67 Melanoma
Melanoma Epidemiology
In the United States, the melanoma incidence rate has risen faster than for any other cancer and is nearly double that of liver, lung, or prostate cancer. Between 1950 and 2000, the incidence of melanoma among Caucasians increased by 619%, an average of 4.3% per year, although the rate of increase has declined in the last decade, suggesting a potential positive impact of primary prevention campaigns.1 Rapid increases in melanoma incidence have been attributed to environmental risk factors and increased sun exposure behaviors, but controversy exists over whether these incidence trends are simply a result of expanded skin screening,2 biopsy rates,3 and reporting of low-risk, biologically indolent tumors to cancer registries.4 Persistent high proportions of thicker melanomas in U.S. Surveillance Epidemiology and End Results (SEER) data and worldwide would seem to mitigate the impact of improved screening and suggest the increased burden of melanoma is a global reality.5
Invasive cutaneous melanoma occured in an estimated 68,720 Americans in 2009 (39,080 men and 29,640 men); an additional 53,120 or more cases of melanoma in situ were diagnosed.6 While the median age of diagnosis is 53 years, melanoma is the most common cancer in women and second only to breast cancer in women in their early 30s.
Melanoma is currently the fifth most common cancer in men and the sixth in women, but it is not a major cause of death in either gender.6 Case-based fatality has declined sharply for melanoma over the past several decades as a result of earlier detection of thinner melanomas that lack or have lower biological potential to metastasize. Current 5-year survival rates exceed 90% in the United States, Australia, and Sweden and are uniformly higher in developed countries than developing countries, where relative survival may be as low as 40%.7,8
Although melanoma accounts for roughly 4% of all skin cancers, it is responsible for more than 75% of skin cancer deaths.6 The most striking differences in melanoma mortality occur in individuals aged 50 or older, who should be a primary target for early detection and screening efforts. Of the estimated 8650 deaths from melanoma in the United States in 2009, more than 64% occured in men (5550 men compared to 3100 women).9 Analysis of SEER data has demonstrated a disproportionate burden of melanoma deaths among middle-aged and older Caucasian men. Between 1973 and 2002, mortality rates rose 64% in U.S. Caucasian men aged 55 through 64 years and 130% (8.6 to 19.8 per 100,000) in men 65 or older.10 This is in contrast to middle-aged women of the same age group, who experienced markedly lower mortality increases (15%), and to both younger men and women (aged 20 to 54 years), who showed declining mortality rates over this period (11% and 23%, respectively).10
Etiology
The process by which normal melanocytes transform into melanoma cells is poorly understood but likely involves progressive genetic mutations that alter cell proliferation, differentiation, and death and impact cellular susceptibility to the carcinogenic effects of ultraviolet radiation.11 It has been estimated that 65% of melanomas in Caucasian populations worldwide is in some way attributable to sun exposure.12 The highest melanoma incidence rates worldwide are observed in fair-complexioned individuals residing in sunny climates, lending credence to the role of excessive ultraviolet radiation (UVR) in melanoma pathogenesis.
A complex relationship among environmental exposures, phenotypic characteristics, and genotypic traits is believed to predispose an individual to melanoma. Multiple risk factors have been identified, including both natural and artificial UVR, other extrinsic factors (occupational exposures, ionizing radiation, and potential dietary deficiencies), host immunosuppression, presence of atypical nevi, and genetic factors.1 Evidence-based analyses support the hypothesis that melanoma risk is affected primarily by intermittent, intense sun exposure, particularly in childhood or adolescence.13,14 In contrast, chronic occupational exposure does not appear to confer increased risk, except perhaps for the more UV-related melanoma subtypes (lentigo maligna and lentigo maligna melanoma).
Ultraviolet B radiation (UVB; wavelengths 290 to 320 nanometers) appears more closely linked with development of melanoma than ultraviolet A (UVA; wavelengths 320 to 400 nanometers).14 This is supported by statistically higher incidence of melanoma in equatorial regions where UVB radiation is most intense compared to latitudes farther from the equator. UVA intensity varies less across latitudes.15,16
Excessive exposure to UV radiation in childhood appears to be particularly important in that 25% and 80% of lifetime sun exposure is estimated to occur before age 20.17 Individuals with five or more severe sunburns in childhood or adolescence have an estimated twofold greater risk of developing melanoma.13 Furthermore, the incidence of melanoma is higher among people who move from northern to more equatorial latitudes, particularly if they were children at the time of relocation.18
Commercial tanning beds, which mainly emit UVA light, have been strongly associated with the development of skin cancer, including melanoma.19 Early studies revealed an elevated risk for melanoma with regular sunbed tanning after adjusting for additional risk factors, with higher risk for those who began sunbed tanning earlier in life.20 A subsequent systematic review by the International Agency for Research on Cancer of pooled data from 19 international studies demonstrated a 15% higher melanoma risk in both men and women who had ever used tanning beds (summary relative risk of 1.15; 95% CI, 1.00-1.31).21 More alarming was the finding of a 75% increased melanoma risk in individuals who first used tanning beds prior to age 35 (summary relative risk 1.75; 95% CI, 1.35-2.26).21 In the United States, adolescent access to indoor tanning is currently regulated in only 25 states, and where regulations exist, compliance is generally low. Of additional concern, businesses actively market their product to minors, which contributes in part to the estimated 35% regular use of indoor tanning facilities by adolescent girls.22 Recent reductions in melanoma incidence in younger individuals (age 20 to 44) may be offset by indoor tanning use in decades to come.
Exposure to oral methoxsalen (psoralen) and UVA radiation (PUVA) is associated with a delayed increase in the risk of melanoma, demonstrating both long latency period and dose-related melanoma risk. In one study, the incidence rate ratio for invasive or in situ melanomas was elevated 15 years following PUVA treatment, increasing to 5.4 by years 16 to 20 and to 9.3 beyond 20 years of follow-up.23 Individuals with a history of nonmelanoma (keratinocytic) skin cancer (basal cell and squamous cell carcinomas) have an estimated threefold increased melanoma risk, likely related to common etiologic factors such as excessive UVR and sun-sensitive phenotype.24,25
Exposure to occupational hazards has been examined in a number of studies focusing on polychlorinated biphenyls (PCBs), petroleum products, ionizing radiation, and selenium. Although initial analyses showed patterns of increased incidence, no statistically significant occupational risk factors were found after adjustment for known risk factors such as nevus count and sun exposure.26,27
The majority of studies addressing the role of dietary factors (e.g., antioxidants, retinoids, vitamin C, and vitamin E) have not shown a consistent effect on melanoma incidence. Case-control studies of diets rich in vitamin D and carotenoids and low in alcohol suggest an associated reduction in melanoma risk.28 Multiple studies addressing the role of oral contraceptives or hormone replacement therapy have found no evidence of increased risk for melanoma.29
Cutaneous melanoma occurs more commonly in immunosuppressed patients, such as organ transplant recipients, and immunosuppression appears to be related to worse prognosis (increased recurrence and death) in patients with higher-risk tumors. In patients with melanoma treated prior to transplantation, recurrences were estimated at 19% in one series, and usually within 5 years.30 Waiting at least 5 years between the treatment of thicker melanomas and transplantation may reduce the risk of recurrent disease resulting from transplant-associated immunosuppression.31 It remains controversial whether an increased incidence of melanoma occurs in HIV-infected populations, although recent studies have suggested an increased risk of melanoma in immunosuppressed cohorts.32
The presence of clinical atypical nevi (CAN), also termed dysplastic nevi, is the most important clinical risk factor for melanoma. Compared with the general population, patients with CAN have a 3- to 20-fold elevated risk of developing malignant melanoma, and risk increases with the number of CAN present and for those who also have a personal or family history of melanoma.33,34 Importantly, only about one-third of all melanomas are estimated to arise from nevus precursors, including congenital, common, and dysplastic nevi, and the estimated percentage of melanomas that arise within atypical nevi is in the range of 10% to 30%.35,36 Since CAN are best considered as risk markers for melanoma rather than precursor lesions, their widespread removal is generally not indicated. However, patients with CAN constitute one of the highest risk groups for melanoma and should receive routine dermatologic surveillance and education regarding melanoma warning signs as well as importance of regular skin self-examination to enhance early detection.
Studies suggesting that high levels of sun exposure are inversely associated with death from melanoma seem to corroborate the novel concept of at least two pathways of melanoma pathogenesis.37 This theory asserts that melanomas in chronically sun-exposed skin (i.e., head and neck) may be more prevalent in older patients with higher cumulative doses of sun exposure and low nevus count, whereas intermittent UV exposure may be more closely linked to truncal melanoma and high nevus count in younger patients.38
This dichotomy in mole phenotype, melanoma site, age of onset, and UVR pattern is supported on a molecular level as well. Recent data have shown that differences in frequency of BRAF or NRAS mutations are also related to patterns of sun exposure, with BRAF mutations more common in intermittently UV-exposed skin compared with chronically sun-damaged skin or relatively unexposed skin, such as acral or mucosal sites, which demonstrate KIT mutations more frequently.39 Further elucidation of this etiologic, phenotypic, and genetic heterogeneity will not only improve the public health messages for melanoma prevention but also result in more effective targeted therapies.
