Managing physiological change in the surgical patient
Systemic responses
Factors responsible for systemic responses (Box 2.1)
• Major operations—anaesthesia (particularly head-down + pneumoperitoneum for laparoscopic surgery), tissue trauma, blood and fluid loss, healing and repair
• Major trauma including fractures and burns; head, abdominal and chest injuries
• Major cardiovascular events, e.g. myocardial infarction, pulmonary embolism, stroke
• Haemorrhage and fluid infusion including blood; fluid and electrolyte abnormalities
The way the body responds to major systemic insults depends on several factors—the physiological reserve of the patient’s chief organ systems (i.e. basic fitness), the nature of the injurious process, the severity of physiological disruption, the duration of delay before resuscitation, and the virulence of any microorganisms involved. Most patients are remarkably resilient given good basic care but in a deteriorating patient, several physiological systems are likely to be impacted upon simultaneously, evoking a range of complex homeostatic mechanisms.
Management of the deteriorating patient
Management requires careful monitoring, often in a high-dependency or intensive care unit, and repeated checks on organ function and dysfunction. In most elective operations, many of the responses discussed below can be mitigated by good preoperative preparation, accurate fluid replacement, ensuring oxygenation, adequate analgesia, reducing psychological stress, preventing infection and using careful operative technique to minimise tissue trauma, blood loss and complications. Enhanced recovery programmes are gradually being introduced which give special attention to these factors before, during and after operation (see: NHS Enhanced Recovery Partnership Programme document: Delivering enhanced recovery—Helping patients to get better sooner). The individual variables responsible for potentially excessive systemic responses to severe injury or major surgery are summarised in Box 2.1.
Stressors in the surgical patient
Direct and indirect tissue trauma: Tissue disruption (whether surgical or traumatic) leads to activation of local cytokine responses more or less in proportion to the damage. Responses are exaggerated if wounds are contaminated (e.g. debris, foreign bodies, faeces) or associated with tissue ischaemia.
Fall in intravascular volume: This is a key factor in initiating systemic responses. Hypovolaemia results from:
• Excess fluid loss (see Box 2.2)
• Interstitial sequestration of fluid as oedema in damaged tissues and generally as a result of systemic hormonal responses. This process is amplified in systemic sepsis
• Restricted oral intake during any perioperative period or whilst in intensive care
Falling intravascular volume stimulates sympathetic activity by removing baroreceptor inhibition in an attempt to maintain blood pressure by increasing cardiac output and peripheral resistance. This also explains the mild tachycardia commonly seen in postoperative patients. Compensation is most effective in young fit individuals, but decompensation is often sudden and rapid. Catecholamines also have profound metabolic effects, increasing the turnover of carbohydrates, proteins and lipids. Falling renal perfusion activates the renin–angiotensin–aldosterone system, increasing renal reabsorption of sodium and water. A centrally mediated increase in antidiuretic hormone (ADH) secretion promotes further conservation of water.
Reduced cardiac output and peripheral perfusion: Circulatory efficiency may be impaired by hypovolaemia, and myocardial contractility may be depressed by anaesthetic agents and other drugs. Anaesthetic drugs generally cause peripheral dilatation and positive-pressure ventilation impairs venous return. Head-down positioning and artificial pneumoperitoneum for laparoscopic surgery further stress cardiovascular physiology. Major events such as sepsis (septic shock), pulmonary embolism or myocardial infarction may precipitate cardiovascular collapse.
Stress: Psychological stress associated with injury, severe illness or elective surgery has an effect similar to pain on sympathetic function and hypothalamic activity.
Excess heat loss: This can occur during long operations and after extensive burns. Heat loss imposes enormous demands upon energy resources; if body core temperature falls, physiological processes such as blood clotting are impaired. Small babies are very vulnerable to heat loss. Heat loss in the operating theatre is counteracted as far as possible by raising the ambient temperature, insulating exposed parts of the body, using warm water underblankets or warm air ‘bear-huggers’ and by warming fluids during intravenous infusion.
