Chapter 226 Management of Traumatic Bilateral Jumped Cervical Facet Joints in a Patient with Incomplete Myelopathy
Closed Traction Reduction Then Surgery
Epidemiology of Cervical Spine Trauma and Spinal Cord Injury
Fractures and dislocations of the spinal column can be devastating injuries, with 10% to 25% of patients experiencing neurologic deficits.1,2 In patients with cervical spine trauma, up to 40% may have neurologic deficits.3 The NEXUS (National Emergency X-Radiography Utilization Study) group reported a 2.4% incidence of cervical spine injury with a heavy male predominance (64.8%).4,5 There is a bimodal age distribution for cervical spine injury, with the highest incidences at ages 15 to 45 and 65 to 85.6 Motor vehicle accidents, falls, and sporting accidents with direct loading of the head are the most common causes of injury.7
The majority of cervical spine injuries occur in the subaxial spine. Two thirds of cervical spine fractures and 75% of dislocations occur in the subaxial cervical spine. Bilateral facet dislocation is a potentially devastating injury and most commonly occurs at C5-6 and C6-7.8 Facet dislocations are a major risk factor for spinal cord injury, with complete spinal cord injury and quadriplegia occurring in 50% to 84% of cases with bilateral facet dislocations.9–13 This highlights the importance of accurate diagnosis and timely treatment of cervical spine injuries.
Neurologic and spinal cord injuries are common in patients with cervical spine trauma; the high cost to society prompted the U.S. Centers for Disease Control and Prevention to establish a system to monitor spinal cord injuries.14 There were an estimated 12,000 new spinal cord injuries in 2008,15 and the incidence is expected to increase to 13,400 in 2010.16 Patients with spinal cord injuries have a shortened life expectancy, with a 10-year survival rate of 86%.17 The most common causes of death are diseases of the pulmonary and cardiovascular system.15,18
Advancements in medical treatment and automobile safety have resulted in fewer complete spinal cord injuries and a higher percentage of incomplete injuries.15,19–21 In the past, diagnosis was often delayed or missed due to poor or inadequate radiographic visualization. Improved and increased use of CT has contributed to more accurate diagnosis and prompt treatment of cervical spine trauma.5 Superior seat belt designs and automobile airbags have reduced mortality and injury severity in motor vehicle accidents.19–24 Fewer cervical fractures and spinal cord injuries occur with proper use of airbags and three-point mechanical restraints.25
Spinal Cord Injuries
Various classification systems have been developed to describe spinal cord injuries. A general method is to describe spinal cord injuries as incomplete or complete. Incomplete injuries have preserved motor or sensory function below the level of injury. However, patients can present only with signs of sacral sparing. Sacral sparing signifies a structural continuity between the sacral motor neurons in the conus medullaris and the cerebral cortex and manifests as perianal sensation, rectal motor function, or great toe flexor activity. Complete spinal cord injuries have total loss of motor and sensory function below the level of injury. A diagnosis of complete spinal cord injury, however, cannot be made until the resolution of spinal shock, a transient state of complete spinal areflexia that typically resolves after 24 hours.26 During spinal shock no spinal cord function is present, including sacral sparing. Return of sacral reflexes such as the S3-4–mediated bulbocavernosus reflex, or anal wink, heralds the end of spinal shock. If no sacral reflexes return after resolution of spinal shock, the injury can be termed a complete spinal cord injury.
Spinal cord injuries can be graded by the American Spinal Injury Association (ASIA) scale.27 The ASIA scale, a modification of the Frankel scale,28 ranks spinal cord injuries from ASIA A through ASIA E. ASIA A designates a complete spinal cord injury with no motor or sensory function below the level of injury. ASIA B, C, and D are categories of incomplete spinal cord injuries. ASIA B indicates an injury with preservation of distal sensory function but no motor function below the level of spinal injury. ASIA C represents a spinal cord injury with a motor function grade of less than 3 out of 5, whereas an ASIA D injury has a motor function grade of 3 or greater. Patients in the ASIA E category have a normal neurologic examination (Table 226-1).
TABLE 221-1 American Spinal Injury Association Impairment Scale
| Grade | Spinal Cord Injury | Description of Spinal Cord Injury Pattern |
|---|---|---|
| A | Complete | No motor or sensory function below neurologic level |
| B | Incomplete | No motor function below neurologic level; sensory function present below neurologic level |
| C | Incomplete | Motor function preserved below neurologic level; at least half of key muscle groups below injured level have a grade less than 3 |
| D | Incomplete | Motor function preserved below neurologic level; at least half of key muscle groups below injured level have a grade 3 or better |
| E | Normal | Motor and sensory function normal |
Adapted from American Spinal Injury Association: Standards for neurological and functional classification of spinal injury—revised, Chicago, 1992, American Spinal Injury Association.
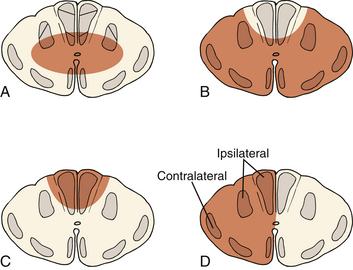
Incomplete spinal cord injuries can also be described by one of several syndromes. These syndromes are based on the anatomic location of the lesion within the spinal cord parenchyma and on clinical presentation (Fig. 226-1).
