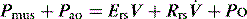Chapter 27 Mechanical ventilation
Mechanical ventilation for acute respiratory failure (ARF) is now a routine aspect of patient management in the intensive care unit (ICU). The 1952 Copenhagen polio epidemic introduced the notion of organised areas (ICU) for the provision of positive-pressure ventilation,1 which was usually applied through a tracheostomy that had been inserted to allow suction of secretions. However, methods of ventilatory assistance without intubation had proliferated prior to the polio epidemic (both negative-pressure chest wall devices and positive-pressure face mask devices), and current trends are to an increased use of non-invasive ventilation (NIV) in patients with respiratory failure.2
A PHYSIOLOGICAL APPROACH
Inertial work is negligible, and usually ignored. Further, Equation (1) does not explicitly describe the elastic work required to initiate inspiration when intrinsic positive end-expiratory pressure (PEEPi) is present.
Because volume is constant in Equation (1), it can be simplified to:
MODES OF VENTILATION
CONTROLLED MECHANICAL VENTILATION
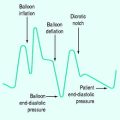
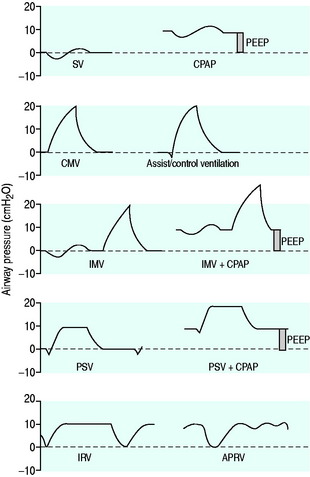
The simplest form of positive-pressure breath occurs in a relaxed subject, and the ventilator provides a constant gas flow during inspiration. The volume delivered will depend upon the inspiratory time (Ti), and Pao during inspiration will reflect Ers and Rrs (Figure 27.1). Expiration is a passive, and usually exponential, decline in volume to the relaxation volume of the respiratory system, equal to the functional residual capacity (FRC).
CMV is the most basic form of mechanical ventilation; however, it is an extremely useful baseline, and is still commonly used. A preset minute ventilation is made up from a fixed respiratory rate (f) and tidal volume (VT). Provided that there are not large variations in alveolar dead space, this maintains a preset alveolar ventilation (VA) and CO2 clearance. Consequently, CMV is useful in conditions where there is alveolar hypoventilation (e.g. respiratory muscle weakness), when PaCO2 needs to be maintained in a fixed range (e.g. raised intracranial pressure) or when the work of breathing must be minimised (e.g. severe cardiorespiratory failure). Because CMV may not match respiratory drive, and spontaneous, supported or assisted breaths are not possible during CMV, sedation, and sometimes muscle paralysis, may be needed. CMV is usually combined with PEEPe, which can recruit collapsed lung and reduce intrapulmonary shunt. The components are discussed below.
TIDAL VOLUME (vT)
Although traditional CMV VT has been 12–15 ml/kg, this may result in excessive lung stretch, particularly in patients with acute lung injury (ALI), leading to ventilator-induced (VILI) – also described as ventilator-associated – lung injury (VALI).3 The basis for this larger VT can be traced back to progressive atelectasis and intrapulmonary shunt, when physiologic VT was used during general anaesthesia. This could be reversed by larger VT ventilation or intermittent sigh breaths.4 In patients with ALI, VT of 6 ml/kg versus 12 ml/kg predicted body weight (i.e. often 4–5 ml/kg versus 9–10 ml/kg true weight) reduced mortality from 40% to 31%.5 Consequently, lower VT should be strongly considered during CMV, and other forms of ventilatory assistance in patients with ALI. However, greater levels of PEEP are usually required; similar data are not available for other respiratory diseases. Indeed, although the reduction in VT is particularly applicable to ALI, excessive lung stretch will be less likely in other patient cohorts that are able to ventilate a greater proportion of the lung (see Chapter 29).
RESPIRATORY RATE (f)
The desired minute ventilation can be selected from the product of VT and f. Common CMV rates are 10–20 breaths/min in adults. Sufficient expiratory time (Te) must be allowed to minimise dynamic hyperinflation and PEEPi. Although high f (up to 35 breaths/min) were allowed in the Acute Respiratory Distress Syndrome (ARDS) Network protocol, and the low-VT, low-mortality group had a mean f of ∼30 breaths/min,5 laboratory data suggest a possible additive role of high f in VILI.6
INSPIRATORY FLOW PATTERN
There are no convincing outcome data differentiating these different modes of CMV and PCV. Although the peak airway pressure (Ppk) is lower with PCV than constant-flow CMV, the alveolar distending pressure, which is usually inferred from the plateau pressure (Pplat), is no different provided that Ti and VT are the same.7 During PCV, Pres is dissipated during inspiration so Ppk and Pplat are equal, and during CMV, Pres accounts for the difference between Ppk and Pplat (Figure 27.2). Similarly, different CMVI patterns will alter Ppk without changing Pplat or mean airway pressure (Pmean) when Ti and VT are constant. In ARDS patients, comparing VCV and PCV, there is no difference in haemodynamics, oxygenation, recruited lung volume or distribution of regional ventilation;7,8 however, PCV may dissipate viscoelastic strain earlier.8 However, high  may cause or exacerbate VILI,9 which may explain why some animal models have found PCV to be injurious compared to VCV, as PCV inherently has a high early
may cause or exacerbate VILI,9 which may explain why some animal models have found PCV to be injurious compared to VCV, as PCV inherently has a high early  .
.
