Chapter 24 Medical and Endovascular Treatment of Renal Artery Disease
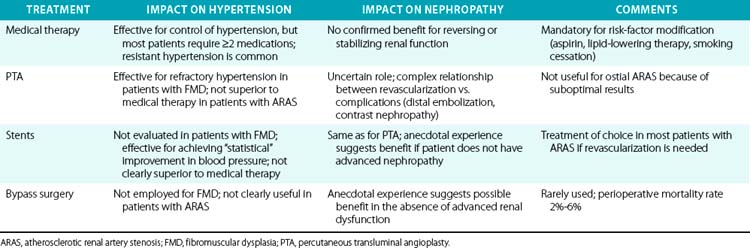
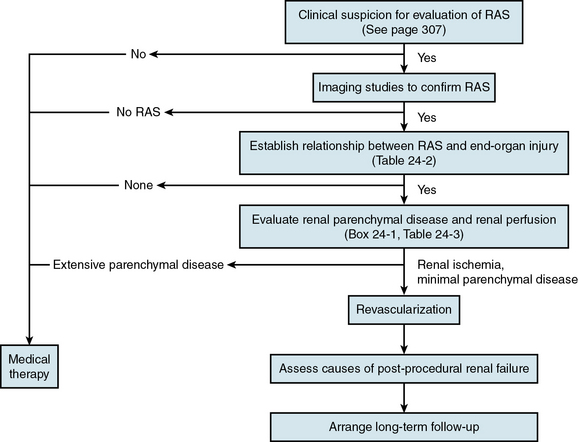
The clinical diagnosis of renal artery stenosis (RAS) relies on a high index of suspicion and confirmation by noninvasive and invasive imaging modalities (see Chapter 23). There are interrelated syndromes associated with RAS, including renovascular (renin-dependent) hypertension, essential hypertension, reversible ischemic renal dysfunction, and irreversible ischemic nephropathy. Clinical features that heighten suspicion for RAS include abrupt-onset or accelerated hypertension at any age, unexplained acute or chronic azotemia, azotemia induced by angiotensin-converting enzyme inhibitors (ACEIs), asymmetrical renal dimensions, and sudden pulmonary edema in the setting of normal left ventricular (LV) systolic function. Therapeutic considerations, alone or in combination, include medical therapy, percutaneous revascularization with angioplasty (PTA) or stenting, and surgical revascularization with bypass surgery or endarterectomy (Table 24-1). Revascularization of RAS with the goal of improving renal function and blood pressure remains controversial, so patient selection is extremely important (Fig. 24-1).
General Considerations for Treatment
Atherosclerosis accounts for more than 90% of cases of RAS, whereas the remaining 10% are associated with fibromuscular dysplasia (FMD) or inflammatory diseases of the renal arterial circulation. Whereas FMD is typically a disease of young and middle-aged females and usually involves the distal two thirds of the renal artery and its branches, atherosclerotic RAS (ARAS) is a disease of the elderly, particularly those with diabetes, aortoiliac occlusive disease, coronary artery disease (CAD), and hypertension. Atherosclerotic RAS usually involves the ostium and proximal one third of the renal artery, and it is a common manifestation of progressive atherosclerosis.1
Despite the prevalence and progressive nature of ARAS, it is likely many cases are never detected. Most patients with ARAS are identified during evaluation for refractory hypertension or progressive renal failure, or fortuitously as part of angiographic evaluation for aneurysmal or occlusive diseases of the aorta and lower-extremity arterial circulation. In general, decisions about treatment of patients with ARAS are usually based on blood pressure control, preservation of renal excretory function, and modification of risk factors for atherosclerosis.2
Identification of Renovascular Syndromes
There are five interrelated renovascular syndromes associated with RAS that can be broadly classified as anatomical RAS, renin-dependent hypertension, essential hypertension, reversible renal ischemic dysfunction, and irreversible ischemic nephropathy. These syndromes may occur alone or in combination with each other and with other nonvascular renal diseases. Furthermore, although the type of RAS (FMD, ARAS) is influenced by age, gender, and other patient-related risk factors for atherosclerosis, clinical manifestations (regarding effects on the kidney, heart, and brain) may be similar. Renin-dependent hypertension is much more likely to be caused by FMD in young patients, whereas ARAS in elderly patients is more likely to be associated with essential hypertension. Although both FMD and ARAS can be associated with similar manifestations of injury to the kidneys, heart, and brain (Table 24-2), renal revascularization is more likely to cure hypertension in FMD patients, whereas ARAS patients are likely to require lifelong antihypertensive medical therapy, despite revascularization. The key point is that patients with anatomical RAS without other clinical manifestations may be treated conservatively without revascularization. Prior to revascularization, patients with RAS and other clinical manifestations should undergo assessment of renal perfusion and the extent of parenchymal disease to determine the likelihood of clinical benefit.2
Table 24-2 Vital Organ Injury That May Be Caused by Hemodynamically Significant Renal Artery Stenosis
| Organ System | Injury |
|---|---|
| Renal | Ischemia/hypoperfusion |
| Cardiovascular | Hypertensive crisis ACS Unexplained pulmonary edema Aortic dissection |
| Cerebrovascular | Hypertensive crisis TIA Stroke ICH Severe retinopathy |
ACS, acute coronary syndrome; ICH, intracerebral hemorrhage; TIA, transient ischemic attack.
