Chapter 141 Management of Traumatic Intracranial Aneurysms
The injury of intracranial arterial wall may result in immediate hemorrhage, thrombosis, dissection, or development of traumatic intracranial aneurysms (TICAs).1 Intracranial vasculature results virtually injured in all cases of severe head injury, which explains the almost constant presence of subarachnoid hemorrhage due to lesion of small pial vessels.1 However, TICAs may develop even following mild or trivial head trauma.2 Despite the frequency of head injury, the incidence of TICAs is quite low, accounting for less than 1% of all intracranial aneurysms.2–6 Indeed, the actual rate of TICAs could be underestimated, since nowadays diagnostic angiograms are rarely performed for head injuries2,5,6 and computed tomography (CT) angiography is not sensitive enough to replace digital subtraction angiography.7 TICAs have been reported in patients of any age, but they are more common in the pediatric population.2,8,9 Moreover, there is a clear male preponderance,2 which is presumably related to the greater frequency of head injury in males.8
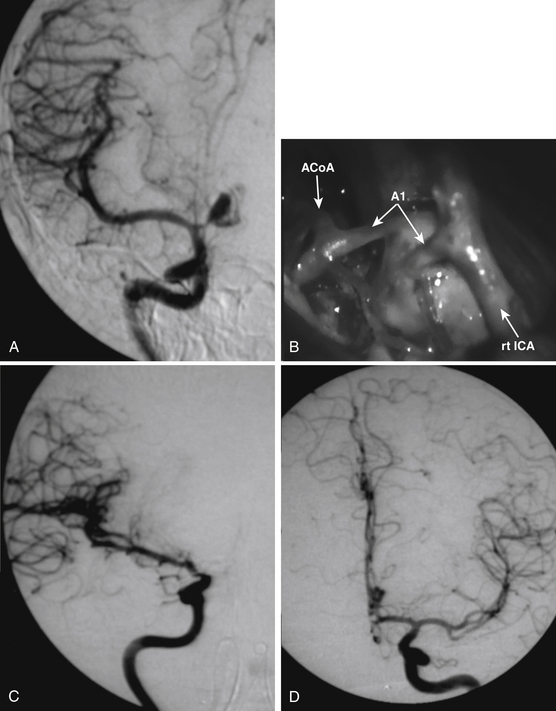
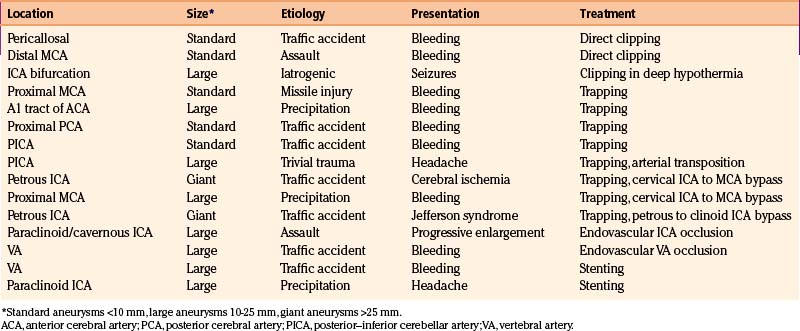
In the last two decades, we managed about 1800 cerebral aneurysms and 1600 severe head injuries (21 missile injuries): we found just 14 TICAs (Figs. 141-1 to 141-4). During the same period, we performed about 8000 brain surgery procedures, which were responsible for just 1 case of iatrogenic aneurysm (Fig. 141-5; also see video). Our series is summarized in Table 141-1.