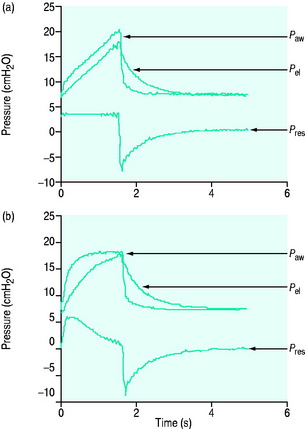
Figure 27.2 Actual pressure–time data from a patient with acute lung injury ventilated with volume-controlled ventilation (panel A), and then with pressure-controlled ventilation (panel B); tidal volume, I:E ratio and respiratory rate are constant. The airway pressure (Paw, bold line) has been broken down to its components, Pel and Pres (see Figure 27.1). Although there is no inspiratory pause, there is marked similarity between panel A and Figure 27.1, with the inspiratory difference between Paw and Pel due to a constant Pres. In panel B, the decelerating inspiratory flow pattern seen with pressure-controlled ventilation results in dissipation of Pres by end-inspiration. Consequently, during pressure-controlled ventilation Paw ≈ Pplat obtained during volume-controlled ventilation. In other words, for the same ventilator settings there is no difference in the elastic distending pressure.
Pressure-regulated volume control (PRVC) is a form of CMV where the VT is preset, and achieved at a minimum pressure using a decelerating flow pattern.
INSPIRATORY PAUSE
An end-inspiratory pause allows examination of the decay in Ppk, and measurement of Pplat. If Ti and V are maintained constant there is no improvement in oxygenation, and a more rapid 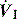 will be needed. This may alter the distribution of ventilation. Theoretically, an end-inspiratory pause will allow a 32% dissipation of the total energy loss within the respiratory system,10 which may significantly reduce the forces driving expiration, and this could exacerbate gas trapping in patients with severe airflow limitation.
will be needed. This may alter the distribution of ventilation. Theoretically, an end-inspiratory pause will allow a 32% dissipation of the total energy loss within the respiratory system,10 which may significantly reduce the forces driving expiration, and this could exacerbate gas trapping in patients with severe airflow limitation.
INSPIRATORY TIME, EXPIRATORY TIME, I:E RATIO
The combination of VT and inspiratory 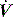 will determine Ti, which in combination with f sets Te. A typical Ti is 0.8–1.2 s; with a VT of 500 ml and inspiratory
will determine Ti, which in combination with f sets Te. A typical Ti is 0.8–1.2 s; with a VT of 500 ml and inspiratory 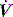 of 0.5 l/s, the Ti is 1.0 s. During spontaneous ventilation, the distribution of ventilation at low
of 0.5 l/s, the Ti is 1.0 s. During spontaneous ventilation, the distribution of ventilation at low 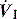 is determined by regional E, and at high inspiratory
is determined by regional E, and at high inspiratory 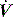 by regional R, leading to greater ventilation of non-dependent lung. In patients with severe airflow limitation high inspiratory flow rates may be used in order to prolong Te and minimise dynamic hyperinflation.11
by regional R, leading to greater ventilation of non-dependent lung. In patients with severe airflow limitation high inspiratory flow rates may be used in order to prolong Te and minimise dynamic hyperinflation.11
The I:E ratio is usually set at or below 1:2 to allow an adequate Te for passive expiration. During inverse-ratio ventilation (IRV), the I:E ratio is greater than 1:1. The putative benefits of a prolonged Ti include recruitment of long time-constant alveoli, and a short Te results in gas trapping and PEEPi. Early reports of clinical benefit did not control for PEEPi, and when total PEEP, f and VT are constant,7,8 pressure control (PC) IRV tends to reduce PaCO2 but does not improve oxygenation, may have deleterious haemodynamic effects and may exaggerate regional overinflation.8
POSITIVE END-EXPIRATORY PRESSURE
PEEP is an elevation in the end-expiratory pressure upon which all forms of mechanical ventilation may be imposed. When PEEP is maintained throughout the respiratory cycle in a spontaneously breathing subject, the term ‘constant positive airway pressure’ (CPAP) is used. The primary role of PEEP is to maintain recruitment of collapsed lung, increase FRC and minimise intrapulmonary shunt. PEEP may also improve oxygenation by redistributing lung water from the alveolus to the interstitium, and although there is no direct effect of PEEP to reduce extravascular lung water, this may occur in patients with left ventricular failure due to a reduction in venous return and left ventricular afterload. Further, inadequate PEEP may contribute to VILI by promoting tidal opening and closing of alveoli.3 PEEP levels of 5–15 cmH2O are commonly used, and levels up to 25 cmH2O may be required in patients with severe ARDS. Although a large multicentre study found no benefit of higher PEEP levels when VT was 6 ml/kg,12 individual titration may need to be considered.
PEEP titration in ARDS is complex (see Chapter 29), and should aim to improve oxygenation and minimise VILI. Since PEEP reduces venous return, cardiac output and O2 delivery may fall despite an improvement in PaO2; indeed, this concept has been used to optimise PEEP in ARF.13 However, in addition to recruitment, increasing PEEP may lead to overinflation of non-dependent alveoli which are already aerated at end-expiration.14,15 This will be less likely if alveolar distending pressure is kept < 30–35 cmH2O, or the change in driving pressure is < 2 cmH2O when VT is constant.16
PEEPe is applied by placing a resistance in the expiratory circuit (Figure 27.3), with most ventilators using a solenoid valve. Independent of the technique, a threshold resistor is preferred since it offers minimal resistance to flow once its opening P is reached. This will minimise expiratory work, and avoid barotrauma during coughing or straining.
