22 Meningeal Tumors
Epidemiology
Meningiomas constitute approximately 20% of all primary intracranial tumors.1,2 Reported incidence rates are between 1 and 5 per 100,000,2–10 with the range perhaps reflecting differences in study design and population exposure to etiologic factors. The Central Brain Tumor Registry of the United States estimated approximately 8600 new cases of meningioma in the United States in 2002.2 Most meningiomas are grossly unifocal. In computed tomography (CT)-era series, multiple synchronous lesions are seen in 40% or fewer of cases,11–16 with the majority of authors reporting multiplicity rates between 5 and 9%.12–15 Intracranial sites for meningioma are listed in order of incidence in Table 22-1. Metastases are extremely rare.17,18 The female/male ratio is approximately 2 : 1 for all meningiomas,1,7,8 and 1 : 1 for anaplastic meningiomas.19 The incidence of benign meningioma increases with age, peaking in the seventh decade of life. The incidence of anaplastic meningioma rises to a plateau in the third decade, where it remains relatively constant thereafter.19 Patients with benign and anaplastic meningioma present at a mean age of 58 ± 15 years (standard error of the mean), and 57 ± 14 years, respectively.19 In the pediatric population, meningiomas are less common, constituting 1% to 4% of all intracranial tumors. There is no female preponderance as seen with adult meningiomas, and there is a common (24%) association with neurofibromatosis.20,21
| Site | Incidence, % |
|---|---|
| Cerebral convexity | 34 |
| Parasagittal | 22 |
| Sphenoid ridge | 17 |
| Lateral ventricle | 5 |
| Cerebellar convexity | 5 |
| Tentorium | 4 |
| Tuberculum sellae/parasellar | 3 |
| Olfactory groove | 3 |
| Intraorbital | 2 |
| Cerebellopontine angle | 2 |
| Foramen magnum | 1 |
| Clivus | 1 |
| Other | 1 |
Data from Rohringer, et al: Incidence and clinicopathologic features of meningioma, J Neurosurg 71:665, 1989.
Ionizing Radiation
Several case reports have identified intracranial meningioma in patients with a history of previous scalp irradiation for tinea capitis,22–24 suggesting an etiologic role. From 1910 to 1959, an estimated 20,000 children in the United States and 200,000 worldwide received such treatments.25 Ron and associates26 compared the incidence of meningioma in a cohort of 10,834 children treated with ionizing radiation in Israel for tinea capitis with that of a nonirradiated control group. With an estimated 1.5 Gy delivered to the brain surface, the relative risk (RR) was 9.5 times that of the control group (95% confidence interval [CI], 3.5-25.7). The mean interval between irradiation and diagnosis with meningioma was 20 years. Rubinstein and associates27 retrospectively analyzed 201 patients with intracranial meningioma, 43 of whom had received childhood radiation for tinea capitis, and identified several distinctive clinical and histologic features in the irradiated subgroup. A statistically significant (P < .001) difference in location was seen favoring falx cerebri, parasagittal, or convexity regions in the irradiated group, corresponding to the site of scalp treatment. Presentation with seizures was more common in the irradiated group (P < .005), perhaps reflecting the predominantly supratentorial location of the tumors. Histologically, the radiation-associated meningiomas (RAMs) had a significantly higher incidence of (1) high cellularity, (2) pleomorphic nuclei, and (3) numerous giant cells (P < .001). Following resection, recurrence rates were 25% versus 11% (P < .05) in the RAM and non-RAM groups, respectively.27 In a comparison between 16 RAM and 17 non-RAM tumors, Rienstein et al28 found no significant differences in the number of genetic changes and the extent and frequency of chromosomes 1 and 22 losses, suggesting similar molecular pathways for tumorogenesis, regardless of radiation exposure (See “Neurofibromatosis and Genetics”). Of note, radiofrequency electromagnetic field exposure, such as cell phone use, has not been associated with meningioma.29
Female Sex Hormones
Several findings suggest a role of female sex hormones in the etiology and regulation of growth of meningiomas: (1) incidence in women is twice that in men;1,7,8 (2) relationship between pregnancy and increased rate of meningioma symptom progression30; (3) approximately three quarters of meningiomas are progesterone receptor (PR) positive;31–36 (4) meningioma is less common in women with history of premenopausal bilateral oophorectomy;37 and more common among postmenopausal women with ever use of hormone replacement therapy (RR = 1.7; 95% CI, 1.0-2.8) or long-term (≥10 years) use of long-acting hormonal contraceptives such as subdermal implants, injections, or hormonal intrauterine devices (RR = 2.7; 95% CI, 0.9-7.5).38 Of note, receptor-negative and estrogen receptor (ER)-positive tumors are associated with higher proliferative index, worse histopathology, and more chromosomal abnormalities than PR-positive meningioma.39
Trauma
Head trauma, hypothesized by some to promote tumorogenesis during the increased mitotic activity of the reparative process, has not been convincingly shown to cause meningioma. In a multicenter study of 330 meningioma cases matched to 1132 controls, Preston-Martin and colleagues40 found no statistically significant relationship between trauma and meningioma—results which refute the authors’ earlier observations41–43 in Los Angeles County. No increased incidence of meningioma was found in a Mayo Clinic review of 3587 residents of Olmstead County who sustained head trauma between 1935 and 1974 associated with loss of consciousness, amnesia, or skull fracture,44 and a case-control study from Schlehofer and colleagues45 actually found a history of head trauma protective, with an RR of 0.5.
Viral Infection
Viral antigen and DNA have been found in meningioma by several investigators.46,47 It has been hypothesized that infection with papovavirus may promote meningioma by causing the loss of a tumor suppressor gene on chromosome 22.48,49 However, the etiologic role of viral infection has not yet been examined epidemiologically.
Neurofibromatosis and Genetics
Losses on 22q are the most frequent genomic alterations observed in meningioma.50,51 Type 2 neurofibromatosis (bilateral acoustic neurofibromatosis, NF2), characterized cytogenetically by the loss of the NF2 gene from 22q12, is associated with an increased incidence of meningioma.52 Based on the most common chromosomal aberrations identified in the various grades of meningioma, a model for progression has been proposed by Weber et al50 that initiates the clonal expansion from normal meningeal cells to benign World Health Organization (WHO) Grade I meningioma with loss on 22q, and continues with cumulative genetic alterations on other chromosomes in higher grade tumors (loss on 1p, 6q, 10, 14q, 18q, and gain on 1q, 9q, 12q, 15q, 17q, and 20 in atypical WHO Grade II; followed by loss on 9p and amplification on 17q in anaplastic WHO Grade III). Quantitative measure of accumulation of chromosome change correlates highly with early tumor recurrence.53 Perhaps contradicting stepwise clonal evolution, Al-Mefty et al have demonstrated deletions of 22, 1p, and 14q ab initio in histologically lower grade tumors that later recur and progress to higher grade, suggesting that benign meningioma presenting with complex genetic alterations are potentially aggressive and require closer follow up.54
Breast Cancer
Both the risk of developing meningioma in patients who have had breast cancer and the risk of developing breast cancer in patients who have had meningioma are higher than in the general population.55 The association may be explained by a hormonal effect or possibly by a shared chromosome 22 abnormality.56–58
Pathology
Meningiomas are thought to arise from arachnoidal cap cells that are found on the outer surface of the arachnoidal membrane between the dura and pia mater. Cells of the arachnoidal membrane uniquely display both mesenchymal and epithelial characteristics, capable of fibro-lipo-chondro-osteoblastic differentiation as well as of well-formed desmosomal connections between tumor cells and other structural and histochemical features usually attributed to epithelial neoplasms. Though much meningioma literature is based on legacy classification systems, most neurooncology communities have now adopted the WHO classification.59
Conventional histologic subtypes, including meningothelial, fibrous, transitional, psammomatous, angiomatous, microcystic, secretory, lymphoplasmacyte-rich, and metaplastic, have no apparent prognostic importance.60 By contrast, four rare variants (each constituting <1% of all meningiomas) have characteristically aggressive behavior:
Clear cell, composed of sheets of clear, glycogen-rich, polygonal cells forming only a few vague whorls; often with extensive stromal and perivascular hyalinization; frequently deceptively benign appearance with few mitotic figures.61
Chordoid, mimicking chordoma with lobular low-power architecture and production of stroma mucosubstances, but with scant reactivity for chordoma-typical epithelial membrane antigen, cytokeratin, and S-100.62
Rhabdoid, usually seen in association with conventional histological subtypes, with increasing prominence with each recurrence; characterized by sheets of loosely cohesive cells with eccentric nuclei and hyaline paranuclear inclusions.63
Papillary, seen both as the predominant morphology and in association with one of the conventional histologic subtypes; characterized by a striking papillary pattern with foci of necrosis, numerous mitotic figures, and local invasiveness.64
Since the 1993 WHO revision,62 characteristically aggressive hemangiopericytomas and hemangioblastomas have been deemed nonmeningothelial in origin and are no longer classified as meningiomas.
2007 WHO Classification59
Grade I: does not fulfill criteria for grades II (atypical) or III (anaplastic).
Grade II (atypical): Any of the following criteria:
Grade III (anaplastic): Either of the following criteria:
 Obviously malignant cytologic characteristics such that tumor cell resembles carcinoma, sarcoma, or melanoma.
Obviously malignant cytologic characteristics such that tumor cell resembles carcinoma, sarcoma, or melanoma.Most meningiomas are grade I. From a series of 581 consecutively resected patients from the Mayo Clinic, 81% were grade I and 19% grade II (data modified to reflect 2007 WHO classification).65 Grade III meningiomas are rare.
Proliferation indices based on MIB-1 labeling of Ki-67 nuclear protein or PC10 labeling of proliferating cell nuclear antigen (PCNA) are powerful adjuncts that offer additional prognostic information. High indices reflect increased proliferative activity and recurrence potential.66–71 Identification and quantitative measure of accumulation of chromosome change correlate highly with early tumor recurrence.53,72
At the time of this chapter’s preparation, the 2007 WHO classification has not yet been examined for prognostic utility in new radiotherapy studies. However, meningioma grade as defined by similar legacy systems,62,73,74 has been established of clear prognostic importance, with atypia and anaplasia associated with more frequent and earlier recurrence.73–76 For example, postradiotherapy 5-year progression-free survival (PFS) rates of 94% versus 78% for 1993 WHO Grade I versus Grade II (P = .02),75 95% versus 0% for 1993 WHO Grade I versus Grade II/III (P < .01),76 and 89% versus 49% for University of California, San Francisco (UCSF) system benign versus malignant (P = .001)73 have been reported.
