Chapter 230 Medical Management of Acute Spinal Cord Injury
Administration of High-Dose Steroids
Trials of neuroprotection and pharmacotherapy in acute spinal cord injury (SCI) have focused on counteracting the multitude of secondary injury mechanisms involved in the pathophysiology of acute SCI. There have been approximately 10 randomized prospective control trials (RPCT) in acute SCI, and virtually all have been based on counteracting one or more of these mechanisms of secondary injury.1 Fundamental laboratory research in SCI has discovered at least 25 secondary injury mechanisms.2,3 Therefore, the drugs that have been selected for trial in human SCI have been those with positive effects on one or more of these potentially damaging secondary injury processes elucidated in laboratory animals. For these reasons, steroids were often selected for human trials, either alone or in combination with another strategy such as surgery or another drug. The major attraction of steroids was the experimental evidence that they significantly affect a large number of secondary injury mechanisms. Many reviews of the pharmacotherapy-neuroprotection trials in acute SCI document the extensive series of studies of steroids.1,4,5 In contrast to the reviews by Bracken that support steroid use in acute SCI in humans,6,7 the review by Short et al. reached the opposite conclusion and, furthermore, flagged the potential for early morbidity and mortality associated with steroid use in acute SCI.8
Steroids Counteract the Secondary Mechanisms of Injury after Acute Spinal Cord Injury and Experimental Evidence for the Effectiveness of Steroids
There have been many studies of the vascular, biochemical, electrolyte, and inflammatory changes after experimental SCI that involved the therapeutic administration of steroids. Most of the early experimental animal studies were in large animals, especially cats,9 but after 1990, most of the studies were in rats. There was intense interest in the 1980s in oxygen free radicals and lipid peroxidation as major mechanisms of secondary injury and evidence arose that these processes could be beneficially altered by steroids.10,11 Many of the papers showed beneficial effects in terms of improved histology, such as reduced tissue cavitation and functional improvement.12,13 Of note is the fact that many of the beneficial results in cats were not seen in other species, including a study in rats in my laboratory.14
Vascular and Blood Flow Changes
One of the principal mechanisms of secondary injury after SCI is posttraumatic ischemia, and steroids have been shown to counteract the posttraumatic reduction in spinal cord blood flow. The early experiments on steroids in cats produced very impressive effects in terms of preservation of posttraumatic spinal cord blood flow.15,16 In 1911, Allen first postulated the concept of the secondary mechanism of injury in SCI.17 He found that myelotomy and removal of the posttraumatic hematomyelia in the central aspect of the injured dog cord resulted in improvement of neurologic function after weight-drop injury. He theorized that there was a noxious agent present in the hemorrhagic necrotic material that caused further damage to the cord. Since 1911, numerous injurious pathophysiologic processes have been discovered,18 and similar theories were postulated to explain the progressive damage in head injury, cerebral ischemia, and subarachnoid hemorrhage. One of the strikingly common and dramatic effects of SCI in all experimental models and in human cord injury is the early and often progressive development of hemorrhages in the central region of the injured cord, especially in the gray matter, followed by ischemia. Angiographic studies in humans and microangiographic studies in experimental animals have consistently shown a major loss of the microcirculation involving the capillaries and venules at the injury site and rostrally and caudally.19,20 Spinal cord blood flow worsens over time after injury.21,22 The exact cause of the ischemia is unknown and is probably a combination of mechanical and biochemical causes producing vasospasm and intravascular thrombosis. Therefore, it was remarkable that steroids such as MP improved the metabolism, electrolyte imbalance and spinal cord blood flow in cats.9,13,16 Unfortunately, the effects of MP on spinal cord blood flow and neurologic recovery were not consistently positive. For example, of the 21 studies that I reviewed, steroids produced positive results in only about half.14
Biochemical Changes
One of the most compelling biochemical derangements in the injured spinal cord is the damage caused by the excitatory amino acid neurotransmitter glutamate.23,24 It has also been hypothesized that cell membrane receptor activation by glutamate may play a key role in the development of ischemic damage,25 the mechanism of which is an early intracellular accumulation of sodium, producing cytotoxic edema and a concomitant elevation of intracellular calcium. Raised levels of intracellular calcium can in turn activate calcium-dependent proteases or lipases that cause further damage due to breakdown of cytoskeletal components, including neurofilaments, and dissolution of cell membranes. Steroids in SCI reduced lipid peroxidation and the production of oxygen free radicals, and it was postulated that inhibition of lipid peroxidation was the main cytoprotective mechanism of action of steroids.26,27
Electrolyte Shifts
There is considerable evidence that there are major electrolyte shifts between the extracellular and intracellular compartments after SCI, and these electrolyte imbalances were also improved with steroids. There was evidence that steroids reversed the accumulation of sodium and calcium in damaged neurons and the loss of intracellular potassium from damaged neurons.15 One of the best-defined electrolyte changes is the marked increase of intracellular calcium.28,29 An excess of free intracellular calcium ions plays a fundamental role in mediating the pathogenesis of all neural injuries but especially ischemia and traumatic injuries. After trauma, calcium shifts into neurons in a variety of ways, including through disrupted cell membranes, by depolarization and entry through voltage-sensitive calcium channels, or through receptor-mediated calcium channels activated by glutamate, such as the AMPA/kainate channels. Ischemia can also increase intracellular calcium through glutamate release.
Edema
Significant and progressive edema can follow SCI,30 but it is not known whether the edema is injurious in itself or an epiphenomenon of another injury mechanism such as ischemia or glutamate toxicity. For example, as was noted previously, the latter causes sodium to enter neurons with resulting cytotoxic edema. Edema can spread in the cord from the site of injury for a considerable distance rostrally and caudally in both experimental models31 and clinical cases. Steroids were shown to reduce posttraumatic swelling of the spinal cord.32
Inflammation
A complex series of inflammatory changes occurs in the spinal cord after SCI, and certain components of the inflammatory reaction may add to the secondary injury.33 Specific components of the inflammatory reaction, such as macrophages and microglial cells and interleukins, may contribute to secondary injury.34,35 Steroids have a profound anti-inflammatory effect on nervous tissue.36
Clinical Evidence for the Effectiveness of Steroids in Acute Spinal Cord Injury
The main trials of steroids for acute SCI were conducted in the United States and Canada in the 1980s and 1990s. The first trials were named the National Acute Spinal Cord Injury Study (NASCIS); and to underline the basis for the selection of MP, the NASCIS papers cited the long list of positive studies of steroids in experimental SCI.37,38 The NASCIS investigators included myself and were led by a number of prominent neurosurgeons including William Collins and neuroscientists including Wise Young. The NASCIS group made many contributions to SCI, including the refinement of the Frankel system for scoring neurologic function, which became known as the NASCIS system but was ultimately replaced by the American Spinal Injury Association (ASIA) system.1 The NASCIS expert in trials design and analysis was Michael Bracken, and the NASCIS studies were funded by the National Institutes of Health (NIH) and by the Upjohn Corporation, the pharmaceutical company that made and supplied MP, which was delivered intravenously in very large doses comparable to the doses found to be effective in experimental SCI. In total, there have been five RPCT MP trials, including three NASCIS trials and two trials in Japan. The first NASCIS MP trial, reported in 1984, encompassed 306 patients in nine centers, showed no difference between low-dose and high-dose MP, and had no placebo group.39 There was an increase in wound infections in the high-dose MP group. NASCIS 2, reported in 1990, included a placebo group; a second pharmacotherapeutic agent, Naloxone, an opioid antagonist; and 487 patients in 10 centers.38 This trial generated much controversy for a number of reasons, beginning with the announcement of the trial as an NIH alert to practitioners in the United States. Partly because of this apparent endorsement of MP use in SCI by the NIH, there was a tendency from then onward to consider MP a standard of therapy for acute SCI. However, the SCI guidelines group emanating from the two major North American neurosurgical organizations40 rejected MP as a standard of therapy for SCI. Nevertheless, in some countries, fear of legal liability for nonusage of MP in acute SCI became a factor in clinicians’ decision to use the drug.41 In NASCIS 2, the rates for wound infections and gastrointestinal bleeding were approximately twice those in the placebo group. The third and last NASCIS trial, reported in 1997, involved 499 patients in 16 centers; showed that the new 21-aminosteroid, tirilazad, was not as effective as MP; and added very little new information.42 The first Japanese MP study essentially confirmed NASCIS 2 but involved only 177 patients in 42 centers.43 The French nimodipine trial also tested MP in a much smaller number of patients, as discussed later, and MP did not improve neurologic recovery over the placebo or nimodipine groups.44
The NASCIS 2 and NASCIS 3 trials engendered considerable controversy concerning the use of MP in acute SCI and produced many very thoughtful and thorough analyses of the results of these two studies.41,45–50 The criticisms of these trials included problems with the selection and interpretation of the statistical tests, lack of impact of the minimal neurologic improvements on functional deficits of importance to SCI patients such as loss of bladder control, and lack of reproducible results by subsequent studies (outlined later). There was also criticism of the use of neurologic scores from only one side of the body. The critics also stated that insufficient consideration was given to the increased complications in the steroid groups, including the increased incidence of pneumonia, wound infections, and sepsis. Many critics concluded that these increased risks do not warrant the use of MP, which, at best, only marginally improves neurologic recovery. Also, it is now recognized that large doses of MP such as those used in SCI can cause acute myopathy in SCI patients.51 These issues have resulted in a major decline in some countries in the use of steroids in acute SCI. For example, it was reported in 2008 that three quarters of neurosurgeons and orthopedic surgeons in Canada involved in the care of acute SCI patients do not use steroids, representing a complete reversal of the results from 5 years previously.52 Many authors have defended the results of the NASCIS studies and provided additional evidence of the effectiveness of steroids.5,7,53 Bracken’s Cochrane review of steroids in SCI has not dispelled the controversy.7
Therapeutic Time Window for Steroids after Acute Spinal Cord Injury
Unfortunately, the optimal time window after SCI for steroid therapy has not been completely elucidated in either laboratory animals or patients. Some secondary injury mechanisms such as ischemia and glutamate toxicity develop within minutes of injury, but others such as edema and apoptosis develop more slowly and may continue for months. Thus, the onset and duration of therapy with neuroprotective agents such as steroids are complex issues and may be factors responsible for the disparity in some of the therapeutic results obtained with steroids. In general, it has been recommended that the agent should be administered as soon as possible after SCI in humans, certainly within 8 hours.38,42 Treatment initiated after 8 hours was discouraged because it was ineffective. Continuing treatment beyond 24 hours has also been discouraged because of the toxicity of prolonged treatment.