The most common incomplete spinal cord injury pattern is central cord syndrome. Central gray matter destruction with sparing of the peripheral sacral spinothalamic and corticospinal tracts causes the characteristic findings of greater motor deficits in the upper extremities than in the lower extremities. Central cord syndrome usually occurs in elderly patients with underlying cervical spondylosis who sustain a hyperextension injury during a fall. Most patients present as quadraplegics with perianal sensation. Patients first regain bowel and bladder function followed by motor function. Motor function recovers in a caudal to cranial fashion with sacral motor elements returning first followed by lumbar motor elements. Recovery of upper extremity function is usually minimal and depends on the degree of central gray matter destruction. Functional recovery in central cord syndrome is moderate, with 75% achieving independent ambulatory status and bowel and bladder function.29
Anterior cord syndrome occurs with injury to the anterior two thirds of the spinal column, causing complete loss of motor and sensory function below the level of injury. Patients often retain pressure sense and proprioception of the lower extremities since the dorsal spinal tracts are spared.30 This syndrome has the worst prognosis for functional recovery, with only 10% of patients regaining ambulatory status.31
Posterior cord syndrome involves loss of proprioception and vibrational sensation. This rare syndrome involves injury to the dorsal spinal columns with relative preservation of anterior and lateral column function. Patients have difficulty coordinating movement but maintain motor power, pain, and temperature sensation.32
Brown-Séquard syndrome results from hemisection of the spinal cord from penetrating injuries such as knife and gunshot wounds. This hemisection causes an ipsilateral motor deficit in combination with contralateral loss of pain and temperature sensation. Patients sustain ipsilateral motor loss because motor fibers in the corticospinal tract run ipsilaterally after decussating in the lower medulla. Brown-Séquard syndrome has the best prognosis, with almost all patients regaining bowel and bladder function and the ability to ambulate.33,34
Mechanisms of Cervical Spine Trauma
Mechanisms of cervical spine injury include flexion, extension, axial loading, and rotation. Most injuries occur from rapid head deceleration or indirectly as the result of a blow to the cranium. Fracture patterns depend on the vertebral alignment at the time of injury and the direction of force. A description of the mechanism of injury is based on the magnitude and direction of the forces applied to the head and neck.34 Most injuries are the result of a complex combination of forces.35
Imaging Studies
Radiographic films for evaluating cervical spine injuries include anteroposterior, lateral, and open mouth odontoid views to visualize from the occiput to the first thoracic vertebra. Visualization of the cervicothoracic junction is critical because fractures of C7 and dislocations at the C7-T1 junction account for almost 17% of all injuries.36 Noncontiguous spinal column injuries occur in 10% to 15% of patients; subtle findings such as soft tissue swelling, disc space narrowing or widening, and abnormal interspinous distances may indicate additional injuries.37
CT use is increasingly popular because it provides superior bony anatomy resolution and excellent visualization in regions of the cervical spine that are difficult to assess with plain radiographs, such as the cervicothoracic junction. Fractures of the posterior elements are common; CT scans can easily identify fractures of the pedicle, facet joints, and lamina. The presence of these fractures may influence surgical management. The widespread availability of CT and its superior resolution of bony anatomy have led some radiologists to suggest CT before or in lieu of plain radiographs.38 The medicolegal and economic implications of using CT scans to evaluate and identify cervical spine injuries, however, are considerable and its use remains controversial.39,40
MRI is a valuable tool that can reveal injuries to the intervertebral disc, posterior ligament complex, and vertebral arteries. From 10% to 40% of patients with bilateral facet dislocations have associated disc herniations.41–43 Fortunately, disc herniations with significant cord compression are infrequent and most herniations are clinically insignificant. It is now recognized that much of the stability of the cervical spine is conferred by the posterior ligamentous complex. The ability to detect subtle soft tissue injuries of the posterior capsuloligamentous structures makes MRI an invaluable tool in evaluating and guiding treatment of cervical spine injuries.44 Vertebral artery injuries occur frequently in cervical spine fractures, with reports as high as 44% in traumatic subluxations.45 Although optimal management of vertebral artery injuries remains controversial, MRI and CT angiography are becoming popular methods of evaluating for these injuries in the setting of subaxial cervical trauma.46
Classification of Cervical Spine Injuries
Sir Frank Holdsworth developed the first comprehensive classification system for the spinal column, based on his experience with more than 2000 spinal column injuries. Fractures were categorized based on radiographic appearance. Injuries were described as wedge fractures, shear fractures, burst injuries, extension injuries, dislocations, or rotational fracture-dislocations. Although this system did not differentiate between cervical and thoracolumbar injuries, Holdsworth was the first to recognize the importance of the posterior ligamentous complex.47
The most widely used classification system was proposed by Allen and Ferguson in 1982. Their mechanistic system was developed from a review of 165 patients. Fractures were classified based on static radiograph appearance and documented mechanisms of injury. Six phylogenies were identified based on the position of the cervical spine at the moment of injury and the principle mechanism of load to failure. The categories proposed by the authors were distractive extension, compressive extension, lateral flexion, vertical compression, compressive flexion, and distractive flexion. Each category was further staged based on the severity of the anatomic disruption. Allen and Ferguson placed bilateral facet dislocations in the distraction flexion phylogeny.48
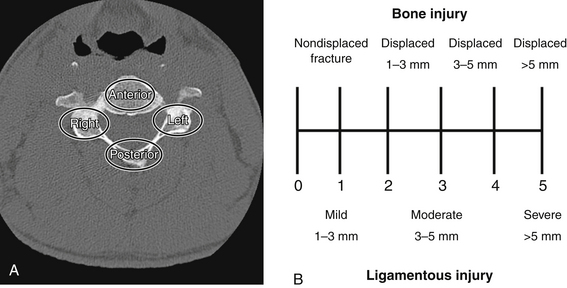
Moore’s Cervical Spine Injury Severity Score evaluates the four columns of the cervical spine to assess stability.49 The four-column model is a modification of the three-column model proposed by Louis50 and includes the anterior column, right pillar, left pillar, and the posterior osseous ligamentous complex. Injury to each of the four columns is assessed on plain radiographs and CT scans and is assigned a score based on the degree of bony displacement and ligamentous disruption (Fig. 226-2). The sum of these four scores is used to determine stability, with higher scores signifying more unstable injuries.
The Subaxial Injury Classification scoring system (SLIC) was developed to guide clinical treatment. This system incorporates the injury morphology, integrity of the discoligamentous complex (DLC), and the patient’s neurologic status into the scoring. Injury morphology is divided into three main categories. The morphologic categories of compression, distraction, and rotation/translation are assigned points according to injury severity. The anatomic components of the DLC, including the intervertebral disc, anterior and posterior longitudinal ligaments, ligamentum flavum, interspinous and supraspinous ligaments, and the facet capsules, are evaluated on radiography, CT, or MRI. Points are given to any potential or definitive DLC injuries. Neurologic status is a key component to the SLIC system; point values are assigned for root injuries, cord injuries, and continuous cord compression with a neurologic deficit. If the total score in the three categories is between 1 and 3, then the injury can be managed nonoperatively. The authors recommend operative treatment for injuries with scores greater than or equal to 551 (Table 226-2).
| Components | Points |
|---|---|
| Morphology | |
| No abnormality | 0 |
| Compression | 1 |
| Burst | +1–2 |
| Distraction (facet perch, hyperextension) | 3 |
| Rotation/Translation (facet dislocation, advanced stage flexion compression injury) | 4 |
| Discoligamentous Complex | |
| Intact | 0 |
| Indeterminate (isolated interspinous widening, MRI signal change only) | 1 |
| Disrupted (widened disc space, facet perch, dislocation) | 2 |
| Neurologic Status | |
| Intact | 0 |
| Root injury | 1 |
| Complete cord injury | 2 |
| Incomplete cord injury | 3 |
| Continuous cord compression in setting of neuro deficit | +1 |
Total score derived from three main components: injury morphology, integrity of the discoligamentous complex, and the neurologic status.
Adapted from Vaccaro AR, Hulbert RJ, Patel AA, et al: The Subaxial Cervical Spine Injury Classification system: a novel approach to recognize the importance of morphology, neurology, and integrity of the disco-ligamentous complex. Spine (Phila Pa 1976) 32:2367, 2007.
Bilateral Facet Dislocations
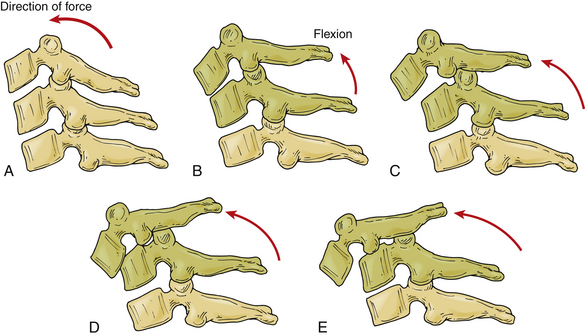
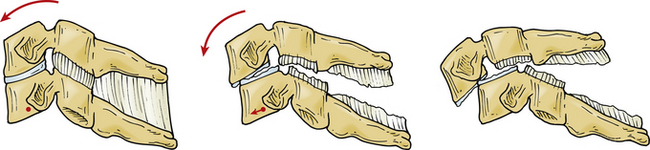
Facet dislocation and fracture-dislocations represent a spectrum of osteoligamentous injury. Allen and Ferguson placed these injuries in the distraction flexion phylogeny of their classification system. Facet subluxations are classified as stage I injuries and unilateral facet dislocations are classified as stage II injuries. Anterior translation of the vertebra indicates a bilateral facet dislocation. Injuries with 50% anterior vertebral translation are stage III injuries; injuries with complete dislocation are classified as stage IV48 (Fig. 226-3).
