Chapter 28 Masqueraders of Spinal Pathology
Musculoskeletal System
A variety of conditions involving the musculoskeletal system are known to mimic spine pathology.1–6 Typically, degenerative conditions in the upper and lower extremities such as arthritis, tendonitis, and bursitis are the primary culprits that bring the patient to the spine specialist, suspecting that their complaints are of spinal origin (Box 28-1). Many musculoskeletal structures in the extremities can present with signs and symptoms that seem to be originating from the spine; however, the most common extraspinal musculoskeletal masqueraders of spine disease are the shoulder, hip, and sacroiliac joint.
Shoulder
Impingement Syndrome
Impingement syndrome is a condition caused by repeated mechanical insult to the rotator cuff, with the tendons forced against the overlying coracoacromial arch as the arm is elevated overhead. A variety of anatomic, biomechanical, and neurologic factors either narrow the space available for the rotator cuff or cause abnormal arthrokinematics, leading to impingement of the tendons. Classically, there are three stages of impingement (described by Neer). Stage 1 is characterized by subacromial edema and hemorrhage; stage 2 progresses to tendonitis and fibrosis; and, ultimately, stage 3 results in tearing of the rotator cuff, either partially or completely.7
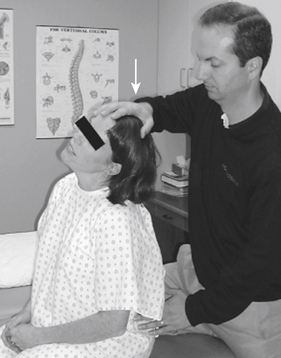
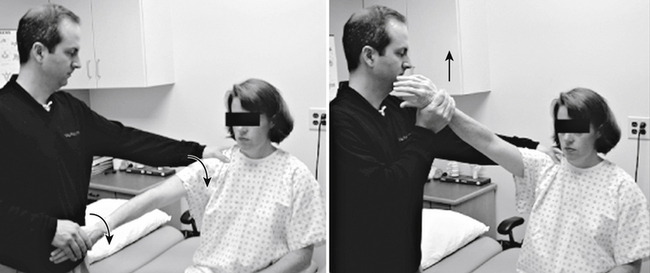
The patient with impingement syndrome generally complains of activity-related shoulder pain that extends from the top of the shoulder into the arm laterally—to the level of the deltoid tuberosity. This is especially true with overhead activities. Night pain and the inability to lie on the affected side may be additional complaints, particularly if a rotator cuff tear is present. Ultimately, the patient develops movement compensation patterns that place abnormal stresses on associated structures, leading to symptoms such as scapular and cervical muscle strain. Although cervical spine provocative maneuvers such as the Spurling test are negative8 (Fig. 28-1), a variety of physical examination maneuvers have been developed to diagnose shoulder impingement and are designed to reproduce the patient’s symptoms by compressing the rotator cuff under the subacromial arch.7 The Neer impingement sign and the Hawkins sign are two such tests (Figs. 28-2 and 28-3). The Neer impingement sign can be particularly useful; this overhead position is not well tolerated in the patient with impingement syndrome but may bring relief to the patient with cervical radiculopathy, because it decreases neural tension (shoulder abduction relief sign).9 Palpation may elicit tenderness over the rotator cuff tendons or the acromioclavicular joint. This joint can be a source of impingement due to degenerative changes, such as spurring and synovitis. In the presence of a rotator cuff tear, weakness of abduction (supraspinatus) and external rotation (infraspinatus) may be noted. However, other structures innervated by C5 and C6, such as the deltoid and bicep muscles, remain intact. In addition, impingement syndrome is not typically associated with sensory loss or reflex changes that may be found with cervical radiculopathy.
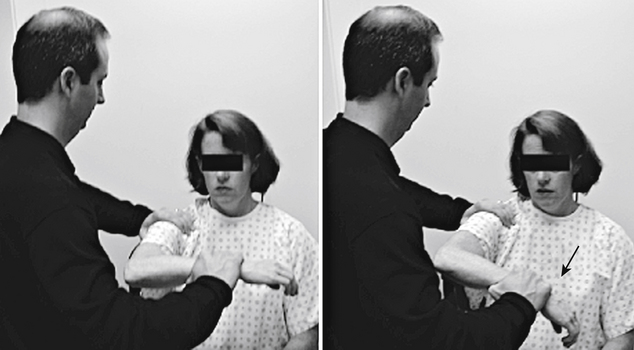
FIGURE 28-3. The Hawkins impingement test causes rotator cuff impingement against the coracoacromial ligament in this position.
A subacromial injection of local anesthetic can implicate or rule out impingement as the cause of the patient’s symptoms. Plain radiographs, including anterior-posterior, lateral, axillary, and scapular outlet views, as well as advanced imaging techniques such as MRI, can be helpful. Care must be taken in interpreting the MRI scans in the absence of a quality physical examination because the prevalence of abnormal studies in asymptomatic subjects ranges from 15% to 60% for the cervical spine and from 4% to 54% for rotator cuff tears, depending on the age of the patient.10,11
Glenohumeral Osteoarthritis
Osteoarthritis (OA), also known as degenerative joint disease of the glenohumeral joint, is not as common as knee or hip OA but is by no means rare. Causes of glenohumeral OA may be primary (idiopathic) or secondary. Examples of secondary causes are posttraumatic, infectious, metabolic, and inflammatory. The patient with early glenohumeral OA presents with activity-related pain that is relieved by rest. The pain is generally located about the shoulder, and there may be mild range of motion (ROM) restrictions. The distribution of pain is similar to that which a patient with a C5 or C6 radiculopathy might present.12 As the disease progresses, pain may persist at rest, and night pain may become a complaint. ROM becomes more restricted, particularly external rotation, due to anterior capsular and subscapularis contracture. Motion restrictions are similar for active and passive ROM. Rotator cuff strength is usually maintained but may seem limited secondary to pain. Palpation may demonstrate tenderness, especially about the posterior glenohumeral joint line, and crepitus may be noted with ROM activities. Plain radiographs (anteroposterior [AP], lateral, and axillary views) of the shoulder are usually conclusive in the case of glenohumeral OA. Diagnostic intra-articular injections can help differentiate shoulder OA from cervical spine disease.
Hip
Hip Osteoarthritis
OA of the hip, as in the shoulder, can be primary or secondary. It can be unilateral or bilateral. When systemic or inflammatory disease processes are responsible for the condition, involvement is more likely to be bilateral. Primary OA is by far the most common form of hip OA. It increases in frequency with increasing age, although the precise interrelationship between aging and arthritis is not clear. Patients with primary hip arthritis complain of activity-related pain that is relieved by rest. Those with severe degenerative changes may even have pain at rest. Their ambulation distance is decreased due to pain, and they may develop an antalgic gait pattern. As the disease progresses, patients may complain of difficulty with tasks such as donning shoes and socks due to limitation in hip ROM. As with any intra-articular hip pathology, OA causes groin pain that typically radiates down the ventromedial thigh to the knee. However, hip arthritis may refer pain distal to the knee in more than 40% of patients.13 In addition to mimicking lumbar radiculopathy, hip arthritis can mimic somatically referred pain from the intervertebral disc, lower lumbar facet joints, and sacroiliac joints. Secondary involvement of the gluteus musculature and trochanteric bursa due to an altered gait pattern may cause lateral hip and thigh pain as well.