Blood coagulation changes: General metabolic responses to injury activate thrombotic mechanisms and initially depress intrinsic intravascular thrombolysis. Thus the patient is in a prothrombotic state and may suffer intravenous thrombosis and consequent thromboembolism.
If substantial haemorrhage occurs, clotting factors eventually become exhausted, causing failure of clotting. The systemic inflammatory response syndrome (SIRS, see Ch. 3, p. 51) may initiate widespread intravascular thrombosis, using up clotting factors and precipitating disseminated intravascular coagulation (DIC), with failure of normal clotting.
Starvation and stress-induced catabolism: Patients with major surgical conditions are often malnourished before operation (see Nutritional management, below). Most are starved for 6–12 hours preoperatively and often do not start eating for 12–24 hours after surgery. After major GI surgery, food may be withheld for several days, or much longer with complications such as anastomotic breakdown or fistula formation.
Metabolic responses to pathophysiological stress
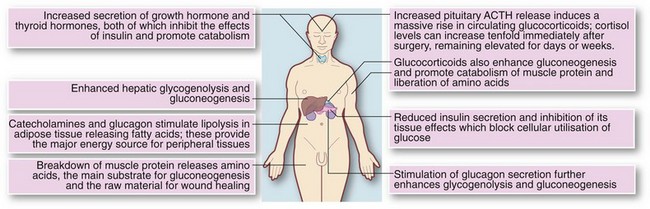
The sum of these factors is to cause inevitable catabolism and potentially extreme changes in fluid balance and electrolytes. These metabolic changes are shown in Figure 2.1.
Effects on carbohydrate metabolism: The overall effect is rising blood glucose (levels may reach 20 mmol/L), often resulting in hyperglycaemia and a pseudodiabetic state, and glucose may appear in the urine. This is in marked contrast to simple fasting, in which glucose levels are normal or low and glycosuria does not occur.
Effects on body proteins and nitrogen metabolism: In the normal healthy adult, nitrogen balance is constantly maintained. Protein turnover results in daily excretion of 12–20 g of urinary nitrogen which is made good by dietary intake. In a hypercatabolic state, nitrogen losses can increase three- or four-fold. Most importantly, the metabolic environment prevents proper utilisation of food or intravenous nutrition. There is therefore huge destruction of skeletal muscle. This state of negative nitrogen balance contrasts markedly with simple starvation in which body protein is preserved.
Fluid, electrolyte and acid–base management
Normal fluid and electrolyte homeostasis
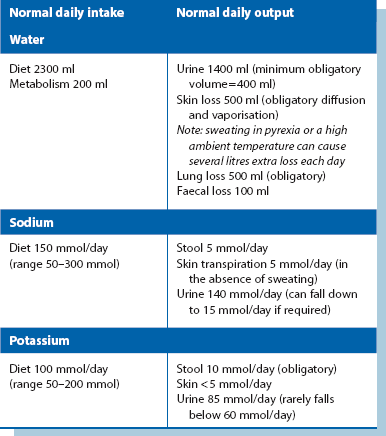
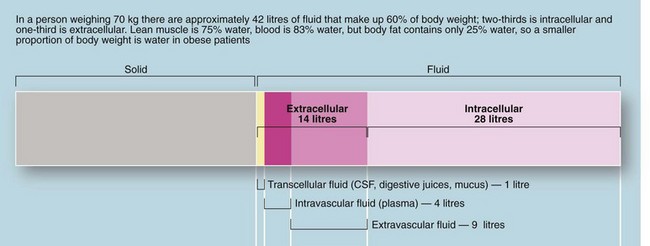
The body of an average 70 kg adult contains 42 litres of fluid, distributed between the intracellular compartment, the extracellular space and the bloodstream (see Fig. 2.2). Fluid input is mainly by oral intake of fluids and food but about 200 ml/day of water is produced during metabolism. Normal adult losses are between 2.5 and 3 litres/day. About one litre is lost insensibly from skin and lungs, 1300–1800 ml are passed as urine (about 60 ml/hour or 1 ml/kg/hour) and 100 ml are lost in faeces. About 100–150 mmol of sodium ions and 50–100 mmol of potassium ions are lost each day in urine and this is balanced by the normal dietary intake (see Table 2.1).