Pathomechanics of Bilateral Facet Dislocation
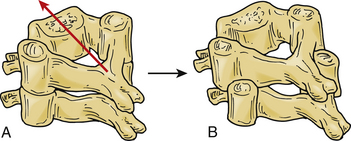
The dynamics of cervical spine injuries are extraordinarily complex.52,53 Bilateral facet dislocations are most often caused by hyperflexion in combination with some rotation35,54 (Fig. 226-4). These injuries are frequently the result of motor vehicle accidents, falls, or head-first dives into shallow water.7 Flexion is resisted by the posterior structures, including the supraspinous and interspinous ligaments, the ligamentum flavum, and the facet capsules. Hyperflexion injuries occur when the head is flexed beyond the physiologic limits of the cervical bone and ligamentous complex. The distraction force during hyperflexion creates tension in the posterior structures and causes rupture to occur in a posterior to anterior direction. Cadaveric dissections of bilateral facet dislocations show significant injury to all the posterior ligamentous components, including the ligamentum flavum, facet capsules, and disc anulus. Often the only remaining intact structure is the anterior longitudinal ligament.54,55 Injuries to the anulus are of particular importance because the nucleus pulposus and portions of the anulus can retropulse into the spinal canal and compress the spinal cord and neural elements.41–43
Bilateral Facet Dislocation and Spinal Cord Injury
Cervical facet dislocations have a high association with spinal cord injury. Bilateral facet dislocations result in complete spinal cord injury in 50% to 84% of cases.9–13 These injuries cause dynamic spinal cord compression and narrowing of the space available for the spinal cord. The spinal cord can experience more compression than postinjury radiographs demonstrate.56 Ivancic et al. simulated dynamic cord compression during the time of injury and measured significant cord compression of 35% to 88%.57 Ebraheim et al. investigated the effect of anterior vertebral translation on spinal canal area. Anterior translation of 6 mm, approximately 50% anterior vertebral translation, decreased spinal canal area by more than 50%.58 Kang et al. demonstrated that spinal cord injury is associated with the space available for the cord after the injury. A sagittal canal diameter of less than 13 mm at the level of injury was highly associated with spinal cord injury.59
Management of Bilateral Facet Dislocations
Treatment of patients with potential spine trauma begins before arrival at the hospital. The cervical spine is immediately placed in a collar and the patient evaluated with Advanced Trauma Life Support protocol.60 The patient should be moved and transported on a rigid spine board. A detailed history of the injury events can provide valuable insight on the energy level and mechanism of injury. During the primary survey, the ABCs of basic life support are assessed and a thorough physical examination is performed.61 A detailed neurologic examination will reveal any neurologic deficits. Careful attention to hemodynamic status may reveal neurogenic shock. Neurogenic shock is vascular hypotension with bradycardia caused by traumatic disruption of sympathetic outflow and unopposed vagal tone.61,62 Hypotensive, bradycardic patients who do not respond to fluid resuscitation have neurogenic shock and should be treated with vasopressive agents such as epinephrine. Evaluation of the cervical spine begins with removal of the collar and palpation of the posterior neck. Any midline or paraspinal tenderness may indicate a cervical injury.4,63 The relative position of the head and neck should be noted; any angular or rotational deformities may suggest a unilateral dislocation. The remainder of the axial spine can be palpated after logrolling the patient.
The role of pharmacologic treatment in patients with suspected spinal cord injury is controversial. Administration of high-dose steroids has been the standard of practice in North America for more than a decade. The National Acute Spinal Cord Injury Study (NASCIS) II established a dosing schedule of an initial intravenous bolus of 30 mg/kg, followed by a continuous infusion of 5.4 mg/kg/hour for an additional 23 hours. The data showed that significantly better neurologic outcomes occurred when high-dose methylprednisolone (MPPS) was administered within 8 hours of injury.64,65 Data from NASCIS III found that patients who received a bolus of MPSS within 3 hours of injury benefited from a 24-hour infusion of MPPS. Patients treated from 3 to 8 hours after injury benefited from a prolonged 48-hour infusion of MPPS.66,67 Recently the data and methodology of the NASCIS II and III studies have been placed under scrutiny. Criticism of these studies has led some Canadian institutions to discontinue high-dose MPPS for spinal cord injuries. Although there is continued controversy over the use of MPPS, new neuroprotective agents are under investigation.
Celthrin is a Rho pathway antagonist that may have neuroprotective and neuroregenerative properties. Rho, a GTPase-associated signaling protein, plays a key role in the pathways that inhibit neuronal survival and regeneration. Rho inhibition leads to decreased apoptosis and astroglial scar formation. Celthrin is currently in clinical trials investigating its use in ASIA A patients undergoing operative treatment within 2 weeks of injury.68
Riluzole is a benzothiazole anticonvulsant sodium channel blocker that has already received approval from the U.S. Food and Drug Administration for the treatment of amyotrophic lateral sclerosis. Riluzole also demonstrates neuroprotective and neuroregenerative properties in rat models and is under investigation for use in spinal cord injuries.69,70
Magnesium within a polyethylene glycol formulation has shown promise as a neuroprotective agent in a variety of neurotrauma settings, including stroke and brain injury. Its use in a thoracic spinal cord injury rat model has shown significant neuroprotective effects; human clinical trials are currently being planned.71
Most cervical injuries can be diagnosed with a thorough and careful evaluation of plain radiographs. In bilateral facet dislocations, the cervical spine often assumes a kyphotic alignment with widening of the spinous processes signifying a posterior ligamentous injury. Widened spinous processes combined with anterior vertebral translation suggest a bilateral facet dislocation. Bilateral facet dislocations have 50% or greater anterolisthesis on lateral radiographs (Fig. 226-5). Subtle disc space narrowing may indicate an associated disc herniation.54 CT scans can help define the bony anatomy. Fractures of the pedicle, facets, and lamina as well as cervical malalignment are easily visualized on CT. MRI can provide information about the soft tissues, including signal change in the spinal cord, the integrity of the posterior ligamentous complex, and the presence of disc herniations.44,72
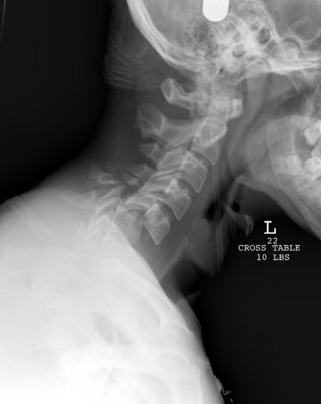
Initial treatment for a cooperative, awake patient with a bilateral facet dislocation consists of closed reduction with tong traction.73 Gardner-Wells tongs are applied to the skull after the skin is prepared with a povidone-iodine solution and infiltrated to the level of the skull periosteum with a local anesthetic. Apply MRI-compatible tongs if an MRI is anticipated. The pins are positioned 1 cm above the tips of the ears and in line with the external auditory meatus and tightened until the spring-loaded plunger protrudes 1 mm. After tightening the locking nuts, the weights are applied with a rope and pulley. An initial traction weight of 5 to 10 pounds is added to counter the weight of the head, and traction weight is increased in increments of 5 to 10 pounds at 15-minute intervals. After each successive weight a neurologic examination is performed and a lateral radiograph is obtained to assess the reduction. Radiographs should be scrutinized for signs of overdistraction, such as widening of the intervertebral discs and facet joints. The head can be gently flexed and rotated to disengage a locked facet. Weight is increased until the dislocation is reduced. There is great disparity in the literature regarding the safe weight limit for a closed reduction.74 Successful reductions may require weights up to 140 pounds (63.6 kg); Sabiston et al. demonstrated that traction totaling up to 70% of body weight is safe.75