Current Experimental Studies of Steroids in Acute Spinal Cord Injury
Current experimental studies have focused on combined therapy, with a variety of agents being paired with steroids in an attempt to produce additive effects. For example, the combination of MP and an anti-Nogo receptor antibody produced better results in rats with acute SCI than either agent alone.54 This chapter has concentrated on MP, but there are other steroids such as progesterone derivatives that may have therapeutic potential in SCI.
Non-NASCIS and Current Clinical Trials with MP in Acute SCI
Gerndt et al.55 analyzed consecutive cohorts of patients treated with and without steroids and found a 2.6-fold increase in pneumonia and an increase in ventilated and intensive-care days but no increase in mortality with NASCIS 2 doses of MP. However, there was a decrease in the duration of rehabilitation days, and these authors advised “the continued but cautious use” of MP. Tsutsumi et al.56 performed a retrospective study of a small case series of acute cervical SCI patients and found that high-dose MP improved ASIA motor scores only in patients with incomplete SCI; the authors stated that only this group should receive MP. They also cautioned that patients should be screened for potential side effects such as serious infections and diabetes and recommended that these groups should not receive the drug. Matsumoto et al.57 performed the fifth RPCT study of MP using a NASCIS 2 dosing regimen in a small number of cervical cord injuries and added prophylactic antibiotics to the treatment protocol. There were significantly more respiratory and gastrointestinal complications in the MP-treated group, especially in patients over 60 years of age. The neurologic results were not reported.
Some of the more recent steroid trials in patients with acute SCI have combined MP with other therapies. Ito et al. recently completed a trial in a small number of patients using a novel consecutive cohort design in which all patients in both cohorts also received early surgical decompression.58 MP was administered according to the NASCIS 2 protocol, with high doses administered within 8 hours of trauma to only one cohort. There was no difference in recovery, but there were significantly more cases of pneumonia in the MP cohort. Pointillart et al.44 devised an excellent RPCT study containing four groups of acute SCI patients treated with either MP alone, nimodipine alone, MP plus nimodipine, or placebo. Unfortunately, there were only 104 patients distributed across these four groups. There was an attempt to operate early, but there was no randomization for surgical groups. There were no differences in neurologic recovery based on the ASIA system, but there were more infectious complications and more hyperglycemia in the MP-treated group.
Inclusion of MP in Future Trials of Neuroprotection in SCI
Because of the unwarranted assumption that MP is a standard of therapy, as discussed previously, and the presumed risk of liability for failure to administer this drug in acute SCI, some investigators have felt obliged to allow inclusion of MP as either an option or a requisite in therapeutic trials of other drugs or strategies. This is indeed unfortunate and should be resisted, as it makes evaluation of other agents more difficult. For example, if one believes that there is a small possible therapeutic value of steroids, such as in incomplete cervical cases, it would make it more difficult to establish that there is an additional value to the new drug or strategy. The large number of patients required for such a study and the costs involved may prevent the trial from being undertaken. The lack of trials of neuroprotective agents in the past 20 years may be partly due to this factor.
Bracken M.B., Collins W.F., Freeman D.F., et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA. 1984;251(1):45-52.
Bracken M.B., Shepard M.J., Collins W.F., et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322(20):1405-1411.
Bracken M.B., Shepard M.J., Holford T.R., et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277(20):1597-1604.
Hurlbert R.J. Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J Neurosurg. 2000;93(Suppl 1):1-7.
Tator C.H. Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations. Neurosurgery. 2006;59(5):957-982. discussion 982–957
Hall E.D., Wolf D.L., Braughler J.M. Effects of a single large dose of methylprednisolone sodium succinate on experimental posttraumatic spinal cord ischemia. Dose-response and time-action analysis. J Neurosurg. 1984;61(1):124-130.
Young W., Flamm E.S. Effect of high-dose corticosteroid therapy on blood flow, evoked potentials, and extracellular calcium in experimental spinal injury. J Neurosurg. 1982;57(5):667-673.
1. Tator C.H. Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations. Neurosurgery. 2006;59(5):957-982. discussion 982–957
2. Tator C.H. Strategies for recovery and regeneration after brain and spinal cord injury. Injury Prevention. 2002;Suppl iv:iv33-iv36.
3. Ramer L.M., Ramer M.S., Steeves J.D. Setting the stage for functional repair of spinal cord injuries: a cast of thousands. Spinal Cord. 2005;43(3):134-161.
4. Lammertse D.P. Update on pharmaceutical trials in acute spinal cord injury. J Spinal Cord Med. 2004;27(4):319-325.
5. Hall E.D., Springer J.E. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. Jan 2004;1(1):80-100.
6. Bracken M.B. Methylprednisolone and acute spinal cord injury: an update of the randomized evidence. Spine. Dec 15 2001;26(Suppl 24):S47-S54.
7. Bracken M.B. Steroids for acute spinal cord injury. The Cochrane Database of Systematic Reviews. The Cochrane Collaboration. New York: John Wiley and Sons; 2006.
8. Short D.J., El Masry W.S., Jones P.W. High dose methylprednisolone in the management of acute spinal cord injury-a systematic review from a clinical perspective. Spinal Cord. 2000;38:273-286.
9. Young W., DeCrescito V., Flamm E.S., et al. Pharmacological therapy of acute spinal cord injury: studies of high dose methylprednisolone and naloxone. Clin Neurosurg. 1988;34:675-697.
10. Hall E.D., Braughler J.M. Central nervous system trauma and stroke. II. Physiological and pharmacological evidence for involvement of oxygen radicals and lipid peroxidation. Free Radic Biol Med. 1989;6(3):303-313.
11. Hall E.D., Yonkers P.A., Horan K.L., Braughler J.M. Correlation between attenuation of posttraumatic spinal cord ischemia and preservation of tissue vitamin E by the 21-aminosteroid U74006F: evidence for an in vivo antioxidant mechanism. J Neurotrauma. 1989;6(3):169-176.
12. Hansebout R.R., Kuchner E.F., Romero-Sierra C. Effects of local hypothermia and of steroids upon recovery from experimental spinal cord compression injury. Surg Neurol. 1975;4(6):531-536.
13. Braughler J.M., Hall E.D., Means E.D., et al. Evaluation of an intensive methylprednisolone sodium succinate dosing regimen in experimental spinal cord injury. J Neurosurg. 1987;67(1):102-105.
14. Koyanagi I., Tator C.H. Effect of a single huge dose of methylprednisolone on blood flow, evoked potentials, and histology after acute spinal cord injury in the rat. Neurol Res. 1997;19(3):289-299.
15. Young W., Flamm E.S. Effect of high-dose corticosteroid therapy on blood flow, evoked potentials, and extracellular calcium in experimental spinal injury. J Neurosurg. Nov 1982;57(5):667-673.
16. Hall E.D., Wolf D.L., Braughler J.M. Effects of a single large dose of methylprednisolone sodium succinate on experimental posttraumatic spinal cord ischemia. Dose-response and time-action analysis. J Neurosurg. Jul 1984;61(1):124-130.
17. Allen. A.R. Surgery of experimental lesions of the spinal cord equivalent to crush injury of fracture dislocation of the spinal column. A preliminary report. JAMA. 1911;57:878-880.