Evaluation of Renal Perfusion
As is true in the coronary circulation, there is poor correlation between angiographic RAS severity and hemodynamic significance, even when quantitative angiography is used.3 As a result, angiography alone is insufficient to establish the presence of renal hypoperfusion, regardless of stenosis severity. Several noninvasive and invasive methods are available to assess the physiological impact of ARAS and identify renal hypoperfusion (Box 24-1; also see Chapter 23). Nuclear scintigraphy and direct glomerular filtration rate (GFR) measurements can assess single- and total-kidney blood flow; diminished renal blood flow ipsilateral to a stenotic renal artery provides reliable evidence of renal hypoperfusion.4–6 Invasively, renal hypoperfusion can be identified with fractional flow reserve or translesional pressure gradients.3,7,8
![]() Box 24-1 Clinical Evaluation of Renal Artery Stenosis and Renal Hypoperfusion
Box 24-1 Clinical Evaluation of Renal Artery Stenosis and Renal Hypoperfusion
Invasive Assessment of Significance of Renal Artery Stenosis
Adapted from Safian RD, Madder RD: Refining the approach to renal artery revascularization. JACC Cardiovasc Interv 2:161–174, 2009.2
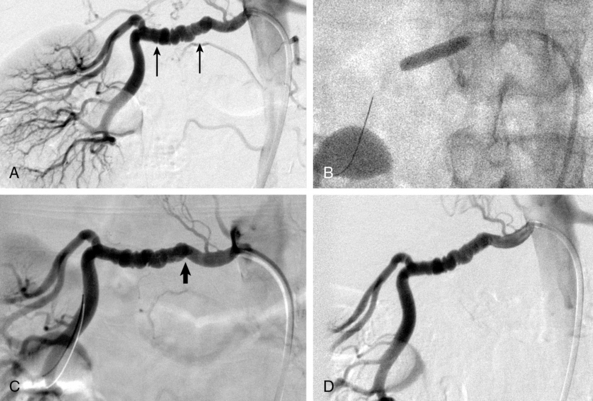
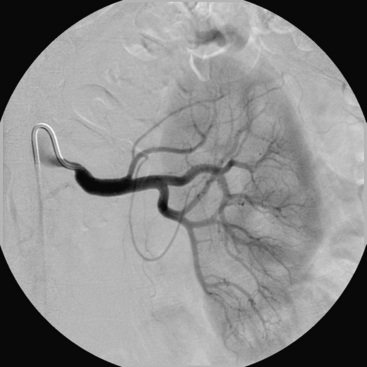
In patients with FMD, angiography alone is nearly useless for assessment of stenosis severity before or after revascularization, or for assessment of renal perfusion. In FMD patients, translesional pressure gradients are extremely useful for localizing the site of critical stenosis and assessing results after intervention (Fig. 24-2). Intravascular ultrasound (IVUS) can also be used to assess intraluminal and vessel dimensions, which are nearly impossible to assess by angiography alone.
