Chapter 68 Management of Unruptured Intracranial Aneurysms
Subarachnoid hemorrhage, when caused by the rupture of an intracranial aneurysm, has a mortality rate near 50% at 30 days, and approximately half of the survivors sustain irreversible brain damage.1 To avoid such a catastrophic event, it is important to identify and treat patients who harbor aneurysms that carry a significant risk of rupture. With the increased use of brain imaging in recent medical practice, including noninvasive tests like CT and MR angiography, a growing number of unruptured and usually asymptomatic intracranial aneurysms are being diagnosed. The decision of whether such lesions should be treated, and if so, whether by surgical or endovascular therapy, has been the subject of great controversy. This chapter evaluates the data available for making management decisions for unruptured aneurysms.
Natural History
Intracranial aneurysms are common, and may generally be classified as saccular (hemodynamic or “berry”) and fusiform (dissecting, infectious, arteriosclerotic, or traumatic) types. The saccular type is by far the most common, and is the focus of this chapter.2–7 Autopsy studies have shown that the overall prevalence of intracranial aneurysms in the general population ranges from 0.2% to 9.9%.6–10 The population-based incidence of aneurysmal subarachnoid hemorrhage varies from 6 to 21.6 cases per 100,000 persons per year.11–16
The decision of whether to treat an unruptured aneurysm is based on the likelihood of its rupture during the patient’s lifetime. The natural history study with the longest follow-up comes from Helsinki, Finland, where Juvela and colleagues reviewed a series of 142 patients with unruptured aneurysms followed without treatment from 1956 to 1978 (median follow-up of 19.7 years).17 An advantage of the study is that it avoids treatment selection bias, because no unruptured aneurysms were treated in Helsinki before 1979. During 2575 person-years, 33 of the 142 patients (23%) had subarachnoid hemorrhage, resulting in an annual rupture rate of 1.3%. The cumulative rates of rupture were 10.5% at 10 years, 23% at 20 years, and 30.3% at 30 years.17 Twenty-nine of 33 aneurysms that eventually ruptured were smaller than 10 mm in diameter at the time of the original diagnosis (18 were ≤6 mm).8,17,18 Those aneurysms that ruptured were more likely to have increased in size (≥1 mm) compared to those that did not rupture. Notably, the majority of the patients (131 of 142) had previous subarachnoid hemorrhage from another aneurysm, thus comprising a group of patients which may have had a higher rupture rate compared to those with no prior history of subarachnoid hemorrhage. Nevertheless, the study by Juvela and colleagues provided substantial long-term data on the natural history of unruptured aneurysms, and the annual rupture rate of 1.3% was similar to previously published reports (1% to 2.3%).19–21
Between 1976 and 1997, Tsutsumi and colleagues observed 62 patients who had noncalcified unruptured intradural aneurysms and no prior history of subarachnoid hemorrhage.22 For small aneurysms (<10 mm), the 5- and 10-year rupture risks were 4.5% and 13.9%, respectively, an annual rupture rate similar to that reported by Juvela and colleagues.8,17,18 For large aneurysms (>10 mm), the 5- and 10-year rupture risks were several-fold higher, 33.5% and 55.9%, respectively.
From 2003 to 2006, Ishibashi and colleagues elected to observe unruptured intracranial aneurysms at their institution. Of a total of 419 patients with 529 aneurysms, 19 aneurysms ruptured during the observation period, resulting in a 1.4% annual rupture rate.23 Eight of the 19 aneurysms that ruptured were under 5 mm in size.
In 1998, a large retrospective international study, Phase I of the International Study of Unruptured Intracranial Aneurysms (ISUIA), evaluated the natural history of 1937 unruptured aneurysms in 1449 patients.24 Patients harboring at least one unruptured aneurysm were divided into two groups: those with no history of subarachnoid hemorrhage (group 1) and those with a history of subarachnoid hemorrhage from another aneurysm (group 2). Patients in these two groups were not selected for surgical repair for various and often unknown reasons. The mean duration of follow-up was 8.3 years. As shown in Table 68-1, for aneurysms smaller than 10 mm, the annual rupture rate was 0.05% in group 1 (727 patients) and 0.5% in group 2 (722 patients). For aneurysms 10 to 24 mm in size, the annual rupture rate was approximately 1% in both groups. For giant aneurysms (25 mm or larger), the rupture rate was 6% in the first year, and declined thereafter. Aneurysms of the posterior circulation (the vertebrobasilar system) were significantly more likely to rupture than aneurysms of the anterior circulation, with basilar apex aneurysms carrying a relative risk of 13.8 compared to other locations. The study concluded that small unruptured aneurysms, particularly those in the anterior circulation with no history of prior aneurysmal subarachnoid hemorrhage, should be left untreated, especially when the morbidity and mortality rates of surgical repair were considered. This study generated significant controversy because its results were substantially different from previously published reports of a 1% to 2% annual rupture risk.8,17–23
In 2003, prospective data from the ISUIA was published as Phase II.25 Aneurysms in this study were categorized by size into four groups: under 7 mm, 7 to 12 mm, 13 to 24 mm, and 25 mm or larger. Noncavernous anterior circulation aneurysms had 5-year cumulative rupture rates of 0% to 1.5%, 2.6%, 14.5%, and 40%, by size category, respectively. Posterior circulation aneurysms (including [Pcom] aneurysms, which are usually considered part of the anterior circulation) had cumulative 5-year rupture rates of 2.5%, 14%, 18.4%, and 50%. Although the two ISUIA studies stratified aneurysm size differently, ISUIA II reported rupture rates that were generally higher than ISUIA I (Table 68-1). For aneurysms in the 7-10-mm size range, ISUIA II suggested an annual rupture risk (0.5%–2.9%) that was several-fold greater than the rate of 0.05% to 0.5% purported by ISUIA I, which grouped together all aneurysms under 10 mm in size for the analysis.
1. Selection bias: To be included in either study, a patient first had to be recommended for conservative management by a neurosurgeon. It is quite possible that those aneurysms included in the studies were judged to be very low risk, based on their benign morphology or location (i.e., intracavernous). In ISUIA I, internal carotid artery (ICA) aneurysms represented 42% of the total in group 1 and 27% in group 2; of these, cavernous-segment ICA aneurysms represented 16.9% in group 1 and 9.5% in group 2. In addition, a significant number of patients (32.7% in group 1 and 61.2% in group 2) had very small aneurysms (2–5 mm in size) which could also carry a lower rupture risk. Thus, the ISUIA I study population included large numbers of patients who harbored intracranial aneurysms considered to have little risk of rupture, at least during the study duration.
2. Crossover: In both ISUIA phases I and II, some patients who were initially chosen for observation were later advised to have treatment. In phase II, of the 1692 patients in the observation cohort, 534 patients were switched to therapeutic intervention. It is possible that the management strategy changed because of new symptoms or increased aneurysm size, both of which are risk factors for rupture. If those patients were left untreated, the observation cohort rupture rate may have been much higher.
3. Incomplete follow-up: Mean follow-up for the retrospective phase I study was 8.3 years, and for the prospective phase II study was 4.1 years. Of the 193 patients (11%) who died of causes other than subarachnoid hemorrhage, 52 patients died of intracranial hemorrhage. These patients were excluded from the analysis. It is unknown how these hemorrhages were determined to result from causes other than aneurysm rupture.
Given the disparity between the ISUIA data and other published reports, a population analysis based on prevalence of unruptured aneurysms and incidence of subarachnoid hemorrhage would be helpful. To estimate the prevalence of aneurysms, Winn and colleagues reviewed 3684 cerebral angiography studies performed at the University of Virginia between April 1969 and January 1980.10 During the pre-CT era, the cerebral angiogram was a commonly performed neuroimaging test for a variety of indications. The authors found 24 asymptomatic unruptured aneurysms in 3684 patients, yielding a prevalence rate of 0.65%. Nearly 80% of the aneurysms were smaller than 10 mm. Because only 53% of the patients underwent a complete angiography study, the authors estimated that the true prevalence ranged from 0.65% to 1.3%. That would mean that in a population of 100,000, between 650 and 1300 people would have an unruptured intracranial aneurysm. Given an annual incidence of subarachnoid hemorrhage of 11 per 100,000 people,26 a person with an unruptured aneurysm can be estimated to have a 0.85% to 1.7% yearly risk of rupture.