18. Anderson D.K., Hall E.D. Pathophysiology of spinal cord trauma. Ann Emerg Med. 1993;22(6):987-992.
19. Koyanagi I., Tator C.H., Lea P.J. Three-dimensional analysis of the vascular system in the rat spinal cord with scanning electron microscopy of vascular corrosion casts. Part 2: Acute spinal cord injury. Neurosurgery. 1993;33(2):285-291. discussion 292
20. Koyanagi I., Tator C.H., Theriault E. Silicone rubber microangiography of acute spinal cord injury in the rat. Neurosurgery. 1993;32(2):260-268. discussion 268
21. Tator C.H. Review of experimental spinal cord injury with emphasis on the local and systemic circulatory effects. Neurochirurgie. 1991;37(5):291-302.
22. Tator C.H., Fehlings M.G. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75(1):15-26.
23. Faden A.I., Simon R.P. A potential role for excitotoxins in the pathophysiology of spinal cord injury. Ann Neurol. 1988;23(6):623-626.
24. Panter S.S., Yum S.W., Faden A.I. Alteration in extracellular amino acids after traumatic spinal cord injury. Ann Neurol. 1990;27(1):96-99.
25. Rothman S.M., Olney J.W. Glutamate and the pathophysiology of hypoxic-ischemic brain damage. Ann Neurol. 1986;19(2):105-111.
26. Braughler J.M., Pregenzer J.F., Chase R.L., et al. Novel 21-amino steroids as potent inhibitors of iron-dependent lipid peroxidation. J Biol Chem. 1987;262(22):10438-10440.
27. Hall E.D. Effects of the 21-aminosteroid U74006F on posttraumatic spinal cord ischemia in cats. J Neurosurg. 1988;68(3):462-465.
28. Stokes B.T., Fox P., Hollinden G. Extracellular calcium activity in the injured spinal cord. Exp Neurol. 1983;80(3):561-572.
29. Young W., Yen V., Blight A. Extracellular calcium ionic activity in experimental spinal cord contusion. Brain Res. 1982;253(1-2):105-113.
30. Wagner F.C.Jr., Stewart W.B. Effect of trauma dose on spinal cord edema. J Neurosurg. 1981;54(6):802-806.
31. Wang R., Ehara K., Tamaki N. Spinal cord edema following freezing injury in the rat: relationship between tissue water content and spinal cord blood flow. Surg Neurol. 1993;39(5):348-354.
32. Lewin M.G., Hansebout R.R., Pappius H.M. Chemical characteristics of traumatic spinal cord edema in cats. Effects of steroids on potassium depletion. J Neurosurg. 1974;40(1):65-75.
33. Popovich P.G., Wei P., Stokes B.T. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377(3):443-464.
34. McTigue D.M., Popovich P.G., Morgan T.E., Stokes B.T. Localization of transforming growth factor-beta1 and receptor mRNA after experimental spinal cord injury. Exp Neurol. 2000;163(1):220-230.
35. McTigue D.M., Popovich P.G., Jakeman L.B., Stokes B.T. Strategies for spinal cord injury repair. Prog Brain Res. 2000;128:3-8.
36. Nadeau S., Rivest S. Glucocorticoids play a fundamental role in protecting the brain during innate immune response. J Neurosci. Jul 2 2003;23(13):5536-5544.
37. Bracken M.B., Shepard M.J., Hellenbrand K.G., et al. Methylprednisolone and neurological function 1 year after spinal cord injury. Results of the National Acute Spinal Cord Injury Study. J Neurosurg. 1985;63(5):704-713.
38. Bracken M.B., Shepard M.J., Collins W.F., et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322(20):1405-1411.
39. Bracken M.B., Collins W.F., Freeman D.F., et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA. 1984;251(1):45-52.
40. Guidelines for Management of Acute Cervical Spinal Injuries. Neurosurgery. 2002;50(3):S1-S199.
41. Hurlbert R.J. Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J Neurosurg. 2000;93(Suppl 1):1-7.
42. Bracken M.B., Shepard M.J., Holford T.R., et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277(20):1597-1604.
43. Otani K., Abe H., Kadoya S., et al. Beneficial effect of methylprednisolone sodium succinate in the treatment of acute spinal cord injury. Sekitsui Sekizui J. 1994;7:633-647.
44. Pointillart V., Petitjean M.E., Wiart L., et al. Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord. 2000;38(2):71-76.
45. Hurlbert R.J. The role of steroids in acute spinal cord injury: an evidence-based analysis. Spine (Phila Pa 1976). 2001;26(Suppl 24):S39-S46.
46. Nesathurai S. Steroids and spinal cord injury: revisiting the NASCIS 2 and NASCIS 3 trials. J Trauma. 1998;45(6):1088-1093.
47. Bracken M.B. Steroids for acute spinal cord injury. Cochrane Database Syst Rev. 2002;3:CD001046.
48. Coleman W.P., Benzel D., Cahill D.W., et al. A critical appraisal of the reporting of the National Acute Spinal Cord Injury Studies (II and III) of methylprednisolone in acute spinal cord injury. J Spinal Disord. 2000;13(3):185-199.
49. Hugenholtz H. Methylprednisolone for acute spinal cord injury: not a standard of care. CMAJ. 2003;168(9):1145-1146.
50. Hugenholtz H., Cass D.E., Dvorak M.F., et al. High-dose methylprednisolone for acute closed spinal cord injury—only a treatment option. Can J Neurol Sci. 2002;29(3):227-235.
51. Qian T., Guo X., Levi A.D., et al. High-dose methylprednisolone may cause myopathy in acute spinal cord injury patients. Spinal Cord. 2005;43(4):199-203.
52. Hurlbert R.J., Hamilton M.G. Methylprednisolone for acute spinal cord injury: 5-year practice reversal. Can J Neurol Sci. 2008;35(1):41-45.
53. Young W., Bracken M.B. The Second National Acute Spinal Cord Injury Study. J Neurotrauma. 1992;9(Suppl 1):S397-S405.
54. Wu J., Yang H., Qiu Z., et al. Effect of combined treatment with methylprednisolone and Nogo-A monoclonal antibody after rat spinal cord injury. J Int Med Res. 2010;38(2):570-582.
55. Gerndt S.J., Rodriguez J.L., Pawlik J.W., et al. Consequences of high-dose steroid therapy for acute spinal cord injury. J Trauma. Feb 1997;42(2):279-284.
56. Tsutsumi S., Ueta T., Shiba K., et al. Effects of the Second National Acute Spinal Cord Injury Study of high-dose methylprednisolone therapy on acute cervical spinal cord injury—results in spinal injuries center. Spine (Phila Pa 1976). 2006;31(26):2992-2996. discussion 2997
57. Matsumoto T., Tamaki T., Kawakami M., et al. Early complications of high-dose methylprednisolone sodium succinate treatment in the follow-up of acute cervical spinal cord injury. Spine (Phila Pa 1976). 2001;26(4):426-430.
58. Ito Y., Sugimoto Y., Tomioka M., et al. Does high dose methylprednisolone sodium succinate really improve neurological status in patient with acute cervical cord injury? A prospective study about neurological recovery and early complications. Spine (Phila Pa 1976). 2009;34(20):2121-2124.
No Administration of Steroids
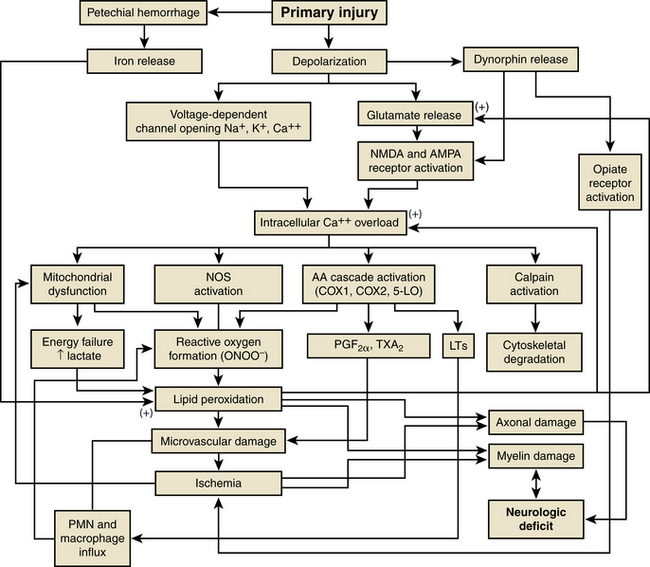
Acute spinal cord injury (SCI) is a devastating traumatic injury with significant impact on a patient’s psychological and physical well-being as well as substantial associated societal costs. According to Yeo et al., the mean life expectancy of a person with SCI compared to that of the entire population is estimated at 70% for patients with complete tetraplegia, 84% for patients with complete paraplegia, and 92% for patients with an incomplete injury and spared motor function.1 The potential for postinjury therapeutic interventions exist because most cases of traumatic SCI involve a severe contusion of the spinal cord rather than spinal cord transection.2 Following the mechanical disruption of local tissues during the primary spinal cord insult, secondary injury occurs. Although the exact pathogenesis of secondary injury is still being elucidated, it represents a complex interaction of intracellular and extracellular processes that begin within minutes following the initial injury3 (Fig. 230-1).