Evaluation of Nephropathy
Assessment of baseline parenchymal disease is essential in selecting patients for renal revascularization (Table 24-3), since the presence of parenchymal disease prior to intervention is the most important predictor of adverse outcome. Even if renal hypoperfusion is present, identification of advanced parenchymal disease suggests that renal dysfunction maybe irreversible regardless of revascularization.9 The exception is the patient with advanced parenchymal disease, bilateral RAS, and recent dialysis, in whom a small increase in renal blood flow may permit separation from dialysis.2,10
| Factor | Comment |
|---|---|
| Serum Cr | Easy to measure and inexpensive. Relatively insensitive to degree of renal dysfunction and not reliable for differentiating nephropathy from renal ischemia. |
| Proteinuria | Easy to measure and inexpensive. Proteinuria ≥1 g/24 h is a good indication of nephropathy, but lesser degrees of proteinuria are less reliable. |
| Renal dimensions | Renal length 10-12 cm is generally favorable. Renal length ≤6 cm indicates irreversible renal injury (atrophic kidney). |
| RRI | RRI <70 is a good measure of reversibility. Although RRI >80 indicates parenchymal disease, it should not be used as the sole indicator of irreversible renal dysfunction. |
| Renal arteriogram | Preservation of cortical blood flow and absence of intrarenal arteriolar disease are indicators of reversible renal dysfunction. Poor cortical blood flow and severe diffuse intrarenal arteriolar disease are markers of advanced nephropathy. |
| Renal biopsy | Reliable for histological confirmation of nephropathy, but not practical for most patients. |
Cr, creatinine; RRI, renal resistive index.
Adapted from Safian RD, Madder RD: Refining the approach to renal artery revascularization. JACC Cardiovasc Interv 2:161–174, 2009.2
Initial clinical evaluation of parenchymal disease includes serum creatinine (Cr), urinalysis for proteinuria, and renal duplex ultrasound to measure renal resistive index (RRI) and kidney dimensions. When evaluating baseline renal function in patients with ARAS, it is important to realize that serum creatinine–based GFR estimates demonstrate good sensitivity but only modest specificity for identifying a measured GFR below 60 mL/min/1.73 m2 in individuals with ARAS.11 As a result, additional testing besides serum Cr should be performed to evaluate the presence of underlying nephropathy. Renal resistive index is obtained by averaging values obtained in the upper, middle, and lower intrarenal segmental arteries according to the formula 100 × [1 − (EDV/PSV)], where EDV and PSV are Doppler-derived end-diastolic and peak-systolic velocities, respectively. Compared to patients with RRI less than 80, those with RRI above 80 are older and have more extensive atherosclerosis and worse baseline renal function, consistent with more parenchymal disease. Additionally, baseline RRI greater than 80 is associated with inadequate blood pressure control, worsening Cr clearance, more frequent progression to dialysis, and higher mortality after renal revascularization.9 Several factors (renal dimensions, serum creatinine, presence of collaterals, and intact glomeruli by renal biopsy) have been proposed to suggest reversible renal failure, but the predictive value of these factors has not been validated, and renal biopsy is rarely employed for this purpose. Factors that identify irreversible dysfunction include severe diffuse intrarenal arteriopathy, proteinuria greater than 1 g/24 h (especially in a diabetic patient), and marked atrophy of the renal cortex.2
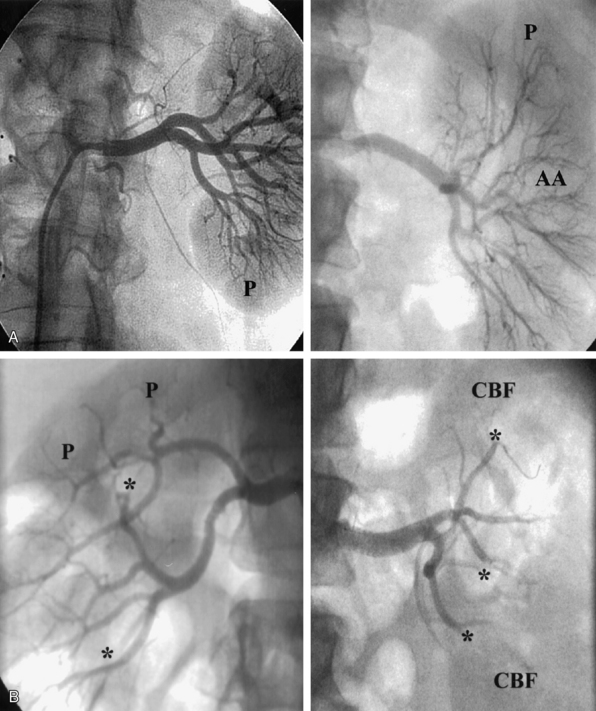
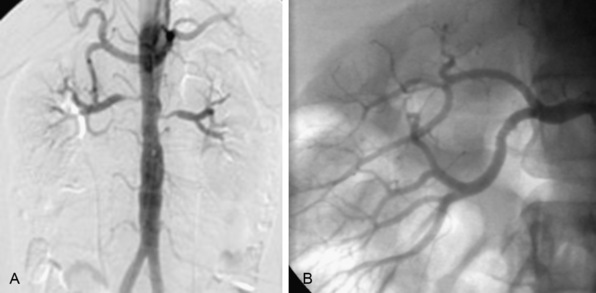