Risk Factors for Rupture
Aneurysm Size
It is now generally accepted that there is a strong correlation between aneurysm size and risk of rupture. ISUIA I used a historical classification scheme to separate aneurysms into small (<10 mm), large (10–24 mm), and giant (≥25 mm) categories (ISUIA 1998). ISUIA II separated nongiant aneurysms into three groups: <7 mm, 7 to 12 mm, and 13 to 24 mm (ISUIA 2003). Both studies demonstrated increased rupture rates with larger sizes. The exact size beyond which an aneurysm becomes “dangerous,” however, is unclear. In both clinical and autopsy series, aneurysms that present with hemorrhage are most commonly between 7 and 10 mm in size, and many are smaller than 7 mm.27–29 Aneurysms smaller than 7 mm in size account for 55% of the aneurysms that ruptured in Juvela’s series, 58% of the aneurysms that ruptured in the study by Ishibashi and colleagues, and 22% of the aneurysms that ruptured in ISUIA II.18,23,25 Some aneurysms may enlarge prior to rupture, allowing for the premise that unruptured aneurysms can be followed until a change in size is noted.30 Unfortunately, enlargement may more often occur near the time when the aneurysm ruptures, a quite unpredictable (and potentially fatal) moment.
Aneurysm Location
Both phase I and phase II ISUIA studies showed that posterior circulation aneurysms, especially basilar apex aneurysms, have a higher relative rupture rate compared to those at other sites.24,25 A similar increased risk was also noted for lesions arising from the Pcom artery, a site traditionally considered to be within the anterior circulation. The distribution of aneurysms described in these series differs markedly from that encountered in ruptured aneurysm series. In the ISUIA studies, cavernous and small parasellar ICA aneurysms are highly represented, while in series dealing with ruptured aneurysms, anterior communicating (Acom) and Pcom aneurysm sites predominate.27 In our personal experience, proximal (paraclinoid) ICA aneurysms are much more common than previously described, and may in fact be the most common aneurysm site, particularly in females. In series of ruptured aneurysms, however, lesions at this site are far less frequent, perhaps indicating that their relationship to and reinforcement by the parasellar dura provides some protection against subarachnoid hemorrhage.
Aneurysm Shape
Several reports suggest that aneurysms with irregular morphology, particularly those that are multilobed with daughter domes, have a significantly higher hemorrhage risk compared to smooth-walled, more spherical lesions.20,31,32 In recent years, as aneurysm shapes and origins have become more important in determining “coilability,” several quantifiable parameters have been evaluated for their contribution to rupture risk, including aspect ratio, ellipticity index, nonsphericity index, and undulation index. Of these parameters, aspect ratio (aneurysm height/neck width) has correlated best with rupture risk.27,33 Several studies have shown that ruptured aneurysms have higher aspect ratios than unruptured aneurysms, but there is no consensus on a threshold value for increased risk.34–38
Symptoms Other than Rupture
Unruptured aneurysms may present with cranial neuropathy (particularly oculomotor nerve or optic nerve/chiasmal deficits), ischemia, or other symptoms related to mass effect. New nonhemorrhagic symptoms suggest an acute change in the aneurysm (i.e., expansion), indicating a higher risk of imminent rupture compared to asymptomatic lesions. Data supporting this assertion is scant and retrospective, but in general, symptomatic aneurysms are treated with relative urgency, especially small Pcom aneurysms that cause oculomotor deficits.27
Significant Family History
In families that have multiple members with intracranial aneurysms, an aneurysm’s risk of rupture is higher, and rupture may occur at an earlier age, compared to aneurysms that arise in individuals with no known family history.39 Familial intracranial aneurysms are discussed later in this chapter.
Prior History of Aneurysmal Subarachnoid Hemorrhage
Phase I of ISUIA showed that a small unruptured aneurysm had a tenfold increase in rupture risk if it occurred in a patient with a history of subarachnoid hemorrhage from a different aneurysm, rather than in a patient with no history of subarachnoid hemorrhage.24
Risk Factors for Aneurysm Formation
Age and Gender
Female gender seems to be a risk factor affecting both aneurysm formation and growth, with aneurysms 1.6 times more likely to occur in women than in men.17,40 A series of 1230 autopsies showed two peaks in the prevalence of aneurysms in women, ages 40 to 49 and ages 60 to 69, which correlate with a peak incidence of subarachnoid hemorrhage between ages 40 and 60.15 Interestingly, in this series, the prevalence of aneurysms in men was unchanged across the range of age groups.
Smoking
Cigarette smoking may hasten the growth of a preexisting aneurysm, and may contribute to an increased rupture rate, with hemorrhage occurring at smaller sizes.17 In smokers, there is an increased ratio of elastase to alpha1-antitrypsin in the walls of cerebral arteries, which may contribute to aneurysm formation or rupture.18,41
Genetic Conditions
In families in which two people have known intracranial aneurysms, first-degree relatives have a 9% to 11% chance of having an aneurysm in adulthood. Autosomal dominant polycystic kidney disease (ADPKD) is associated with a 15% prevalence of intracranial aneurysms.40 Genetic conditions with at least some evidence of having an increased incidence of intracranial aneurysms are outlined in Table 68-2.
Table 68-2 Genetic Conditions Associated with Increased Incidence of Aneurysm Formation
| Autosomal dominant polycystic kidney disease |
| Type IV Ehlers-Danlos syndrome |
| Hereditary hemorrhagic telangiectasia |
| Neurofibromatosis type 1 |
| Alpha1-antitrypsin deficiency |
| Klinefelter’s syndrome |
| Tuberous sclerosis |
| Noonan’s syndrome |
| Alpha-1,4-glucosidase deficiency |
Aneurysm Detection
Transfemoral Cerebral Angiography
Digital subtraction angiography (DSA), with selective injections of dye into the intracranial arteries, has long been the gold standard for imaging intracranial aneurysms. Since cerebral angiography is invasive (procedural stroke risk ranges from 0.07% to 0.5%) and requires a 2 to 6 hour hospital stay after the procedure, it is generally not advocated as a screening procedure.42,43 A more recent innovation in angiography is three-dimensional reconstruction (3D-DSA). This technology permits rotation of the virtual image in any direction, allowing neurosurgeons and endovascular practitioners to evaluate specific anatomic characteristics of the aneurysm, including the neck width and the relationship of the aneurysm to the parent artery and branch vessels. Both conventional and 3D-DSA reveal only the patent lumen of an aneurysm; heavy calcification or intraluminal thrombosis are not visualized, and CT and MRI provide useful complementary information.44
Magnetic Resonance Angiography
The quality and the spatial resolution of noninvasive imaging have significantly improved in recent years, approaching that of DSA. Magnetic resonance angiography (MRA) is useful for screening and follow-up, and in some cases, it is sufficient for treatment planning.45 Aneurysms with diameters as small as 2 mm and vessels as small as 1 mm can be detected.46 Aneurysms 6 mm or more in diameter have been detected with 100% sensitivity. The sensitivity decreased to 87.5%, 68.2%, 60%, and 55.6% for aneurysms with a diameter of 5, 4, 3, and 2 mm, respectively. Three-dimensional reconstructions are valuable for anatomic evaluation; 3D contrast-enhanced MRA may be superior to 3D time-of-flight MRA in the detection of aneurysms.44,47,48 Imaging of an aneurysm’s morphology and relationship to branch vessels may be improved with 3-Tesla and 7-Tesla time-of-flight MRAs.49,50 MRA does not expose a patient to radiation risks, and thus its utility is quite attractive in those patients with anticipated multiple studies during their follow-up, as long as the area of interest is seen in sufficient detail by this technology.
Computed Tomography Angiography
Multislice CT scanners allow simultaneous acquisition of as many as 64 slices by using multirow detector systems.49 The concurrent acquisition of multiple slices results in a dramatic reduction of scan time. The major advantages of multislice CT are a longer scanning range, shorter scanning times, and a higher two-axis resolution.51 The high scan speed of multislice helical CT permits scanning with a smaller slice thickness than is possible with conventional helical CT.52 As a result, volumetric data with superior resolution in the z-axis can be obtained. Acquired data can be reformatted to provide 3D angiographic images, called CT angiography (CTA). Many physicians routinely use CTA in clinical practice. A recent review reported that the sensitivity of the CTA ranged from 53% for 2-mm aneurysms (Fig. 68-1) to 95% for 7-mm aneurysms. The overall specificity was 98.9%, but there was interstudy heterogeneity.53 A meta-analysis comparing CTA with DSA in the diagnosis of cerebral aneurysms revealed that CTA had an overall sensitivity of 93.3% and a specificity of 87.8%.54
The advantages of CTA over DSA are the following:
1. Data can be obtained more quickly and less expensively.
2. CTA provides additional anatomic information (information on surrounding bony structures and the presence of calcium or atheromas).
3. CTA can be used for the rapid planning of craniotomies for clip obliteration, and the preliminary determination of whether aneurysms are suitable for coil embolization.
4. CTA subjects patients to virtually no stroke risk and negligible discomfort.
The disadvantages of CTA are as follows:
1. Less sensitive and specific than the standard method, DSA, for the detection of cerebral aneurysms.
2. Difficulty detecting aneurysms at the skull base, such as those of clinoidal or cavernous segments of the ICA, due to the aneurysms’ proximity to bone.