Genetics
The majority of melanomas are not hereditary in nature, and only 5% to 10% estimated to be due to a familial predisposition.40 Germline mutations in the cyclin-dependent kinase inhibitor 2A (CDKN2A/p16)/cyclin-dependent kinase 4 (CDK4) cell-cycle and tumor-suppressor gene axis and in the melanocortin-1 receptor (MCR1) pigmentation-associated gene have been associated with melanoma pathogenesis, as have somatic mutations in the PTEN and BRAF genes.41,42 Melanoma in association with other tumors, including pancreatic, breast, and brain tumors, may be associated with germline mutations in CDKN2A or potentially BRCA2.43 Additional melanoma risk occurs in CDKN2A mutation carriers who express variants of the MCR1 gene, which is associated with red hair, fair skin, and freckling.44
About 40% of hereditary melanoma is attributed to germline mutations in CDKNA/p16, making it the most important melanoma susceptibility gene identified to date.45 Commercial availability for CDKN2A/p16 genetic testing has heightened the need to develop evidence-based guidelines for genetic assessment in appropriate individuals. While a positive or negative p16 mutation result does not often change the need for ongoing dermatologic surveillance in patients with strong family or personal history of melanoma and/or atypical mole phenotype, it may affect the need for surveillance for pancreatic cancer in individuals with familial predisposition to develop this cancer.43
Pathology
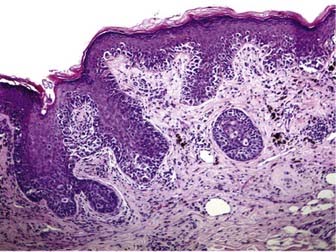
Melanoma in situ is characterized histologically by a poorly circumscribed proliferation of markedly atypical melanocytes, which may be nested or solitary along the basal layer of the epidermis, and which may or may not exhibit pagetoid upward scatter, depending on histologic subtype.46 Superficial spreading melanoma has an in situ (radial growth) phase characterized by increased numbers of intraepithelial melanocytes, which are large and atypical, arranged haphazardly at the dermoepidermal junction, and show upward (pagetoid) migration (Fig. 67-1). Melanomas in situ of the lentigo maligna and acral lentiginous subtypes demonstrate predominant “lentiginous” growth at the dermoepidermal junction (e.g., solitary atypical melanocytes at the epidermal basal layer) and less intraepidermal pagetoid scatter of cells, although lentigo maligna may exhibit nested aggregates of melanocytes and histologic features similar to those observed in dysplastic nevi.47 Lateral intraepidermal extension of melanoma cells occurs in all subtypes except nodular melanoma, which exhibits a prominent dermal nodular aggregate of atypical melanocytes and a relatively small in situ component, typically limited to the area directly over or just beyond the invasive dermal component.46
Dermal invasion of cutaneous melanoma confers metastatic potential, with the greatest risk believed to occur in the setting of a vertical growth (tumorigenic) phase.48,49 Tumorigenicity is characterized by a distinct population of melanoma cells with evidence of proliferation (mitoses, MIB-1 staining) and nuclear pleomorphism within the dermis and possibly involving the subcutaneous fat. Failure of melanocyte maturation and dispersion as the tumor extends downward into the dermis is characteristic of invasive melanoma (Fig. 67-2).
Tumor thickness, as defined by the Breslow depth, is the most important histologic determinant of prognosis and is measured vertically in millimeters from the top of the granular layer (or base of superficial ulceration) to the deepest point of tumor involvement. Increased tumor thickness confers a higher metastatic potential and a poorer prognosis.50,51 Analysis of the American Joint Committee on Cancer (AJCC) collaborative melanoma database for both the 6th and 7th edition of the AJCC Cancer Staging Manual melanoma staging guidelines (published in 2001 and 2009, respectively) showed that histologic ulceration of the primary tumor is the next most important determinant of patient prognosis, and its presence upstages individuals to the next more advanced AJCC stage.52,53 The most recent worldwide analysis of the AJCC melanoma staging database also confirmed that elevated mitotic rate (≥1 mitosis/mm2) confers worse overall survival, and it will now be used to classify thin melanomas (≤1 mm) as T1b. While not incorporated into current 2010 AJCC microstaging for melanoma, lymphovascular invasion of the primary tumor has also been reported to have both prognostic value and potential correlation with sentinel lymph node positivity.54
Clinical Presentation
Primary cutaneous melanoma may occur anywhere on the body, although in the United States, it is most commonly diagnosed on the lower extremities and back in women and the trunk in men.55 Certain melanoma subtypes, such as lentigo maligna melanoma and acral lentiginous melanoma, occur in characteristic locations as discussed later. In addition to clinical warning signs such as asymmetry, border irregularity, color variegation, and diameter larger than 6 mm (the “ABCD” criteria), surface features such as elevation and ulceration may be useful in predicting whether melanoma is early or advanced.56 Recognition of evolving (E) features of a skin lesion is an additional criterion to assist in early detection.57
Histogenetic Subtypes
Controversy persists regarding the validity of subtyping melanoma, but recent evidence of divergent pathways of melanomagenesis according to anatomic location, sun exposure, nevus count, age of onset, and various mutations (e.g., BRAF, KIT) suggest that distinct phenotypic and genotypic patterns of melanoma exist.38,39 Most multivariate analysis have shown that histogenetic type is not an independent prognostic variable for survival after controlling for tumor thickness; however, there is little doubt that the nodular subtype accounts for most newly diagnosed deep primaries, and that it is a subtype that may elude early detection.5 With the exception of nodular melanoma, all growth patterns are characterized by a preceding in situ (intraepithelial) phase that lacks the biological potential to metastasize and may last from months to years before invasion occurs. As such, melanoma in situ is completely cured following excisional surgery.51
Superficial Spreading Melanoma
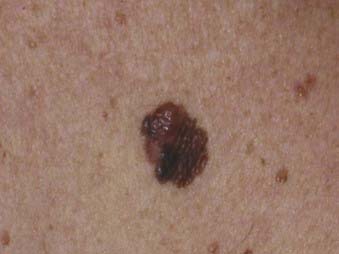
Superficial spreading melanoma is the most common subtype of melanoma, accounting for about 70% of all cases, particularly between the ages of 30 and 50.58 It occurs most frequently on the upper back of men and women as well as the lower extremities of women. The clinical lesion typically shows irregular, asymmetric borders with color variegation (e.g., black, blue, or pink), and its size is generally larger than 6 to 8 mm (Fig. 67-3). Superficial spreading melanoma characteristically exhibits some or all of the ABCD criteria, and widespread use of these clinical warning signs over the past 2 decades is believed to have contributed to significantly thinner melanomas of this subtype being diagnosed.5
Nodular Melanoma
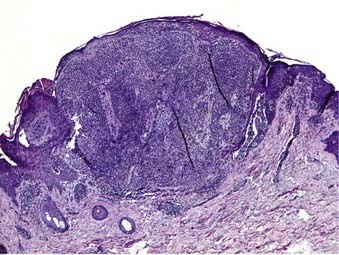
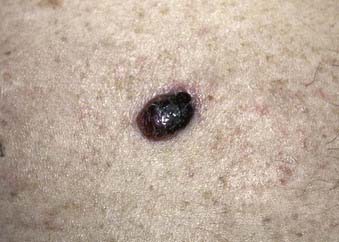
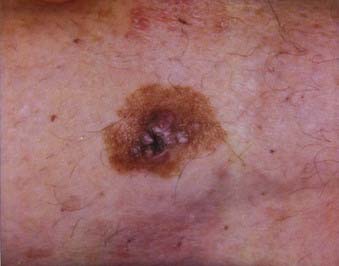
Nodular melanoma is the second most common subtype of melanoma, accounting for 15% to 30% of all types and is more common in men than women.58 The median age of diagnosis is 53 years; however, thicker nodular melanomas are associated with older age.5 Like superficial spreading melanoma, legs and trunk are the most frequent sites of involvement. Clinically, the lesion presents as a raised, dark brown to black papule or nodule, and ulceration and bleeding are common (Fig. 67-4). Rapid growth over weeks to months is a hallmark of nodular melanoma, typically resulting in greater thickness at diagnosis compared to other melanoma subtypes. Despite its seemingly more aggressive clinical behavior, nodular melanoma has a prognosis similar to superficial spreading melanoma when matched for tumor thickness.59
Lentigo Maligna Melanoma
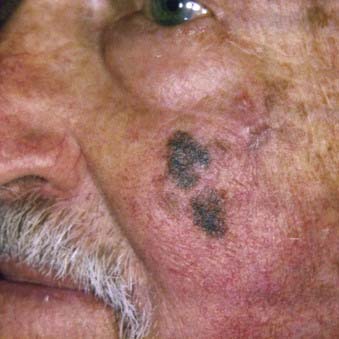
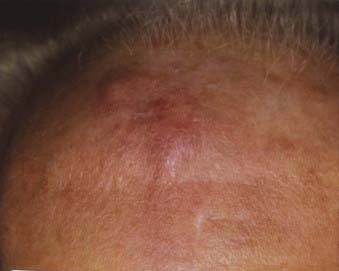
Lentigo maligna melanoma accounts for 4% to 15% of cutaneous melanoma and is typically located on the head, neck, and arms (sun damaged skin) of elderly, fair-skinned individuals (mean age 65).58 Recent characterization of melanoma subtype incidence has suggested increasing rates of both in situ and invasive lentigo maligna subtypes, particularly in individuals older than 50.60 Like nonmelanoma (keratinocytic) skin cancers (e.g., basal cell and squamous cell carcinomas), lentigo maligna melanoma is linked to cumulative rather than intermittent sun exposure. The face is the most common site of involvement, particularly the nose and cheeks. The precursor in situ lesion, lentigo maligna, is usually present for over 5 to 20 years and often attains large size (>1 to 3 cm diameter) before progression to lentigo maligna melanoma occurs. Lentigo maligna appears as a tan to brown macule or patch with variation in pigment or areas of regression that appear hypopigmented (Fig. 67-5). Only 5% to 8% of lentigo maligna are estimated to evolve to invasive melanoma, and this event is characterized by nodule development within the flat precursor lesion (Fig. 67-6).61
Acral Lentiginous Melanoma
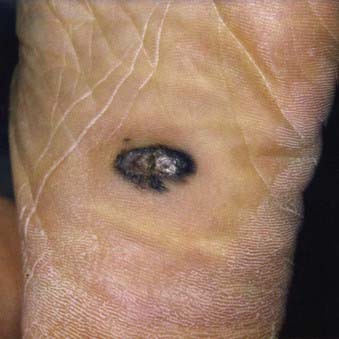
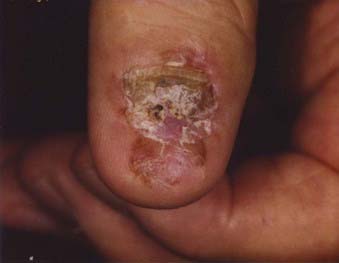
Acral lentiginous melanoma is the least common subtype in the United States, representing only 2% to 8% of melanoma in Caucasians, although it accounts for 29% to 72% of melanoma in dark-complexioned individuals (African Americans, Asians, and Hispanics).58,62 It typically occurs on the palms or soles or beneath the nail plate (subungual variant). A small percentage of superficial spreading and nodular melanoma may also be located acrally. Patients are generally middle-aged to elderly, with an average onset in the sixth decade. Irregular pigmentation, large size (≥3 cm diameter), and plantar location are characteristic features of acral lentiginous melanoma (Fig. 67-7). Subungual melanoma occurs most commonly on the great toe or thumb and is characterized by rapid onset of diffuse nail discoloration or a longitudinal pigmented band within the nail plate, although nail dystrophy may also occur. The additional presence of pigmentation extending into the proximal or lateral nail folds (Hutchinson’s sign) strongly suggests subungual melanoma and warrants biopsy of the nail matrix from which these melanomas arise (Fig. 67-8). Subungual melanoma may be confused with a benign junctional nevus, pyogenic granuloma, infectious process (bacterial or fungal), or subungual hematoma. If subungual hematoma is suspected, a history of trauma should be elicited and the lesion followed to ensure resolution with continued growth of the nail plate.