Maintenance of water and sodium
For most patients, the daily water and sodium requirements are best met by using appropriate quantities of normal saline solution (0.9% sodium chloride) and 5% dextrose (glucose) solution. Normal saline contains 154 mmol each of sodium and chloride ions per litre. One litre will thus satisfy the daily sodium requirement of uncomplicated patients. The additional requirement for water is made up with 2–2.5 litres of 5% glucose (see Box 2.3). The small amount of glucose this contains contributes little to nutrition but renders the solution isotonic. This prescription is altered for patients with electrolyte abnormalities by varying the volume of normal saline given.
In children, water excretion is markedly reduced in the postoperative period as a result of increased ADH secretion. Maintenance fluids requirements are based on published guidelines and formulae (see: http://www.nda.ox.ac.uk/wfsa/html/u19/u1914_01.htm).
Physiological changes in response to surgery and trauma
Effects of a fall in renal perfusion: Any substantial reduction in effective circulating volume may cause a fall in renal perfusion. In addition, aortic surgery involving aortic clamping may alter the dynamics of renal artery flow, whilst raised intra-abdominal pressure (see Abdominal compartment syndrome, below) disrupts renal blood flow.
Other factors in water conservation: Water conservation is further enhanced by stress-mediated secretion of antidiuretic hormone (ADH), also known as vasopressin, from the posterior pituitary (neurohypophysis). Loss of water alone increases the plasma osmolality, stimulating ADH release, mediated by osmoreceptors in the hypothalamus. ADH binds to receptors in the distal renal tubules and promotes reabsorption of water. Release of ADH is also stimulated by falls in blood pressure and volume, sensed by stretch receptors in the heart and large arteries. Changes in blood pressure and volume are not nearly as sensitive a stimulator as increased osmolality, but are potent in extreme conditions (e.g. loss of over 15% volume in acute haemorrhage). Stress and pain probably also promote ADH release via other hypothalamic pathways.
Postoperative situation: At the site of trauma or major surgery, fluid is effectively removed from the circulation in the form of inflammatory oedema (isotonic local third space losses). This displaced volume is compensated by fluid retained by the hormonal changes described above. More potassium is released from damaged cells than the excess lost by exchange in the kidney. Thus, the postoperative plasma potassium level tends to rise in the first day or two. This is particularly true if stored blood has been transfused as this releases potassium from elderly red cells, so potassium supplements are not usually needed for the first few days after operation provided preoperative plasma potassium is normal and potassium-losing diuretics are not prescribed.
Abdominal compartment syndrome: (see http://www.ncbi.nlm.nih.gov/pmc/articles/PMC137242/)
• Oliguria due to renal hypoperfusion and collapsed renal veins
• Respiratory embarrassment due to restriction and elevation of the diaphragm, and compression of alveoli. This results in increased peak airways pressure, decreased tidal volume, hypoxaemia and hypercarbia
• Decreased venous return leading to falling cardiac output and hypotension
In patients with a distended and taut abdomen, measuring abdominal compartment pressure can help early recognition. Treatment involves reopening the abdomen and leaving it open until the risk of rising pressure subsides.
Problems of fluid and electrolyte depletion
Loss of whole blood or plasma: Rapid and copious blood loss in traumatic injury or operative surgery initially depletes the intravascular compartment and loss of only 1 litre may cause hypotension or even hypovolaemic shock. When haemorrhage is less rapid, there is time to replace fluid loss from the extracellular compartment, so greater volumes can be lost before the cardiovascular system becomes compromised, although losses still need to be restored physiologically or by transfusion.
In severe burns, the amount of plasma likely to be lost is easily underestimated and should be calculated using a standard formula based on the burnt area to guide fluid replacement (see Ch. 17).