Pathology
Basing on the pathology and the mechanism of formation, four types of TICAs have been described: true, false (or pseudoaneurysms), mixed, and dissecting.2,4,5,10–15 True TICAs consist of localized dilatation of the artery with an expanded lumen lined by stretched remnants of the arterial wall: in these cases, only the adventitia is intact and represents the single layer of the aneurysm sac.2,8,10,14,15 This would be the main difference between a true TICA and a common saccular aneurysm, which has a wall consisting of both intima and adventitia.10,14 Other authors4 think true TICAs originate from the outpouching of the intima through a fragmented media, resulting in an aneurysm wall consisting of intima separated from adventitia by fibrous tissue. False TICAs originate from the rupture of all arterial layers with perivascular hematoma formation and organization.8 These lesions consist of encapsulated hematoma in communication with the lumen of the ruptured vessel, and sometimes, the surrounding structures contribute to the aneurysm wall formation.5,10 Mixed TICAs derive from contained post-traumatic rupture of true aneurysms with local hematoma formation, false lumen development, and production of secondary false aneurysms.4,5,8,10 Dissecting TICAs originate when trauma provokes the splitting of the arterial wall layers with blood entering through intimal tears inside the arterial wall and forming a false lumen between the intima and the elastica.5,10 In such conditions, aneurysm outpouching may easily occur and arterial dissection may evolve to dissecting aneurysm. Dissecting aneurysms also may break and become false aneurysms. The term mixed aneurysm has also often been used to describe saccular aneurysms associated with dissection of the parent vessels.2
Although false aneurysms have been considered the most frequent TICAs,2,4,5,8 indeed the relative incidence of the four histologic types is not known.1,2 Furthermore, the term pseudoaneurysm, which should refer only to false aneurysm, is often used as a synonym of TICA.4 However, all types of TICAs carry a high hemorrhagic risk, and their distinction is often impossible on angiography. Therefore, the aforementioned classification is academically relevant but of little benefit in clinical practice, because intervention is required regardless the type.2,4,5
Physiopathology
Both penetrating and nonpenetrating injuries may be responsible for TICA formation.3,10 TICAs are most frequently reported following blunt injuries.1,2,16 As for penetrating wounds, low-velocity penetrations (from stab or shotgun wounds) are more risky than high-velocity penetrations (from bullet wounds):2,4,5 14.9% versus 3.6%, respectively.17,18 The prevalence of blunt trauma could be explained by penetrating injuries more likely being responsible for complete arterial disruption with immediate hemorrhage and without subsequent aneurysm formation. When projectile injuries are responsible of TICAs, these are always false aneurysms with complete lesion of the arterial wall.1,19
The main mechanisms of TICA formation are direct injury to the vessel or stretching of the vessel by adjacent forces.2 The mechanisms are different in penetrating and in nonpenetrating injuries. In the first case, the proximity of the missile track to the location of the TICA indicates that this is the result of direct contact to the vessel by the missile, shrapnel, or secondary bone fragments.1,4,5 This could result in arterial laceration or avulsion of perforating branches or produce enough shearing forces to cause a rent in the arterial wall.1,17,20 Penetrating fragments around the face or pterion have the high probability to produce TICAs: 65% of these aneurysms are reported in association with facial, orbital, basifrontal, or pterional injury.1,19–21 But in case of nonpenetrating injuries, there is a frequent association (60%-90% of cases) between TICA development and presence of skull fractures.1,10,22 Otherwise, the arterial disruption triggering TICA formation may result from differential motion between the brain and the skull during acceleration/deceleration.1,2,8