Clinical Presentation and Diagnostic Evaluation
Evaluation begins with a detailed history and physical examination. Presenting complaints and physical findings, listed in order of frequency, are found in Tables 22-2 and 22-3, respectively. Patients with visual complaints should be evaluated with formal visual field and acuity testing before surgery or radiation therapy. Patients with hearing loss should be tested with formal audiometry.
| Complaints | Incidence, % |
|---|---|
| Headache | 37 |
| Personality change/confusion | 22 |
| Paresis | 19 |
| Generalized seizures | 19 |
| Visual impairment | 16 |
| Focal seizures | 15 |
| Ataxia | 15 |
| Aphasia | 10 |
| Decreasing level of consciousness | 7 |
| Paresthesia | 6 |
| Diplopia | 3 |
| Vertigo | 1 |
| Decreased hearing | 1 |
Data from Rohringer, et al: Incidence and clinicopathologic features of meningioma, J Neurosurg 71:665, 1989.
| Finding | Incidence, % |
|---|---|
| Paresis | 30 |
| Nonfocal examination | 26 |
| Memory deficit | 15 |
| Cranial nerve deficit other than CN II | 12 |
| Visual field deficit | 10 |
| Paresthesia | 9 |
| Aphasia | 9 |
| Papilledema | 8 |
| Decreased visual acuity | 6 |
| Altered level of consciousness | 5 |
| Nystagmus | 3 |
| Decreased hearing | 2 |
Data from Rohringer, et al: Incidence and clinicopathologic features of meningioma, J Neurosurg 71:665, 1989.
Pediatric patients with convexity or parasagittal meningiomas often present with seizures, focal deficits, or signs of increased intracranial pressure (ICP), and those with intraventricular lesions typically present with elevated ICP, hemiparesis, and hemianopia. In addition to these typical presenting signs and symptoms, increasing head circumference as a first sign of meningioma is also seen in children.77
Adult patients with spinal meningioma most often present with pain (72%), which usually antedates other symptoms by months to years, followed by sensory loss and weakness, followed by bowel and bladder dysfunction.78 Children with spinal meningioma, unlike adults, most often present with extremity weakness (57%), followed by back pain, bowel and bladder dysfunction, and extremity pain. In children, back and extremity pain may be expressed as increased irritability.79
CT and magnetic resonance imaging (MRI) with contrast yield information regarding tumor size, location, potential resectability, and proximity to critical neurologic structures. Meningiomas are generally well circumscribed and smooth in contour. Most are homogeneously high density on unenhanced CT scans, 25% to 33% are isodense, about 1% are hypodense, and a few demonstrate mixed attenuation. The majority demonstrate moderate to intense homogeneous enhancement following administration of contrast, with the degree of enhancement dependent on tumor vascularity and extracellular accumulation of the contrast material. Peritumoral edema is seen in 60% of cases, and associated bony changes (destruction or hyperostosis) in 15% to 20%.80 The degree of preoperative peritumoral has been reported to correlate positively with probability of brain invasion81 and postoperative neurologic deficit.82
On MRI, meningiomas are typically isointense with gray matter on both T1– and T2-weighted images, and intensely enhanced when contrast is used. A radiographic feature that is suggestive of, but not specific for, meningioma is linear meningeal thickening and enhancement adjacent to a peripherally located cranial mass—the so-called “dural tail sign.”80,83 This has been reported in 60% of meningiomas, but can also been seen in chloroma, lymphoma, and sarcoidosis. The enhancement of the meningioma dural tail usually represents reactive changes and does not necessarily indicate neoplastic involvement.84–86 Intratumoral inhomogeneities are often seen on MRI, associated with calcifications, central necrosis, pseudocysts, and vascular spaces. Intratumoral hemorrhage is unusual. Displacement of the gray-white interface, peritumoral edema, and adjacent encephalomalacic changes are better seen with MRI than CT, whereas osseous changes and calcification are better seen with CT than MRI.80,87
Meningiomas also show high expression of the somatostatin-receptor subtype 2, enabling visualization with [68Ga]-DOTATOC-PET with very high meningioma to background ratios.88 Milker-Zabel et al have demonstrated complementary spatial information in a comparison of planning tumor volume (PTV) obtained with CT-MRI versus CT-MRI-[68Ga]-DOTATOC-PET, with the later smaller than CT-MRI in 35%, similar in 26%, and larger in 39%.89
Standard Therapeutic Approaches
Benign meningiomas are well managed with total excision if achievable with minimal morbidity, resulting in 5-, 10-, and 15-year PFS rates of 93%, 80%, and 75%, and 5-, 10-, and 15-year cause-specific survival (CSS) rates of 95%, 93%, and 88%, respectively.90 Approximately one in three meningiomas is not fully resectable,91 however, because of tumor location, size, and proximity to adjacent critical central nervous system (CNS) and vascular structures. In the skull base, total resection is accomplished about half the time91,92 and is less likely in the setting of previous irradiation, vessel encasement, multiple fossa involvement, and cranial nerve palsies.92 Although subtotal resection is an appropriate goal when decompression is expected to result in amelioration of symptoms, it is inadequate as a sole modality, with inferior 5-, 10-, and 15-year PFS of approximately 50%, 40%, and 30%, and CSS of 80%, 66%, and 50%,90 respectively.
Fractionated radiotherapy93 or single fraction radiosurgery,94 as primary management without surgery or after subtotal resection of benign meningioma, yields long-term PFS superior to subtotal resection and comparable to total excision, with low morbidity.
Atypical meningioma has an intermediate prognosis, with a 5-year PFS of 59% to 87%.65,95 Characteristics associated with higher likelihood of recurrence include subtotal resection,95,96 as well as brain invasion,65,95 high proliferative indices,97 numerous mitoses,98 and chromosomal change accumulation53,72,99 regardless of degree of resection. Adjuvant irradiation is recommended for subtotally resected or brain-invasive atypical meningioma, and should be considered for completely resected tumors with the above high-risk features (Fig. 22-1).100
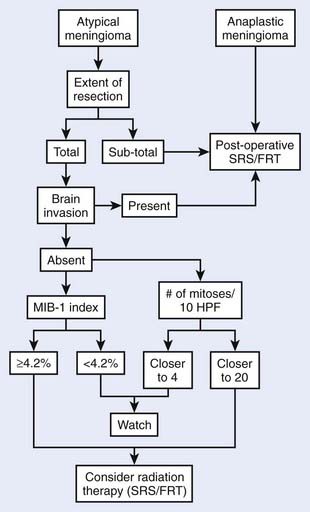
FIGURE 22-1 • The atypical and anaplastic meningioma treatment algorithm.
(From Modha A, Gutin PH: Diagnosis and treatment of atypical and anaplastic meningiomas: a review, Neurosurgery 57(3):538–550, 2005.)
Anaplastic meningiomas are likely to recur postoperatively, with a median PFS of 32 months and 5-year PFS rate of 29% to 44%.65,95 Adjuvant irradiation for anaplastic tumor is independently associated with an improved PFS rate95 and is recommended regardless of degree of resection.95,100
Fractionated Radiotherapy
Technique
Optimal radiation therapy requires meticulous attention to tumor volume as delineated by the neurosurgeon’s description as well as CT and MRI findings. MRI is superior to CT, with its exquisite resolution, absence of bone artifact, intense tumor contrast enhancement, and coronal and sagittal viewing advantages. In a comparison studied by Khoo and colleagues,87 however, MRI-defined meningioma volumes were larger but not inclusive of CT-defined volumes, suggesting complementary spatial information and a role for treatment planning with composite CT/MRI volumes. Milker-Zabel et al have also demonstrated complementary tumor imaging with CT/MRI and [68Ga]-DOTATOC-PET,89 suggesting composite use of all three modalities when available. Pre- and postoperative studies should be utilized in treatment planning when available. The PTV consists of the clinical tumor volume (CTV) plus a margin of 5 mm for conventional mask immobilization or 2 to 3 mm for stereotactic frame-based immobilization technique.75,101,102 For benign (WHO Grade I) meningioma, the CTV includes the composite gross tumor volume (GTV) in the setting of primary radiotherapy, and residual composite GTV in the adjuvant setting. After surgery, the CTV may be expanded beyond the remaining radiographic GTV to encompass microscopic residua based on the neurosurgeon’s description of intraoperative findings and an appreciation of the preoperative dural base. Because (1) the majority of benign meningioma recurrences following stereotactic fractionated radiotherapy75 are not marginal, and (2) microscopic involvement of the adjacent dura is typically confined to the first millimeter or two from the gross tumor,84–86 routine inclusion of the entire dural tail in the CTV has not been demonstrated to be necessary. Atypical and anaplastic meningioma also rarely fail marginally when treated with a CTV 10 mm beyond GTV, operative bed, hyperostotic bone, and dural thickening.103 For anaplastic tumors the CTV also includes peritumoral edema if present,103 or a rim of adjacent normal brain parenchyma if not.
Five-mm set-up error precision is achieved by use of an immobilization system, consisting of a head support, a thermoplastic mask that is molded to the patient’s head, and a frame that locks the mask and head in a reproducible position. Fused CT/MRI computerized treatment planning should be used, with treatment-planning scans obtained with the patient in the treatment position, wearing the immobilization device with radio-opaque fiducials. The use of such careful tumor localization and immobilization techniques has been shown to have greater prognostic importance than tumor size or radiotherapy dose, and has been associated with a 5-year PFS for benign meningioma which is significantly better (98% vs 77%, P = .002) than that achieved without such techniques.73 With the addition of a stereotactic frame and localization system, set-up error is reduced to 2 mm, allowing for a tighter PTV and less normal tissue irradiation.75,101
Optic nerve sheath meningiomas require precision conformal therapy, such as described by Eng and colleagues.104 The patient is positioned supine with the head rotated laterally, so that the affected optic nerve is approximately perpendicular to the horizontal plane. The appropriate angle of lateral head rotation can be estimated from diagnostic CT scans. In the series from Eng’s group, this value ranged from 15 to 27 degrees. Neck flexion should establish an imaginary vertical line on the lateral simulation film from the center of the orbit to a point just superior to the pituitary fossa. In this position, the patient’s head is immobilized with a head support, thermoplastic mask, and locking frame as just described. The treatment-planning CT scan is then obtained with the patient in this position, wearing the immobilization mask. Three small half-beam blocked fields are set up isocentrically with axes lying in the horizontal plane. Treatment table rotation is used to produce a vertex field and two superior-lateral fields. The superior-lateral fields are angled to avoid the contralateral eye and optic nerve. The beams are split perpendicular to the vertical axis and the anterior half is blocked in order to prevent anterior divergence toward the globe. Differential field weighting is used to optimize the isodose distribution, and the prescribed isodose volume (with 5% or less heterogeneity) should encompass the PTV. Stereotactic technique is particularly appropriate in this setting in order to minimize set-up error and PTV and maximize organ-at-risk (OAR) sparing.105,106
For other intracranial sites, multiple static fields should be selected to minimize dose to the uninvolved CNS tissues (Figs. 22-2 and 22-3). Opposed lateral fields should be avoided in practically all cases to prevent unnecessarily irradiating lateral CNS structures such as the temporal lobes. Intensity-modulated radiotherapy (IMRT), consisting of objective weighting, inverse planning, and fluence modulation, yields target coverage, target conformity, and OAR sparing superior to conventional radiotherapy, particularly for complex tumor volumes, at the expense of larger volumes of normal tissue receiving a low dose (Fig. 22-4).107,108 Careful conformal technique should limit inhomogeneity of dose to less than 10% (ideally less than 5%). No portion of the optic nerve or chiasm should receive greater than 54 Gy at 1.8 Gy per fraction.109 A dose of 54 Gy at 1.8 Gy per fraction for 5 days a week is generally given to benign (WHO Grade I) meningiomas,73,93 and for atypical and anaplastic (WHO Grade II and III) lesions, the dose is increased to 59.4 to 61.2 Gy103,110 Dose-response relationships have been demonstrated,73,103,111,112 with 54 Gy and 60 Gy conventionally fractionated yielding a significantly better PFS than lower dose schedules for benign112 and atypical or anaplastic103 meningiomas, respectively. Aggressive fractionated radiotherapy beyond 60 Gy is more toxic and has not been demonstrated to improve local control.110,113 Anaplastic lesions may be considered for radiosurgery boost following external beam radiotherapy, if the tumor volume is well defined and small.114
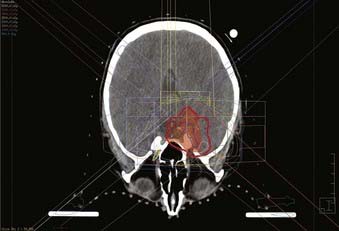
FIGURE 22-2 • Axial plane intensity-modulated radiation therapy isodose plan for right cavernous sinus meningioma.