Surgery following spine trauma is targeted at preventing further mechanical damage to the spinal cord by reestablishing the stability of the vertebral column.4 Pharmacologic therapies, on the other hand, are most often targeted at the secondary injury cascade, which can aggravate primary injury and worsen neurologic deficits. The concept of pharmacologic intervention to improve outcomes following acute SCI is therefore both appealing and feasible. From a treatment standpoint, organized trauma care provides an opportunity to administer drugs within hours of sustaining the initial SCI. Many agents have been studied in animal models of acute SCI, but very few have had potential application to human SCI patients, including corticosteroids.5
The precise mechanism of corticosteroids is unknown, but they have the potential to stabilize membrane structures and maintain the blood–spinal cord barrier, thereby potentially reducing vasogenic edema. Corticosteroids can also enhance spinal cord blood flow, alter electrolyte concentrations at the site of injury, inhibit endorphin release, scavenge damaging free radicals, and limit the inflammatory response following injury.5–10 Methylprednisolone (MP) is one corticosteroid that has been studied extensively both in animal models and in controlled randomized blinded clinical trials of patients with SCI. Few medical management strategies, however, have generated as much conflict in the literature as the utilization of MP following nonpenetrating acute SCI. Even though it has been more than two decades since the initial reports on the beneficial effects of MP in SCI patients, according to the National Acute Spinal Cord Injury Studies (NASCIS), the use of steroids remains highly controversial.4,11–16
In this chapter, the NASCIS publications, the controversy surrounding the NASCIS studies, and comment on other studies that have evaluated the role of MP in acute SCI are briefly reviewed. Because of the highly controversial nature of this topic, health-care providers are encouraged to review some of the pertinent literature themselves to help formulate their own opinion.
History of National Acute Spinal Cord Injury Study
The NASCIS was established in 1975 under the leadership of Michael Bracken in the department of epidemiology and public health at Yale University.4 The goal of NASCIS was to evaluate pharmacologic therapies that could potentially improve recovery following traumatic injury to the spinal cord. Early experiments compared placebo, hypothermia, and corticosteroids in the treatment of SCI in animals. Initial findings demonstrated improved neurologic recovery in animals treated with hypothermia and steroids when treatment was delivered immediately following injury.17–20 Many preclinical experiments also demonstrated that functional recovery and tissue preservation occurred as a result of inhibition of posttraumatic spinal cord lipid peroxidation by large doses of MP shortly following injury.20,21
National Acute Spinal Cord Injury Study
A prospective randomized double-blind clinical phase II NASCIS trial was initiated in 1979 at nine hospitals in seven states.22,23 Patients with acute SCI were randomized within 48 hours of SCI to one of two treatment arms: low-dose (100 mg bolus followed by 25 mg every 6 hours for 10 days) (n = 165) and high-dose (1000 mg bolus followed by 250 mg every 6 hours for 10 days) (treatment group) intravenous MP. The bolus MP dose was given over 10 minutes, and there was no placebo group in this study. A total of 330 patients were entered, of whom 179 (54%) were available for 6-month follow-up. Motor scores were assessed on a six-point scale and determined from examination of seven muscle groups on each side of the body. Sensory function was assessed on a three-point scale of dermatomal light touch and pinprick sensation. Motor and sensory scores were reported only from the right side of the body, and the study did not require a minimum motor impairment for inclusion.
Results of this study demonstrated no significant difference in any of the primary neurologic outcome measures between the low-dose and high-dose MP groups at any of the 6-week, 6-month, or 1-year follow-ups. There was, however, increased risk of wound infection in the high-dose (9.3%) regimen group compared to the low-dose group (2.6%). There was also a trend toward a higher incidence of sepsis, pulmonary embolus, and death in the high-dose group that was not statistically significant.14
National Acute Spinal Cord Injury Study 2
The lack of any benefit from MP utilization in NASCIS was attributed to treatment doses that were considered subtherapeutic as determined previously in animal studies. NASCIS 2 was started in the mid-1980s and consisted of the prospective randomized double-blind use of MP, naloxone, or placebo in 10 hospitals in eight states.24,25 Naloxone is an opiate antagonist that had previously shown promise in animal studies.23 A total of 487 patients were randomized into one of the three treatment groups within 12 hours of acute SCI. The control group (n = 171) was given a placebo infusion, one treatment group received a naloxone 5.4 mg/kg body weight bolus with a 4 mg/kg/hour infusion for 23 hours (n = 154), and another treatment group received a MP 30 mg/kg body weight bolus in the first hour followed by a 5.4 mg/kg/hour intravenous infusion for 23 hours (n = 162). Motor scores were assessed on a five-point scale and determined from the examination of seven muscle groups on each side of the body. Motor scores were reported only from the right side of the body, and no minimum motor impairment was necessary for inclusion. Sensory function was assessed by using a three-point scale of dermatomal light touch and pinprick sensation. Follow-up was 97% at 6 months and 95% at 1 year, not including deaths.
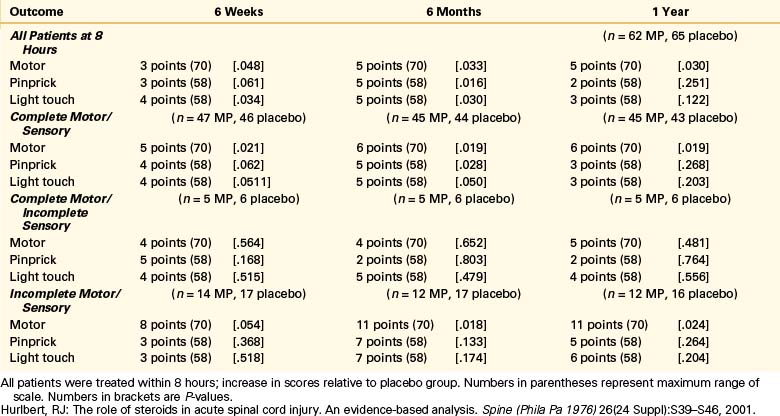
Patients in NASCIS 2 demonstrated no differences in neurologic outcome measures between any of the treatment groups before stratification. When patients were stratified according to time to initiate treatment (≤8 hours vs. >8 hours), however, some significant results were obtained. Patients who received MP within 8 hours of acute SCI were reported to demonstrate significant improvement in motor and sensory function at the 6-month follow-up compared to patients who received naloxone, placebo, or MP more than 8 hours after injury. When patients were further subdivided into groups based on complete and incomplete injuries, motor improvement was reported significantly improved at 1 year in patients who demonstrated motor and sensory complete deficits as well as those who were motor and sensory incomplete. There were no significant differences in sensory scores for any of the treatment groups or categories of patients despite the difference reported at the 6-month follow-up for patients who received MP within 8 hours of injury. There was a twofold increase in wound infection in patients treated with steroids compared with controls (7.1% vs. 3.6%, respectively) and a higher rate of gastrointestinal bleeding in the steroid-treated patients (4.5% vs. 3.0%, respectively).5,14,26
National Acute Spinal Cord Injury Study 3
NASCIS 3 was a prospective randomized double-blind trial conducted in 1991 to compare the efficacy of MP administered for 24 hours versus MP administered for 48 hours versus tirilazad mesylate (TM), a potent lipid peroxidation inhibitor.27,28 A total of 499 patients in 16 centers in Canada and the United States were randomized to one of the three treatment groups: MP 5.4 mg/kg/hour infusion for 24 hours (n = 166) or 48 hours (n = 167) or TM 2.5 mg/kg every 6 hours for 48 hours (n = 166). Only patients who presented within 8 hours of SCI were included, and all pregnant woman and patients with serious illness were excluded. All patients were given a 30 mg/kg intravenous bolus of MP prior to randomization, and there was no placebo group. Entry criteria were similar to those of NASCIS 2, and patients were assessed neurologically according to the NASCIS and NASCIS 2 (motor and sensory changes) protocols as well as change in Functional Independence Measure (FIM) scores. Follow-up was 95% at 6 months and 92% at 1 year, not including deaths.
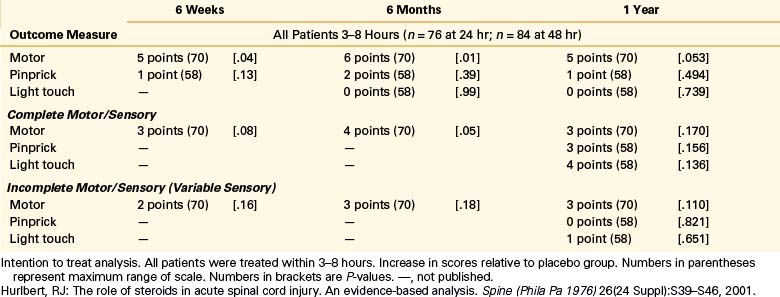
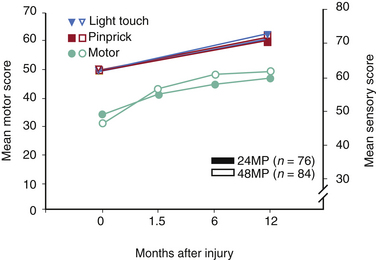
NASCIS 3 results demonstrated improved motor recovery on the 48-hour MP protocol compared with the 24-hour MP or 48-hour TM protocol at 6 weeks and 6 months. When treatment was initiated between 3 and 8 hours after SCI, significant motor function recovery found was on the 48-hour MP regimen at 6 weeks and 6 months compared to the 24-hour MP or 48-hour TM protocols. FIM scores were reported improved in the areas of self-care and sphincter control for the 48-hour group at the 6-month follow-up. All treatment groups saw the same recovery pattern when therapy was initiated less than 3 hours after injury. On the basis of these findings, the authors concluded that acute SCI patients who get MP within 3 hours should be maintained on the 24-hour protocol, whereas patients who receive MP 3 to 8 hours after injury should be maintained on the 48-hour protocol.