SIGH
Many ventilators have the ability to deliver a breath intermittently at least twice VT. Sighs may reduce atelectasis, in part through release of pulmonary surfactant,17 resulting in recruitment and improved oxygenation in ARDS.18 However, if sighs or recruitment manoeuvres are used, care must be taken to avoid recurrent excessive lung stretch.
ASSIST-CONTROL VENTILATION (ACV)
During ACV, in addition to the set f, patient effort can trigger a standard CMV breath (Figure 27.4). This allows greater patient comfort; however, there may be little reduction in respiratory work compared to an unassisted breath at low 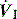 , because the respiratory muscles continue to contract through much of the breath.19 The equivalent PCV breath is termed pressure assist-control ventilation (PACV). Differences between triggering modes will be discussed below, in the section on patient–ventilator interaction.
, because the respiratory muscles continue to contract through much of the breath.19 The equivalent PCV breath is termed pressure assist-control ventilation (PACV). Differences between triggering modes will be discussed below, in the section on patient–ventilator interaction.
INTERMITTENT MANDATORY VENTILATION (IMV), SYNCHRONISED IMV (SIMV)
IMV was introduced over 20 years ago to aid weaning from CMV by allowing the patient to take unimpeded breaths while still receiving a background of controlled breaths. Proposed advantages include a reduction in sedation, lower mean intrathoracic pressure with less barotrauma and adverse haemodynamic consequences, improved intrapulmonary gas distribution, continued use of respiratory muscles and faster weaning. During SIMV Ti is partitioned into patient-initiated and true spontaneous breaths to avoid breath-stacking. However, during spontaneous breaths the work of breathing imposed by the endotracheal tube, circuit and ventilator must be overcome.20 In weaning studies comparing SIMV with T-piece trials and pressure support ventilation (PSV), SIMV is the slowest.21 Many clinicians add PSV during gradual reduction in respiratory rate with SIMV to overcome the added respiratory work imposed by the circuit and endotracheal tube; however, this approach has not been formally compared with other weaning techniques.
PRESSURE SUPPORT VENTILATION
During PSV, each patient-triggered breath is supported by gas flow to achieve a preset pressure, usually designated to be above the PEEPe. This can be explained by referring to the equation of motion where:
During PSV, Pao is the targeted variable by the ventilator, which leads to a significant and important reduction in Pmus and work of breathing.22 The detection of neural expiration varies between ventilators, but commonly relies upon a fall in the inspiratory 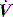 to either 25% of the initial flow rate or to less than 5 l/min: some ventilators allow titration of the percentage reduction in initial flow to allow improved patient–ventilator synchrony. PSV may also be titrated to offset the work imposed by the circuit and endotracheal tube. The absolute level required to offset this will vary with endotracheal tube size and inspiratory
to either 25% of the initial flow rate or to less than 5 l/min: some ventilators allow titration of the percentage reduction in initial flow to allow improved patient–ventilator synchrony. PSV may also be titrated to offset the work imposed by the circuit and endotracheal tube. The absolute level required to offset this will vary with endotracheal tube size and inspiratory 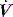 ,23 but is commonly 5–10 cmH2O.24 PSV can be used during weaning, or as a form of variable ventilatory support, with pressures of 15–20 cmH2O commonly used. Disadvantages include variable VT, and hence minute ventilation, the potential to deliver an excessive VT (common in patients recovering from ARDS), and patient-ventilator dyssynchrony (see below).
,23 but is commonly 5–10 cmH2O.24 PSV can be used during weaning, or as a form of variable ventilatory support, with pressures of 15–20 cmH2O commonly used. Disadvantages include variable VT, and hence minute ventilation, the potential to deliver an excessive VT (common in patients recovering from ARDS), and patient-ventilator dyssynchrony (see below).
PROPORTIONAL ASSIST VENTILATION (PAV)
PAV is a form of partial ventilatory support where inspiratory P is applied in proportion to patient effort. Because this allows the breathing pattern and minute ventilation to be matched to patient effort, it is only suitable if respiratory drive is normal or elevated. In concept this should optimise the patient–ventilator interaction; however, the prescription of PAV requires a greater level of physiological understanding than similar forms of partial ventilatory support such as PSV, since there is no target P,V or 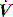 . PAV is usually prescribed using volume assist (VA) and flow assist (FA), with V and
. PAV is usually prescribed using volume assist (VA) and flow assist (FA), with V and 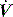 measured continuously. VA generates greater P as V increases, leading to elastic unloading, and FA generates greater P as
measured continuously. VA generates greater P as V increases, leading to elastic unloading, and FA generates greater P as 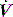 increases, leading to resistive unloading. Not surprisingly, the units of VA are cmH2O/L (i.e. an elastance term) and those for FA are cmH2O/l per second (i.e. a resistance term). This can be illustrated by referring to Equation (6):
increases, leading to resistive unloading. Not surprisingly, the units of VA are cmH2O/L (i.e. an elastance term) and those for FA are cmH2O/l per second (i.e. a resistance term). This can be illustrated by referring to Equation (6):
where Pao is determined by PAV, where 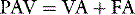 , so:
, so:
If Ers and Rrs are known, PAV can, at least in principle, be targeted to reduce a specified proportion of either, or both, elastic and resistive respiratory work. For example, when VA and FA are adjusted to counterbalance Ers and Rrs so as to achieve normal values, minute ventilation increases, and respiratory drive and work decrease; if PEEPi is present, work can be further reduced by applying PEEPe.25 Estimates of respiratory mechanics are relatively hard to measure in spontaneously breathing patients; however, they are now offered on some ventilators. Consequently, PAV is often titrated to patient comfort. Despite a growing body of data demonstrating reduced work of breathing and improved patient–ventilator synchrony with PAV, it is a more difficult technique to use, and definitive studies showing a clinically important outcome difference are awaited.