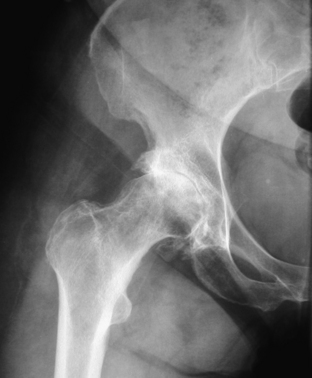
Physical examination may demonstrate gait abnormalities such as a trunk lean over the affected hip during weight-bearing (to decrease the abductor moment about the hip, thus reducing compressive load across the joint). Patients may display apparent and/or true leg length discrepancy due to hip flexor/adductor contractures and/or hip joint cartilage loss, respectively. There may be weakness of the hip abductors, and they may be tender to palpation. ROM tends to be limited, primarily in hip internal rotation. The most provocative maneuver with hip OA is to flex the hip of the supine patient to at least 90 degrees and then apply adduction and internal rotation. In the case of unilateral disease, this motion is restricted and painful on the involved side. Patients with OA of the hip do not typically have sensory or reflex changes as part of their clinical picture unless there is coexisting neuropathy or spinal pathology. Occasionally, a diagnostic, intra-articular hip injection with local anesthetic is required to differentiate hip and spine symptomatology with a calculated sensitivity of 88% and specificity of 100%.14 The standard radiographic workup for primary hip OA is an AP pelvis radiograph and AP and lateral radiographs of the involved hip. This is usually confirmatory (Fig. 28-4).
Greater Trochanteric Pain Syndrome
Greater trochanteric pain syndrome (GTPS) is a term that has been used to describe lateral hip pain of soft tissue origin in the region of the greater trochanter. Trochanteric bursitis is the most common etiology of this syndrome. However, other conditions have been described such as tendonitis or tears of the gluteus medius and iliotibial band irritation.2,15 These conditions are likely related to overuse, abnormal biomechanics, or local trauma. Patients with GTPS complain of pain about the hip laterally that may radiate proximally to the buttock and distally along the lateral aspect of the thigh to the lateral knee. Several lumbar dermatomes share this distribution, allowing GTPS to mimic lumbar radiculopathy. It has been reported that up to 20% to 25% of patients being evaluated for lumbar spine pathology have GTPS as the source of their complaints. In addition, greater trochanteric bursitis has been found in 25% to 35% of patients with low back pain.2,5
Physical examination of the patient with GTPS reveals local tenderness to palpation of the involved structure (tendon or bursa). Tenderness may be ventral, dorsal, rostral, or directly over the greater trochanter, depending on the structure involved. Similarly, patients complain of increased symptoms when sleeping on the involved side due to direct pressure on the inflamed structures. Resisted hip abduction or external rotation also compresses or stresses the tissues in GTPS, reproducing the symptoms. Once the physical examination has implicated a particular structure but lumbar radiculopathy has not been ruled out, an injection of a local anesthetic can help in the differentiation between local pathology and referred pain. Some authors suggest the use of fluoroscopy for the injection to ensure that the anesthetic reaches the site of pathology.15 Plain radiographs are useful to help to rule out hip arthritis and other local pathology such as a fracture of the greater trochanter. Often, patients complain of tenderness on palpation in this region, even when this is not the presenting complaint. Recognizing the existence of the various conditions under the umbrella of GTPS, coupled with a careful history and physical examination, is usually sufficient to differentiate between hip and lumbar spine disease. One must realize, however, that these conditions can coexist.
Sacroiliac Joint
The sacroiliac joint (SIJ) is certainly capable of producing pain locally. SIJ pain is also in the differential diagnosis as an extraspinal cause of low back and lower extremity pain. A variety of pathologic processes can affect the SIJ, including SIJ pain syndrome, OA, sacroiliitis (as in ankylosing spondylitis and Reiter syndrome), septic arthritis, and traumatic SIJ instability or dislocation. Many of these conditions have an obvious history as well as objective radiographic findings, implicating the SIJ as a pain generator. However, in the absence of “hard” findings on examination or radiographs, attributing a patient’s pain to the SIJ has in recent decades been met with skepticism due to the controversy over the joint’s ability to be a source of pain and the thought that the SIJ does not move in most individuals. Recent injection studies have demonstrated the SIJ to be a potential source of pain in the low back, buttock, and lower extremity, and studies involving radiostereometric analyses have shown small but definite motion at the SIJ.4,16
Sacroiliac Joint Dysfunction
The clinical presentation of the patient with SIJ dysfunction can be highly variable. In patients with a diagnosis of low back pain, the prevalence of this condition has been reported to range from 13% to 30%. Injection studies of the SIJs of asymptomatic volunteers produced pain in the low back, posterior superior iliac spine (PSIS) area, buttock, and thigh. Further studies on symptomatic subjects demonstrated that SIJ injection with a local anesthetic relieved a variety of pain patterns, including pain in the low back, PSIS region, abdomen, buttock, groin, thigh, leg, and foot.4 Clearly, SIJ dysfunction can mimic lumbar spine pathology, including discogenic pain, lumbosacral facet joint arthropathy, and radiculopathy.
Patients with SIJ dysfunction typically present with pain in the lateral low back, in the parasacral region with radiation to the buttock and posterolateral proximal thigh. They may complain of tenderness in the area of the sacral sulcus. Some authors have suggested that a painful SIJ can refer pain into the calf and foot, although much less commonly. There are many tests described in the literature to test for SIJ dysfunction; to review all of these is not within the scope of this chapter. Two categories of SIJ testing include tests designed to stress the SIJ and reproduce the patient’s symptoms and those designed to detect abnormal or asymmetrical motion by palpation of certain anatomic landmarks about the pelvis.17 Some tests used to stress the SIJ include the Gaenslen and Faber tests and SIJ compression/distraction maneuvers. Motion palpation tests include the standing flexion test and Gillet test. However, using physical examination tests to provoke SIJ pain through manually stressing the joint, detecting motion abnormalities by palpation, or relying on features of the patient history have traditionally all correlated poorly with the response of fluoroscopically guided intra-articular SIJ injections.4 However, more recent data suggest that by using a composite of manual tests, a practitioner can reliably implicate or rule out the SIJ as a source of low back pain.18 Even with these new data, the diagnostic test of choice for SIJ dysfunction seems to be a fluoroscopically guided intra-articular SIJ injection of a local anesthetic.
Nervous System
Conditions of both the central and peripheral nervous systems can mimic spine disease. Pathology of the peripheral nervous system is more likely than that of the central nervous system to mimic spine pathology, but occasionally, an intracranial condition will do so. Intracranial conditions known to have signs and symptoms overlapping those of spine pathology include intracranial neoplasms, Chiari malformation, cerebrovascular accidents, normal pressure hydrocephalus, and spontaneous intracranial hypotension.19–21
As previously stated, peripheral neuropathy is one of the more common masqueraders of spinal pathology, particularly radiculopathy and myelopathy. There are many etiologies of peripheral neuropathy, including compression or entrapment; metabolic, nutritional, toxic, hereditary, autoimmune, neoplastic disorders; and disorders associated with neuromuscular disease22–27 (Box 28-2). Even in the face of an extensive workup, the etiology of peripheral neuropathy is frequently not identified. When this is the case, the initial serologic studies should include vitamin B12, folate, hemoglobin A1C, erythrocyte sedimentation rate, and thyroid-stimulating hormone. The following section will concentrate on the more common peripheral nervous system conditions mimicking spine disease. There are certainly many more causes of peripheral neuropathy than are discussed in this section. The list is too exhaustive to review in this chapter, and the authors suggest a neurology text for further detail.