3. Sensitive to bolus timing, and opacified veins may be incorporated into the reconstructed image,44 making interpretation of the arterial tree anatomy difficult.
4. May be inadequate in patients with left ventricular failure due to suboptimal opacification of the intracranial vasculature.55
5. Like standard CT, it is subject to motion artifact.
6. Provides no information regarding flow in all phases of the bolus transit in the cerebral vessels.
7. Has not been validated to detect vasospasm or other flow-limiting lesions with the same reliability as DSA.
Indications for Treatment
Aneurysmal subarachnoid hemorrhage carries a high fatality rate. In the retrospective part of ISUIA, 66% of the patients whose aneurysms ruptured died (83% in group 1 and 55% in group 2).24 In the natural history study by Juvela and colleagues, 52% of patients whose aneurysms ruptured died.8 Tsutsumi and colleagues reported a mortality rate of 86% after subarachnoid hemorrhage.22 Thus, there are clear reasons to obliterate an unruptured aneurysm that has a significant risk of rupture.
Before deciding to treat an unruptured aneurysm, the cumulative risk of rupture over the patient’s expected lifetime needs to be estimated and weighed against the risks of treatment.56–60 Juvela advocated that all young and middle-aged patients with unruptured aneurysms should be surgically treated, regardless of the size of the aneurysm.17 White and Wardlaw suggest that in patients under age 50 with no prior history of subarachnoid hemorrhage, all posterior circulation aneurysms and those anterior circulation aneurysms 7 mm or larger should be treated.61 In patients over age 50 with no prior history of subarachnoid hemorrhage, they favor treatment for posterior circulation aneurysms larger than 7 mm and anterior circulation aneurysms larger than 12 mm.
In 2000, after the publication of phase I of ISUIA, the Stroke Council of the American Heart Association issued the following recommendations for the management of patients with unruptured intracranial aneurysms.62
1. The treatment of small incidental intracavernous ICA aneurysms is not generally indicated. For large symptomatic intracavernous aneurysms, treatment decisions should be individualized on the basis of patient age, severity, and progression of symptoms, and treatment alternatives. The higher risk of treatment and shorter life expectancy in older individuals must be considered in all patients and favors observation in older patients with asymptomatic aneurysms.
2. Symptomatic intradural aneurysms of all sizes should be considered for treatment, with relative urgency for the treatment of acutely symptomatic aneurysms. Symptomatic large or giant aneurysms carry higher surgical risks that require a careful analysis of individualized patient and aneurysmal risks and surgeon and center expertise.
3. Coexisting or remaining aneurysms of all sizes in patients with SAH due to another treated aneurysm carry a higher risk for future hemorrhage than do similar sized aneurysms without a prior SAH history and warrant consideration for treatment. Aneurysms located at the basilar apex carry a relatively high risk of rupture. Treatment decisions must take into account the patient’s age, existing medical and neurologic condition, and relative risks of repair. If a decision is made for observation, re-evaluation on a periodic basis with CT/MRA or selective contrast angiography should be considered, with changes in aneurysmal size sought, although careful attention to technical factors will be required to optimize the reliability of these measures.
4. In consideration of the apparent low risk of hemorrhage from incidental small (<10 mm) aneurysms in patients without previous SAH, treatment rather than observation cannot be generally advocated. However, special consideration for treatment should be given to young patients in this group. Likewise, small aneurysms approaching the 10-mm diameter size, those with daughter sac formation and other unique hemodynamic features, and patients with a positive family history for aneurysms or aneurysmal SAH deserve special consideration for treatment. In those managed conservatively, periodic follow-up imaging evaluation should be considered and is necessary if a specific symptom should arise. If changes in aneurysm size or configuration are observed, this should lead to special consideration for treatment.
5. Asymptomatic aneurysms of ≥10 mm in diameter warrant strong consideration for treatment, taking into account patient age, existing medical and neurologic conditions, and relative risks for treatment.
After the above recommendations were issued, prospective results from phase II of ISUIA were published, which showed that 7- to 10-mm aneurysms had a higher rupture rate than was suggested by the phase I study. In light of those results, most neurosurgeons and endovascular practitioners give strong consideration to treating aneurysms ≥7 mm in diameter in patients who are not elderly. As stated in recommendation (4) above, treatment may be favored for some smaller aneurysms, with worrisome anatomic features, young patient age, or significant family history (Table 68-3).
Table 68-3 Factors That Influence Management of Unruptured Intracranial Aneurysm
| Favoring Treatment | Favoring Observation |
|---|---|
| Patient Factors | |
| Age <70 | Age >70 |
| Prior SAH from another aneurysm | Significant medical comorbidities |
| Family history of intracranial aneurysms | Patient preference |
| Symptoms caused by aneurysm | |
| Size | |
| Size approaching ≥7 mm | Size much <7 mm |
| Location | |
| Within the subarachnoid space | Intracavernous or clinoidal segment |
| Posterior circulation aneurysm | Small superior hypophyseal aneurysm |
| Shape | |
| Irregular with bleb | Regular |
| Daughter dome | Unilobed |
| High aspect ratio | Low aspect ratio |
SAH, subarachnoid hemorrhage.
Treatment Options
Observation
If intervention is not recommended for an unruptured intracranial aneurysm, periodic follow-up imaging with either CTA or MRA should be considered. In patients of advanced age or a limited life expectancy, no further imaging may be necessary for an asymptomatic aneurysm. A subsequent change in aneurysm size or morphology warrants consideration for treatment. In a retrospective series of 191 unruptured aneurysms with median follow-up of 47 months, 10% of aneurysms showed enlargement on serial MRAs.30 In the time course of the study, aneurysms smaller than 8 mm had a 6.9% risk of growth, while those 8 mm or larger had a 44% chance of growth. The authors report that at least one aneurysm ruptured during the study, but they state that their follow-up information regarding aneurysm rupture was incomplete.
No noninvasive measures have been shown to prevent aneurysm rupture. However, since smoking has been associated with an increased rate of aneurysm growth and rupture, cessation is generally advocated. Among smokers, the number of cigarettes smoked daily seems to correlate with aneurysm growth more than the lifetime history of tobacco use. Patients who had quit smoking had no increased risk of aneurysm growth.17,41
Questions commonly arise about whether patients with unruptured aneurysms should be anticoagulated for other disorders (i.e., atrial fibrillation, pulmonary embolus), or whether they should take aspirin or NSAIDs. There is no evidence that anticoagulation increases the chance of subarachnoid hemorrhage in a patient with an unruptured aneurysm. However, should an aneurysm rupture occur, patients on anticoagulation have a twofold increase in mortality.40 On the other hand, aspirin or NSAID use preceding aneurysmal subarachnoid hemorrhage does not significantly affect outcome.
Surgical Treatment
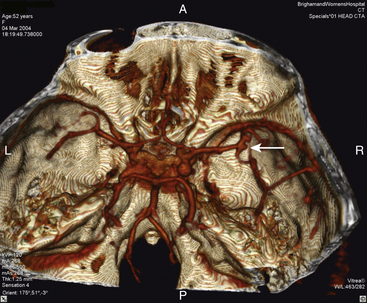
Since the operating microscope revolutionized neurosurgery over 40 years ago, the microsurgical clipping of aneurysms has been the mainstay of treatment, and is considered the time-tested way to obliterate aneurysms63–65 (Fig. 68-2A and B). Over the years, microsurgical techniques have been developed and refined, and surgical instrumentation has been innovated and modified. Intraoperative angiography has been used to verify occlusion of the aneurysm, and preservation of the parent and branch arteries. Recently, fluorescence videoangiography has provided real-time intraoperative visualization of blood flow through small arteries that would not be visible on standard angiography.
The various innovations have been designed to reduce surgical risks. The decision to proceed with surgery requires an adequate assessment of these risks. High-volume centers and specialized neurosurgeons with cerebrovascular expertise offer better outcomes and fewer complications.59
Risks of Surgery
A meta-analysis of surgical treatment for unruptured aneurysms identified 61 studies published between 1966 and 1996, with a total of 2460 patients (57% female; mean age 50 years) and at least 2568 unruptured aneurysms.56 Mean follow-up was 24 weeks, 27% of the aneurysms were over 25 mm in size, and 30% were located in the posterior circulation (vertebrobasilar system). Overall postoperative mortality and morbidity were 2.6% and 10.9%, respectively. Mortality and morbidity for nongiant aneurysms and anterior circulation aneurysms were significantly lower in more recent years; surgery for small anterior circulation aneurysms had a 0.8% mortality and 1.9% morbidity, whereas surgery for large posterior circulation aneurysms had a 9.6% mortality and 37.9% morbidity.