HIGH-FREQUENCY VENTILATION (HFV)
HFV encompasses techniques where small VT (1–3 ml/kg) are delivered at high f (100–300/min). Hazards include inadequate humidification and gas trapping in patients with severe airflow limitation. High-frequency jet ventilation (HFJV) utilises dry gas from a high-pressure source delivered into an intratracheal catheter or specifically manufactured endotracheal tube. High-frequency oscillation (HFO) uses oscillatory flow within the airway to provide active inspiration and expiration at rates of 3–20 Hz. HFO offers benefit over conventional ventilation in neonates and children with respective infant and acute respiratory distress syndrome; data in adults are inconclusive.26 HFJV has been used with improvement in gas exchange in adults with ARDS.27
LIQUID VENTILATION
Small clinical studies have reported improved gas exchange and respiratory mechanics after administration of perfluorocarbons;28 however, a moderately large clinical study (n = 311) found that patients receiving conventional ventilation compared to both low- and high-dose perfluorocarbons had more ventilator-free days and tended to reduce mortality.29 Consequently, liquid ventilation cannot be recommended.
INDICATIONS AND OBJECTIVES OF MECHANICAL VENTILATION30
Institution of mechanical ventilation is a clinical decision; it can only be supported by parameters such as blood gases or measures of respiratory muscle function. Even then, the decision to choose IV over NIV will be influenced by numerous factors, including the likely course of the ARF and its response to treatment. Often there will be an indication for intubation (Table 27.1) and mechanical ventilation; however, if intubation is required to overcome upper-airway obstruction, no ventilatory assistance may be needed despite the increase in respiratory work imposed by the endotracheal or tracheostomy tube.18Once the decision has been made to proceed to ventilatory support the choice of mode should be based on a physiological approach, local expertise and simplicity.
Table 27.1 Indications and objectives of intubated mechanical ventilation
| Endotracheal intubation or tracheostomy |
Patients who are likely to need ventilatory assistance (e.g. acute severe asthma) should be considered for early ICU admission since this will allow faster responses and avoid cardiorespiratory arrest. Specific issues and methods of ventilatory assistance are dealt with in Chapters 29, 31 and 33. In patients with traumatic brain injury IV is commonly required to protect the airway and control ICP; similarly, patients with severe pancreatitis or serious abdominal infection may need prolonged IV to maintain an adequate FRC, reduce work of breathing, protect their airway and allow suctioning of secretions.
INITIATION OF INTUBATED MECHANICAL VENTILATION
A manual resuscitation circuit, mechanical ventilator and equipment for safe endotracheal intubation (see Chapter 25) should be available. Initial ventilator settings are commonly set to achieve adequate oxygenation and VA; however, this will depend upon the patient’s condition. Common settings are: VT 6–10 ml/kg, f 10–20 breaths/min, PEEP 5 cmH2O and FiO2 of 1.0, and these will need to be adjusted according to a specific patient’s pathophysiology and response.
MANUAL RESUSCITATION CIRCUITS
Manual resuscitation circuits are primarily used to provide emergency ventilation when spontaneous effort is absent or inadequate. They may be used with a face or laryngeal mask, or an endotracheal tube. Occasionally they are used to provide a high inspired O2 concentration during spontaneous breathing; however, this may impose significant additional respiratory work.31 In the ICU they are commonly used for preoxygenation and manual lung inflation.
Self-inflating reservoir bags use a series of one-way valves to allow fresh gas flow oxygen and entrained air to fill the bag. Inspired oxygen fractions as high as 0.8 may be achieved with neonatal or paediatric bags when an additional reservoir bag is used to allow fresh gas flow filling during expiration, after the bag has refilled.32 However, lower FiO2s (∼0.6) will be obtained with both conventional O2 flow rates of 8–15 l/min, and usual VT and f, with an adult bag. Generally the valves are simple flap or duck-bill in nature, and both positive-pressure and spontaneous ventilation are possible. The reservoir bag volume in adults is typically 1600 ml, and VT can be judged from chest wall movement. It is essential that these devices use standard 15/22-mm connectors to allow rapid connection to standard endotracheal tubes and ventilator circuits.
COMPLICATIONS OF MECHANICAL VENTILATION (Table 27.2)26
Although mechanical ventilation may be vital, it also introduces numerous potential complications. Monitoring includes a high nurse-to-patient ratio (usually 1:1), ventilator alarms and pulse oximetry. Capnography is recommended to confirm endotracheal tube placement, and may be used to monitor the adequacy of VA;however, expired CO2 is strongly influenced by factors that alter alveolar dead space, such as cardiac output. Intermittent blood gases, PEEPi, airway pressures in volume-preset modes and VT in pressure-preset modes should be recorded. Individual patients may benefit from more extensive monitoring of their respiratory mechanics or tissue oxygenation.
Table 27.2 Complications of intubation and mechanical ventilation
| Equipment |
Mechanical ventilation is also associated with a marked increase in the incidence of nosocomial pneumonia due to a reduction in the natural defence of the respiratory tract, and this represents an important advantage offered by NIV. In patients successfully managed with NIV, Girou and colleagues reported a reduction in the incidence of nosocomial pneumonia, associated with improved survival, compared to IV.33 Erect versus semirecumbent posture34 also reduces the incidence of ventilator-associated pneumonia.