Compression Neuropathy
Compression or entrapment neuropathy is a type of mononeuropathy resulting from local compression on a peripheral nerve. One well-known example is carpal tunnel syndrome. Compression neuropathies can present with varying degrees of motor, sensory, and autonomic disturbances and can be confused with myelopathy and radiculopathy. However, a careful history, physical examination, and special studies such as electromyography and nerve conduction velocity (EMG/NCV) help localize the site of compression. Injections of local anesthetics at the suspected site of compression can be performed easily in the office and can often help differentiate between several diagnoses. There are numerous peripheral nerve compression syndromes of the upper and lower extremities. Therefore, only a select few are discussed in detail, with others represented in table format (Tables 28-1 and 28-2).
| Compressive Syndrome | Anatomy | Masquerading As |
|---|---|---|
| Thoracic outlet syndrome | Brachial plexus compression at the level of the scalenes, clavicle, first rib, or coracoid process | Cervical radiculopathy, primarily C8, T1 |
| Suprascapular nerve compression | Suprascapular nerve compression at the transverse scapular ligament or at the spinoglenoid notch | C5, (C6) radiculopathy |
| Carpal tunnel syndrome | Median nerve compression at the wrist | C6 radiculopathy |
| Pronator syndrome | Median nerve compression about the anteromedial elbow | C6 radiculopathy |
| Anterior interosseous nerve (AIN) syndrome | AIN compression in the proximal volar forearm | Not commonly confused with cervical radiculopathy because there are no sensory disturbances, only motor abnormalities (weakness of the flexor digitorum profundus to the index finger, flexor pollicis longus, and pronator quadratus) |
| Cubital tunnel syndrome | Ulnar nerve compression about the medial elbow | C8, T1 radiculopathy |
| Ulnar tunnel syndrome | Ulnar nerve compression in the canal of Guyon at the wrist | C8, T1 radiculopathy |
| Wartenberg syndrome | Superficial radial nerve compression between the brachioradialis and extensor carpi radialis longus in forearm | C6 radiculopathy |
| Posterior interosseous nerve (PIN) syndrome | PIN compression in the proximal forearm | C6, C7 radiculopathy |
| Radial tunnel syndrome | Radial nerve compression at or distal to the elbow | C6 radiculopathy |
TABLE 28-2 Compression Syndromes of Lower Extremity
| Compressive Syndrome | Anatomy | Masquerading As |
|---|---|---|
| Lumbosacral plexopathy | Compression of the lumbosacral plexus, (e.g., tumor, hematoma) | L1 through S4 radiculopathy |
| Piriformis syndrome | Sciatic nerve compression at the level of the piriformis muscle | S1 radiculopathy |
| Meralgia paresthetica (LFCN) | LFCN compression at the level of the inguinal ligament | L2, L3 radiculopathy |
| Obturator neuropathy | Compression due to many intrapelvic and hip pathologies | L1, L2, L3 radiculopathy |
| Saphenous neuropathy | Saphenous nerve compression at Hunter’s canal or from direct trauma | L4 (L3, L5) radiculopathy |
| Peroneal neuropathy | Common peroneal nerve compression at the level of the fibular head | L4, L5 radiculopathy |
| Tarsal tunnel syndrome (tibial nerve) | Tibial nerve compression at the posteromedial ankle | L5, S1 radiculopathy |
LFCN, lateral femoral cutaneous nerve.
Upper Extremity Compression Neuropathies
Thoracic Outlet Syndrome
Thoracic outlet syndrome (TOS) is a controversial diagnosis. Some authors even question the existence of this condition. The terminology describing this entity is confusing, as well. In fact, more than 10 different terms are used to describe this syndrome in the literature.28 In any event, TOS has persisted as the most widely recognized term for this condition and will therefore be used in the following discussion.
Coursing from proximal to distal, the neurovascular bundle first passes between the anterior and middle scalene muscles. This is referred to as the scalene triangle, with the first rib forming the base of the triangle. The subclavian vein is the exception, because it usually passes ventral to the anterior scalene muscle. Next, the neurovascular structures pass through the costoclavicular space, bordered by the clavicle rostrally and the first rib caudally. Finally, the neurovascular bundle passes beneath the coracoid process of the scapula through the subcoracoid space. This space is bordered by the coracoid process rostrally, the scapula dorsally, and the tendon of the pectoralis minor and costocoracoid ligament ventrally.
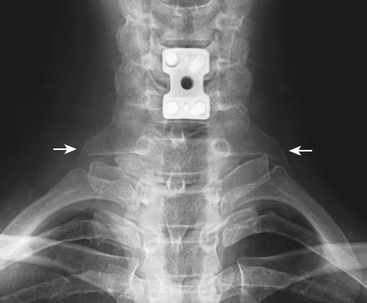
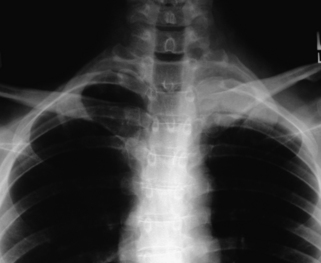
Etiologies of TOS can be divided into congenital, traumatic, and acquired categories.28 An example of a congenital anomaly causing TOS is the presence of a cervical rib or fibrous cervical band. The presence of this anomaly causes the neurovascular structures to be stretched and kinked as they are draped over the extra rib or cervical band on their way to the upper extremity. Cervical ribs can be noted on careful inspection of plain radiographs of the cervical spine (Fig. 28-5). An unusually large C7 transverse process seen on AP cervical spine radiographs suggests the presence of a cervical band29 (Fig. 28-6). Traumatic factors can also play a role in developing TOS. One example is a clavicle fracture that heals in a malreduced position or one that develops a significantly large callus. This can decrease the size of the costoclavicular space, leading to compression of the neurovascular structures. The most common cause of TOS, which falls into the acquired category, is posture related. Upper thoracic and cervical spine posture influences the flexibility and tone of the scalene muscles. Forward shoulder posture with scapular depression narrows the costoclavicular space and causes adaptive shortening of the pectoralis minor muscle and the costocoracoid ligament.
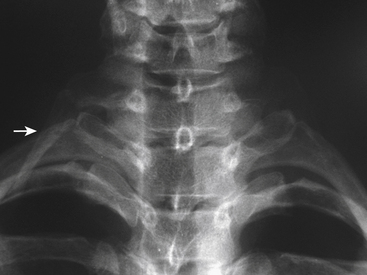
FIGURE 28-5. Anteroposterior cervical spine radiograph demonstrating a unilateral cervical rib (arrow).
Signs and symptoms in the patient with TOS include pain, paresthesias, motor weakness, and autonomic disturbances in the involved upper extremity. Pain can be reported from the supraclavicular and periscapular regions down the arm to the fingers, not necessarily in a dermatomal pattern. Paresthesias, including tingling or numbness, may be more often described in the C8-T1 distribution. Motor deficits may be noted in the hand, particularly the intrinsic muscles. Even without objective weakness or visible atrophy, patients may complain of hand weakness and poor dexterity. Chronic cases of TOS have been known to cause trophic changes in the hand. Vasomotor signs and symptoms, including periodic pallor or cyanosis, may indicate involvement of the sympathetic fibers or vascular compression.28
Median Neuropathy
The most common peripheral neuropathy of the upper extremity is compression of the median nerve at the level of the wrist, known as carpal tunnel syndrome. The median nerve is formed by the lateral and medial cords of the brachial plexus and therefore carries fibers from C5, C6, C7, C8, and T1. The carpal tunnel is a fibro-osseous channel containing nine flexor tendons and the median nerve. The causes of carpal tunnel syndrome are too numerous to mention but can be divided into two major categories: (1) those conditions that decrease the size of the carpal tunnel such as distal radius fracture; and (2) those that take up space in the tunnel such as flexor tenosynovitis and ganglion cysts. (Additional median nerve compression neuropathies that occur about the elbow and proximal forearm include the pronator syndrome and the anterior interosseous syndrome [see Table 28-1].)