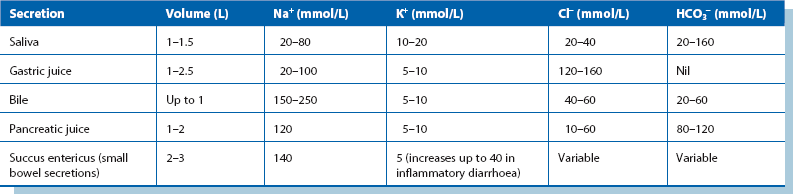
Gastrointestinal fluid loss: Between 5 and 9 litres of electrolyte-rich fluid is normally secreted into the upper GI tract each day as saliva, gastric juice, bile, pancreatic fluid and succus entericus (small bowel secretions; see Table 2.2). Most of the fluid is reabsorbed in the large intestine.
From Table 2.2, it can be seen that vomitus, nasogastric aspirate and diarrhoea are variably rich in sodium and potassium. As a general rule, GI fluid losses should be replaced by an equivalent volume of normal saline, with potassium chloride added as needed. In intestinal obstruction or adynamic ileus, fluid sequestrated in bowel is replaced in a similar manner, although volume requirements have to be estimated. Fistulae and overactive ileostomies cause chronic loss of fluid that is high in chloride and bicarbonate.
Intra-abdominal accumulation of inflammatory fluid: Severe intra-abdominal inflammation may cause several litres of fluid rich in plasma proteins and electrolytes to be lost into the peritoneal cavity. This typically occurs in peritonitis or acute pancreatitis and often in the context of the systemic inflammatory response syndrome (SIRS). This is best replaced (as well as can be estimated) by physiological saline or other suitable crystalloids.
Systemic sepsis (SIRS and multiple organ dysfunction syndrome): Systemic sepsis is associated with widespread endothelial damage and a large increase in capillary permeability mediated by a range of cytokines and other circulating mediators. The result is extensive loss of protein and electrolyte-rich fluid from the circulation into the extravascular space (‘third space loss’), which, combined with loss of peripheral resistance, results in cardiovascular collapse and shock.
The required fluid volume is difficult to estimate and replacement is usually given so as to maintain cardiovascular stability (pulse rate and blood pressure) and urinary output (at least 0.5 ml/kg body weight/hour) whilst avoiding fluid overload and cardiac failure. In the severely ill patient, in whom the volume requirements are particularly difficult to judge, a central venous pressure line or transoesophageal Doppler provide a more accurate method of assessing precise fluid replacement needs (see Enhanced recovery programmes, below).
Abnormal insensible fluid loss: Abnormal insensible fluid loss can greatly increase overall fluid loss, particularly in the seriously ill or elderly patient and must be included in the fluid balance equation. Pyrexia increases insensible loss by approximately 20% for each degree Celsius rise in body temperature, mainly in the form of exhaled water vapour. A pyrexia of 38.5°C for 3 days would therefore cause an extra litre of fluid loss. Sweating causes loss of sodium-rich fluid which can be easily overlooked in patients with fever and when the ambient temperature rises.
Preventing acute renal failure: Maintaining fluid balance in surgical patients depends on anticipating problems before they cause adverse effects and risk acute renal failure. Acute renal failure is a serious complication with a high mortality in surgical patients. Prevention involves similar strategies in all patients at risk, namely:
• Observing changes in vital signs—pulse rate, blood pressure and CVP if appropriate
• Checking hourly urine output is adequate
• Measuring fluid losses to guide replacement
• Seeking clinical signs of fluid imbalance (both dehydration and overload)
In patients with cardiac failure or shock, monitoring and treatment is best carried out in an intensive care or high-dependency/critical care unit, using invasive monitoring to help determine the required volume of fluid replacement.
Common fluid and electrolyte problems
Major operations
• Patients are often elderly and are likely to have a diminished cardiovascular reserve. They may have pre-existing fluid and electrolyte abnormalities
• Preoperative vomiting and restricted fluid intake may have caused dehydration and electrolyte abnormalities
• Blood loss during and after operation may be substantial
• Operations may take several hours with consequent insensible losses from the open wound
• Third space losses of 500–1000 ml can occur after major surgery or trauma as a result of systemic responses to trauma
• The recovery period when oral intake is nil or restricted may become extended—several days following complicated bowel surgery or peritonitis (e.g. perforated diverticulitis or an anastomotic leak)
Careful pre- and postoperative assessment of patients is crucial so problems can be anticipated. This should include clinical examination for dehydration (dry mouth and loss of normal skin turgor) or overhydration (elevated jugular venous pressure or cardiac failure). Plasma urea and electrolytes, creatinine and full blood count should be measured daily. Elevated urea concentration with little elevation of creatinine is characteristic of dehydration. An abnormally high haemoglobin concentration (providing polycythaemia is not present) also indicates dehydration, especially if it was normal beforehand.