TICAs are frequently reported in the peripheral vascular tree, and the anterior circulation is more commonly involved with infratentorial TICAs representing less than 5% of the total.2,5,6,10,16 The location of TICAs closely depends on the mechanism of injury.2 Intrapetrous and/or infraclinoid carotid artery, as well as basilar artery, aneurysms are usually related to skull base fractures, and para- and supraclinoid carotid artery aneurysms may result from fractures of the anterior clinoid process or stretching of the artery across the process during the impact. Otherwise, injury to the paraclinoid carotid artery may originate by the stretching of the artery at the level of the transitional zone between the relatively fixed intracavernous segment and the relatively mobile cisternal segment,2,15,23 pericallosal artery aneurysms may develop from the injury of the artery against the falx edge,2,9,24 posterior cerebral artery aneurysms may be the result of trauma against the tentorium,2 and cortical artery aneurysms are usually associated with cranial vault fractures.2,5,24,25 TICAs of the meningeal artery are infrequently reported, which is curious considering the intimate relationships of this vessel with the vault bone.6,10
Besides traditional traumas, TICAs may be iatrogenic. Injuries of intracranial arteries with subsequent TICA formation have been reported in skull base surgery, paranasal sinuses surgery, trans-sphenoidal surgery, ventriculostomy, and endoscopic neurosurgery and even following repeated subdural taps.1,2,4,5,10,12,14,26,27 In particular, basilar artery injury with possible TICA development represents the unique, serious (but rare) risk following endoscopic treatment of hydrocephalus.27
In summary, the relative incidences of TICAs would be 60% to 70% of blunt traumas, 16% to 26% of penetrating injuries, and 10% of iatrogenic lesions.8
Clinical Presentation
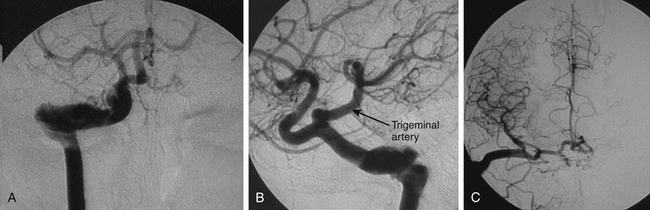
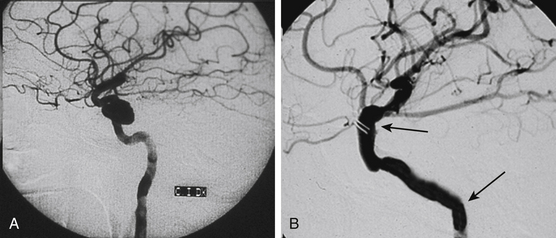
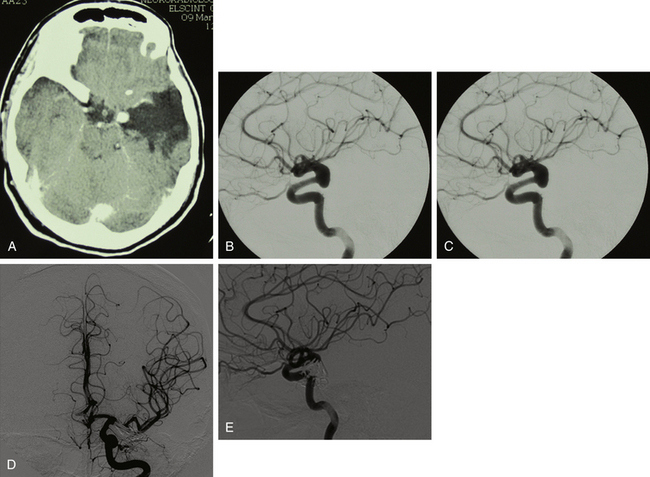
Spontaneous regression of the aneurysm is possible but improbable.3,5,,8,10,13,22 A study of 40 patients, who did not receive immediate treatment, revealed that 40% of TICAs bled and 21% enlarged.1 We assume that the probable destiny of TICAs is progressive enlargement until rupture.19 This could explain the relative rarity of giant subarachnoid TICAs, which instead may be found at intrapetrous level (Figs. 141-1 and 141-2).
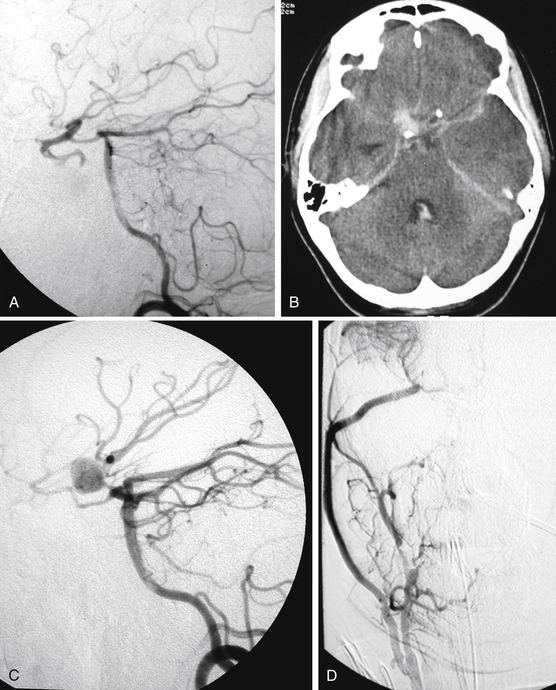
The most common clinical presentation consists of acute neurologic deterioration due to delayed intracranial hemorrhage following head injuries or surgical procedures1,2,4,5,10,12–14,26 (Figs. 141-3 and 141-4). However, warning signs such as headache, memory disturbance, and progressive visual loss were reported years before TICA rupture.2,25 Other clinical manifestations of TICAs are epistaxis, cranial nerve palsy, diabetes insipidus, unilateral blindness, symptoms of carotid–cavernous fistula, hydrocephalus, growing fractures, and severe headache.2,4,9,10,14,22,23 Cerebral ischemia and seizures are possible, such as in our patients in Figs. 141-1 and 141-5. Most TICAs present within 2 or 3 weeks, and Ahmadi et al.1 calculated that the mean time for angiographic diagnosis is about 20 days (standard deviation 13 days). On the one hand, TICA may develop within a few hours after injury,19 but on the other hand, hemorrhages have been reported 10 years after a penetrating wound.18
Secondary hemorrhages from TICAs in already-injured brains usually represent dramatic events and carry ominous consequences.4 The morbidity and mortality rate following TICA rupture is about 40% to 70% in untreated patients and 15% to 30% in operated ones.2,5,6,8 Therefore, early identification and timely intervention to prevent bleeding are tantamount.4,5,16 Unfortunately, only 10% to 20% of TICAs are discovered when they are still asymptomatic.8
Diagnosis