Carpal Tunnel Syndrome
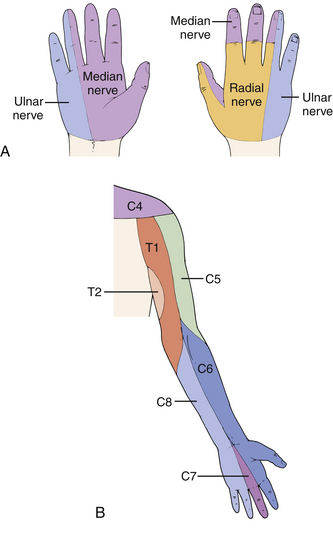
The clinical picture of carpal tunnel syndrome includes pain and paresthesias in the palmar-radial aspect of the hand, which are often worse at night and aggravated by repetitive use of the hand. Occupational and recreational risk factors should be elicited in the history, including vibration exposure and repetitive use of the hands and wrists. The most common pattern of median nerve sensory distribution in the hand is shown in Figure 28-7A, and comparison with the typical C6 and C7 dermatomes is shown in Figure 28-7B. EMG/NCV testing can help delineate anomalies such as Martin-Gruber anastomosis. The physical examination may demonstrate decreased sensation in the median nerve distribution, particularly with threshold testing using Semmes-Weinstein monofilament. A late finding may be thenar muscle atrophy and weakness, particularly of the abductor pollicis brevis. Additional physical examination maneuvers are directed at reproducing the patient’s hand paresthesias. The Tinel percussion test is probably the most commonly performed test. The examiner uses a finger to lightly tap over the carpal tunnel in an effort to reproduce paresthesias in the median nerve distribution; the occurrence of paresthesias indicates a positive test (sensitivity 0.60, specificity 0.67). The tapping should be performed from the wrist to the proximal forearm to rule out more proximal sites of median nerve compression. The Phalen test requires maintaining the wrist flexed to 90 degrees. Numbness and tingling in the median nerve distribution within 60 seconds is considered a positive test (sensitivity 0.75, specificity 0.47). The carpal compression test is performed when the examiner applies direct pressure over the median nerve at the wrist. The occurrence of paresthesias within 30 seconds is considered a positive response (sensitivity 0.87, specificity 0.90).30
When the diagnosis of carpal tunnel syndrome is suspected, cervical radiculopathy and proximal median nerve compression should be ruled out. Cervical radiculopathy can be placed higher or lower on the differential diagnosis list by a careful examination of hand and forearm sensation, wrist extensor strength, deep tendon reflexes (brachioradialis), the Spurling test, and cervical spine ROM. Signs and symptoms have been shown to be very useful in differentiating carpal tunnel syndrome from cervical spondylosis.31 Certainly cervical radiculopathy and carpal tunnel syndrome can coexist, making EMG/NCV testing an invaluable part of the workup. When a patient presents with signs and symptoms of bilateral carpal tunnel syndrome, systemic and metabolic etiologies such as hypothyroidism, diabetes, and amyloidosis (to mention a few) should be considered.
Ulnar Neuropathy
The ulnar nerve is prone to compression at several sites in the upper extremity and can mimic lower cervical spine radiculopathy. The ulnar nerve is a terminal branch of the medial cord of the brachial plexus and usually carries spinal levels C8 and T1. The most common site of ulnar nerve compression in the upper extremity is at the dorsomedial elbow, referred to as cubital tunnel syndrome. Cubital tunnel syndrome can be caused by fascial and anomalous muscle tissue, various soft tissues such as tumors and ganglion cysts, subluxating ulnar nerve, bone spurs, and cubitus valgus deformity. The ulnar nerve can also be compressed in the canal of Guyon at the wrist, a condition called ulnar tunnel syndrome. The canal of Guyon is a fibro-osseous tunnel where the ulnar nerve and artery pass from the forearm into the hand. Several of the more common pathologies implicated are ganglion cysts, ulnar artery aneurysm (or thrombosis), and fractures of the hook of the hamate.30
Cubital Tunnel Syndrome
Patients with cubital tunnel syndrome complain of numbness and/or paresthesias in the ulnar side of the hand. There is also numbness in the distribution of the dorsal sensory branch of the ulnar nerve but a lack of sensory disturbance in the medial forearm, which is supplied by the C8 dermatome (see Fig. 28-7B). This helps differentiate cubital tunnel syndrome from cervical radiculopathy. Patients may also complain of worsening symptoms when the elbow is in a flexed position for a prolonged period, such as when driving or sleeping. This is due to increased intraneural pressure and decreased cubital tunnel space with elbow flexion or from subluxation of the ulnar nerve over the medial epicondyle as the elbow is flexed. Tapping over the cubital tunnel (Tinel sign) can often reproduce the patient’s paresthesias. In addition to sensory changes, there may also be interosseous wasting and related weakness, which is usually a late finding. The more common sites of ulnar nerve compression about the elbow, from proximal to distal, are arcade of Struthers, medial intermuscular septum, medial epicondyle, cubital tunnel, and proximal edge of the pronator aponeurosis. A careful physical examination can usually differentiate cervical radiculopathy from cubital tunnel syndrome. EMG/NCV can confirm the level of compression, and plain radiographs of the elbow can rule out bony pathology as a source of compression.30
Radial Neuropathy
The radial nerve has several regions of potential compression in the upper extremity, rarely proximal to the elbow and more commonly in the forearm. The radial nerve carries fibers from C5, C6, C7, C8, and inconsistently T1, and it is one of the terminal branches of the posterior cord. Syndromes involving compression of the radial nerve include radial tunnel syndrome, Wartenberg syndrome, and posterior interosseous nerve compression syndrome. These are compression neuropathies of the forearm, which can usually, but not always, be differentiated based on patient complaints and careful physical examination. Radial neuropathy is most likely to be confused with a C6 or C7 radiculopathy (see Fig. 28-7).
Lower Extremity Compression Neuropathies
Piriformis Syndrome
Piriformis syndrome refers to entrapment of the sciatic nerve as it passes beneath or through the piriformis muscle, causing pain along the distribution of the sciatic nerve (buttock, dorsal thigh, and dorsal leg). This distribution is also a common source of pain in a patient with an L5 or S1 intervertebral disc herniation, thereby causing a diagnostic dilemma. The piriformis syndrome is a controversial diagnosis; some authors deny its existence, others believe that it should be a diagnosis of exclusion, and yet others consider it a primary diagnostic entity.32 The sciatic nerve is the largest nerve in the body and is actually composed of two nerves: the common peroneal nerve and the tibial nerve. The peroneal portion arises from the ventral rami of spinal levels L4-S2, and the tibial portion arises from the ventral rami of L4-S3. The sciatic nerve exits the pelvis, coursing through the greater sciatic foramen, usually caudal to the piriformis muscle; however, great variability exists in the relationship between the piriformis muscle and the sciatic nerve.
Many reported etiologic factors are associated with sciatic nerve compression at the level of the piriformis muscle. As mentioned, anatomic anomalies altering the relationship between the piriformis muscle and the sciatic nerve may increase the potential for nerve entrapment.33 Abnormal mechanics about the lumbar spine, pelvis, hips, and lower extremities have also been implicated in piriformis syndrome due to chronic stretching of the piriformis muscle or chronic overuse of the muscle, both of which can increase compression of the nerve. Patients’ symptoms can be intermittent and dynamic, precipitated by particular activities and positions.34
Meralgia Paresthetica
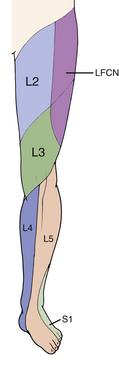
Meralgia paresthetica is a term used to describe pain, paresthesias, and/or numbness in the ventrolateral thigh due to neuropathy of the lateral femoral cutaneous nerve (LFCN) and is also referred to as Bernhardt disease. The term meralgia comes from two Greek words, meros (thigh) and algos (pain).35 The LFCN typically originates from the dorsal branches of the ventral rami of L2, L3, and occasionally L1 and therefore has the ability to mimic upper lumbar radiculopathy. The most common site of entrapment is thought to be the site at which the nerve passes above, below, or through the inguinal ligament just medial to the anterior superior iliac spine. There is considerable anatomic variation as the nerve courses past the inguinal ligament from the pelvis to the thigh.16
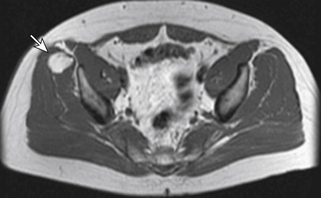
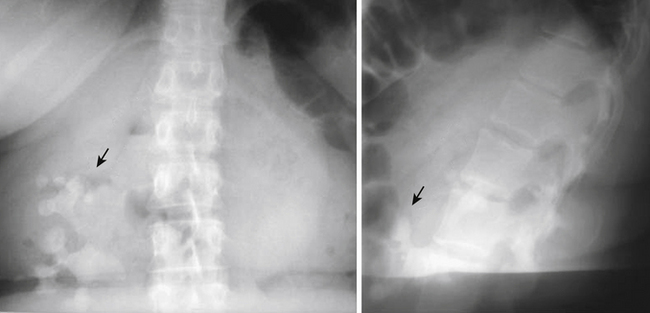
Some authors have divided the etiology of meralgia paresthetica into spontaneous and iatrogenic forms, but this section will discuss only the spontaneous etiologies. Most noniatrogenic causes of LFCN neuropathies are either mechanical compression or metabolic neuropathy. Mechanical compression can result from external causes such as obesity and pregnancy due to increased intra-abdominal pressure and direct compression at the level of the inguinal ligament, as well as from wearing tight, low-lying pants or belts. Compression can also come from within by way of intra-abdominal and intrapelvic tumors, for example (Fig. 28-8). The LFCN bypasses many potentially entrapping structures prior to reaching the level of the inguinal ligament. Several metabolic conditions, including diabetes mellitus and alcoholism, can be causative or at least associated with neuropathy of the LFCN.