The nephrogram is often overlooked during selective renal arteriography, since many operators tend to focus on the renal artery itself. However, there are several arteriographic features that can indicate the presence of nephropathy, including intrarenal arteriolar narrowing, pruning (cut-off) of interlobar arterioles, and diminished cortical blood flow (Fig. 24-3). It is important to understand that it is best to use all the variables discussed to obtain a “nephropathy profile” because individual variables alone are not sufficiently reliable to assess the degree of nephropathy.2
Medical Therapy for Renal Artery Disease
The risk of cardiovascular events in hypertensive adults is most dependent on the degree of hypertension rather than its cause. True renovascular hypertension (i.e., renin-dependent hypertension) is much more likely in young patients with FMD than in elderly patients with ARAS. In fact, most hypertensive patients with ARAS have essential hypertension, whereas those with accelerated or malignant hypertension may have a renovascular component superimposed on a background of essential hypertension.2
Patients with FMD rarely have excretory dysfunction, and hypertension generally responds to ACEIs. In contrast, there are a number of issues concerning medical management of patients with ARAS. First, although never specifically studied in patients with ARAS, it is reasonable to treat all patients with aggressive risk-factor modification to limit atherosclerosis. These measures include aspirin, lipid-lowering therapy, smoking cessation, and aggressive treatment of diabetes mellitus to limit diabetic nephropathy.12 Second, medical therapy for hypertensive patients with ARAS is similar to that for patients with essential hypertension. Even after renal artery revascularization, antihypertensive medical therapy is necessary in most ARAS patients, since revascularization cures hypertension (i.e., normal blood pressure off all medications) in less than 10% of patients.13,14 Third, patients with ischemic renal dysfunction represent a particularly high-risk group with a poor prognosis, and there are no studies demonstrating benefit of medical therapy for reversing or stabilizing renal function. The impact of medical therapy on long-term renal function in ARAS is controversial. One study reported a rise in serum Cr concentration in 5% to 10% of patients,15 whereas another showed a progressive rise in Cr despite excellent blood pressure control.16
Use of an ACEI or angiotensin receptor blocker (ARB) is controversial in hypertensive patients with ARAS. Important considerations relate to extent of RAS and degree of baseline renal impairment. Patients with hypertension, unilateral ARAS, and normal baseline renal function are good candidates for an ACEI or ARB. In fact, ACEIs appear to be more effective than other antihypertensive agents in this setting. In patients with hypertension, unilateral RAS, and abnormal baseline renal function, ACEIs exert a beneficial impact on survival without affecting renal function. In these patients, long-term renal function is influenced most by the degree of baseline renal dysfunction and proteinuria, not by pharmacological treatment. In diabetic patients with hypertension, unilateral renal artery stenosis, proteinuria, and normal or abnormal renal function, ACEIs and ARBs are effective antihypertensive agents, and drug-induced reduction of intraglomerular capillary pressure decreases proteinuria and renal injury.17 It is interesting to speculate whether renal revascularization in this subgroup of patients could offset the benefit of ACEIs by increasing intraglomerular capillary pressure, proteinuria, and renal injury.