Outcome
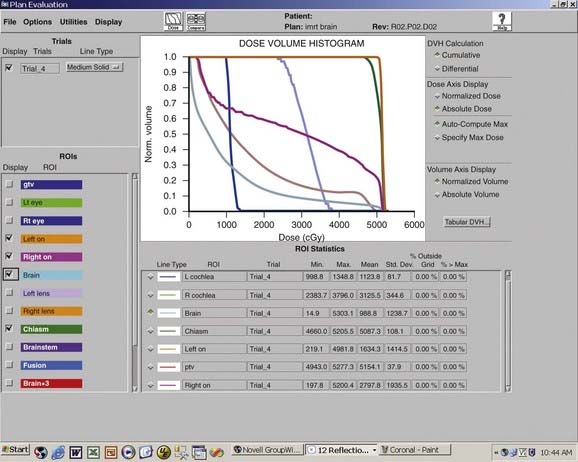
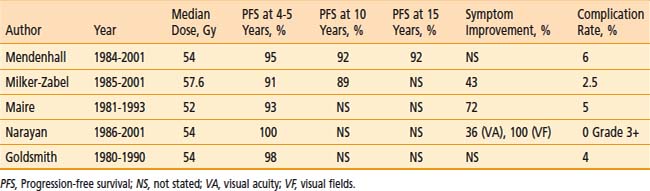
Fractionated radiotherapy as primary management without surgery75,93,115 or after subtotal resection of benign meningioma73,75,93 yields a 5-year PFS between 91% and 98%, which is superior to subtotal resection and comparable to total excision, and symptom improvement in 43% to 72% (Table 22-4). (Note that Table 22-4 excludes reports published before 1994, because those series did not use CT- and/or MRI-based targeting and dose planning, in contradistinction to all contemporary single- or multifraction radiation treatments of meningiomas.) Multivariate analyses demonstrate no relationship between PFS and degree of resection or tumor size.73,75,93,116
Mendenhall and colleagues93 treated 101 patients with skull base meningiomas between 1984 and 2001with fractionated radiotherapy alone (n = 66) or after subtotal resection (n = 35). The median dose was 54 Gy. After median follow-up of 5 years, 5-, 10-, and 15-year PFS rates were 95%, 92%, and 92%, respectively, and the corresponding CSS rates were 97%, 92%, and 92%, respectively, both independent of previous surgery.
Milker-Zabel and coworkers117 reported the University of Heidelberg’s experience in treating 317 patients (153 benign tumors, 26 atypical tumors, 138 unbiopsied tumors) between 1985 and 2001 with stereotactic fractionated radiotherapy to a median dose of 57.6 Gy. After a median follow-up of 5.7 years, 5- and 10-year PFS rates were 91% and 89%, respectively, for benign and unbiopsied tumors. Pretreatment neurologic deficits improved in 43% of patients and worsened in 8%. Improvement was seen in diplopia in 39 (37%) of 106 patients, exophthalmos in 17 (38%) of 45 patients, trigeminal hypoesthesia or dysesthesia in 20 (24%) of 82 patients, and trigeminal neuralgia in nine (33%) of 27 patients. Twenty-two percent of patients with preexisting cranial nerve deficits experienced complete resolution. A previously published analysis of University of Heidelberg skull base meningiomas75 (a subset of those described by Milker-Zabel’s group) found no difference in PFS between primary radiotherapy without surgery and postoperative radiotherapy.
Maire and colleagues116 have described the Hôpital Saint-André experience with radiotherapy alone for 44 inoperable or unresectable patients between 1981 and 1993. The median dose was 52 Gy. After a median follow-up of 3 years, the crude PFS rate was 93%. When reported in combination with 51 patients treated for other indications (subtotal resection, salvage after recurrence, and total resection with unfavorable histology) overall neurologic performance improved in 72% of patients.
Optic nerve sheath meningioma is frequently irradiated without previous surgery. Narayan and coworkers115 described 14 such patients treated (12 without surgery) between 1986 and 2001 with three-dimensional conformal fractionated radiotherapy to a median dose of 54 Gy and followed for a median 4 years. The radiographic PFS rate was 100%. Visual acuity significantly improved, remained stable, and worsened in 36%, 50%, and 14% of patients, respectively. Of nine patients with complete baseline and follow-up visual fields testing, all significantly improved.
Goldsmith and colleagues73 reported on 117 patients irradiated after surgery to a median dose of 54 Gy for benign meningioma. After a median follow-up of 3 years, 5-year PFS rate for patients treated after 1980, when CT or MRI imaging was used for treatment planning, was 98%. Local control of orbital, parasellar, and posterior fossa tumors, notoriously difficult to completely resect and therefore associated with higher recurrence rates in the surgical literature,91 did not differ significantly from all other sites.
Reflecting the relative rarity of atypical and anaplastic histology, the treatment of higher grade meningiomas is described in studies that include small numbers of patients. The University of Heidelberg series cited above117 included 26 patients with atypical tumors. Stereotactic fractionated radiotherapy to a median dose of 57.6 Gy yielded 5- and 10-year PFS rates of 89% and 67%, respectively. From the University of Florida, Katz and coworkers110 reported a 45% 5-year PFS rate for 36 atypical and anaplastic tumors combined, after conventionally fractionated radiotherapy (55 to 60 Gy) or accelerated radiotherapy (1.5 Gy twice daily to 60 Gy +/− 12.5-Gy radiosurgery boost), with outcome independent of dose, fractionation, and radiosurgery boost.
Toxicity
Common acute toxicities of fractionated radiotherapy for meningioma are, in general, mild and include fatigue, skin erythema, and varying degrees of dose-related alopecia (which may be transient or permanent). If the external auditory canal and middle ear are not excluded from the prescription volume and irradiated field, an external otitis and serous otitis media may result. Rarely seen are acute transient symptoms suggestive of increased ICP (e.g., headache, nausea, vomiting) and exacerbation of preradiation therapy neurologic deficits.118 The risk of radiation induced edema is low.73,93,117
Late effects are dependent on the region irradiated and uncommon when OAR dose tolerances are respected. Chronic otitis119 and decreased hearing,75,119,120 have been reported after irradiation of the ear. Retinopathy73,120,121 and optic neuropathy* have been reported after irradiation of the globe to doses above 45 Gy and after irradiation of the anterior visual pathway to doses above 54 Gy, respectively. Cerebral necrosis73,74,103,113,119,124 and focal neurologic deficits suggestive of late brain injury have been seen, particularly after doses greater than 60 Gy, a dose per fraction greater than 1.8 to 2 Gy, and opposed lateral technique.103,118 Hypopituitarism manifested by hyposecretion of growth hormone, luteinizing hormone, follicle stimulating hormone, corticotropin, and thyrotropin (in order of radiosensitivity) is expected after irradiation of the hypothalamic-pituitary axis region and has been reported after radiation therapy for meningiomas.122 Mechanisms of injury and time-dose analyses for radiation injury to the visual apparatus,* brain,118,127 and pituitary gland118,128 are available to the interested reader.
Goldsmith and colleagues73 reported a crude incidence of radiation complications of 4% (five of 140 patients treated to a wide range of target volumes between 1967 and 1990). Two patients experienced retinopathy, and one experienced optic neuropathy. One of two patients with cerebral necrosis recovered after decompressive craniotomy, and the second died of bronchopneumonia secondary to chronic brain syndrome. As a consequence of the recognition of these complications and a subsequent analysis of optic neuropathy risk,109 UCSF established an institutional policy to avoid using the high-dose opposed lateral technique and exceeding a maximum dose of 54 Gy to the optic nerve whenever possible.
Mendenhall and coworkers93 described a crude incidence of late radiation complications of 6% (six of 101 patients treated to a wide range of target volumes between 1984 and 2001). Two patients experienced gradual progressive dementia. One developed edema after radiotherapy as primary management and subsequently underwent resection of the meningioma. Three suffered significant peritumoral edema refractory to steroids, resulting in progressive neurologic compromise and death.
Milker-Zabel and colleagues117 reported a crude incidence of late grade 3 toxicity of 2.5% (eight of 317 patients treated between 1985 and 1998). Two patients experienced reduced vision, one had trigeminal neuropathy, and five developed intermittent tinnitus.
Single-Fraction Radiosurgery
Technique
For several delivery systems, a stereotactic frame is fixed to the patient’s head under local anesthesia, and imaging is acquired with a stereotactic localizer to define tumor and normal tissue anatomy relative to the frame. Some robotically controlled radiosurgery systems do not require application of a stereotactic frame, but rather rely on thermoplastic mask fixation and plain x-ray/CT scan image coregistration to define stereotactic space for treatment. The necessity of including the entire dural tail within the target volume is unclear. Although recommended by DiBiase and colleagues129 on the basis of univariate association between tail inclusion and local control, this factor was also significantly associated with target conformity and insignificant on multivariate analysis. Patterns of failure were not reported, complicating interpretation. Available data on radiosurgery failure location is conflicting, with Stafford and coworkers94 and Rowe and colleagues130 describing marginal failure as infrequent (three of 16 cases) and common (12 of 16 cases), respectively. Because microscopic involvement of the dura is typically confined to a distance of 1 to 2 mm beyond the gross tumor,84–86 inclusion of the first several millimeters of adjacent dura within the target volume may be sufficient.
Dose prescriptions are based on tumor size, location, and history of previous radiotherapy. At the Mayo Clinic,94 tumor margin doses (TMDs) of 16, 18, and 20 Gy have generally been selected for isodose volumes more than 14.1, 4.2 to 14.1, and less than 4.2 cm3, respectively. At the University of Pittsburgh,131,132 TMDs between 12 and 18 Gy have been used, and lower doses between 12 and 14 Gy have been typical since 1993.132,133 Shin and coworkers134 have reported a local failure rate of 20% associated with TMDs between 10 and 12 Gy versus 0% with a TMD greater than 14 Gy. In the treatment of atypical and anaplastic meningiomas, higher TMDs up to 20 Gy have been employed.135 The maximum dose to the optic nerve and chiasm is limited to 8 to 12 Gy to a small portion (Mayo Clinic),136 8 Gy (University of Pittsburgh),137 10 Gy (Medical University Graz),138 and 8 Gy (UCSF) (Fig. 22-5).