• Preoperative assessment and improved education and preparation of patients so that they understand what will happen and cope with planned early discharge
• Home care arrangements to cope with early discharge
• Improved methods of calculating fluid replacement that ensure the patient remains normovolaemic within narrow limits, preventing central hypovolaemia and fluid overload. NICE have recommended the use of transoesophageal Doppler ultrasound monitoring of left ventricular stroke volume to enable intraoperative assessment of fluid status and provide individualised goal-directed fluid therapy. Trials have shown this can reliably shorten hospital stays and reduce complication rates
• Planned and assisted early postoperative mobilisation
• Early enteral nutrient challenge and the use of gut-specific nutrients such as glutamine, antioxidants and synbiotics (nutritional supplements that improve the balance of intestinal microflora). Methods that enable earlier return of gut function may be fundamental to rapid recovery. Gastrointestinal gut-associated lymphoid tissue (GALT) forms more than half the body’s immunological cell mass and is believed to play a key role in stress responses to surgery. Sustaining nutrition of the small bowel wall from within the lumen may prevent breakdown of intestinal barrier function. Healthy bowel function enables earlier tolerance of food, less postoperative ileus and less postoperative nausea and vomiting
Abnormalities of individual electrolytes
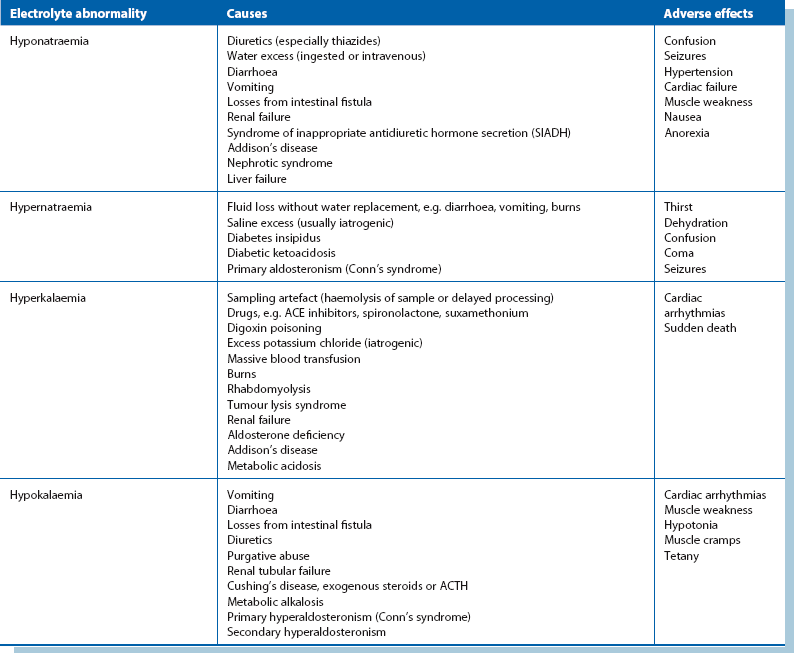
(see Table 2.3 for a summary of causes and effects)
Abnormalities of plasma sodium concentration: Plasma sodium abnormalities are usually discovered incidentally on regular measurement of electrolytes.
Hyponatraemia: A low plasma sodium level may be real or spurious. Spurious results commonly arise when blood is taken from an arm receiving an intravenous infusion; less commonly, false laboratory results can occur if there is lipaemia resulting from parenteral nutrition. If in doubt, the test should be repeated with appropriate precautions.