Patients with meralgia paresthetica complain of pain, burning, paresthesias, numbness, or hypersensitivity in the distribution of the LFCN, which overlaps the dermatomal distribution supplied by L2 and L3 (Fig. 28-9). The LFCN carries only sensory fibers. Therefore, weakness on examination should raise suspicion of conditions other than meralgia paresthetica to explain the patient’s symptoms.36 Physical examination may demonstrate decreased sensation in the LFCN distribution, which is different than the true L2 or L3 dermatome distribution. Extending the hip, which places the nerve under tension, increases intraneural pressure and exacerbates the compressive pathology; this maneuver may reproduce patients’ paresthesias and pain. Additionally, palpation or Tinel percussion about the site of compression may also reproduce their symptoms. If the history and physical examination are equivocal, electrodiagnostic testing can be helpful. As with most peripheral compression neuropathies, injection of a local anesthetic at the site of compression can be used as a diagnostic test.
Metabolic Neuropathy: Diabetic Neuropathy
Patients with diabetes mellitus may present with a wide variety of signs and symptoms with respect to peripheral neuropathy because their disease process can affect sensory, motor, and autonomic fibers. Although the causative factors for neuropathy in patients with diabetes are multiple and complex, several studies support hyperglycemia as playing an important role in the development of neuropathy.37 Classifying neuropathy in diabetes is difficult. However, one simple classification distinguishes between mononeuropathy (isolated or multiple) and polyneuropathy. This section will discuss the three most common presentations: sensory mononeuropathy, proximal motor neuropathy, and distal sensory neuropathy.
Proximal motor neuropathy (also known as diabetic amyotrophy or diabetic polyradiculopathy) is another clinical presentation in patients with diabetes, typically in older patients with type 2 diabetes. Presenting signs and symptoms include pain, weakness, and atrophy of the proximal lower extremity musculature.28 It can present unilaterally or bilaterally, with bilateral findings being asymmetrical. These clinical findings can be similar to those found in patients with lumbar spinal stenosis, and coexisting pathology makes diagnosis and treatment more difficult.
Distal sensory neuropathy is the most common neuropathy found in the diabetic population. These patients can present with a wide variety of symptoms ranging from significant burning pain, paresthesias, and numbness to an insensate foot with Charcot arthropathy. Distal sensory neuropathy usually develops gradually. However, an acute form may develop during a period of poor glycemic control. The legs and feet are commonly involved in a stocking-like distribution, and less commonly the hands are affected in a glovelike distribution. Proprioception may also be impaired; patients may complain of progressively worsening balance with increasing falls as well as demonstrating increased sway with Romberg testing.37 Many of these signs and symptoms overlap with those of spinal stenosis.
Visceral Organs and Related Systems
The visceral organs of the thoracic, abdominal, and pelvic cavities are associated with pathologic conditions that may present with signs and symptoms that can be confused with spine pathology (Box 28-3). The mechanism responsible for this is generally thought to involve a referred pain mechanism. Although the majority of patients with these conditions have signs and symptoms related to the organ system involved, back pain or radiculopathy could be the sole presenting complaint. This section will discuss spinal masqueraders as they relate to the cardiovascular, pulmonary, gastrointestinal, genitourinary, and gynecologic systems.
BOX 28-3 Visceral Organ Conditions Known to Mimic Spine Pathology
Cardiovascular System
Myocardial Ischemia
Cardiac ischemia can result in multiple signs and symptoms, occurring either together or in isolation. Patients with ischemic heart disease can present with any combination of the following signs and symptoms: retrosternal pain; pressure, tightness, or burning; nausea; diaphoresis; epigastric discomfort; pain and numbness in the shoulder, arm, or hand; and interscapular, neck, jaw, or face pain. Many of these signs and symptoms are referred to as angina “equivalents.”38 In fact, there are several case reports of patients presenting to their dentist with complaints of jaw or tooth pain, which is ultimately discovered to be of cardiac origin.
Of particular interest to the spine surgeon is the upper extremity and interscapular back pain that can be of cardiac origin. In patients with myocardial ischemia, the prevalence of arm pain or numbness has been reported as high as 46% to 67% and that of back pain approximately 6%.39,40 When arm pain does occur due to cardiac ischemia, it is more common on the left side and typically refers to the shoulder, medial arm, and forearm. An additional useful aspect of the patient’s history is that the symptoms are exertional. Fortunately, the majority of patients with ischemic heart disease have more than just arm pain, and an appropriate history and review of systems help lead to the correct diagnosis.
Aortic Aneurysm
Patients with AAAs may have no symptoms. Studies have indicated the prevalence of asymptomatic AAAs (>3 cm) is 3% to 10% in patients older than 50 years of age in the Western world, with an increasing incidence partly related to an aging population and improved detection methods.41 When patients do have symptoms, this is likely related to leakage, rupture, or acute expansion of the aneurysm. Most AAAs rupture dorsally, and the retroperitoneal space provides a tamponade effect to slow or contain the hemorrhage, which can produce chronic low back pain. When they rupture ventrally, the peritoneal cavity provides little tamponade, resulting in significant hemorrhage and acute patient deterioration. The classic triad of abdominal or back pain, pulsatile abdominal mass, and hypotension is present in only about 25% of cases with confirmed AAA rupture.42 AAAs increase in diameter approximately 0.2 to 0.5 cm per year on average.43
The most common complaint is an acute onset of abdominal or back pain, which may radiate to the groin or flank. Other symptoms include those related to compression of neighboring structures by the aneurysm. Duodenal compression can lead to nausea and vomiting, and compression of the ureter(s) may cause hydronephrosis and urinary tract symptoms. Dorsal aneurysm expansion can erode adjacent vertebral bodies, causing back pain. Additionally, aortic aneurysms may compromise blood flow to the spinal cord by aneurysm location and/or thrombus formation. Affected patients can present with back pain, radicular complaints, and varying degrees of myelopathy or paralysis from spinal cord ischemia.44 Even in the absence of bony erosion and spinal cord ischemia, AAA can cause chronic back pain that is quite vague.