Outcome
Single-fraction radiosurgery as primary management without surgery94,139,140 or after subtotal resection94,140 of benign meningioma yields a 5-year PFS rate between 86% and 98%, which is superior to subtotal resection and comparable to total excision, and symptom improvement in 13% to 42% of patients (Table 22-5).
Table 22-5 Stereotactic Radiosurgery for Benign Meningiomas: Selected Series Since 2000 With 5-Year Progression-Free Survival
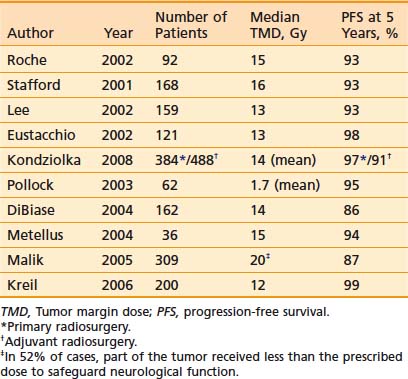
Kreil and colleagues138 treated 200 patients with meningioma (99 after surgery with benign histology and 101 without previous surgery) between 1992 and 1999 to a median TMD of 12 Gy, with a median isodose volume of 6.5 cm3. After a median follow-up of almost 8 years, the 5- and 10-year PFS rates were 99% and 97%, respectively. Preexisting neurologic symptoms improved in 42% of patients, with most improvements in visual fields, trigeminal neuralgia, and diplopia.
Stafford and coworkers94 described radiosurgery for 190 patients with meningiomas treated between 1990 and 1998 to a median TMD of 16 Gy, with a median isodose volume of 8.2 cm3. Forty-one percent were treated primarily without surgery and 59% had had at least one previous operation. After a median follow-up of 3 years, benign meningioma 5-year PFS and CSS rates were 93% and 100%, respectively, independent of previous surgery. Preexisting neurologic symptoms (diplopia or facial pain) improved in 8% of patients. Subsequently, Pollock and colleagues139 from the same institution compared contemporary patients managed with primary radiosurgery to a mean TMD of 18 Gy (n = 62) or resection (n = 136; 57 Simpson grade 1, 57 grade 2, and 22 grades 3 and 4). After a median follow-up of 5 years, tumor recurrence and/or progression was more frequent in the surgical group (11%) than in the radiosurgical group (2%; P < .05). The 7-year PFS rate achieved with radiosurgery (95%) was equivalent to that after Simpson grade 1 excision (all tumor, attached dura, involved bone: 96%) and superior to anything less than this (Simpson grades 2 and 3-4: 82% and 34%, respectively). Symptoms improved in 13% of patients after resection (visual fields, trigeminal neuralgia, and gait) and 13% of patients after radiosurgery (trigeminal neuralgia, diplopia, facial hypoesthesia, and visual fields). Subsequent tumor treatments were less common after primary radiosurgical management, and there were fewer complications as well. The authors suggested that if the long-term tumor control rates remain high and morbidity remains low, radiosurgery can be considered as the primary form of treatment for small- to medium-sized meningiomas without symptomatic mass effect.
Kondziolka and coworkers141 reported on the treatment of 872 meningiomas with a mean volume of 7.4 cm3. Fifty-one percent of the patients were treated primarily, and 49% had had at least one previous operation. The mean TMD was 14 Gy. After a median follow-up of 4 years, the 5- and 10-year PFS rates for the primary radiosurgery group were 97% and 87%, respectively, and for the adjuvant radiosurgery benign meningioma group were 91% and 86%, respectively. In the patients treated primarily, 215 (44%) of 488 tumors shrank, 256 (52%) were unchanged, and 19 (4%) enlarged. Recurrences did not typically arise from the dural tail. Eighty-seven (17%) of 502 primarily treated patients neurologically improved, 380 (76%) were unchanged, and 35 (7%) worsened. In the patients treated adjuvantly, 172 (45%) of 384 tumors shrank, 186 (48%) were unchanged, and 26 (7%) enlarged. Twenty-one (5%) of 398 primarily treated patients neurologically improved, 341 (86%) were unchanged, and 36 (9%) worsened. Combining primarily and adjuvantly treated patents, local control probability correlated with tumor volume (P = .01) and multiplicity (P = .03), but not TMD (P = .61).
Malik and colleagues140 treated 309 meningiomas (44% without previous surgery, 56% after surgery) with a mean volume of 7.3 cm3 between 1994 and 2000 with single-fraction radiosurgery. The median TMD was 20 Gy, but in 52% of cases part of the tumor received less than the prescribed dose to safeguard neurological, most commonly visual, function. After a mean follow-up of almost 4 years, 5- and 8-year benign meningioma PFS rates were 87% and 75%, respectively. Neither conformity nor the volume of tumor receiving less than 5, 10, or 15 Gy influenced outcome.
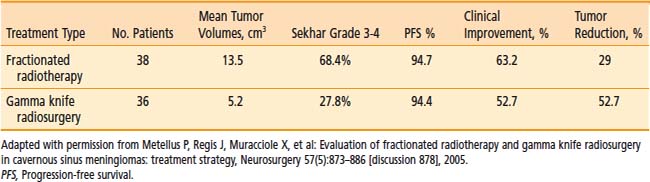
Although no randomized trial of radiotherapy treatment techniques for meningiomas has been published, Metellus and coworkers142 reviewed their own experience with a small series of cavernous sinus meningioma patients treated with fractionated radiotherapy or radiosurgery at a single institution (Table 22-6). Radiosurgery was performed in accordance with commonly accepted techniques, whereas radiotherapy in this retrospective study involved several different fractionation schemes, including some that are associated with a higher risk of complication. The median follow-up was 88.6 months for the fractionated radiotherapy group and 63.6 months for the radiosurgery group. Selection of treatment modality was based on the availability of radiosurgery (after 1992) or on the lesion size, shape, or proximity to critical structures. Although tumor configuration, size, and Sekhar grade were less favorable in the fractionated radiotherapy group, actuarial PFS and clinical improvement rates were not different between the two treatment groups. The radiographic shrinkage was higher in the radiosurgery group (52.7% versus 29%) and was noted to increase in longer follow-up from a subset of patients previously analyzed out to only 30 months (31% shrinkage at 30 months of mean follow-up). In any case, the degree of postradiation shrinkage does not seem to be correlated with any other outcome variable. The authors concluded that for cavernous sinus meningiomas, fractionated radiotherapy and radiosurgery were both safe and effective techniques for securing long-term tumor control.
Table 22-6 Retrospective Comparison of Fractionated Radiotherapy and Gamma Knife Radiosurgery for Cavernous Sinus Meningiomas
As is the case with fractionated radiotherapy, the higher grade meningioma radiosurgery literature is limited in terms of numbers of patients treated. Harris and colleagues114 described the University of Pittsburgh experience treating 18 patients with atypical tumors and 12 patients with anaplastic tumors, with mean TMD and isodose volumes of 15 Gy and 15 cm3 for atypical tumors and 16 Gy and 12 cm3 for anaplastic tumors. Twenty-four (80%) of these 30 patients also received fractionated radiotherapy to a mean dose of 54 Gy. Sixteen patients were treated early with adjuvant intent, and 14 were treated late, after radiographic progression. With a median follow-up of 3.8 years, the 5-year PFS rates for atypical and anaplastic meningioma were 83% and 72%, respectively. The median PFS rates were 38 months in the early treatment group versus 13 months in the late group (P = .06). Kondziolka and colleagues141 subsequently updated the University of Pittsburgh experience, reporting on the treatment of 54 atypical and 29 anaplastic meningiomas (which may have included those previously described by Harris and colleagues). With a median follow-up of 2 and 1.3 years for atypical and anaplastic meningiomas, respectively, the 5-year PFS rates were 45% and 13%, respectively.
Kano and coworkers135 treated 10 patients with atypical meningioma after progression to a median TMD of 19 Gy (range: 12 to 20 Gy) with a median isodose volume of 2.9 cm3. On univariate analysis, only TMD was predictive of local recurrence: after a mean follow-up of 43 months, the 5-year PFS rates were 63% for patients treated to 20 Gy TMD versus 29% for patients treated to lower doses (P = .01). Huffmann and colleagues143 treated 15 patients with atypical meningioma to a median TMD of 16 Gy (range: 14 to 18 Gy) with a median isodose volume of 5 cm3. After a median follow-up of 35 months, 4 (27%) of 15 patients recurred locally or marginally. Stafford and coworkers94 treated 13 patients with atypical meningioma and nine patients with anaplastic meningioma (see above for description of technique), with 5-year PFS rates of 68% and 0%, respectively.
Toxicity
Stafford and coworkers94 described a crude complications rate of 13% (24 of 190 patients) after treatment to a median TMD of 16Gy, including 15 new or worsened cranial neuropathies with a median onset of 6 months (two resolved), five cases of symptomatic edema, two internal carotid artery (ICA) occlusions, and two peritumoral cysts (Table 22–7). Pollock and colleagues139 reported complications in 6% (6 of 62 patients) after treatment to a mean TMD of 18 Gy, including diplopia in one patient, trigeminal neuralgia and/or hypoesthesia in three patients, cyst formation requiring a shunt in one patient, and ICA occlusion in one patient, respectively.
Table 22-7 Complication Rate After Radiosurgery for Benign Mengiomas: Series of More Than 100 Patients
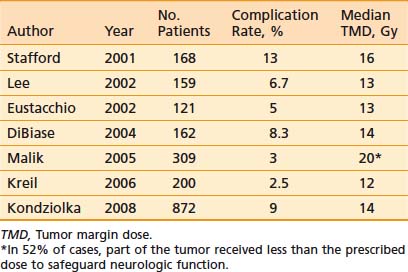
Kodziolka and coworkers141 reported a 9% 10-year complication rate after treatment to a mean TMD of 14 Gy, including hydrocephalus in 0.4% of patients, cranial nerve deficit in 3.4% of patients, headache in 2.2% of patients, seizure in 2.4% of patients, a motor deficit in 1.4% of patients, and a sensory deficit in 0.3% of patients. A ventriculoperitoneal shunt was placed in 0.5% of patients. Symptoms completely resolved in 35% of those who sustained morbidity. On multivariate analysis, tumor volume correlated with risk of complications (P < .001, RR = 1.1 per cm3), but not TMD (P = .95).
Malik and colleagues140 described a crude complication rate of 3% (nine of 277 patients) after treatment to a median TMD of 20 Gy but with part of the tumor receiving less than the prescribed dose in 52% of cases to safeguard neurologic function. One patient experienced trigeminal hypoesthesia exacerbation, and three patients developed new transient trigeminal symptoms. Three patients developed new or altered diplopia, and two patients experienced motor weakness.
Kreil and coworkers138 reported a crude complication rate of 3% (five of 200 patients) after treatment to a median TMD of 12 Gy. Two patients experienced transient increased seizure activity and headache associated with peritumoral edema; two developed trigeminal neuralgia at 12 and 16 months, which resolved months later; and one had permanent optic neuropathy. Of note in the small series reported by Kim and colleagues144 on the results of radiosurgery for superficially located meningiomas, 43% of patients experienced the development of new or worsening edema. The volume of increased signal on T2-weighted images was measured in three dimensions and used to define the “edema index” as the ratio of the volume of T2 signal to the volume of tumor. Edema after radiosurgery occurred at a mean interval of 6 months after treatment (range: 2 to 11 months). In nine of 11 patients, the edema resolved after a mean period of 11 months (range: 5 to 23 months). The edema index was 16.6 for parasagittal meningiomas, 2.5 for falx meningiomas, and 1.5 for convexity meningiomas; however, there was no significant relationship between location and the development of edema. In univariate analysis, high integral tumor dose and larger tumor volume were associated with the development of edema. In tumors larger than 4.2 cm3, edema occurred more frequently. Interestingly, tumor shrinkage occurred more frequently in those patients who had developed edema after treatment.
