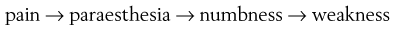Mechanical disorders
Mechanical disorders of the cervical spine affect the structures of the locomotor system. They are often related to the consequences of the ageing spine. Degenerative changes at the level of the intervertebral discs may lead to disc displacements and also to anatomical and biomechanical changes in ligaments, capsules, nervous tissue and vascular structures. In addition to this degeneration being responsible for the development of certain disorders, injuries and overuse may also lead to soft tissue lesions in the region of the neck (Box 8.1).
Degeneration and anatomical changes
Degenerative changes in the cervical spine are part of the normal ageing process and are almost ubiquitous in older people.1,2 Very often they remain asymptomatic.3,4 Therefore diagnoses such as ‘degeneration’, ‘arthrosis’, ‘osteophytosis’, ‘spondylosis’ or ‘spondylarthrosis’ based on incidental radiological findings are still too easily made. Throughout the literature, a discrepancy between the discovery of structural changes and the presence of symptoms is a consistent finding.5
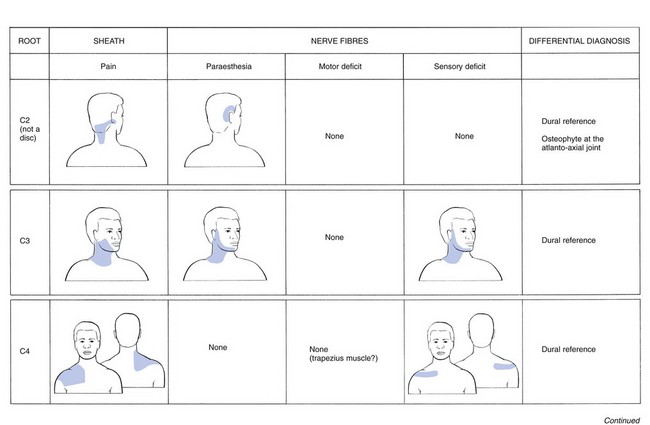
Ageing of the cervical spine
Ageing of the cervical disc
It has been shown that, in the first two decades of life, lateral tears occur in the annulus fibrosus. They tend to develop joint-like structures – the uncovertebral joints – that then begin to undergo transformation.6 In the second and third decades, the lateral tears enlarge towards the medial part of the disc, often ending in a complete transverse splitting of the disc into equal halves. These anatomical changes cause instability of the disc and favour the possibility of cartilaginous displacements, either of the annulus fibrosus or of the nucleus pulposus (see below: disc displacements).
See Chapters 31 and 32 for an extensive explanation of the function and behaviour of the lumbar disc, which is highly comparable to the situation in the cervical spine.
Consequences of disc ageing for surrounding structures
Effects on the facet joints
The prevalence of cervical facet joint degeneration is very high in individuals aged 50 years and more, with a tendency to increase in severity with age. Diminution of the height of the disc and the subsequent reversal from lordosis to kyphosis result in a greater transmission of shearing forces to the facet joints. They start to bear weight and this furthers the degenerative process. This is characterized by fibrillation and erosion of the articular cartilage, partial or complete denudation of the cartilage, and new bone formation.7 Later in life, subperiosteal osteophytes with periarticular fibrosis develop, resulting in a gross decrease in mobility.8
All levels of the middle and lower cervical spine are affected to almost the same degree, whereas in the lumbar spine there is an increase in degeneration towards the lower levels.9
Effects on the spinal canal
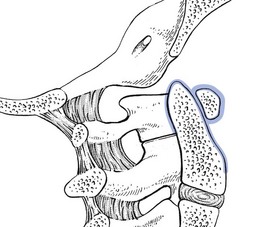
Circular disc displacement, as is seen when the disc has degenerated entirely, may further the development of osteophytic outcrops on all sides, and thus also into the spinal canal, threatening the spinal cord.10 Once the bony parts formed by the uncovertebral joints touch each other, further loss of height can only happen anteriorly. Because of the decrease in height in the anterior column of the cervical spine, cervical lordosis may diminish and even disappear, resulting in the typical position of the degenerated neck – the chin projected forwards. Retrolisthesis in the mid-cervical area (C3–C6) is a common finding in spondylarthrosis. Also, buckling of an enlarged ligamentum flavum narrows the spinal canal, especially during extension of the neck.11,12
Effects on the intervertebral foramen
The association of diminished intervertebral space, osteophyte formation at both the uncovertebral and zygapophyseal joints, and hypertrophy of the facet joint capsules causes diminution of the intervertebral foramen and eventual neural foraminal encroachment.13 Further diminution of the foraminal space is caused by the backwards slip of the upper vertebra during extension.14
Radiological changes
Degenerative changes of the cervical spine are common in both symptomatic and asymptomatic adults. At the age of 50, spondylosis is visible on plain X-rays in more than 50% of asymptomatic people.15 A magnetic resonance imaging (MRI) study on 500 asymptomatic subjects could detect disc degeneration in 17% of discs in men and in 12% of those of women in their twenties, and 86% and 89% of discs in both men and women over 60 years of age. Posterior disc protrusion with demonstrable compression of the spinal cord was observed in 7.6% of subjects over 50 years of age.16,1 The clinical question is whether the patient’s symptoms are caused by the changes that are seen on imaging. Unfortunately, even the most sophisticated imaging studies do not show pain, but only structural abnormalities that may or may not be the cause of the patient’s symptoms.4,17
Pathology
Disorders of the disc: disc displacements
Symptomatic disc displacements in the cervical spine are more common than is generally believed. They can occur at any age from childhood onwards but present with different clinical syndromes, depending on the age group.18
Cartilaginous displacements are most frequent at the C6–C7 and C5–C6 joints. They are occasionally found at the C4–C5 or C7–T1 joints, and rarely at the C2–C3 or C3–C4 joints.19 Nuclear prolapses are very uncommon, except in young people. It was to Cyriax’s credit that he recognized the clinical pictures associated with disc displacements and made an inventory of symptoms and signs. A distinction can be made between posterocentral and posterolateral displacements resulting respectively in discodural and discoradicular interactions. It is important to remember that diagnoses are made on clinical grounds, following the patient’s history and the functional examination. Technical investigations that rely only on anatomical findings lead very easily to misinterpretation.17 They can sometimes be used to confirm a diagnosis, rather than to make one. Discs may displace in two ways: either they move posteriorly to compress dura and/or cord, or they move posterolaterally to compress the nerve root.
Posterocentral displacement
Posterior migration of disc tissue beyond the posterior limits of the intervertebral joint space causes pressure on the posterior longitudinal ligament and, via the ligament, on the anterior part of the dura mater. This produces multisegmental pain with dural reference, mostly in the trapezius and scapular area but possibly towards the head and/or pectoral area (see Ch. 7). The pain is the result of a discodural interaction: irritation of the anterior structures in the spinal canal is responsible for the dural symptoms. Pure posterocentral compression gives rise to pain felt centrally and/or bilaterally. Compression just laterally from the midline will result in pain that is felt unilaterally but is still multisegmental. The internal derangement in the intervertebral joint provokes articular signs demonstrable on functional examination.
Posterolateral displacement
Stages of disc displacement
Disc displacements also behave according to their actual status of degeneration. Cyriax described seven stages of disc lesions, each one typical of a certain age group and characterized by a typical history (Fig. 8.1).20
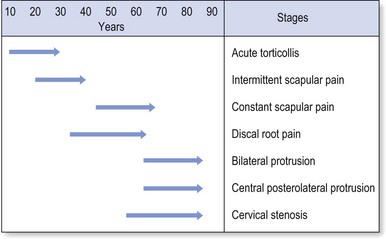
Fig 8.1 Stages of disc displacement.
Findings on examination
It is believed that disc degeneration and disc displacements are of themselves painless events. It is only when pain-sensitive structures in the neighbourhood pick up on the disc abnormality and translate mechanical pressure and inflammatory changes into pain that signs and symptoms originate. The reader is referred to Chapter 33 – the ‘dural concept’ – for a thorough description of this dural hypothesis.
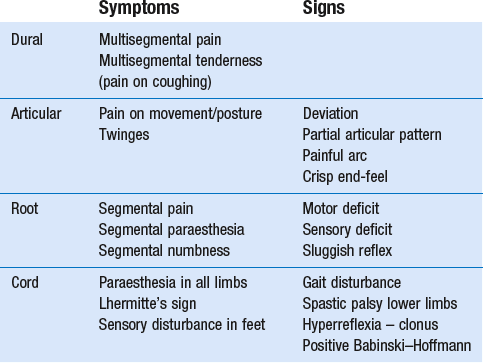
Dural symptoms
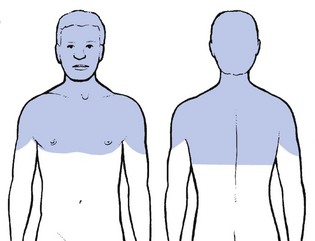
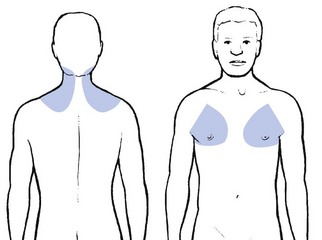
When a fragment of disc (at any level) protrudes posterocentrally, it may compress the dura mater either in the midline, which results in central or bilateral pain, or slightly to one side, leading to pain felt unilaterally. The dura mater, being a multisegmentally innervated tissue, translates this compression into multisegmental pain (see Ch. 1). Pain reference from the cervical dural mater may thus spread up to the head and down to the mid-thoracic region, and may be felt anywhere in this area (Fig. 8.2). However, the pain is most commonly experienced in the region of neck, trapezius and scapula (Fig. 8.3a). Occasionally it is felt in the clavipectoral area or in the axilla (Fig. 8.3b).
Patients often mention a tender spot, usually somewhere at the superior border of the trapezius, which they identify as the source of their pain. On palpation the examiner indeed finds localized tenderness. However, during the examination, movements designed to test the trapezius muscle are found to be negative. Furthermore, the tender spot may shift during neck manipulation and disappears when a full and painless range is obtained. This confirms that the tenderness is clearly a referred phenomenon (see Ch. 1) without localizing or diagnostic value. This multisegmental tenderness is one of the dural symptoms and results from dural compression at any level.
Dural signs
Dural signs are not found on examination. Although neck flexion stretches the dura mater, the pain very often experienced during this movement must be regarded as an articular sign rather than a dural one. Neck flexion can only be regarded as being a dural test if it stretches the dura from a distance, which is not the case here. There is also no equivalent to the straight-leg raising test in the lumbar examination where a simple movement (hip flexion with an extended knee) allows differentiation between dural pain, root pain and muscular tightness. Some nerve roots (C4–C6) are tethered to the gutter of their respective transverse process,21 but apparently not to the foramen.22 Some authors have developed and discussed ‘upper limb tension tests’ (ULTT) as a valuable examination procedure for patients with neck problems with or without radiation down the upper limb.23,24 The aim would be to create stress, transmitted to the structures in the spinal canal via the peripheral nerves (median–radial–ulnar). The peripheral nerves in the upper limb however, have, unlike those in the lower limb, a more complex course. It is therefore more difficult to carry out ‘pure’ movements and, although these tests stress the neurological tissues, they also stress some contractile and inert tissues in neck, shoulder girdle and arm. Because of their low specificity, 25–27 they are not integrated into the standard functional examination of the cervical spine.
Articular symptoms and signs
On examination, a partial articular pattern of internal derangement is found: some active movements are painful and limited or cause pain at the end of range, while others are negative or clearly less positive. The asymmetrical pattern that occurs may vary from very subtle, with hardly any limitation, to very pronounced with some movements completely blocked, forcing the head in a deviated position. A minimal partial articular pattern (Fig. 8.4a) emerges when the internal derangement is minor and the discodural interaction slight. It is hypothesized that pronounced signs result from a severe discodural interaction (Fig. 8.4b). Passive movements usually hurt more than active ones, though cases may be encountered in which passive movements ease the pain. Resisted movements are negative, except perhaps resisted flexion. The latter may slightly increase the pain in more acute cases, presumably as a result of the consequent compression strain on the affected joint.