In a cohort of 1917 patients who underwent aneurysm surgery, ISUIA phase II reported surgical mortality to be 0.6% in patients who had a history of subarachnoid hemorrhage from another aneurysm (group 2), and 2.7% in patients with no history of subarachnoid hemorrhage (group 1).25 Neurologic outcome was graded at 1 year using the modified Rankin scale, and cognitive status was included in the assessment of morbidity, and the rates of morbidity were 9.8% and 9.9% for groups 1 and 2, respectively.
A review of the Nationwide Inpatient Sample hospital discharge database from 1996 to 2000 showed that surgical outcomes were better at high-volume hospitals (treating 20 or more cases per year) than at low-volume hospitals (treating less than four cases per year).59 In recent years, specialized vascular neurosurgeons have reported low complication rates in appropriately selected patients. There are also various other predictors of surgical outcome besides hospital case volume and the expertise of surgeons, including patient age, aneurysm size, and aneurysm location.
Factors Associated with Surgical Outcome
Age
In ISUIA phase II, patients age 50 or older had an increased rate of adverse outcomes, with a relative risk of 2.4.25 Takahashi found that patients age 80 or older had the worst surgical outcomes.66 Khanna and colleagues report a that a 70-year-old patient has a sixfold higher risk of a poor outcome than a 30-year-old patient, keeping aneurysm size and location constant.67 This increased complication incidence could be due to an increased frequency of atherosclerotic and/or calcified aneurysm necks in the elderly, as well as medical comorbidities in the older age group.
Aneurysm Size
Solomon and colleagues found that aneurysm size had an important influence on surgical outcome.68 The combined morbidity and mortality of unruptured aneurysms was 0% for aneurysms 10 mm or smaller, 6% for aneurysms between 10 and 25 mm, and 20% for aneurysms greater than 25 mm. Drake reported 15% morbidity and mortality in nongiant posterior circulation aneurysms, compared with 39% for giant posterior circulation aneurysms, although ruptured aneurysms were also included in his series.69 The ISUIA Phase II study showed a 2.6 relative risk of poor surgical outcome for an aneurysm greater than 12 mm in diameter.25
Aneurysm Location
In the ISUIA studies, posterior circulation aneurysms were associated with worse surgical outcomes.25 Solomon and colleagues observed 50% morbidity and mortality after surgery for unruptured giant basilar aneurysms, compared with 13% for anterior circulation giant aneurysms.68 Drake reported a 14.3% morbidity for unruptured asymptomatic posterior circulation aneurysms, compared with a 0% morbidity in the anterior circulation.69
Some anterior circulation aneurysms may have a higher morbidity and mortality compared to others, due to the technical challenges of surgical exposure and treatment. These include Acom aneurysms that project posterosuperiorly70 and ICA aneurysms of the clinoidal and cavernous segments.71,72
Endovascular Treatment
In 1991, Guglielmi and colleagues introduced the detachable coil for treating intracranial aneurysms.73,74 The coiling procedure involves passing a catheter through the arterial tree to the aneurysm, and then deploying platinum wire coils into the aneurysm to pack and occlude it (Fig. 68-2C to F). The Guglielmi detachable coil (GDC) system (Boston Scientific/Target) received U.S. Food and Drug Administration approval in 1995. Aneurysms considered unsuitable for surgery were the initial candidates for GDC coil embolization. In 1999, Guglielmi’s group reported the coiling of 120 unruptured aneurysms, and in subsequent years, criteria for endovascular therapy have broadened to make it the first line of treatment at many centers.75
Experience with Ruptured Aneurysms
In 2002, the International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group published the first prospective randomized trial comparing endovascular treatment with surgery for 2143 patients with ruptured intracranial aneurysms.76 Inclusion in the trial was based on a pretreatment estimation that the ruptured aneurysm could be treated successfully by either coiling or clipping. At the 1-year follow-up, the combined rates of death and disability were 23.7% after endovascular therapy, and 30.6% after surgical clipping.
For ruptured aneurysms, ISAT achieved prominence as the only randomized trial comparing the two modalities of treatment. The study may have implications for the types of patients that were selected for randomization, and for neurosurgeons and endovascular practitioners with similar outcomes as the participating providers. Unfortunately, no information is provided on the level of expertise of the neurosurgeons who participated in the study, almost all of whom practiced in Europe. Furthermore, there is limited information about what constituted a randomizable aneurysm for the trial. The spectrum of aneurysms that was considered coilable at the time of the trial was much narrower than it is now. While newer techniques may allow the endovascular treatment of previously uncoilable aneurysms, these techniques would be associated with different morbidity and mortality rates than those represented in ISAT.
Certain aneurysm morphologies and locations favor surgical clipping over endovascular treatment. Younger patients with subarachnoid hemorrhage, and patients with good clinical status, could potentially benefit from the long-term aneurysm occlusion that clipping provides. Recognizing the conclusions and the limitations of ISAT, the Stroke Council of the American Heart Association issued the following recommendation in the 2009 Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage77:
Risks of Endovascular Treatment
In 2008, initial results were published for the first prospective multicenter study of the endovascular treatment of unruptured aneurysms (Analysis of Treatment by Endovascular approach of Non-ruptured Aneurysms [ATENA]).78 During the 17-month study period, a total of 649 patients in Canada and France underwent coil embolization of 739 unruptured aneurysms, all less than 15 mm in size. Anterior circulation aneurysms accounted for 92% of the total, while 8% were in the posterior circulation. Balloon remodeling was employed in 37.3%, and stenting in 7.8%. The report describes the immediate clinical outcome of patients in the study. Endovascular treatment was attempted but aborted in 4.3% of aneurysms, because of anatomic and technical problems. Complications were encountered in 15.4% of patients, including infarction (7.1% risk per procedure), intraprocedural aneurysm rupture (2.6% risk per procedure), device-related problems such as coil stretching and inappropriate coil detachment (2.9% risk per procedure), and nonspecific complications (2.3% risk per procedure). With intraprocedural rupture, there was 50% risk of death or disability. The 30-day mortality of the study population was 1.4%. The rate of infarction was higher in middle cerebral artery (MCA) (9.6%) and anterior communicating artery (ACA)/Acom (8.8%) aneurysms, and lower in ICA (4.6%) and posterior circulation lesions (3.3%). Intraprocedural rupture occurred in 4.1% of MCA aneurysms, 2.2% of ACA/Acom aneurysms, 1.9% of ICA aneurysms, and 0.0% of posterior circulation aneurysms.
The complication rates in the ATENA study are similar to those in a recent multicenter retrospective study.79 Gallas and colleagues reported 321 unruptured aneurysms treated by coiling, with a treatment-related morbidity and mortality of 14.4% and 1.7%, respectively. Infarctions occurred in 9% of patients, and intraprocedural aneurysm rupture in 2.6%.
A few studies have used diffusion-weighted MRI to show that the true rate of ischemic complications after endovascular treatment may be higher than is clinically apparent. In a prospective evaluation of 66 patients who underwent coiling of their unruptured aneurysms, Soeda and colleagues found new hyperintense diffusion-weighted imaging (DWI) lesions in 61% of patients.80 Grunwald and colleagues found a 42% incidence of new DWI lesions after coiling in their 68 patients.81 Both series reported a much lower rate of permanent neurologic morbidity, similar to other studies of endovascular treatment. For comparison, there is very sparse prospective data from the microsurgical literature. In a prospective surgical series with preoperative and postoperative MRIs, Krayenbuhl and colleagues report a 9.8% occurrence of new DWI lesions after the clipping of 51 aneurysms, both unruptured and ruptured, with a 2% risk of symptomatic infarction.82
Efficacy of Coiling
The immediate and long-term efficacy of the endovascular treatment of unruptured aneurysms is an issue under investigation. Excluding those patients in whom coiling attempts failed, Gallas and colleagues report complete occlusion of unruptured aneurysms in 70% of 302 patients on initial post-treatment angiogram.79 In aneurysms that were initially completely occluded, a 16.5% rate of recurrence or recanalization was observed at final follow-up, which ranged from 3 months to 2 years. Choi and colleagues report that 26.4% of completely occluded aneurysms recanalized after an average follow-up of 26.4 months, and noted that aneurysms with wide necks (4 mm or larger) had a higher rate of recanalization.83 Secondary endovascular and surgical treatments of recanalized aneurysms have been described, but the success rate of re-treatment is unknown.79,83,84 Clearly, longer follow-up is necessary to determine the durability of endovascular treatment. Proposed in 2008, the TEAM trial (Trial on Endovascular Aneurysm Management) plans to be a 14-year, large, randomized controlled trial comparing endovascular treatment to observation for unruptured aneurysms.85 The ultimate results of that study may show whether, and under what conditions, coiling improves long-term outcome compared with the natural history of the disease.