The incidence of complications after meningioma radiosurgery is low, and these are most frequently cranial nerve injury and symptomatic edema. Generally, over the years, there has been a trend toward using lower TMDs132,133,138 or compromising conformality to safeguard neurologic function130,140 for benign meningioma, with a decrease in complications. Flickinger and colleagues133 reported a significantly lower (RR = 4.5) complication rate in patients treated after 1991, which was associated with the use of lower doses and MRI for treatment planning. Selection of smaller treatment volumes133 and restricting maximum dose to the optic nerve and chiasm131 are also expected to result in a decline in the incidence of radiosurgery complications.
Spinal Meningioma
The optimal treatment of spinal meningioma is total excision. A large series from the Cleveland Clinic78 reported only one recurrence among 80 patients managed with complete tumor removal. Subtotal resection in their experience was unusual (7% of all spinal meningioma cases), and disease recurred in only two of seven such cases, at 13 and 16 years. The authors concluded that, unlike intracranial meningiomas, subtotally resected spinal meningiomas often pursue an indolent course. This characteristic indolence agrees with observation of relatively low proliferative index in spinal meningioma.145 No radiation oncology literature has addressed the role of radiation therapy in such patients, so treatment recommendations are lacking. The UCSF policy is to treat subtotally resected spinal meningiomas with a total dose of 5040 to 5400 cGy, since in general it is felt that the potential complications of recurrence or further surgery outweigh the risks of radiation therapy.
Meningioma in the Pediatric Population
Pediatric meningiomas are rare, and their treatment with irradiation is even more uncommon. Most pediatric patients are managed with surgery alone, resulting in an overall 20-year survival rate of 62%.21 As a consequence, there is little radiotherapy literature addressing this topic, and there are no established treatment recommendations.20,146 Leibel and coworkers146 reported on the treatment of three patients with intracranial meningiomas irradiated after subtotal resection. The first patient, with a convexity anaplastic meningioma, had a minimal response to 50 Gy with concurrent cytoxan chemotherapy and died 4 months later of uncontrolled disease. The second patient, also with a convexity anaplastic meningioma, received 32 Gy after a subtotal resection, followed by complete excision 6 months later, but this patient died because of postoperative complications. The third patient, with a benign meningioma of the posterior fossa, was treated to 50.4 Gy and recurred 4 years later. The recurrent tumor was then subtotally resected, and the patient was followed for an additional 9 years without progression. Irradiation for recurrence has also been reported by Leibel and colleagues146 All three patients had benign meningiomas which had recurred after subtotal resection. The first patient was treated to 50.6 Gy and was free of recurrence at 6 years follow-up. The second patient was treated to 52.9 Gy, followed 6 months later by total excision, and was free of disease 9 years later. The third patient was treated to 50 Gy, but died of uncontrolled disease 3 months later. The unimpressive results and small number of patients make interpretation of this data difficult. The authors concluded that when the tumor location and potential neurologic sequelae of recurrence and salvage surgery permit, radiotherapy may be withheld. Reasonable indications for radiotherapy (to a dose of 50 to 54 Gy) would include: (1) anaplastic tumor, (2) incompletely resected or brain-invasive atypical tumor, (3) benign meningioma at a site where the potential complications of recurrence or further surgery outweigh the risks of radiotherapy, and (4) progressive unresectable disease.
Medical Treatment of Meningiomas
Medical therapy for patients with progressive meningioma following maximal resection and radiation modalities remains investigational. Chemotherapeutic as well as other drug strategies that have been evaluated in meningioma are reviewed.147
Standard Chemotherapeutic Agents
Hydroxyurea is a cell cycle–specific urea analog that inhibits the enzyme ribonucleotide diphosphate reductase, thereby interfering with DNA synthesis by reducing the available deoxyribinucleotides pool.148 Controlled clinical trials of hydroxyurea are lacking. Newton and associates149,150 reported on the use of hydroxyurea (20 mg/kg/day) in two studies with overlapping cohorts. The expanded cohort consisted of 21 patients in whom at least one resection had been attempted. Nine patients had had previous external-beam irradiation; none of the patients had had any previous chemotherapy. All of the tumors were WHO Grade I, except for one patient with an atypical meningioma. Eighteen of 20 evaluable patients (90%) had stable disease after treatment with hydroxyurea, with a median time to disease progression of 176 weeks (range: 20 to 328 weeks). Overall, eight of 20 evaluable patients remained stable on MR imaging for longer than 4 years.149,150 Rosenthal and colleagues treated 15 consecutive patients harboring recurrent or high-risk meningiomas with hydroxyurea. Eleven patients (85%) had stable disease for a median of 11 months (range: 3 to 24 months), and two had progressive disease.151
Temozolomide is an alkylating agent that is effective in the treatment of patients with malignant glioma; however, it failed to show any benefit when evaluated in a prospective Phase II study in 16 patients with refractory meningioma. These patients had previous treatment with surgery and radiotherapy but no chemotherapy.152
Combination chemotherapy regimens administered both intravenously and intra-arterially have been evaluated especially in the more aggressive malignant meningioma population. Younis reviewed 25 patients with aggressive malignant meningiomas, 10 of whom received chemotherapy but did not derive any objective benefit. Typical regimens included intra-arterial or intravenous cisplatin, intravenous dacarbazine, and intravenous doxorubicin.153,154 Other regimens have included ifosphamide/mesna or adriamycin/DTIC.155 No effective combinations have been described and the toxicities of therapy do not warrant standard use in meningioma.
Because of the poor prognosis of malignant meningioma despite maximal surgery and radiation, concurrent and adjuvant chemotherapy strategies have been described. Fourteen patients were treated in phase II clinical trial with resective surgery followed by adjuvant limited-field radiotherapy and cyclophosphamide-adriamycin-vincristine (CAV). There were three patients with a partial response to treatment and 11 with stable disease. The median time to tumor progression was 4.6 years, with a median survival of 5.3 years.156 The small number of patients and lack of a concurrent control group limits the determination of the benefit of this strategy.
Immunotherapy
The most frequently recommended immunotherapy for meningiomas is interferon-alpha (IFN-α), a cytokine produced by activated lymphocytes. It is basically nontoxic in low dosages and is easily tolerated.157 The major side effects include fever, flu-like symptoms, and fatigue. The mechanism of action of IFN-α includes direct cytotoxic effect, activation of natural killer cells, modulation of antibody production, antiangiogenesis, and induction of major histocompatibility complex antigens on the tumor cell surface.157 There is in vitro evidence that IFN inhibits the proliferation of meningioma cells.158 In a phase II clinical trial by Kaba et al159 six patients with recurrent unresectable meningioma were treated with IFN-α administered subcutaneously and five exhibited positive response to treatment. Stabilization of the size of the tumor occurred in four patients and slight regression in one, with responses lasting from 6 to 14 months. Prospective studies of larger cohorts of patients need to be performed using IFN-α to confirm the benefit of this treatment.
Hormonal Treatment
Although several investigators reported encouraging preliminary results with salvage hormonal therapy for unresectable meningioma, subsequent trials have been disappointing. The Southwest Oncology Group (SWOG) studied the antiestrogen tamoxifen160 in 21 patients with unresectable meningioma. All patients received tamoxifen 40 mg per m2 bid for 4 days, then 10 mg bid thereafter until progression. Of 19 evaluable patients, 1 (5%) achieved an MRI documented partial response, 2 had a minor response of short duration (4 and 20 months), 6 (32%) remained stable for a median of 31 months, and 10 (53%) progressed.
Medroxyprogesterone acetate (MPA) is a semisynthetic progestin that acts as a competitive agonist for PR. Although it has been shown to reduce the growth of menigioma cells in vitro, it failed to show any benefit in clinical studies.161–163 Another approach to hormone therapy is to use antiprogestins such as mifepristone (RU486) a derivative of the progestin norethindrone to block potential mitogenic stimulation mediated via PRs. Several in vitro and animal studies have shown growth inhibition in meningioma cells after treatment with RU486.164,165 Early clinical studies demonstrated encouraging responses to mifepristone ranging from stabilization of disease to minor responses.31,166 On the basis of those results, the SWOG and Eastern Cooperative Oncology Group (ECOG) subsequently conducted a phase III placebo-controlled, randomized trial evaluating mifepristone (RU486) for the treatment of unresectable nonanaplastic meningioma. One hundred and ninety-three patients were enrolled, of whom 160 were evaluable. Patients received RU486 versus placebo daily over a 2-year period. The median time to progression was 10 months for RU486 and 12 months for placebo (P = .44). The study was prematurely closed and a final report has yet to be published.167 Treatment was well tolerated with reported toxicities consisting mainly of fatigue, headache, and hot flushes but a significant number of female patients who received RU486 developed endometrial hyperplasia.168
Targeting Surface Receptors
Studies have demonstrated the presence of IGF (insulin-like growth factor)-1 receptors in virtually all meningioma specimens. Growth factor and IGF-I have been identified as potent inducers of cell growth in many types of neoplasms. It was demonstrated that the growth hormone (GH)/IGF-I axis is also important in modulating meningioma growth.169 In in vivo tumor models, downregulation of the GH/IGF-I axis significantly reduced meningioma growth and, in some instances, caused tumor regression with growth factor receptor antagonist pegvisomant treatment.170 Yin et al showed that pegvisomant was well-tolerated at subcutaneous doses and was efficacious in suppressing the GH axis, resulting in substantial and sustained inhibition of circulating IGF-I, IGF-II, and IGFBP-3 concentrations.171 These results provide evidence in favor of further testing the hypothesis that pegvisomant, through blocking the GH receptor-mediated signal transduction pathways, could be effective in treating tumors, such as meningioma, that may be GH, IGF-I, and/or IGF-II dependent.
Somatostatin (sst) receptors are expressed in meningioma cells suggesting that there may be a therapeutic role for somatostatin inhibitors.172 An in vitro study on 16 surgically removed meningiomas showed that sst2A expression was readily detectable in 75% of the tumors, with particularly high levels in 50% of cases.173 The addition of somatostatin inhibited meningioma growth in vitro in some preclinical studies. A pilot prospective trial of 16 patients with recurrent meningioma were treated with long-acting somatostatin (Sandostatin LAR) and four partial responses, five stable disease, and seven progressive disease patterns were seen. The overall PFS was 44% at 6 months.174 Further studies need to be performed to confirm the benefit of this treatment.