Selecting Patients for Renal Artery Endovascular Revascularization
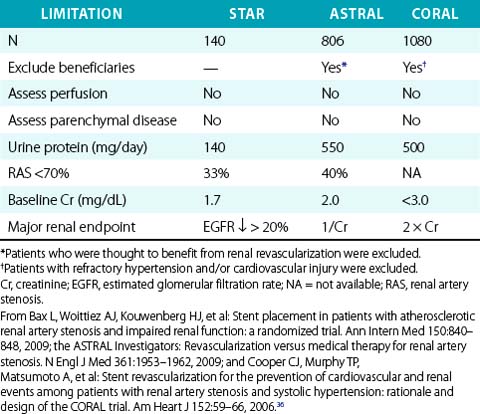
The major challenge in selecting patients for renal revascularization is the absence of compelling data supporting doing so. A review of worldwide studies of ARAS identified approximately 5000 studies of renal revascularization,18 including only two randomized controlled trials (RCTs) of PTA (not stenting) versus medical therapy.19,20 A subsequent review nearly led to withdrawal of Center for Medicare and Medicaid Services (CMS) reimbursement for renal stenting.21 Those two RCTs, two subsequent RCTs,22,23 and another comparative trial24 reported no benefit of renal revascularization (PTA or stenting) compared to best medical therapy with respect to hypertension control and estimated GFR during 1- to 2-year follow-up. In contrast, numerous observational studies reported stabilization or improvement in renal function4,25–27 and hypertension control8,28,29 after renal stenting. There are several potential explanations for the negative results of RCTs and discrepant findings among studies, including treatment of patients with anatomical stenosis but normal renal perfusion (i.e., oculostenotic reflex), failure to differentiate reversible ischemic renal dysfunction from irreversible parenchymal disease (i.e., nephropathy), and unrealistic expectations that ARAS patients have renin-dependent (i.e., renovascular hypertension) rather than essential hypertension.2 In addition, many RCTs have significant methodological flaws that limit their ability to measure changes in renal function,11 raising doubts about the validity of their results and conclusions (Table 24-4). Finally, the medical literature is filled with ambiguous and inconsistent terminology regarding renovascular syndromes.2
The 2005 American College of Cardiology/American Heart Association (ACC/AHA) guidelines propose recommendations for renal artery revascularization,12 even though the recommendations have not been established by any randomized clinical trials. In general, ACC/AHA revascularization guidelines are based on the assumption that the RAS is hemodynamically significant, and that revascularization will improve blood pressure control, preserve renal function, or have a favorable impact on cardiovascular manifestations of severe hypertension. Accordingly, patient selection can be enhanced by identification of clear clinical syndromes that link RAS to reversible injury to the heart, brain, or kidneys; by demonstration that RAS causes renal hypoperfusion; and by assessment of baseline renal parenchymal disease.
The best candidates for revascularization are patients with RAS, vital organ injury, renal hypoperfusion, and no underlying nephropathy. Conversely, the worst patients for revascularization are those with RAS, advanced nephropathy, and normal renal perfusion.2 Vital organ injury includes functional impairment of the heart, brain, or kidneys attributable to renal artery stenosis; such manifestations include hypertensive crisis (nonischemic pulmonary edema, acute coronary syndrome (ACS), aortic dissection, or neurological impairment) and renal insufficiency (rising Cr due to ACEIs, bilateral ARAS and rising Cr or declining nuclear GFR, and unilateral ARAS and fractional GFR ≤40%).
Patients with Renal Artery Stenosis and Refractory Hypertension
In patients with ARAS, refractory hypertension, and normal renal perfusion, it is reasonable to intensify the antihypertensive regimen, seek alternative etiologies for refractory hypertension, and follow patients clinically for development of vital organ injury. For patients with unilateral or bilateral ARAS, refractory hypertension, and objective evidence of renal hypoperfusion, revascularization is reasonable if advanced baseline nephropathy is not present (Fig. 24-4). For patients with hypertension and renal FMD, PTA should be performed if patients do not respond to ACEIs or ARBs.2 Additionally, such patients should undergo carotid duplex ultrasound and intracranial magnetic resonance angiography (MRA) or computed tomographic angiography (CTA), because carotid FMD and berry aneurysms of the circle of Willis are common.