There are three possibilities:
• Water deficit with a larger sodium deficit (clinical signs—dry mouth, poor skin turgor, poor urine output, high urine osmolality): sodium insufficiency is usually due to diuretic therapy, vomiting, diarrhoea or other excessive losses of body fluids with inadequate replacement. Treatment involves rehydration with appropriate sodium-containing intravenous fluids
• Sodium normal with a larger water excess (clinical signs—weight gain, ankle swelling, raised jugular venous pressure): this usually results from organ dysfunction. Cardiac failure is the most common cause, followed by renal, liver and respiratory failure. Overhydration is compounded by excessive intravenous fluid administration. Management is based primarily on treating the organ failure, e.g. diuretics for cardiac failure
• Water excess: this is uncommon and is usually due to inappropriate antidiuretic hormone (ADH) secretion. This is rare on a surgical ward except for TUR syndrome in which excess fluid is absorbed during transurethral resection of the prostate. It can also occur following head injury or neurosurgery, or may occur in pneumonia, empyema, lung abscess or oat-cell carcinoma of the lung. Excess ADH increases water reabsorption by the renal tubules independently of sodium. The result is water overload and dilutional hyponatraemia. Inappropriate ADH secretion is the most likely diagnosis if the urine osmolality is found to be high and the plasma osmolality low. Hyponatraemia caused by inappropriate ADH secretion is managed by restricting fluid intake to 1 litre per day
Hypernatraemia: This is uncommon and is often iatrogenic in the surgical patient. The usual cause is either excess administration of sodium via intravenous fluids or inadequate water replacement. Hypernatraemia is more likely to occur after operation because increased aldosterone secretion causes sodium to be conserved by the kidney. Very rarely, hypernatraemia is caused by Conn’s syndrome (primary hyperaldosteronism).
Abnormalities of plasma potassium concentration: Acid–base abnormalities (see below) can have a profound effect on plasma potassium concentration but are likely to correct spontaneously as the acid–base problem is treated.
Hypokalaemia: In the preoperative patient, hypokalaemia usually results from poor dietary intake, diuretic therapy, chronic diarrhoea, losses from a malfunctioning ileostomy or, rarely, excess mucus secretion from a rectal villous adenoma. Rarely, hypokalaemia may be caused by primary hyperaldosteronism (Conn’s syndrome).
Hyperkalaemia: This is less common than hypokalaemia in surgical patients but may require urgent correction. In the preoperative patient, it is most commonly caused by chronic renal failure, high doses of ACE inhibiting drugs or potassium-sparing diuretics. Occasionally, non-steroidal anti-inflammatory drugs cause hyperkalaemia. Postoperative hyperkalaemia is usually iatrogenic, caused by excessive intravenous potassium administration, although it may be associated with acute renal failure or blood transfusion.
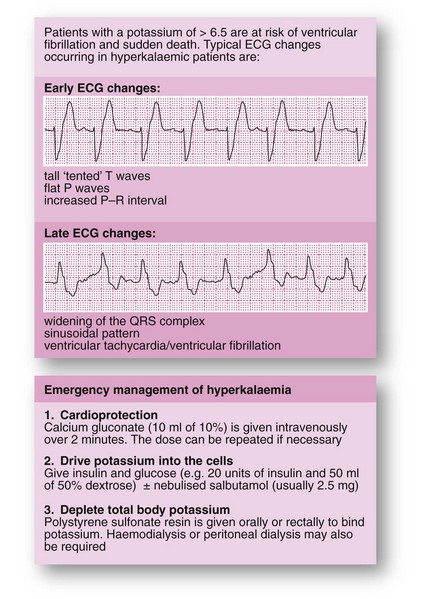
Hyperkalaemia is asymptomatic in its early stages but there is a high risk of sudden death from asystole when plasma potassium concentration reaches about 7.0 mmol/L. The emergency management of hyperkalaemia is shown in Figure 2.3.