Since the development of balloon-assisted and stent-assisted coiling, various technologies have emerged, designed to increase the efficacy of endovascular procedures. Self-expanding stents permit the coiling of wide-necked aneurysms that would otherwise be unsuitable for endovascular treatment. Recent studies show an increased morbidity of stent-assisted coiling. Use of the Neuroform stent (Boston Scientific) has been associated with a 5.8% risk of delayed in-stent thrombosis, and a 4.6% rate of delayed thromboembolic events, despite the combined use of antiplatelet agents and heparinization.86,87 As a multicenter study of the newer Enterprise stent (Cordis) shows, there are also immediate procedural complications associated with this technique, including failed or inaccurate stent deployment, and a high mortality (12%) when used to treat ruptured aneurysms.88
Coils with a bioactive coating have been designed to induce thrombosis and reduce recanalization after the initial treatment. However, a study of 165 aneurysms treated exclusively with the bioactive Matrix coils (Boston Scientific) showed no better recanalization rates than those reported for bare platinum coils.89 In addition, the authors noted a 3.3% rate of delayed infarction with the bioactive coils.
As newer techniques and materials are developed for endovascular treatment, careful analysis with standardized reporting will help determine their efficacy and safety.90 Each new technology will add to the high device cost of coils. The endovascular treatment of unruptured aneurysms is associated with shorter hospitalization but higher hospital costs than surgical treatment, particularly because of the high cost of coils.91
A Multidisciplinary Approach to Treating Aneurysms
A review of the Nationwide Inpatient Sample hospital discharge database from 1993 to 2003 showed that the number of endovascular procedures for ruptured and unruptured aneurysms doubled, while the number of surgeries for clipping remained the same.92 In-hospital mortality rates for endovascular therapy showed no significant change during the 11-year period. In-hospital mortality rates for surgical clipping decreased by 30% throughout the study, reaching the mortality rates of endovascular therapy. Teaching hospitals were associated with better outcomes and lower mortality rates, especially in patients who underwent aneurysm clipping. From 1993 to 2003, the number of admissions for unruptured aneurysms doubled, while the in-hospital mortality for this group decreased by 50%.
• Relationship of the aneurysm to the parent or branch arteries
• Presence of mass effect or thrombus
• Accessibility of the aneurysm
• Patient’s age and medical comorbidities
1. Location: Aneurysms in some locations are associated with a high surgical risk. During surgical repair of posteriorly projecting basilar apex aneurysms, thalamoperforating arteries may be injured. When these aneurysms warrant treatment, the endovascular alternative is generally safer.93 The clipping of cavernous segment ICA aneurysms may result in cranial nerve palsies, while these aneurysms carry very little or no risk of rupture when left untreated. Paraclinoid aneurysms and posterosuperior-projecting ACA aneurysms have intermediate surgical risks.70,94,95 MCA aneurysms are associated with a high endovascular failure and complication rate, and are usually more safely treated with surgical clipping.96,97
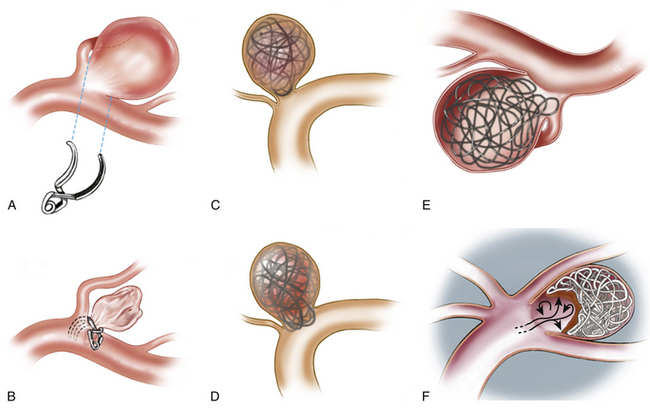
2. Relationship to parent or branch arteries: Aneurysms with branch vessels originating from the neck are not suitable for endovascular treatment. Surgical clip reconstruction generally allows the parent and branch vessels to be preserved while the aneurysm is obliterated (Fig. 68-3).
3. Dome-to-neck ratio: An aneurysm with a neck larger than 4 mm, or a dome-to-neck ratio of less than 2, is generally not amenable to simple coiling, and surgical clipping may be preferable.98–100 The use of stents has made many wide-necked aneurysms coilable, but such treatment is associated with higher complication rates. Clipping may be the better alternative if the surgical risk is low.
4. Presence of Mass Effect or Thrombus: Aneurysms that are symptomatic from mass effect, such as those that present with vision loss or oculomotor palsy, can be decompressed with surgical clipping, but not with endovascular therapy. Partially thrombosed aneurysms are likely to recur after coiling, due to shifting and compaction of the coil mass. Surgical clipping offers definitive treatment, and the complete recovery of cranial nerve deficits is more often seen after clipping than after coiling.101
5. Endovascular/surgical accessibility: Tortuosity or occlusion anywhere along the arterial pathway to the aneurysm may prohibit endovascular treatment. It is not infrequent to find tortuosity of the aortic arch, carotid or vertebral arteries; or chronic occlusion of the carotid or vertebral arteries. In contrast, aneurysms of the midbasilar artery require an extensive and complex skull base approach for surgical treatment.
6. Age and medical condition: Elderly patients, and those who are at high risk for prolonged general anesthesia, are generally better candidates for endovascular treatment.77 With very advanced age, the long-term durability of treatment is less of an issue. A few recent studies have reported good outcomes after the coiling of symptomatic unruptured aneurysms in septuagenarians and octogenarians.102–104 Surgery may be preferable for patients with renal insufficiency who might not tolerate the significant dye load required for endovascular treatment.
Follow-Up After Treatment
Risk of Aneurysm Regrowth
In a surgical series with long-term angiographic follow-up, David and colleagues report 135 aneurysms that were clipped without residual. Of these aneurysms that were completely treated at surgery, two aneurysms (1.5%) recurred at long-term follow-up (2.6 to 9.6 years).105 Of the 12 aneurysms in their series that were known to have residuals after clipping, 3 (25%) enlarged. In contrast, endovascular treatment is associated with a relatively high rate of aneurysm recanalization. In 145 aneurysms that were completely occluded by coiling, Raymond and colleagues found recurrences in 20%, which appeared on follow-up angiography at a mean of 12.3 months after treatment.106 Of the 187 aneurysms in their series that had residual necks after coiling, enlargement occurred in 40%. Of the 47 aneurysms that had a dome residual after coiling, 51% enlarged. Of the recurrent aneurysms that underwent re-treatment with endovascular therapy, 48.6% showed a second recurrence. Data from a longer duration of follow-up is needed to determine the long-term recurrence rates after coiling.
De Novo Aneurysms
In a series of 142 patients with unruptured aneurysms followed for 20 years, Juvela found that 19 new aneurysms developed in 15 patients, of which 2 caused subarachnoid hemorrhage.17 The chance that an aneurysm would form de novo was 0.84% per year. A similar annual rate of 0.89% was reported by Tsutsumi and colleagues.107 The annual risk of de novo aneurysm formation may be higher (1.8%) in patients with multiple aneurysms.105
Screening for Intracranial Aneurysms
Patients with a Family History of Intracranial Aneurysms
Adults who have two first-degree family members with known intracranial aneurysms, or who have one first-degree and one second-degree affected family members, have a 9% to 11% chance of harboring an aneurysm.56,57,108 In families with multiple affected individuals, first-degree relatives who have a history of smoking or hypertension have a 20.6% chance of harboring an unruptured intracranial aneurysm, according to early results from the Familial Intracranial Aneurysm Study.39 This frequency is much higher than the prevalence in the general population. The rupture risk is also likely higher for familial aneurysms than for sporadic aneurysms of similar size. Subarachnoid hemorrhage may occur at younger ages in subsequent generations.109 In siblings, aneurysm rupture tends to occur within the same decade of life.110,111 It is therefore reasonable to screen individuals with multiple affected family members, with either MRA or CTA, beginning in early adulthood. The optimal age and subsequent frequency to screen these individuals is unknown.
Autosomal Dominant Polycystic Kidney Disease
Approximately 500,000 persons in the United States carry a genetic mutation for autosomal dominant polycystic kidney disease (ADPKD), making it one of the most common inherited disorders.112 ADPKD is associated with an increased prevalence of intracranial aneurysms and an increased risk of subarachnoid hemorrhage. The prevalence of asymptomatic aneurysms in patients with ADPKD is 14% to 16% by autopsy and angiography studies.113 When aneurysm rupture occurs in these patients, it happens at a mean age of 35 to 40 years,114–116 which is 10 to 20 years earlier than the mean age for patients with sporadic intracranial aneurysms. Therefore, MRA screening is reasonable for young adults with this disease.
Andaluz N., Zuccarello M. Recent trends in the treatment of cerebral aneurysms: analysis of a nationwide inpatient database. J Neurosurg. 2008;108:1163-1169.
Bederson J.B., Awad I.A., Wiebers D.O., et al. Recommendations for the management of patients with unruptured intracranial aneurysms: a statement for healthcare professionals from the Stroke Council of the American Heart Association. Stroke. 2000;31:2742-2750.
