3 Mechanical Ventilation
• Noninvasive positive pressure ventilation reduces the need for endotracheal intubation.
• Rapid titration and adjustment of the noninvasive ventilator to reduce the work of breathing are essential for the success of noninvasive positive pressure ventilation.
• Monitoring of airway pressure and use of a low–tidal volume strategy minimize the risk for ventilator-induced lung injury.
• Appropriate selection of ventilator mode, tidal volume, positive end-expiratory pressure, fraction of inspired oxygen, and inspiratory flow rate is essential to minimize the work of breathing and enhance correction of hypoxemia.
• Correction of hypoxemia is critical, but correction of hypercapnia is not.
Epidemiology
Patients with severe respiratory complaints account for about 12% of emergency department (ED) visits.1 Almost 800,000 hospitalizations per year involve mechanical ventilation, which costs nearly $27 billion and represents 12% of all hospital costs. Although the overall number of patients requiring mechanical ventilation is small (2.8%), the relative mortality is as high as 34%.2 Twenty-six percent of asthmatic patients who required intubation reported the ED as their primary source of health care.3 Thorough knowledge of noninvasive and invasive mechanical ventilation, lung-protective ventilation strategies, and methods to enhance patient-ventilator synchrony is essential in the practice of emergency medicine.
Modes of Invasive Mechanical Ventilation
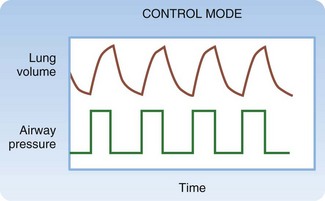
Control Mode
Control mode ventilation (CMV) is used almost exclusively in anesthesia, but knowledge of this mode’s limitations aids in comprehension of other modes’ features (Fig. 3.1). In CMV, all breaths are triggered, limited, and cycled by the ventilator. In volume-targeted mode, the physician selects a tidal volume (VT), RR, inspiratory flow rate (IFR), fraction of inspired oxygen (FIO2), and positive end-expiratory pressure (PEEP). The machine then delivers positive pressure and applies as much pressure as required to deliver the set VT at the set IFR. (In pressure-targeted mode the physician sets the pressure high, RR, FIO2 and pressure low or PEEP.) Note that patients can set their own flow rate in pressure-targeted modes. The machine then delivers positive pressure and applies as much pressure as required to reach the set pressure high. The VT values generated are a function of respiratory system compliance. The patient is not able to initiate or terminate a breath. If inspiratory effort is initiated before the machine is triggered to deliver a breath, airflow would not occur regardless of the patient’s inspiratory effort. If exhalation is incomplete and the time for the machine to deliver a breath has occurred, the ventilator would provide as much pressure as necessary to cause inhalation. Imagine forcibly exhaling, or coughing, when the ventilator begins to deliver a breath. This lack of synchrony would cause distress and risk structural lung or airway injury. For these reasons, CMV is never used except for apneic, paralyzed, or anesthetized patients.
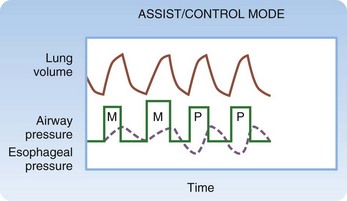
Assist/Control Mode
AC mode usually provides the greatest level of ventilatory assistance (Fig. 3.2). In volume-targeted ventilation, the physician sets VT, RR, IFR, FIO2, and PEEP. (In pressure-targeted mode, the physician sets the pressure high, RR, FIO2 and pressure low or PEEP.) In contrast to all other modes, the trigger that initiates inspiration can be either an elapsed time interval (determined by the set RR) or the patient’s spontaneous inspiratory effort. When either occurs, the machine delivers the set VT (in volume-targeted mode) or pressure high (in pressure-targeted mode). The machine follows a time algorithm that synchronizes the machine with patient-initiated breaths. If the patient is breathing at or above the set RR, all breaths are initiated by the patient. If the patient breathes below the set RR, machine-initiated breaths are interspersed among the patient’s breaths. Work of breathing (WOB) is primarily the effort that the patient exerts to cause airway pressure to drop to the threshold that triggers onset of the ventilator. (Manipulating the sensitivity of the ventilator sets this threshold.) Furthermore, WOB may be performed to a variable degree during inspiration, depending on how much the respiratory muscles are activated. WOB with the volume-targeted AC mode may be extreme in two situations: when the VT drawn by the patient is greater than the set VT and when the patient inspires at a rate that exceeds the set IFR (see later).