Targeting Other Signal Transduction Pathways
Compared to the extensive knowledge of the molecular pathogenesis and biology of malignant gliomas or other systemic cancer, relatively little is known about the molecular pathogenesis of meningiomas and the critical molecular changes driving tumor growth. Overexpression of various growth factors—including platelet derived growth factor, epidermal growth factor, and vascular endothelial growth factor—and their receptors, as well as aberrations of signal transduction pathways—such as the Ras/MAPK, PI3K-Akt, and PLC-g1-PKC pathways—have been implicated, but their relative significance is largely unknown.175 Some targeted therapeutic agents might be effective as single agents or in combination with chemotherapy in meningioma, but further studies are needed to confirm that hypothesis. Examples of targeted agents include: imatinib, erlotinib, cetuximab, sorafenib, tipifarnib, mammalian target of rapamycin inhibitors, and bevacizumab.176
Novel Medical Treatment Approaches
Suppressive therapy with calcium channel antagonists (CCAs) represents an attractive adjunctive treatment for unresectable or recurrent meningiomas, because they are safe drugs with well-known side effect profiles that lend themselves to long-term chronic therapy.177 In a meningioma mouse xenograft flank model, diltiazem and verapamil treatment resulted in dose-dependent decreased tumor growth over time compared with control groups. Drug treatment was not curative, however, indicating a growth-suppressive effect of CCAs.178 Addition of diltiazem or verapamil to hydroxyurea or RU486 intensified in vitro meningioma growth inhibition by 20 to 60% by inducing apoptosis and G1 cell cycle arrest.179 A clinical trial of this approach is ongoing.
In in vitro studies, celecoxib, a COX-2 inhibitor, showed a dose-dependent inhibition of meningioma cell growth in a malignant cell line (IOMM-Lee) and in six benign cell lines.180 An in vivo mouse meningioma flank tumor model was used to show a statistically significant decrease in tumor size with high-dose celecoxib treatment in two of three cell lines, with a mean tumor volume reduction between 25% and 66%. Also tumors showed decreased microvascular density and diminished COX-2 and VEGF staining as well as increased apoptosis.181 It has thus been hypothesized that the use of celecoxib as a chronic therapy for meningiomas could be effective and easily tolerated.182,183 Clinical confirmation of the benefit of COX-2 inhibitors remains to be shown.
Radiographic Follow-Up Recommendations
At the UCSF, posttreatment scans (CT or MRI) are obtained annually for non–optic nerve sheath benign meningiomas, and twice a year for benign optic nerve sheath, atypical, and anaplastic meningiomas, largely based on mean tumor volume doubling times reported by Jaaskelainen.184 Such careful follow-up is warranted because the potential for salvage is substantial.
1 Longstreth WTJr, et al. Epidemiology of intracranial meningioma. Cancer. 1993;72(3):639-648.
2 Surawicz TS, et al. Descriptive epidemiology of primary brain and CNS tumors: results from the Central Brain Tumor Registry of the United States, 1990–1994. Neurooncol. 1999;1(1):14-25.
3 Preston-Martin S. Descriptive epidemiology of primary tumors of the brain, cranial nerves and cranial meninges in Los Angeles County. Neuroepidemiol. 1989;8(6):283-295.
4 Sutherland GR, et al. Epidemiology of primary intracranial neoplasms in Manitoba, Canada. Can J Neurol Sci. 1987;14(4):586-592.
5 Kurland LT, et al. The incidence of primary intracranial neoplasms in Rochester, Minnesota, 1935–1977. Ann N Y Acad Sci. 1982;381:6-16.
6 Sankila R, et al. Long-term survival of 1986 patients with intracranial meningioma diagnosed from 1953 to 1984 in Finland. Comparison of the observed and expected survival rates in a population-based series. Cancer. 1992;70(6):1568-1576.
7 Helseth A, et al. Neoplasms of the central nervous system in Norway. IV. A population-based epidemiological study of meningiomas. Apmis. 1989;97(7):646-654.
8 Staneczek W, Janisch W. Epidemiologic data on meningiomas in East Germany 1961–1986: incidence, localization, age and sex distribution. Clin Neuropathol. 1992;11(3):135-141.
9 Sant M, et al. Incidence and survival of brain tumors: a population-based study. Tumori. 1988;74(3):243-252.
10 Lovaste MG, Ferrari G, Rossi G. Epidemiology of primary intracranial neoplasms. Experiment in the Province of Trento, (Italy), 1977-1984. Neuroepidemiology. 1986;5(4):220-232.
11 Andrioli GC, et al. Multiple meningiomas. Neurochirurgia (Stuttg). 1981;24(2):67-69.
12 Butti G, et al. Multiple meningiomas: a clinical, surgical, and cytogenetic analysis. Surg Neurol. 1989;31(4):255-260.
13 Domenicucci M, et al. Multiple intracranial meningiomas. J Neurosurg. 1989;70(1):41-44.
14 Locatelli D, et al. Multiple meningiomas evaluated by computed tomography. Neurochirurgia (Stuttg). 1987;30(1):8-10.
15 Lusins JO, Nakagawa H. Multiple meningiomas evaluated by computed tomography. Neurosurgery. 1981;9(2):137-141.
16 Borovich B, et al. The incidence of multiple meningiomas–do solitary meningiomas exist? Acta Neurochir (Wien). 1988;90(1–2):15-22.
17 Shuangshoti S, Hongsaprabhas C, Netsky MG. Metastasizing meningioma. Cancer. 1970;26(4):832-841.
18 Batsakis JG. Pathology consultation. Extracranial meningiomas. Ann Otol Rhinol Laryngol. 1984;93(3 Pt 1):282-283.
19 Rohringer M, et al. Incidence and clinicopathological features of meningioma. J Neurosurg. 1989;71(5 Pt 1):665-672.
20 Shah MV, Haines SJ. Pediatric skull, skull base, and meningeal tumors. Neurosurg Clin N Am. 1992;3(4):893-924.
21 Deen HGJr, Scheithauer BW, Ebersold MJ. Clinical and pathological study of meningiomas of the first two decades of life. J Neurosurg. 1982;56(3):317-322.
22 Munk J, Peyser E, Gruszkiewicz J. Radiation induced intracranial meningiomas. Clin Radiol. 1969;20(1):90-94.
23 Beller AJ, Feinsod M, Sahar A. The possible relationship between small dose irradiation to the scalp and intracranial meningiomas. Neurochirurgia (Stuttg). 1972;15(4):135-143.
24 Modan B, et al. Radiation-induced head and neck tumours. Lancet. 1974;1(7852):277-279.
25 Shore RE, Albert RE, Pasternack BS. Follow-up study of patients treated by x-ray epilation for tinea capitis: resurvey of post-treatment illness and mortality experience. Arch Environ Health. 1976;31:21.
26 Ron E, et al. Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med. 1988;319(16):1033-1039.
27 Rubinstein AB, et al. Radiation-induced cerebral meningioma: a recognizable entity. J Neurosurg. 1984;61(5):966-971.
28 Rienstein S, et al. Comparative genomic hybridization analysis of radiation-associated and sporadic meningiomas. Cancer Genet Cytogenet. 2001;131(2):135-140.
29 Schuz J, et al. Cellular phones, cordless phones, and the risks of glioma and meningioma (Interphone Study Group, Germany). Am J Epidemiol. 2006;163(6):512-520.
30 Goldberg M, Rappaport ZH. Neurosurgical, obstetric and endocrine aspects of meningioma during pregnancy. Isr J Med Sci. 1987;23(7):825-828.
31 Grunberg SM, et al. Treatment of unresectable meningiomas with the antiprogesterone agent mifepristone. J Neurosurg. 1991;74(6):861-866.
32 Poisson M, et al. Steroid hormone receptors in human meningiomas, gliomas and brain metastases. J Neurooncol. 1983;1(3):179-189.
33 Schnegg JF, et al. Presence of sex steroid hormone receptors in meningioma tissue. Surg Neurol. 1981;15(6):415-418.
34 Magdelenat H, et al. Progestin and oestrogen receptors in meningiomas. Biochemical characterization, clinical and pathological correlations in 42 cases. Acta Neurochir (Wien). 1982;64(3–4):199-213.
35 Yu ZY, et al. Estrogen and progestin receptors in intracranial meningiomas. J Steroid Biochem. 1982;16(3):451-456.
36 Blankenstein MA, Blaauw G, Lamberts SW. Progestin and estrogen receptors in human meningioma. Clin Neuropharmacol. 1984;7(4):363-367.
37 Schlehofer B, Blettner M, Wahrendorf J. Association between brain tumors and menopausal status. J Natl Cancer Inst. 1992;84(17):1346-1349.
38 Wigertz A, et al. Risk of brain tumors associated with exposure to exogenous female sex hormones. Am J Epidemiol. 2006;164(7):629-636.
39 Pravdenkova S, et al. Progesterone and estrogen receptors: opposing prognostic indicators in meningiomas. J Neurosurg. 2006;105(2):163-173.
40 Preston-Martin S, et al. An international case-control study of adult glioma and meningioma: the role of head trauma. Int J Epidemiol. 1998;27(4):579-586.
41 Preston-Martin S, Mack W, Henderson BE. Risk factors for gliomas and meningiomas in males in Los Angeles County. Cancer Res. 1989;49(21):1637-1643.
42 Preston-Martin S, et al. Case-control study of intracranial meningiomas in women in Los Angeles County, California. J Natl Cancer Inst. 1980;65(1):67-73.
43 Preston-Martin S, et al. Risk factors for meningiomas in men in Los Angeles County. J Natl Cancer Inst. 1983;70(5):863-866.
44 Annegers JF, et al. Head trauma and subsequent brain tumors. Neurosurgery. 1979;4(3):203-206.
45 Schlehofer B, et al. Medical risk factors and the development of brain tumors. Cancer. 1992;69(10):2541-2547.
46 Martini F, et al. SV40 early region and large T antigen in human brain tumors, peripheral blood cells, and sperm fluids from healthy individuals. Cancer Res. 1996;56(20):4820-4825.
47 Ibelgaufts H, et al. Adenovirus-related RNA sequences in human neurogenic tumours. Acta Neuropathol (Berl). 1982;56(2):113-117.
48 Zang KD, May G, Fischer H. Expression of SV 40-related T-antigen in cell cultures of human meningiomas. Naturwissenschaften. 1979;66(1):59.
49 Weiss AF, et al. Simian virus 40-related antigens in three human meningiomas with defined chromosome loss. Proc Natl Acad Sci USA. 1975;72(2):609-613.
50 Weber RG, et al. Analysis of genomic alterations in benign, atypical, and anaplastic meningiomas: toward a genetic model of meningioma progression. Proc Natl Acad Sci USA. 1997;94(26):14719-14724.
51 Zang KD. Meningioma: a cytogenetic model of a complex benign human tumor, including data on 394 karyotyped cases. Cytogenet Cell Genet. 2001;93(3–4):207-220.
52 Collins VP, Nordenskjold M, Dumanski JP. The molecular genetics of meningiomas. Brain Pathol. 1990;1(1):19-24.
53 Ketter R, et al. Application of oncogenetic trees mixtures as a biostatistical model of the clonal cytogenetic evolution of meningiomas. Int J Cancer. 2007;121(7):1473-1480.
54 Al-Mefty O, et al. Malignant progression in meningioma: documentation of a series and analysis of cytogenetic findings. J Neurosurg. 2004;101(2):210-218.
55 Helseth A, Mork SJ, Glattre E. Neoplasms of the central nervous system in Norway. V. Meningioma and cancer of other sites. An analysis of the occurrence of multiple primary neoplasms in meningioma patients in Norway from 1955 through 1986. Apmis. 1989;97(8):738-744.