Diagnosing an asymptomatic AAA on physical examination is difficult. Variables that make this problematic include the skill and experience of the examiner, size of the patient, size of the aneurysm, and whether or not the examination is focused. Diagnosing a symptomatic AAA can also be difficult. In a study looking specifically at misdiagnoses of ruptured AAAs, 30% were initially misdiagnosed. Misdiagnoses included renal colic, diverticulitis, gastrointestinal hemorrhage, myocardial infarction, and idiopathic back pain. Fifty-four percent of patients complained of back pain, and it was the second most common complaint after abdominal pain.42
Because physical examination for diagnosing AAA is not reliable, imaging becomes crucial. Ultrasound, CT, and MRI are all useful in certain situations, but CT is the most used because of its rapidity, availability, cost, and ability to provide detail for surgical planning. Plain radiographs occasionally demonstrate an AAA (Fig. 28-10). The calcified aneurysmal vessel wall may be seen, as well as softer signs such as loss of the psoas or kidney shadow. If an AAA is discovered or suspected, a prompt vascular surgery consult is in order. Aneurysms less than 5 cm in diameter can be followed with ultrasound every 4 to 6 months, with a risk of rupture ranging from 0% in 5 years to 6% per year. In aneurysms greater than 5 cm, the risk of rupture is approximately 25% at 5 years.43
Vascular Claudication
Generally, either disease can present with a variety of lower extremity complaints, including numbness, weakness, cramping sensation, pain, and a feeling of tiredness in the legs. In the case of foraminal stenosis, the findings may include a dermatomal distribution of sensory changes and a myotomal pattern of weakness. Numbness is a common complaint in either condition. A complaint of weakness in arterial insufficiency is typically a sense of hip and thigh weakness, especially with proximal occlusion. Cramping in the calves is a classic complaint of vascular insufficiency but occurs in either disease, as does the vague complaint of lower extremity fatigability and tiredness with ambulation. The lower extremity symptoms caused by arterial insufficiency are relieved with rest (stopping ambulation), whether standing or sitting. That is to say, a change in patients’ spine posture does not affect their symptoms. In patients with spinal stenosis, standing itself may produce symptoms and sitting (lumbar spine flexion) relieves the symptoms.45 Some patients with neurogenic claudication volunteer that they can walk further when shopping and leaning on a cart. This position allows the lumbar spine to flex, which increases the dimensions of the spinal canal and intervertebral foramen, thereby relieving neural element compression. When impotence is a complaint, it is commonly associated with buttock and thigh claudication as a result of aortoiliac insufficiency.
In addition to presenting symptoms, some physical examination findings are unique to each condition. In neurogenic claudication, the involved spinal levels may correspond to diminished deep tendon reflexes and positive nerve root tension tests. Physical examination maneuvers associated with lumbar spinal stenosis include a wide-based gait, abnormal Romberg test, thigh pain after 30 seconds of lumbar extension, and neuromuscular deficits.45 Intermittent vascular claudication may present with diminished pulses (femoral, popliteal, dorsalis pedis, and posterior tibial) and bruits. Ankle-brachial indices (ABI) will also be diminished. Normal ABI is 1, whereas vascular “claudicators” have an ABI between 0.6 and 0.9. An ABI less than 0.5 may be associated with rest pain and ulceration. It is necessary to realize that the calcified vessels in patients with diabetes yield a falsely elevated ABI. In those patients with vascular claudication, there may also be trophic changes in the feet and distal legs (thin, shiny, atrophic skin, thickened and ridged nails, and loss of hair) and they may feel cool. One may also find that elevating the limb produces pallor (cadaveric) of the foot and a dusky rubor when placing the foot in the dependent position (Buerger sign). Finally, the van Gelderen bicycle test can help differentiate vascular and neurogenic claudication.46 Exercising on a stationary bike should reproduce the symptoms of vascular disease more rapidly and reliably because the disease is one of ischemia, so the type of exercise (walking or cycling) should not matter. However, in spinal stenosis, sitting or positions of lumbar spine flexion (as on a stationary bike) often relieve the lower extremity symptoms, and therefore patients with spinal stenosis are able to ride a stationary bike without necessarily reproducing their symptoms. Certainly, patients can have both spinal stenosis and arterial occlusive disease; therefore, consultation with a vascular surgeon may be warranted in this situation.
Infective (Bacterial) Endocarditis
The term infective endocarditis (IE) is used to describe an infection of the endocardial surface of a heart valve. The clinical picture of IE can be extremely varied, and of all the potential presenting symptoms, musculoskeletal complaints are frequent and often the initial complaints.47–49 Several series have reported that more than 40% of patients with IE manifest musculoskeletal signs and symptoms, and as many as 25% of patients have musculoskeletal complaints as the initial symptoms.47 Of particular interest to the spine specialist are the complaints of neck and back pain and lower extremity myalgias in the thighs and calves. As many as 5% to 20% of patients with IE have back pain as their presenting symptom, and this may be the only complaint for several months, delaying the correct diagnosis. Lower extremity myalgias, although not radicular in distribution, may be severe enough to make the clinician consider radiculopathy in the differential diagnosis, particularly when accompanied by back pain.48
The low back pain associated with IE can be quite severe and can present with paraspinal tenderness, muscle spasm, and decreased ROM. The pain may be accentuated by straight-leg raising and Valsalva maneuvers and may be accompanied by lower extremity myalgias, all suggesting a herniated lumbar intervertebral disc.47 Generally, in patients with complaints of back pain, they are unable to obtain positional relief. Some patients with low back pain and IE have a disc space infection or vertebral osteomyelitis; however, the majority of musculoskeletal manifestations are thought to be related to arterial emboli containing bacteria and immune complexes causing a vasculitic reaction. It has also been suggested that the low back pain in some patients may be a nonspecific manifestation of the infection. Other common symptoms of IE include fever, chills, weakness, anorexia, and headache. Additional signs include heart murmur (changing or new) and dermatologic manifestations such as splinter hemorrhages and petechiae.
Pulmonary System: Pancoast Tumor
The most significant pulmonary condition to present with signs and symptoms, seemingly of spinal origin, is a tumor at the superior pulmonary sulcus (Pancoast tumor). This region lies in close proximity to the C8 and T1 nerve roots and the lower trunk of the brachial plexus, making cervical radiculopathy as well as peripheral neuropathy high on the differential diagnosis in the patient with a Pancoast tumor. Several signs and symptoms can help raise suspicion of a superior sulcus tumor. The majority of patients with a Pancoast tumor are smokers. Unfortunately, respiratory symptoms rarely dominate the initial clinical picture. One of the first and most significant symptoms is shoulder pain, and this is the presenting symptom in more than 90% of patients.50 The lower trunk of the brachial plexus, the subclavian artery and vein, and the sympathetic chain and stellate ganglia are a few of the important structures in close proximity to the superior pulmonary sulcus that help explain some of the signs and symptoms in these patients. In addition to shoulder pain and lower plexus neuropathy, other findings include Horner syndrome, supraclavicular fullness, upper extremity swelling or discoloration, and hand intrinsic muscle wasting. Many authors state that Pancoast tumor should be in the differential diagnosis whenever lower brachial plexopathy exists, and some include this in their differential diagnosis even with C8 and T1 radiculopathy. If the diagnosis of Pancoast tumor is entertained, chest imaging beginning with routine chest radiographs, including an apical lordotic view, remains a crucial step in the workup (Figs. 28-11 and 28-12). Unfortunately, the average lag time between the onset of symptoms and definitive diagnosis is 7 to 7.5 months.50
Gastrointestinal System
Although not common, patients with gastrointestinal pathology present with pain referred to the spine. Pathology of the stomach/small intestine (ulcer disease), gallbladder, pancreas, and large intestine can all mimic spine pathology.51 Peptic ulcer disease, cholecystitis, and pancreatic conditions can all refer pain to the thoracic region and thoracolumbar junction, whereas pathology of the colon can refer pain to the lumbar region or can cause compression on the lumbosacral plexus, mimicking lumbar radiculopathy.51–57 Although signs or symptoms related to the primary structure involved are usually present, there are occasions when back pain or radiculopathy is the presenting symptom.
The patient with peptic ulcer disease and biliary colic due to cholelithiasis may present with back pain, from the interscapular to the thoracolumbar region. In fact, a German gastroenterologist named Ismar Boas (1858–1938) described a tender spot (Boas point) to the left of the T12 vertebra dorsally in patients with gastric ulcer disease. The literature reflects this with cases of patients being treated for musculoskeletal thoracic spine problems only to have resolution of their pain after diagnosis and treatment for peptic ulcer disease. In patients with cholelithiasis and biliary colic, 50% complain of periscapular back pain. Often, these patients complain of intermittent abdominal pain and nausea and may have a positive Murphy sign.5
Genitourinary System
The kidneys, ureters, and bladder can be a source of referred pain to the abdomen, flank, back, and groin and can therefore mimic spine disease.51,58 Several conditions known to cause either flank, back, or groin pain include urolithiasis, pyelonephritis, urinary tract infection, renal artery occlusion, and neoplasm.59 Disease of the prostate such as prostatitis has also been associated with low back pain, often radiating to the rectum. As with most organ systems, these conditions usually have presenting complaints other than referred pain such as abdominal pain, abdominal mass, dysuria, hematuria, nausea, and/or fever, depending on the underlying process.