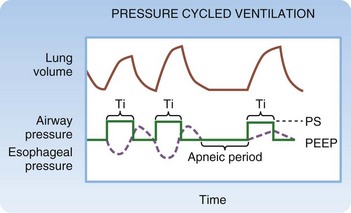
In the majority of situations, AC mode is used as described earlier and is termed volume-targeted or volume-cycled ventilation. As an alternative, some ventilators allow pressure-targeted (cycled) ventilation (PCV, not to be confused with PSV, described later) (Fig. 3.3). Instead of IFR, the limit during PCV is a set airway pressure. Instead of VT, the cycle during PCV is a set inspiratory time (TI). On some ventilator models, RR and the inspiratory-to-expiratory (I : E) ratio are set, and TI is calculated from these settings. On other models, TI is available as a setting. Because VT is not set, the VT delivered varies slightly from breath to breath, depending on lung compliance, airway resistance, and patient effort. PCV may offer a slight advantage over VCV in clinical scenarios that require control of the I : E ratio, but a body of literature investigating this concept does not exist. Historically, PCV was commonly used in neonates and infants, although modern ventilators that precisely measure small VT are currently favored. PCV may be the only mode available on some portable and transport ventilators.
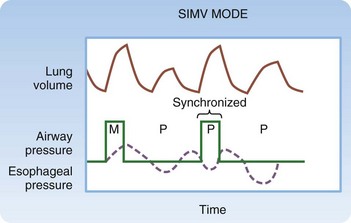
Synchronized Intermittent Mandatory Ventilation and Pressure Support
Synchronized intermittent mandatory ventilation (SIMV) is probably the most commonly misunderstood mode of mechanical ventilation (Fig. 3.4). The physician sets VT, RR, IFR, FIO2, and PEEP, as in AC mode. In contrast to AC mode, however, the trigger that initiates inspiration depends on the patient’s RR relative to the set RR. When the patient breathes at or below the set RR, the trigger can be either elapsed time or the patient’s respiratory effort. In this case, WOB is equivalent to AC. When the patient breathes above the set RR, the ventilator is not triggered to assist in making spontaneous breaths in excess of the set RR. The work associated with such breaths may be quite high because the patient must generate enough negative force to pull air through the ventilator and overcome the resistance to airflow caused by the ventilator circuit tubing and the endotracheal tube (ETT), in addition to the WOB required as a result of the underlying disease process.
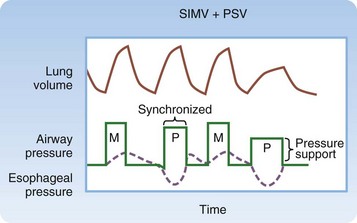
This limitation of SIMV can be diminished by the addition of PSV (Fig. 3.5). PSV causes inspiratory positive pressure to be applied during patient-initiated breaths that exceed the set RR. The patient initiates and terminates inspiration, thereby determining VT. Once the patient triggers pressure support, it is maintained until the machine detects cessation of patient effort, as indicated by a fall in inspiratory airflow. VT, IFR, and TI are not controlled but instead are determined by patient effort. The WOB performed during PSV involves triggering the ventilator to deliver the pressure and maintaining inspiratory effort throughout inhalation. Contrast this with machine-assisted ventilation in AC or SIMV, where WOB involves triggering the ventilator but lung inflation continues regardless of the patient’s inspiratory effort. WOB during PSV also depends on the set level of pressure support. Insufficient pressure support is associated with high WOB, which leads to a small VT and a high RR. Adequate pressure support reduces WOB and improves VT and RR. Many experts view RR as the best index of the adequacy of the level of pressure support. It should be adjusted to maintain an acceptable RR of less than 30 but preferably less than 24 breaths per minute.