Although lung overdistension may result in alveolar rupture leading to pulmonary interstitial air, pneumomediastinum or pneumothorax, it may also lead to diffuse alveolar damage similar to that found in ALI and ARDS. Both are termed VILI, and VT reduction leads to a marked decrease in ALI mortality, due to a reduction in multiple-organ dysfunction.5 There are also laboratory data suggesting that inadequate PEEP with tidal recruitment and derecruitment of alveoli leads to VILI; however, this has not been proven in a clinical trial. Finally, patient–ventilator asynchrony may result in wasted respiratory work, impaired gas exchange and respiratory distress (see below).
Sleep disturbance, and agitation and discomfort are common in mechanically ventilated patients. These effects may be reduced with sedation until weaning is planned; however, it is important not to prolong mechanical ventilation due to excessive use of sedatives, which may also depress blood pressure and spontaneous respiratory effort. Finally, complex neuropsychological sequelae have been described in recovering ARDS patients.35,36 These do not appear to reflect the severity of the acute illness since ARDS patients have a poorer quality of life than patients with a similar severity of illness without ARDS.37 However, they do correlate with their duration of hypoxaemia. Clearly, this is an important issue that needs further research.
WITHDRAWAL (WEANING) FROM MECHANICAL VENTILATION
Once the underlying process necessitating mechanical ventilation has started to resolve, withdrawal of ventilatory support should be considered; increased duration of ventilation leads to a progressive rise in complications such as ventilator-associated pneumonia. However, other important parameters that must be considered include the neuromuscular state of the patient (ability to initiate a spontaneous breath), adequacy of oxygenation (typically low requirements for PEEP (5–8 cmH2O) and FiO2 < 0.4–0.5) and cardiovascular stability.38 Once a patient is considered suitable to wean, a secondary question is whether an artificial airway is still required for airway protection or suction of secretions. Many patients can rapidly make the transition from mechanical ventilation to extubation, but ∼20% of patients fail weaning despite meeting clinical criteria.21 Advanced age, prolonged mechanical ventilation and chronic obstructive pulmonary disease all increase the likelihood that weaning will be difficult.21
Weaning failure is usually associated with an increase in respiratory drive and respiratory rate and a fall in VT which contributes to hypercapnoea;39 about 10% of patients fail due to central respiratory depression. Various indices such as maximal inspiratory pressure (MIP), minute ventilation (VE), f,VTf/VT ratio and the compliance, respiratory rate, oxygenation, maximum inspiratory pressure (CROP) index have been investigated as predictors of weaning failure (Table 27.3). They are rarely used alone and careful clinical assessment is often adequate, yielding a reintubation rate as low as 3%,40 and none of these indices assess airway function following extubation. Although the typical threshold value for the rapid shallow-breathing index (f/VT ratio) is > 105, a large recent multicentre study reported progressive increase in risk as this increased with a threshold of 57; in addition apositive fluid balance immediately prior to extubation was a significant risk factor for reintubation.41 Consequently, weaning indices should not necessarily delay extubation or a weaning trial. However, they may quantitate important issues in the general clinical assessment, and may be directly relevant for a given patient. For example, frequent small VT, an inadequate vital capacity (less than 8–12 ml/kg), large minute ventilation (= 15 l/min), depressed respiratory drive and reduced respiratory muscle strength (MIP = –15 cmH2O) or drive should be strongly factored into deciding whether a patient is ready to undergo a trial of weaning safely.
Table 27.3 Sample of measurements that have been used to predict successful outcome from weaning in critically ill patients*
| Parameter | Typical threshold value† | Comment |
|---|---|---|
| VE | ≤ 15 l/min | Moderate to high sensitivity, low specificity |
| MIP | ≤ −15 cmH2O | High sensitivity, low specificity |
| CROP index# | ≥ 13 | Moderate to high sensitivity, modest specificity |
| During a spontaneous breathing trial | ||
| f | ≤ 38 breaths/min | High sensitivity, low specificity |
| VT | ≥ 325 ml (4 ml/kg) | High sensitivity, low specificity |
| f/VT | ≤ 105 | High sensitivity, moderate specificity |
MIP, maximal inspiratory pressure; CROP, compliance, respiratory rate, oxygenation, maximum inspiratory pressure.
* See reference 38 for further detail.
† Although threshold values are often used, the data are not dichotonous. For example, as the f/VT ratio increases, so does the rate of reintubation.41
# CROP index = (Cdyn × MIP × [PaO2/PAO2]/f).21
Direct comparisons between T-piece trials, PSV and SIMV as weaning techniques have been performed in patients previously failing a 2-hour trial of spontaneous breathing. In the study by Brochard and colleagues,42 PSV led to fewer failures and a shorter weaning period. In contrast, Esteban and coworkers43 found that a once-daily trial of spontaneous breathing resulted in the shortest duration of mechanical ventilation; however, a relatively high proportion of patients required reintubation (22.6%). Viewed together these studies suggest that weaning was slower with SIMV,19 although SIMV with PSV was not studied, and that either PSV or a T-piece trial is the preferred method for weaning. Since low levels of PSV can help compensate for the additional work of breathing attributable to the endotracheal tube and circuit, some clinicians use low levels of PSV (5–7 cmH2O) during weaning or during a spontaneous breathing trial. However, the work of breathing following extubation is usually higher than expected, probably due to upper-airway oedema and dysfunction, so that work is similar to that during a T-piece trial.21
Reintubation is associated with a 7–11-fold increased risk of hospital death.19 A number of factors may account for this, including selection of previously unaccounted-for severity of illness as the mortality rate associated with reintubation is much higher in patients whose primary diagnosis was respiratory failure. In addition, complications which may (pneumonia, heart failure) or may not be attributable to extubation contribute to this poor outcome. Hence, an important goal during mechanical ventilation will be to proceed to early and expeditious extubation with a low reintubation rate.