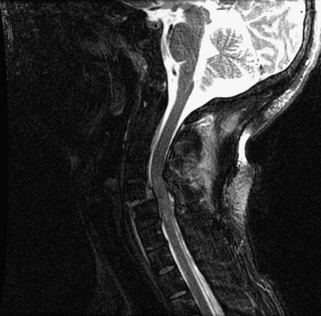
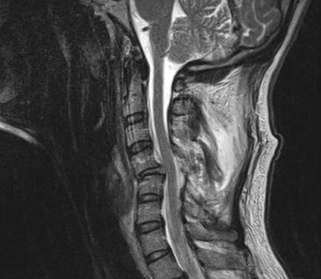
Prompt closed reduction can prevent neurologic deterioration and improve neurologic status. Reduction within the first few hours can lead to dramatic improvement, and reversal of tetraplegia has been reported with successful reduction within 2 hours of injury.76–79 Any neurologic deterioration during closed reduction in an awake, cooperative patient warrants an MRI, which can provide invaluable information, including any signal change in the spinal cord and the presence of disc herniations.44 Obtaining an MRI to assess for disc herniation before closed reduction is controversial.41,42,78,80,81 If a patient is awake, alert, and cooperative for neurologic examinations, closed reduction with sequential weights and intermittent neurologic examinations has been shown to be safe if the closed reduction is aborted and a MRI obtained at the first sign of neurologic abnormality.82 Any patient who is obtunded, anesthetized, unreliable, or uncooperative for a neurologic examination should undergo MRI before a closed reduction is initiated.43 If the MRI in either of these scenarios demonstrates extruded disc material, surgical decompression may be preferable before a reduction procedure. What constitutes a disc herniation in the setting of facet dislocations is controversial.83 Vaccaro et al. defined disc herniation as material with signal consistent with nucleus pulposus protruding posterior to the cortical wall of the subjacent vertebral body.84 However, most surgeons regard any disc material that may result in spinal cord compression on reduction as significant and warranting consideration of anterior discectomy (Fig. 226-6).
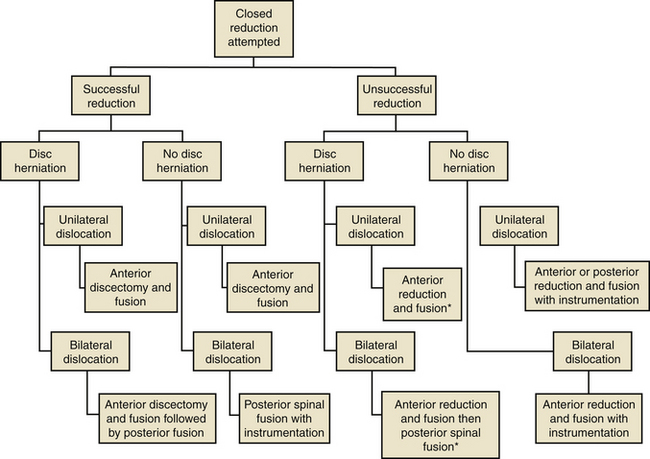
Open reduction is indicated if closed reduction is unsuccessful or if the patient experiences neurologic deterioration during closed reduction. Open reduction is also indicated in obtunded patients and in those with delayed presentations, in whom closed reduction is likely unsuccessful. Surgical treatment of cervical facet dislocations is highly variable, and several techniques have been described for achieving open reduction, including anterior approach alone, posterior approach alone, and combined approaches. The choice of approach is guided by whether an MRI demonstrates disc herniation85 (Fig. 226-7).
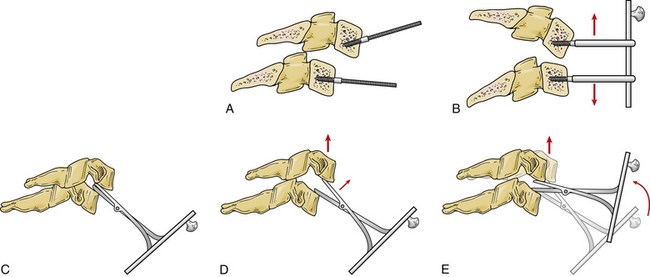
Unsuccessful closed reductions with a herniated disc should be first addressed with an anterior approach. Anterior open reduction is technically more challenging but allows for removal of any extruded disc herniation before reduction. After discectomy, a reduction is performed under spinal cord monitoring with distraction and gentle manipulation. Distraction is obtained through traction via the skull tongs or distraction across Caspar pins placed in the vertebral bodies. Alternatively, the distractor can be placed directly into the disc space after discectomy86,87 (Fig. 226-8). Once there is adequate distraction to disengage the facets, reduction is achieved with gentle pressure or levering of the anteriorly translated vertebrae back into position. A study by Reindl et al. demonstrated that most cervical dislocations can be safely reduced through an anterior approach.87 After reduction, stabilization involves arthrodesis with a structural graft and plate fixation. Anterior discectomy and fusion for bilateral facet dislocation results in a high rate of arthrodesis; Ordonez et al. reported a fusion rate of 90% with anterior decompression, reduction, and fusion.88 However, failure with loss of reduction requiring revision surgery with anterior approach alone occurs in 13% of cases, and the addition of posterior fixation may be considered.89
If anterior reduction is unsuccessful, the anterior wound is closed and a posterior reduction and fusion is performed followed by a return to the anterior spine for grafting and fusion. Several surgical techniques can avoid the need to return to the anterior exposure. The graft can be placed in the anterior interspace in an exaggerated anterior position with the expectation that the posterior reduction maneuver will pull the graft into an acceptable position. Another method places a junction plate on the superior vertebral body in a position to hold the bone graft. The anterior surface of the graft is placed in line with the anterior cortex of the superior vertebral body, and a posterior reduction maneuver will reduce the superior vertebral body, plate, and bone graft into proper position.90
In the absence of a herniated disc on MRI, a safe reduction can be approached posteriorly. Facet dislocations have historically been treated with posterior procedures. The advantages of a posterior approach include the ability to decompress and expand the spinal canal indirectly, the relative ease of reduction, and the ability to visualize the dislocated facet joints directly.85 A reduction is achieved by applying a distracting force across the dislocated level to disengage the facet joints. Bone tenaculum or towel clips placed at the spinolaminar junction can provide a distractive force. A nerve hook or Penfield placed in the medial aspect of the dislocated facets can help maneuver and reduce the facet joints. Distraction can also be achieved by placing a modified laminar spreader across the dislocated segment.91 Alternatively the superior aspect of the superior articular process can be removed with a high-speed burr or a Kerrison punch if the facets are locked. However, resection of the facet joint may decrease stability once reduction is achieved. After reduction, arthrodesis with instrumentation is performed. Instrumentation techniques, including lateral mass screw fixation, pedicle screw fixation, and posterior wiring, are effective in maintaining reduction and long-term stability, and result in good patient outcomes.92–94 Posterior spinal fusion with instrumentation following reduction is effective, with excellent fusion rates.95 However, significant postoperative kyphosis with posterior fixation alone has been reported.96,97 Elgafy et al. suggested that disc disruption occurs with distraction injuries and leads to disc space collapse and progressive kyphosis.98 The tendency of some posterior stand-alone constructs to fall into kyphosis has led some authors to recommend anterior plating for bilateral facet dislocations.96
Timing of surgical intervention in cervical dislocations is controversial. Although no definitive data demonstrates that early treatment results in improved neurologic recovery in humans, data from NASCIS II and studies by Belanger and Levi suggest that optimal timing of surgical decompression is less than 24 hours from time of injury.64,99,100 Early decompression and removal of spinal cord compression can significantly improve neural recovery. Many researchers believe that optimal timing of surgical intervention is within 3 hours; several case reports note complete reversal of spinal cord injury when reduction and decompression were performed within the first 3 hours.78 Various animal studies also support early treatment. Delamarter et al. found that early (within 1 hour) removal of constrictive bands placed in dog spinal canals resulted in full clinical recovery.101 Carlson et al. demonstrated effective recovery of electrophysiologic function in dogs when spinal cord compression was removed within 1 to 3 hours.102 These animal experiments have shown that early decompression can result in improved neurologic outcomes; currently the Surgical Treatment of Spinal Cord Injury Study (STACIS), a prospective, multicenter study, has begun to investigate surgical timing in humans.103
Allen B.L.Jr., Ferguson R.L., Lehmann T.R., O’Brien R.P. A mechanistic classification of closed, indirect fractures and dislocations of the lower cervical spine. Spine (Phila Pa 1976). 1982;7:1-27.