Bederson J.B., Connolly E.S., Batjer H.H., et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a Special Writing Group of the Stroke Council, American Heart Association. Stroke. 2009;40:994-1025.
Broderick J.P., Brown R.D., Sauerbeck L., et al. Greater rupture risk for familial as compared to sporadic unruptured intracranial aneurysms. Stroke. 2009;40:1952-1957.
Chappell E.T., Moure F.C., Good M.C. Comparison of computed tomographic angiography with digital subtraction angiography in the diagnosis of cerebral aneurysms: a meta-analysis. Neurosurgery. 2003;52:624-631.
Choi D.S., Kim M.C., Lee S.K., et al. Clinical and angiographic long-term follow-up of completely coiled intracranial aneurysms using endovascular technique. J Neurosurg. 2010;112(3):575-581. erratum 2010;112(3):690
Fiorella D., Albuquerque F.C., Woo H., et al. Neuroform in-stent stenosis: incidence, natural history, and treatment strategies. Neurosurgery. 2006;59:34-42.
Gallas S., Drouineau J., Gabrillargues J., et al. Feasibility, procedural morbidity and mortality, and long-term follow-up of endovascular treatment of 321 unruptured aneurysms. Am J Neuroradiol. 2008;29:63-68.
Grunwald I.Q., Papanagiotou P., Politi M., et al. Endovascular treatment of unruptured intracranial aneurysms: occurrence of thromboembolic events. Neurosurgery. 2006;58:612-618.
International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. N Engl J Med. 1998;339:1725-1733.
Ishibashi T., Murayama Y., Urashima M., et al. Unruptured intracranial aneurysms: incidence of rupture and risk factors. Stroke. 2009;40:313-316.
Juvela S. Natural history of unruptured intracranial aneurysms: risks for aneurysm formation, growth, and rupture. Acta Neurochir Suppl. 2002;82:27-30.
Juvela S., Porras M., Heiskanen O. Natural history of unruptured intracranial aneurysms: a long-term follow-up study. J Neurosurg. 1993;79:174-182.
Krayenbuhl N., Erdem E., Oinas M., et al. Symptomatic and silent ischemia associated with microsurgical clipping of intracranial aneurysms: evaluation with diffusion-weighted MRI. Stroke. 2009;40:129-133.
Lall R.R., Eddleman C.S., Bendok B.R., et al. Unruptured intracranial aneurysms and the assessment of rupture risk based on anatomical and morphological factors: sifting through the sands of data. Neurosurg Focus. 2009;26(E2):1-7.
Mocco J., Snyder K.V., Albuquerque F.C., et al. Treatment of intracranial aneurysms with the Enterprise stent: a multicenter registry. J Neurosurg. 2009;110:35-39.
Molyneux A., Kerr R., Stratton I., et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267-1274.
Pierot L., Spelle L., Vitry F., et al. Immediate clinical outcome of patients harboring unruptured intracranial aneurysms treated by endovascular approach: results of the ATENA study. Stroke. 2008;39:2497-2504.
Piotin M., Spelle L., Mounayer C., et al. Intracranial aneurysms coiling with Matrix: immediate results in 152 patients and midterm anatomic follow-up from 115 patients. Stroke. 2009;40:321-323.
Raaymakers T.W., Rinkel G.J., Limburg M., Algra A. Mortality and morbidity of surgery for unruptured intracranial aneurysms: a meta-analysis. Stroke. 1998;29:1531-1538.
Raymond J., Guilbert F., Weill A., et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34:1398-1403.
Soeda A., Sakai N., Sakai H., et al. Thromboembolic events associated with Guglielmi detachable coil embolization of asymptomatic cerebral aneurysms: evaluation of 66 consecutive cases with use of diffusion-weighted MR imaging. Am J Neuroradiol. 2003;24:127-132.
Tsutsumi K., Ueki K., Morita A., Kirino T. Risk of rupture from incidental cerebral aneurysms. J Neurosurg. 2000;93:50-53.
Wiebers D.O., Whisnant J.P., Huston J.3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103-110.
Yahia A.M., Gordon V., Whapham J., et al. Complications of Neuroform stent in endovascular treatment of intracranial aneurysms. Neurocrit Care. 2008;8:19-30.
1. Graves E.J. Detailed diagnoses and procedures, National Hospital Discharge Survey, 1990. Vital Health Stat. 13, 1992. 1–225
2. Chason J.L., Hindman W.M. Berry aneurysms of the circle of Willis: results of a planned autopsy study. Neurology. 1958;8:41-44.
3. Housepian E.M., Pool J.L. A systematic analysis of intracranial aneurysms from the autopsy file of the Presbyterian Hospital, 1914 to 1956. J Neuropathol Exp Neurol. 1958;17:409-423.
4. Stehbens W.E. Aneurysms and anatomical variation of cerebral arteries. Arch Pathol. 1963;75:45-64.
5. McCormick W.F., Acosta-Rua G.J. The size of intracranial saccular aneurysms: an autopsy study. J Neurosurg. 1970;33:422-427.
6. Jellinger K. Pathology of intracerebral hemorrhage. Zentralbl Neurochir. 1977;38:29-42.
7. Jakubowski J., Kendall B. Coincidental aneurysms with tumours of pituitary origin. J Neurol Neurosurg Psychiatry. 1978;41:972-979.
8. Juvela S., Porras M., Heiskanen O. Natural history of unruptured intracranial aneurysms: a long-term follow-up study. J Neurosurg. 1993;79:174-182.
9. Menghini V.V., Brown R.D.Jr., Sicks J.D., et al. Incidence and prevalence of intracranial aneurysms and hemorrhage in Olmsted County, Minnesota, 1965 to 1995. Neurology. 1998;51:405-411.
10. Winn H.R., Jane S.r. J.A., Taylor J., et al. Prevalence of asymptomatic incidental aneurysms: review of 4568 arteriograms. J Neurosurg. 2002;96:43-49.
11. Ingall T.J., Whisnant J.P., Wiebers D.O., et al. Has there been a decline in subarachnoid hemorrhage mortality? Stroke. 1989;20:718-724.
12. Sarti C., Tuomilehto J., Saloman V., et al. Epidemiology of subarachnoid hemorrhage in Finland from 1983 to 1985. Stroke. 1991;22:848-853.
13. Broderick J.P., Brott T., Tomsick T., et al. The risk of subarachnoid and intracerebral hemorrhages in blacks as compared with whites. N Engl J Med. 1992;326:733-736.
14. Mayberg M.R., Batjer H.H., Dacey R., et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1994;25:2315-2328.
15. Iwamoto H., Kiyohara Y., Fujishima M., et al. Prevalence of intracranial saccular aneurysms in a Japanese community based on a consecutive autopsy series during a 30-year observation period: the Hisayama study. Stroke. 1999;30:1390-1395.
16. Hamada J., Morioka M., Yano S., et al. Incidence and early prognosis of aneurysmal subarachnoid hemorrhage in Kumamoto Prefecture, Japan. Neurosurgery. 2004;54:31-38.
17. Juvela S. Natural history of unruptured intracranial aneurysms: risks for aneurysm formation, growth, and rupture. Acta Neurochir Suppl. 2002;82:27-30.
18. Juvela S., Porras M., Poussa K. Natural history of unruptured intracranial aneurysms: probability of and risk factors for aneurysm rupture. J Neurosurg. 2000;93:379-387.
19. Winn H.R., Almaani W.S., Berga S.L., et al. The long-term outcome in patients with multiple aneurysms: incidence of late hemorrhage and implications for treatment of incidental aneurysms. J Neurosurg. 1983;59:642-651.
20. Wiebers D.O., Whisnant J.P., Sundt T.M.Jr., O’Fallon W.M. The significance of unruptured intracranial saccular aneurysms. J Neurosurg. 1987;66:23-29.
21. Yasui N., Suzuki A., Nishimura H., et al. Long-term follow-up study of unruptured intracranial aneurysms. Neurosurgery. 1997;40:1155-1160.
22. Tsutsumi K., Ueki K., Morita A., Kirino T. Risk of rupture from incidental cerebral aneurysms. J Neurosurg. 2000;93:50-53.
23. Ishibashi T., Murayama Y., Urashima M., et al. Unruptured intracranial aneurysms: incidence of rupture and risk factors. Stroke. 2009;40:313-316.
24. International Study of Unruptured Intracranial Aneurysms Investigators. unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. N Engl J Med. 1998;339:1725-1733.
25. Wiebers D.O., Whisnant J.P., Huston J.3rd, et al. Unruptured intracra-nial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103-110.
26. Phillips L.H.2nd, Whisnant J.P., O’Fallon W.M., Sundt T.M.Jr. The unchanging pattern of subarachnoid hemorrhage in a community. Neurology. 1980;30:1034-1040.