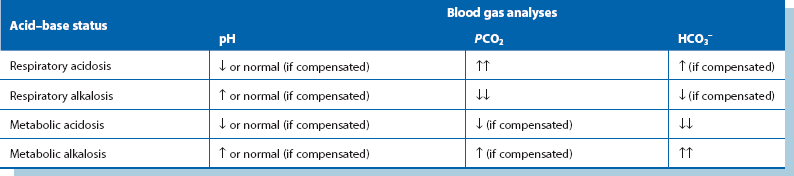
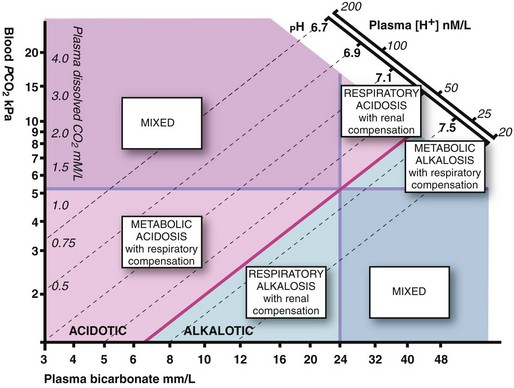
Acid–base disturbances (see Fig. 2.4 and Table 2.4)
Metabolic acidosis: Metabolic acidosis usually follows an episode of severe tissue hypoxia resulting from hypovolaemic shock, myocardial infarction or systemic sepsis. The most common cause is inadequate tissue oxygenation leading to accumulation of lactic acid. In surgical patients, the onset of metabolic acidosis is often an indicator of serious intra-abdominal problems such as an anastomotic leak. Metabolic acidosis is also seen in acute renal failure and uncontrolled diabetic ketoacidosis. Clinically, patients have rapid, deep, sighing ‘Kussmaul’ respirations as they hyperventilate to blow off carbon dioxide (a respiratory compensatory mechanism). Arterial blood gas estimations show the characteristic picture of raised hydrogen ion concentration and low standard bicarbonate with a low arterial PCO2. Plasma potassium concentration is elevated because of a shift from the intracellular compartment to the extracellular compartment. Treatment is directed at the underlying cause.
Respiratory acidosis: This results from carbon dioxide retention in respiratory failure. The usual causes in surgical patients are underlying chronic respiratory disease made worse by postoperative chest complications or prolonged respiratory depression due to sedative, hypnotic or narcotic drugs. Plasma hydrogen ion concentrations and PCO2 are elevated but standard bicarbonate is initially normal. A degree of metabolic compensation may occur as the kidneys excrete excess hydrogen ions and retain bicarbonate. Treatment is directed at the underlying cause and to providing assisted ventilation.
Metabolic alkalosis: Metabolic alkalosis is usually caused by severe and repeated vomiting or prolonged nasogastric aspiration for intestinal obstruction. The latter classically occurs in pyloric stenosis with gross loss of gastric acid. The patient becomes severely dehydrated and depleted of sodium and chloride ions; the condition is thus known as hypochloraemic alkalosis. The kidney attempts to compensate by conserving hydrogen ions but this occurs at the expense of potassium ions lost into the urine. Patients become hypokalaemic not only from excess urinary loss but also because potassium shifts into the cells in response to the alkalosis. Treatment of hypochloraemic hypokalaemic alkalosis involves rehydration with normal saline infusion including potassium supplements; large volumes (up to 10 litres) are often required. Renal excretion of bicarbonate ions eventually corrects the alkalosis.
Nutritional management in the surgical patient
Malnutrition is a wasting condition resulting from deficiencies in energy (i.e. calorie), protein and sometimes vitamins and trace elements. Recognising and treating pre-existing malnutrition and preventing postoperative starvation are important aspects of surgical management that are often neglected. Basic evaluation for malnutrition should be a standard part of assessing surgical patients (see Box 2.4) because untreated malnutrition predisposes to a range of problems that substantially increase morbidity and mortality rates and delay recovery (see Box 2.5).
Recognising the patient at risk
Malnutrition is common in surgical patients and often goes unrecognised. Studies have shown that as many as 50% of surgical inpatients suffer from mild malnutrition and 30% from severe malnutrition. Simple clinical assessment is the best determinant of the state of nutrition, although other indices can also be used (see Box 2.4).