Rare Melanoma Variants
Unusual subtypes of primary melanoma include desmoplastic/neurotropic melanoma, mucosal (lentiginous) melanoma, malignant blue nevus, melanoma arising in giant congenital nevus, and melanoma of soft parts (clear cell sarcoma). Together, these variants account for less than 5% of primary melanomas.58 Desmoplastic melanoma may occur in association with macular, lentigo maligna-type pigmentation or present de novo as a firm, amelanotic nodule or scar (Fig. 67-9). Desmoplastic melanoma predominantly occurs on sun-exposed areas of the head and neck, with a mean age between 60 and 65 years.63 Lack of pigmentation and clinical features more suggestive of keratinocytic skin cancer may result in delay in detection and thicker tumors at diagnosis. Desmoplastic melanoma frequently exhibits perineural extension and has a predilection for local recurrence, somewhat analogous to a skin sarcoma. Wide excisional margins (≥2 cm) and adjuvant radiation therapy are frequently recommended for improved local control of this uncommon melanoma subtype.
Diagnostic and Staging Studies
The most important aspects of the initial workup for patients diagnosed with primary cutaneous melanoma are a careful history, review of systems, and physical examination.64 Published data have shown that baseline and surveillance laboratory studies (e.g., lactate dehydrogenase [LDH] level, liver function tests), chest radiography (CXR), and other imaging studies (computed tomography [CT], positron emission tomography [PET], bone scintigraphy, magnetic resonance imaging [MRI]) are not typically beneficial for stage I/II (cutaneous) melanoma patients who have no signs or symptoms of metastasis.65–68
National Comprehensive Cancer Network (NCCN) practice guidelines support the concept that most melanoma recurrences are diagnosed clinically. Current recommendations advise against the practice of obtaining baseline studies in patients with asymptomatic cutaneous melanoma, including melanoma in situ (stage 0) and stages I and II invasive primary melanoma. The 2009 guidelines emphasize that imaging (CT scan, PET, MRI) should be obtained to evaluate specific signs or symptoms suspicious for disease recurrence. Routine surveillance laboratory tests and radiologic imaging to screen for asymptomatic metastasis are not recommended for stage IA to stage IIA melanoma (invasive primaries ≤4 mm depth). However, surveillance CXR, CT and/or PET/CT may be considered to screen for metastasis in asymptomatic patients with stage IIB (>4 mm) to stage IV disease, although there is not uniform consensus of the NCCN melanoma panel for this recommendation (category 2B).69
While abnormal laboratory tests are rarely the sole indicator of metastatic disease, elevated serum LDH levels are associated with worse survival in patients with stage IV (distant) disease and have thus been incorporated since 2002 into the AJCC melanoma staging guidelines.70 Serum S-100 protein levels may also be useful as a tumor marker in patients with metastatic disease, but this practice is not widely employed in the United States.71
Sentinel lymph node biopsy (SLNB) is typically performed for pathologic staging of the regional nodal basin(s) for primary tumors 1 mm depth or more and may be considered in thinner melanomas when certain high-risk histologic features (e.g., ulceration, high mitotic rate, lymphovascular invasion) are present.69 A metastatic workup (CT, PET or PET/CT, brain MRI) should be initiated if physical findings or symptoms suggest disease recurrence, or if the patient has documented sentinel lymph node metastasis, although the low tumor burden in the setting of microscopic regional nodal metastasis is generally not associated with synchronous detection of visceral metastasis.
Imaging Studies
Primary Cutaneous Melanoma
As previously noted, imaging studies such as CT scans, MRI, PET, ultrasonography, and bone scans have an extremely low yield in asymptomatic patients with primary cutaneous melanoma (AJCC stages I and II) and are generally not indicated. However, maintaining a low threshold for obtaining symptom-directed tests is important for melanoma surveillance. Baseline metastatic staging for melanoma patients with primary tumors greater than 4 mm in depth may include CXR but remains optional in the absence of signs or symptoms of metastatic disease.69
Sentinel node status (positive or negative) is the most important prognostic factor for recurrence and is the most powerful predictor of survival in patients with melanoma.72 Current AJCC melanoma staging and NCCN clinical practice guidelines advocate pathologic staging of the regional lymph nodes for cutaneous melanoma of greater than 1 mm depth (T1b to T4b and for thinner (T1a) melanomas with high-risk histologic features, along with microstaging of the primary melanoma, as the most complete means of staging.69,73
Metastatic Melanoma
Melanoma surveillance for stage III (regional nodal) or stage IV (distant) disease often involves imaging with CT, PET, combined PET/CT, and/or MRI and is generally indicated for determining the extent of disease in patients with recurrent melanoma to guide management and assess whether surgical resection is appropriate. Whole body PET has shown increased sensitivity, specificity, and accuracy for detection of metastasis compared with CT (chest, abdomen, and pelvis), although combined PET/CT provides vital anatomic information not available with PET alone.74,75 As with cutaneous melanoma, NCCN guidelines promote the use of more extensive imaging studies to follow up suspicious signs and symptoms, but surveillance CT scans for asymptomatic patients with stage IIB and IIC high-risk primaries or stage III/IV melanoma may be considered at the clinician’s discretion.69
Melanoma Staging System
The AJCC staging system for melanoma underwent a major modification in 2002, following an evidence-based review of prognostic information collected on 17,600 melanoma patients from 13 international cooperative groups and major cancer centers.52,70 This analysis identified novel factors for incorporation into the staging classification of melanoma for the T, N, and M categories. As indicated previously, in the T category, primary tumor thickness and ulceration were found to be the most important prognostic factors of those analyzed by the AJCC staging committee in patients with localized, cutaneous melanoma. As a result, these two factors have been incorporated into the T classification, with tumor thickness being the predominant stratification factor, with cutoffs of 1.0 mm, 2.0 mm, and 4.0 mm. Thus, the T1 category includes melanomas 1.0 mm thick or less; T2, 1.01 to 2.0 mm; T3, 2.01 to 4.0 mm; and T4 greater than 4.0 mm thick. Each T category is further classified by the absence (a) or presence (b) of ulceration, which denotes a histologic loss of epidermal continuity overlying the primary melanoma. In the prognostic analysis presented, the presence of ulceration within a given tumor thickness stratum upstaged the tumor to the next stratum without ulceration.
The 2010 version of the AJCC staging classification has largely abandoned the use of the Clark level, which measures the anatomic depth of penetration into the skin by the melanoma and was incorporated into the prior version of the staging classification.54 In 2002, Clark level was retained only in thin, T1 tumors in which the presence of either ulceration or a deep tumor (i.e., Clark level IV or V) would upstage the tumor to T1b. Effective 2010, the AJCC melanoma staging system will instead incorporate mitotic rate 1/mm2 or more to upstage T1a to T1b tumors. The T classification described five substages with worsening survival based on tumor thickness and histologic ulceration: stage IA, with an estimated 97% 5-year survival; stage IB, with 91% to 94% 5-year survival; stage IIA, with 79% to 82% 5-year survival; stage IIB, with 68% to 71% 5-year survival; and stage IIC, with estimated 53% 5-year survival.
In the nodal (N) category, the 2002 AJCC system defined nodal involvement as presence of metastatic melanoma within the lymph node on routine hematoxylin and eosin staining.70 Thus, patients with only immunohistochemical positive staining of the lymph node or those with positive detection of tumor markers using quantitative polymerase chain reaction were not included in the node-positive category. However, the 2009 iteration will now include sentinel lymph node metastasis detected by immunohistochemical staining alone.53
Finally, the revised staging classification incorporated patients with satellite and in-transit metastases into stage III (N2c), given that the survival of such patients was similar to those with nodal involvement. Patients with satellite/in-transit metastases and concomitant nodal involvement were upstaged to stage IIIC. The staging classification divided stage III into three substages with worsening survival based on number of involved nodes and histologic ulceration of the primary melanoma: IIIA, with a 5-year survival of approximately 78%, IIIB, with a 5-year survival of approximately 59%, and stage IIIC, with an estimated 5-year survival of 40%.53
Stage IV melanoma includes patients with distant metastasis and is associated with a poor prognosis, with a median survival under 12 months. The metastasis (M) category in the current classification encompasses two primary factors: location of the metastasis and serum LDH levels. The AJCC analysis was unable to identify differential survival in any subgroups of patients with distant metastasis, but the M classification was stratified into three subgroups: M1a, characterized by distant soft-tissue metastases only (with a normal LDH); M1b, limited to patients with lung metastases only (with a normal LDH); and M1c, including patients with any other visceral organ metastasis or elevated LDH.70 These substages have been validated through an updated analysis of patients with stage IV melanoma and are maintained in the 2010 AJCC version.53
It is important to note that numerous other histologic prognostic factors have been described that may be useful in identifying subsets of patients at higher risk of relapse or death.76 As noted earlier, factors such as mitotic index and lymphovascular invasion have also been shown to have an independent impact on survival, with an impact in certain studies that may be greater than that of ulceration. Other histologic factors that may be useful in certain settings include microsatellites, tumor vascularity, lymphatic invasion, regression, and tumor-infiltrating lymphocytes.