When the 1-year follow-up data were analyzed, patients in all treatment groups who were treated within 3 hours of injury were shown to have similar rates of recovery. In patients who received treatment between 3 and 8 hours following injury, patients on the 24-hour MP protocol had diminished motor recovery, whereas patients on the 48-hour MP protocol had increased motor recovery (P = .053). At 1 year, there was no significant difference in FIM between the treatment groups. However, there was double the incidence of severe pneumonia and a fourfold higher incidence of severe sepsis in the 48-hour MP group compared with the 24-hour MP group patients (5.8% vs. 2.6% and 2.6% vs. 0.6%, respectively). Although overall mortality rates were comparable between groups, there was a sixfold higher incidence of death due to respiratory complications in the 48-hour MP patients compared with the 24-hour MP cohort (P = .056)14 The authors again concluded that patients with acute SCI who were started on MP within 3 hours of injury should be maintained on the 24-hour protocol and that initiation of MP therapy in acute SCI patients 3 to 8 hours following injury should be maintained on the 48-hour protocol. These final recommendations appeared to be based on the motor recovery score improvement alone, despite the fact that the difference between groups was not statistically significant.5
Controversy
There have been, in essence, three NASCIS studies of MP in acute SCI, as summarized in the preceding sections. Although NASCIS is typically not a focal point of commentators, it is nonetheless important because it laid the foundation for the design and analysis of the subsequent NASCIS 2 and NASCIS 3 trials. NASCIS 2 and 3 have been criticized repeatedly in the literature for flaws in methodology, scientific and statistical analyses, and conclusions14 (Table 230-1).
| Study Characteristics | NASCIS II (24 hours) | NASCIS III (48 hours) |
|---|---|---|
| Prospective randomized | + | + |
| Well-designed | + | + |
| Well-executed | + | + |
| Compelling results | − | − |
| Appropriate statistics | − | − |
| Meaningful outcomes | − | − |
| (Harmful?) | ||
| Reproducible | − | − |
Hurlbert, RJ: The role of steroids in acute spinal cord injury. An evidence-based analysis. Spine (Phila Pa 1976) 26(24 Suppl):S39–S46, 2001.
One criticism of the NASCIS 2 trial is the mechanism by which the trial results were publicized. The 6-month findings were released to the public and medical community simultaneously, prior to any scientific, peer-reviewed publication of the study. On the basis of their results, the authors of NASCIS 2 concluded that (1) treatment with the study doses of MP administered within 8 hours of SCI improved neurologic outcome and was therefore indicated in the treatment of patients with acute SCI and (2) study doses of MP were not associated with harmful side effects in comparison with patients in other treatment groups.5 The initial 1990 National Institutes of Neurological Disorders and Stroke press release of these conclusions was widely publicized in the news media with enthusiasm and fervor over the reported positive results.13 Abbreviated versions of the results also made their way into the popular press.7 Despite little opportunity for the implications of the NASCIS 2 results to be discussed among the members of the medical community and prompted by unusual medical and lay publicity, neurosurgeons felt considerable pressure to adopt this method of treatment even if they had reservations about efficacy and, more important, potential side effects.
Many clinicians have questioned the authors of the NASCIS trials and their ability to provide sufficient evidence to support the use of MP in acute SCI patients.4,14,15,29 One such article, published by Coleman et al.,13 outlined weaknesses of the NASCIS 2 conclusions:
The article reported a positive result only for a secondary post hoc analysis in a subgroup of patients who received treatment within 8 hours and had, apparently, only 62 patients taking MP and 67 receiving placebo. (Data published later30 show further that most of the combined improvement from all patients in this subgroup was due to differences in the changes in the patients with incomplete lesions. This comparison involved only 22 patients in the MP group and 24 patients in the placebo group.) The reported recovery was for a motor scale rather than for a functional scale that might have been more indicative of clinical recovery.
Thus, the result that was publicized and reported as primary was found only in a small subgroup. This fact is still poorly appreciated because of the confusing way in which the results were presented. The article abstract gave sample sizes for the entire patient group but does not mention the negative result of the analysis for this group. It reports the positive result for the subgroup but does not mention its size. The size of this subgroup is nowhere given explicitly but apparently must be inferred by adding the sizes, given in Table 230-5 [in orig. article], of some of its subgroups.31
In response to a letter32 in The Medical Journal of Australia, Dr. Michael Bracken31 gave seven reasons for the decision to release the study findings before their publication. These reasons might be convincing were it not for the disparity between the strength of the conclusions reported in the early publicity and those reported in the New England Journal of Medicine article, and were it not for the potentially misleading vagueness of the reporting in the article.
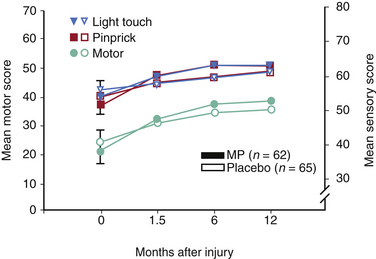
In essence, none of the NASCIS studies achieved their primary end point. All primary outcomes, defined before patient enrollment, were negative in NASCIS 2 and NASCIS 3, and the only interesting findings were encountered when post hoc analyses were performed14 (Tables 230-2 and 230-3). In both NASCIS 2 and 3, the mean improvement that was noted in treatment groups was not significant when compared as a function of the entire measurement scale (representing complete quadriplegia to normalcy) rather than as a function of baseline14 (Figs. 230-2 and 230-3). In his analysis of nine clinical studies that have been published on the effects of steroids in acute SCI, Hurlbert14 concluded that (1) neither NASCIS 2 nor NASCIS 3 provided acceptable evidence (level 1 or otherwise) to support the use of MP and (2) there exists no convincing class II or even class III evidence that makes a reasonable argument in favor of the use of MP for acute SCI. Furthermore, neither the efficacy nor the safety of MP in acute SCI was established, as there was a noted trend toward higher infectious complications and death from respiratory compromise in steroid-treated patients.
Although the NASCIS trials represent landmark clinical trials because of the sheer volume of patients recruited, many in the medical community could not understand why MP treatment was advocated on the basis of conclusions drawn from a selected post hoc subgroup analysis in one clinical trial. As Short et al.16 stated, promoted practices require a more thorough search for pertinent studies, appropriate inclusion criteria, and adequate assessment of the validity of included and defined frameworks for interpreting results and making practice recommendations. The 2001 Spine Focus Panel summary statement stated that “no clear consensus could be reached on the appropriate use of steroids” in acute SCI.33 Further, in the summary statement, Fehlings acknowledged that while many members felt that MP is clearly indicated in acute SCI because of its favorable risk-benefit profile and the lack of alternative therapies, a significant minority felt that the evidence supporting the use of steroids was weak and did not justify its use.33 Of historical note, the authors of the NASCIS trials finally agreed to release the primary data from the NASCIS trials around the time of the Spine Focus Panel’s summary statement.