Urolithiasis (Kidney Stones)
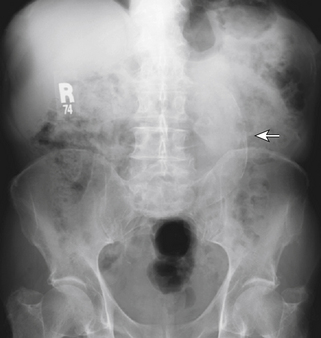
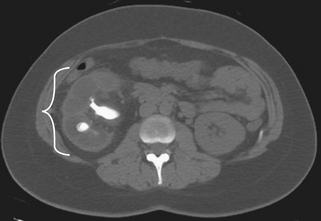
Urolithiasis is a condition describing the presence of calculi within the urinary system. This condition affects 2% to 5% of individuals in their lifetime, with males affected more often than females. Depending on the location of the calculi in the urinary system, patients may complain of dermatomal pain about the thoracolumbar region, flank and back pain, or groin pain. Kidney pain of visceral origin follows a dermatomal pattern from T10 to L1 and tends to be dull and poorly localized because it is mediated by slow C-type fibers. Parietal pain is usually located adjacent to the affected organ, so in the case of kidney pathology the pain is located about the flank and back with the presence of costovertebral angle tenderness. This pain is usually sharp and well localized. Pain originating from the ureters may refer to the low back and groin.58 The onset of pain in patients with urolithiasis is usually sudden and is not mechanical; that is, they cannot find a comfortable position. There may be urinary symptoms such as urgency, frequency, and dysuria, as well as signs such as hematuria (most if not all have at least microhematuria),51 but back pain may be the initial and only presenting symptom (Figs. 28-13 and 28-14). Plain radiographs such as studies of the kidney, ureter, and bladder may demonstrate calculi, but radiolucent stones are not visualized. The diagnostic test of choice for patients with suspected urolithiasis is a noncontrast helical CT scan, which is significantly more sensitive than an intravenous pyelogram.59
Gynecologic System
Although the pelvic organs are an infrequent cause of low back pain, various pathologic conditions of the ovaries, fallopian tubes, and uterus have been associated with low back and sacral pain, including endometriosis, pregnancy (normal and ectopic), and neoplastic disease.51,54,60,61 Thoracolumbar pain may be present due to L1 and L2 innervation of pelvic viscera. Conditions that involve the uterosacral ligament are thought to be an important source of low back pain. This can occur due to ligament strain from a malpositioned uterus or from carcinoma invading the ligament. Menstrual pain may be felt in the sacral region as a dull, cramping, poorly localized pain and may radiate into the lower extremities.62 In this case, the pain has a temporal relationship with the menstrual cycle.
Cases of uterine fibroids mimicking lumbar radiculopathy have been reported.63 The fibroid tumor can be positioned in such a way as to compress a portion of the lumbosacral plexus, thus presenting as lumbar spine pathology.
Summary
The evaluation of patients with neck or back pain, radiculopathy, or myelopathy usually reveals a spinal source to explain their complaints. It is not rare, however, to have their symptoms stemming from a condition unrelated to the spine. This chapter is not intended to be all-inclusive, but hopefully it will serve as an initial source of information and as a reminder that not all patients present in the same way and that there are many conditions masquerading as spine disease.
Borenstein D.G., Wiesel S.W., Boden S.D., editors. Low back pain: medical diagnosis and comprehensive management. Philadelphia: WB Saunders, 1995.
Kleiner J.B., Donaldson W.F., Curd J.G., Thorne R.P. Extraspinal causes of lumbosacral radiculopathy. J Bone Joint Surg [Am]. 1991;73(6):817-821.
Lauder T.D. Musculoskeletal disorders that frequently mimic radiculopathy. Phys Med Rehabil Clin North Am. 2002;13:469-485.
Mazanec D.J. Pseudospine: conditions that mimic spine pain. In: Cole A.J., Herring S.A., editors. The low back pain handbook: a guide for the practicing clinician. ed 2. Philadelphia: Hanley & Belfus; 2003:117-131.
Mazanec D.J. Differential diagnosis of low back pain and sciatica. Semin Spine Surg. 1994;6(3):180-185.
Szabo R.M. Nerve compression syndromes. In: Manske P.R., editor. Hand surgery update. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1996:221-231.
1. Lauder T.D. Musculoskeletal disorders that frequently mimic radiculopathy. Phys Med Rehabil Clin North Am. 2002;13:469-485.
2. Mazanec D.J. Differential diagnosis of low back pain and sciatica. Semin Spine Surg. 1994;6(3):180-185.
3. Slipman C.W., Rogers D.P., Lipetz J.S. An unusual extraspinal cause of bilateral leg pain. Arch Phys Med Rehabil. 1999;80:721-724.
4. Slipman C.W., Jackson H.B., Lipetz J.S., et al. Sacroiliac joint pain referral zones. Arch Phys Med Rehabil. 2000;81:334-338.
5. Tortolani P.J., Carbone J.J., Quartararo L.G. Greater trochanteric pain syndrome in patients referred to orthopedic spine specialists. Spine J. 2002;2:251-254.
6. Uppal G.S., Uppal J.A., Dwyer A.P. Glenoid cysts mimicking cervical radiculopathy. Spine (Phila Pa 1976). 1995;20(20):2257-2260.
7. Tifford C.D., Plancher K.D. Nonsurgical treatment of rotator cuff tears. In: Norris T.R., editor. Orthopaedic knowledge update shoulder and elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1997:135-149.
8. Tong H.C., Haig A.J., Yamakawa K. The Spurling test and cervical radiculopathy. Spine (Phila Pa 1976). 2002;27(2):156-159.
9. Davidson R.L., Dunn E.J., Metzmaker J.N. The shoulder abduction test in the diagnosis of radicular pain in cervical extradural compressive mononeuropathies. Spine (Phila Pa 1976). 1981;6(3):441-446.
10. Boden S.D., McCowin P.R., Davis D.O., et al. Abnormal magnetic resonance scans of the cervical spine in asymptomatic subjects: a prospective investigation. J Bone Joint Surg [Am]. 1990;72:1178-1184.
11. Sher J.S., Uribe J.W., Posada A., et al. Abnormal findings on magnetic resonance images of asymptomatic shoulders. J Bone Joint Surg [Am]. 1995;77:10-15.
12. Slipman C.W., Plastaras C.T., Palmitier R.A., et al. Symptom provocation of fluoroscopically guided cervical nerve root stimulation: are dynatomal maps identical to dermatomal maps? Spine (Phila Pa 1976). 1998;23(20):2235-2242.
13. Kahn A.M., McLoughlin E., Giannakas K., et al. Hip osteoarthritis: where is the pain? Ann R Coll Surg Engl. 2004;86(2):119-121.
14. Faraj A.A., Kumaraguru P., Kosygan K. Intra-articular bupivacaine hip injection in differentiation of coxarthrosis from referred thigh pain: a ten year study. Acta Orthop Belg. 2003;69(6):518-521.
15. Cohen S.P., Narvaez J.C., Lebovits A.H., et al. Corticosteroid injections for trochanteric bursitis: is fluoroscopy necessary? A pilot study. Br J Anesth. 2005;94(1):100-106.
16. Harrison D.E., Harrison D.D., Troyanovich S.J. The sacroiliac joint: a review of anatomy and biomechanics with clinical implications. J Manipulative Physiol Ther. 1997;20(9):607-617.
17. Dreyfuss P., Michaelsen M., Pauza K., et al. The value of medical history and physical examination in diagnosing sacroiliac joint pain. Spine (Phila Pa 1976). 1996;21(22):2594-2602.
18. Laslett M., Aprill C., McDonald B., et al. Diagnosis of sacroiliac pain: validity of individual provocation tests and composites of tests. Man Ther. 2005;10:207-218.