Standard Therapeutic Approaches for Melanoma
Surgical Techniques for Staging and Treatment
Wide Local Excision
Wide local excision is the primary treatment for cutaneous melanoma. Several studies have looked at what constitutes an adequate margin. For in situ melanoma, a margin of 0.5 cm is indicated, although wider margins may be necessary to achieve histologic clearance of larger melanomas in situ of the lentigo maligna type. For melanomas with a depth of 1 mm or less, 1-cm margins are indicated. For melanomas with a depth 1.01 to 2.0 cm, a margin of either 1 cm or 2 cm is acceptable. For melanomas with a depth of more than 2.0 cm, a margin of 2 cm is indicated. Randomized controlled trial data do not support wider margins for local control of thicker cutaneous melanomas. For invasive primary melanoma, excision should extend to but not include the deep fascia.69
Clinical margins may be difficult to determine with melanoma in situ of the lentigo maligna subtype. Often there is subclinical extension of atypical melanocytes. Different techniques in biopsy and margin control have been proposed to address this issue. These include techniques for staged excision and Mohs micrographic surgery.77–79 Some advocate using topical imiquimod 5% cream to treat these in situ melanomas with indistinct margins in patients who are not surgical candidates, but this therapy has not been subjected to a randomized controlled trial.80
Sentinel Lymph Node Biopsy
Sentinel lymph node biopsy was first introduced for staging of melanoma in the 1990s.81 It is now considered a standard staging procedure for cutaneous melanoma worldwide. Pathologic staging of the regional nodes is recommended for clinically node-negative patients with T1b to T4b melanomas by both the AJCC and the NCCN, although its impact on overall survival remains unclear. The SLNB is based on the theory that a given area of skin will initially drain via afferent cutaneous lymphatics to a specific lymph node or nodes, which can then be examined to determine occult regional spread of the melanoma, without the need to remove other nodes. By reducing the amount of nodal tissue to study, the SLNB allows closer examination of the tissue and greater diagnostic accuracy. It also reduces the morbidity associated with ELND.
Current indications for SLNB are patients with clinically negative (nonpalpable) regional lymph node and melanoma with a Breslow depth 1 mm or greater.69 In addition, patients with thinner melanomas (T1a) with adverse histology may be considered for SLNB. Individuals with melanoma 0.76 to 1.0 mm depth and increased mitotic rate greater than 1/mm2 (T1b per 2010 AJCC staging criteria) may demonstrate a higher risk for SLN positivity. Other histologic features such as extensive regression and lymphovascular invasion are still being investigated regarding their predictive value for SLN positivity. Clark’s level IV/V in T1 melanomas is no longer considered an indication for SLNB.
In 10% to 15% of cases, more than one nodal basin may be involved on lymphoscintigraphy.82 In addition, one or more sentinel nodes may be present in a nodal basin.83,84 Axillary and inguinal basins average slightly more than 1 sentinel node per basin, and neck basins average more than 2 sentinel nodes per basin.85,86
After removal of the sentinel node, the background radioactivity of the basin is assessed with the gamma probe. If there is still elevated radioactivity, a search for further sentinel node(s) is performed. Generally, a further search for nodes should be performed if the ratio of the resection bed to the background radioactivity remains higher than 3 : 1. A sentinel node is defined as a node with a 10 : 1 ratio of ex vivo counts compared to the resection bed.87
Completion Lymphadenectomy
If the sentinel node is positive, a complete lymph node dissection (CLND) is generally recommended. In up to 20% of cases, additional positive (nonsentinel) lymph nodes are identified.88 Lymphadenectomy is also performed when patients present with clinically palpable (macroscopic) nodes and when patients with initial negative SLNB develop clinically apparent nodes, which is generally classified as a false-negative SLNB. Axillary dissection includes level I to III nodes. Inguinal dissection includes deep nodes when there is involvement of 3 or more superficial nodes, Cloquet’s node, or iliac or obturator nodes on pelvic CT.
Treatment of Metastatic Melanoma
Effective therapy of metastatic melanoma remains elusive. Not since interleukin-2 was approved by the U.S. Food and Drug Administration (FDA) in 199889 have any new drugs been approved for treatment of advanced disease. In the current AJCC staging system, 5-year survival continues to be dismal at less than 18%.53,70,90 It is worth noting that the stage IV category can be broken down into three groups, M1a to c, with groups M1a (skin and lymph node) and M1b (lung) surviving longer than M1c (visceral disease, with or without LDH elevation).
Treatment of metastatic disease can be divided broadly into three categories: chemotherapy, immunotherapy, and targeted therapies. The following discussion is most relevant to cutaneous metastatic melanoma, although it can be applied to mucosal melanomas as well.91,92 Uveal melanomas are generally thought of as more refractory to the standard cutaneous melanoma therapies and are known to have different biology.93–95 Data for application of these therapies to both mucosal and uveal melanomas are scarce.
Dacarbazine (DTIC) is the only FDA-approved chemotherapy agent for melanoma, but there are other active agents.96–98 Dacarbazine remains the standard chemotherapeutic agent for comparison of newer regimens in melanoma, with a response rate that averages around 10% to 15%, although responses tend to be partial, short-lived, and limited to skin, soft-tissue, lymph node, and lung metastases. Combination chemotherapy regimens have been utilized, but survival in the studies to date show no superiority over single-agent DTIC.99 Despite a lack of alteration in survival, combination regimens such as Dartmouth (DTIC, cisplatinum, BCNU, and tamoxifen) do show increased response rate. It is worth noting that the trial comparing DTIC and the Dartmouth regimen did use a lower dose of tamoxifen than in earlier versions of this regimen. Temozolomide,100 an analog of dacarbazine, has been widely used in metastatic melanoma because of the ease of oral administration and the potential antitumor effects in the central nervous system (CNS).100,101 Temozolomide still awaits FDA approval for melanoma. Taxane-based regimens continue to show activity, and Abraxane is now also being studied for treatment of melanoma, although no published data regarding its efficacy are available.102,103 The combination of taxanes with carboplatinum is emerging as an alternative chemotherapeutic regimen for stage IV melanoma, with a reproducible progression-free survival exceeding 4 months (PRISM, BEAM trials).
In terms of immunotherapy for stage IV melanoma, interleukin-2 is the only FDA-approved agent based on long-term, durable survival of a small proportion of patients, with an overall response rate of 16%.89 This regimen involves a specific protocol known as high-dose bolus interleukin-2 and is generally offered to younger patients who can tolerate its associated severe toxicity, although smaller volume of metastatic disease is believed to be more predictive of response than performance status.89 Because of the serious side-effect profile of interleukin-2, it is generally only administered at centers where the physicians and staff have a great deal of experience with its use. The serious nature of the cardiovascular effects of the drug requires cardiac stress testing and selective pulmonary function testing prior to treatment, making this therapy applicable to only a small subset of patients. Management guidelines of the capillary leak syndrome induced by interleukin-2 are available.104
The success of interleukin-2 led investigators to design a combined regimen. Although there is variability in the combinations employed, they are generally classified under the umbrella of biochemotherapy.105 In the United States, the most common combination includes cisplatinum, vinblastine, temozolomide or DTIC, interferon alpha-2b, and a much lower dose of interleukin-2 than the FDA-approved regimen. Although initial single-center results were very exciting, showing responses in the range of 50%, Eastern Cooperative Oncology Group (ECOG) 3695, a multicenter randomized intergroup trial, failed to show a survival benefit.106 A European Organisation for Research and Treatment of Cancer (EORTC) trial was also negative.107 A single-center trial at M.D. Anderson108 has shown survival benefit. Recently both M.D. Anderson and California Pacific Medical Center have reviewed their non-randomized results and shown similar long-term survival to high-dose bolus interleukin-2.109,110 Based on ECOG 3695 data, the use of biochemotherapy has become controversial, and many centers have stopped using it. Nevertheless, biochemotherapy may still have a role in cytoreduction for patients with large tumor volumes and rapidly growing or symptomatic disease. All of the trials demonstrate that the response rate is higher, and there may be progression-free survival benefit.
Vaccine therapy has yet to show any efficacy, although numerous randomized trials have been performed.111,112 Despite these results, vaccine studies continue with the hope of improvements with new research applications in immunology.111,113–116
Anti-CTLA4 antibodies have shown promise as an immunotherapy, and two different antibodies are currently undergoing extensive testing.117–120 Overall response rates remain low and comparable to interleukin-2 and DTIC, but the optimal schedule and assessment of anti-CTLA4 strategies have yet to be determined.120–122 Problems encountered with immunotherapy assessment include the observation of delayed responses. Traditional imaging assessment is based on chemotherapy regimens where imaging is done at 5 to 6 weeks after the beginning of chemotherapy. Anti-CTLA4 has been noted to show responses long after this time period.120,122 Some patients who respond are showing long-term control of the disease.123 One small phase III trial is negative for survival but has yet to be published. Anti-CTLA4 is also being studied in brain metastasis, along with agents such as temozolomide. Side effects are mainly autoimmune in nature, including colitis. As new immunotherapy agents continue to be tested, there will likely be an attempt to combine agents proven to be safe and effective.
Promising immune therapies are on the horizon, and some involve intensive conditioning regimens. Recently Dudley and Rosenberg124 and colleagues published very impressive results with an intensive conditioning-based immunotherapy program based on adoptive gene transfer. Generalizability of these results remains to be proven. Hunder and colleagues have explored similar strategies and also published on T-cell immunotherapy.125
Numerous reviews are available on new areas of melanoma research and include targeted therapies126–128 and stem cells.129 Targeted therapies127,130 are now involved in many randomized and earlier-phase trials. Thus far, results have been disappointing, with phase III trials not demonstrating survival benefit. Agents showing negative survival in phase III trials include sorafenib131 and oblimerson.132 Imatinib has shown promise in selected cases of metastatic disease (generally acral or mucosal in origin) expressing activating KIT mutations133–135 and is currently under investigation for subsets of melanoma patients. Owing to advances in the understanding of melanoma pathogenesis and lack of efficacy of standard therapies, numerous investigational agents are being assessed. Other promising agents include bevacizumab,136 synta 41813, and dasatinib. Although results have been disappointing overall, some preliminary results are inspiring cautious optimism.
Given the high proportion of BRAF mutations in melanoma, the development of selective small-molecule inhibitors of mutant BRAF have generated intense interest recently. One such molecule, PLX4032, has shown impressive activity in a recent phase I trial in patients with BRAF-mutant metastatic melanoma.137 Further trials of this promising agent and other selective BRAF inhibitors are clearly warranted and currently underway.