Patients with Isolated Atherosclerotic Renal Artery Stenosis
In patients with unilateral or bilateral ARAS and no evidence of baseline nephropathy or cardiovascular injury, renal perfusion should be evaluated by noninvasive or invasive techniques. If renal perfusion is normal, revascularization is not indicated regardless of stenosis severity; such patients should be followed for development of vital organ injury. If renal hypoperfusion is documented, such patients may be considered to have “unilateral” renal injury. This form of renal injury is not mentioned in existing guidelines and has not been studied in randomized controlled trials, but we generally consider such patients candidates for renal revascularization to preserve renal function.2,4
Patients with Atherosclerotic Renal Artery Stenosis and Chronic Kidney Disease
Decisions regarding revascularization of patients with ARAS and renal dysfunction are often challenging.2 In general, patients without renal hypoperfusion should not be revascularized. For patients with unilateral ARAS, renal hypoperfusion, and underlying nephropathy, decisions regarding revascularization should be individualized; revascularization may not improve renal function but might be beneficial if other cardiovascular injury is present. Patients with renal dysfunction and bilateral ARAS or ARAS of a solitary kidney may have global renal ischemia; such patients should be considered for renal revascularization unless advanced parenchymal disease is identified (Fig. 24-5). In nondiabetic patients who have been on dialysis for less than 1 year, it is reasonable to perform diagnostic testing for bilateral ARAS, since some may benefit from renal revascularization and separate from dialysis.2,10
Type of Revascularization
Percutaneous revascularization is now widely accepted as the best technique for renal revascularization in most patients. For patients with refractory hypertension and FMD, PTA is preferred and results in renal artery patency rates of 90% at 10 years.30 Although PTA achieves excellent results in patients with FMD, stenting is the endovascular procedure of choice in patients with ARAS. Stenting in ARAS can be accomplished with procedural success rates exceeding 95%, major complications in less than 5%, and restenosis in 10% to 15%.
Impact of Endovascular Revascularization on Hypertension
The impact of revascularization on hypertension depends on the type of RAS, presence of renal hypoperfusion, and degree of renal parenchymal disease.2 Although numerous observational studies and two small randomized trials of PTA and medical therapy for ARAS demonstrated a significant decrease in blood pressure and fewer medications after PTA,9,20 a more recent randomized trial in hypertensive patients with ARAS showed no difference in outcomes between PTA and medical therapy.24 Published studies on the impact of renal revascularization on hypertension have numerous limitations including inclusion of patients with heterogeneous causes of hypertension, varying degrees of renal dysfunction, inconsistent techniques for revascularization, and ambiguous terminology and endpoints to assess clinical benefit. For example, hypertension has been classified as “cured” (i.e., blood pressure is normal without the need for medication), improved (number or dose of medications is reduced, blood pressure is better controlled, or both), or unchanged. Interpretation of data is limited by the uncertain clinical relevance of these classification groups. Furthermore, there are several potential reasons why renal artery revascularization for ARAS may not result in dramatic improvement or cure of hypertension. First, many patients with ARAS probably have essential, not renovascular hypertension. Experimental Goldblatt models demonstrate renin-angiotensin activation due to RAS, but hypertension in humans is more complex than indicated by these models.31 Hypertension in ARAS may be confounded by the presence of sympathetic and cerebral nervous system activation, vasoactive oxygen species, abnormalities in endothelial-dependent relaxation, and ischemic and hypertensive intrarenal injury.32 A more complex milieu than suggested by Goldblatt models is suggested by similar degrees of renin activation in hypertensive patients with and without ARAS and by the low cure rates demonstrated after successful revascularization. Second, many patients with hypertension have intrarenal parenchymal disease, leading to hypertensive nephropathy and self-perpetuating hypertension. In these patients, hypertension is sustained by intrarenal mechanisms including increased sympathetic nerve activity, renin-angiotensin system activity, and impaired sodium excretion regardless of patency of the proximal renal artery.
Despite these limitations, it is apparent that hypertension is more likely to be cured after revascularization in patients with FMD than in those with ARAS (75% vs. < 20%), regardless of the type of revascularization.20,30 With respect to differences in unilateral and bilateral ARAS, one study suggested that improvement in hypertension after renal stenting was more likely in the presence of severe bilateral ARAS and severe baseline hypertension (mean arterial pressure >110 mmHg),33 especially when parenchymal disease is absent.9,34 A contemporary study reports substantial improvement in blood pressure when renal hypoperfusion was sought and corrected by renal stenting.8
Impact of Revascularization on Renal Function
Observational studies suggest that renal artery revascularization can stabilize or improve renal function (Fig. 24-6). Surgical revascularization results in significant improvement in postoperative total- and single-kidney nuclear GFR, a slower decline in GFR, and improvements in renal dimensions and hyperconcentration of urinary creatine. Improvement in renal function after stenting occurred in 8% to 22% of patients in a systematic review,18 and 20 of 22 cohort studies reported improvement or stabilization of renal function.