Administration of Steroids: Not a “Standard of Care”
Although the scientific publication of NASCIS 2 in the New England Journal of Medicine was less conclusive than the public was led to believe, the administration of MP was considered by many to represent a “standard of care.”20,24 The problem for treating physicians subsequently became difficult because of the possibility that failure to administer MP to a spine-injured patient could lead to medicolegal problems.34 The notion that MP is a standard of care in the United States certainly requires further clarification. The common definition of “standard of care” relates to its use in medical malpractice litigation, in which a standard of care is defined loosely as the care that most physicians would provide in a similar clinical situation. This definition places no value on the actual worth of a practice but simply refers to its common occurrence. The promotion of MP by the NASCIS investigators created a perception of a standard of care that was not based on solid scientific data. The phrase “treatment standard” has also been used in some clinical practice guidelines to indicate practices that are supported by high-quality medical evidence. These recommendations are made in contrast to treatment “options,” which are made on the basis of lower-quality evidence. In fact, an evidence-based review of the use of MP was performed by the American Association of Neurological Surgeons/Congress of Neurological Surgeons (AANS/CNS) guidelines committee. In the 2002 Guidelines for the Management of Acute Cervical Spinal and Spinal Cord Injuries by the Section on Disorders of the Spine and Peripheral Nerves of the AANS/CNS, the committee gave the following recommendations with regards to the use of corticosteroids for spinal cord injury5:
The guidelines committee clearly felt that methodologic flaws in the analysis and presentation of the NASCIS data devalued the author’s conclusions about the relative worth of MP administration in acute SCI. Furthermore, the complications associated with MP use were thought to be significant and worthy of consideration given the limited clinical benefit.5,35
Other Studies on Corticosteroid in Spinal Cord Injury
In 1993, Galandiuk et al. reported the results of 32 patients with cervical or upper thoracic acute SCI who had been treated at an urban trauma center between January 1987 and February 1993. Eighteen patients treated with no MP were compared to 14 patients who received MP according to the NASCIS 2 protocol within 8 hours of injury. Although no significant differences were found between the two groups, the patients who were treated with MP were found to have a higher rate of pneumonia (79% vs. 50%), longer hospital stays (44.4 days vs. 27.7 days), and altered immune response (lower percentage and density of monocyte class II antigen expression and lower T-cell helper/suppressor cell ratios).34
Gerhart et al. reported a concurrent cohort comparison study in 1995 of acute SCI patients treated in Colorado. They included 218 patients managed from May 1990 through December 1991 and another 145 patients treated in 1993. Of the patients treated in the earlier group, 51 patients received no medication; 100 patients received MP according to the NASCIS 2 protocol; and 67 patients received a different corticosteroid, were given an incorrect dose, or had insufficient data. Of the latter treatment date group from 1993, 39 patients received no medication; 88 patients received MP according to the NASCIS 2 protocol; and 18 patients received no medication, received an incorrect dose, or had insufficient data. When the 188 patients who received MP were compared to those that did not receive any MP (n = 90), no significant differences were found in outcomes as assessed by the Frankel scale at the time of hospital discharge.36
In 1994, Otani et al. completed a randomized prospective multicenter trial involving 158 patients in Japan. Eighty-two patients received MP treatment according to the NASCIS 2 protocol, and 76 patients did not receive MP. Forty-one patients were excluded from the analysis for protocol violations, leaving 117 patients (n = 70 MP, n = 47 non-MP) for analysis. Neurologic outcome measures were similar to those used in NASCIS studies except for a modified sensory scale. None of the neurologic outcomes measures differed significantly between the treatment groups. In a post hoc analysis, significantly more patients treated with MP were shown to have some degree of sensory improvement compared to patients not treated with MP.37
George et al. completed a retrospective evaluation in 1995 comparing the outcomes of 80 acute SCI patients treated with MP per the NASCIS 2 protocol to 65 acute SCI patients treated with no MP. The non–MP-treated group in this study was significantly older than the MP-treated group (38 years vs. 30 years), and the MP-treated patients had a significantly lower injury severity score than the non–MP-treated group even though the mean trauma scores were similar between the two groups. The authors did not find any statistically significant differences in mortality or neurologic improvement between the two groups. At the time of hospital discharge, the patients who did not receive MP treatment had better mobility even though they had a higher injury severity score and older age. It was not clear in the study why some patients did not receive corticosteroid therapy.38
In 1997, Gerndt et al. completed a retrospective review of 140 patients who were admitted within 8 hours of suffering an SCI. Ninety-three patients were treated with MP according to the NASCIS 2 protocol, and 47 patients from a historical control group received no corticosteroid therapy. Ninety-one patients who received corticosteroids outside the NASCIS protocol were not included in the study. There were no differences in injury severity between the two groups. Although the authors reported a reduction in duration of rehabilitation, they also found a 2.6-fold higher rate of pneumonia (statistically significant) as well as ventilated days and intensive-care length of stay in the MP-treated group. There was also a higher incidence of urinary tract infections in the non–MP-treated group, but they did not find any difference in other outcome parameters or mortality between the treated and untreated patients.39
A European study from the National Spinal Trauma Unit in Dublin, Ireland, was published in 1997 by Poynton et al. In their case-control analysis, they evaluated 71 consecutive patients with acute SCI over a mean follow-up of 30 months (range, 13 to 57 months). A total of 63 patients were available for follow-up; 38 patients treated with MP according to NASCIS 2 were compared with 25 patients who did not receive MP. The patients who did not receive MP were referred to the trauma center more than 8 hours following injury. The American Spinal Injury Association (ASIA) scoring system was used on admission and at follow-up to determine changes in neurologic status. The authors found no difference in neurologic outcome between patients treated with MP and patients who did not receive MP.40
In 2000, Pointillart et al. conducted a prospective clinical trial in which they randomized 106 patients with acute SCI into one of four treatment groups: MP, nimodipine, MP plus nimodipine, and no pharmacologic therapy. MP was given according to the NASCIS 2 protocol, and the goal of the study was to evaluate the safety and efficacy of pharmacologic therapy in acute SCI patients. Patients were evaluated prior to initiation of treatment and at their 1-year follow-up via a blinded neurologic assessment via ASIA scoring. Although there was no statistically significant difference between any of the groups in terms of ASIA motor, sensory, or pain scores, patients in all groups demonstrated significant improvement at the 1-year follow-up compared to admission. A significant improvement was also noted in patients below the level of injury among patients with complete SCI compared to patients with incomplete injuries. The authors noted a nonsignificant higher rate of infectious complications in patients treated with MP compared with those that did not receive corticosteroids (66% vs. 45%) and a high incidence of hyperglycemia in almost half of the MP-treated patients who were analyzed for complications.41
In 2001, Matsumoto et al. conducted a prospective, randomized double-blind clinical trial in 46 patients with cervical acute SCI. Twenty-three patients treated with MP according to the NASCIS 2 protocol were compared with 23 patients in a placebo treatment group. The authors were specifically looking at potential medical complications following acute SCI. They found that patients with acute SCI treated with the MP protocol had a nonsignificant trend toward sepsis as well as a greater incidence of respiratory and gastrointestinal complications compared to the placebo-treated group.42 In 2003, Pollard and Apple retrospectively studied 412 patients with incomplete acute SCI over an 18-year period and found no evidence to support the administration of high-dose MP.43
In 2005, Aito et al. retrospectively analyzed 61 patients who sustained complete and incomplete SCI from diving accidents. They found a total of 30 patients with acute SCI over an 8-year period. Ten of the patients did not receive MP, and 20 patients received high-dose steroids. The degree of improvement was not reported in this study, but the authors reached statistical significance by applying logistical regression to the multivariate analysis and concluded that neurologic outcome was influenced positively by MP treatment during the initial 8 hours following trauma.44
In 2006, Tsutsumi et al. assessed neurologic recovery 6 weeks and 6 months after high-dose MP treatment according to the NASCIS 2 protocol to patients within 8 hours of suffering a traumatic cervical acute SCI. They compared improvements in ASIA motor scores (of patients with incomplete SCI) and myotomal levels (in complete SCI patients) between patients treated with MP (n = 37) and patients not administered MP (n = 33). They also evaluated early complications of administering high-dose MP between the two groups. They found the ASIA motor scores significantly improved in the incomplete paralysis patients at 6 weeks and 6 months in the MP group compared to the non-MP group. No significant improvement was seen in either ASIA motor or myotomal level among patients with complete paralysis. There were no significant differences in rates of early complications between the two groups, but the non-MP group had more early complications than the MP group.45
In 2008, Suberviola et al. retrospectively reviewed the data from all their patients who were admitted to the ICU within 8 hours of acute SCI over an 11-year period. Their focus was to evaluate the early complications and effect on neurologic outcome of MP treatment in SCI patients during the acute phase. Eligible patients were over 14 years and patients were grouped according to the medical treatment they received: those who received MP according to the NASCIS 2 protocol (n = 59) and patients who did not receive MP (n = 23). They found that while MP use in acute SCI patients was not associated with an improvement in outcome or neurologic function at ICU discharge compared with patients who did not receive MP, there was an increased risk of infectious and metabolic complications during the ICU stay.46
Other Effects of Corticosteroids
Aside from neurologic recovery, several authors have reviewed other glucocorticoid actions that could potentially counteract or even complicate any potential neuroprotective properties. See Box 230-1.
Risk of glucocorticoid steroid receptor-mediated side effects
Infection: pneumonia and septic shock
Avascular necrosis of the femoral head
Biphasic dose-response curve requires care in dose calculation and administration
Duration of treatment dosing (24 vs. 48 hours) depends on time of treatment initiation
Initiation of treatment beyond 8-hour window can exacerbate damage
Potential negative effect on neuronal survival and plasticity
Hall ED, Springer J: Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx 1:80–100, 2004.