Adjuvant therapies are limited for resected metastatic melanoma, and only interferon alfa has shown efficacy.138,139 The main benefit appears to be limited to relapse-free survival, although a recent meta-analysis published in abstract form does show a small survival benefit.140 The FDA-approved regimen is for high-dose interferon administered for 1 year; patients at high risk for relapse (stage IIC and III) are generally considered the best candidates for this intensive regimen. The first month involves 20 million units/m2 daily for 5 days every week. The remaining 11 months require injection of 10 million units/m2 3 times per week. Some preliminary data suggest the first month of the regimen may be enough for this benefit. GM-CSF has been evaluated in a promising single-arm study, and a randomized, placebo-controlled intergroup trial assessing its use with and without concomitant peptide vaccine is currently being analyzed (ECOG 4697). Based on this, the standard adjuvant regimen remains high-dose interferon alpha-2b. Numerous reviews detail safe administration of this drug, which is associated with significant adverse side effects throughout its year-long course.141,142
Treatment of brain metastasis continues to be a challenging problem and is one of the major causes of failure of systemic therapies.143,144 Currently, radiation therapy is considered to be the primary modality for treatment of brain metastases. It appears that highly selected patients may benefit from stereotactic radiosurgery to specific lesions.101 Two widely used devices include the Gamma-knife101 and Cyberknife.145 Selective use of whole brain radiotherapy is useful but results are not generally dramatic. Other than a few successful anecdotal reports with temozolomide and anti-CTLA4 antibodies, there is little to offer from a systemic standpoint. Imatinib146 and anti-CTLA4 antibodies are being specifically studied in this population with CNS metastasis. Treatment standardization is clearly needed, as are new agents and strategies.145,147
Radiotherapy in Melanoma
Radiotherapy at the present time has a relatively limited role in primary melanoma management except in a few selected sites such as choroidal melanoma (see Chapter 64), lentigo maligna of the face, and for postoperative treatment of patients treated surgically for advanced nodal disease in the neck and axilla. It has a major role in treatment of brain metastases from melanoma. Radiotherapy also has a place in the palliative treatment of skin and nodal metastases, particularly in conjunction with hyperthermia148,149 (see Chapter 74).
Techniques of Radiotherapy
Simulation Details
Portals for postoperative treatment in the head, neck, and axilla are as follows. For primaries in the head and neck, the port should include the pre- and postauricular node basins, the cervical node basins, and the supraclavicular area. Treatment of the axillary nodes alone was compared to extended fields including the supraclavicular area, and extending fields beyond the immediate draining groups does not seem to confer any advantage.150
Dose Fractionation
For the past 20 years, fraction size has been a major radiobiological question in the treatment of melanoma. In a classic paper, Bentzen et al. derived the α/β ratio for melanoma from clinical data. The value was low, similar to late effects in normal tissues at 0.57 Gy (95% CL, 1.07-2.5).151 This was consistent with values derived from some cell lines in culture. For more discussion of the biology of fractionation see Chapter 3.
Based on radiobiological data, the RTOG (Radiation Therapy Oncology Group) conducted a trial reported in 1991.152 A group of 137 patients with measurable lesions was assigned to two hypofractionated arms, either 8 Gy × 4 or 2.5 Gy × 20. The complete response rate was 24.2% for the 8-Gy arm and 23.4 % for the 2.5-Gy arm. The general approach to melanoma treatment has evolved, with hypofractionation using 6-Gy doses per fraction.153
Definitive Treatment
Currently, radiotherapy is indicated as initial treatment only for selected lentigo maligna lesions of the face where cosmetic or functional factors preclude surgery, although topical imiquimod cream has been more recently used as first-line treatment for nonsurgical in situ cases.154 Harwood reported on 51 patients with lentigo maligna and lentigo maligna melanoma treated with fractionated radiotherapy.155 Among lentigo maligna patients, 10/23 were controlled, and among lentigo maligna melanoma patients, 23/29. Orthovoltage x-rays were used. Doses for lesions less than 3 cm were 35 Gy in 5 fractions, for lesions 3 to 5 cm, 45 Gy in 10 fractions, and for larger lesions, 50 Gy in 15 to 20 fractions. This remains an option for patients who fail imiquimod therapy.
Adjuvant Radiotherapy
Beginning in 1983, the M.D. Anderson group began a trial of adjuvant radiotherapy for cutaneous melanoma of the head and neck region.156 This trial involved postoperative irradiation for patients in any of three clinical scenarios. Group 1, patients with Clark’s level IV to V melanoma, status post wide local excision, received postoperative treatment without neck dissection (79 patients). Group 2 were given adjuvant radiotherapy after wide local excision and limited neck dissection (32 patients). Group 3 were treated for recurrent neck disease by surgery and postoperative radiotherapy (63 patients). Treatment consisted of 5 fractions of 6 Gy delivered twice a week in most cases with electron beams to ports described earlier. Junction lines were moved twice. This trial was prospective but did not involve a randomized control group.
Melanoma recurred above the clavicles in only six patients and in distant sites in 58. Five-year locoregional control was 88%, and survival was 47% for the whole group. Depth of invasion was the main factor influencing survival. The authors concluded the treatment was safe, with only three moderate late complications of fibrosis. They felt the local control was better than in historical controls (50%), as was survival, and that a randomized trial was indicated.156
Since that study was reported, numerous reports and updates have appeared. Based on retrospective reviews, some have claimed adjuvant radiotherapy of definite benefit,157–159 whereas others note it does not improve local control.160 The Trans Tasman Radiation Oncology Group (TROG) has just reported on a phase III trial for postoperative patients treated after definitive resection of palpable nodal metastases. This trial involved a randomization of 250 patients between surgery alone and surgery plus postoperative radiotherapy to the nodal region of 48 Gy in 20 fractions. There was a statistically significant reduction of local failure in the irradiated group (HR = 1.77).161 Berk has concluded that postoperative radiotherapy is indicated for patients with nodes greater than 3 cm, more than three involved nodes, or extracapsular extension.162
For patients without nodal disease on clinical or imaging studies, elective radiotherapy to the regional nodes has been evaluated as a replacement for elective or selective node dissection in patients who were poor operative candidates. Bonnen and colleagues reported on 157 patients treated after wide local excision for stage I or II cutaneous melanoma of the head and neck.163 None had received sentinel node biopsy or dissection. The actuarial regional control rate was 89% at 5 and 10 years. They advocated radiotherapy as an alternative for patients who were not good candidates for sentinel node biopsy, neck dissection, or systemic therapy.
Mucosal Melanoma
Mucosal melanoma of the head and neck has a dismal prognosis. Disseminated disease is common, as is local failure.164 One retrospective report showed improved local control with postoperative radiotherapy but no change in distant metastases.165 Carbon ion radiotherapy was used in 72 patients in Japan, yielding a 5-year local control rate of 84% and survival of 27%. The authors concluded that carbon ion radiotherapy was superior to conventional radiotherapy and similar in results to surgery.166
Palliative Radiotherapy
The data presented support the use of radiotherapy in melanoma when surgery is not indicated or not possible. Palliation at all symptomatic sites can be achieved with radiotherapy. Although the RTOG trial did not show a difference between 800 cGy × 3 and 250 cGy × 20 for palliation, larger fractions are preferred. Radiosurgical techniques are also ideal for melanoma palliation (see Chapters 25 and 74).
Brachytherapy
There is little evidence for a role for brachytherapy for melanoma, with the exception of radioactive plaque therapy for choroidal melanoma (see Chapter 64). For head and neck and gynecologic mucosal sites, brachytherapy may be considered for unresectable lesions (see specific head and neck and gynecologic chapters).
Critical Normal Tissues: Radiation Injury
Since melanoma may receive radiotherapy at any site, no specific tissues are limiting except skin and subcutaneous tissue (see Chapter 66). Hypofractionation has been shown to cause only minimal fibrosis at doses of 30 Gy in five fractions using electron beams.
Selected Outcome
Role of Vascular Factors in Progression of Melanoma and Development of Ulceration
Given the incorporation of ulceration into the AJCC staging classification for melanoma, understanding the biological basis of melanoma and identifying clinically relevant cases of ulceration emerged as important issues. Using the University of California, San Francisco, (UCSF) Melanoma Center database, a number of analyses were performed to address these research questions.167–170 A retrospective survival analysis of a cohort of 526 patients with primary cutaneous melanoma identified the interactions between tumor cells and the tumor vasculature as playing an important role in the progression and prognostic assessment of melanoma patients and development of ulceration.167 The vascular interactions examined included tumor vascularity and vascular involvement. Tumor vascularity referred to the level of vascularity present at the base of vertical growth phase and was graded morphologically. Vascular involvement was defined as both (a) vascular invasion, with tumor cells within the wall or lumens of endothelial-lined vessels; and (b) uncertain or incipient vascular invasion, with melanoma cells immediately adjacent to the endothelium, without perithelial cell or fibrous stroma suggestive of incipient invasion. Tumor vascularity and vascular involvement were both found to be independently predictive of survival in this melanoma cohort, surpassing tumor thickness and ulceration.167,168 The combination of increased vascularity and the presence of vascular involvement resulted in a worse survival and predisposed to the development of ulceration. In multivariate analysis, with the inclusion of these vascular factors, ulceration was no longer significant in the prediction of overall survival of this melanoma cohort.169 Interestingly, in ulcerated cases without increased vascularity, the presence of ulceration did not adversely affect survival.
These results suggest that biologically meaningful ulceration in melanoma occurs as a result of interactions between tumor cells and the tumor vasculature (as defined by these two prognostic factors), and that these interactions play an important role in the progression of melanoma. In additional studies, the extent of ulceration was analyzed to identify cases with an adverse impact on survival or sentinel lymph node positivity.170 Extent of ulceration was defined as the diameter of the ulcerated area in millimeters over the diameter of the entire vertical growth phase (resulting in percentage of tumor ulceration). There was a significant relationship between increasing percentage of tumor ulceration and both sentinel lymph node status and overall survival, with a cutoff of 5% for overall survival and 2% for sentinel lymph node status. These results suggested that minimal ulceration is required to have a prognostic impact on melanoma survival.
1 Swetter SM, Curiel-Lewandrowski C, Kirkwood JM. Organ specific cancer control: melanoma. Alexandria, VA: American Society of Clinical Oncology Cancer Prevention Curriculum, American Society of Clinical Oncology; 2007. pp 24:1–25
2 Swerlick RA, Chen S. The melanoma epidemic. Is increased surveillance the solution or the problem? Arch Dermatol. 1996;132:881-884.