In the past decades, CT scan almost completely replaced cerebral angiography in the workup of the head-injured patients. At the same time, the rate of early recognition of TICAs fell proportionally, and these lesions have become quite unusual.1,5–7,10 Some TICAs may remain unrecognized because of superimposed brain injury.1 Because of the potential lethality of these lesions, they instead should be recognized early.16 The term stroke-after-head-injury syndrome has been coined just to emphasize the importance of early diagnosis and prevention.1 A high index of suspicion should exist in the following cases: (1) facio-orbital–pterional injuries; (2) penetrating fragments, especially if they cross the midline or traverse into another dural compartment; (3) severe subarachnoid hemorrhage and subdural or intracerebral hematomas, particularly if they have unusual location; and (4) retained fragment associated with hematoma in the distal part of its trajectory.1,16,19,28 Some authors5,10 advise traditional angiography in the setting of all severe head injuries. The introduction of CT and magnetic resonance angiography has provided a powerful noninvasive diagnostic screening method, but these assessments may be hampered by artifacts from retained metal fragments, and generally their sensitivity is lower than digital subtraction angiography, which remains the gold standard in the diagnosis of TICAs.5,7,8 However, the recent and continuous evolution of more sophisticated, high-quality CT angiographies is progressively changing this situation. In our present clinical practice, when an intracranial vascular injury is suspected, spiral CT angiography with three-dimensional reconstructions has become the first-choice assessment, and digital subtraction angiography is performed just in patients whose vascular injury has been already screened. Apart from the greater sensitivity in comparison with CT angiography,5,7 there is another reason to obtain digital subtraction angiography: This is still the only way to obtain reliable information about the collateral circulation, as well as visualization of the arterial supply and the venous drainage, which means it is the only way to understand whether the parent artery may be sacrificed and a bypass is indicated.
There is no complete agreement upon the timing of angiography: overly early angiography could miss the TICA because this has not yet grown16,17,19,22 (Fig. 141-3), but significantly better outcomes have been reported following early rather than delayed angiography.29 Dubey et al.5 recommended early angiography and follow-up angiogram if the initial study was negative. Particularly, all cases of cerebral stab wounds should be routinely restudied angiographically 2 weeks after the initial negative angiography.3,13,17 Clinically stable patients with penetrating stab wounds and the weapon in place may deserve immediate angiography to check for any possible vascular injury before the weapon removal. However, “not actively bleeding” patients, presenting with the weapon already removed, could theoretically undergo angiography without urgency. Nevertheless, even in these patients, the outcomes are better following early angiography.29
Several angiographic features have been reported to differentiate TICAs from traditional cerebral aneurysms,5,10,30 but such a distinction remains difficult.8 Characteristic features of TICAs are similar to those reported for spontaneous dissecting aneurysms and do not allow differentiation among true, false, mixed, or dissecting TICAs. Following severe head injury, a TICA is more probable than a spontaneous aneurysm when it is located on a peripheral artery at a site other than a branching point, when the parent artery presents abrupt changes of caliber and/or irregular walls, when the neck is poorly defined or absent, when the dome has an irregular contour and strange shape, and when the aneurysm presents delayed filling and emptying5,8,10,25,30 (Figs. 141-1 to 141-5). Vasospasm is uncommon in TICAs and does not indicate the ruptured aneurysm in presence of multiple lesions.19 It has been reported that most TICAs range between 2 and 15 mm,8,19 which means larger aneurysms are likely not traumatic. However, we cannot confirm these findings. In our experience, we found giant TICAs only at the petrous level (maybe because the surrounding bone prevented rupture and the petrous aneurysm kept growing), but large subarachnoid TICAs were not unusual (Figs. 141-1 to 141-5 and Table 141-1). Moreover, when surgically exposed, TICAs were often larger than we were expecting based on the angiography.