PATIENT–VENTILATOR INTERACTION
TRIGGERING OF INSPIRATION
 effects of cardiac oscillations, hiccups, circuit rainout or mask leak with NIV, and has been reported as a cause of apparent respiratory effort in brain-dead patients.48 Flow triggering reduces the risk of autocycling at a given trigger sensitivity, and reduces respiratory effort a small amount compared to pressure triggering; however, it does not alter the frequency of ineffective efforts,49 or patient effort following triggering.40
effects of cardiac oscillations, hiccups, circuit rainout or mask leak with NIV, and has been reported as a cause of apparent respiratory effort in brain-dead patients.48 Flow triggering reduces the risk of autocycling at a given trigger sensitivity, and reduces respiratory effort a small amount compared to pressure triggering; however, it does not alter the frequency of ineffective efforts,49 or patient effort following triggering.40INSPIRATION
Pmus will reflect the difference between Pao due to E,R and Po and the observed Pao. In contrast, during P-cycled ventilation (PACV), greater patient effort is rewarded, and inspiratory work is lower than during equivalent ACV.50
Modern ventilators allow adjustment of inspiratory  and
and  pattern, and the rate of rise of Pao. Low inspiratory rates during ACV result in significant inspiratory work, but this may be markedly reduced by increasing inspiratory
pattern, and the rate of rise of Pao. Low inspiratory rates during ACV result in significant inspiratory work, but this may be markedly reduced by increasing inspiratory 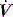 to 65 l/min.51 However, these issues are quite complex, and f increases (probably due to a lower respiratory tract reflex) as
to 65 l/min.51 However, these issues are quite complex, and f increases (probably due to a lower respiratory tract reflex) as 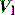 increases,52 reducing Te, which may contribute to dynamic hyperinflation in patients with severe airflow obstruction. During PSV and PACV, many ventilators allow adjustment of the rate of rise of P to its target. A steeper P ramp leads to earlier attainment of the P target, greater early rates and reduction of inspiratory drive and work.53,54
increases,52 reducing Te, which may contribute to dynamic hyperinflation in patients with severe airflow obstruction. During PSV and PACV, many ventilators allow adjustment of the rate of rise of P to its target. A steeper P ramp leads to earlier attainment of the P target, greater early rates and reduction of inspiratory drive and work.53,54
CESSATION OF INSPIRATION
During PSV, an increase in airways resistance will result in a delayed fall in 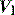 . Since this is the trigger for cycling to expiration, the ventilator may continue to provide
. Since this is the trigger for cycling to expiration, the ventilator may continue to provide 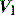 while the patient desires to exhale. This commonly leads to recruitment of expiratory muscles, detected both clinically and as a transient rise in the end-inspiratory Pao.55 Some modern ventilators allow control of the fall in
while the patient desires to exhale. This commonly leads to recruitment of expiratory muscles, detected both clinically and as a transient rise in the end-inspiratory Pao.55 Some modern ventilators allow control of the fall in 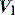 that is sensed as end-inspiration. High levels of PSV (≥ 20 cmH2O), weak respiratory muscles and mask leak with NIV are other common causes of dyssynchrony at the termination of inspiration. In this last group, PACV, which is time-cycled, allows improved patient–ventilator synchrony at end-inspiration compared to PSV.56
that is sensed as end-inspiration. High levels of PSV (≥ 20 cmH2O), weak respiratory muscles and mask leak with NIV are other common causes of dyssynchrony at the termination of inspiration. In this last group, PACV, which is time-cycled, allows improved patient–ventilator synchrony at end-inspiration compared to PSV.56
1 Lassen HC. A preliminary report on the 1952 epidemic of poliomyelitis in Copenhagen with special reference to the treatment of acute respiratory insufficiency. Lancet. 1953;Jan 3:37-41.
2 Mehta S, Hill NS. Noninvasive ventilation. Am J Respir Crit Care Med. 2001;163:540-547.
3 International Consensus Conference in Intensive Care Medicine. Ventilator-associated lung injury in ARDS. Am J Respir Crit Care Med. 1999;160:2118-2124.
4 Bendixen HH, Hedley-White J, Laver MB. Impaired oxygenation in surgical patients during general anesthesia with controlled ventilation: a concept of atelectasis. N Engl J Med. 1963;269:991-996.
5 Ventilation with lower tidal volumes as compared with traditional volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301-1308.
6 Hotchkiss JR, Blanch L, Murias G, et al. Effects of decreased respiratory frequency on ventilator-induced lung injury. Am J Respir Crit Care Med. 2000;161:463-468.
7 Lessard MR, Guerot E, Lorino H, et al. Effects of pressure-controlled with different I:E ratios versus volume-controlled ventilation on respiratory mechanics, gas exchange, and hemodynamics in patients with adult respiratory distress syndrome. Anesthesiology. 1994;80:983-991.
8 Edibam C, Rutten AJ, Collins DV, et al. Effect of inspiratory flow pattern and inspiratory to expiratory ratio on nonlinear elastic behavior in patients with acute lung injury. Am J Respir Crit Care Med. 2003;167:702-707.
9 Bersten AD, Bryan DL. Ventilator-induced lung injury: do dynamic factors also play a role? Crit Care Med. 2005;33:907-909.
10 Jonson B, Beydon L, Brauer K, et al. Mechanics of respiratory system in healthy anesthetized humans with emphasis on viscoelastic properties. J Appl Physiol. 1993;75:132-140.
11 Tuxen DV, Lane S. The effects of ventilatory pattern on hyperinflation, airway pressures, and circulation in mechanical ventilation of patients with severe airflow obstruction. Am Rev Respir Dis. 1987;136:872-879.