When neck flexion elicits pain in the upper thoracic area, further examination of neck, shoulder girdle and thorax must differentiate between a cervical lesion with pain reference to the upper thorax and a lesion in the shoulder girdle or upper thoracic spine. The movement is an articular sign for the cervical spine but is also regarded as a dural sign for the thoracic spine. During neck flexion the dura mater is stretched and drawn forward against an eventual upper thoracic protrusion (see Ch. 25).
Root symptoms and signs
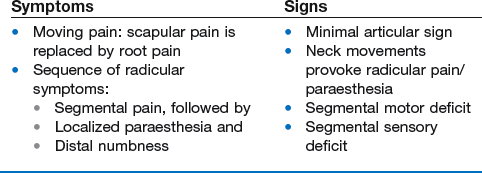
By analogy with the lumbar spine, where backache may evolve into sciatica, cervicoscapular pain may be replaced by brachial pain. The original multisegmental cervicoscapular pain disappears and severe and strictly segmental pain down the arm supervenes. The examiner must be aware that patients sometimes omit to mention the original pain in the trapezius or scapular area but concentrate only on the severe symptoms at presentation. This change in pain localization is the result of the disc moving laterally (Fig. 8.5). Pressure on the dura mater ceases when the dural sleeve of the nerve root becomes compressed and radicular pain appears. Increasing compression of the nerve root is translated into increasing symptoms and signs. Paraesthesia and/or numbness point towards involvement of the nerve root fibres and are usually felt in the distal part of the corresponding dermatome.
Additional tests to provoke radicular pain can be added. During the Spurling test, the patient side-flexes the head to the affected side first, followed by the unaffected side. Meanwhile the examiner presses straight down on the head.28 A test result is classified as positive if pain radiates into the arm towards which the head is side-flexed during compression. Alternatively the head is rotated and side-flexed to the same side during compression.29 This is called the ‘maximal cervical compression test’. Great care is needed while performing this test as it also compresses the vertebral artery.
Common syndromes
Acute torticollis: unilateral pain with asymmetrical picture
History
Most patients wake in the morning with a stiff neck. Pain and stiffness increase as soon as they adopt an upright position. Any attempt to move the neck results in unilateral pain, radiating to trapezius and/or upper scapular area. Occasionally the symptoms come on suddenly as the result of a certain movement (e.g. bending over a washbasin), trivial trauma (e.g. a blow to the head30) or coughing.
Examination
On examination, gross and obvious articular signs are found: a striking and extremely painful limitation of one rotation and one lateral flexion towards the same side, usually the painful side. Often no movement is possible in either of these directions (Fig. 8.6). The other movements are much less painful and are scarcely limited, except possibly extension. The end-feel is empty or one of severe muscle guarding.
Diagnosis
The pattern of limitation is diagnostic. Acute torticollis is the most striking example of a partial articular pattern, which indicates that part of the joint is blocked by an intra-articular displacement. In most instances the displacement is nuclear: the nucleus has oozed out slowly during the night, while the patient has being lying for some hours with the neck in side flexion or rotation. The dura mater becomes compressed and irritated, which causes the cervicoscapular pain. A sudden onset suggests an annular displacement. It usually occurs in patients over 30. This mechanism was hypothesized by Cyriax more than 50 years ago and was recently proven by two MRI studies. Maigne et al performed MRI in a 15-year-old male adolescent, a few hours after the onset of torticollis. Increased signal intensity compatible with a fluid collection was seen in the right uncovertebral region at C2–C3, causing excessive lateral pressure and pushing C2 outwards. A new MRI taken 3 weeks after resolution of symptoms was unremarkable.31 Another MRI study performed by Gubin et al in ten children and adolescents with acute torticollis showed similar findings: high-intensity zones were seen near uncovertebral zones C2–C3 or C3–C4. The zones were always on the side where the patients felt pain and disappeared spontaneously after a few days.32
The characteristics of acute unilateral torticollis are listed in Box 8.2.
Differential diagnosis
Torticollis in infancy and childhood
Osseous lesions are mostly anomalies of the atlas,33 such as hemiatlas, partial atlantic aplasia34 or partial atlanto-occipital fusion,35 and Klippel–Feil syndrome. Klippel–Feil syndrome is congenital fusion of two or more cervical vertebrae. The classic clinical triad is: lower neckline, short neck, and restriction of head and neck movements.36 In patients with moderate involvement, this classic triad may not be seen and sometimes there is only some scoliosis deformity or moderate torticollis.37
Congenital muscular torticollis (CMT) is caused by shortening of the sternocleidomastoid muscle. This leads the head to bend toward the affected side and the chin to turn to the opposite side, the so-called cock robin position of the neck. CMT is the third most common congenital musculoskeletal anomaly after dislocation of the hip and clubfoot,38 with a reported incidence of 0.3–1.9%.39 Various theories have been proposed but the true aetiology of torticollis remains uncertain.40 As a result of fibrotic changes of the sternocleidomastoid muscle, the unilateral contracture may subsequently result in plagiocephaly, skull and facial asymmetry.41 When diagnosed early, CMT can be managed conservatively and seldom requires surgery.42 In fact, a simple stretching programme leads to spontaneous resolution in most patients.43 In children older than 1 year, corrective surgery has both cosmetic and functional benefits, the best outcomes being obtained between the ages of 1 and 4. After the age of 5, the form and efficacy of treatment are controversial.44
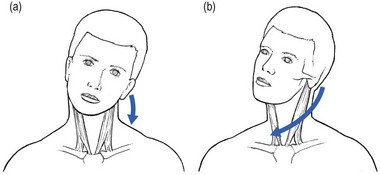
Acquired torticollis in children should always be taken seriously, as it may result from a number of underlying causes, some of which are severe and life-threatening. Spontaneous subluxation of the atlantoaxial joint following peripharyngeal inflammation is known as Grisel’s syndrome. Children between 5 and 12 years are most frequently affected. However, the condition has been reported in patients ranging from infancy to the seventh decade of life.45 Torticollis may occur shortly after the onset of the underlying infection or it may follow mild trauma to the neck. The syndrome has been reported in association with rhinopharyngitis,46 cervical osteomyelitis, rheumatic conditions, and following surgical procedures such as tonsillectomy or adenoidectomy,47 choanal atresia repair and mastoidectomy. It is hypothesized that distension and abnormal laxity of the ligaments surrounding the atlantoaxial articulation result from direct spread of inflammation from the pharynx and nasopharynx.48 This leads to C1–C2 instability with subluxation and (sometimes) devastating neurological sequelae (between 10 and 15% of patients have neurological signs or symptoms, with serious consequences including quadriplegia and sudden death49). The condition begins with a spasm of the irritated sternocleidomastoid muscle. This contraction leads to a typical position of the head, which is easily differentiated from the position in acute discogenic torticollis. In a disc lesion, the deviation is purely in lateral flexion or flexion or a combination of both. When a spasm of the sternocleidomastoid muscle is responsible, the neck is held in flexion, side flexion towards and rotation away from the painful side (signe du merle or ‘blackbird’s sign’) (Fig. 8.7).50
Benign paroxysmal torticollis is a relatively uncommon, self-limiting condition occurring during infancy. It resolves by the age of 2–3 years. Periodic episodes of torticollis may randomly alternate from side to side and be associated with vegetative symptoms. The aetiology is unknown and no treatment is effective.51
Spasmodic torticollis or cervical dystonia is the most frequently occurring focal dystonia, with an incidence of 8.9 per 100 000 people.52 Dystonia is defined as sustained, involuntary muscle contractions, which often cause twisting or repetitive movements or abnormal postures. The origin of the symptoms is still unclear. The condition often results in cervical pain and disability as well as impairments affecting postural control.53
Therapy of spasmodic torticollis includes behaviour modification, such as training the patient to readjust the position of the head, and biofeedback, hypnosis and anticholinergic drugs.54 When these procedures are not successful, local application of botulinum toxin A offers a highly effective treatment technique. Botulinum toxin A is injected into the overactive, dystonic cervical musculature to provide a graded, reversible denervation of the neuromuscular junction by preventing the release of acetylcholine from the presynaptic axon of the motor end plate. Botulinum treatment showed success rates ranging from approximately 60 to 90%.55,56 Surgical intervention is used when a patient has not responded to other interventions. Although a variety of surgical techniques have been studied, the most commonly used approach is selective peripheral denervation.57
The presence of a compensatory thoracic curve following cervical scoliosis indicates the possibility of adolescent scoliosis, unilateral cervical rib, Klippel–Feil deformity58 or previous thoracoplasty.
Treatment
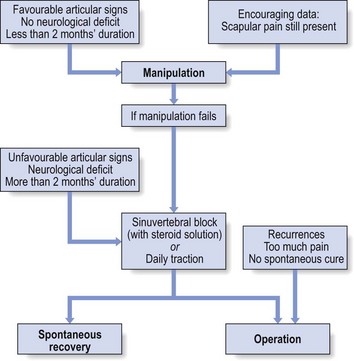
Treatment is by manipulation. The technique is adapted according to the clinical picture. Acute torticollis in patients under 30 often behaves in a nuclear manner; these patients should be treated with ‘nuclear’ techniques – progressive sustained rotation and/or lateral flexion, whereby an attempt is made to push the displaced nuclear material slowly back. The less common annular cases should be treated with the usual ‘annular’ manipulation techniques (see Ch. 11).
Unilateral cervicoscapular pain
Examination
A mild partial articular pattern is found (Fig. 8.8). As a rule, two, three or four movements out of the six are painful, and four, three or two prove painless. Passive movements are usually more painful than active ones. There may be minor limitation, or a painful arc is found. The end-feel is rather crisp, indicating the muscle guarding typical of a disc lesion, but it is not uncommon to find a normal, capsular end-feel. Root and cord signs are absent. It is not easy to determine the level at which the protrusion lies, because any disc at any level gives rise to the same symptoms (multisegmental pain) and signs (partial articular pattern). Oscillatory techniques on a sitting or prone lying patient or anteroposterior gliding movements with the patient lying supine may sometimes provoke the pain and give an idea as to the level but this cannot always be relied upon.
Differential diagnosis
The differential diagnosis includes lesions in the shoulder girdle (e.g. posterior sternoclavicular syndrome, see online chapter Interpretation of the clinical examination of the shoulder girdle), in the upper thoracic area (see Ch. 26), internal disorders (e.g. in the heart, lungs or diaphragm, see Ch. 28) and neurological conditions (e.g. long thoracic or spinal accessory neuritis, see online chapter Nerve lesions and entrapment neuropathies of the upper limb).
Treatment
Unilateral pain in the cervicoscapular area is highly suitable for manipulation. Usually a few sessions suffice to restore a full and painless range of movement. The patient must be informed that the disc may move again, however, and that recurrences are not uncommon. Each new attack should be treated in a similar way. However, when there is a tendency to recurrence, prophylactic measures should be taken (i.e. position at work, sleeping position, collar, exercises – see Ch. 13).
See Box 8.3 for a summary of unilateral pain in the neck, trapezius or scapular area.
Unilateral root pain
Root pain as the result of a disc protrusion pressing on the dural investment of a cervical nerve root is quite common. It has an incidence rate of 1/1000 per year with a peak age between 45 and 54. The lesion very seldom occurs in adolescence. In younger patients suffering from cervical root pain, other disorders must be excluded before a disc lesion is considered. Over the age of 60, root pain caused by a discoradicular interaction is also rare, whereas an osteophytic or metastatic compression becomes more likely (Box 8.4).59
History
As a rule, the patient has had a history of recurrent bouts of unilateral cervicoscapular pain over the past years. The present symptoms start with the usual scapular pain, but this time they do not resolve spontaneously. On the contrary, one day the scapular pain may have become much worse and strictly unilateral, subsequently shifting to shoulder and arm (Box 8.5). The scapular pain then disappears or diminishes to a degree that it is no longer worthy of mention, whereas pain in the arm increases and remains severe for 1–2 months. The ache is sharp and permanent, and prevents the patient from sleeping. Sometimes the patient can only achieve rest by bringing the arm above the head – a position that relieves tension on the roots but also widens the intervertebral foramen and diminishes the pressure.60,61 This sign is highly suggestive of a discoradicular conflict, but is unlikely to be present in radiculopathy caused by spondylosis.62 The arm pain may be accompanied by pins and needles in some fingers, later followed by numbness. In order to make the differential diagnosis with other lesions that provoke paraesthesia, it is vital to collect more information about its behaviour: how far proximally does the paraesthesia spread, which fingers and what parts of them are involved, and when precisely are the pins and needles felt? A typical feature is that the paraesthesia has neither edge nor aspect within the fingers. Stroking the skin may provoke or increase pins and needles but moving the digits has no influence. Paraesthesia comes and goes, day or night, in an erratic fashion, appears without any definite reason and does not last for more than an hour at a time. In difficult cases, diagnostic traction may help: if the paraesthesia disappears during manual traction at the head and comes back when traction is released, the cause is clearly cervical.63 In most cases pain and paraesthesia disappear spontaneously, 6–10 weeks after the onset of the brachial pain. It may take months for the numbness to disappear completely.
Examination
The patient is asked about the precise location of the pain, which will enable the examiner to determine the dermatome and affected root. However, it is important to mention that the localization of the arm pain is not always a reliable localizing sign. Due to the high incidence of intradural connections between the dorsal rootlets of C5, C6 and C7 segments, there can be overlap between dermatomes, with one dermatome encompassing one or two adjacent segments.64,65 It may therefore be possible for an individual to have a dermatomal distribution that fails to match the classic pattern precisely. A better pointer to the level of compression is paraesthesia, and therefore, if the patient mentions pins and needles, more details should be sought about their localization.
Articular signs may be present but are not paramount. Usually only a slight partial articular pattern of internal derangement is seen; as the protrusion lies more outside the joint and the articular movements no longer interfere with it, articular signs are less important than root signs. Some active and passive movements may provoke or increase pain in the scapular area. The end-feel is crisp. It is possible, although not at all common, for neck movements to influence the pain and/or the pins and needles down the arm. The Spurling manœuvre (a downwards pressure on an extended and rotated neck) may also elicit radicular pain and paraesthesia.28 Resisted movements of the head are negative.
Weakness indicates which nerve root has become compressed. The degree of motor deficit can be very subtle. The examiner should therefore compare both sides carefully.65 Gross weakness (3+ or 4) is a warning sign, indicating that the lesion is very probably non-discogenic in nature. Cutaneous analgesia is sought at the fingers and the reflexes are tested. An uncomplicated discoradicular interaction does not cause cord signs.
Different root syndromes and their differential diagnosis
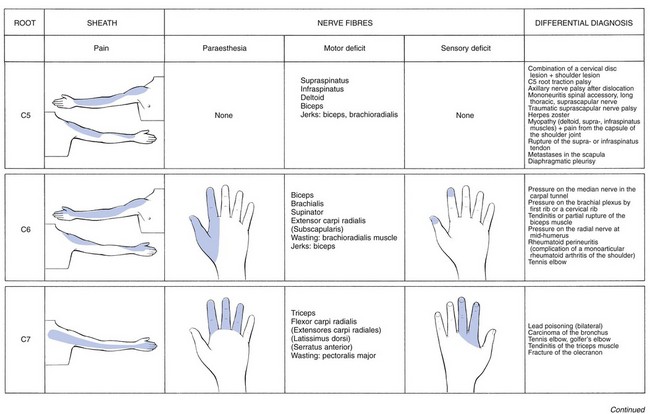
The nerve roots most frequently compressed by a disc are, in order of frequency, C7 (56–70%), C6 (19–25%), C5 (2–14%) and C8 (4–10%).66 Each nerve root lesion, whatever its cause may be, gives rise to pain felt in the skin area corresponding with the segment to which the nerve root belongs. The pain is the result of involvement of the dural sheath surrounding the nerve fibres. When the nerve parenchyma becomes affected too, paraesthesia will be felt down the nerve distribution in the arm. However, variations in the nerve distribution may occur,67–69 and pain and paraesthesia may be felt in areas which do not totally correspond to one specific dermatome. More involvement of the nerve fibres causes weakness of the muscles belonging to the same segment and cutaneous analgesia in some (mostly the distal) part of the dermatome. One should bear in mind that other (non-neural) soft tissue lesions may lead to exactly the same segmental pain.70 Therefore resisted tests, undertaken to examine nerve root conduction, also serve to test for alternative causes of pain down the limb.
The absence of discs at the first two cervical levels implies that nerve root compression by discs cannot occur. Reference of pain to the C1 or C2 dermatomes (headache; Fig. 8.9) does not always mean that C1 or C2 nerve roots are involved; the pain may be multisegmental and the result of compression of the lower cervical dura by a posterocentral disc protrusion.71,72
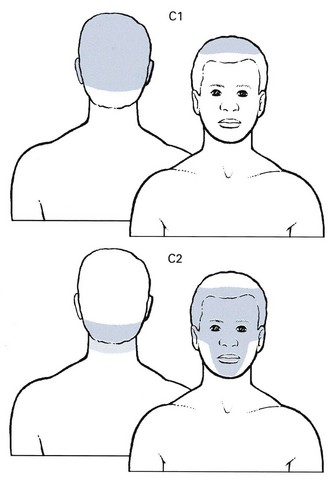
Fig 8.9 The C1 and C2 dermatomes.
The C2 spinal nerve has a close proximity to the lateral capsule of the atlantoaxial (C1–2) zygapophyseal joint and innervates the atlantoaxial and C2–3 zygapophyseal joints; therefore, trauma to or pathologic changes around these joints can be a source of referred headache. This occurs especially in elderly patients with gross unilateral arthrosis of the atlantoaxial joint. It causes unilateral deep or dull pain that usually radiates from the occipital to parietal, temporal, frontal and periorbital regions. Ipsilateral eye lacrimation and conjunctival injection are common associated signs.73 Clinical examination shows a marked full articular pattern in which rotation is especially limited.
Compression of the third cervical nerve root by a posterolateral disc displacement is very rare.
Pain is felt at the lateral aspect of the neck and is referred to the occipital and temporal regions and the posterior part of the cheek. Pins and needles or numbness may be present (Fig. 8.10). Weakness is clinically not detectable. Cutaneous analgesia is uncommon, but if it occurs, it occupies any part of the lateral aspect of the neck.
Fig 8.10 The C3 dermatome.
Most C3 pain stems from the C2–3 zygapophyseal joint. This joint and the third occipital nerve appear most vulnerable to trauma from acceleration–deceleration (‘whiplash’) injuries of the neck.74 Pain in this region may also result from pressure on the dura mater at any level and is then multisegmental. Heart disease may also give rise to reference of pain in the C3 dermatome. Another possibility is a trigeminal neuritis, which is characterized by paroxysms of severe, lancinating, electric bouts of pain restricted to the distribution of the trigeminal nerve.75
Discogenic lesions of the fourth cervical nerve root are rare. Pain spreads from the lower half of the neck towards the point of the shoulder (Fig. 8.11). Paraesthesia seems not to occur at this level. In theory, weakness of the trapezius muscle should be found but in practice muscular weakness is not detectable. A horizontal band of cutaneous analgesia may be found along the spine of the scapula, the mid-deltoid area and the clavicle.
Fig 8.11 The C4 dermatome.
Pain is felt along the lateral aspect of the arm and forearm as far as the base of the thumb (Fig. 8.12). Pins and needles do not seem to occur in C5 nerve root compression.76 Weakness may be found in supraspinatus, infraspinatus, deltoid and brachial biceps. A C5 sensory palsy from a disc is rare. The biceps jerk may be sluggish or absent; the brachioradialis jerk is sluggish, absent or inverted.
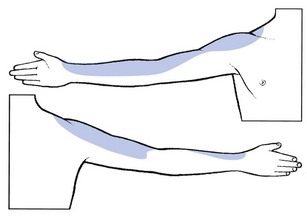
Fig 8.12 The C5 dermatome.
The differential diagnosis (see online chapter Nerve lesions and entrapment neuropathies of the upper limb) includes some peripheral nerve lesions and all pathology of the shoulder (see Chapters 14 and 15 ):
• Axillary nerve palsy after dislocation of the shoulder: this results in painless weakness and atrophy of the deltoid muscle and consequent weakness of abduction of the shoulder. Rotation movements remain strong.
• Mononeuritis of the spinal accessory nerve: there is painless weakness of the trapezius muscle which results in slight limitation of active elevation of the arm.
• Mononeuritis of the long thoracic nerve: this presents with painless weakness of the serratus anterior muscle. Active elevation of the arm is grossly limited.
• Mononeuritis or traumatic palsy of the suprascapular nerve: there is painless weakness of the infraspinatus (and supraspinatus) muscles.
• Neuralgic amyotrophy: this leads to gross weakness in several muscles.
• Herpes zoster: there is pain and a typical skin eruption in the C5 dermatome.
• Shoulder arthritis, supraspinatus tendinitis or infraspinatus tendinitis: pain down the C5 dermatome can be referred from the joint capsule or from a rotator cuff tendon.
• Rupture of the supraspinatus muscle: partial rupture leads to painful weakness and complete rupture to painless weakness of abduction at the shoulder.
• Rupture of the infraspinatus muscle: lateral rotation is painful and weak in partial rupture, and weak only in complete rupture.
• Metastases or fracture in the scapula: passive mobility of the scapula is limited and the muscles attached to it are weak.
• Myopathy: bilateral wasting of the supraspinatus and infraspinatus muscles is suggestive of myopathy. Arthritis from immobilization of the shoulder is then a painful complication.
Pain runs along the anterior aspect of the arm, the volar aspect of the forearm and the radial side of the hand as far as the thumb and index finger (Fig. 8.13). Pins and needles are felt in the thumb and index finger.77 There is weakness of the biceps, brachialis, supinator brevis and the extensors of the wrist. Sometimes the subscapularis muscle is affected too. Cutaneous analgesia is sometimes detectable at the tips of thumb and index finger. The biceps jerk is sluggish or absent.
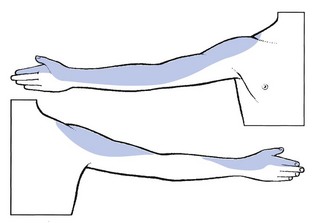
Fig 8.13 The C6 dermatome.
The differential diagnosis is from the following disorders:
• Thoracic outlet syndrome: nocturnal pins and needles in one or both hands are often a conspicuous feature. Searching for the release phenomenon by scapular testing provides the clue (see online chapter Nerve lesions and entrapment neuropathies of the upper limb).
• Compression of the median nerve in the carpal tunnel: there are pins and needles in the three and a half radial fingers. The history is indicative (see online chapter Nerve lesions and entrapment neuropathies of the upper limb).
• Palsy of the radial nerve from compression at mid-humerus: there is paralysis of the extensors of wrist and fingers, together with weakness of supination of the elbow and of extension of the thumb (see online chapter Nerve lesions and entrapment neuropathies of the upper limb).
• Tendinitis or partial rupture of the biceps muscle: there is pain or painful weakness on resisted flexion of the elbow (see Ch. 15).
• Tennis elbow: tendinitis at the origin of the extensor carpi radialis brevis may be so acute that the patient often winces and lets the hand go when asked to extend it against resistance (see Ch. 19).
Compression of the seventh cervical nerve root by the C6 disc is far more common than compression at any other level.66
Pain is felt at the posterior aspect of the arm and the dorsal aspect of the forearm as far as the second, third and fourth fingers (Fig. 8.14). Rarely, pain is experienced at the anterior and upper aspect of the chest and not in the arm. Pins and needles are mostly felt in the index, middle and ring fingers. The most conspicuous feature is weakness of extension of the elbow (triceps muscle). Flexion of the wrist may also be weak (flexor carpi radialis). In severe cases, resisted adduction of the arm is slightly weak and there may even be some visible wasting of the mid-portion of the pectoralis major. The examiner also notices that voluntary elevation of the arm is lacking over the final 5°. In the unusual circumstance of a larger part than usual of the serratus anterior muscle being derived from the C7 segment, winging of the scapula may be seen.78 Cutaneous analgesia is sought at the dorsal aspect of the index and middle fingers. The triceps jerk is seldom affected, even in quite severe cases.
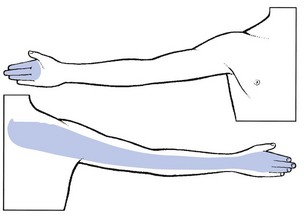
Fig 8.14 The C7 dermatome.
Differential diagnosis is from the following disorders:
• Lead poisoning: one of the first signs is often bilateral weakness of extension of the wrist (see online chapter Nerve lesions and entrapment neuropathies of the upper limb).
• Carcinoma of the bronchus: this may also lead to bilateral weakness of extension of the wrist.
• Tennis elbow: a painful twinge with weakness of extension of the wrist may be found in acute cases (see C6 root).
• Golfer’s elbow: pain on resisted flexion of the wrist is the primary sign (see Ch. 19).
• Tendinitis of the triceps muscle: there is pain on resisted extension of the elbow (see Ch. 19).
• Fracture of the olecranon: resisted extension of the elbow is painful and weak but this picture is complicated by articular signs at the elbow joint (see Ch. 18).
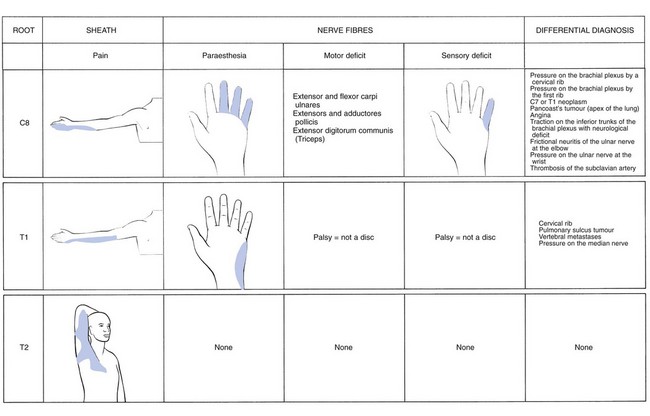
Pain is felt at the inferior part of the scapular region and the ulnar aspect of the hand, over the middle, ring and little fingers and the ulnar and distal aspect of the forearm (Fig. 8.15). Pins and needles are experienced in the middle, ring and little fingers). The condition is easily confused with ulnar nerve problems (‘pseudo ulnar palsy’).79
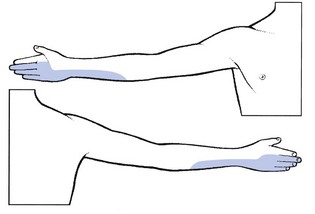
Fig 8.15 The C8 dermatome.
The differential diagnosis includes:
• Thoracic outlet syndrome: this may result in pins and needles in the ulnar fingers and sometimes in weakness of the intrinsic muscles of the hand too (see online chapter Nerve lesions and entrapment neuropathies of the upper limb).
• Metastases in the C1 or T1 vertebra: involvement of the C7 and C8 nerve roots at the same time is very suggestive of malignant disease.
• Angina pectoris: heart disease should always be considered when pain radiates to the left arm, especially to the ulnar aspect of the hand and forearm.
• Traction palsy of the lower trunk of the brachial plexus: this also affects the fibres derived from the C8 nerve root (see online chapter Nerve lesions and entrapment neuropathies of the upper limb).
• Frictional neuritis of the ulnar nerve at the elbow or pressure on the ulnar nerve at the wrist (see online chapter Nerve lesions and entrapment neuropathies of the upper limb).
• Thrombosis of the subclavian artery: there is pain in the upper limb and an absent radial pulse.
T1 pain is felt in two distinct sites: the pectoroscapular area and the ulnar aspect of the forearm (Fig. 8.16). The thoracic pain may be influenced by coughing and neck flexion. The T1 root is stretched by a forward movement of the scapulae, and by abducting the arm and flexing the elbow so that the hand comes to lie behind the neck.80 Pins and needles are felt at the ulnar side of the hand. A T1 palsy is characterized by weakness of the intrinsic hand muscles, but is never caused by a disc lesion. Also, numbness in the T1 area is always the result of a non-discal condition. A first thoracic palsy, together with Horner’s syndrome, is one of the main symptoms in Pancoast’s tumour81 and also vertebral metastases may involve the T1 nerve root.
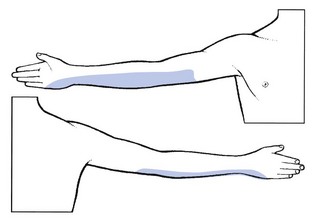
Fig 8.16 The T1 dermatome.
Differential diagnosis of a T1 lesion should be made from the following conditions (see online chapter Nerve lesions and entrapment neuropathies of the upper limb):
• Cervical rib: this may compress the lower trunk of the brachial plexus, which is partly derived from the T1 nerve root.
• Compression of the median nerve: in carpal tunnel syndrome, pain may sometimes spread to the forearm. Most of the muscles in the hand that are supplied by the median nerve are also partly derived from the T1 nerve root.
• Compression of the ulnar nerve: this may cause pins and needles in the ulnar border of the hand. All the small muscles of the hand that are innervated by the ulnar nerve are also partly derived from the T1 nerve root.
• Amyotrophic lateral sclerosis: weakness and atrophy of the small intrinsic muscles of the hand is often one of the first signs of amyotrophic lateral sclerosis.82
A disc lesion at this level is extremely rare. If it does occur, it gives rise to pectoroscapular pain radiating down the inner aspect of the arm as far as the elbow (Fig. 8.17). The pain is influenced by neck flexion, approximation of the scapulae and sometimes stretching of T1.
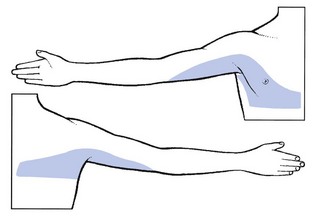
Fig 8.17 The T2 dermatome.
Natural history
In discoradicular interactions, spontaneous cure is the rule for both pain and neurological deficit.83 Patients with neurological deficit usually recover in about 3 months, and those without in 4 months, reckoned from the onset of the pain down the arm. The more marked the palsy, the more quickly the pain abates. Muscle strength will almost certainly have returned to normal 3–6 months after the pain ceased. The only exception is the C8 root, which may take 6 months or more to recover. The sensory disturbance recovers very gradually over a year. Mochida et al performed follow-up studies, both clinical and with MRI, on 38 patients with lateral cervical disc herniations. All of them were treated with conservative therapy. The authors’ conclusion was: ‘migrating, lateral-type herniations regress so frequently that conservative treatment should be chosen not only for patients with radicular pain, but also for those with upper limb amyotrophy.’84
Treatment
There are a number of approaches to treatment of nerve root problems.
In those patients in whom manipulation fails or is no longer indicated, 1–6 infiltrations with 2 mL of a steroid solution at the nerve root may afford considerable relief. Pain and inflammation are diminished during the period of spontaneous healing.85
Neck surgery for discoradicular interactions is performed far too often. The condition has such a favourable spontaneous cure rate that surgical intervention should only be considered in exceptional situations: when the condition recurs, when pain is intolerable or when spontaneous cure fails to occur. Furthermore, the long-term results of surgery are no better than when spontaneous cure has been awaited.86
Less common syndromes
The syndromes described in this section are also based on disc lesions that, in contrast to the lumbar spine, may imperil the spinal cord. Cyriax believed that posterocentral disc protrusions may form the first step in the evolution towards spinal stenosis. An unreduced central disc displacement exerts continuous pressure on the posterior longitudinal ligament. The latter draws the periosteum away from the vertebral bone. Bone grows towards the periosteum and osteophytes form. These diminish the anteroposterior diameter of the spinal canal and spinal stenosis syndrome develops (see p. 164).
Acute torticollis with central pain and a symmetrical picture
Central or bilateral pain in the neck, trapezius or scapular area
The condition causes central neck pain, with or without bilateral radiation to occiput, trapezii and scapulae, although slow painless development also occurs. Pain is the result of the pressure exerted on the dura mater (Fig. 8.20). Cord symptoms indicate involvement of the spinal cord. Cord signs point to irreversible impairment of conduction. The evolution from pain, through cord symptoms to irreversible cord signs may take several years to develop.
Fig 8.20 Posterocentral disc displacement.
Examination
Increased compression leads to the manifestation of the clinical syndrome of cervical myelopathy (see p. 165): a positive plantar reflex, incoordination and spasticity of the lower limbs and weakness of the upper limbs.87
Natural history
There is a tendency towards spontaneous cure but regression of the disc protrusion is slow.88
Degenerative disorders
Arthrosis of the cervical spine is usually referred to as cervical spondylosis or spondylarthrosis. It starts very early in life with disintegration of the disc. The ageing of the cervical disc and the consequences for the surrounding structures have been discussed earlier in this chapter. Degenerative changes in vertebrae and discoligamentous structures are found in 75–90% of people aged over 50.15,16,89 From middle age on, degeneration is evident on plain X-rays and on MRI in more than 50% of asymptomatic people.90,91 Therefore, most authors emphasize that there is no consistent correlation between abnormal radiographic appearances and symptoms.92,93 The conclusion that pain originates from degeneration of the cervical spine is all too often made by looking at imaging alone. The importance of arthrotic changes is overestimated because, in most instances, the degenerative condition is not painful in itself and leads only to some stiffness. It is the clinician’s task to determine the possible relationship between the degenerative changes and the patient’s symptom.94
Symptomatic cervical spondylosis should be diagnosed purely on clinical grounds, through history and clinical examination. When cervical movements influence the patient’s symptoms, the problem clearly lies within the spine but this does not necessarily imply that arthrosis is responsible. Although degeneration is not painful of itself, it can lead to symptomatic conditions such as: ligamentous contracture, overstretching of a facet joint, radicular compression by osteophytes or cervical spinal stenosis. The diagnosis of these lesions should be made on clinical grounds and not on imaging.95
Local pain
Morning headache in the elderly
‘Morning headache’ in the elderly is believed to stem from ligamentous contracture at the atlanto-occipital and atlantoaxial complexes. The hypothesis relies on the typical distribution of pain in the C1–C2 dermatomes,96,97 the leathery end-feel on clinical examination and the uniformly good results with mobilization.
As age advances, increasing pain develops in the C1–C2 dermatomes: the middle of the upper neck and the occiput, the temples and the forehead (C2). Local pain may be totally absent, so that the patient complains of occipitofrontal headache only.98 The headache is only on waking and happens without fail every morning. After some hours the pain begins to diminish and by midday all symptoms have ceased, only to return the next morning. Later in the course, the pain may last longer into the day.
The differential diagnosis is between traumatic arthrosis and postconcussional headache. In both, the history is distinctive (see pp. 164 and 168).
The condition is very easily cured by manipulation: 1–4 sessions of slow capsular stretching make all symptoms disappear. Age is not a contraindication to manipulation but the older the patient, the more gradually the therapist must work: fewer manœuvres are carried out in one session and the interval between the sessions is longer (see Ch. 11 and summary in Box 8.7).
Subacute arthritis of the atlantoaxial joint
Subacute arthritis of the atlantoaxial joint is rare. The patient, aged between 25 and 40, develops stiffness and discomfort in the middle of the upper neck, and over the following weeks these symptoms gradually increase. On clinical examination, extension, both side flexions and flexion movements are full and painless. Both rotations are equally painful and very limited – only 10–20% is possible. Even in a supine position, the range remains unchanged. The end-feel is soft. This combination of symptoms and signs is clearly a warning sign but further investigations, such as blood tests and imaging, remain negative. The aetiology remains unclear but the disorder recovers in a couple of months with anti-inflammatory therapy.99
Arthrosis of a facet joint
The prevalence of cervical facet joint degeneration is very high in individuals aged 50 years and more, with a tendency to increase in severity with age. Contrary to the lumbar spine, where there is an increase in degeneration towards the lower segments, in the cervical spine all levels of the middle and lower cervical spine are affected to almost the same degree.100 However, the relation between the high prevalence of radiographic zygapophyseal arthrosis and neck pain is not clear.101 It is not well understood why arthrosis at a facet joint becomes symptomatic in one person and not in another. Cyriax hypothesized that pain results from the formation of adhesions and a ‘self-perpetuating inflammation’, which follows overstretching of the arthrotic joint capsule.20
Diagnosis of a facet joint lesion is not easy and is always tentative. Some elements from the history and clinical examination may help in differentiating a facet joint lesion from a discodural conflict. In doubtful cases, local infiltration with lidocaine may prove diagnostic.102
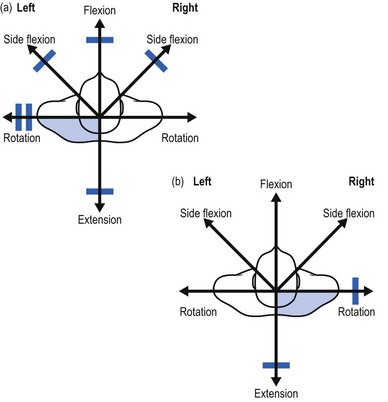
On examination, a partial articular pattern is found: some movements, especially passive ones, hurt. The end-feel is rather hard. The fact that the pain is quite localized and does not radiate indicates the possibility of a facet joint lesion. Troisier82 distinguishes a ‘convergent’ and a ‘divergent’ pattern of painful movements (Figs 8.21 and 8.22). In the first, there is pain on extension and on lateral flexion and rotation towards the painful side. In the second, the opposite is found: flexion, lateral flexion and rotation away from the painful side hurt. Such a pattern is compatible with facet joint involvement but does not exclude a discodural interaction. Differentiation between these lesions is always difficult.
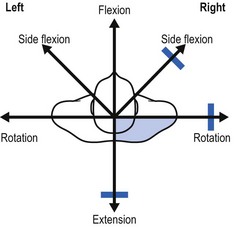
Fig 8.21 The convergent partial articular pattern in arthrosis at a facet joint (colour indicates pain).
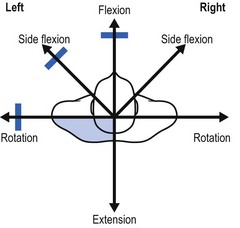
Fig 8.22 The divergent partial articular pattern in arthrosis at a facet joint (colour indicates pain).
This condition responds to deep transverse massage at the joint capsule and slow stretching manipulation. Infiltration with a steroid suspension is an alternative (Box 8.8).
Radicular complaints: osteophytic root compression
A nerve root can become compressed by osteophytes and bony spurs in the neural foramen. The root normally occupies about one-third of the space in the foramen and is accompanied by radicular arteries and veins. The spinal nerve root is vulnerable to compression, anteriorly by an osteophyte derived from the uncovertebral joint and posteriorly by one originating from the facet joints, or from both (Fig. 8.23). These phenomena are secondary to degeneration of the intervertebral disc, with subsequent dehydration and collapse. This disintegration causes increased mechanical stress at the uncovertebral joints and the facet joints, leading to the formation of subperiosteal bone and osteophytic outgrowth, and finally resulting in narrowing of the intervertebral foramen and encroachment of the nerve.103,104
Fig 8.23 Osteophytic nerve root compression.
On examination, a full but painless articular pattern with a hard end-feel is found, indicating arthrosis. If any movement does hurt, it is usually side flexion towards the affected side, and a twinge may travel down the arm. Alternatively, waves of paraesthesia may be felt in the distal part of the relevant dermatome and are clearly the result of the osteophyte being pushed against the nerve root. The Spurling manœuvre (a downward pressure on an extended and rotated neck) may also elicit radicular pain and paraesthesia.28 Isometric testing of the upper limb shows segmental weakness. The most frequent localization is C4–C5. A combination of higher uncinate process, smaller anteroposterior diameter of the intervertebral foramen, and a longer course of the nerve root in close proximity to the uncovertebral joint may explain the predilection of nerve root compression at this level.105,106 The C5 nerve root thus becomes compressed, identified by the difficulty or sometimes even inability to bring the arm up. In severe cases, the deltoid may become wasted.107
The differential diagnosis is from root compression by a disc protrusion or by a neuroma or metastases, and can usually be made on a clinical basis (Table 8.2). The osteophyte grows slowly and lies quite far laterally so that it does not exert pressure on the dural investment of the nerve root. Therefore intense pain, as would occur in a discoradicular interaction, is seldom present. The patient complains mainly of paraesthesia and of a progressive weakness of the upper limb developing over the course of a few months. Nerve root compression by a disc protrusion is characterized by a definite chronology: a period of bouts of multisegmental pain, referred to the scapular area, is followed by the occurrence of segmental pain, followed by segmental paraesthesia and often moderate neurological deficit of the muscles belonging to the same segment. The condition undergoes spontaneous cure in 2–3 months. A neuroma usually starts in the opposite way: distal paraesthesia first, followed by pain that starts distally and spreads in a proximal direction. Root pain due to a neuroma typically occurs in younger people, while root pain from a disc lesion occurs more frequently in the middle-aged. A tumour or metastasis leading to root compression usually has a quicker progression than an osteophyte. Further differential diagnosis includes all the upper limb entrapment syndromes and neuralgic amyotrophy (see online chapter Nerve lesions and entrapment neuropathies of the upper limb).
Table 8.2
Differential diagnosis between discal and osteophytic root compression
| Disc | Osteophyte | |
| History | > 35 years of age | Elderly: > 50 years of age |
| Shifting pain (scapula > arm) | Arm pain | |
| Typical evolution | No evolution | |
| Intense pain | Slight pain | |
| Pain > weakness | Weakness > pain | |
| Examination | Partial articular pattern | Full articular pattern |
| Compression pain | ||
| Subtle weakness | Serious weakness | |
| C7 root | C5 root |
The diagnosis can be confirmed by radiography. Anteroposterior and oblique views show the encroachment. The uncovertebral osteophytes are best seen on an anterior view and the posterior osteophytes on an oblique one. Computed tomography (CT) is often used to complement radiography because it provides superior imaging of bone and better defines the anatomy and size of the neural foramen.108 When the clinical diagnosis of an osteophytic root palsy has been made and the X-ray confirms its presence, the diagnosis is clear. Nevertheless, it should be borne in mind that the prevalence of asymptomatic osteophytes is very high. Therefore, the clinical examination is decisive.
Manipulation is, of course, contraindicated. Two infiltrations of triamcinolone suspension around the nerve root – nerve root block – at an interval of 2 weeks may be tried. It alleviates the inflammation at the level of the compressed nerve root, so that the pressure diminishes.109,110 The discomfort disappears but the anatomical situation has not changed. When there is gross muscular weakness or weakness has a tendency to increase, surgical treatment is indicated. During the last few decades, a minimally invasive posterior cervical foraminotomy technique has been developed111 that is highly effective and results in long-lasting pain relief.112
Compression phenomenon: the mushroom phenomenon
This phenomenon is extensively described in later chapters on the thoracic and lumbar spine (see Chs 27 and 35) and is summarized in Box 8.9. A cervical mushroom phenomenon is very rare. It occurs in advanced degeneration of the disc, which displaces mainly in the anterior and anterolateral directions. The intervertebral space becomes so narrow that the vertebral bodies lie in apposition. This phenomenon, together with folding of the posterior longitudinal ligament and enlargement of the arthrotic facet joints, can cause considerable narrowing of the spinal canal and the lateral recess, which may result in compression of dura or nerve root during axial loading.
The patient can be helped either by a weight-relieving collar or by surgery (arthrodesis).
Cervical spondylotic myelopathy
Cervical spinal myelopathy (CSM) is defined as spinal cord dysfunction, secondary to intrinsic compression from degenerative disease of the cervical spine. It is the most common cause of spinal cord dysfunction in patients who are aged over 55.113 The presence of a congenitally narrow spinal canal is a critical predisposing factor in patients with spondylotic myelopathy.114 The anteroposterior diameter of the cervical spinal cord is 10 mm on average and normally the sagittal diameter of the spinal canal averages ± 18 mm. It is therefore generally accepted that a spinal canal with a diameter between 10 and 13 mm is at risk and can only sustain a narrowing of 2–4 mm before developing myelopathy.115,116
Pathogenesis
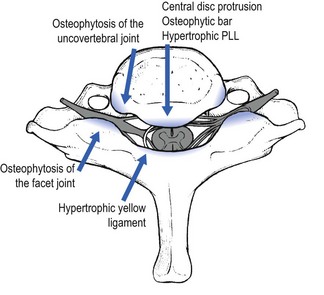
Symptoms of myelopathy occur in a wide variety of combinations. In its most severe form the clinical presentation is a spastic gait. There may be atrophy, sensory disturbance and spasticity in the hands. Sometimes impairment of sphincter function also occurs.117 The variable clinical picture reflects the many complex factors that may affect the spinal cord. The primary pathophysiologic abnormality is a reduced sagittal diameter of the spinal canal. In that narrowed canal, two different mechanisms – a mechanical and a vascular one – can cause pathological changes in the spinal cord. White and Panjabi118 divide the mechanical factors involved in the pathogenesis of CSM into two groups: static and dynamic. Static factors include the well-known arthrotic changes that narrow the canal: a spondylotic bar anteriorly, degenerative osteophytosis of the uncovertebral joints and facet joints laterally, and hypertrophic ligamenta flava posteriorly (Fig. 8.24).119,120 Dynamic factors are abnormal forces placed on the spinal column and spinal cord during flexion and extension. Diminution of the diameter of the spinal canal occurs during extension (because of folding of the posterior longitudinal ligament and of the yellow ligament121) and during flexion (the spinal cord is pulled against the osteophytes).122 Also degenerative cervical spondylolisthesis may cause excessive movement.123,124 During extension, backward gliding of the vertebrae, in combination with folding of the posterior ligaments, may result in cervical cord encroachment (the pincer effect) (see Fig. 8.24).125
Another mechanical theory is that CSM is caused by tensile stresses transmitted to the spinal cord from the dura via the dentate ligaments.126 A spondylotic bar pushes the spinal cord posteriorly, but this displacement is resisted by the dentate ligaments that are anchored by the dural root sleeves in the lateral foramen.127 This theory could explain the neuropathological findings in CSM where damage to the spinal cord is most severe at the lateral columns, and in them the involved areas are often wedge-shaped, with the apex medial and the base lateral.128
Other authors consider spinal cord ischaemia to be a major cause of CSM, either through compression of the anterior spinal artery and its branches in the spinal cord, or through the radicular arteries in the intervertebral foramina. This vascular theory is not widely accepted and most believe that a combination of both mechanical and vascular causes has to be considered (Box 8.10).129,130
Diagnosis
CSM usually develops insidiously, although episodes of abrupt deterioration can occur.
A subtle gait disturbance is usually the first and most common presentation.131 Although CSM can cause a variety of signs and symptoms, spastic gait occurs first, followed by upper extremity numbness and clumsiness. There is loss of fine motor control of the hands and difficulty in writing. Surprisingly, neck pain is not as common a symptom as one might expect, maybe because some pain and stiffness is accepted as normal for the person’s age.
After some years, when the situation gets worse, patients develop a typical ‘spastic gait’ that is broad-based, hesitant and jerky. This gait is one of the main characteristics of cervical myelopathy. Nurick has classified CSM largely on the basis of gait abnormality – typically the most common clinical concern of the patient (Table 8.3).132
Table 8.3
Nurick’s classification of disability in spondylotic myelopathy
| Grade | Symptoms and signs |
| 0 | Root symptoms and signs |
| No evidence of cord involvement | |
| I | Signs of cord involvement |
| Normal gait | |
| II | Mild gait impairment |
| Able to be employed | |
| III | Gait abnormality prevents employment |
| IV | Able to ambulate only with assistance |
| V | Chair-bound or bedridden |
There are also sensory changes in the lower extremities, which the patient typically describes as ‘walking on cotton wool’. Bowel and bladder dysfunction may supervene; according to Epstein et al, 20% of patients with CMS over the age of 65 have bladder dysfunction, mostly associated with urinary retention.133
Examination of the neck is usually not very painful but reveals a gross full articular pattern of limitation with a hard end-feel, suggesting severe arthrosis. Flexion and extension may elicit electric shock sensations in the extremities (Lhermitte’s sign134), which is present in about one-quarter of myelopathic patients.135,136
Other functional tests can be performed and, when positive, are very suggestive of CSM, though none of these tests in itself has pathognomonic value. Ono et al, for instance, observed a characteristic abnormality in the hands of patients with CSM. There was inability to extend the ulnar two or three fingers, despite the relatively well-preserved function of the wrist, thumb and index finger. Rapid extension of the fingers was impossible, even in patients with less advanced disease.137 Also Hoffman’s sign is very often positive. The sign is described as follows.138 The pronated hand is supported so that it is completely relaxed and the fingers partially flexed. The middle finger is firmly grasped and partially extended, and the nail is flicked by the examiner’s thumbnail. The flicking should be done with considerable force. The sign is present if quick flexion of both the thumb and the index finger results. A positive sign is highly indicative of possible pyramidal tract pathology.139 Shimizu et al described the scapulohumeral reflex, which is positive in more than 95% of patients with high cervical cord compression.140 The scapulohumeral reflex is elicited by tapping the tip of the spine of the scapula and acromion in a caudal direction and is positive when elevation of the scapula or abduction of the humerus is seen. The reflex centre of the scapulohumeral reflex is clinically presumed to be located between the posterior arch of C1 and the caudal edge of the body of C3. A hyperactive scapulohumeral reflex therefore provides useful information about dysfunction of the upper motor neurones cranial to the level of the C3 vertebral body. As the disease develops, Babinski’s sign and its variants, Chaddock (stimulation below the external malleolus causes extension of the great toe) and Oppenheim (stroking down the medial side of the tibia causes extension of the great toe), become positive.141
Differential diagnosis
The differential diagnosis of CSM is not easy. It is important to exclude both multiple sclerosis and upper motor neurone disease (amyotrophic lateral sclerosis), as their presentations are very similar to that of CSM.142 A thorough neurological examination is therefore necessary. Most importantly, CSM does not affect the cranial nerves or the normal jaw reflex, whereas the other two disorders may. Other disorders in the differential diagnosis include spinal tumours, cerebrovascular disorders that cause spastic hemiplegia and peripheral neuropathy.
Amyotrophic lateral sclerosis (ALS) is a disorder of the anterior horn cells and of the pyramidal tract (first motor neurone).143 The disease starts between the ages of 40 and 70 in one (commonly upper) limb and advances progressively to the contralateral upper and later the lower limbs. Depending on the localization in the upper or lower motor neurone, spastic or weak paralysis will predominate. The classic triad is atrophic weakness of the hand and forearm muscles, minor spasticity of the legs and generalized hyperreflexia, but without sensory changes; as a pure motor neurone disease ALS does not affect sensation. A diagnosis of ALS is further favoured if there are muscular fasciculations in the face, tongue or lower extremities. The diagnosis is supported by typical findings on electromyography (EMG).144
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system with the morphological hallmarks of inflammation, demyelination, axonal loss and gliosis. The spinal form of MS in particular may mimic the clinical course of CSM: there is a generalized spastic paralysis and clonus, and the patient also experiences paraesthesia. However, the patient is younger – the average age of onset is 30 years. Diagnosis relies on EMG findings, typical appearances on MRI145 and cerebrospinal fluid analysis.
Radiographic studies
In the past, the radiological diagnosis of CSM was dependent on radiography, myelography, CT and CT myelography. More recently, MRI has proven to be the most valuable tool, not only to depict anatomically how the spinal cord is compressed, but also to demonstrate the pathologic changes in the spinal cord.146 Takahashi et al described the MRI findings of intramedullary high signal intensity (SI) on T2-weighted MR images in CSM. They include oedema, ischaemia, demyelination, gliosis and cavities.147
Natural history
The natural history seems to be one of static neurological deficit or episodic progression. The course is quite variable. Often, symptoms are mild and do not progress. Such cases are best treated conservatively with a cervical collar and physical therapy. However, once clinical CSM is evident, progression may occur despite the best of treatments. Elderly patients often experience faster progression of symptoms and more serious neurological impairment.148 It was found that those who deteriorated were more likely to be female and to have significantly more cervical mobility when compared to those patients whose disability remained static. It is proposed that measurement of cervical mobility may help to select patients who are more likely to deteriorate and thus more likely to benefit from surgical intervention.149
Treatment
Minimal symptoms without hard evidence of gait disturbance or pathologic reflexes warrant non-operative treatment, but surgery should be recommended to arrest progression of myelopathic symptoms in patients whose general condition is satisfactory.150 Both anterior and posterior approaches have been utilized for surgical treatment of cervical myelopathy. Anterior decompression frequently requires corpectomy at one or more levels and strut grafting with bone from the ilium or fibula. Multilevel laminectomies were initially used for posterior decompression but now are either combined with fusion or replaced by laminoplasty.151
Patients who are treated by surgery seem to have fair outcomes. The progression of myelopathy is arrested in about 95% and the improvement rate of the gait is estimated to be around 51–85%.152,153
Some recommend preventive decompression surgery in individuals who have spondylotic encroachment on the cervical spinal cord without clinical signs and symptoms of myelopathy. The reasoning is that these patients are at increased risk of spinal cord injury if they experience minor trauma. A recent meta-analysis of the literature, however, concluded that there is insufficient evidence for an increased risk and that recommendations for preventive decompression surgery cannot be made.154
Disorders causing pain on resisted movements of the neck
Musculotendinous lesions
Lesion of the longus colli muscle (retropharyngeal tendinitis)
Retropharyngeal calcific tendonitis, also known as acute calcific prevertebral tendinitis, is a clinical syndrome that was described originally by Hartley in 1964,155 and was later demonstrated to be secondary to calcium hydroxyapatite deposition in the longus colli muscle.156 This muscle is a paired neck flexor that comprises the prevertebral space (Fig. 8.25). Classically, calcification affects the superior oblique portion of the longus colli muscle at the C1–C2 level (Fig. 8.26). The condition affects adults within a reported age range of 21–81 years, with its greatest distribution between 30 and 60 years. The incidence of this condition is rare, although its true incidence is probably higher than previously thought.157
CT shows the pathognomonic tendinous calcifications within the longus colli and can also demonstrate sterile fluid smoothly expanding the retropharyngeal space.158 In the presence of a fluid collection, the possibility of infection must be considered, specifically abscess from lymphadenitis.159
Disorders causing symptoms on active and/or resisted shrugging of the shoulders
Examination of the shoulder girdle is part of the scan examination of the cervical spine. Some conditions in the scapular area and shoulder girdle may provoke positive signs during this examination. These lesions are extensively discussed in the online section The shoulder girdle. They are:
References
1. Matsumoto, M, Fujimara, Y, Suzuki, N, et al, MRI of cervical intervertebral discs in asymptomatic subjects. J Bone Joint Surg 1998; 80B:19–24. ![]()
2. Gore, DR, Roentgenographic findings in the cervical spine in asymptomatic persons a ten-year follow-up. Spine (Phila Pa 1976) 2001; 26:2463–2466. ![]()
3. Pallis, C, Jones, A, Spiliane, J, Cervical spondylosis. Incidence and implications. Brain 1954; 77:274. ![]()
4. Okada, E, Matsumoto, M, Ichihara, D, Chiba, K, Aging of the cervical spine in healthy volunteers: a 10-year longitudinal magnetic resonance imaging study. Spine (Phila Pa 1976). 2009;34(7):706–712. ![]()
5. Friedenberg, ZB, Miller, WT, Degenerative disc disease of the cervical spine – a comparative tudy of symptomatic and asymptomatic patients. J Bone Joint Surg (Am) 1963; 45:1171–1178. ![]()
6. Parke, WW, Sherk, HH. Normal adult anatomy. In: Sherk HH, ed. The Cervical Spine. 2nd ed. Philadelphia: Lippincott; 1989:14–15.
7. Fletcher, G, Haughton, VM, Ho, KC, Yu, SW, Age-related changes in the cervical facet joints: studies with cryomicrotomy, MR, and CT. AJR Am J Roentgenol. 1990;154(4):817–820. ![]()
8. Bogduk, N, Marsland, A, The cervical zygapophysial joints as a source of neck pain. Spine 1988; 13:610–617. ![]()
9. Kettler, A, Werner, K, Wilke, HJ, Morphological changes of cervical facet joints in elderly individuals. Eur Spine J. 2007;16(7):987–992. ![]()
10. Morishita, Y, Naito, M, Hymanson, H, et al, The relationship between the cervical spinal canal diameter and the pathological changes in the cervical spine. Eur Spine J. 2009;18(6):877–883. ![]()
11. Levine, DN, Pathogenesis of cervical spondylotic myelopathy. J Neurol Neurosurg Psychiatry. 1997;62(4):334–340. ![]()
12. Kitagawa, T, Fujiwara, A, Kobayashi, N, et al, Morphologic changes in the cervical neural foramen due to flexion and extension: in vivo imaging study. Spine (Phila Pa 1976). 2004;29(24):2821–2825. ![]()
13. Lu, J, Ebraheim, NA, Huntoon, M, Haman, SP, Cervical intervertebral disc space narrowing and size of intervertebral foramina. Clin Orthop Relat Res 2000; 370:259–264. ![]()
14. Ebraheim, NA, An, HS, Xu, R, et al, The quantitative anatomy of the cervical nerve root groove and the intervertebral foramen. Spine (Phila Pa 1976). 1996;21(14):1619–1623. ![]()
15. Gore, DR. Radiological evaluation of the degenerative cervical spine. In: Clark CR, ed. The Cervical Spine. 3rd ed. Philadelphia: Lippincott-Raven; 1998:765–778.
16. Lehto, IJ, Tertti, MO, Komu, ME, Paajanen, HE, et al, Age-related MRI changes at 0.1 T in cervical discs in asymptomatic subjects. Neuroradiology. 1994;36(1):49–53. ![]()
17. Boden, SD, McCowin, PR, Davis, DO, et al, Abnormal magnetic resonance scans of the cervical spine in asymptomatic subjects. J Bone Joint Surg 1990; 72A:1178–1184. ![]()
18. Cyriax, JH. Textbook of Orthopaedic Medicine, vol I. Diagnosis of Soft Tissue Lesions, 8th ed. London: Baillière Tindall; 1982.
19. Connell, MD, Wiegel, SW, Natural history and pathogenesis of cervical disc disease. Orthop Clin North Am. 1992;23(3):369–380. ![]()
20. Cyriax, JH. Textbook of Orthopaedic Medicine, vol I. Diagnosis of Soft Tissue Lesions, 8th ed. London: Baillière Tindall; 1982.
21. Alleyne, CH, Jr., Cawley, CM, Barrow, DL, Bonner, GD, Microsurgical anatomy of the dorsal cervical nerve roots and the cervical dorsal root ganglion/ventral root complexes. Surg Neurol. 1998;50(3):213–218. ![]()
22. Sunderland, S, Meningeal-neural relations in the intervertebral foramen. J Neurosurg 1974; 40:756–763. ![]()
23. Elvey, RL. The investigation of arm pain. In Boyling JD, Palastanga N, eds.: Grieve’s Modern Manual Therapy: The Vertebral Column, 2nd ed, Edinburgh: Churchill Livingstone, 1994.
24. Butler, DS. Mobilisation of the Nervous System. Melbourne: Churchill Livingstone; 1991.
25. Wainner, RS, Fritz, JM, Irrgang, J, et al, Reliability and diagnostic accuracy of the clinical examination and patient self-report measures for cervical radiculopathy. Spine. 2003;28(1):52–62. ![]()
26. Rubinstein, SM, Pool, JJ, van Tulder, MW, et al, A systematic review of the diagnostic accuracy of provocative tests of the neck for diagnosing cervical radiculopathy. Eur Spine J. 2007;16(3):307–319. ![]()
27. Davis, DS, Anderson, IB, Carson, MG, et al, Upper limb neural tension and seated slump tests: the false positive rate among healthy young adults without cervical or lumbar symptoms. J Man Manip Ther. 2008;16(3):136–141. ![]()
28. Spurling, RG, Scoville, WB. Lateral rupture of the cervical intervertebral disc. Surg Gynec Obstet. 1944; 78:350–358.
29. Mendel, T, Wink, CS, Zimny, ML, Neural elements in human cervical intervertebral discs. Spine 1992; 17:132–135. ![]()
30. McNair, JFS. Acute locking of the cervical spine. In: Grieve GP, ed. Modern Manual Therapy of the Vertebral Column. London: Churchill Livingstone; 1986:351.
31. Maigne, JY, Mutschler, C, Doursounian, L, Acute torticollis in an adolescent: case report and MRI study. Spine (Phila Pa 1976). 2003;28(1):E13–E15. ![]()
32. Gubin, AV, Ulrich, EV, Taschilkin, AI, Yalfimov, AN, Etiology of child acute stiff neck. Spine (Phila Pa 1976). 2009;34(18):1906–1909. ![]()
33. Jones, ET. Congenital anomalies of the atlas. In: Clark CR, ed. The Cervical Spine. 3rd ed. Philadelphia: Lippincott-Raven; 1998:325–329.
34. Saltzman, CL, Hensinger, RN, Blane, CE, Philips, WA, Familial cervical dysplasia. J Bone Joint Surg 1991; 73A:163–171. ![]()
35. Hensinger, RN, Osseous anomalies of the craniovertebral junction. Spine 1986; 11:323–333. ![]()
36. Van Kerckhoven, MF, Fabry, G, The Klippel–Feil syndrome: a constellation of deformities. Acta Orthop Belg 1989; 55:107–118. ![]()
37. Guille, JT, Sherk, HH, Congenital osseous anomalies of the upper and lower cervical spine in children. J Bone Joint Surg (Am) 2002; 84:277–288. ![]()
38. Bredenkamp, JK, Hoover, LA, Berke, GS, Shaw, A, Congenital muscular torticollis. A spectrum of disease. Arch Otolaryngol Head Neck Surg 1990; 116:212–216. ![]()
39. Wei, JL, Schwartz, KM, Weaver, AL, Orvidas, LJ, Pseudotumor of infancy and congenital muscular torticollis: 170 cases. Laryngoscope 2001; 111:688–695. ![]()
40. Robin, NH, Congenital muscular torticollis. Pediatr Rev 1996; 17:374–375. ![]()
41. Hollier, L, Kim, J, Grayson, BH, McCarty, JG, Congenital muscular torticollis and the associated craniofacial changes. Plast Reconstr Surg 2000; 105:827–835. ![]()
42. Do, TT, Congenital muscular torticollis: current concepts and review of treatment. Curr Opin Pediatr. 2006;18(1):26–29. ![]()
43. Emery, C, The determinants of treatment duration for congenital muscular torticollis. Phys Ther. 1994;74(10):921–929. ![]()
44. Omidi-Kashani, F, Hasankhani, EG, Sharifi, R, Mazlumi, M, Is surgery recommended in adults with neglected congenital muscular torticollis? A prospective study. BMC Musculoskelet Disord 2008; 9:158. ![]()
45. Akpinar, G, Tekkok, IH, Sumer, M, Grisel’s syndrome: a case of potentially lethal spinal cord injury in the adult. Br J Neurosurg 2002; 16:592–596. ![]()
46. Fernandez Cornejo, VJ, Martinez-Lage, JF, Piqueras, C, et al, Inflammatory atlanto-axial subluxation (Grisel’s syndrome) in children: clinical diagnosis and management. Childs Nerv Syst 2003; 19:342–347. ![]()
47. Kraft, M, Tschopp, K, Evaluation of persistent torcicollis following adenoidectomy. J Laryngol Otol 2001; 115:669–672. ![]()
48. Wetzel, FD, La Rocca, H, Grisel’s syndrome: a review. Clin Orthopaed Related Res 1989; 240:141–152. ![]()
49. Grobman, LR, Stricker, S, Grisel’s syndrome. Ear Nose Throat J 1990; 69:799–801. ![]()
50. Grisel, P. Enucléation de l’atlas et torcicolis nasopharyngien. Presse Médicale. 1930; 38:50–53.
51. Bratt, HD, Menelaus, MB, Benign paroxysmal torticollis of infancy. J Bone Joint Surg. 1992;74B(3):449–457. ![]()
52. Nutt, JG, Muenter, MD, Melton, LJ, III., et al, Epidemiology of dystonia in Rochester, Minnesota. Adv Neurol 1988; 50:361–365. ![]()
53. Crowner, BE, Cervical dystonia: disease profile and clinical management. Phys Ther. 2007;87(11):1511–1526. ![]()
54. Smith, DL, DeMario, MC, Spasmodic torticollis: a case report and review of therapies. J Am Board Fam Pract. 1996;9(6):435–441. ![]()
55. Costa, J, Espirito-Santo, C, Borges, A, et al, Botulinum toxin type B for cervical dystonia. Cochrane Database Syst Rev. 2005;(1). ![]()
56. Haussermann, P, Marczoch, S, Klinger, C, et al, Long-term follow-up of cervical dystonia patients treated with botulinum toxin A. Mov Disord 2004; 19:303–308. ![]()
57. Cohen-Gadol, AA, Ahlskog, JE, Matsumoto, JY, et al, Selective peripheral denervation for the treatment of intractable spasmodic torticollis: experience with 168 patients at the Mayo Clinic. J Neurosurg 2003; 98:1247–1254. ![]()
58. Thomsen, MN, Schneider, U, Weber, M, et al, Scoliosis and congenital anomalies associated with Klippel–Feil syndrome types I–III. Spine 1997; 22:396–401. ![]()
59. Radhakrishnan, K, Litchy, WJ, O’Fallon, WM, Kurland, LT, Epidemiology of cervical radiculopathy. A population-based study from Rochester, Minnesota, 1976 through 1990. Brain. 1994;117(Pt 2):325–335. ![]()
60. Fast, A, Parikh, S, Marin, EL, The shoulder abduction relief sign in cervical radiculopathy. Arch Phys Med Rehabil. 1989;70(5):402–403. ![]()
61. Farmer, JC, Wisneski, RJ, Cervical spine nerve root compression. An analysis of neuroforaminal pressures with varying head and arm positions. Spine (Phila Pa 1976). 1994;19(16):1850–1855. ![]()
62. Beatty, RM, Fowler, FD, Hanson, EJ, Jr., The abducted arm as a sign of ruptured cervical disc. Neurosurgery. 1987;21(5):731–732. ![]()
63. Abdulwahab, SS, Sabbahi, M, Neck retractions, cervical root decompression, and radicular pain. J Orthop Sports Phys Ther. 2000;30(1):4–9. ![]()
64. Tanaka, N, Fujimoto, Y, An, HS, et al, The anatomic relation among the nerve roots, intervertebral foramina, and intervertebral discs of the cervical spine. Spine (Phila Pa 1976). 2000;25(3):286–291. ![]()
65. Viikari-Juntura, E, Porras, M, Laasonen, EM, Validity of clinical tests in the diagnosis of root compression in cervical disc disease. Spine. 1989;14(3):253–257. ![]()
66. Levin, KH, Maggiano, HJ, Wilbourn, AJ, Cervical radiculopathies: comparison of surgical and EMG localization of single-root lesions. Neurology. 1996;46(4):1022–1025. ![]()
67. Moriishi, J, Otani, K, Tanaka, K, et al, The intersegmental anastomoses between spinal nerve roots. Anat Rec 1989; 224:110–116. ![]()
68. Anderberg, L, Annertz, M, Rydholm, U, et al, Selective diagnostic nerve root block for the evaluation of radicular pain in the multilevel degenerated cervical spine. Eur Spine J. 2006;15(6):794–801. ![]()
69. Murphy, DR, Hurwitz, EL, Gerrard, JK, Clary, R, Pain patterns and descriptions in patients with radicular pain: does the pain necessarily follow a specific dermatome? Chiropr Osteopat 2009; 17:9. ![]()
70. Abbed, KM, Coumans, JV, Cervical radiculopathy: pathophysiology, presentation, and clinical evaluation. Neurosurgery. 2007;60(1 Suppl 1):S28–S34. ![]()
71. Persson, LC, Carlsson, JY, Anderberg, L, Headache in patients with cervical radiculopathy: a prospective study with selective nerve root blocks in 275 patients. Eur Spine J. 2007;16(7):953–959. ![]()
72. Michler, RP, Bovim, G, Sjaastad, O, Disorders in the lower cervical spine. A cause of unilateral headache? Headache 1991; 31:550–551. ![]()
73. Pikus, HJ, Phillips, JM, Outcome of surgical decompression of the second cervical root for cervicogenic headache. Neurosurgery. 1996;39(1):63–70. ![]()
74. Lord, SM, Barnsley, L, Wallis, BJ, Bogduk, N, Chronic cervical zygapophyseal joint pain after whiplash. A placebo-controlled prevalence study. Spine 1996; 21:1737–1744. ![]()
75. Nurmikko, TJ, Eldridge, PR, Trigeminal neuralgia: pathophysiology, diagnosis and current treatment. Br J Anaesth. 2001;87(1):117–132. ![]()
76. Benini, A. Clinical features of cervical root compression C5–C8 and their variations. Neuro Orthop. 1987; 4:74–88.
77. Schimsheimer, RJ, de Visser, BW, Kemp, B, The flexor carpi radialis H-reflex in lesions of the sixth and seventh cervical nerve roots. J Neurol Neurosurg Psychiatry. 1985;48(5):445–449. ![]()
78. Makin, GJ, Brown, WF, Ebers, GC, C7 radiculopathy: importance of scapular winging in clinical diagnosis. J Neurol Neurosurg Psychiatry. 1986;49(6):640–644. ![]()
79. Wallace, D, Disc compression of the eighth cervical nerve: pseudo ulnar palsy. Surg Neurol. 1982;18(4):295–299. ![]()
80. Evans, RC. Illustrated Essentials in Orthopedic Physical Assessment. St Louis: Mosby Year Books; 1994.
81. Arcosoy, SM, Jett, JR, Superior sulcus tumors and Pancoast’s syndrome. N Engl J Med 1997; 337:1370–1376. ![]()
82. Ross, MA, Miller, RG, Berchert, L, et al, Toward earlier diagnosis of amyotrophic lateral sclerosis. Revised criteria. Neurology 1998; 50:768–772. ![]()
83. Saal, JS, Saal, JA, Yurth, EF, Nonoperative management of herniated cervical intervertebral disc with radiculopathy. Spine 1996; 21:1877–1883. ![]()
84. Mochida, K, Komori, H, Okawa, A, et al, Regression of cervical disc herniation observed on magnetic resonance images. Spine (Phila Pa 1976). 1998;23(9):990–995. ![]()
85. Bush, K, Hillier, S, Outcome of cervical radiculopathy treated with periradicular/epidural corticosteroid injections: a prospective study with independent clinical review. Eur Spine J 1996; 5:319–325. ![]()
86. Heckmann, JG, Lang, CJ, Zöbelein, I, et al, Herniated cervical intervertebral discs with radiculopathy: an outcome study of conservatively or surgically treated patients. J Spinal Disord. 1999;12(5):396–401. ![]()
87. Clarke, E, Robinson, PK, Cervical myelopathy. A complication of cervical spondylosis. Brain 1956; 92:1607–1610. ![]()
88. LaRocca, H, Cervical spondylotic myelopathy: natural history. Spine 1988; 13:854–855. ![]()
89. Abdulkarim, JA, Dhingsa, R, L Finlay, DB, Magnetic resonance imaging of the cervical spine: frequency of degenerative changes in the intervertebral disc with relation to age. Clin Radiol. 2003;58(12):980–984. ![]()
90. Matsumoto, M, Fujimura, Y, Suzuki, N, et al, MRI of cervical intervertebral discs in asymptomatic subjects. J Bone Joint Surg Br. 1998;80(1):19–24. ![]()
91. Boden, SD, McCowin, PR, Davis, DO, et al, Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72(8):1178–1184. ![]()
92. Gore, DR, Sepic, SB, Gardner, GM, Roentgenographic findings of the cervical spine in asymptomatic people. Spine (Phila Pa 1976). 1986;11(6):521–524. ![]()
93. Okada, E, Matsumoto, M, Ichihara, D, Chiba, K, et al, Aging of the cervical spine in healthy volunteers: a 10-year longitudinal magnetic resonance imaging study. Spine (Phila Pa 1976). 2009;34(7):706–712. ![]()
94. Friendeberg, ZB, Broder, HA, Edeiken, JE, Spencer, HN, Degenerative disk disease of cervical spine. Clinical and roentgenographic study. JAMA 1960; 174:375–380. ![]()
95. Boden, SD, Wiesel, SW, Laws, ER, et al. The Ageing Spine, Essentials of Pathophysiology, Diagnosis and Treatment. Philadelphia: Saunders; 1991.