56 Larsson C, Bystian C, Skoog L. Chromosomal mutations in human breast carcinoma. Genes Chromosomes Cancer. 1990;2:181.
57 Iida A, Kurose K, Isobe R. Mapping of a new target region of allelic loss to a 2-cM interval at 22q13.1 in primary breast cancer. Genes Chromosomes Cancer. 1998;21(2):108-112.
58 Hartikainen JM, et al. An autosome-wide scan for linkage disequilibrium-based association in sporadic breast cancer cases in eastern Finland: three candidate regions found. Cancer Epidemiol Biomarkers Prev. 2005;14(1):75-80.
59 Louis DN, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97-109.
60 Kleihues P, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61(3):215-225.
61 Zorludemir S, et al. Clear cell meningioma. A clinicopathologic study of a potentially aggressive variant of meningioma. Am J Surg Pathol. 1995;19(5):493-505.
62 Kleihues P, Burger PC, Scheithauer BW. The new WHO classification of brain tumours. Brain Pathol. 1993;3(3):255-268.
63 Perry A, et al. “Rhabdoid” meningioma: an aggressive variant. Am J Surg Pathol. 1998;22(12):1482-1490.
64 Ludwin SK, Rubinstein LJ, Russell DS. Papillary meningioma: a malignant variant of meningioma. Cancer. 1975;36(4):1363-1373.
65 Perry A, et al. Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol. 1997;21(12):1455-1465.
66 Fukui M, et al. Proliferative activity of meningiomas as evaluated by bromodeoxyuridine uptake examination. Acta Neurochir (Wien). 1986;81(3–4):135-141.
67 Shibuya M, et al. Meningiomas: clinical implications of a high proliferative potential determined by bromodeoxyuridine labeling. Neurosurgery. 1992;30(4):494-497.
68 Spaar FW, Ahyai A, Blech M. DNA-fluorescence-cytometry and prognosis (grading) of meningiomas–a study of 104 surgically removed tumors. Neurosurg Rev. 1987;10(1):35-39.
69 Cobb MA, et al. Significance of proliferating cell nuclear antigen in predicting recurrence of intracranial meningioma. J Neurosurg. 1996;84(1):85-90.
70 Takeuchi H, et al. Prediction of recurrence in histologically benign meningiomas: proliferating cell nuclear antigen and Ki-67 immunohistochemical study. Surg Neurol. 1997;48(5):501-506.
71 Colvett KT, et al. High PCNA index in meningiomas resistant to radiation therapy. Int J Radiat Oncol Biol Phys. 1997;38(3):463-468.
72 Carvalho LH, et al. Molecular signatures define two main classes of meningiomas. Mol Cancer. 2007;6:64.
73 Goldsmith BJ, et al. Postoperative irradiation for subtotally resected meningiomas. A retrospective analysis of 140 patients treated from 1967 to 1990. J Neurosurg. 1994;80(2):195-201.
74 Glaholm J, Bloom HJ, Crow JH. The role of radiotherapy in the management of intracranial meningiomas: the Royal Marsden Hospital experience with 186 patients. Int J Radiat Oncol Biol Phys. 1990;18(4):755-761.
75 Debus J, et al. High efficacy of fractionated stereotactic radiotherapy of large base-of-skull meningiomas: long-term results. J Clin Oncol. 2001;19(15):3547-3553.
76 Pourel N, et al. Efficacy of external fractionated radiation therapy in the treatment of meningiomas: a 20-year experience. Radiother Oncol. 2001;61(1):65-70.
77 Herz DA, Shapiro K, Shulman K. Intracranial meningiomas of infancy, childhood and adolescence. Review of the literature and addition of 9 case reports. Childs Brain. 1980;7(1):43-56.
78 Levy WJJr, Bay J, Dohn D. Spinal cord meningioma. J Neurosurg. 1982;57(6):804-812.
79 Murovic J, Sundaresan N. Pediatric spinal axis tumors. Neurosurg Clin N Am. 1992;3(4):947-958.
80 Sheporaitis LA, et al. Intracranial meningioma. AJNR Am J Neuroradiol. 1992;13(1):29-37.
81 Mantle RE, et al. Predicting the probability of meningioma recurrence based on the quantity of peritumoral brain edema on computerized tomography scanning. J Neurosurg. 1999;91(3):375-383.
82 Kozler P, et al. Preoperative neuroimage findings as a predictor of postoperative neurological deficit in intracranial meningiomas. Zentralbl Neurochir. 2007;68(4):190-194.
83 Tien RD, Yang PJ, Chu PK. “Dural tail sign”: a specific MR sign for meningioma? J Comput Assist Tomogr. 1991;15(1):64-66.
84 Tokumaru A, et al. Prominent meningeal enhancement adjacent to meningioma on Gd-DTPA-enhanced MR images: histopathologic correlation. Radiology. 1990;175(2):431-433.
85 Kawahara Y, et al. Dural congestion accompanying meningioma invasion into vessels: the dural tail sign. Neuroradiology. 2001;43(6):462-465.
86 Nagele T, et al. The “dural tail” adjacent to meningiomas studied by dynamic contrast-enhanced MRI: a comparison with histopathology. Neuroradiology. 1994;36(4):303-307.
87 Khoo VS, et al. A comparison of clinical target volumes determined by CT and MRI for the radiotherapy planning of base of skull meningiomas. Int J Radiat Oncol Biol Phys. 2000;46(5):1309-1317.
88 Henze M, et al. PET imaging of somatostatin receptors using [68GA]DOTA-D-Phe1-Tyr3-octreotide: first results in patients with meningiomas. J Nucl Med. 2001;42(7):1053-1056.
89 Milker-Zabel S, et al. Improved target volume definition for fractionated stereotactic radiotherapy in patients with intracranial meningiomas by correlation of CT, MRI, and [68Ga]-DOTATOC-PET. Int J Radiat Oncol Biol Phys. 2006;65(1):222-227.
90 Condra KS, et al. Benign meningiomas: primary treatment selection affects survival. Int J Radiat Oncol Biol Phys. 1997;39(2):427-436.
91 Mirimanoff RO, et al. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62(1):18-24.
92 Levine ZT, et al. Proposed grading system to predict the extent of resection and outcomes for cranial base meningiomas. Neurosurgery. 1999;45(2):221-230.
93 Mendenhall WM, et al. Radiotherapy alone or after subtotal resection for benign skull base meningiomas. Cancer. 2003;98(7):1473-1482.
94 Stafford SL, et al. Meningioma radiosurgery: tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery. 2001;49(5):1029-1037.
95 Yang SY, et al. Atypical and anaplastic meningiomas: prognostic implications of clinicopathological features. J Neurol Neurosurg Psychiatry. 2008;79(5):574-580.
96 Goyal LK, et al. Local control and overall survival in atypical meningioma: a retrospective study. Int J Radiat Oncol Biol Phys. 2000;46(1):57-61.
97 Bruna J, et al. Ki-67 proliferative index predicts clinical outcome in patients with atypical or anaplastic meningioma. Neuropathology. 2007;27(2):114-120.
98 Perry A, et al. “Malignancy” in meningiomas: a clinicopathologic study of 116 patients, with grading implications. Cancer. 1999;85(9):2046-2056.
99 Ketter R, et al. Predictive value of progression-associated chromosomal aberrations for the prognosis of meningiomas: a retrospective study of 198 cases. J Neurosurg. 2001;95(4):601-607.
100 Modha A, Gutin PH. Diagnosis and treatment of atypical and anaplastic meningiomas: a review. Neurosurgery. 2005;57(3):538-550.
101 Schlegel W, et al. Stereotactically guided fractionated radiotherapy: technical aspects. Radiother Oncol. 1993;29(2):197-204.
102 Gross MW, Spahn U, Engenhart-Cabillic R. Assessment of the accuracy of a conventional simulation for radiotherapy of head and skull base tumors. Technol Cancer Res Treat. 2003;2(4):345-351.
103 Hug EB, et al. Management of atypical and malignant meningiomas: role of high-dose, 3D-conformal radiation therapy. J Neurooncol. 2000;48(2):151-160.
104 Eng TY, et al. Precision radiation therapy for optic nerve sheath meningiomas. Int J Radiat Oncol Biol Phys. 1992;22(5):1093-1098.
105 Baumert BG, et al. Early improvements in vision after fractionated stereotactic radiotherapy for primary optic nerve sheath meningioma. Radiother Oncol. 2004;72(2):169-174.
106 Becker G, et al. Stereotactic fractionated radiotherapy in patients with optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2002;54(5):1422-1429.
107 Baumert BG, Norton IA, Davis JB. Intensity-modulated stereotactic radiotherapy vs stereotactic conformal radiotherapy for the treatment of meningioma located predominantly in the skull base. Int J Radiat Oncol Biol Phys. 2003;57(2):580-592.
108 Pirzkall A, et al. Comparison of intensity-modulated radiotherapy with conventional conformal radiotherapy for complex-shaped tumors. Int J Radiat Oncol Biol Phys. 2000;48(5):1371-1380.
109 Goldsmith BJ, et al. Optic neuropathy after irradiation of meningioma. Radiology. 1992;185(1):71-76.
110 Katz TS, et al. Pushing the limits of radiotherapy for atypical and malignant meningioma. Am J Clin Oncol. 2005;28(1):70-74.
111 Milosevic MF, et al. Radiotherapy for atypical or malignant intracranial meningioma. Int J Radiat Oncol Biol Phys. 1996;34(4):817-822.
112 Haie-Meder C, et al. [Role of radiotherapy in the treatment of meningioma]. Bull Cancer Radiother. 1995;82(1):35-39.
113 Wenkel E, et al. Benign meningioma: partially resected, biopsied, and recurrent intracranial tumors treated with combined proton and photon radiotherapy. Int J Radiat Oncol Biol Phys. 2000;48(5):1363-1370.
114 Harris AE, et al. The effect of radiosurgery during management of aggressive meningiomas. Surg Neurol. 2003;60(4):298-305.
115 Narayan S, et al. Preliminary visual outcomes after three-dimensional conformal radiation therapy for optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2003;56(2):537-543.
116 Maire JP, et al. Fractionated radiation therapy in the treatment of intracranial meningiomas: local control, functional efficacy, and tolerance in 91 patients. Int J Radiat Oncol Biol Phys. 1995;33(2):315-321.
117 Milker-Zabel S, et al. Fractionated stereotactic radiotherapy in patients with benign or atypical intracranial meningioma: long-term experience and prognostic factors. Int J Radiat Oncol Biol Phys. 2005;61(3):809-816.
118 Capo H, Kupersmith MJ. Efficacy and complications of radiotherapy of anterior visual pathway tumors. Neurol Clin. 1991;9(1):179-203.
119 Miralbell R, et al. The role of radiotherapy in the treatment of subtotally resected benign meningiomas. J Neurooncol. 1992;13(2):157-164.
120 Forbes AR, Goldberg ID. Radiation therapy in the treatment of meningioma: the Joint Center for Radiation Therapy experience 1970 to 1982. J Clin Oncol. 1984;2(10):1139-1143.
121 Parsons JT, et al. Radiation retinopathy after external-beam irradiation: analysis of time-dose factors. Int J Radiat Oncol Biol Phys. 1994;30(4):765-773.