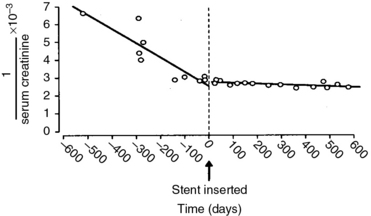
(Reproduced with permission from Harden PN, MacLeod MJ, Rodger RS, et al: Effect of renal artery stenting on progression of renovascular renal failure. Lancet 349:1133, 1997.)26
In contrast to these observational data, five prospective randomized trials of renal revascularization failed to demonstrate improvement in renal outcomes after intervention.19,20,22–24 Interpretation of these studies is confounded by failure to assess renal perfusion and the extent of baseline nephropathy prior to revascularization. Additionally, these studies relied on reciprocal serum Cr or creatinine-based GFR estimates as major renal endpoints, which have been shown to be unreliable for serial assessment of renal function in ARAS patients.11 Taken collectively, these studies suggest that patient selection for revascularization of ARAS that is based on the oculostenotic reflex, and without assessment of renal ischemia and parenchymal disease, is likely not beneficial.
Impact of Revascularization on Cardiovascular Outcome
Understanding the impact of renal revascularization on long-term outcome is hampered by selection bias and poorly designed randomized controlled trials. The survival of medically treated patients with renovascular disease has not been defined, but most late deaths are due to cardiovascular events rather than progressive renal failure. After revascularization, predictors of 5- and 10-year mortalities include age older than 60 years, CAD, baseline renal insufficiency, and persistent elevation of postoperative creatinine. Baseline Cr above 1.5 mg/dL is the strongest independent predictor of late mortality at 4 years (relative risk [RR] 5.0); is a stronger correlate of late mortality than diabetes (RR 2.5) or age older than 70 years (RR 1.9); and is associated with a greater risk of deterioration in renal function.35 Together, these data suggest that elderly patients with advanced generalized atherosclerosis, manifested by occlusive diseases in multiple vascular beds and baseline renal insufficiency, have a worse prognosis than patients with limited atherosclerosis and normal renal function. These data also suggest that the outcomes of renal revascularization are better when revascularization is performed before the development of advanced parenchymal disease.
To address the impact of renal artery revascularization on overall cardiovascular outcomes, best medical therapy alone is being compared to medical therapy plus stenting in the CORAL trial (Cardiovascular Outcomes with Renal Atherosclerotic Lesions). Enrollment of approximately 1100 randomized patients is expected by 2012, and the primary endpoint is a composite of death, myocardial infarction (MI), stroke, hospitalization for congestive heart failure, need for renal replacement, and doubling of serum Cr at 5 years.36
1 Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344:431.
2 Safian RD, Madder RD. Refining the approach to renal artery revascularization. JACC Cardiovasc Interv. 2009;2:161–174.
3 Subramanian R, White CJ, Rosenfield K, et al. Renal fractional flow reserve: a hemodynamic evaluation of moderate renal artery stenoses. Catheter Cardiovasc Interv. 2005;64:480–486.
4 Hanzel G, Balon H, Wong O, et al. Prospective evaluation of aggressive medical therapy for atherosclerotic renal artery stenosis, with renal artery stenting reserved for previously injured heart, brain, or kidney. Am J Cardiol. 2005;96:1322–1327.
5 Leertouwer TC, Derkx FH, Pattynama PM, et al. Functional effects of renal artery stent placement on treated and contralateral kidneys. Kidney Int. 2002;62:574–579.
6 La Batide-Alanore A, Azizi M, Froissart M, et al. Split renal function outcome after renal angioplasty in patients with unilateral renal artery stenosis. J Am Soc Nephrol. 2001;12:1235–1241.
7 Tonino PAL, De Bruyne B, Pijls NHJ, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224.
8 Mangiacappra F, Trana C, Sarno G, et al. Translesional pressure gradients to predict the blood pressure response after renal artery stenting in patients with renovascular hypertension. Circ Cardiovasc Interv. 2010;3:537–542.
9 Radermacher J, Chavan A, Bleck J, et al. Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N Engl J Med. 2001;344:410–417.
10 Textor SC, Wilcox CS. Renal artery stenosis: a common treatable cause of renal failure. Annu Rev Med. 2001;52:421–442.
11 Madder RD, Hickman L, Crimmins GM, et al. Validity of estimated glomerular filtration rates for assessment of baseline and serial renal function in patients with atherosclerotic renal artery stenosis: implications for clinical trials of renal revascularization. Circ Cardiovasc Interv. 2011;4:219–225.
12 Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice guidelines for the management of patients with peripheral arterial disease. Circulation. 2006;113:463–654.