Belanger E., Levi A.D. The acute and chronic management of spinal cord injury. J Am Coll Surg. 2000;190:603-618.
Hoffman J.R., Mower W.R., Wolfson A.B., et al. Validity of a set of clinical criteria to rule out injury to the cervical spine in patients with blunt trauma. N Engl J Med. 2000;343:94-99.
Nassr A., Lee J.Y., Dvorak M.F., et al. Variations in surgical treatment of cervical facet dislocations. Spine (Phila Pa 1976). 2008;33:E188-E193.
Ordonez B.J., Benzel E.C., Naderi S., et al. Cervical facet dislocation: techniques for ventral reduction and stabilization. J Neurosurg. 2000;92:18-23.
O’Brien P.J., Schweigel J.F., Thompson W.J. Disclocations of the lower cervical spine. J Trauma. 1982;22:710-714.
Roaf R. A study of the mechanics of spinal injury. J Bone Joint Surg [Br]. 1960;42:810-823.
Vaccaro A.R., Daugherty R.J., Sheehan T.P., et al. Neurological outcome of early versus late surgery for cervical spinal cord injury. Spine (Phila Pa 1976). 1997;22:2609-2613.
1. D.R. Benson, T.L. Keenen, J. Antony. Unsuspected associated findings in spinal fractures. J Orthop Trauma. 1989;3:160.
2. Riggins R.S., Kraus J.F. The risk of neurologic damage with fractures of the vertebrae. J Trauma. 1977;7:126-133.
3. Bohlman H.H. Acute fractures and dislocations of the cervical spine. J Bone Joint Surg [Am]. 1979;61:1119-1142.
4. Hoffman J.R., Mower W.R., Wolfson A.B., et al. Validity of a set of clinical criteria to rule out injury to the cervical spine in patients with blunt trauma. N Engl J Med. 2000;343:94-99.
5. Jackson R.S., Banit D.M., Rhyne A.L.III, Darden B.V. Upper cervical spine injuries. J Am Acad Orthop Surg. 10, 2002. 71–280
6. Lowery D.W., Wald M.M., Browne B.J., et al. Epidemiology of cervical spine injury victims. Ann Emerg Med. 2001;38:12-16.
7. Stover S.L., Fine P.R. Spinal cord injury: the facts and figures. Birmingham: The University of Alabama at Birmingham; 1986.
8. Goldberg W., Mueller D., Panacek E., et al. Distribution and patterns of blunt traumatic cervical spine injury. Ann Emerg Med. 2001;38:17-21.
9. Wolf A., Levi L., Mirvis S., et al. Operative management of bilateral facet dislocation. J Neurosurg. 1991;75:883-890.
10. O’Brien P.J., Schweigel J.F., Thompson W.J. Disclocations of the lower cervical spine. J Trauma. 1982;22:710-714.
11. Lintner D.M., Knight R.Q., Cullen J.P. The neurologic sequelae of cervical spine facet injuries: the role of canal diameter. Spine (Phila Pa 1976). 1993;18:725-729.
12. Hadley M.N., Fitzpatrick B.C., Sonntag V.K., et al. Facet fracture-dislocation injuries of the cervical spine. Neurosurgery. 1992;30:661-666.
13. Bedbrook G.M., Sakae T. A review of cervical spine injuries with neurological dysfunction. Paraplegia. 1982;20:321-333.
14. Centers for Disease Control. Acute traumatic spinal cord injury surveillance—U.S.: 1987. MMWR CDC Surveill Summ. 1987;37:285-286.
15. Spinal cord injury: the facts and figures at a glance 2008. Birmingham: The University of Alabama at Birmingham; 2008.
16. Stripling T.E. The cost of economic consequences of traumatic spinal cord injury. Paraplegia News. 1990:50-54.
17. Stover S.L., Fine P.R. The epidemiology and economics of spinal cord injury. Paraplegia. 1987;25:225-228.
18. Wood G.W.2nd. Fractures, dislocations, and fracture-dislocations of the spine. Campbell’s Operative Orthopaedics. 2008;11(2):1761-1850.
19. Lund A.K., Ferguson S.A. Driver fatalities in 1985–1993 cars with airbags. J Trauma. 1995;38:469-475.
20. O’Neill B., Lund A.K. The effectiveness of air bags in preventing driver fatalities in the United States. Presented at The International Conference on Air Bags and Seat Belts: Evaluation and Implications for Public Policy. Montreal: Canada; 1992.
21. Zador P.L., Ciccone M.A. Automobile driver fatalities in frontal impacts: airbags compared with manual belts. Am J Public Health. 1993;83:661-666.
22. National Highway Traffic Safety Administration. FMVSS 208 regulatory impact analysis. Washington, DC: Department of Transportation; 1984.
23. Evans L. Fatality risk reduction from safety belt use. J Trauma. 1987;27:746-749.
24. Orsay E.M., Turnbull T.L., Dunne M., et al. Prospective study of the effect of safety belts on morbidity and health care costs in motor-vehicle accidents. JAMA. 1988;260:3598-3603.
25. Donaldson W.F., Hanks S.E., Ahmad N., et al. Cervical spine injuries associated with the incorrect use of airbags in motor collisions. Spine (Phila Pa 1976). 2008;33:631-634.
26. Stauffer E.S. Diagnosis and prognosis of the acute cervical spinal cord injury. Clin Orthop. 1975;112:9-15.
27. American Spinal Injury Association. Standards for neurological and functional classification of spinal injury. Chicago: American Spinal Injury Association; 1992. —revised
28. Frankel H., Hancock D.O., Hyslop G., et al. The value of postural reduction in the initial management of closed injuries to the spine with paraplegia and tetraplegia. Paraplegia. 1969;7:179-192.