27. Ecker R.D., Hopkins L.N. Natural history of unruptured intracranial aneurysms. Neurosurg Focus. 2004;17:1-5.
28. Ferguson G.G., Peerless S.J., Drake C.G. Natural history of intracranial aneurysms. N Engl J Med. 1981;305:99.
29. Locksley H.B., Sahs A.L., Knowler L. Report on the cooperative study of intracranial aneurysms and subarachnoid hemorrhage. J Neurosurg. 1966;24:922-932.
30. Burns J.D., Huston J., Layton K.F., et al. Intracranial aneurysm enlargement on serial magnetic resonance angiography: frequency and risk factors. Stroke. 2009;40:406-411.
31. Asari S., Ohmoto T. Natural history and risk factors of unruptured cerebral aneurysms. Clin Neurol Neurosurg. 1993;95:205-214.
32. Sampei T., Mizuno M., Nakajima S., et al. Clinical study of growing up aneurysms: report of 25 cases. No Shinkei Geka. 1991;19:825-830.
33. Lall R.R., Eddleman C.S., Bendok B.R., et al. Unruptured intracranial aneurysms and the assessment of rupture risk based on anatomical and morphological factors: sifting through the sands of data. Neurosurg Focus. 2009;26(E2):1-7.
34. Ujiie H., Tachibana H., Hiramatsu O., et al. Effects of size and shape (aspect ratio) on the hemodynamics of saccular aneurysms: a possible index for surgical treatment of aneurysms. Neurosurgery. 1999;45:119-129.
35. Weir B., Amidei C., Kongable G., et al. The aspect ratio (dome/neck) of ruptured and unruptured aneurysms. J Neurosurg. 2003;99:447-451.
36. Nader-Sepahi A., Casimiro M., Sen J., et al. Is aspect ratio a reliable predictor of intracranial aneurysm rupture? Neurosurgery. 2004;54:1343-1347.
37. Hoh B.L., Sistrom C.L., Firment C.S., et al. Bottleneck factor and height-width ratio: association with ruptured aneurysms in patients with multiple cerebral aneurysms. Neurosurgery. 2007;61:716-722.
38. Sadamoto T., Yuki K., Migita K., et al. Morphological differences between ruptured and unruptured cases in middle cerebral artery aneurysms. Neurosurgery. 2008;62:602-609.
39. Broderick J.P., Brown R.D., Sauerbeck L., et al. Greater rupture risk for familial as compared to sporadic unruptured intracranial aneurysms. Stroke. 2009;40:1952-1957.
40. Rinkel G.J., Djibuti M., Algra A., Van Gijn J. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke. 1998;29:251-256.
41. Juvela S., Poussa K., Porras M. Factors affecting formation and growth of intracranial aneurysms: a long-term follow-up study. Stroke. 2001;32:485-491.
42. Heiserman J.E., Dean B.L., Hodak J.A., et al. Neurologic complications of cerebral angiography. AJNR Am J Neuroradiol. 1994;15:1401-1411.
43. Cloft H.J., Joseph G.J., Dion J.E. Risk of cerebral angiography in patients with subarachnoid hemorrhage, cerebral aneurysm, and arteriovenous malformation: a meta-analysis. Stroke. 1999;30:317-320.
44. Adams W.M., Laitt R.D., Jackson A. The role of MR angiography in the pretreatment assessment of intracranial aneurysms: a comparative study. AJNR Am J Neuroradiol. 2000;21:1618-1628.
45. Graves M.J. Magnetic resonance angiography. Br J Radiol. 1997;70:6-28.
46. Huston J.3rd, Nichols D.A., Luetmer P.H., et al. Blinded prospective evaluation of sensitivity of MR angiography to known intracranial aneurysms: importance of aneurysm size. AJNR Am J Neuroradiol. 1994;15:1607-1614.
47. Metens T., Rio F., Baleriaux D., et al. Intracranial aneurysms: detection with gadolinium-enhanced dynamic three-dimensional MR angiography initial results. Radiology. 2000;216:39-46.
48. Suzuki I.M., Matsui Ueda F., et al. Contrast-enhanced MR angiography (enhanced 3-D fast gradient echo) for diagnosis of cerebral aneurysms. Neuroradiology. 2002;44:17-20.
49. Hiratsuka Y., Miki H., Kiriyama I., et al. Diagnosis of unruptured intracranial aneurysms: 3T MR angiography versus 64-channel multi-detector row CT angiography. Magn Reson Med Sci. 2008;7:169-178.
50. Monninghoff C., Maderwald S., Theysohh J.M., et al. Evaluation of intracranial aneurysms with 7 T versus 1.5 T time-of-flight MR angiography—initial experience. Rofo. 2009;181:16-23.
51. Fuchs T., Kachelriess M., Kalender W.A. Technical advances in multi-slice spiral CT. Eur J Radiol. 2000;36:69-73.
52. Dillon E.H., van Leeuwen M.S., Fernandez M.A., Mali W.P. Spiral CT angiography. AJR Am J Roentgenol. 1993;160:1273-1278.
53. van Gelder J.M. Computed tomographic angiography for detecting cerebral aneurysms: implications of aneurysm size distribution for the sensitivity, specificity, and likelihood ratios. Neurosurgery. 2003;53:597-606.
54. Chappell E.T., Moure F.C., Good M.C. Comparison of computed tomographic angiography with digital subtraction angiography in the diagnosis of cerebral aneurysms: a meta-analysis. Neurosurgery. 2003;52:624-631.
55. Vieco P.T. CT angiography of the intracranial circulation. Neuroimaging Clin North Am. 1998;8:577-592.
56. Raaymakers T.W., Rinkel G.J., Limburg M., Algra A. Mortality and morbidity of surgery for unruptured intracranial aneurysms: a meta-analysis. Stroke. 1998;29:1531-1538.
57. Raaymakers T.W., Rinkel G.J., Ramos L.M. Initial and follow-up screening for aneurysms in families with familial subarachnoid hemorrhage. Neurology. 1998;51:1125-1130.
58. Johnston S.C., Zhao S., Dudley R.A., et al. Treatment of unruptured cerebral aneurysms in California. Stroke. 2001;32:597-605.
59. Barker F.G.2nd, Amin-Hanjani S., Butler W.E., et al. In-hospital mortality and morbidity after surgical treatment of unruptured intracranial aneurysms in the United States, 1996–2000: the effect of hospital and surgeon volume. Neurosurgery. 2003;52:995-1009.
60. Raymond J., Guilbert F., Weill A., et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34:1398-1403.
61. White P.M., Wardlaw J.M. Unruptured intracranial aneurysms. J Neuroradiol. 2003;30:336-350.
62. Bederson J.B., Awad I.A., Wiebers D.O., et al. Recommendations for the management of patients with unruptured intracranial aneurysms: a statement for healthcare professionals from the Stroke Council of the American Heart Association. Stroke. 2000;31:2742-2750.
63. Yasargil M.G., Antic J., Laciga R., et al. Microsurgical pterional approach to aneurysms of the basilar bifurcation. Surg Neurol. 1976;6:83-91.
64. Jane J.A., Winn H.R., Richardson A.E. The natural history of intracranial aneurysms: rebleeding rates during the acute and long term period and implication for surgical management. Clin Neurosurg. 1977;24:176-184.
65. Asgari S., Wanke I., Schoch B., Stolke D. Recurrent hemorrhage after initially complete occlusion of intracranial aneurysms. Neurosurg Rev. 2003;26:269-274.
66. Takahashi T. The treatment of symptomatic unruptured aneurysms. Acta Neurochir Suppl. 2002;82:17-19.
67. Khanna R.K., Malik G.M., Qureshi N. Predicting outcome following surgical treatment of unruptured intracranial aneurysms: a proposed grading system. J Neurosurg. 1996;84:49-54.
68. Solomon R.A., Fink M.E., Pile-Spellman J. Surgical management of unruptured intracranial aneurysms. J Neurosurg. 1994;80:440-446.
69. Drake C.G. Progress in cerebrovascular disease. Management of cerebral aneurysm. Stroke. 1981;12:273-283.
70. Proust F., Debono B., Hannequin D., et al. Treatment of anterior communicating artery aneurysms: complementary aspects of micro-surgical and endovascular procedures. J Neurosurg. 2003;99:3-14.
71. Guidetti B., La Torre E. Carotid-ophthalmic aneurysms. A series of 16 cases treated by direct approach. Acta Neurochir (Wien). 1970;22:289-304.
72. Guidetti B., La Torre E. Management of carotid-ophthalmic aneurysms. J Neurosurg. 1975;42:438-442.
73. Guglielmi G., Vinuela F., Dion J., Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: Preliminary clinical experience. J Neurosurg. 1991;75:8-14.