Monitoring Dynamic Pressure During Invasive Ventilation
Modes of Noninvasive Mechanical Ventilation
The cause of the respiratory failure is the best predictor of whether a patient will respond to noninvasive techniques. The literature supports the application of NPPV for certain conditions—COPD,4,5 asthma,6 congestive heart failure (CHF),7,8 pneumonia, trauma, cancer, and neuromuscular disease—as well as for pediatric patients.
Specific Disease Processes
Controlling Airway Pressure—Lung-Protective Ventilator Strategies
Causes of difficulty with mechanical ventilation fall into four general categories:
• High airway pressure during lung inflation
• High PEEPi because of obstructive airways disease
Acute Respiratory Distress, Acute Lung Injury, and Pulmonary Edema
Initial studies compared a conventional ventilation strategy (VT of 10 to 15 mL/kg IBW with a goal of obtaining normal PaO2 and PaCO2) with a lung-protective ventilation strategy (VT of 6 to 8 mL/kg IBW with correction of hypoxia, but allowing hypercapnia in favor of avoiding high airway pressure). The results were conflicting.9–13 A landmark study, the ARDS Network Trial,14 prospectively compared a conventional strategy (VT of 12 mL/kg and a Pplat limit of 50 cm H2O) with a protective strategy (VT of 6 mL/kg IBW and a Pplat limit of 30 cm H2O). After enrollment of 861 patients with ARDS and an interim analysis, the trial was stopped early because of a 22% reduction in mortality, 20% fewer days requiring mechanical ventilation, and fewer cases of organ system failure in the group receiving the lung-protective strategy.
Obstructive Airways Disease
Exacerbation of obstructive airways disease requiring mechanical ventilation is often associated with air trapping and dynamic hyperinflation of the lungs. High Ppeak arises as a result of inspiratory airflow resistance, a phenomenon more common in patients with severe asthma than in those with COPD. High Pplat is caused by lung overdistention and consequent diminished compliance. Patients with both high Ppeak and Pplat comprise a group of high-risk patients with both obstruction and overdistention who are at high risk for complications, including pneumothorax, tension pneumothorax, pneumomediastinum, dysrhythmias, and hemodynamic collapse. No prospective trials comparing ventilation strategies in such patients have been conducted. It is common practice to use a strategy of permissive hypercapnia to eliminate PEEPi and avoid high Pplat. This strategy makes use of low VT, low RR, and high IFR to shorten the inspiratory time and prolong the expiratory time. Although this strategy often leads to hypercapnia, it is considered safer to allow respiratory acidosis to develop than to ventilate at excessive airway pressure. A lower limit of acceptable pH has not been established, but general recommendations have been to allow pH values as low as 7.15 to 7.2. Permissive hypercapnia is required more often in the management of status asthmaticus than in the management of COPD. Evidence in support comes from retrospective studies. Current recommendations include VT less than 8 mL/kg IBW, an initial RR of 8 to 10 cycles/min, and FIO2 adjusted to obtain a PaO2 of approximately 60 mm Hg or a PaO2 of 85% to 88%. The concept of permissive hypercapnia and controlled hypoventilation in the management of acute asthma exacerbation has been widely accepted.15 Some reports suggest that Ppeak and Pplat are not adequate indicators of pulmonary hyperinflation16 and recommend that expiratory volumes be measured in these patients.17 This latter technique has not gained widespread acceptance, however.