Biphasic (U-Shaped) Neuroprotective Dose Response
The administration of MP can be difficult because of a sharp U-shaped dose-response curve requiring repeated dosing, such as continuous infusion, to maintain effects. While optimal dosing of MP lessens posttraumatic anaerobic metabolism and lactic acid accumulation, inadequate or excessive MP dosing can aggravate posttraumatic lactate accumulation.9 The potential for dosing miscalculations and lack of compliance to MP protocols is a very real problem.12 Molloy et al. found that, of 100 consecutive patients with acute SCI, only 25% of patients were given MP correctly according to NASCIS 2 or NASCIS 3 protocols. An additional 10 patients in this study were given MP but not according to NASCIS protocols; 4 of the patients were maintained on the wrong duration of MP infusion, and the other 6 patients were given MP more than 8 hours after their injury.47 This problem was also found in a study completed by McCutcheon et al. in 2004. They retrospectively reviewed the administration of MP for acute SCI in South Carolina between 1993 and 2000 and found that only 48.7% of the 1227 acute SCI patients they randomly reviewed had received MP and that the distribution of MP was not uniform.48
Duration of Treatment Dependent on Time of Treatment Initiation
Attention to the time of injury versus the treatment initiation time adds another layer of complexity and difficulty to the utilization of MP in acute SCI. According to the NASCIS studies, patients need to receive MP continuous infusion (according to the NASCIS 3 protocol) for 24 hours if treatment was initiated within the first 3 hours after the initial insult. However, if therapy is initiated after 3 hours from time of injury, the infusion protocol is guided by the NASCIS 3 study and extended to 48 hours.9
Late Treatment May Exacerbate Damage
According to Hall and Springer, initiation of MP treatment beyond the 8-hour therapeutic window can actually lead to inhibition of membrane phospholipase A2 and subsequent aggravated peroxidative damage.9 This means that the precise time of injury needs to be noted for initiation of treatment and that any attempts to start MP protocols should be avoided beyond the 8-hour window.
Potential Negative Effects on Neuronal Survival and Plasticity
Corticosteroids have been shown to attenuate the regenerative response by inhibiting axonal sprouting and synaptogenesis.9 Although it has not been specifically studied in spinal neurons, the effect of high-dose MP on posttraumatic plasticity requires further investigation. Merola et al., in their histologic tissue analysis following high-dose MP therapy, found that while high-dose MP reduced the development of severe edema and preserved spinal cord structure adjacent to the site of injury, it did not alter the development of spinal cord necrosis or astrocytic response at the area of injury.49
Leypold et al. evaluated the role of MP on lesion severity following acute SCI. They compared patients with complete cervical acute SCI treated with MP according to NASCIS 2 protocol initiated within 8 hours of injury to historical controls that did not receive MP treatment. All patients underwent MRI evaluation to determine the length of spinal cord edema, presence/absence of intramedullary hemorrhage, and length of intramedullary hemorrhage between the two groups. The authors found a significant reduction in the length of intramedullary hemorrhage in MP-treated patients and a nonsignificant reduction in the number of patients exhibiting spinal cord hemorrhage and mean length of spinal cord edema between the MP-treated and untreated patients.50
Potential Development of Femoral Head Avascular Necrosis
Wing et al. compared the effect of MP administration on the development of avascular necrosis of the femoral heads in 59 patients who received MP according to the NASCIS 2 protocol with 32 patients who did not receive corticosteroids. The authors estimated the relative risk of avascular necrosis with high-dose 24-hour MP therapy to be less than 5%.51
Potential Development of Acute Corticosteroid Myopathy
Qian et al. completed a prospective cohort study to evaluate for acute corticosteroid myopathy in SCI patients treated with MP. They performed muscle biopsies on five patients who were treated with MP according to the NASCIS 3 protocol and three patients who did not receive MP. They found muscle damage consistent with acute corticosteroid myopathy in four out of the five patients treated with MP and in none of the non–MP-treated patients. Electromyography studies were also completed and were normal in the patients who did not receive MP but were consistent with myopathic changes in the MP-treated patients.52
Shifting Practice Paradigms
In 2001, Molloy et al. published the results of a questionnaire survey of delegates at a European Cervical Spine Research Society Meeting. They found that 75% of the respondents gave MP according to NASCIS 2 and NASCIS 3 protocols in acute SCI patients. Further, two-thirds of these delegates had reservations about the efficacy of the research regarding the use of MP but felt that the potential benefits outweighed the risks of MP treatment.53 In 2002, the results of the first Canadian survey on steroid administration by orthopedic and neurologic spine surgeons were published by Hurlbert and Moulton. Their goal was to determine the practice patterns of MP administration for patients with acute SCI and the reasons behind the patterns of treatment. They found that approximately 76% of treating physicians were prescribing MP for acute SCI. Even more interesting, however, was the fact that only 17% of Canadian spine surgeons were prescribing MP for acute SCI because they felt that it was clinically effective, whereas 70% prescribed it either because of peer pressure or out of fear of litigation. On the basis of these results, the authors concluded that there was a need for evidence-based practice guidelines.54
A committee of Canadian neurosurgical and orthopedic spine specialists, emergency physicians, and physiatrists reviewed the evidence and concluded that high-dose MP infusion was not an evidence-based standard of care for patients with acute SCI.55,56 The Canadian Neurosurgical Society, the Canadian Spine Society, and the Canadian Association of Emergency Physicians all adopted the committee’s recommendations that a high-dose, 24-hour infusion of MP started within 8 hours following an acute SCI is not a standard treatment or a guideline for treatment but rather is a treatment option for which there is very weak level 2 and 3 evidence.56,57
Hurlbert and Hamilton reevaluated the practice patterns for MP administration in patients with acute SCI among orthopedic and neurologic spine surgeons across Canada 5 years after publishing practice recommendations. In sharp contrast to 2002, they found that 76% of spine surgeons did not prescribe MP for acute SCI, compared to the 76% who had prescribed it 5 years earlier. The NASCIS 2 dosing regimen was followed by 24% of prescribing practitioners. Interestingly, about one third of those who continued to prescribe MP did so because of fear of litigation.58
Why was there such a dramatic shift in treatment over just a few years? As discussed by Hurlbert in his 2008 paper, very little new evidence was published with respect to the efficacy of MP in SCI over the 5-year interval between surveys. The reason for this shift was identified by the respondents as stemming from information disseminated through journal publications, subspecialty meetings, and discussions with colleagues.58
Conclusion
The controversy and editorials over the NASCIS outcomes and conclusions will undoubtedly continue. The bottom line is that additional studies to clarify the efficacy of MP in acute SCI patients have yet to be completed while enduring the rigors of the scientific process. Despite previous attempts to clarify the role of MP in SCI, there has not yet been a single publication demonstrating significant clinical benefit or a superiority of MP over other pharmacologic agents. High-dose MP therapy in SCI is appropriately referred to as a “two-edged sword” because of the fact that potential neuroprotective properties of MP are offset by the risk of harmful side-effects and complications in a very vulnerable patient population.9,34 The use of MP in acutely injured patients is a treatment option that may be employed on the basis of the particular characteristics of an individual patient, the SCI type and severity, associated injuries, and physician experience. However, there is no “treatment standard” with regard to the use of MP in acute SCI.
Coleman W.P., Benzel E., Cahill D.W., et al. A critical appraisal of the reporting of the National Acute Spinal Cord Injury Studies (II and III) of the methylprednisolone in acute spinal cord injury. J Spinal Disord. 2000;13(3):185-199.
Hadley M.N., Walters B.C., et al. Guidelines for the management of acute cervical spine and spinal cord injuries. Clin Neurosurg. 2002;49:407-498.
Hall E.D., Springer J. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004;1:80-100.
Hurlbert R.J. The role of steroids in acute spinal cord injury: an evidence-based analysis. Spine (Phila Pa 1976). 2001;26(24S):S39-S46.
Sayer F.T., Kronvall E., Nilsson O.G. Methylprednisolone treatment in acute spinal cord injury: the myth challenged through a structured analysis of published literature. Spine J. 2006;6:335-343.
Short D.J., El Masry W.S., Jones P.W. High dose methylprednisolone in the management of acute spinal cord injury. a systematic review from a clinical perspective. Spinal Cord. 2000;38:273-286.
1. Yeo J.D., Walsh J., Rutkowski S., et al. Mortality following spinal cord injury. Spinal Cord. 1998;36:329-336.
2. Kakulas B.A. The clinical neuropathology of spinal cord injury: a guide to the future. Paraplegia. 1987;25:212-216.
3. Rozet I. Methylprednisolone in acute spinal cord injury—is there any other ethical choice? J Neurosurg Anesthesiol. 2008;20(2):137-139.
4. Sayer F.T., Kronvall E., Nilsson O.G. Methylprednisolone treatment in acute spinal cord injury: the myth challenged through a structured analysis of published literature. Spine J. 2006;6:335-343.
5. Hadley M.N., Walters B.C., et al. Guidelines for the management of acute cervical spine and spinal cord injuries. Clin Neurosurg. 2002;49:407-498.
6. Amar A.P., Levy M.L. Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery. 1999;44:1027-1039. discussion 1039-1040
7. Molloy S., Middleton F., Casey A.T.H. Failure to administer methylprednisolone for acute traumatic spinal cord injury: a prospective audit of 100 patients from a regional spinal injuries unit. Injury. 2002;33:575-578.
8. Fehlings M.G., Baptiste D.C. Current status of clinical trials for acute spinal cord injury. Injury. 2005;36(Suppl 2):B113-B122.