29. Stauffer ES. A quantitative evaluation of neurologic recovery following cervical spinal cord injuries. Presented at the Third Annual Meeting of the Federation of Spine Associates, Paper 39. Atlanta, Georgia, February 1988.
30. Schneider R.C. The syndrome of acute anterior cervical spinal cord injury. J Neurosurg. 1955;12:95-122.
31. Stauffer E.S. Neurologic recovery following injuries to the cervical spinal cord and nerve roots. Spine. 1984;9:532-534.
32. McKinley W., Santos K., Meade M., Brooke K. Incidence and outcomes of spinal cord injury clinical syndromes. J Spinal Cord Med. 2007;30(3):215-224.
33. Bosh A., Stauffer E.S., Nickel V.L. Incomplete traumatic quadriplegia: a ten-year review. JAMA. 1971;216:473-478.
34. White A.A.III, Panjabi M.M. Clinical biomechanics of the spine. Philadelphia: Lippincott; 1978.
35. Roaf R. A study of the mechanics of spinal injury. J Bone Joint Surg [Br]. 1960;42:810-823.
36. Kwon B.K., Vaccaro A.R., Grauer J.N., et al. Subaxial cervical spine trauma. J Am Acad Orthop Surg. 2006;14:78-89.
37. Vaccaro A.R., An H.S., Lin S., et al. Noncontiguous injuries of the spine. J Spinal Disord. 1992;5:320-329.
38. LeBlang S.D., Nunez D.B.Jr. Helical CT of cervical spine and soft tissue injuries of the neck. Radiol Clin North Am. 1999;37:515-532.
39. Rybicki F., Nawfel R.D., Judy P.F., et al. Skin and thyroid dosimetry in cervical spine screening: two methods for evaluations and a comparison between a helical CT and radiographic trauma series. Am J Roentgenol. 2002;179:933-937.
40. Fisher A., Young W.F. Is the lateral cervical spine x-ray obsolete during the initial evaluation of patients with acute trauma? Surg Neurol. 2008;70:53-57.
41. Rizzolo S.J., Piazza M.R., Cotler J.M., et al. Intervertebral disc injury complicating cervical spine trauma. Spine (Phila Pa 1976). 1991;16(Suppl 6):187-189.
42. Doran S.E., Papadopoulos S.M., Ducker T.B., Lillehei K.O. Magnetic resonance imaging documentation of coexistent traumatic locked facets of the cervical spine and disc herniation. J Neurosurg. 1993;79:341-345.
43. Eismont F.J., Arena M.J., Green B.A. Extrusion of an intervertebral disc associated with traumatic subluxation or dislocation of cervical facets. Case report. J Bone Joint Surg [Am]. 1991;73:1555-1560.
44. Schaefer D.M., Flanders A., Northrup B.E., et al. Magnetic resonance imaging of acute cervical spine trauma. Correlation with severity of neurologic injury. Spine (Phila Pa 1976). 1989;14:1090-1095.
45. Miller P.R., Fabian T.C., Croce M.A., et al. Prospective screening for blunt cerebrovascular injuries: analysis of diagnostic modalities and outcomes. Ann Surg. 2002;236:386-395.
46. Fassett D.R., Dailey A.T., Vaccaro A.R. Vertebral artery injuries associated with cervical spine injuries: a review of the literature. J Spinal Disord Tech. 2008;21:252-258.
47. Holdsworth F. Fractures, dislocations, and fracture-dislocations of the spine. J Bone Joint Surg [Am]. 1970;52:1534-1551.
48. Allen B.L.Jr., Ferguson R.L., Lehmann T.R., O’Brien R.P. A mechanistic classification of closed, indirect fractures and dislocations of the lower cervical spine. Spine (Phila Pa 1976). 1982;7:1-27.
49. Moore T.A., Vaccaro A.R., Anderson P.A. Classification of lower cervical spine injuries. Spine (Phila Pa 1976). 2006;31:S37-S43.
50. Louis R. Spinal stability as defined by the three-column spine concept. Anat Clin. 1985;7:33-42.
51. Vaccaro A.R., Hulbert J., Patel A.A., et al. The subaxial cervical spine injury classification system: a novel approach to recognize the importance of morphology, neurology, and integrity of the disco-ligamentous complex. Spine (Phila Pa 1976). 2007;32:2365-2374.
52. Nightingale R.W., McElhaney J.H., Richardson W.J., Myers B.S. Dynamic responses of the head and cervical spine to axial impact loading. J Biomech. 1996;29:307-318.
53. Reynen P.D., Clancy W.G.Jr. Cervical spine injury, hockey helmets, and face masks. Am J Sports Med. 1994;22:167-170.
54. White A.A.III, Panjabi M.M. The problem of clinical instability in the human spine: a systematic approach. In: White III A.A., Panjabi M.M., editors. Clinical biomechanics of the spine. Philadelphia: Lippincott; 1990:277-378.
55. Beatson T.R. Fractures and dislocations of the cervical spine. J Bone Joint Surg [Br]. 1963;45:21-35.
56. Carter J.W., Mirza S.K., Tencer A.F., Ching R.P. Canal geometry changes associated with axial compressive cervical spine fracture. Spine (Phila Pa 1976). 2000;25:46-54.
57. Ivancic P.C., Pearson A.M., Tominaga Y., et al. Mechanism of cervical spinal cord injury during bilateral facet dislocation. Spine (Phila Pa 1976). 2007;32:2467-2473.
58. Ebraheim N.A., Xu R., Ahmad M., et al. The effect of anterior translation of the vertebra on the canal size in the lower cervical spine: a computer-assisted anatomic study. J Spinal Disord. 1997;10:162-166.
59. Kang J.D., Figgie M.P., Bohlman H.H. Sagittal measurements of the cervical spine in subaxial fractures and dislocations: an analysis of 288 patients with and without neurological deficits. J Bone Joint Surg [Am]. 1994;76:1617-1628.
60. Advanced trauma life support student manual. Chicago: American College of Surgeons; 1989.
61. Grundy D., Swain A., Russell J. ABC of spinal cord injury: early management and complications. I. BMJ. 1986;292:44-47.
62. Piepmeier J.M., Lehmann K.B., Lane J.G. Cardiovascular instability following acute cervical spinal cord trauma. Cent Nerv Sys Trauma. 1985;2:153-160.
63. Stiell I.G., Wells G.A., Vandemheen K.L., et al. The Canadian C-spine rule for radiography in alert and stable trauma patients. JAMA. 2001;286:1841-1848.
64. Bracken M.B., Shepard M.J., Collins W.F., et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405-1411.
65. Bracken M.B., Shepard M.J., Collins W.F.Jr., et al. Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the second National Acute Spinal Cord Injury Study. J Neurosurg. 1992;76:23-31.
66. Bracken M.B., Shepard M.J., Holford T.R., et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597-1604.
67. Bracken M.B., Shepard M.J., Holford T.R., et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up. Results of the third National Acute Spinal Cord Injury randomized controlled trial. J Neurosurg. 1998;89:699-706.
68. Fehlings M.G., Baptiste D.C. Current status of clinical trials for acute spinal cord injury. Injury. 2005;36(Suppl 2):B113-B122.
69. Baptiste D.C., Fehlings M.G. Pharmacological approaches to repair the injured spinal cord. J Neurotrauma. 2006;23:318-334.