3 Welch HG, Woloshin S, Schwartz LM. Skin biopsy rates and incidence of melanoma: population based ecological study. Br Med J. 2005;331:481.
4 Hall HI, Jamison P, Fulton JP, Clutter G, Roffers S, Parrish P. Reporting cutaneous melanoma to cancer registries in the United States. J Am Acad Dermatol. 2003;49:624-630.
5 Demierre MF, Chung C, Miller DR, Geller AC. Early detection of thick melanomas in the United States: beware of the nodular subtype. Arch Dermatol. 2005;141:745-750.
6 Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249.
7 Geller AC, Swetter SM, Brooks K, et al. Screening, early detection, and trends for melanoma: Current status (2000–2006) and future directions. J Am Acad Dermatol. 2007;5:555-572.
8 Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108.
9 American Cancer Society. Cancer facts and figures, 2008. Atlanta, GA: American Cancer Society; 2008.
10 Ries LAG, Eisner MP, Kosary CL, et al, SEER Cancer Statistics Review; 1975–2002; National Cancer Institute, Bethesda MD. http://seer.cancer.gov/csr/1975_2002/
11 Demierre MR, Nathanson L. Chemoprevention of melanoma: an unexplored strategy. J Clin Oncol. 2003;21:158-165.
12 Armstrong BK, Kricker A. How much melanoma is caused by sun exposure? Melanoma Res. 1993;3:395-401.
13 Elwood JM, Jopson J. Melanoma and sun exposure: an overview of published studies. Int J Cancer. 1997;73:198-203.
14 Gilchrest BA, Eller MS, Geller AC, et al. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341-1348.
15 Pennello G, Devesa S, Gail M. Association of surface ultraviolet B radiation levels with melanoma and non-melanoma skin cancer in United States blacks. Cancer Epidemiol Biomarkers Prev. 2000;9:291-297.
16 Eide MJ, Weinstock MA. Association of UV index, latitude, and melanoma incidence in nonwhite populations–US Surveillance, Epidemiology, and End Results (SEER) Program, 1992 to 2001. Arch Dermatol. 2005;141(4):477-481.
17 Thieden E, Philipsen PA, Sandby-Moller J, Heydenreich J, Wulf HC. Proportion of lifetime UV dose received by children, teenagers and adults based on time-stamped personal dosimetry. J Invest Dermatol. 2004;123:1147-1150.
18 Mack TM, Floderus B. Malignant melanoma risk by nativity, place of residence at diagnosis, and age at migration. Cancer Causes Control. 1991;2:401-411.
19 Gallagher RP, Spinelli JJ, Lee TK. Tanning beds, sunlamps, and risk of cutaneous malignant melanoma. Cancer Epidemiol Biomarkers Prev. 2005;14(3):562-566.
20 Westerdahl J, Ingvar C, Måsbäck A, et al. Risk of cutaneous malignant melanoma in relation to use of sunbeds: further evidence for UV-A carcinogenicity. Br J Cancer. 2000;82:1593-1599.
21 International Agency for Research on Cancer Working Group on artificial ultraviolet (UV) light and skin cancer. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: A systematic review. Int J Cancer. 2007;120:1116-1122.
22 Demko CA, Borawski EA, Debanne SM, Cooper KD, Stange KC. Use of indoor tanning facilities by white adolescents in the United States. Arch Pediatr Adolesc Med. 2003;157:854-860.
23 Stern RS, PUVA Follow up study. The risk of melanoma in association with long-term exposure to PUVA. J Am Acad Dermatol. 2001;44:755-761.
24 Levi F, Randimbison L, La Vecchia C, Erler G, Te VC. Incidence of invasive cancers following squamous cell skin cancer. Am J Epidemiol. 1997 Nov 1;146:734-739.
25 Friedman GD, Tekawa IS. Association of basal cell skin cancers with other cancers (United States). Cancer Causes Control. 2000;11:891-897.
26 Bergomi M, Pellacani G, Vinceti M, et al. Trace elements and melanoma. J Trace Elem Med Biol. 2005;19:69-73.
27 Dupuy A, Shamsaldin A, Quiniou E, et al. Risk of melanoma following adulthood cancer: a case-control study. Eur J Cancer. 2005;41:2904-2910.
28 Millen AE, Tucker MA, Hartge P, et al. Diet and melanoma in a case-control study. Cancer Epidemiol Biomarkers Prev. 2004;13:1042-1051.
29 Smith MA, Fine JA, Barnhill RL. Hormonal and reproductive influences and risk of melanoma in women. Int J Epidemiol. 1998;27:751-757.
30 Penn I. The effect of immunosuppression on pre-existing cancers. Transplantation. 1993;55:742-747.
31 Penn I. Malignant melanoma in organ allograft recipients. Transplantation. 1996;61:274-278.
32 Burgi A, Brodine S, Wegner S. Incidence and risk factors for the occurrence of non-AIDS-defining cancers among human immunodeficiency virus-infected individuals. Cancer. 2005;104:1505-1511.
33 Tucker MA, Halpern A, Holly EA, et al. Clinically recognized dysplastic nevi. A central risk factor for cutaneous melanoma. JAMA. 1997;277:1439-1444.
34 Carey WPJr, Thompson CJ, Synnestvedt M, et al. Dysplastic nevi as a melanoma risk factor in patients with familial melanoma. Cancer. 1994;74:3118-3125.
35 Sagebiel RW. Melanocytic nevi in histologic association with primary cutaneous melanoma of superficial spreading and nodular types: effect of tumor thickness. J Invest Dermatol. 1993;100:3225-3255.
36 Tsao H, Bevona C, Goggins, et al. The transformation rate of moles (melanocytic nevi) into cutaneous melanoma: a population-based estimate. Arch Dermatol. 2003;139:282-288.
37 Berwick M, Armstrong BK, Ben-Porat L, et al. Sun exposure and mortality from melanoma. J Natl Cancer Inst. 2005;9:195-199.
38 Whiteman DC, Watt P, Purdie DM, et al. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst. 2003;95:806-812.
39 Maldonado JL, Fridlyand J, Patel H, et al. Determinants of BRAF mutations in primary melanomas. J Natl Cancer Inst. 2003;95(24):1878-1890.
40 Florell SR, Boucher KM, Garibotti G, et al. Population-based analysis of prognostic factors and survival in familial melanoma. J Clin Oncol. 2005;23:168-177.
41 Aitken J, Welch J, Duffy D, et al. CDKN2A variants in a population-based sample of Queensland families with melanoma. J Natl Cancer Inst. 1999;91:446-452.
42 Celebi JT, Ward KM, Wanner M, et al. Evaluation of germline CDKN2A, ARF, CDK4, PTEN, and BRAF alterations in atypical mole syndrome. Clin Exp Dermatol. 2005;30:68-70.
43 Tsao H, Niendorf K. Genetic testing in hereditary melanoma. J Am Acad Dermatol. 2004;51:803-808.
44 Box NF, Duffy DL, Chen W, et al. MC1R genotype modifies risk of melanoma in families segregating CDKN2A mutations. Am J Hum Genet. 2001;69:765-773.
45 Goldstein AM, Chan M, Harland M, et al. Features associated with germline CDKN2A mutations: a GenoMEL study of melanoma-prone families from three continents. J Med Genet. 2007;44:99-106.
46 Elder DE, Murphy GF. Melanocytic tumors of the skin, fascicle 2. In: Rosai J, Sobin LH, editors. Atlas of tumor pathology, third series. Washington (DC): Armed Forces Institute of Pathology; 1991:146-149.
47 Farrahi F, Egbert BM, Swetter SM. Histologic similarities between lentigo maligna and dysplastic nevus: importance of clinicopathologic distinction. J Cutan Pathol. 2005;32:405-412.
48 Elder DE, Guerry DIV, Epstein MN, et al. Invasive malignant melanomas lacking competence for metastasis. Am J Dermatopathol. 1984;6:55S-61S.
49 Guerry DIV, Synnestvedt M, Elder DE, Schultz D. Lessons from tumor progression: the invasive radiation growth phase of melanoma is common, incapable of metastasis, and indolent. J Invest Dermatol. 1993;100:342S-345S.
50 Clark WH, Elder DE, Guerry DIV, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81:1893-1904.
51 Balch CM. Cutaneous melanoma: prognosis and treatment results worldwide. Semin Surg Oncol. 1992;8:400-414.
52 Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622-3634.
53 Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors; Melanoma of the skin. In: AJCC Cancer Staging Manual. ed 7. 2009; Springer, New York:325-340.
54 Doeden K, Ma Z, Narasimhan B, Swetter SM, Detmar M, Dadras SS. Lymphatic invasion in cutaneous melanoma is associated with sentinel lymph node metastasis. J Cutan Pathol. 2009;36:772-780. Epub 2009 Nov 5
55 Sober AJ, Fitzpatrick TB, Mihm MCJr, et al. Early recognition of cutaneous melanoma. JAMA. 1979;242:2795-2799.
56 Friedman RJ, Rigel DS, Kopf AW. Early detection of malignant melanoma: the role of physician examination and self-examination of the skin. CA, Cancer J Clin. 1985;35:130-151.
57 Abassi NR, Shaw HM, Rigel DS, et al. Early diagnosis of cutaneous melanoma: revisiting the ABCD criteria. JAMA. 2004;292:2771-2776.
58 Langley RG, Fitzpatrick TB, Sober AJ. Clinical characteristics. In: Balch CM, Houghton AN, Sober AJ, Soong S-J, editors. Cutaneous melanoma. ed 3. St Louis: Quality Medical Publishing; 1998:81-101.
59 Balch CM, Soong S-J, Shaw HM, et al. An analysis of prognostic factors in 8,500 patients with cutaneous melanoma. In: Balch CM, Houghton AN, Milton GW, editors. Cutaneous melanoma. ed 2. Philadelphia: JB Lippincott; 1992:165-187.
60 Swetter SM, Boldrick JC, Jung S, Harvell JD, Egbert BM. Increasing incidence of lentigo maligna melanoma subtypes: northern California and national trends 1990–2000. J Invest Dermatol. 2005;125:685-691.