13 Cooper CJ, Murphy TP. Is renal artery stenting the correct treatment of renal artery stenosis? the case for renal artery stenting for treatment of renal artery stenosis. Circulation. 2007;115:263–270.
14 Dworkin LD, Jamerson KA. Case against angioplasty and stenting of atherosclerotic renal artery stenosis. Circulation. 2007;115:271–276.
15 Caps MT, Zierler RE, Polissar NL, et al. Risk of atrophy in kidneys with atherosclerotic renal artery stenosis. Kidney Int. 1998;53:735.
16 Toto RD, Mitchell HC, Lee HC, et al. Reversible renal insufficiency due to angiotensin converting enzyme inhibitors in hypertensive nephrosclerosis. Ann Intern Med. 1991;115:513.
17 Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med. 1998;339:1448.
18 Balk E, Raman G, Chung M, et al. Effectiveness of management strategies for renal artery stenosis: a systematic review. Ann Intern Med. 2006;145:901–912.
19 Webster J, Marshall F, Abdalla M, et al. Randomised comparison of percutaneous angioplasty vs. continued medical therapy for hypertensive patients with atheromatous renal artery stenosis. J Hum Hypertens. 1998;12:329–335.
20 Plouin PF, Chatellier G, Darne B, et al. Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: a randomized trial. Hypertension. 1998;31:823–829.
21 Balk EM, Raman G. Comparative effectiveness of management strategies for renal artery stenosis: 2007 update, comparative effectiveness review no. 5 update. (Prepared by Tufts New England Medical Center under Contract No. 290-02-0022). Rockville, MD: Agency for Healthcare Research and Quality. 2007.
22 Bax L, Woittiez AJ, Kouwenberg HJ, et al. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med. 2009;150:840–848.
23 The ASTRAL Investigators. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009;361:1953–1962.
24 Van Jaarsveld BC, Krijnen P, Pieterman H, et al. The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. N Engl J Med. 2000;342:1007–1014.
25 Muray S, Martín M, Amoedo ML, et al. Rapid decline in renal function reflects reversibility and predicts the outcome after angioplasty in renal artery stenosis. Am J Kidney Dis. 2002;39:60–66.
26 Harden PN, MacLeod MJ, Rodger RS, et al. Effect of renal-artery stenting on progression of renovascular renal failure. Lancet. 1997;349:1133–1136.
27 Watson PS, Hadjipetrou P, Cox SV, et al. Effect of renal artery stenting on renal function and size in patients with atherosclerotic renovascular disease. Circulation. 2000;102:1671–1677.
28 De Bruyne B, Manharan G, Pijls NHJ, et al. Assessment of renal artery stenosis severity by pressure gradient measurements. J Am Coll Cardiol. 2006;48:1851–1855.
29 Mahmud E, Smith TWR, Palakodeti V, et al. Renal frame count and renal blush grade: quantitative measures that predict the success of renal stenting in hypertensive patients with renal artery stenosis. J Am Coll Cardiol Interv. 2008;1:286–292.
30 Slovut DP, Olin JW. Fibromuscular dysplasia. N Engl J Med. 2004;350:1862–1871.
31 Krum H, Sobotka P, Mahfoud F, et al. Device-based antihypertensive therapy: therapeutic modulation of the autonomic nervous system. Circulation. 2011;123:209–215.
32 Higashi Y, Sasaki S, Nakagawa K, et al. Endothelial function and oxidative stress in renovascular hypertension. N Engl J Med. 2002;346:1954–1962.
33 Rocha-Singh KJ, Mishkel GJ, Katholi RE, et al. Clinical predictors of improved long-term blood pressure control after successful stenting of hypertensive patients with obstructive renal artery atherosclerosis. Catheter Cardiovasc Interv. 1999;47:167.
34 Zeller T, Ulrich F, Muller C, et al. Predictors of improved renal function after percutaneous stent-supported angioplasty of severe atherosclerotic ostial renal artery stenosis. Circulation. 2003;108:2244–2249.
35 Dorros G, Jaff M, Mathiak L, et al. Four-year follow-up of Palmaz-Schatz stent revascularization as treatment for atherosclerotic renal artery stenosis. Circulation. 1998;98:642.
36 Cooper CJ, Murphy TP, Matsumoto A, et al. Stent revascularization for the prevention of cardiovascular and renal events among patients with renal artery stenosis and systolic hypertension: rationale and design of the CORAL trial. Am Heart J. 2006;152:59–66.