70. Schwartz G., Fehlings M.G. Evaluation of neuroprotective effects of sodium channel blockers after spinal cord injury: improved behavioral and neuroanatomical recovery with riluzole. J Neurosurg. 2001;94:245-256.
71. Kwon B.K., Roy J., Lee J., Stammers A., et al. Magnesium in a polyethylene glycol formulation provides neuroprotection after acute spinal cord injury. Spine J. 2008;8:51S.
72. Copes W.S., Champion H.R., Sacco W.J., et al. Progress in characterizing anatomic injury. J Trauma. 1990;30:1200-1207.
73. Star A.M., Jones A.A., Cotler J.M., et al. Immediate closed reduction of cervical spine dislocations using traction. Spine (Phila Pa 1976). 1990;15:1068-1072.
74. Initial closed reduction of cervical spine fracture-dislocation injuries. Neurosurgery. 2002;50:S44-S50.
75. Sabiston C.P., Wing P.C., Schweigel J.F., et al. Closed reduction of dislocations of the lower cervical spine. J Trauma. 1988;28:832-835.
76. Shrosbree R.D. Neurological sequelae of reduction of fracture dislocations of the cervical spine. Paraplegia. 1979;17:212-221.
77. Gillingham J. Letter. J Neurosurg. 1976;44:766-767.
78. Lee A.S., MacLean J.C., Newton D.A. Rapid traction for reduction of cervical spine dislocations. J Bone Joint Surg [Br]. 1994;76:352-356.
79. Bohlman H.H., Anderson P.A. Anterior decompression and arthrodesis of the cervical spine: long-term motor improvement. Part I—improvement in incomplete traumatic quadriparesis. J Bone Joint Surg [Am]. 1992;74:671-682.
80. Tribus C.B. Cervical disk herniation in association with traumatic facet dislocation. Tech Orthop. 1994;9:5-7.
81. Hart R.A., Vaccaro A.R., Nachwalter R.S. Cervical facet dislocation: when is magnetic resonance imaging indicated? Spine (Phila Pa 1976). 2002;27:116-117.
82. Cotler H.B., Miller L.S., DeLucia F.A., et al. Closed reduction of cervical spine dislocations. Clin Orthop. 1987;214:185-199.
83. Grant G.A., Mirza S.K., Chapman J.R., et al. Risk of early closed reduction in cervical spine subluxation injuries. J Neurosurg. 1999;90(1 Suppl):13-18.
84. Vaccaro A.R., Falatyn S.P., Flanders A.E., et al. Magnetic resonance evaluation of the intervertebral disc, spinal ligaments, and spinal cord before and after closed traction reduction of cervical spinal dislocations. Spine (Phila Pa 1976). 1999;24:1210-1217.
85. Nassr A., Lee J.Y., Dvorak M.F., et al. Variations in surgical treatment of cervical facet dislocations. Spine (Phila Pa 1976). 2008;33:E188-E193.
86. De Oliveira J.C. Anterior reduction of interlocking facets in the lower cervical spine. Spine (Phila Pa 1976). 1979;4:195-202.
87. Reindl R., Ouellet J., Harvey E.J., et al. Anterior reduction for cervical spine dislocation. Spine (Phila Pa 1976). 2006;31:648-652.
88. Ordonez B.J., Benzel E.C., Naderi S., et al. Cervical facet dislocation: techniques for ventral reduction and stabilization. J Neurosurg. 2000;92:18-23.
89. Johnson M.G., Fisher C.G., Boyd M., et al. The radiographic failure of single segment anterior cervical plate fixation in traumatic cervical flexion distraction injuries. Spine (Phila Pa 1976). 2004;29:2815-2820.
90. Allred C.D., Sledge J.B. Irreducible dislocations of the cervical spine with a prolapsed disc: preliminary results from a treatment technique. Spine (Phila Pa 1976). 2001;26:1927-1931.
91. Fazl M., Pirouzmand F. Intraoperative reduction of locked facets in the cervical spine by use of a modified interlaminar spreader: Technical note. Neurosurgery. 2001;48:444-445.
92. Abumi K., Shono Y., Kotani Y., et al. Indirect posterior reduction and fusion of the traumatic herniated disc by using a cervical pedicle screw system. J Neurosurg. 2000;92:30-37.
93. Brodke D.S., Anderson P.A., Newell D.W., et al. Comparison of anterior and posterior approaches in cervical spinal cord injuries. J Spinal Disord Tech. 2003;16:229-235.
94. Weiland D.J., McAfee P.C. Posterior cervical fusion with triple-wire strut graft technique: 100 consecutive patients. J Spinal Disord. 1991;4:15-21.
95. Wiseman D.B., Bellabarba C., Mirza S.K., Chapman J. Anterior versus posterior surgical treatment for traumatic cervical spine dislocation. Curr Opin Orthop. 2003;14:174-181.
96. Lifeso R.M., Colucci M.A. Anterior fusion for rotationally unstable cervical spine fractures. Spine (Phila Pa 1976). 2000;25:2028-2034.
97. Fehlings M.G., Cooper P.R., Errico T.J. Posterior plates in the management of cervical instability: long-term results in 44 patients. J Neurosurg. 1994;81:341-349.
98. Elgafy H., Fisher C.G., Zhao Y., et al. The radiographic failure of single segment posterior cervical instrumentation in traumatic cervical flexion distraction injuries. Top Spinal Cord Inj Rehabil. 2006;12:20-29.
99. Janssen L., Hansebout R.R. Pathogenesis of spinal cord injury and newer treatments. A review. Spine. 1989;14:23-32.
100. Belanger E., Levi A.D. The acute and chronic management of spinal cord injury. J Am Coll Surg. 2000;190:603-618.
101. Delamarter R.B., Sherman J., Carr J.B. Pathophysiology of spinal cord injury. Recovery after immediate and delayed decompression. J Bone Joint Surg [Am]. 1995;77:1042-1049.
102. Carlson G.D., Minato Y., Okada A., et al. Early time-dependent decompression of spinal cord injury: vascular mechanisms of recovery. J Neurotrauma. 1997;14:951-962.
103. Vaccaro A.R., Daugherty R.J., Sheehan T.P., et al. Neurological outcome of early versus late surgery for cervical spinal cord injury. Spine (Phila Pa 1976). 1997;22:2609-2613.
Open Reduction
Cervical facet subluxations and dislocations with locked facets account for a significant portion of cervical spine injuries. These are highly unstable injuries that can lead to spinal cord injury. The mechanism of injury is usually due to a severe flexion-distraction injury (Fig. 226-9). The inferior facets of the rostral vertebrae move ventrally over the superior facets of the caudal vertebrae. Neurologically, most patients with bilateral dislocations present with either complete or incomplete spinal cord injuries.1,2 There is often significant dorsal cervical ligamentous damage associated with this injury. Occasionally, a herniated disc is present at the level of injury (Fig. 226-10). Due to their highly unstable nature, reducing and stabilizing this injury is usually necessary. Such can often be accomplished with minimal to moderate manipulation of the spine.