61 Weinstock MA, Sober AJ. The risk of progression of lentigo maligna to lentigo maligna melanoma. Br J Dermatol. 1987;116:303-310.
62 Reintgen DS, McCarty KMJr, Cox E, et al. Malignant melanoma in black American and white American populations. A comparative review. JAMA. 1982;248:1856-1859.
63 Jain S, Allen PW. Desmoplastic malignant melanoma and its variants: a study of 45 cases. Am J Surg Pathol. 1989;13:358-373.
64 Swetter SM. Malignant melanoma. eMedicine Journal [serial online]. Available at http://www.emedicine.com/derm/topic257.htm, 2002. (last update 12/07)
65 Weiss M, Loprinzi CL, Creagan ET, et al. Utility of follow-up tests for detecting recurrent disease in patients with malignant melnaomas. JAMA. 1995;274:1703-1705.
66 Johnson TM, Bradford CR, Bruber SB, et al. Staging workup, sentinel node biopsy, and follow-up tests for melanoma: update of current concepts. Arch Dermatol. 2004;140:107-113.
67 Wang TS, Johnson TM, Cascade PN, et al. Evaluation of staging chest radiographs and serum lactate dehydrogenase for localized melanoma. J Am Acad Dermatol. 2004;51:399-405.
68 Hafner J, Schmid MH, Kempf W, et al. Baseline staging in cutaneous malignant melanoma. Br J Dermatol. 2004;150:677-686.
69 National Comprehensive Cancer Care Network, Clinical practice guidelines in oncology—v.1.2010, 10/19/09: melanoma, Available at. http://www.nccn.org/professionals/physician_gls/PDF/melanoma.pdf, 2009. Jenkintown, PA: ©National Comprehensive Care Network, Inc
70 Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635-3648.
71 Kaskel P, Berking C, Sander S, et al. S-100 protein in peripheral blood: a marker for melanoma metastases: a prospective 2-center study of 570 patients with melanoma. J Am Acad Dermatol. 1999;41:962-969.
72 Gershenwald JE, Thompson W, Mansfield PF, et al. Multi-institutional melanoma lymphatic mapping experience: the prognostic value of sentinel lymph node status in 613 stage I or II melanoma patients. J Clin Oncol. 1999;17:976-983.
73 Balch CM, Soong SJ, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131-149.
74 Swetter SM, Carroll LA, Johnson DL, Segall GM. Positron emission tomography is superior to computed tomography for metastatic detection in melanoma patients. Ann Surg Oncol. 2002;9:646-653.
75 Krug B, Crott R, Lonneux M, et al. Role of PET in the initial staging of cutaneous malignant melanoma: a systematic review. Radiology. 2008;249:836-844.
76 Zettersten E, Shaikh L, Ramirez R, Kashani-Sabet M. Prognostic factors in primary cutaneous melanoma. Surg Clin North Am. 2003;83:61-75.
77 Johnson TM, et al. Usefulness of the staged excision for lentigo maligna and lentigo maligna melanoma: the “square” procedure. J Am Acad Dermatol. 1997;37(5):758-764.
78 Dawn ME, et al. Mohs surgery for the treatment of melanoma in situ: a review. Dermatol Surg. 2007;33(4):395-402.
79 Walling HW, et al. Staged excision versus Mohs micrographic surgery for lentigo maligna and lentigo maligna melanoma. J Am Acad Derm. 2007;57(4):659-664.
80 Wolf IH, et al. Treatment of lentigo maligna (melanoma in situ) with the immune response modifier Imiquimod. Arch Dermatol. 2005 April;141:510-514.
81 Morton DL, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392-399.
82 Sumner WE, et al. Implications of lymphatic drainage to unusual sentinel node sites in patients with primary cutaneous melanoma. Cancer. 2002;95:354-360.
83 Thompson JF, et al. Sentinel lymph node status as indicator of the presence of melanoma in regional lymph nodes. Melanoma Res. 1995;5:255-260.
84 Norman J, et al. Redefinition of cutaneous lymphatic drainage with the use of lymphoscintigraphy for malignant melanoma. Am J Surg. 1991;162:432-437.
85 Carlson GW, et al. Sentinel lymph node biopsy in the management of cutaneous head and neck melanoma. Plast Reconstr Surg. 2005;115:721-728.
86 Wagner JD, et al. Cervical sentinel lymph node biopsy for melanomas of the head and neck and upper thorax. Arch Otolaryngol Head Neck Surg. 2000;126:313-321.
87 McMasters KM, et al. Lessons learned from the sunbelt melanoma trial. J Surg Oncol. 2004;86(4):212-223.
88 Lee JH, et al. Factors predictive of tumor-positive nonsentinel lymph nodes after tumor-positive sentinel lymph node dissection for melanoma. J Clin Oncol. 2004;22:3677-3684.
89 Atkins MB, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105-2116.
90 Balch CM, et al. A new American Joint Committee on Cancer staging system for cutaneous melanoma. Cancer. 2000;88(6):1484-1491.
91 Yanagi T, et al. Mucosal malignant melanoma of the head and neck treated by carbon Ion radiotherapy. Int J Radiat Oncol Biol Phys. 2008.
92 Bartell HL, et al. Biochemotherapy in patients with advanced head and neck mucosal melanoma. Head Neck. 2008;30(12):1592-1598.
93 Vajdic C, et al. Ocular melanoma is not associated with CDKN2A or MC1R variants—a population-based study. Melanoma Res. 2003;13(4):409-413.
94 Skalicky SE, et al. Australian Cancer Network clinical practice guidelines for the management of ocular and periocular melanoma: an evidence-based literature analysis. Clin Experiment Ophthalmol. 2008;36(7):646-658.
95 Triozzi PL, Eng C, Singh AD. Targeted therapy for uveal melanoma. Cancer Treat Rev. 2008;34(3):247-258.
96 Flaherty KT. Chemotherapy and targeted therapy combinations in advanced melanoma. Clin Cancer Res. 2006;12(7 Pt 2):2366s-2370s.
97 Tawbi HA, Kirkwood JM. Management of metastatic melanoma. Semin Oncol. 2007;34(6):532-545.
98 Bajetta E, et al. Metastatic melanoma: chemotherapy. Semin Oncol. 2002;29(5):427-445.
99 Chapman PB, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol. 1999;17(9):2745-2751.
100 Agarwala SS, Kirkwood JM. Temozolomide, a novel alkylating agent with activity in the central nervous system, may improve the treatment of advanced metastatic melanoma. Oncologist. 2000;5(2):144-151.
101 Mathieu D, et al. Gamma knife radiosurgery in the management of malignant melanoma brain metastases. Neurosurgery. 2007;60(3):471-481.
102 Lobo C, et al. Paclitaxel albumin-bound particles (Abraxane) in combination with bevacizumab with or without gemcitabine: early experience at the University of Miami/Braman Family Breast Cancer Institute. Biomed Pharmacother. 2007;61(9):531-533.
103 Green MR, et al. Abraxane, a novel Cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann Oncol. 2006;17(8):1263-1268.
104 Schwartzentruber DJ. Guidelines for the safe administration of high-dose interleukin-2. J Immunother. 2001;24(4):287-293.
105 Atkins MB. Cytokine-based therapy and biochemotherapy for advanced melanoma. Clin Cancer Res. 2006;12(7 Pt 2):2353s-2358s.
106 Atkins MB, Lee S, Cohen GI, et al. Phase III Trial Comparing Concurrent Biochemotherapy With Cisplatin, Vinblastine, Dacarbazine, Interleukin-2, and Interferon Alfa-2b With Cisplatin, Vinblastine, and Dacarbazine Alone in Patients With Metastatic Malignant Melanoma (E3695): A Trial Coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2008. (epub)
107 Keilholz U, et al. Dacarbazine, cisplatin, and interferon-alfa-2b with or without interleukin-2 in metastatic melanoma: a randomized phase III trial (18951) of the European Organisation for Research and Treatment of Cancer Melanoma Group. J Clin Oncol. 2005;23(27):6747-6755.
108 Eton O, et al. Sequential biochemotherapy versus chemotherapy for metastatic melanoma: results from a phase III randomized trial. J Clin Oncol. 2002;20(8):2045-2052.
109 Bedikian AY, et al. Systemic therapy for unresectable metastatic melanoma: impact of biochemotherapy on long-term survival. J Immunotoxicol. 2008;5(2):201-207.
110 Minor DR, et al. A retrospective study of biochemotherapy for metastatic melanoma: the importance of dose intensity. Cancer Biother Radiopharm. 2005;20(5):479-486.
111 Chapman PB. Melanoma vaccines. Semin Oncol. 2007;34(6):516-523.
112 Riley LB, Agarwala SS. Melanoma vaccines. Expert Rev Vaccines. 2008;7(7):937-949.
113 Engell-Noerregaard L, et al. Review of clinical studies on dendritic cell-based vaccination of patients with malignant melanoma: assessment of correlation between clinical response and vaccine parameters. Cancer Immunol Immunother. 2009;58(1):1-14.
114 Agostino NM, et al. Current immunotherapeutic strategies in malignant melanoma. Surg Oncol Clin N Am. 2007;16(4):945-973. xi
115 Amaravadi RK, Flaherty KT. Targeted therapy for metastatic melanoma. Clin Adv Hematol Oncol. 2007;5(5):386-394.
116 Lejeune FJ, Rimoldi D, Speiser D. New approaches in metastatic melanoma: biological and molecular targeted therapies. Expert Rev Anticancer Ther. 2007;7(5):701-713.
117 Wolchok JD, Saenger Y. The mechanism of anti-CTLA-4 activity and the negative regulation of T-cell activation. Oncologist. 2008;13(Suppl 4):2-9.
118 Langer LF, Clay TM, Morse MA. Update on anti-CTLA-4 antibodies in clinical trials. Expert Opin Biol Ther. 2007;7(8):1245-1256.
119 Maker AV, et al. Intrapatient dose escalation of anti-CTLA-4 antibody in patients with metastatic melanoma. J Immunother. 2006;29(4):455-463.
120 Lens M, Ferrucci PF, Testori A. Anti-CTLA4 monoclonal antibody ipilimumab in the treatment of metastatic melanoma: recent findings. Recent Pat Anticancer Drug Discov. 2008;3(2):105-113.