Treatment
Taking into account the high probability of catastrophic aneurysm rupture, treatment of TICAs should be performed as soon as such lesions are found.5,16 Surgical mortality accounts for 15% to 16% of patients and mainly depends on the critical preoperative conditions of these patients and on the propensity of TICAs to rupture during treatment because of the absence of a true wall.1,8,31 This means that, unlike for spontaneous aneurysms, significant reduction in mortality and morbidity of TICAs cannot be expected by the introduction of endovascular therapy. Notwithstanding that, the recent progress in endovascular therapy undoubtedly offers an effective and less invasive alternative to open surgical treatment.8,31,32
In the first scenario, the surgeon must be prepared to deal with an unexpected or inadequately studied TICA and to face its possible intraoperative rupture. Sometimes, the presence of TICA is intraoperatively heralded just because of unexpected arterial bleeding during clot removal.1 In this scenario, the unique option often is arterial occlusion. In emergency surgery, cerebral revascularization is difficult or even contraindicated: on the one hand, the surgeon cannot be sure the bypass is indicated due to the lack of preoperative digital subtraction angiography; on the other hand, revascularization could even be responsible for severe hyperemia because of the impaired vasoregulation triggered by the head injury. If possible, the surgeon should remove the life-threatening clots and explore the TICA. In many cases, the TICA is superficially located but hidden by clots and can be found using intraoperative angiography with metallic references.10 Once the lesion is exposed, the surgeon must decide about clipping, wrapping, or trapping.1,5,10 The surgeon should not feel required to solve the case at any cost, and if the injured vessel might be preserved or better handled by endovascular treatment following clot removal and clinical stabilization, the patient should be referred for the endovascular therapy.31
In the second scenario, the clinical stability permits adequate preoperative angiographic study. In this case, the treatment should be tailored based on the TICA features. For instance, endovascular treatment may be the treatment of choice for many skull base and posterior circulation TICAs that are not due to missile injury.1,5 In such cases, the parent vessel may be even preserved by the modern stenting techniques.32 Otherwise, if the injured vessel cannot be preserved and the collateral circulation is adequate, it may be directly occluded by coiling or a balloon.31 However, the lack of aneurysm wall may lead to severe hemorrhage during balloon or coil placement.31 Furthermore, sometimes the sac/neck ratio makes coiling impossible.31 Finally, distal circulation aneurysms may be difficult to reach by the endovascular route, whereas their surgical exposure usually does not represent a major problem.5,10 Despite the endovascular evolution, there remain cases in which endovascular treatment is impossible or dangerous due to the lack of a true aneurysm wall. In such cases, if proximal endovascular occlusion is not feasible, open surgery remains the only therapeutic option.
The neurosurgical techniques to deal with TICAs are the classical ones to face cerebral aneurysms: clipping, vascular reconstruction/revascularization, wrapping, and trapping. Aneurysm clipping leaving the parent vessel patent is preferable,8 but this would be possible in just 10% to 15% of cases because of the lack of aneurysm neck.16 Nonetheless, sometimes the serial application of tangential clips adequately remodels the vessel1,5 (Fig. 141-5). In selected cases, the injured vessel may be treated by arteriotomy and wall repair, aneurysmectomy and aneurysmorraphia, removal of the injured segment and end-to-end anastomosis, or arterial occlusion and revascularization.1,5,20,33 Most often, simple excision with or without an arterial bypass seems to be the procedure of choice1,8,20,29,33 (Fig. 141-3). Discussion of methods to assess the adequacy of the collateral circulation and the indication for revascularization (balloon test occlusion, electroencephalogram monitoring, evaluation of the times of venous visualization, etc.) is beyond the scope of this chapter; however, some arteries cannot be occluded. In these cases, a procedure of cerebral revascularization must be considered. Depending on the artery to be replaced, the revascularization may be through arterial transposition, a superficial temporal artery–middle cerebral artery (MCA) bypass, the interposition of a “short” vascular graft just to bypass the injured arterial segment, or the interposition of a “long” saphenous vein or radial artery graft between the cervical internal carotid artery (ICA) and the intracranial ICA or MCA20,33 (Figs. 141-1, 141-2, and 141-4).
