CHAPTER 209 Management of Severe Head Injury in Children
Pediatric traumatic brain injury (TBI) is a significant public health issue throughout the world. Although efforts at prevention and new safety technologies have led to a slight reduction in the rates of significant head injury in the United States, the rate of TBI is increasing in developing countries. TBI also remains the most common cause of death and disability in children in our country. In this review we discuss the background and epidemiology of pediatric TBI, followed by a review of management with use of a critical pathway. This pathway follows the 2003 “Guidelines for the Acute Management of Severe Traumatic Brain Injury in Infants, Children, and Adolescents,” a jointly sponsored guideline that addresses multiple facets of the management of severe brain injury in an evidenced-based fashion. In the guidelines, “standards” are accepted principles that reflect a high degree of certainty, “guidelines” reflect moderate clinical certainty, and “options” are strategies for which there is unclear clinical benefit.1
Background
Pediatric head trauma is an important public health issue with both high mortality and lifelong physical, cognitive, behavioral, and social implications. TBI accounts for 435,000 visits to the emergency department (ED), 37,000 hospital admissions, and approximately 2,500 deaths annually in the United States.2 These numbers do not include patients treated in offices or those who do not seek medical care for mild TBI.
The incidence of TBI is not consistent across sexes or age groups, with an overall male-to-female ratio of about 2:1. This gender difference does not start until after the age of 5; boys’ risk for TBI increases as they reach their teens, whereas girls’ risk declines after the age of 10.3 No group has a higher incidence of head injury than male adolescents aged 15 to 19, and only adults older than 75 years have a higher hospitalization rate.1 Studies have also shown that approximately 40% to 50% of all mortality after trauma is secondary to brain injury.4
Mild TBI is now being recognized in epidemic proportions. Ninety percent of patients with mild head injury (Glasgow Coma Scale [GCS] score of 13 to 15 and loss of consciousness for <30 minutes) seen in the ED are released.2,5 There are many more children who are never evaluated in the ED after a mild TBI. Although most obvious postconcussive symptoms improve within 1 month after injury and the majority of behavioral or attention problems resolve within 6 months after a mild head injury, a smaller subset of children will have prolonged symptoms.
Studies have shown that children with moderate (GCS score of 9 to 12) to severe (GCS score or 8 or less) head injuries are at significant risk for long-term problems with behavior and cognition. Although it had previously been thought that infants were at lower risk for long-term sequelae, it appears likely that the developing brain is even more susceptible to injury. Several recent studies have shown infants to have less positive acceleration in IQ than older children after similar head injuries and that children younger than 6 years had more difficulty in executive control and memory after head injuries than did older children. In addition, 40% of children have a persistent change in personality after severe head injury, and the incidence of behavioral problems correlates with increasing severity of head injury (36% in those with severe injury and 22% in those with moderate injury).6–12 Clearly, pediatric head injury is a significant public health issue not only in terms of the immediate hospitalization and health care costs but also with regard to the potential long-term implications on the neurocognitive development of these children.
Classification of Head Injuries
Hematomas
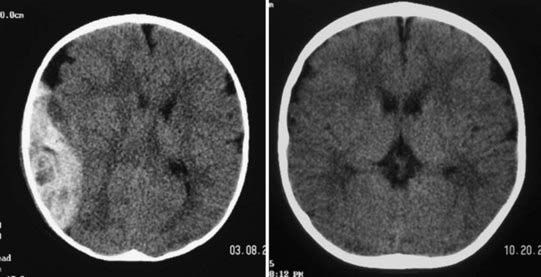
Epidural hematomas (EDHs) are collections of blood outside the dura that are usually arterial in origin, but they can be venous in up to 10% of patients. Very commonly, EDHs are associated with skull fractures, with the fracture resulting in an underlying injury to blood vessels. They often result from direct impact on the pterion of the temporal bone with the fracture injuring the middle meningeal artery, and they can enlarge rapidly and reach their peak size within 6 to 8 hours after injury. EDH forms a convex shape on imaging because the hematoma is contained by attachments of the dura to the skull at the cranial sutures. Classically, patients are described as having brief loss of consciousness initially because of a “concussive”-type injury, a lucid period during which the hematoma is expanding, and then neurological decline as intracranial pressure (ICP) increases. Unlike other head injuries, patients with poor GCS scores can have good outcomes postoperatively if there is no major concomitant brain injury and if the EDH is evacuated quickly. For patients with milder injuries and stable results on examination, it is possible that the EDH can be handled nonoperatively under the care and decision making of a neurosurgeon (Fig. 209-1).