121 Weber J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist. 2007;12(7):864-872.
122 Saenger YM, Wolchok JD. The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun. 2008;8:1.
123 Weber J. Overcoming immunologic tolerance to melanoma: targeting CTLA-4 with ipilimumab (MDX-010). Oncologist. 2008;13(Suppl 4):16-25.
124 Dudley ME, Rosenberg SA. Adoptive cell transfer therapy. Semin Oncol. 2007;34(6):524-531.
125 Hunder NN, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358(25):2698-2703.
126 Haluska F, et al. The RTK/RAS/BRAF/PI3K pathways in melanoma: biology, small molecule inhibitors, and potential applications. Semin Oncol. 2007;34(6):546-554.
127 Hocker TL, Singh MK, Tsao H. Melanoma genetics and therapeutic approaches in the 21st century: moving from the benchside to the bedside. J Invest Dermatol. 2008;128(11):2575-2595.
128 Singh M, et al. Genetics of melanoma tumorigenesis. Br J Dermatol. 2008;158(1):15-21.
129 Schatton T, Frank MH. Cancer stem cells and human malignant melanoma. Pigment Cell Melanoma Res. 2008;21(1):39-55.
130 Sosman JA, Puzanov I. Molecular targets in melanoma from angiogenesis to apoptosis. Clin Cancer Res. 2006;12(7 Pt 2):2376s-2383s.
131 Egberts F, et al. Metastatic melanoma: scientific rationale for sorafenib treatment and clinical results. Onkologie. 2008;31(7):398-403.
132 Frankel SR. Oblimersen sodium (G3139 Bcl-2 antisense oligonucleotide) therapy in Waldenstrom’s macroglobulinemia: a targeted approach to enhance apoptosis. Semin Oncol. 2003;30(2):300-304.
133 Quintas-Cardama A, et al. Complete response of stage IV anal mucosal melanoma expressing KIT Val560Asp to the multikinase inhibitor sorafenib. Nat Clin Pract Oncol. 2008;5(12):737-740.
134 Rivera RS, et al. C-kit protein expression correlated with activating mutations in KIT gene in oral mucosal melanoma. Virchows Arch. 2008;452(1):27-32.
135 Hodi FS, et al. Major response to imatinib mesylate in KIT-mutated melanoma. J Clin Oncol. 2008;26(12):2046-2051.
136 Mahabeleshwar GH, Byzova TV. Angiogenesis in melanoma. Semin Oncol. 2007;34(6):555-565.
137 Flaherty K, Puzanov I, Sosman J, et al. Phase I study of PLX4032: proof of concept for V600E BRAF mutation as a therapeutic target in human cancer. J Clin Oncol. 27(15S), 2009.
138 Kirkwood JM, et al. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: results of intergroup trial E1694/S9512/C509801. J Clin Oncol. 2001;19(9):2370-2380.
139 Eggermont AM, Gore M. Randomized adjuvant therapy trials in melanoma: surgical and systemic. Semin Oncol. 2007;34(6):509-515.
140 Wheatley K, Hancock B, Gore M, et al. on behalf of International Malignant Melanoma Collaborative Group: Interferon-a as adjuvant therapy for melanoma: an individual patient data meta-analysis of randomised trials. J Clin Oncol. 2007;25:8526.
141 Hauschild A, et al. Practical guidelines for the management of interferon-alpha-2b side effects in patients receiving adjuvant treatment for melanoma: expert opinion. Cancer. 2008;112(5):982-994.
142 Kirkwood JM, et al. Mechanisms and management of toxicities associated with high-dose interferon alfa-2b therapy. J Clin Oncol. 2002;20(17):3703-3718.
143 Agarwala SS, et al. Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J Clin Oncol. 2004;22(11):2101-2107.
144 Weller M. Radiochemotherapy for brain metastasis: how to define a role for temozolomide. Onkologie. 2007;30(7):350-351.
145 Gerszten PC, et al. CyberKnife frameless single-fraction stereotactic radiosurgery for tumors of the sacrum. Neurosurg Focus. 2003;15(2):E7.
146 Narayana A, et al. Feasibility of using bevacizumab with radiation therapy and temozolomide in newly diagnosed high-grade glioma. Int J Radiat Oncol Biol Phys. 2008;72(2):383-389.
147 Atkins MB, et al. Temozolomide, thalidomide, and whole brain radiation therapy for patients with brain metastasis from metastatic melanoma: a phase II Cytokine Working Group study. Cancer. 2008;113(8):2139-2145.
148 Jones EL, Oleson JR, Prosnitz LR, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol. 2005 May 1;23(13):3079-3085. PubMed PMID: 15860867
149 Schmidt-Ullrich RK, Johnson CR. Role of radiotherapy and hyperthermia in the management of malignant melanoma. Semin Surg Oncol. 1996 Nov-Dec;12(6):407-415. Review. PubMed PMID: 8914205
150 Beadle BM, Guadagnolo BA, Ballo MT, et al. Radiation therapy field extent for adjuvant treatment of axillary metastases from malignant melanoma. Int J Radiat Oncol Biol Phys. 2009 Apr 1;73(5):1376-1382. Epub 2008 Sep 5. PubMed PMID: 18774657
151 Bentzen SM, Overgaard J, Thames HD, et al. Clinical radiobiology of malignant melanoma. Radiother Oncol. 1989 Nov;16(3):169-182. PubMed PMID: 2587808
152 Sause WT, Cooper JS, Rush S, et al. Fraction size in external beam radiation therapy in the treatment of melanoma. Int J Radiat Oncol Biol Phys. 1991 Mar;20(3):429-432. PubMed PMID: 1995527
153 Guadagnolo BA, Zagars GK. Adjuvant radiation therapy for high-risk nodal metastases from cutaneous melanoma. Lancet Oncol. 2009 Apr;10(4):409-416. Review. PubMed PMID:19341972
154 Powell AM, Robson AM, Russell-Jones R, Barlow RJ. Imiquimod and lentigo maligna: a search for prognostic features in a clinicopathological study with long-term follow-up. Br J Dermatol. 2009 May;160(5):994-998. Epub 2009 Feb 16. PubMed PMID: 19222462
155 Harwood AR. Conventional fractionated radiotherapy for 51 patients with lentigo maligna and lentigo maligna melanoma. Int J Radiat Oncol Biol Phys. 1983 Jul;9(7):1019-1021. PubMed PMID: 6863069
156 Ang KK, Peters LJ, Weber RS, et al. Postoperative radiotherapy for cutaneous melanoma of the head and neck region. Int J Radiat Oncol Biol Phys. 1994 Nov 15;30(4):795-798. PubMed PMID: 7960981
157 Agrawal S, Kane JM3rd, Guadagnolo BA, Kraybill WG, Ballo MT. The benefits of adjuvant radiation therapy after therapeutic lymphadenectomy for clinically advanced, high-risk, lymph node-metastatic melanoma. Cancer. 2009 Aug 21. [Epub ahead of print] PubMed PMID: 19701906
158 Hamming-Vrieze O, Balm AJ, Heemsbergen WD, Hooft van Huysduynen T, Rasch CR. Regional control of melanoma neck node metastasis after selective neck dissection with or without adjuvant radiotherapy. Arch Otolaryngol Head Neck Surg. 2009 Aug;135(8):795-800. PubMed PMID: 19687401
159 Mendenhall WM, Amdur RJ, Grobmyer SR, et al. Adjuvant radiotherapy for cutaneous melanoma. Cancer. 2008 Mar 15;112(6):1189-1196. Review. PubMed PMID: 18260153
160 Moncrieff MD, Martin R, O’Brien CJ, et al. Adjuvant postoperative radiotherapy to the cervical lymph nodes in cutaneous melanoma: is there any benefit for high-risk patients? Ann Surg Oncol. 2008 Nov;15(11):3022-3027. Epub 2008 Oct 30. PubMed PMID: 18958539
161 Burmeister B, Henderson M, Thompson J, et al. Adjuvant radiotherapy improves regional (lymph node field) control in melanoma patients after lymphadenectomy: results of an Intergroup randomized trial (TROG02.01/ANZMTG01.02). Int J Radiat Oncol Biol Phys. 75(3), 2009. Supplement
162 Berk LB. Radiation therapy as primary and adjuvant treatment for local and regional melanoma. Cancer Control. 2008 Jul;15(3):233-238. Review. PubMed PMID:18596675
163 Bonnen MD, Ballo MT, Myers JN, et al. Elective radiotherapy provides regional control for patients with cutaneous melanoma of the head and neck. Cancer. 2004 Jan 15;100(2):383-389. PubMed PMID: 14716775
164 Bachar G, Loh KS, O’Sullivan B, et al. Mucosal melanomas of the head and neck: experience of the Princess Margaret Hospital. Head Neck. 2008 Oct;30(10):1325-1331. PubMed PMID: 18704964
165 Meleti M, Leemans CR, de Bree R, Vescovi P, Sesenna E, van der Waal I. Head and neck mucosal melanoma: experience with 42 patients, with emphasis on the role of postoperative radiotherapy. Head Neck. 2008 Dec;30(12):1543-1551. PubMed PMID:18704960
166 Yanagi T, Mizoe JE, Hasegawa A, et al. Mucosal malignant melanoma of the head and neck treated by carbon ion radiotherapy. Int J Radiat Oncol Biol Phys. 2009 May 1;74(1):15-20. Epub 2008 Nov 27. PubMed PMID: 19046826
167 Kashani-Sabet M, Sagebiel RW, Ferreira CM, Nosrati M, Miller JRIII. Vascular involvement in the prognosis of primary cutaneous melanoma. Arch Dermatol. 2001;137:1169-1173.
168 Kashani-Sabet M, Sagebiel RW, Ferreira CMM, Nosrati M, Miller JRIII. Tumor vascularity in the prognostic assessment of primary cutaneous melanoma. J Clin Oncol. 2002;20:1826-1831. Reprinted in Classic Papers and Current Comments, Highlights Melanoma Res 7:470–477, 2002
169 Kashani-Sabet M, Shakh L, Sagebiel RW, Nosrati M, Ferreira CMM, Miller JRIII. NF-κB in the vascular progression of melanoma. J Clin Oncol. 2004;22:617-623.
170 Grande Sarpa H, Reinke K, Shaikh L, et al. Prognostic significance of extent of ulceration in primary cutaneous melanoma. Am J Surg Pathol. 2006;30:1396-1400.