19. Westhout F.D., Pará L.S., Linskey M.E. Central causes of foot drop: rare and underappreciated differential diagnosis. J Spinal Cord Med. 2007;30(1):62-66.
20. Bejjani GK, Cockerham KP: Adult Chiari malformation. Contemp Neurosurg 23(26):1–8, 2002.
21. Kleopa K.A., Natsiopoulos K. Acute cervical radiculopathies in spontaneous intracranial hypotension. J Neurol. 2009;256:499-501.
22. Busis N.A. Femoral and obturator neuropathies. Neurol Clin. 1999;17(3):633-653.
23. Dropcho E.J. Remote neurological manifestations of cancer. Neurol Clin. 2002;20(1):85-122.
24. Harvey G., Bell S. Obturator neuropathy: an anatomic perspective. Clin Orthop. 1999;363:203-211.
25. Kleiner J.B., Donaldson W.F., Curd J.G., et al. Extraspinal causes of lumbosacral radiculopathy. J Bone Joint Surg [Am]. 1991;73(6):817-821.
26. Krarup C., Crone C. Neurophysiologic studies in malignant disease with particular reference to involvement of peripheral nerves. J Neurol. 2002;249:651-661.
27. Saal J.A., Dillingham M.F., Gamburd R.S., Fanton G.S. The pseudoradicular syndrome: lower extremity peripheral nerve entrapment masquerading as lumbar radiculopathy. Spine (Phila Pa 1976). 1988;13(8):926-930.
28. Sheth R.N., Belzberg A.J. Diagnosis and treatment of thoracic outlet syndrome. Neurosurg Clin North Am. 2001;12(2):295-309.
29. Liu J.E., Tahmoush A.J., Roos D.B., Schwartzman R.J. Shoulder-arm pain from cervical bands and scalene muscle anomalies. J Neurol Sci. 1995;128:175-180.
30. Szabo R.M. Nerve compression syndromes. In: Manske P.R., editor. Hand surgery update. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1996:221-231.
31. Chow C.S., Hung L.K., Chin C.P. Is symptomatology useful in distinguishing between carpal tunnel syndrome and cervical sponylosis? Hand Surg. 2005;10:1-5.
32. Fishman L.M., Dombi G.W., Michaelsen C., et al. Piriformis syndrome: diagnosis, treatment, and outcome—a 10-year study. Arch Phys Med Rehabil. 2002;83:295-301.
33. Sayson S.C., Ducey J.P., Maybrey J.B., et al. Sciatic entrapment neuropathy associated with an anomalous priformis muscle. Pain. 1994;59:149-152.
34. Rodrique T., Hardy R.W. Diagnosis and treatment of piriformis syndrome. Neurosurg Clin North Am. 2001;12(2):311-319.
35. Grossman M.G., Ducey S.A., Nadler S.S., Levy A.S. Meralgia paresthetica: diagnosis and treatment. J Am Acad Orthop Surg. 2001;9(5):336-344.
36. Erbay H. Meralgia paresthetica in differential diagnosis of low back pain. Clin J Pain. 2002;18:132-135.
37. Boulton A.J.M., Malik R.A. Diabetes mellitus; neuropathy. In: DeGroot L.J., Jameson J.L., editors. Endocrinology. ed 4. Philadelphia: WB Saunders; 2001:868-876.
38. Topol E.J. The history. In: Topol E.J., editor. Textbook of cardiovascular medicine. ed 2. Philadelphia: Lippincott Williams & Wilkins; 2002:265-272.
39. Goff D.C., Sellers D.E., McGovern P.G., et al. Knowledge of heart attack symptoms in a population survey in the United States: the REACT trial. Rapid Early Action for Coronary Treatment. Arch Intern Med. 1998;158(21):2329-2338.
40. Goldberg R., Goff D., Cooper L., et al. Age and sex differences in presentation of symptoms among patients with acute coronary disease: the REACT trial. Rapid Early Action for Coronary Treatment. Coron Artery Dis. 2000;11(5):399-407.
41. Cronenwett J.L., Krupski W.C., Rutherford R.B. Abdominal aortic and iliac aneurysms. In: Rutherford R.B., editor. Vascular surgery. ed 5. Philadelphia: WB Saunders; 2000:1246-1280.
42. Marston W.A., Ahlquist R., Johnson G., et al. Misdiagnosis of ruptured abdominal aortic aneurysms. J Vasc Surg. 1992;16:17-22.
43. Nevitt M.P., Ballard D.J., Hallett J.W. Prognosis of abdominal aortic aneurysms: a population study. N Engl J Med. 1989;321:1009-1014.
44. Larsson E.M., Heiling M., Holtas S. Aortic pathology revealed by MRI in patients with clinical suspicion of spinal disease. Neuroradiology. 1993;35:499-502.
45. Katz J.N., Dalgas M., Stucki G., et al. Degenerative lumbar spinal stenosis: diagnostic value of the history and physical examination. Arthritis Rheum. 1995;38(9):1236-1241.
46. Gray J.C. Diagnosis of intermittent vascular claudication in a patient with a diagnosis of sciatica. Phys Ther. 1999;79:582-590.
47. Churchill M.A., Geraci J.E., Hunder G.G. Musculoskeletal manifestations of bacterial endocarditis. Ann Intern Med. 1977;87:754-759.
48. Härkönen M., Olin P., Wennström J. Severe backache as a presenting sign of bacterial endocarditis. Acta Med Scand. 1981;210:329-331.
49. Meyers O.L., Commerford P.J. Musculoskeletal manifestations of bacterial endocarditis. Ann Rheum Dis. 1977;36:517-519.
50. Vargo M.M., Flood K.M. Pancoast tumor presenting as cervical radiculopathy. Arch Phys Med Rehabil. 1990;71:606-609.
51. Borenstein D.G., Wiesel S.W., Boden S.D., editors. Low back pain: medical diagnosis and comprehensive management. Philadelphia: WB Saunders, 1995.
52. Doran F.S. The sites to which pain is referred from the common bile-duct in man and its implication for the theory of referred pain. Br J Surg. 1967;54(7):599-606.
53. Horton J.D., Bilhartz L.E. Gallstone disease and its complications. In: Feldman M., Friedman L.S., Sleisenger M.H., editors. Sleisenger and Fordtran’s gastrointestinal and liver disease: pathophysiology/diagnosis/management. ed 7. Philadelphia: WB Saunders; 2002:1065-1090.
54. Mazanec D.J. Pseudospine: conditions that mimic spine pain. In: Cole A.J., Herring S.A., editors. The low back pain handbook: a guide for the practicing clinician. ed 2. Philadelphia: Hanley & Belfus; 2003:117-131.
55. Murtagh J. Three unusual presentations of interscapular back pain. Aust Fam Physician. 1993;22(6):1000-1004.
56. Spoelhof G.D., Bristow M. Back pain pitfalls. Am Fam Physician. 1989;40(4):133-138.
57. Weiss D.J., Conliffe T., Tata N. Low back pain caused by a duodenal ulcer. Arch Phys Med Rehabil. 1998;79(9):1137-1139.
58. Quast M.S., Goldflies M.L. A new differential diagnosis for musculoskeletal posterior thoracic wall pain: a case report. Orthop Rev. 1989;18(4):461-465.
59. Smith R.C., Rosenfeld A.T., Choe K.A., et al. Acute flank pain: comparison of non-contrast-enhanced CT and intravenous urography. Radiology. 1995;194:789-794.
60. Klineberg E., Mazanec D., Orr D., et al. Masquerade: medical causes of back pain. Cleve Clin J Med. 2007;74(12):905-913.
61. Goff B.A., Mandel L.S., Melancon C.H., et al. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291(22):2705-2712.
62. Engstrom J.W. Back and neck pain. In: Fauci A.S., Braunwald E., Jameson J.L., et al, editors. Harrison’s principles of internal medicine. ed 15. New York: McGraw-Hill; 2001:79-90.
63. Murphy D.R., Bender M.I., Green G. Uterine fibroid mimicking lumbar radiculopathy: a case report. Spine (Phila Pa 1976). 2010;35(24):E1435-E1437.