12 The National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressure in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327-336.
13 Suter PM, Fairley B, Isenberg MD. Optimum end-expiratory airway pressure in patients with acute pulmonary failure. N Engl J Med. 1975;292:284-289.
14 Gattinoni L, Pelosi P, Crotti S, et al. Effects of positive end-expiratory pressure on regional distribution of tidalvolume and recruitment in adult respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:1807-1814.
15 Vieira SRR, Puybasset L, Richecoeur J, et al. A lung computed tomographic assessment of positive end-expiratory pressure-induced lung overdistension. Am J Respir Crit Care Med. 1998;158:1571-1577.
16 Bersten AD. Measurement of overinflation by multiple linear regression analysis in patients with acute lung injury. Eur Respir J. 1998;12:526-532.
17 Nicholas TE, Power JHT, Barr HA. The pulmonary consequences of a deep breath. Respir Physiol. 1982;49:315-324.
18 Pelosi P, Cardringher P, Bottino N, et al. Sigh in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;159:872-880.
19 Marini JJ, Rodriguez M, Lamb V. The inspiratory workload of patient-initiated mechanical ventilation. Am Rev Respir Dis. 1986;134:902-909.
20 Bersten AD, Rutten AJ, Vedig AE, et al. Additional work of breathing imposed by endotracheal tubes, breathing circuits and intensive care ventilators. Crit Care Med. 1989;17:671-680.
21 Esteban A, Alia I. Clinical management of weaning from mechanical ventilation. Intens Care Med. 1998;24:999-1008.
22 Brochard L, Harf A, Lorino H, et al. Inspiratory pressure support prevents diaphragmatic fatigue during weaning from mechanical ventilation. Am Rev Respir Dis. 1989;139:513-521.
23 Bersten AD, Rutten AJ, Vedig AE. Efficacy of pressure support ventilation in compensating for apparatus work. Anaesth Intens Care. 1993;21:67-71.
24 Brochard L, Rua F, Lorino H, et al. Inspiratory pressure support compensates for the additional work of breathing caused by the endotracheal tube. Anesthesiology. 1991;75:739-745.
25 Appendini L, Purro A, Gudjonsdottir M, et al. Physiologic response of ventilator-dependent patients with chronic obstructive pulmonary disease to proportional assist ventilation and continuous positive airway pressure. Am J Respir Crit Care Med. 1999;159:1510-1517.
26 Derdak S, Mehta S, Stewart TE, et al. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med. 2002;166:801-808.
27 Gluck E, Heard S, Patel C, et al. Use of ultrahigh frequency ventilation in patients with ARDS: a preliminary report. Chest. 1993;103:1413-1420.
28 Hirschl RB, Pranikoff T, Wise C, et al. Initial experience with partial liquid ventilation in adult patients with the acute respiratory distress syndrome. JAMA. 1996;275:383-389.
29 Kacmarek RM, Wiedemann HP, Lavin PT, et al. Partial liquid ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006;173:882-889.
30 Slutsky AS. Mechanical ventilation. Chest. 1993;104:1833-1859.
31 Hess D, Hirsch C, Marquis-D’Amico C, et al. Imposed work and oxygen delivery during spontaneous breathing with adult disposable manual ventilators. Anesthesiology. 1994;81:1256-1263.
32 Agarwal KS, Puliyel JM. A simple strategy to improve first breath oxygen delivery by self inflating bag. Resuscitation. 2000;45:221-224.
33 Girou E, Schortgen F, Delclaux C, et al. Association of noninvasive ventilation with nosocomial infections and survival in critically ill patients. JAMA. 2000;284:2361-2367.
34 Drakulovic MB, Torres A, Bauer TT, et al. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354:1851-1858.
35 Hopkins RO, Weaver LK, Opep D, et al. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:50-56.
36 Rothenhausler H, Ehrentraut S, Stoll C, et al. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen Hosp Psychiatry. 2001;23:90-96.
37 Davidson TA, Caldwell ES, Curtis JR, et al. Reduced quality of life in survivors of acute respiratory distress syndrome compared with critically ill control patients. JAMA. 1999;281:354-360.
38 MacIntyre NR. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest. 2001;120:375. –96S
39 Tobin MJ, Perez W, Guenther SM, et al. The pattern of breathing during successful and unsuccessful trials of weaning from mechanical ventilation. Am Rev Respir Dis. 1986;134:1111-1118.
40 Leitch EA, Moran JL, Grealy B. Weaning and extubation in the intensive care unit. Clinical or index-driven approach? Intens Care Med. 1996;22:752-779.
41 Frutos-Vivar F, Ferguson ND, Esteban A, et al. Risk factors for extubation failure in patients following a successful spontaneous breathing trial. Chest. 2006;130:1664-1671.
42 Brochard L, Rauss A, Benito S, et al. Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. Am J Respir Crit Care Med. 1994;150:896-903.
43 Esteban A, Frutos F, Tobin MJ, et al. A comparison of four methods of weaning patients from mechanical ventilation. N Engl J Med. 1995;332:345-350.
44 Smith TC, Marini JJ. Impact of PEEP on lung mechanics and work of breathing in severe airflow obstruction. J Appl Physiol. 1988;65:1488-1499.
45 Petrof BJ, Legare M, Goldberg P, et al. Continuous positive airway pressure reduces work of breathing and dyspnea during weaning form mechanical ventilation in severe chronic obstructive pulmonary disease. Am Rev Respir Dis. 1990;141:281-289.