9. Hall E.D., Springer J. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004;1:80-100.
10. Tsai M.C., Wei C.P., Lee D.Y., et al. Inflammatory mediators of cerebrospinal fluid from patients with spinal cord injury. Surg Neurol. 2008;70(Suppl 1):19-24.
11. Nesathurai S. Steroids and spinal cord injury: revisiting the NASCIS 2 and NASCIS 3 trials. J Trauma. 1998;45:1088-1093.
12. Bracken M.B. Methylprednisolone and acute spinal cord injury: an update of the randomized evidence. Spine (Phila Pa 1976). 2001;26(Suppl):47-54.
13. Coleman W.P., Benzel E., Cahill D.W., et al. A critical appraisal of the reporting of the National Acute Spinal Cord Injury Studies (II and III) of the methylprednisolone in acute spinal cord injury. J Spinal Disord. 2000;13(3):185-199.
14. Hurlbert R.J. The role of steroids in acute spinal cord injury: an evidence-based analysis. Spine (Phila Pa 1976). 2001;26(Suppl 24):S39-S46.
15. Hurlbert R.J. Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J Neurosurg. 2000;93:1-7.
16. Short D.J., El Masry W.S., Jones P.W. High dose methylprednisolone in the management of acute spinal cord injury—a systematic review from a clinical perspective. Spinal Cord. 2000;38:273-286.
17. Ducker T.B., Hamit H.F. Experimental treatments of acute spinal cord injury. J Neurosurg. 1969;30:693-697.
18. Kuchner E.F., Hansebout R.R. Combined steroid and hypothermia treatment of experimental spinal cord injury. Surg Neurol. 1976;6:371-376.
19. Ducker T.B., Zeidman S.M. Spinal cord injury. Role of steroid therapy. Spine (Phila Pa 1976). 1994;19:2281-2287.
20. Hardy R.W. Commentary on spinal cord injury: role of steroid therapy. Neurosurg Q. 1996;6(1):71-72.
21. Hall E.D. Inhibition of lipid peroxidation in central nervous system trauma and ischemia. J Neurol Sci. 1995;134(Suppl):79-83.
22. Bracken M.B., Collins W.F., Freeman D.F., et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA. 1984;251:45-52.
23. Bracken M.B., Shepard M.J., Hellenbrand K.G., et al. Methylprednisolone and neurological function one year after spinal cord injury. J Neurosurg. 1985;63:704-713.
24. Bracken M.B., Shepard M.J., Collins W.F., et al. A randomized controlled trial of mthylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405-1411.
25. Bracken M.B., Shepard M.J., Collins W.F.Jr., et al. Methylprednisolone or naloxone treatment after acute spinal cord injury: 1 year follow-up data. Results of the second National Acute Spinal Cord Injury Study. J Neurosurg. 1992;76:23-31.
26. Bracken M.B., Aldrich E.F., Herr D.L., et al. Clinical measurement, statistical analysis, and controversies from trials of spinal injury. J Trauma. 2000;48(3):558-561.
27. Bracken M.B., Shepard M.J., Holford T.R., et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury: results of the third national acute spinal cord injury randomized controlled trial. JAMA. 1997;277(20):1597-1604.
28. Bracken M.B., Shepard M.J., Holford T.R., et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow-up. J Neurosurg. 1998;89:699-706.
29. Miller S. Methylprednisolone in acute spinal cord injury: a tarnished standard. J Neurosurg Anesthesiol. 2008;20(2):140-142.
30. Bracken M.B., Holford T.R. Effects of timing of methylprednisolone or naloxone administration on recovery of segmental and long-tract neurological function in NASCIS 2 [Comments]. J Neurosurg. 1993;79:500-507.
31. Bracken M.B. Methylprednisolone in the management of acute spinal cord injuries [Letter] [Comment]. Med J Aust. 1990;153:368.
32. Taylor T.K., Ryan M.D. Methylprednisolone in the management of acute spinal cord injuries [Letter] [Comments]. Med J Aust. 1990;153:307-308.
33. Fehlings M. The Spine Focus Panel: Summary statement: the use of methylprednisolone in acute spinal cord injury. Spine (Phila Pa 1976). 2001;26(245):S55.
34. Galandiuk S., Raque G., Appel S., Polk H.C.Jr. The two-edged sword of large-dose steroids for spinal cord trauma. Ann Surg. 1993;218(4):419-427.
35. Geisler F.H., Coleman W.P., Benzel E., et al. Spinal cord injury (correspondence). Lancet. 2002;360:1883.
36. Gerhart K.A., Johnson R.L., Menconi J., et al. Utilization and effectiveness of methylprednisolone in a population-based sample of spinal cord injured persons. Paraplegia. 1995;33:316-321.
37. Otani K., Abe H., Kadoya S., et al. Beneficial effect of methylprednisolone sodium succinate in the treatment of acute spinal cord injury (translation of Japanese). Sekitsui Sekizui J. 1994;7:633-637.
38. George E.R., Scholten D.J., Buechler C.M., et al. Failure of methylprednisolone to improve the outcome of spinal cord injuries. Am Surg. 1995;61:659-664.
39. Gerndt S.J., Rodriguez J.L., Pawlik J.W., et al. Consequences of high-dose steroid therapy for acute spinal cord injury. J Trauma. 1997;42:279-284.
40. Poynton A.R., O’Farrell D.A., Shannon F., et al. An evaluation of the factors affecting neurological recovery following spinal cord injury. Injury. 1997;28:545-548.
41. Pointillart V., Petitjean M.E., Wiart L., et al. Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord. 2000;38:71-76.
42. Matsumoto T., Tamaki T., Kawakami M., et al. Early complications of high-dose methylprednisolone sodium succinate treatment in the follow-up of acute-cervical spinal cord injury. Spine (Phila Pa 1976). 2001;26:426-430.
43. Pollard D.E., Apple D.F. Factors associated with improved neurological outcomes in patients with incomplete tetraplegia [clinical case series]. Spine. 2003;28(1):33-38.
44. Aito S., D’Andrea M., Werghagen L. Spinal cord injuries due to diving accidents. Spinal Cord. 2005;43(2):109-116.
45. Tsutsumi S., Ueta T., Shiba K., et al. Effects of the Second National Acute Spinal Cord Injury Study of high-dose methylprednisolone therapy on acute cervical spinal cord injury-results in spinal injury centers. Spine (Phila Pa 1976). 2006;31(26):2992-2996.
46. Suberviola B., González-Castro A., Llorca J., et al. Early complications of high-dose methylprednisolone in acute spinal cord injury patients. Injury. 2008;39:748-752.
47. Molloy S., Middleton F., Casey A.T. Failure to administer methylprednisolone for acute traumatic spinal cord injury: a prospective audit of 100 patients from a regional spinal injuries unit. Injury. 2002;33:575-578.
48. McCutcheon E.P., Selassie A.W., Gu J.K., et al. Acute traumatic spinal cord injury, 1993–2000: a population-based assessment of methylprednisolone administration and hospitalization. J Trauma. 2004;56:1076-1083.
49. Merola A., O’Brien M.F., Castro B.A., et al. Histologic characterization of acute spinal cord injury treated with intravenous methylprednisolone. J Orthop Trauma. 2002;16(3):155-161.
50. Leypold B.G., Flanders A.E., Schwartz E.D., Burns A.S. The impact of methylprednisolone on lesion severity following spinal cord injury. Spine (Phila Pa 1976). 2007;32(3):373-378.
51. Wing P.C., Nance P., Connell D.G., Gagnon F. Risk of avascular necrosis following short-term megadose methylprednisolone treatment. Spinal Cord. 1998;36:633-636.
52. Qian T., Guo X., Levi A.D., et al. High-dose methylprednisolone may cause myopathy in acute spinal cord injury patients. Spinal Cord. 2005;43:199-203.
53. Molloy S., Price M., Casey A.T. Questionnaire survey of the views of the delegates at the European cervical spine research society meeting on the administration of methylprednisolone for acute traumatic spinal cord injury. Spine (Phila Pa 1976). 2001;26:E562-E564.
54. Hurlbert R.J., Moulton R. Why do you prescribe methylprednisolone for acute spinal cord injury? A Canadian perspective and a position statement. Can Journal of Neurol Sci. 2002;29:236-239.
55. Hugenholtz H., Cass D.E., Dvorak M.F., et al. High-dose methylprednisolone for acute closed spinal cord injury—only a treatment option. Can J Neurol Sci. 2002;29(3):227-235.
56. Hugenholtz H. Methylprednisolone for acute spinal cord injury: not a standard of care (commentary). CMAJ. 2003;168(9):1145-1146.
57. Canadian Task Force on Periodic Health Examination. The periodic health examination. 1. Introduction. CMAJ. 1986;134(7):721-729.
58. Hurlbert R.J., Hamilton M.G. Methylprednisolone for acute spinal cord injury: a 5-year practice reversal. Can J Neurol Sci. 2008;35:41-45.