Niguarda’s Experience
Our experience is summarized in Table 141-1. During the last 20 years, we managed a total of 15 traumatic aneurysms: 13 were related to blunt traumas, 1 related to penetrating injury, and 1 was iatrogenic. Direct clipping was possible in two cases, one patient with giant paraclinoid aneurysm underwent clipping in deep hypothermia and cardiocirculatory arrest, eight aneurysms were surgically trapped (four following revascularization), two patients underwent endovascular artery occlusion, and two were treated by a stenting procedure.
Indications, timing, and techniques to be employed in these fragile patients must be carefully analyzed and individualized. The variation in aneurysm geometry, aneurysm location, collateral circulation, and clinical conditions mandate that the most appropriate endovascular or surgical techniques be chosen case by case.2
Conclusions
Intracranial TICAs are considered uncommon lesions, but their real incidence is unknown.5,10 Perhaps they should be suspected in all severely head-injured patients, but patients with penetrating wounds (particularly stab wounds) and/or skull fractures approaching the normal anatomic arterial location should be regarded with suspicion.5,10 TICAs should be considered highly probable in all patients with delayed post-traumatic intracranial hemorrhage. Since TICA rupture is a catastrophic event with high mortality,5 every effort should be made to achieve timely diagnosis and treatment.16
Endovascular therapy may represent a valid therapeutic option for many TICAs.5 However, unlikely spontaneous aneurysms, in many cases endovascular treatment still takes an overly high risk of hemorrhage; otherwise, the injured vessel cannot be easily reached or cannot be safely occluded. Moreover, there are patients who require the immediate removal of their intracranial clots without adequate angiographic study. In all of these cases, open neurosurgery remains the best therapeutic option for these dangerous lesions.
Aarabi B. Traumatic aneurysms of brain due to high velocity missile head wounds. Neurosurgery. 1988;22:1056-1063.
Ahmadi J., Levy M.L., Aarabi B., Giannotta S.L. Vascular lesion resulting from head injury. In: Wilkins R.H., Rengachary S.S. Neurosurgery. New York: McGraw-Hill; 1996:2821-2840.
Britz G.W., Newell D.W., West G.A., Winn H.R. Traumatic cerebral aneurysms secondary to penetrating intracranial injuries. In: Winn R.H., editor. Youmans Neurological Surgery. Philadelphia: Saunders; 2004:2131-2135.
Buckingham M.J., Crone K.R., Ball W.S., et al. Traumatic intracranial aneurysms in childhood: two cases and a review of the literature. Neurosurgery. 1988;22:398-408.
Dario A., Dorizzi A., Scamoni C., et al. Iatrogenic intracranial aneurysm. Case report and review of the literature. J Neurosurg Sci. 1997;41:195-202.
du Trevou M.D., van Dellen J.R. Penetrating stab wounds to the brain: the timing of angiography in patients presenting with the weapon already removed. Neurosurgery. 1992;31:905-911.
Dubey A., Sung W.S., Chen Y.Y., et al. Traumatic intracranial aneurysms: a brief review. J Clin Neurosci. 2008;15:609-612.
Guillaume DJ, Haddad FS, Haddad GF, Hitchon PW. Diagnosis and management of traumatic intracranial aneurysms. In: Schmideck HH and Sweet WH (eds): Operative Neurosurgical Techniques. Philadelphia: Elsevier, 2005, pp 1224-1232.
Haddad F.S., Haddad G.F., Taha J. Traumatic intracranial aneurysms caused by missiles: their presentation and management. Neurosurgery. 1991;28:1-7.
Horowitz M., Albright A.L., Jungreis C., et al. Endovascular management of a basilar artery false aneurysm secondary to endoscopic third ventriculostomy: case report. Neurosurgery. 2001;49:1461-1465.
Kiek C.F., de Villiers J.C. Vascular lesion due to transcranial stab wounds. J Neurosurg. 1984;60:42-46.