46 Ranieri VM, Giuliiani R, Cinnella G, et al. Physiologic effects of positive end-expiratory pressure in patientswith chronic obstructive pulmonary disease during acute ventilatory failure and controlled mechanical ventilation. Am Rev Respir Dis. 1993;147:5-13.
47 Alsanian P, El Atrous S, Isabey D, et al. Effects of flow triggering on breathing effort during partial ventilatory support. Am J Respir Crit Care Med. 1998;157:135-143.
48 Willatts SM, Drummond G. Brainstem death and ventilator trigger settings. Anaesthesia. 2000;55:676-677.
49 Sassoon CSH, Foster GT. Patient-ventilator asynchrony. Curr Opin Crit Care. 2001;7:28-33.
50 Cinnella G, Conti G, Lofaso F, et al. Effects of assisted ventilation on the work of breathing: volume controlled versus pressure-controlled ventilation. Am J Respir Crit Care Med. 1996;153:1025-1033.
51 Ward ME, Corbeil C, Gibbons W, et al. Optimization of respiratory muscle relaxation during mechanical ventilation. Anesthesiology. 1988;69:29-35.
52 Corne S, Gillespie D, Roberts D, et al. Effect of inspiratory flow rate on respiratory rate in intubated ventilated patients. Am J Respir Crit Care Med. 1997;156:304-308.
53 Bonmarchand G, Chevron V, Chopin CC, et al. Increased initial flow rate reduces inspiratory work of breathing during pressure support ventilation in patients with exacerbation of chronic obstructive pulmonary disease. Intens Care Med. 1996;22:1147-1154.
54 Bonmarchand G, Chevron V, Menard JF, et al. Effects of pressure ramp slope values on the work of breathing during pressure support ventilation in restrictive patients. Crit Care Med. 1999;27:715-722.
55 Parthasarathy S, Jubran A, Tobin MJ. Cycling of inspiratory and expiratory muscle groups with the ventilator in airflow limitation. Am J Respir Crit Care Med. 1998;158:1471-1478.
56 Calderinin E, Confalonieri M, Puccio PG, et al. Patient–ventilator asynchrony during noninvasive ventilation: the role of expiratory trigger. Intens Care Med. 1999;25:662-667.

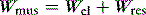
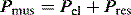
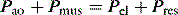
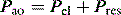
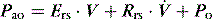
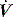 is the gas flow rate and Po is the total PEEP (the sum of extrinsic PEEP [PEEPe] and PEEPi). PEEPi imposes a threshold load – additional elastic work – as inspiratory muscle contraction must occur without
is the gas flow rate and Po is the total PEEP (the sum of extrinsic PEEP [PEEPe] and PEEPi). PEEPi imposes a threshold load – additional elastic work – as inspiratory muscle contraction must occur without 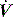 until Pao falls below atmospheric pressure (see section on patient–ventilator interaction, below).
until Pao falls below atmospheric pressure (see section on patient–ventilator interaction, below).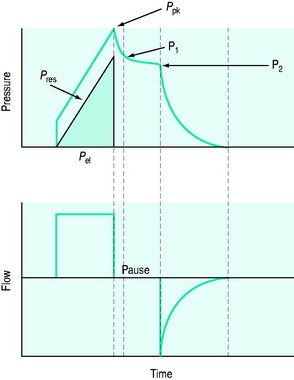
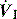 ), and in combination with Ti, a preset volume is delivered. This is also called volume-controlled ventilation (VCV), and some ventilators use VT and Ti to set
), and in combination with Ti, a preset volume is delivered. This is also called volume-controlled ventilation (VCV), and some ventilators use VT and Ti to set 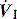 . Alternative
. Alternative 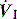 patterns that are commonly available with VCV include a ramped descending flow pattern and a sine pattern. When a time-preset inspiratory pressure is delivered, this is termed pressure-controlled ventilation (PCV).
patterns that are commonly available with VCV include a ramped descending flow pattern and a sine pattern. When a time-preset inspiratory pressure is delivered, this is termed pressure-controlled ventilation (PCV).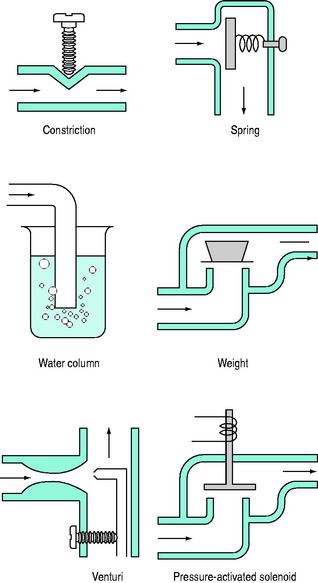
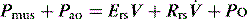
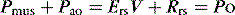
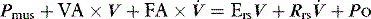
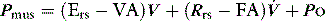
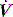 and P may lead to barotrauma or gastric inflation.
and P may lead to barotrauma or gastric inflation.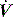 waveforms displayed by the ventilator.
waveforms displayed by the ventilator.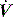 is determined by P,Ti or
is determined by P,Ti or 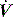 . For example, during PSV ventilation a target P is held until expiration is sensed, and during ACV
. For example, during PSV ventilation a target P is held until expiration is sensed, and during ACV 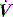 is held for a set Ti. During ACV there may be continued inspiratory effort, and since inspiratory
is held for a set Ti. During ACV there may be continued inspiratory effort, and since inspiratory 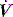 is fixed, this will be reflected by a scalloping of the Pao–T graph if
is fixed, this will be reflected by a scalloping of the Pao–T graph if 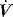 is inadequate. Again this may be illustrated using the equation of motion:
is inadequate. Again this may be illustrated using the equation of motion: