Chapter 23 Massive Pulmonary Embolism
Pulmonary embolism usually occurs secondary to venous thrombosis in the deep veins of the lower limbs, proximal to but including the popliteal veins.1 Risk factors for venous thrombosis are listed in Table 23-1.
Table 23-1 Major Risk Factors for the Development of Venous Thromboembolism
| Risk Factor | Examples |
|---|---|
| Venous stasis | Long-distance travel |
| Postoperative recuperation | |
| Plaster immobilization of the lower limb | |
| Pregnancy | |
| Stroke or spinal cord injury | |
| Injury to the vein | Postsurgical (e.g., knee joint surgery) |
| Posttrauma (e.g., fracture of the lower limb) | |
| Prothrombotic states | Antiphospholipid syndrome |
| Factor V Leiden mutation | |
| Other | Malignancy |
| Increasing age |
Massive pulmonary embolism can be defined anatomically as a greater than 50% thrombotic obstruction of the pulmonary vasculature or the occlusion of two or more lobar arteries.2 However, the clinical impact of this obstruction depends on the size of the embolus and on the patient’s underlying cardiopulmonary function. Therefore, it is preferable to define massive pulmonary embolism as that which causes hemodynamic compromise which, by one definition, is a systolic blood pressure of less than 90 mmHg or a drop of 40 mmHg for at least 15 minutes.3 Massive pulmonary embolism constitutes about 4% of all pulmonary embolisms4 and has a mortality rate of 15%.5 Mortality rates increase to 25% in patients with cardiogenic shock and to 65% in patients who require cardiopulmonary resuscitation.5 Submassive pulmonary embolism may be defined as pulmonary embolism in which there is echocardiographic evidence of right ventricular dysfunction in the absence of hypotension.
PATHOPHYSIOLOGY
Because of the high compliance of the pulmonary vascular bed (see Chapter 1), more than 50% of the cross-sectional area must be occluded for pulmonary arterial pressure to rise. The right ventricle is a thin-walled structure that is better suited to volume work than pressure work, and an acute increase in pulmonary vascular resistance sufficient to require a mean pulmonary artery pressure of more than 40 mmHg is likely to cause right ventricular failure and hemodynamic collapse (see Chapter 24). (Patients with chronically elevated pulmonary vascular resistance who have developed right ventricular hypertrophy are able to generate much higher pulmonary arterial pressures.)
Massive pulmonary embolism causes regions of the lungs to be well ventilated but poorly perfused—that is, to have a high ventilation/perfusion (V/Q) ratio. This constitutes dead-space ventilation. From first principles (see Chapter 1), this would be expected to lead to an increased arterial carbon dioxide tension (Paco2) but have a minimal effect on arterial oxygen tension (Pao2; see Chapter 1). However, patients with massive pulmonary embolism are typically hypocarbic and hypoxemic. Low Paco2 is explained by the fact that most patients have marked increase in minute ventilation (secondary to pulmonary irritation and hypoxemia), which offsets the effect of increased alveolar dead space. Hypoxemia results mainly from relative intrapulmonary shunting (i.e., regions of low V/Q ratio). Although pulmonary embolism per se does not cause intrapulmonary shunting, lung units with low V/Q ratios develop rapidly due to the effects of surfactant loss, bronchoconstriction, pulmonary hemorrhage, and pulmonary edema. In addition, the effects of low mixed venous oxygen saturation (caused by low cardiac output), reduced diffusing capacity due to obstruction of the pulmonary capillary bed, and right-to-left shunting across a patent foramen ovale (PFO) also contribute to hypoxemia.
Each lung has a dual blood supply from the pulmonary and bronchial arteries. Thus, even complete obstruction of a lobar branch of the pulmonary artery does not usually cause pulmonary infarction.
DIAGNOSIS
Clinical Findings
The symptoms and signs of pulmonary embolism are variable (Table 23-2), and only a minority of patients have all the classic findings of marked respiratory distress (dyspnea, tachypnea), pleuritic chest pain, and clinical evidence of deep venous thrombosis.
Table 23-2 Presentation of Pulmonary Embolism in Patients with Syncope or Shock
| Signs and Symptoms | Syncope (n = 19) (percentage) | Shock (n = 21) (percentage) |
|---|---|---|
| Tachycardia (heart rate >100/min) | 58 | 86 |
| Tachypnea (breath rate >20/min) | 89 | 81 |
| Dyspnea | 89 | 71 |
| Apprehension | 74 | 71 |
| Accentuated P2 heart sound | 79 | 62 |
| Crackles on chest auscultation | 47 | 48 |
| Fever (temperature >37.5° C) | 21 | 43 |
| Pleuritic chest pain | 63 | 38 |
| Cough | 42 | 33 |
| Coexistent DVT | 42 | 19 |
| Hemoptysis | 5 | 10 |
Adapted from Stein PD, Henry JW: Clinical characteristics of patients with acute pulmonary embolism stratified according to their presenting symptoms. Chest 112:974-979, 1997. DVT, deep venous thrombosis
Electrocardiography
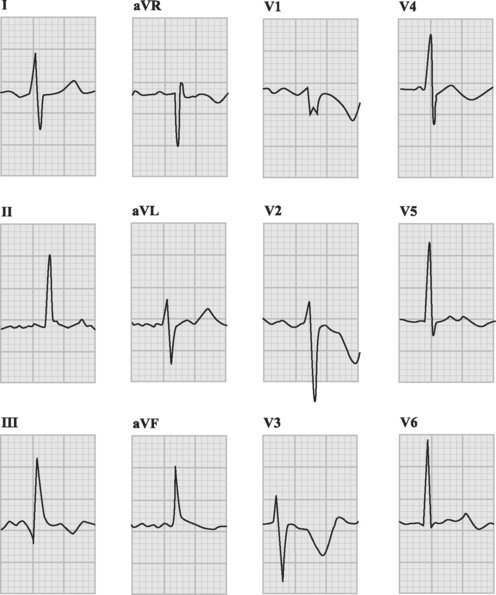
The ECG is usually abnormal but the findings are neither sensitive nor specific for pulmonary embolism. Common abnormalities include sinus tachycardia, anterior (V1 through V4) T wave inversion, partial or complete right bundle branch block, P pulmonale (a tall P wave in lead II, indicative of right atrial enlargement; see Fig. 24-1), and low voltage QRS complexes. The classic findings of an S wave in lead I and a Q wave and T wave inversion in lead III (Fig. 23-1) occur in only 50% of patients.6 T wave inversion in the anterior leads is the most common ECG finding, occurring in 68% of patients with pulmonary embolism overall but in 85% of patients with massive pulmonary embolism.6 The earlier this pattern appears, the greater the size of the embolus. Early normalization of T wave inversion correlates with clinical improvement.
Arterial Blood Gases
Most patients with massive pulmonary embolism have marked hypoxemia, an increased alveolar-arterial Po2 difference (see Chapter 27), and hypocarbia. However, taken in isolation, these blood gas results are neither sensitive nor specific for pulmonary embolism.7 Respiratory alkalosis is common due to hyperventilation, but with massive pulmonary embolism, respiratory and metabolic acidosis can occur.
D-dimer
The product of fibrin cross-linkage, the D-dimer is elevated in any condition in which fibrin cross-links have been cleaved by plasmin. Thus, D-dimer is a nonspecific but highly sensitive test for pulmonary embolism. Several studies have demonstrated its high negative predictive value in pulmonary embolism.8,9 The D-dimer is most commonly used as a screening test in the emergency department, where a normal result virtually excludes pulmonary embolism. Institution of anticoagulation should not be delayed while awaiting this result if there is a high index of clinical suspicion of pulmonary embolism.
Cardiac Biomarkers
Elevated troponin levels (troponin T >0.01 ng/ml) are predictive of a complicated in-hospital course and increased mortality rates in patients with pulmonary embolism.10 High levels of B-type natriuretic peptide (BNP) are also predictive of adverse outcome; a BNP level below 50 pg/ml identifies 95% of patients with benign clinical courses.11 However, the clinical utility of both troponin and BNP in patients with massive pulmonary embolism has yet to be determined.
Scintigraphy
V/Q lung scintigraphy is a noninvasive test for pulmonary embolism that was, until recently, the most commonly performed diagnostic imaging modality. A normal V/Q scan effectively excludes pulmonary embolus. A high-probability scan, as evidenced by multiple segmental mismatches between ventilation and perfusion in the context of a high clinical suspicion, identifies more than 90% of patients with pulmonary embolism.12 However, the utility of V/Q scans is limited by difficulties in interpreting them, especially in patients with underlying lung disease in whom regional variations in ventilation and perfusion confound interpretation of the image. Thus, although agreement among observers is high for normal and high-probability scans, it is substantially lower for intermediate scans, and these are the kinds of scans most commonly encountered in everyday practice. Scans should be performed within 24 hours of clinical suspicion of pulmonary embolus because the changes may revert to normal within a week.
Computed Tomogram Pulmonary Angiography
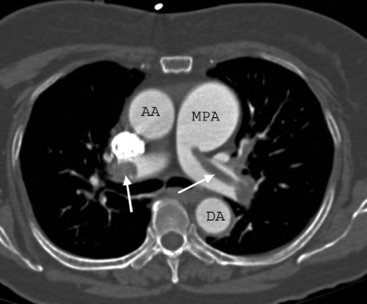
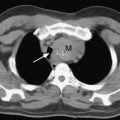
CTPA has become the imaging modality of choice for diagnosing pulmonary embolism (Fig. 23-2). The technique involves the rapid injection of up to 120 ml of radiocontrast into a peripheral vein using a powered injector. Imaging delay and scan thickness are optimized for the pulmonary arteries in the arterial phase. It is relatively noninvasive, very rapid (imaging is completed easily in one breathhold), and highly accurate. In two studies, the sensitivities for CTPA of four-slice scanners were 90% and 100%, with respective specificities of 98% and 89%.13,14 A negative CT scan reliably rules out pulmonary embolism.15,16 CTPA combined with venous phase CT angiography of the lower extremities (using 4-, 8-, or 16-slice scanners) has been shown to be superior to CTPA alone; the sensitivities and specificities of standard imaging were 83% and 96%, respectively, compared with 90% and 95%, respectively, for combined imaging.17 In 5% to 10% of patients, the image quality of CTPA is insufficient for diagnosis, in which case another imaging modality may be required.
Lower Limb Ultrasonography
In patients with contrast allergy who cannot undergo CTPA, ultrasound of the lower limbs may demonstrate thrombus proximal to the popliteal veins within the deep venous system of the leg or pelvis, which provides indirect evidence of pulmonary embolism. However, sometimes all of the thrombus has embolized, in which case the ultrasound scan is negative. Thus, although the specificity of leg ultrasonography is high (97% in one study18), the sensitivity is low (29%18).
Echocardiography
The sensitivity of transthoracic echocardiography in identifying pulmonary embolism is low; two studies showed it to be 56% and 41%, respectively.19,20 Direct visualization of thrombus within the pulmonary artery is difficult, and inferences regarding the presence of pulmonary embolism are based on signs of acute right ventricular pressure overload (Table 23-3). Occasionally, clot is seen in transit within the right atrium or ventricle, in which case the diagnosis of pulmonary embolism is confirmed. Visualization of thrombus within the pulmonary artery is often possible with transesophageal echocardiography,21,22 but this investigation is moderately invasive and is not well suited for routine diagnostic use.
Table 23-3 Echocardiographic Signs of Acute Right Ventricular Pressure Overload Consistent With Acute Massive or Submassive Pulmonary Embolism
| Right ventricular dilatation and hypokinesis |
| Tricuspid regurgitation |
| Tricuspid regurgitant jet velocity of 2 to 3.5 m/sec* |
| Flattening of the interventricular septum and paradoxical septal motion during diastole |
| Compression of the left ventricle |
| Enlarged pulmonary arteries |
| Signs of raised right atrial pressure: |
| Abnormal leftward bowing of the interatrial septum |
| Failure of inferior vena cava to collapse during inspiration (spontaneously breathing patients only) |
Note: See Chapter 7 for details
* From Equation 7-5: velocity of the tricuspid regurgitant jet can be used to estimate pulmonary arterial systolic pressure. Assuming a right atrial pressure of 10 mmHg, tricuspid regurgitant velocities of 2 and 3-5 m/sec equate to pulmonary arterial systolic pressures of 26 mmHg (the upper limit of normal) and 60 mmHg (the approximate maximum systolic pressure that can be achieved by the untrained right ventricle), respectively.(source)
Although limited as a diagnostic tool, echocardiography is useful in stratifying risk levels in patients and in gauging their responses to treatment. Patients with moderate to severely depressed right ventricular systolic function, even if hemodynamically stable at the time of evaluation, are at increased risk for developing cardiogenic shock and of dying.23 Echocardiography is also useful for excluding other potential diagnoses, such as aortic dissection, myocardial infarction, or cardiac tamponade. The presence of a PFO, which may allow paradoxic embolization of thrombus or right-to-left shunting of blood, should be actively sought. Although right-to-left shunting across a PFO may cause clinically significant hypoxemia, it also allows decompression of the right heart, thus improving left ventricular filling and cardiac output.
TREATMENT
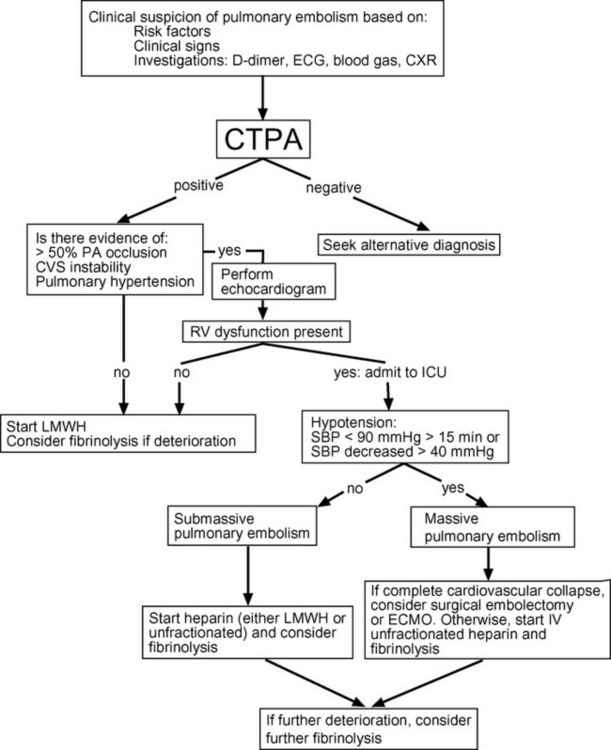
The primary treatment goal is the rapid relief of the pulmonary artery obstruction, by thrombolysis, surgical embolectomy, or catheter-applied mechanical disruption of the central embolism. This should be combined with anticoagulation and supportive treatment of hypoxemia and right heart failure. Right heart failure is the most common cause of death in patients with massive pulmonary embolism, and it usually occurs early in the disease course, before embolectomy can be arranged or thrombolysis has had time to be effective. Abrupt hemodynamic deterioration and cardiac arrest may occur at any time, but they are more likely to occur after institution of mechanical ventilation, which is commonly required for the treatment of hypoxemia. Patients with metabolic acidosis who have compensatory hyperventilation may become markedly acidemic if mechanical ventilation is instituted with a “normal” minute volume. Patients who are hypotensive or have echocardiographic evidence of moderate or severe right ventricular dysfunction are at increased risk for sudden deterioration and should be cared for in the intensive care unit. A management algorithm for massive pulmonary embolism is provided in Figure 23-3.
Anticoagulation
Heparin should be given as soon as pulmonary embolism is suspected unless there are contraindications.3,24,25 The rationale for this recommendation is to prevent clot extension and recurrent pulmonary embolism. Patients with massive pulmonary embolism (i.e., all hypotensive patients) should receive intravenous unfractionated heparin.3 The standard dose is 80 IU/kg followed by an infusion of 18 IU/kg/hr, adjusted to keep the activated partial thromboplastin time within the therapeutic range (see Table 30-7).25 However, it has been suggested that higher doses (10,000 IU followed by an infusion of at least 1250 IU/hr with the goal of keeping the activated partial thromboplastin time above 80 seconds) of unfractionated heparin be used in patients with massive pulmonary embolism.3 The dose of heparin may need to be increased in patients with heavy clot burdens.
For patients with submassive pulmonary embolism, either unfractionated heparin or low molecular weight heparin (LMWH) may be used.25 Recent evidence suggests that high-dose (e.g., enoxaparin 1 mg/kg subcutaneously twice daily) LMWH is associated with fewer complications and provides more effective anticoagulation than unfractionated heparin in this patient population.26 For most patients with pulmonary embolism, therapeutic drug monitoring of LMWH is not required, but in pregnant patients and those with moderate renal dysfunction, antifactor Xa levels should be measured to guide dosing (see Chapter 30). Patients with severe renal failure should receive unfractionated heparin rather than LMWH.
Warfarin is usually started on the same day as heparin, aiming for an International Normalized Ratio (INR; see Chapter 30) of 2 to 3. Heparin should be continued until warfarin has taken effect, which typically takes about 5 days. Patients with massive pulmonary embolism may empirically be given a longer course of heparin (e.g., 7 to 14 days), and the introduction of warfarin may be delayed.
Fibrinolytic Therapy
Debate exists regarding the indications for and benefits of fibrinolysis in patients with pulmonary embolism.27–30 In general, fibrinolytic therapy produces more rapid clot lysis than heparin but has not been convincingly demonstrated to improve survival rates. Currently, fibrinolytic therapy is recommended for patients with massive pulmonary embolism who are at low risk for bleeding.25 The role of fibrinolytic therapy in patients who are not hypotensive but have echocardiographic evidence of severely impaired right ventricular function is unresolved,25 and the decision to use fibrinolytic therapy in this situation must be made on a case-by-case basis.
Either streptokinase (250,000 IU loading dose followed by an infusion of 100,000 IU/hr for 24 hours) or tissue plasminogen activator (TPA; 100 mg infusion over 2 hours) may be used.25 However, because of the reduced duration of infusion and fewer side effects (particularly hypotension), rTPA is preferred over streptokinase (see Table 18-11). Irrespective of the agent used, heparin should be continued during and after the infusion.
Administration of fibrinolytic therapy directly into the pulmonary artery is not recommended because it offers no benefit over peripheral intravenous administration and can result in bleeding at the catheter insertion site. Fibrinolytic therapy is most effective when administered soon after pulmonary embolism, but a benefit extends to as long as 2 weeks after the onset of symptoms. Each patient should have a repeat CTPA following fibrinolysis to document clot resolution. If a patient remains hemodynamically unstable (or has recurrent instability) following fibrinolysis or has persistently elevated right ventricular pressures on echocardiography, a repeat CTPA may guide the decision as to whether a second dose of fibrinolysis should be given. Contraindications to fibrinolysis are listed in Table 18-10. Occasionally, fibrinolytic therapy can precipitate further embolization of thrombus.31 Placement of a temporary inferior vena cava filter (see subsequent material) before administration of fibrinolytic therapy has been reported to prevent this problem,32 but such a strategy is not routine.
Acute Surgical Embolectomy
No randomized trials compare medical with surgical therapy in massive pulmonary embolism; hence, the place of surgical embolectomy remains unclear. Most series report survival rates in excess of 50%, with worse outcome in patients who have had prior cardiopulmonary arrest.33,34 Emergency embolectomy may be considered in patients who have (1) massive pulmonary embolism; (2) persistent hemodynamic instability despite heparin and other resuscitative measures; or (3) a contraindication to or failure of thrombolytic therapy.25,35 Surgery is appropriate only for emboli involving the proximal branches of the pulmonary artery.
Percutaneous Thrombus Disruption
Various percutaneous, catheter-based approaches for the disruption or removal of central pulmonary emboli have been described; they include rheolytic thrombectomy, clot fragmentation, and clot aspiration.36 Most series are small but the results described are encouraging, and the methods are applicable to patients who are unsuitable for surgical embolectomy. Unfortunately, the devices required for percutaneous thrombus disruption are not widely available.
Inferior Vena Cava Filters
Placement of an inferior vena cava filter is recommended in patients with pulmonary embolism in whom anticoagulation is contraindicated or who have recurrent pulmonary emboli despite adequate anticoagulation.25 The most extensive experience has been with the Greenfield and bird’s-nest filters, both of which are designed for permanent placement and have been available for many years. They are inserted into the internal jugular or femoral veins and positioned in the inferior vena cava under fluoroscopic guidance. Anticoagulation should be commenced as soon as practical. Side effects of filter placement include occlusion and perforation of the inferior vena cava and migration of the device.
CHRONIC THROMBOEMBOLIC PULMONARY HYPERTENSION
A minority of patients (3.8% at 2-year follow-up in one study37) with pulmonary embolism go on to develop chronic pulmonary hypertension. The prognosis with this complication is poor: 5-year survival in patients with mean pulmonary artery pressures higher than 30 mmHg is 30%.38 This falls to 10% in patients with mean pulmonary artery pressures higher than 50 mmHg.38 Medical treatment of chronic thromboembolic pulmonary hypertension involves anticoagulation and vasodilator therapy (see Chapter 24). Surgical options include pulmonary thromboendarterectomy or lung transplantation.
Pulmonary Thromboendarterectomy
Pulmonary thromboendarterectomy involves bilateral pulmonary arterial endarterectomy to remove organized thrombus from the proximal pulmonary arterial tree. The procedure is performed under hypothermic cardiopulmonary bypass with periods of circulatory arrest. The procedure is a major undertaking and is performed in only a few institutions. Selection criteria include patients in New York Heart Association functional class III or IV, the appearances of chronic embolic disease on pulmonary angiography, the absence of significant noncardiac disease, and an elevated pulmonary vascular resistance (>4 Wood units or >320 dyne.sec.cm−5).39,40 Many patients referred for surgery have pulmonary vascular resistance greater than 12 Wood units (>1000 dyne.sec.cm−5) and suprasystemic pulmonary arterial pressure.41 In centers with a large experience, mortality rates in this procedure are very low. For instance, Jamieson has reported a mortality rate of 4.4% in his most recent 500 patients undergoing this procedure.42 Mortality rates in centers where there is less experience are likely to be much higher.
1 British Thoracic Society Standards of Care Committee. Suspected acute pulmonary embolism: a practical approach British Thoracic Society, Standards of Care Committee. Thorax. 1997;52(suppl 4):S1-S24.
2 Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. 2002;121:877-905.
3 Kucher N, Rossi E, DeRosa M, et al. Massive pulmonary embolism. Circulation. 2006;113:577-582.
4 Goldhaber SZ, Visani L, DeRosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999;353:1386-1389.
5 Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol. 1997;30:1165-1171.
6 Ferrari E, Imbert A, Chevalier T, et al. The ECG in pulmonary embolism: predictive value of negative T waves in precordial leads—80 case reports. Chest. 1997;111:537-543.
7 Stein PD, Goldhaber SZ, Henry JW, et al. Arterial blood gas analysis in the assessment of suspected acute pulmonary embolism. Chest. 1996;109:78-81.
8 Kelly J, Hunt BJ. Role of D-dimers in diagnosis of venous thromboembolism. Lancet. 2002;359:456-458.
9 Dunn KL, Wolf JP, Dorfman DM, et al. D-dimer levels in emergency department patients suspected of acute pulmonary embolism. J Am Coll Cardiol. 2002;40:1475-1478.
10 Pruszczyk P, Bochowicz A, Torbicki A, et al. Cardiac troponin T monitoring identifies high-risk group of normotensive patients with acute pulmonary embolism. Chest. 2003;123:1947-1952.
11 Kucher N, Printzen G, Goldhaber SZ. Prognostic role of brain natriuretic peptide in acute pulmonary embolism. Circulation. 2003;107:2545-2547.
12 PIOPED Investigators: Value of the ventilation/perfusion scan in acute pulmonary embolism Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED) The PIOPED Investigators. JAMA. 1990;263:2753-2759.
13 Coche E, Verschuren F, Keyeux A, et al. Diagnosis of acute pulmonary embolism in outpatients: comparison of thin-collimation multi-detector row spiral CT and planar ventilation-perfusion scintigraphy. Radiology. 2003;229:757-765.
14 Winer-Muram HT, Rydberg J, Johnson MS, et al. Suspected acute pulmonary embolism: evaluation with multi-detector row CT versus digital subtraction pulmonary arteriography. Radiology. 2004;233:806-815.
15 Quiroz R, Kucher N, Zou KH, et al. Clinical validity of a negative computed tomography scan in patients with suspected pulmonary embolism: a systematic review. JAMA. 2005;293:2012-2017.
16 Perrier A, Roy PM, Sanchez O, et al. Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med. 2005;352:1760-1768.
17 Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354:2317-2327.
18 Turkstra F, Kuijer PM, van Beek EJ, et al. Diagnostic utility of ultrasonography of leg veins in patients suspected of having pulmonary embolism. Ann Intern Med. 1997;126:775-781.
19 Miniati M, Monti S, Pratali L, et al. Value of transthoracic echocardiography in the diagnosis of pulmonary embolism: results of a prospective study in unselected patients. Am J Med. 2001;110:528-535.
20 Jackson RE, Rudoni RR, Hauser AM, et al. Prospective evaluation of two-dimensional transthoracic echocardiography in emergency department patients with suspected pulmonary embolism. Acad Emerg Med. 2000;7:994-998.
21 Wittlich N, Erbel R, Eichler A, et al. Detection of central pulmonary artery thromboemboli by transesophageal echocardiography in patients with severe pulmonary embolism. J Am Soc Echocardiogr. 1992;5:515-524.
22 Krivec B, Voga G, Zuran I, et al. Diagnosis and treatment of shock due to massive pulmonary embolism: approach with transesophageal echocardiography and intrapulmonary thrombolysis. Chest. 1997;112:1310-1316.
23 Ribeiro A, Lindmarker P, Juhlin-Dannfelt A, et al. Echocardiography Doppler in pulmonary embolism: right ventricular dysfunction as a predictor of mortality rate. Am Heart J. 1997;134:479-487.
24 Hyers TM, Agnelli G, Hull RD, et al. Antithrombotic therapy for venous thromboembolic disease. Chest. 1998;114:561S-578S.
25 Buller HR, Agnelli G, Hull RD, et al. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:401S-428S.
26 van Dongen CJ, van den Belt AG, Prins MH, et al. Fixed-dose subcutaneous low molecular weight heparins versus adjusted-dose unfractionated heparin for venous thromboembolism. Cochrane Database of Systematic Reviews. 2004:CD001100.
27 Anderson DR, Levine MN. Thrombolytic therapy for the treatment of acute pulmonary embolism. Can Med Assoc J. 1999;146:1317-1324.
28 Arcasoy SM, Kreit JW. Thrombolytic therapy of pulmonary embolism: a comprehensive review of current evidence. Chest. 1992;115:1695-1707.
29 Dalen JE. Pulmonary embolism: what have we learned since Virchow treatment and prevention. Chest. 2002;122:1801-1817.
30 Konstantinides S, Geibel A, Heusel G, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347:1143-1150.
31 Grimm W, Schwieder G, Wagner T. Fatal pulmonary embolism in venous thrombosis of the leg and pelvis during lysis therapy. Deutsche Medizinische Wochenschrift. 1990;115:1183-1187.
32 Lorch H, Welger D, Wagner V, et al. Current practice of temporary vena cava filter insertion: a multicenter registry. J Vasc Interverv Radiol. 2000;11:83-88.
33 Gray HH, Morgan JM, Paneth M, Miller GA. Pulmonary embolectomy for acute massive pulmonary embolism: an analysis of 71 cases. Br Heart J. 1988;60:196-200.
34 Doerge H, Schoendube FA, Voss M, et al. Surgical therapy of fulminant pulmonary embolism: early and late results. Thorac Cardiovasc Surg. 1999;47:9-13.
35 Meneveau N, Seronde MF, Blonde MC, et al. Management of unsuccessful thrombolysis in acute massive pulmonary embolism. Chest. 2006;129:1043-1050.
36 Ballew KA, Philbrick JT, Becker DM. Vena cava filter devices. Clin Chest Med. 1995;16:295-305.
37 Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257-2264.
38 Riedel M, Stanek V, Widimsky J, et al. Long-term follow-up of patients with pulmonary thromboembolism: late prognosis and evolution of hemodynamic and respiratory data. Chest. 1982;81:151-158.
39 Thistlethwaite PA, Mo M, Madani MM, et al. Operative classification of thromboembolic disease determines outcome after pulmonary endarterectomy. J Thorac Cardiovasc Surg. 2002;124:1203-1211.
40 Riedel M. Indications for pulmonary endarterectomy. J Thorac Cardiovasc Surg. 2003;126:1227-1228. author reply 1228-1229
41 Jamieson SW. Pulmonary thromboendarterectomy. Heart. 1998;79:118-120.
42 Jamieson SW, Kapelanski DP, Sakakibara N, et al. Pulmonary endarterectomy: experience and lessons learned in 1,500 cases. Ann Thorac Surg. 2003;76:1457-1462.
43 Stein PD, Henry JW. Clinical characteristics of patients with acute pulmonary embolism stratified according to their presenting syndromes. Chest. 1997;112:974-979.