et al. Spinal meningiomas: critical review of 131 surgically treated patients. Eur Spine J. 2008;17:1035-1041.
82. Schaller B. Spinal meningioma: relationship between histological subtypes and surgical outcome? J Neurooncol. 2005;75:157-161.
83. Condra K.S., Buatti J.M., Mendenhall W.M., et al. Benign meningiomas: primary treatment selection affects survival. Int J Radiat Oncol Biol Phys. 1997;39(2):427-436.
84. Pourel N., Auque J., Bracard S., et al. Efficacy of external fractionated radiation therapy in the treatment of meningiomas: a 20-year experience. Radiother Oncol. 2001;61(1):65-70.
85. Goldsmith B.J., Wara W.M., Wilson C.B., et al. Postoperative irradiation for subtotally resected meningiomas. A retrospective analysis of 140 patients treated from 1967 to 1990. J Neurosurg. 1994;80(2):195-201.
86. Miralbell R., Linggood R.M., de la Monte S., et al. The role of radiotherapy in the treatment of subtotally resected benign meningiomas. J Neurooncol. 1992;13:155-164.
87. Kokubo M., Shibamoto Y., Takahashi J.A., et al. Efficacy of conventional radiotherapy for recurrent meningioma. J Neurooncol. 2000;48(1):51-55.
88. Igaki H., Maruyama K., Koga T., et al. Stereotactic radiosurgery for skull base meningioma. Neurol Med Chir (Tokyo). 2009;49(10):456-461.
89. Andrews D.W., Faroozan R., Yang B.P., et al. Fractionated stereotactic radiotherapy for the treatment of optic nerve sheath meningiomas: preliminary observations of 33 optic nerves in 30 patients with historical comparison to observation with or without prior surgery. Neurosurgery. 2002;51:890-902.
90. Brell M., Villa S., Teixidor P., et al. Fractionated stereotactic radiotherapy in the treatment of exclusive cavernous sinus meningioma: functional outcome, local control, and tolerance. Surg Neurol. 2006;65:28-33.
91. Cozzi L., Fogliata A., Lomax A., et al. A treatment planning comparison of 3D conformal therapy, intensity modulated photon therapy and proton therapy for treatment of advanced head and neck tumours. Radiother Oncol. 2001;61:287-297.
92. Wenkel E., Thornton A.F., Finkelstein D., et al. Benign meningioma: partially resected, biopsied, and recurrent intracranial tumors treated with combined proton and photon radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48:1363-1370.
93. Chamberlain M.C. Adjuvant combined modality therapy for malignant meningiomas. J Neurosurg. 1996;84:733-736.
94. Kaba S.E., Demonte F., Bruner J.M., et al. The treatment of recurrent unresectable and malignant meningiomas with interferon alpha-2B. Neurosurgery. 1997;40:271-275.
95. Schrell U.M.H., Rittig M.G., Anders M., et al. Hydroxyurea for treatment of unresectable and recurrent meningiomas. II. Decrease in the size of meningiomas in patients treated with hydroxyurea. J Neurosurg. 1997;86:840-844.