Kumar M., Kitchen N.D. Infective and traumatic aneurysms. Neurosurg Clin North Am. 1998;9:577-586.
Larson P.S., Reisner A., Morassutti D.J., et al. Traumatic intracranial aneurysms. Neurosurgical Focus. 8(1), 2000. Article 4
Levy M.L., Rezai A., Masri L.S., et al. The significance of subarachnoid hemorrhage after penetrating craniocerebral injury: correlations with angiography and outcome in a civilian population. Neurosurgery. 1993;32:532-540.
Morand M., de Tribolet N. Traumatic aneurysm of the posterior inferior cerebellar artery: case report. Neurosurgery. 1991;29:438-441.
Nakstad P., Nornes H., Hauge H.N. Traumatic aneurysm of the pericallosal arteries. Neuroradiology. 1986;28:235-240.
Parkinson D: Surgical management of traumatic intracranial aneurysms. In Schmideck HH and Sweet WH (eds): Operative Neurosurgical Techniques. Orlando: Grune & Stratton, 1988, pp 991-995.
Pozzati E., Gaist G., Servadei F. Traumatic aneurysms of the supraclinoid internal carotid artery. Report of two cases. J Neurosurg. 1982;57:418-422.
Redekop G., Marotta T., Weill A. Treatment of traumatic aneurysms and arteriovenous fistulas of the skull base by using endovascular stents. J Neurosurg. 2001;95:412-419.
Spetzler R.F., Owen M.P. Extracranial–intracranial bypass to a single branch of the middle cerebral artery in the management of traumatic aneurysm. Neurosurgery. 1979;4:334-337.
Teksam M., McKinney A., Casey S., et al. Multi-section CT angiography for detection of cerebral aneurysms. AJNR Am J Neuroradiol. 2004;25:1485-1492.
Türeyen K. Traumatic intracranial aneurysms after blunt trauma. Br J Neurosurg. 2001;15:429-431.
Uzan M., Cantasdemir M., Seckin M.S., et al. Traumatic intracranial carotid tree aneurysms. Neurosurgery. 1998;43:1314-1322.
Vishteh A.G., Marciano F.F., David C.A., et al. Long-term graft patency rates and clinical outcomes after revascularization for symptomatic traumatic internal carotid artery dissection. Neurosurgery. 1998;43:761-767.
Yonas H., Dujovny M. “True” traumatic aneurysm of the intracranial internal carotid artery: case report. Neurosurgery. 1980;7:499-502.
1. Ahmadi J., Levy M.L., Aarabi B., Giannotta S.L. Vascular lesion resulting from head injury. In: Wilkins R.H., Rengachary S.S. Neurosurgery. New York: McGraw-Hill; 1996:2821-2840.
2. Larson P.S., Reisner A., Morassutti D.J., et al. Traumatic intracranial aneurysms. Neurosurgical Focus. 8(1), 2000. Article 4
3. Amirjamshidi A., Rahmat H., Abbassioun K. Traumatic aneurysms and arteriovenous fistulas of intracranial vessels associated with penetrating head injuries occurring during war: principles and pitfalls in diagnosis and management. A survey of 31 cases and review of the literature. J Neurosurg. 1996;84:769-780.
4. Britz G.W., Newell D.W., West G.A., Winn H.R. Traumatic cerebral aneurysms secondary to penetrating intracranial injuries. In: Winn R.H., editor. Youmans Neurological Surgery. Philadelphia: Saunders; 2004:2131-2135.
5. Dubey A., Sung W.S., Chen Y.Y., et al. Traumatic intracranial aneurysms: a brief review. J Clin Neurosci. 2008;15:609-612.
6. Parkinson D., West M. Traumatic intracranial aneurysms. J Neurosurg. 1980;52:11-20.
7. Teksam M., McKinney A., Casey S., et al. Multi-section CT angiography for detection of cerebral aneurysms. AJNR Am J Neuroradiol. 2004;25:1485-1492.
8. Guillaume DJ, Haddad FS, Haddad GF, Hitchon PW. Diagnosis and management of traumatic intracranial aneurysms. In: Schmideck HH and Sweet WH (eds): Operative Neurosurgical Techniques. Philadelphia: Elsevier, 2005, pp 1224-1232.
9. Buckingham M.J., Crone K.R., Ball W.S., et al. Traumatic intracranial aneurysms in childhood: two cases and a review of the literature. Neurosurgery. 1988;22:398-408.