Complications
Invasive Mechanical Ventilation
In the ED, complications of mechanical ventilation (Box 3.1) can begin during the preintubation period. Induction agents may cause or worsen hypotension. Overly aggressive bag-valve-mask ventilation may lead to decreased venous return and hypotension. Airway trauma and mechanical complications may be caused by the act of intubation. Initiating mechanical ventilation and transitioning from negative pressure ventilation to positive pressure ventilation may lead to hypotension. Positive pressure ventilation may worsen an existing pneumothorax or give rise to pneumothorax. Auto-PEEP (also known as PEEPi, dynamic hyperinflation, breath stacking) can lead to hypotension and circulatory collapse. Ventilator-associated lung injury can be caused by barotrauma, volutrauma, or trauma related to atelectasis. Long-term complications can include inability to be liberated from the ventilator, ventilator-associated pneumonia, tracheal stenosis, and vocal cord injury.
Intrinsic Positive End-Expiratory Pressure
Prognosis
Because of the wide range of causes of respiratory failure, the prognosis is highly variable and dependent on the cause and severity. The prognosis of patients with respiratory failure caused simply by oversedation from intoxicants can be quite good. Conversely, patients with ARDS as their sole organ dysfunction have a mortality of 20% to 25%. Respiratory failure with multiorgan system failure carries much higher mortality that is based on the severity of the illness. Overall, a requirement for invasive mechanical ventilation carries an approximate 34.5% in-hospital mortality.2
Tips and Tricks
Pitfalls and Pearls for Mechanical Ventilation
1 McCaig LF, Ly N. National hospital ambulatory medical care survey: 2000 emergency department summary. Advance Data. April 22, 2002.
2 Wunsch H, Linde-Zwirble W, Angus D, et al. The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38:1947–1953.
3 Moore BB, Wagner R, Weiss KB. A community based study of near-fatal asthma. Ann Allergy Asthma Immunol. 2001;86:190–195.
4 Ram FS, Picot J, Lightowler J, et al. Noninvasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. (3):2004. CD004104
5 Conti G, Antonelli M, Navalesi P, et al. Noninvasiveness vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Crit Care Med. 2002;28:1701–1707.
6 Soroksky A, Stay D, Shpirer I. A pilot, prospective, placebo-controlled trial of bilevel positive airway pressure in acute asthma attack. Chest. 2003;123:1018–1025.
7 Peter JV, Moran JL, Phillips-Hughes J, et al. Effect of non-invasive positive pressure ventilation (NIPPV) on mortality in patients with acute cardiogenic pulmonary oedema: a meta-analysis. Lancet. 2006;367:1155–1163.
8 Weng CL, Zhao YT, Liu QH, et al. Meta-analysis: noninvasive ventilation in acute cardiogenic pulmonary edema. Ann Intern Med. 2010;152:590–600.
9 Bower RG, Shanholtz CB, Fessler HE, et al. Prospective, randomized, controlled clinical trial comparing traditional versus reduced tidal volume ventilation in acute respiratory distress syndrome patients. Crit Care Med. 1999;27:1492–1498.
10 Stewart TE, Meade MO, Cook DJ, et al. Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. N Engl J Med. 1998;338:355–361.
11 Brochard L, Roudot-Thoraval F, Roupie E, et al. Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1998;158:1831–1838.
12 Amato MBP, Barbas CSV, Medeiros DM, et al. Effect of a protective ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354.
13 Amato MBP, Barbas CSV, Medeiros DM, et al. Beneficial effects of the “open lung approach” with low distending pressures in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;152:1835–1846.
14 The Acute Respiratory Distress Syndrome Network Authors. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308.
15 Slutsky AS. Mechanical ventilation. American College of Physicians’ Consensus Conference. Chest. 1993;104:1833–1859.
16 Williams TJ, Tuxen DV, Scheinkestel CD, et al. Risk factors for morbidity in mechanically ventilated patients with acute severe asthma. Am Rev Respir Dis. 1992;146:607–615.
17 Tuxen DV, Williams TJ, Scheinkestel CD, et al. Use of a measurement of pulmonary hyperinflation to control the level of mechanical ventilation in patients with acute severe asthma. Am Rev Respir Dis. 1992;146:1136–1142.