96. Dreyfuss, P, Michaelsen, M, Fletcher, D, Atlanto-occipital and lateral atlanto-axial joint pain patterns. Spine (Phila Pa 1976). 1994;19(10):1125–1131. ![]()
97. Paluzzi, A, Belli, A, Lafuente, J, Wasserberg, J, Role of the C2 articular branches in occipital headache: an anatomical study. Clin Anat. 2006;19(6):497–502. ![]()
98. Aprill, C, Axinn, MJ, Bogduk, N, Occipital headaches stemming from the lateral atlanto-axial (C1–2) joint. Cephalalgia. 2002;22(1):15–22. ![]()
99. Ogoke, BA, The management of the atlanto-occipital and atlanto-axial joint pain. Pain Physician. 2000;3(3):289–293. ![]()
100. Kettler, A, Werner, K, Wilke, HJ, Morphological changes of cervical facet joints in elderly individuals. Eur Spine J. 2007;16(7):987–992. ![]()
101. Manchikanti, L, Singh, V, Rivera, J, Pampati, V, Prevalence of cervical facet joint pain in chronic neck pain. Pain Physician 2002; 5:243–249. ![]()
102. Sehgal, N, Dunbar, E, Shah, R, Colson, J, Systematic review of diagnostic utility of facet (zygapophyseal) joint injections in chronic spinal pain: an update. Pain Physician 2007; 10:213–228. ![]()
103. Uhrenholt, L, Hauge, E, Charles, AV, Gregersen, M, Degenerative and traumatic changes in the lower cervical spine facet joints. Scand J Rheumatol. 2008;37(5):375–384. ![]()
104. Kumaresan, S, Yoganandan, N, Pintar, FA, et al, Contribution of disc degeneration to osteophyte formation in the cervical spine: a biomechanical investigation. J Orthop Res. 2001;19(5):977–984. ![]()
105. Lu, J, Ebraheim, NA, Huntoon, M, Haman, SP, Cervical intervertebral disc space narrowing and size of intervertebral foramina. Clin Orthop Relat Res 2000; 370:259–264. ![]()
106. Ebraheim, NA, Lu, J, Biyani, A, et al, Anatomic considerations for uncovertebral involvement in cervical spondylosis. Clin Orthop Relat Res 1997; 334:200–206. ![]()
107. Chang, H, Park, JB, Hwang, JY, Song, KJ, Clinical analysis of cervical radiculopathy causing deltoid paralysis. Eur Spine J. 2003;12(5):517–521. ![]()
108. Nasca, RJ, Cervical radiculopathy: current diagnostic and treatment options. J Surg Orthop Adv. 2009;18(1):13–18. ![]()
109. Bush, K, Hillier, S, Outcome of cervical radiculopathy treated with periradicular/epidural corticosteroid injections: a prospective study with independent clinical review. Eur Spine J 1996; 5:319–332. ![]()
110. Slipman, CW, Lipetz, JS, Jackson, HB, et al, Therapeutic selective nerve root block in the nonsurgical treatment of atraumatic cervical spondylotic radicular pain: a retrospective analysis with independent clinical review. Arch Phys Med Rehabil 2000; 81:741–746. ![]()
111. Jho, HD, Microsurgical anterior cervical foraminotomy. A new approach to cervical disc herniation. J Neurosurg 1996; 84:155–160. ![]()
112. Jagannathan, J, Sherman, JH, Szabo, T, et al, The posterior cervical foraminotomy in the treatment of cervical disc/osteophyte disease: a single-surgeon experience with a minimum of 5 years’ clinical and radiographic follow-up. J Neurosurg Spine. 2009;10(4):347–356. ![]()
113. Bernhardt, M, Hynes, R, Blume, HA, White, AA, III., Current Concepts Review: Cervical spondylotic myelopathy. J Bone and Joint Surg 1993; 75A:119–128. ![]()
114. Inoue, H, Ohmori, K, Takatsu, T, et al, Morphological analysis of the cervical spinal canal, dural tube and spinal cord in normal individuals using CT myelography. Neuroradiology. 1996;38(2):148–151. ![]()
115. Yu, YL, du Boulay, GH, Stevens, JM, Kendall, BE, Morphology and measurements of the cervical spinal cord in computer-assisted myelography. Neuroradiology. 1985;27(5):399–402. ![]()
116. Yu, YL, du Boulay, GH, Stevens, JM, Kendall, BE, Computed tomography in cervical spondylotic myelopathy and radiculopathy: visualisation of structures, myelographic comparison, cord measurements and clinical utility. Neuroradiology. 1986;28(3):221–236. ![]()
117. Brain, WR, Northfield, D, Wilkinson, M, The neurological manifestations of cervical spondylosis. Brain. 1952;75(2):187–225. ![]()
118. White, AA, 3rd., Panjabi, MM, Biomechanical considerations in the surgical management of cervical spondylotic myelopathy. Spine (Phila Pa 1976). 1988;13(7):856–860. ![]()
119. Shedid, D, Benzel, EC, Cervical spondylosis anatomy: pathophysiology and biomechanics. Neurosurgery. 2007;60(1 Supp1 1):S7–13. ![]()
120. Meire, D, De Bleecker, J, Vallaeys, J, Van de Velde, E, Ossification of the cervical posterior longitudinal ligament with severe myelopathy and fatal outcome. J Belge Radiol. 1990;73(4):249–252. ![]()
121. Maiuri, F, Gangemi, M, Gambardella, A, et al, Hypertrophy of the ligamenta flava of the cervical spine. Clinico-radiological correlations. J Neurosurg Sci. 1985;29(2):89–92. ![]()
122. Baptiste, DC, Fehlings, MG, Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 Suppl):190S–197S. ![]()
123. Boulos, AS, Lovely, TJ, Degenerative cervical spondylolisthesis: diagnosis and management in five cases. J Spinal Disord. 1996;9(3):241–245. ![]()
124. Woiciechowsky, C, Thomale, UW, Kroppenstedt, SN, Degenerative spondylolisthesis of the cervical spine – symptoms and surgical strategies depending on disease progress. Eur Spine J. 2004;13(8):680–684. ![]()
125. Muhle, C, Metzner, J, Weinert, D, et al, Kinematic MR imaging in surgical management of cervical disc disease, spondylosis and spondylotic myelopathy. Acta Radiol. 1999;40(2):146–153. ![]()
126. Kahn, EA, The role of the dentate ligaments in spinal cord compression and the syndrome of lateral sclerosis. J Neurosurg 1947; 4:191–199. ![]()
127. Levine, DN, Pathogenesis of cervical spondylotic myelopathy. J Neurol Neurosurg Psychiatry. 1997;62(4):334–340. ![]()
128. Mair, WG, Druckman, R, The pathology of spinal cord lesions and their relation to the clinical features in protrusion of cervical intervertebral discs. Brain. 1953;76(1):70–91. ![]()
129. Ferguson, RJ, Caplan, LR, Cervical spondylitic myelopathy. Neurol Clin. 1985;3(2):373–382. ![]()
130. Hohmann, D, Kügelgen, B, Liebig, K, Chronic spondylogenic cervical myelopathy. Pathogenesis, prognosis, therapy. Orthopade. 1985;14(2):101–111. ![]()
131. Gorter, K, Influence of laminectomy on the course of cervical myelopathy. Acta Neurochir (Wien). 1976;33(3–4):265–281. ![]()
132. Nurick, S, The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95(1):87–100. ![]()
133. Epstein, N, Epstein, J, Carras, R. Cervical spondylostenosis and related disorders in patients over 65: current management and diagnostic techniques. Orthotransactions. 1987; 11–15.
134. Gutrecht, JA, Lhermitte’s sign. From observation to eponym. Arch Neurol. 1989;46(5):557–558. ![]()
135. Montgomery, DM, Brower, RS, Cervical spondylotic myelopathy. Clinical syndrome and natural history. Orthop Clin North Am. 1992;23(3):487–493. ![]()
136. Baron, EM, Young, WF, Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery. 2007;60(1 Supp1 1):S35–S41. ![]()
137. Ono, K, Ebara, S, Fuji, T, et al, Myelopathy hand. New clinical signs of cervical cord damage. J Bone Joint Surg Br. 1987;69(2):215–219. ![]()
138. Bendheim, OL. On the history of Hoffmann’s sign. Bull Inst Hist Med. 1937; 5:684–685.
139. Glaser, JA, Curie, JK, Bauley, KL, et al, Cervical spinal cord compression and the Hoffman sign. Iowa Orthop J 2001; 21:49–52. ![]()
140. Shimizu, T, Shimada, H, Shirakura, K, Scapulohumeral reflex (Shimizu). Its clinical significance and testing maneuver. Spine (Phila Pa 1976). 1993;18(15):2182–2190. ![]()
141. Singerman, J, Lee, L, Consistency of the Babinski reflex and its variants. Eur J Neurol. 2008;15(9):960–964. ![]()
142. Srinivasan, J, Scala, S, Jones, HR, et al, Inappropriate surgeries resulting from misdiagnosis of early amyotrophic lateral sclerosis. Muscle Nerve 2006; 34:359–360. ![]()
143. Oliveira, AS, Pereira, RD, Amyotrophic lateral sclerosis (ALS): three letters that change the people’s life. For ever. Arq Neuropsiquiatr. 2009;67(3A):750–782. ![]()
144. de Carvalho, M, Dengler, R, Eisen, A, et al, Electrodiagnostic criteria for diagnosis of ALS: consensus of an International Symposium sponsored by IFCN. Clin Neurophysiol 2008; 119:497–503. ![]()
145. Miller, DH, Grossman, RI, Reingold, SC, McFarland, HF, The role of magnetic resonance techniques in understanding and managing multiple sclerosis. Brain. 1998;121(Pt 1):3–24. ![]()
146. Mehalic, TF, Pezzuti, RT, Applebaum, BI, Magnetic resonance imaging and cervical spondylotic myelopathy. Neurosurgery. 1990;26(2):217–226. ![]()
147. Takahashi, M, Sakamoto, Y, Miyawaki, M, Bussaka, H, Increased MR signal intensity secondary to chronic cervical cord compression. Neuroradiology 1987; 29:550–556. ![]()
148. Swagerty, DL, Jr., Cervical spondylotic myelopathy: a cause of gait disturbance and falls in the elderly. Kans Med. 1994;95(10):226–227. ![]()
149. Barnes, MP, Saunders, M, The effect of cervical mobility on the natural history of cervical spondylotic myelopathy. J Neurol Neurosurg Psychiatry. 1984;47(1):17–20. ![]()
150. Matz, PG, Does nonoperative management play a role in the treatment of cervical spondylotic myelopathy? Spine J. 2006;6(6 Suppl):175S–181S. ![]()
151. Emery, SE, Cervical spondylotic myelopathy: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9(6):376–388. ![]()
152. Naderi, S, Ozgen, S, Pamir, MN, et al, Cervical spondylotic myelopathy: surgical results and factors affecting prognosis. Neurosurgery. 1998;43(1):43–49. ![]()
153. Herkowitz, HN, The surgical management of cervical spondylotic radiculopathy and myelopathy. Clin Orthop Relat Res 1989; 239:94–108. ![]()
154. Murphy, DR, Coulis, CM, Gerrard, JK, Cervical spondylosis with spinal cord encroachment: should preventive surgery be recommended? Chiropr Osteopat 2009; 17:8. ![]()
155. Hartley, J, Acute cervical pain associated with retropharyngeal calcium deposit: a case report. J Bone Joint Surg 1964; 46-A:1753–1754. ![]()
156. Ring, D, Vacarro, AR, Scuderi, G, et al, Acute calcific retropharyngeal tendinitis. J Bone Joint Surg 1994; 76-A:1636–1642. ![]()
157. Hviid, C, Salomonsen, M, Gelineck, J, et al, Retropharyngeal tendinitis may be more common than we think: a report on 45 cases seen in Danish chiropractic clinics. J Manipulative Physiol Ther. 2009;32(4):315–320. ![]()
158. Omezzine, SJ, Hafsa, C, Lahmar, I, Calcific tendinitis of the longus colli: diagnosis by CT. Joint Bone Spine 2008; 75:90–91. ![]()
159. Eastwood, JD, Hudgins, PA, Malone, D, Retropharyngeal effusion in acute calcific prevertebral tendinitis: diagnosis with CT and MR imaging. AJNR Am J Neuroradiol. 1998;19(9):1789–1792. ![]()