10. Parkinson D. Surgical management of traumatic intracranial aneurysms. In: Schmideck HH and Sweet WH (eds): Operative Neurosurgical Techniques. Orlando: Grune & Stratton, 1988, pp 991-995.
11. Chadduck W.M. Traumatic cerebral aneurysm due to speargun injury: case report. J Neurosurg. 1969;31:77-81.
12. Holmes B., Harbaugh R.E. Traumatic intracranial aneurysms: a contemporary review. J Trauma. 1993;35:855-860.
13. Kumar M., Kitchen N.D. Infective and traumatic aneurysms. Neurosurg Clin North Am. 1998;9:577-586.
14. Raimondi A.J., Yashon D., Reyes C., Yarzagaray L. Intracranial false aneurysms. Neurochirurgia (Stuttg). 1968;11:219-233.
15. Yonas H., Dujovny M. “True” traumatic aneurysm of the intracranial internal carotid artery: case report. Neurosurgery. 1980;7:499-502.
16. Türeyen K. Traumatic intracranial aneurysms after blunt trauma. Br J Neurosurg. 2001;15:429-431.
17. Aarabi B. Traumatic aneurysms of brain due to high velocity missile head wounds. Neurosurgery. 1988;22:1056-1063.
18. Kiek C.F., de Villiers J.C. Vascular lesion due to transcranial stab wounds. J Neurosurg. 1984;60:42-46.
19. Courville C.B. Traumatic aneurysm of an intracranial artery: description of a lesion incident to a shotgun wound of the skull and brain. Bull Los Angeles Neurol Soc. 1960;25:48-54.
20. Haddad F.S., Haddad G.F., Taha J. Traumatic intracranial aneurysms caused by missiles: their presentation and management. Neurosurgery. 1991;28:1-7.
21. Spetzler R.F., Owen M.P. Extracranial–intracranial bypass to a single branch of the middle cerebral artery in the management of traumatic aneurysm. Neurosurgery. 1979;4:334-337.
22. Burton R. Massive epistaxis from a ruptured traumatic internal carotid aneurysm. Med J Aust. 1973;1:692-694.
23. Pozzati E., Gaist G., Servadei F. Traumatic aneurysms of the supraclinoid internal carotid artery. Report of two cases. J Neurosurg. 1982;57:418-422.
24. Nakstad P., Nornes H., Hauge H.N. Traumatic aneurysm of the pericallosal arteries. Neuroradiology. 1986;28:235-240.
25. Benoit B.G., Wortzman G. Traumatic cerebral aneurysms: clinical features and natural history. J Neurol Neurosurg Psychiatry. 1973;36:127-134.
26. Dario A., Dorizzi A., Scamoni C., et al. Balcone Grimaldi G: Iatrogenic intracranial aneurysm. Case report and review of the literature. J Neurosurg Sci. 1997;41:195-202.
27. Horowitz M., Albright A.L., Jungreis C., et al. Endovascular management of a basilar artery false aneurysm secondary to endoscopic third ventriculostomy: case report. Neurosurgery. 2001;49:1461-1465.
28. Levy M.L., Rezai A., Masri L.S., et al. The significance of subarachnoid hemorrhage after penetrating craniocerebral injury: correlations with angiography and outcome in a civilian population. Neurosurgery. 1993;32:532-540.
29. du Trevou M.D., van Dellen J.R. Penetrating stab wounds to the brain: the timing of angiography in patients presenting with the weapon already removed. Neurosurgery. 1992;31:905-911.
30. Morand M., de Tribolet N. Traumatic aneurysm of the posterior inferior cerebellar artery: case report. Neurosurgery. 1991;29:438-441.
31. Uzan M., Cantasdemir M., Seckin M.S., et al. Traumatic intracranial carotid tree aneurysms. Neurosurgery. 1998;43:1314-1322.
32. Redekop G., Marotta T., Weill A. Treatment of traumatic aneurysms and arteriovenous fistulas of the skull base by using endovascular stents. J Neurosurg. 2001;95:412-419.
33. Vishteh A.G., Marciano F.F., David C.A., et al. Long-term graft patency rates and clinical outcomes after revascularization for symptomatic traumatic internal carotid artery dissection. Neurosurgery. 1998;43:761-767.