For patients found to be malnourished, studies have shown that simple pretreatment with regular proprietary sip feeds can stave off postoperative muscle weakness, reduce fatigue and markedly lessen complication rates. However, the evidence of clinical benefit is weak for more complex and prolonged endeavours to improve nutrition by the parenteral route (for example, in oesophageal cancer). Even if it is impracticable to improve the preoperative nutritional state, malnutrition still needs to be recognised and attention given in the postoperative period. The duration of starvation should be kept as short as possible and appropriate nutrition provided. This contributes to healing, improves resistance to infection and reduces complications caused by muscle weakness (see Box 2.5).
Effects of starvation
Simple starvation: During simple starvation (i.e. in the absence of illness or trauma), blood glucose concentration is maintained by lowering of insulin secretion and increasing glucagon production. Liver glycogen becomes exhausted within 24 hours but gluconeogenesis in liver and kidneys is enhanced, utilising amino acids from protein breakdown and glycerol from lipolysis as substrates. Much of the glucose thus produced is used by the brain, as most other tissues are able to metabolise fatty acids and ketones derived from adipose tissue. Overall energy demands fall in simple starvation and energy is obtained largely from body fat. Protein is conserved until a late stage.
Trauma, surgery or sepsis: In severe trauma or major surgery, and particularly in sepsis, energy requirements increase by 20–100% of normal. As in simple starvation, lipid becomes a major fuel source; this decreases glucose utilisation, but fatty acids other than glycerol cannot be used for glucose synthesis. Hepatic glucose production increases, but peripheral glucose utilisation is impaired, often leading to hyperglycaemia.
Supplementary nutrition
• That the patient is malnourished or will be deprived of nutrition for at least 5 days
• That the patient is likely to benefit—certain conditions make supplemental nutrition ineffective (e.g. enteral feeding in high output enterocutaneous fistula)
• Whether there is an appropriate route for administration, e.g. suitable gut function
Nutritional support is generally recommended in well-nourished patients who are unable to tolerate oral feeding for 7–10 days, or 5–7 days if already malnourished.
Methods of giving supplementary nutrition: Box 2.6 summarises the range of nutritional regimens and their main surgical indications. The gastrointestinal tract should be used whenever possible because any form of enteral feeding is intrinsically safer than parenteral nutrition and is much cheaper. In addition, the small intestinal mucosa tends to atrophy when not used. Enteral feeding supports the gut-associated immunological shield and prevents microorganisms translocating into the circulation, reducing the chances of blood-borne infection. Contraindications to enteral feeding include intestinal obstruction, high-output intestinal fistula, intractable vomiting or diarrhoea, and severe malabsorption.
Sip feeds: If the patient is able to eat, fluid diets (total or supplementary) can be given orally. Proprietary sip feeds containing easily absorbed calories, protein, minerals and vitamins are available in a variety of formulations and flavours and are well tolerated.
Tube feeds: Certain patients are unsuitable for sip feeding but can be fed by one of several tube feeding routes. Indications include patients with swallowing difficulties (including overspill and lack of cooperation), anorexia, lack of palatability of liquid feeds, the need for a higher volume of feed than the patient can comfortably manage and anticipated substantial delay in resuming oral feeding after operation.
Total parenteral nutrition (TPN)
Indications for TPN: Parenteral nutrition should be reserved for patients who are already malnourished (or are likely to become malnourished), in whom the GI tract is not functional or is inaccessible and is likely to remain so for a substantial period of days or weeks. Note that in major sepsis, the metabolic changes described earlier mean that TPN brings little benefit.
Methods of giving TPN: Parenteral nutrition is usually delivered into the superior vena cava via the internal jugular or subclavian vein. This is so that high venous flow rapidly dilutes the hyperosmolar solution, minimising thrombosis risk. If long periods of nutritional support are anticipated, a designated tunnelled line is usually employed, with the skin access point remote from the venous entry point to minimise risk of line infection.
Parenteral nutrition is costly in materials and staff time and is prone to complications; it should be discontinued as soon as nutrition can be supplied by an enteral route. Patients on TPN need close and regular monitoring for a range of problems including line problems, local and systemic infection, fluid balance and deficiencies of electrolytes (see Box 2.7). Complications of TPN are detailed in Box 2.8.