74. Guglielmi G., Vinuela F., Sepetka I., Macellari V. Electrothrombosis of saccular aneurysms via endovascular approach. Part 1: Electrochemical basis, technique, and experimental results. J Neurosurg. 1991;75:1-7.
75. Murayama Y., Vinuela F., Duckwiler G.R., et al. Embolization of incidental cerebral aneurysms by using the Guglielmi detachable coil system. J Neurosurg. 1999;90:207-214.
76. Molyneux A., Kerr R., Stratton I., et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267-1274.
77. Bederson J.B., Connolly E.S., Batjer H.H., et al. Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage: a Statement for Healthcare Professionals From a Special Writing Group of the Stroke Council, American Heart Association. Stroke. 2009;40:994-1025.
78. Pierot L., Spelle L., Vitry F., et al. Immediate clinical outcome of patients harboring unruptured intracranial aneurysms treated by endovascular approach: results of the ATENA study. Stroke. 2008;39:2497-2504.
79. Gallas S., Drouineau J., Gabrillargues J., et al. Feasibility, procedural morbidity and mortality, and long-term follow-up of endovascular treatment of 321 unruptured aneurysms. Am J Neuroradiol. 2008;29:63-68.
80. Soeda A., Sakai N., Sakai H., et al. Thromboembolic events associated with Guglielmi detachable coil embolization of asymptomatic cerebral aneurysms: evaluation of 66 consecutive cases with use of diffusion-weighted MR imaging. Am J Neuroradiol. 2003;24:127-132.
81. Grunwald I.Q., Papanagiotou P., Politi M., et al. Endovascular treatment of unruptured intracranial aneurysms: occurrence of thromboembolic events. Neurosurgery. 2006;58:612-618.
82. Krayenbuhl N., Erdem E., Oinas M., et al. Symptomatic and silent ischemia associated with microsurgical clipping of intracranial aneurysms: evaluation with diffusion-weighted MRI. Stroke. 2009;40:129-133.
83. Choi D.S., Kim M.C., Lee S.K., et al. Clinical and angiographic long-term follow-up of completely coiled intracranial aneurysms using endovascular technique. J Neurosurg. 2010;112(3):575-581. erratum 2010;112(3):690
84. Pandey A.S., Koebe C., Rosenwasser R.H., et al. Endovascular coil embolization of ruptured and unruptured posterior circulation aneurysms: review of a 10-year experience. Neurosurgery. 2007;60:626-636.
85. Raymond J., Molyneux A.J., Fox A.J., et al. The TEAM trial: safety and efficacy of endovascular treatment of unruptured intracranial aneurysms in the prevention of aneurismal hemorrhages: a randomized comparison with indefinite deferral of treatment in 2002 patients followed for 10 years. Trials. 2008;43:1-11.
86. Fiorella D., Albuquerque F.C., Woo H., et al. Neuroform in-stent stenosis: incidence, natural history, and treatment strategies. Neurosurgery. 2006;59:34-42.
87. Yahia A.M., Gordon V., Whapham J., et al. Complications of Neuroform stent in endovascular treatment of intracranial aneurysms. Neurocrit Care. 2008;8:19-30.
88. Mocco J., Snyder K.V., Albuquerque F.C., et al. Treatment of intracranial aneurysms with the Enterprise stent: a multicenter registry. J Neurosurg. 2009;110:35-39.
89. Piotin M., Spelle L., Mounayer C., et al. Intracranial aneurysms coiling with Matrix: immediate results in 152 patients and midterm anatomic follow-up from 115 patients. Stroke. 2009;40:321-323.
90. Meyers P.M., Schumacher H.C., Higashida R.T., et al. Reporting standards for endovascular repair of saccular intracranial cerebral aneurysms. Stroke. 2009;40:366-379.
91. Hoh B.L., Chi Y.Y., Dermott M.A., et al. The effect of coiling versus clipping of ruptured and unruptured cerebral aneurysms on length of stay, hospital cost, hospital reimbursement, and surgeon reimbursement at the University of Florida. Neurosurgery. 2009;64:614-619.
92. Andaluz N., Zuccarello M. Recent trends in the treatment of cerebral aneurysms: analysis of a nationwide inpatient database. J Neurosurg. 2008;108:1163-1169.
93. Eskridge J.M., Song J.K. Endovascular embolization of 150 basilar tip aneurysms with Guglielmi detachable coils: results of the Food and Drug Administration multicenter clinical trial. J Neurosurg. 1998;89:81-86.
94. Martin N.A. The combination of endovascular and surgical techniques for the treatment of intracranial aneurysms. Neurosurg Clin North Am. 1998;9:897.
95. Park H.K., Horowitz M., Jungreis C., et al. Endovascular treatment of paraclinoid aneurysms: experience with 73 patients. Neurosurgery. 2003;53:14-24.
96. Regli L., Uske A., de Tribolet N. Endovascular coil placement compared with surgical clipping for the treatment of unruptured middle cerebral artery aneurysms: a consecutive series. J Neurosurg. 1999;90:1025-1030.
97. Regli L., Dehdashti A.R., Uske A., de Tribolet N. Endovascular coiling compared with surgical clipping for the treatment of unruptured middle cerebral artery aneurysms: an update. Acta Neurochir Suppl. 2002;82:41-46.
98. Debrun G.M., Aletich V.A., Kehrli P., et al. Selection of cerebral aneurysms for treatment using Guglielmi detachable coils: the preliminary University of Illinois at Chicago experience. Neurosurgery. 1998;43:1281-1297.
99. Debrun G.M., Aletich V.A., et al. Aneurysm geometry: an important criterion in selecting patients for Guglielmi detachable coiling. Neurol Med Chir (Tokyo). 1998;38(Suppl):1-20.
100. Fernandez Zubillaga A., Guglielmi G., Vineula F., Duckwiler G.R. Endovascular occlusion of intracranial aneurysms with electrically detachable coils: correlation of aneurysm neck size and treatment results. AJNR Am J Neuroradiol. 1994;15:815-820.
101. Chen P.R., Amin-Hanjani S., Albuquerque F.C., et al. Outcome of oculomotor nerve palsy from posterior communicating artery aneurysms: comparison of clipping and coiling. Neurosurgery. 2006;58:1040-1046.
102. Bradac G.B., Bergui M., Fontanella M. Endovascular treatments of cerebral aneurysms in elderly patients. Neuroradiology. 2005;47:938-941.
103. Cai Y.L., Spelle L., Wang H., et al. Endovascular treatment of intracranial aneurysms in the elderly: single-center experience in 63 consecutive patients. Neurosurgery. 2005;57:1096-1102.
104. Gizewski E.R., Goricke S., Wolf A., et al. Endovascular treatment of intracranial aneurysms in patients 65 years or older: clinical outcomes. Am J of Neuroradiol. 2008;29:1575-1580.
105. David C.A., Vishteh A.G., Spetzler R.F., et al. Late angiographic follow-up review of surgically treated aneurysms. J Neurosurg. 1999;91:396-401.
106. Raymond J., Guilbert F., Weill A., et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34:1398-1403.
107. Tsutsumi K., Ueki K., Morita A., et al. Risk of aneurysm recurrence in patients with clipped cerebral aneurysms: results of long-term follow-up angiography. Stroke. 2001;32:1191-1194.
108. Ronkainen A., Hernesniemi J., Puranen M., et al. Familial intracranial aneurysms. Lancet. 1997;349:380-384.
109. Bromberg J.E., Rinkel G.J., Algra A., et al. Familial subarachnoid hemorrhage: distinctive features and patterns of inheritance. Ann Neurol. 1995;38:929-934.
110. Leblanc R., Melanson D., Tampieri D., Guttmann R.D. Familial cerebral aneurysms: a study of 13 families. Neurosurgery. 1995;37:633-639.
111. Ronkainen A., Hernesniemi J., Tromp G. Special features of familial intracranial aneurysms: report of 215 familial aneurysms. Neurosurgery. 1995;37:43-47.
112. Parfrey P.S., Bear J.C., Morgan J., et al. The diagnosis and prognosis of autosomal dominant polycystic kidney disease. N Engl J Med. 1990;323:1085-1090.
113. Butler W.E., Barker F.G.2nd, Crowell R.M. Patients with polycystic kidney disease would benefit from routine magnetic resonance angio-graphic screening for intracerebral aneurysms: a decision analysis. Neurosurgery. 1996;38:506-516.
114. Lozano A.M., Leblanc R. Cerebral aneurysms and polycystic kidney disease: a critical review. Can J Neurol Sci. 1992;19:222-227.
115. Chauveau D., Pirson Y., Verellen-Dumoulin C., et al. Intracranial aneurysms in autosomal dominant polycystic kidney disease. Kidney Int. 1994;45:1140-1146.
116. Fick G.M., Johnson A.M., Hammond W.S., Gabow P.A. Causes of death in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1995;5:2048-2056.