122 al-Mefty O, et al. The long-term side effects of radiation therapy for benign brain tumors in adults. J Neurosurg. 1990;73(4):502-512.
123 Parsons JT, et al. Radiation optic neuropathy after megavoltage external-beam irradiation: analysis of time-dose factors. Int J Radiat Oncol Biol Phys. 1994;30(4):755-763.
124 Yamashita J, et al. Recurrence of intracranial meningiomas, with special reference to radiotherapy. Surg Neurol. 1980;14(1):33-40.
125 Parsons JT, Bova FJ, Fitzgerald CR. Tolerance of the visual apparatus to conventional therapeutic irradiation. In: Gutin PH, Leibel SA, Sheline GE, editors. Radiation Injury to the Nervous System. New York: Raven Press; 1991:283.
126 Shrieve DC, et al. Dose fractionation in stereotactic radiotherapy for parasellar meningiomas: radiobiological considerations of efficacy and optic nerve tolerance. J Neurosurg. 2004;101(Suppl 3):390-395.
127 Leibel SA, Sheline GE. Tolerance of the brain and spinal cord to conventional irradiation. In: Gutin PH, Leibel SA, Sheline GE, editors. Radiation Injury to the Nervous System. New York: Raven Press; 1991:239.
128 Littley MD, Shalet SM, Bearswell CG. Radiation and the hypothalamic-pituitary axis. In: Gutin PH, Leibel SA, Sheline GE, editors. Radiation Injury to the Nervous System. New York: Raven Press; 1991:303.
129 DiBiase SJ, et al. Factors predicting local tumor control after gamma knife stereotactic radiosurgery for benign intracranial meningiomas. Int J Radiat Oncol Biol Phys. 2004;60(5):1515-1519.
130 Rowe JG, et al. Radiosurgical planning of meningiomas: compromises with conformity. Stereotact Funct Neurosurg. 2004;82(4):169-174.
131 Kondziolka D, et al. Long-term outcomes after meningioma radiosurgery: physician and patient perspectives. J Neurosurg. 1999;91(1):44-50.
132 Lee JY, et al. Stereotactic radiosurgery providing long-term tumor control of cavernous sinus meningiomas. J Neurosurg. 2002;97(1):65-72.
133 Flickinger JC, et al. Gamma knife radiosurgery of imaging-diagnosed intracranial meningioma. Int J Radiat Oncol Biol Phys. 2003;56(3):801-806.
134 Shin M, et al. Analysis of treatment outcome after stereotactic radiosurgery for cavernous sinus meningiomas. J Neurosurg. 2001;95(3):435-439.
135 Kano H, et al. Stereotactic radiosurgery for atypical and anaplastic meningiomas. J Neurooncol. 2007;84(1):41-47.
136 Stafford SL, et al. A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2003;55(5):1177-1181.
137 Tishler RB, et al. Tolerance of cranial nerves of the cavernous sinus to radiosurgery. Int J Radiat Oncol Biol Phys. 1993;27(2):215-221.
138 Kreil W, et al. Long term experience of gamma knife radiosurgery for benign skull base meningiomas. J Neurol Neurosurg Psychiatry. 2005;76(10):1425-1430.
139 Pollock BE, et al. Stereotactic radiosurgery provides equivalent tumor control to Simpson Grade 1 resection for patients with small- to medium-size meningiomas. Int J Radiat Oncol Biol Phys. 2003;55(4):1000-1005.
140 Malik I, et al. The use of stereotactic radiosurgery in the management of meningiomas. Br J Neurosurg. 2005;19(1):13-20.
141 Kondziolka D, et al. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery. 2008;62(1):53-58.
142 Metellus P, et al. Evaluation of fractionated radiotherapy and gamma knife radiosurgery in cavernous sinus meningiomas: treatment strategy. Neurosurgery. 2005;57(5):873-886.
143 Huffmann BC, Reinacher PC, Gilsbach JM. Gamma knife surgery for atypical meningiomas. J Neurosurg. 2005;102(Suppl):283-286.
144 Kim DG, et al. Gamma knife surgery of superficially located meningioma. J Neurosurg. 2005;102(Suppl):255-258.
145 Roser F, et al. Proliferation potential of spinal meningiomas. Eur Spine J. 2005;15(2):211-215.
146 Leibel SA, et al. The treatment of meningiomas in childhood. Cancer. 1976;37(6):2709-2712.
147 Marosi C, et al. Meningioma. Crit Rev Oncol Hematol. 2008;67(2):153-171.
148 Yarbro JW. Mechanism of action of hydroxyurea. Semin Oncol. 1992;19(3 Suppl 9):1-10.
149 Newton HB, Scott SR, Volpi C. Hydroxyurea chemotherapy for meningiomas: enlarged cohort with extended follow-up. Br J Neurosurg. 2004;18(5):495-499.
150 Newton HB, Slivka MA, Stevens C. Hydroxyurea chemotherapy for unresectable or residual meningioma. J Neurooncol. 2000;49(2):165-170.
151 Rosenthal MA, Ashley DL, Cher L. Treatment of high risk or recurrent meningiomas with hydroxyurea. J Clin Neurosci. 2002;9(2):156-158.
152 Chamberlain MC, Tsao-Wei DD, Groshen S. Temozolomide for treatment-resistant recurrent meningioma. Neurology. 2004;62(7):1210-1212.
153 Younis GA, et al. Aggressive meningeal tumors: review of a series. J Neurosurg. 1995;82(1):17-27.
154 Stewart DJ, et al. Intra-arterial cisplatin plus intravenous doxorubicin for inoperable recurrent meningiomas. J Neurooncol. 1995;24(2):189-194.
155 Kyritsis AP. Chemotherapy for meningiomas. J Neurooncol. 1996;29(3):269-272.
156 Chamberlain MC. Adjuvant combined modality therapy for malignant meningiomas. J Neurosurg. 1996;84(5):733-736.
157 Sondak VK, Daud AI. Pharmacology of cancer biotherapeutics. In: DeVita VT, Laurence TS, Rosenberg SA, editors. Cancer: principals and practice of oncology. Philadelphia: Lippincott Williams & Wilkins; 2008:497-505.
158 Zhang ZJ, Muhr C, Wang JL. Interferon-alpha inhibits the DNA synthesis induced by PDGF and EGF in cultured meningioma cells. Anticancer Res. 1996;16(2):717-723.
159 Kaba SE, et al. The treatment of recurrent unresectable and malignant meningiomas with interferon alpha-2B. Neurosurgery. 1997;40(2):271-275.
160 Goodwin JW, et al. A phase II evaluation of tamoxifen in unresectable or refractory meningiomas: a Southwest Oncology Group study. J Neurooncol. 1993;15(1):75-77.
161 Jaaskelainen J, et al. Hormone treatment of meningiomas: lack of response to medroxyprogesterone acetate (MPA). A pilot study of five cases. Acta Neurochir (Wien). 1986;80(1–2):35-41.
162 Waelti ER, Markwalder TM. Endocrine manipulation of meningiomas with medroxyprogesterone acetate. Effect of MPA on growth of primary meningioma cells in monolayer tissue culture. Surg Neurol. 1989;31(2):96-100.
163 Grunberg SM, Weiss MH. Lack of efficacy of megestrol acetate in the treatment of unresectable meningioma. J Neurooncol. 1990;8(1):61-65.
164 Olson JJ, et al. Hormonal manipulation of meningiomas in vitro. J Neurosurg. 1986;65(1):99-107.
165 Olson JJ, et al. Effect of the antiprogesterone RU-38486 on meningioma implanted into nude mice. J Neurosurg. 1987;66(4):584-587.
166 Lamberts SW, et al. Mifepristone (RU 486) treatment of meningiomas. J Neurol Neurosurg Psychiatry. 1992;55(6):486-490.
167 Grunberg SM, Rankin C, Townsend J, Ahmadi J, Feun L, Fredericks R, et al. Phase III double-blind randomized placebo-controlled study of mifepristone (RU) for the treatment of unresectable meningioma. Proc Am Soc Clin Oncol. 2001;20:222.
168 Newfield RS, et al. Long-term mifepristone (RU486) therapy resulting in massive benign endometrial hyperplasia. Clin Endocrinol (Oxf). 2001;54(3):399-404.
169 Friend KE, Radinsky R, McCutcheon IE. Growth hormone receptor expression and function in meningiomas: effect of a specific receptor antagonist. J Neurosurg. 1999;91(1):93-99.
170 McCutcheon IE, et al. Antitumor activity of the growth hormone receptor antagonist pegvisomant against human meningiomas in nude mice. J Neurosurg. 2001;94(3):487-492.
171 Yin D, et al. Clinical pharmacodynamic effects of the growth hormone receptor antagonist pegvisomant: implications for cancer therapy. Clin Cancer Res. 2007;13(3):1000-1009.
172 Prat R, Banzo J, Diaz FJ. [Somatostatin receptors in meningioma: diagnostic and therapeutic value]. Rev Neurol. 1997;25(148):2002-2005.
173 Schulz S, et al. Immunohistochemical determination of five somatostatin receptors in meningioma reveals frequent overexpression of somatostatin receptor subtype sst2A. Clin Cancer Res. 2000;6(5):1865-1874.
174 Chamberlain MC, Glantz MJ, Fadul CE. Recurrent meningioma: salvage therapy with long-acting somatostatin analogue. Neurology. 2007;69(10):969-973.
175 Johnson M, Toms S. Mitogenic signal transduction pathways in meningiomas: novel targets for meningioma chemotherapy? J Neuropathol Exp Neurol. 2005;64(12):1029-1036.
176 Norden AD, Drappatz J, Wen PY. Targeted drug therapy for meningiomas. Neurosurg Focus. 2007;23(4):E12.
177 Ragel BT, et al. Chronic suppressive therapy with calcium channel antagonists for refractory meningiomas. Neurosurg Focus. 2007;23(4):E10.
178 Ragel BT, et al. Calcium channel antagonists augment hydroxyurea- and ru486-induced inhibition of meningioma growth in vivo and in vitro. Neurosurgery. 2006;59(5):1109-1120.
179 Jensen RL, Wurster RD. Calcium channel antagonists inhibit growth of subcutaneous xenograft meningiomas in nude mice. Surg Neurol. 2001;55(5):275-283.
180 Ragel BT, et al. Ubiquitous expression of cyclooxygenase-2 in meningiomas and decrease in cell growth following in vitro treatment with the inhibitor celecoxib: potential therapeutic application. J Neurosurg. 2005;103(3):508-517.
181 Ragel BT, et al. Celecoxib inhibits meningioma tumor growth in a mouse xenograft model. Cancer. 2007;109(3):588-597.
182 Ragel BT, Jensen RL, Couldwell WT. Inflammatory response and meningioma tumorigenesis and the effect of cyclooxygenase-2 inhibitors. Neurosurg Focus. 2007;23(4):E7.
183 Pfister C, et al. Are there attacking points in the eicosanoid cascade for chemotherapeutic options in benign meningiomas? Neurosurg Focus. 2007;23(4):E8.
184 Jaaskelainen J, et al. The growth rate of intracranial meningiomas and its relation to histology. An analysis of 43 patients. Surg Neurol. 1985;24(2):165-172.