As stated, bilateral facet locking is associated with or caused by significant ligament injury, including rupture of the dorsal ligamentous complex (interspinous and supraspinous ligament injury occurs in 97% of cases), the facet joint capsules, the intervertebral disc and ligamentum flavum in 90%, and usually the anterior (26.7%) and posterior (40%) longitudinal ligaments.3 Most would agree that with this type of injury, rapid realignment and decompression of the spinal cord should give the patient the best chance for neurologic recovery by preventing secondary spinal cord injury. The most common form of treatment, historically, has been to perform an awake closed reduction via cervical traction immediately after establishing the diagnosis, adding general anesthesia, muscle relaxation, and manual traction if necessary, thus reserving open reduction for failed closed reduction. However, closed reduction has been associated with several drawbacks. First, it has failed in a substantial percentage of patients, even when utilizing up to two thirds of the weight of the patient.4 In combined published series for closed reduction, only 75% of closed reductions were successful.1,2,5–7 Second, a traumatic disc herniation has been found in 10% to 80% of bilateral locked facets.8,9 Some studies have shown increased spinal cord compression and worsened neurologic deficit with closed reduction of bilateral facet dislocation associated with disc rupture.10 In attempting closed reduction, the absence of a ligamentous barrier to adverse mechanical tension on the spinal cord should be recognized. Distraction injuries of the spinal cord caused by dislocation may be worsened by the separation of the spine produced by excessive traction weight.6 No upper limit of weight used for reduction has been described in the literature, but clinical, neurologic, and radiographic monitoring should be reassessed throughout the process to avoid overtraction, which might aggravate neurologic deficit. Such occurs in 2% to 4% of patients.11
Early versus delayed reduction remains controversial. Advocacy of early reduction is based on intuition rather than observation. Spinal cord dysfunction is produced by energy transfer at the moment of impact, and by persistent deformity and distortion of the spinal cord. Nothing can be done about the damage produced by energy transfer, and deformity/distortion of the spinal cord should not increase in a properly immobilized patient. Stauffer and Kelly also have noted that early versus late reduction does not seem to affect nerve root recovery.12
Some authors have suggested an immediate open ventral-dorsal fixation/fusion without closed reduction.13 This treatment strategy avoids time loss and patient discomfort from attempted closed reduction by traction, obviates the need for external immobilization, and results in an excellent fusion rate.4 The development of modern internal fixation devices has allowed for early mobilization and has largely replaced halo immobilization. There seems to be a reasonable justification for treating these injuries surgically, given the incidence of associated neurologic injury, frequency of malalignment, failure rates with conservative therapy, and satisfying results with contemporary surgical stabilization and fusion. Several techniques have been described for open reduction, most recently by Fazl and Pirouzmand, who described the use of an interlaminar spreader for intraoperative reduction of locked facets. The interlaminar spreader is a modified vertebral body spreader with revised tips consisting of a double-apposed cup-shaped configuration to fit the laminar borders. They describe its use in 52 patients with bilateral or unilateral dislocations with no complications with its use.14
Displacement of intervertebral disc into the spinal canal can occur during either open or closed reduction and may produce an increase in neurologic deficit. Doran et al., in 1993, illustrated the importance of using MRI to document the presence of a herniated disc during the initial evaluation of a patient with traumatic locked facets of the cervical spine and before attempted reduction of the locked facets. Their experience indicated that closed reduction of facet dislocation that was associated with disc rupture may result in increased spinal cord compression and neurologic deficit. If a herniated disc is discovered, anterior discectomy and fusion would, hence, be favored as the initial therapy over attempts at closed reduction or operative dorsal reduction.8 However, some have found that performing ventral fusion alone in the absence of dorsal ligamentous support has a fairly high rate of failure, thus supporting a 360-fusion for these injuries.12,15,16
Lu K., Lee T.C., Chen H.J. Closed reduction of bilateral locked facets of the cervical spine under general anesthesia. Acta Neurochir (Wien). 1998;140(10):1055-1061.
Maiman D.J., Barolat G., Larson S.J. Management of bilateral locked facets of the cervical spine. Neurosurgery. 1986;18(5):542-547.
Ordonez B.J., Benzel E.C., Nader S., Weller S.J. Cervical facet dislocation: techniques for ventral reduction and stabilization. J Neurosurg. 2000;92:18-23.
Payer M. Immediate open anterior reduction and anterior-posterior fixation/fusion for bilateral cervical locked facet. Acta Neurochir (Wien). 2005;147:509-514.
Robertson P.A., Ryan M.D. Neurological deterioration after reduction of cervical subluxation. Mechanical compression by disc tissue. J Bone Joint Surg [Br]. 1992;74(2):224-227.
Sonntag V.K. Management of bilateral locked facets of the cervical spine. Neurosurgery. 1981;8(2):150-152.
1. Sonntag V.K. Management of bilateral locked facets of the cervical spine. Neurosurgery. 1981;8(2):150-152.
2. Wolf A., Levi L., Mirvis S., et al. Operative management of bilateral facet dislocation. J Neurosurg. 1991;75(6):883-890.
3. Carrino J.A., Manton G.L., Morrison W.B., et al. Posterior longitudinal ligament status in cervical spine bilateral facet dislocations. Skeletal Radiol. 2006;35:510-514.
4. Payer M. Immediate open anterior reduction and anterior-posterior fixation/fusion for bilateral cervical locked facet. Acta Neurochir (Wien). 2005;147:509-514.
5. Lu K., Lee T.C., Chen H.J. Closed reduction of bilateral locked facets of the cervical spine under general anesthesia. Acta Neurochir (Wien). 1998;140(10):1055-1061.
6. Maiman D.J., Barolat G., Larson S.J. Management of bilateral locked facets of the cervical spine. Neurosurgery. 1986;18(5):542-547.
7. Razack N., Green B.A., Levi A.D. The management of traumatic cervical bilateral facet fracture-dislocations with unicortical anterior plates. J Spinal Disord. 2000;13(5):374-381.
8. Doran S.E., Papadopoulos S.M., Ducker T.B., Lillehei K.O. Magnetic resonance imaging documentation of coexistent traumatic locked facets of the cervical spine and disc herniation. J Neurosurg. 1993;79(3):341-345.
9. Eismont F.J., Arena M.J., Green B.A. Extrusion of an intervertebral disc associated with traumatic subluxation or dislocation of cervical facets. Case report. J Bone Joint Surg [Am]. 1991;73(10):1555-1560.
10. Robertson P.A., Ryan M.D. Neurological deterioration after reduction of cervical subluxation. Mechanical compression by disc tissue. J Bone Joint Surg [Br]. 1992;74(2):224-227.
11. Rosenfeld J.F., Vaccaro A.R., Albert T.J., et al. The benefits of early decompression in cervical spinal cord injury. Am J Orthop. 1998;27:23-28.
12. Stauffer E.S., Kelly E.J. Fracture-dislocations of the cervical spine: Instability and recurrent deformities following treatment by anterior interbody fusion. J Bone Joint Surg [Am]. 1977;59:45-48.
13. Ordonez B.J., Benzel E.C., Nader S., Weller S.J. Cervical facet dislocation: techniques for ventral reduction and stabilization. J Neurosurg. 2000;92:18-23.
14. Fazl M., Pirouzmand F. Intraoperative reduction of locked facets in the cervical spine by use of a modified interlaminar spreader: technical note. Neurosurgery. 2001;48:444-446.
15. O’Brien P.J., Schweigel J.F., Thompson W.J. Dislocation of the lower cervical spine. J Trauma. 1982;22:710-714.
16. Van Peteghem P.R., Schweigel J.F. Fractured cervical spine rendered unstable by anterior cervical fusion. J Trauma. 1979;19:110-117.