Management of Traumatic Brain Injury
Prehospital Management
Trauma Systems
Prehospital identification as well as assessment of TBI appears to be an important factor for patient outcomes. There are no definitive outcome data on the effect of time from the injury to arrival at the trauma center, although good data do indicate that patients fare better when treated within a trauma system. Several studies have shown a significant decrease in mortality in adult patients with acute SDH who were operated on within 2 to 4 hours after injury.13,14 Two studies, from Washington, DC, and Pennsylvania, have looked specifically at pediatric TBI patients and found increased survival in those who were transferred directly to a trauma facility with special qualifications to treat children.15,16
Improved survival in a region is a function of not just meticulous postinjury care but also an overall plan that focuses on all aspects of TBI from prevention through community reintegration. A recent study reviewed pediatric deaths as a result of trauma between 2001 and 2003 versus deaths between 1985 and 1987, before the implementation of a trauma system. The system included injury prevention programs, field triage, and designated trauma centers. In that period, the incidence of identified preventable deaths declined from 21% to 7%, a 68% reduction in relative risk, thus showing the importance of not only a coordinated trauma system but also a concomitant focus on prevention programs.17
Based on the 2007 prehospital guidelines of the Brain Trauma Foundation, it is recommended that pediatric patients with suspected severe TBI be transported directly to a facility with specific qualifications to treat children.18
Airway
Hypoxemia has been shown to have a significant impact on the survival and outcome of patients with TBI in studies of both pediatric and adult populations.19,20 Accordingly, the first concern for emergency medical service personnel should be securing an airway. Prospective studies have compared bag-valve mask ventilation and endotracheal intubation and found no difference in outcome.21,22 One such study showed that either intubation or bag-valve mask ventilation provided effective ventilation in patients with head injury, and additional studies have supported these data.23,24
Whether the airway is secured through bag-valve mask ventilation or endotracheal intubation, it is essential to prevent hypoxemia in the prehospital setting. Many studies have shown the negative impact of hypoxemia on outcome in TBI patients, with significantly worsening outcomes and mortality being associated with worsening hypoxemia at an arterial oxygen saturation (SaO2) of greater than 90% (mortality, 14.3%), 60% to 90% (mortality, 27.3%), and less than 60% (mortality, 50%).19
Another consideration in prehospital management of the airway is prevention of significant hypocapnia or hypercapnia. Physiologically, there are clear links between PCO2 and cerebral blood flow (CBF), and currently, it is thought best to maintain normocapnia. Two studies have shown that capnography with ventilator adjustments en route can significantly reduce hypocapnia on arrival at the trauma center in comparison to standard settings.25,26 Because maintenance of normocapnia in transport appears to be important, it is generally recommended that emergency medical teams have the ability to monitor PCO2, especially if they have intubated the patient.27
Resuscitation
Once the airway has been secured, the next step in prehospital evaluation is to address resuscitation. Prehospital recommendations for fluid resuscitation have been evaluated in several studies. The Saline versus Albumin Fluid Evaluation (SAFE) study compared normal saline and albumin for resuscitation and found significantly worse outcomes in patients who received albumin.28 Two studies evaluated hypertonic saline; one compared hypertonic saline with normal saline for the prehospital treatment of hypotension and found an initial survival advantage with hypertonic saline but no significant difference in long-term survival, whereas the second compared treatment with hypertonic saline versus lactated Ringer’s solution and found no difference in survival or outcome at 6 months despite early promising effects on ICP with hypertonic saline.29,30
To summarize, the primary goals of prehospital treatment are to prevent hypoxia and hypotension en route to an appropriate trauma facility. A mortality rate of 55% can occur if hypoxia, hypotension, or hypercapnia is present in the setting of severe TBI; the rate is reduced to just 7.7% when none of these risk factors are present.31 In a prospective study of almost 7000 adult and 2000 pediatric patients with TBI, Luerssen and colleagues found that hypotension had a significantly greater negative impact on the outcomes of pediatric patients.1 The 2003 guidelines recommend that hypotension, defined as systolic blood pressure below the 5th percentile for age, or clinical signs of shock should be corrected as quickly as possible with fluid resuscitation and that the airway should be controlled to avoid hypoxia.32
Clinical and Radiographic Examination
Clinical examination can be unreliable in patients with TBI. Two prospective studies of pediatric trauma patients found that 33% of the patients with abnormal head CT findings had normal results on neurological examination.33,34 Therefore, almost all children who have sustained a significant injury or are suspected of having TBI should undergo imaging. CT remains the test of choice in the acute trauma setting, and MRI and adjuncts such as angiography should also be considered. On the negative side in children with severe TBI, the initial GCS score and pupil response have been significantly correlated with long-term outcomes. If a child is initially seen with bilateral fixed and dilated pupils, multiple studies have shown 100% mortality.35,36
Intensive Care Unit Management
Guidelines for Management of Intracranial Pressure and Cerebral Perfusion Pressure
As with adults, many pediatric studies have now demonstrated improved outcomes in patients who receive aggressive management of ICP. The 2003 guidelines reviewed nine studies that included 518 pediatric patients, all of which showed an association of persistently elevated ICP (usually ICP > 20 mm Hg) with poor outcome or increased mortality (or both) in comparison to patients with well-controlled ICP.37 Several studies using different modalities to control ICP, including early decompressive craniectomy, hypertonic saline, barbiturates, and hyperventilation, have revealed improved outcomes and decreased mortality with aggressive treatment of ICP.38–41
Although there are no specific guidelines in pediatrics on when to insert an ICP monitor, several studies have provided useful information. The presence of an open fontanelle or sutures does not preclude the development of intracranial hypertension, and the fontanelle should not be used as a guideline by which to judge ICP.40 In one study consisting of 22 pediatric patients with severe TBI and an initial GCS score of 8 or less, 86% were found to have ICP higher than 20 mm Hg during the monitoring period.42 In general, a threshold for placing a monitor at a GCS score of 8 or less is what is generally accepted in the pediatric population and supported by the pediatric guidelines.37 Although infant modifiers to the GCS score exist for pediatric patients, the neurological examination can be an unreliable predictor of outcome in infants and toddlers. There have been two studies reviewing pediatric patients who initially appeared well but suffered neurological deterioration, death, or both.
Ultimately, the goal of head injury management is to provide adequate CBF to allow delivery of oxygen and nutrients to the brain. After injury, there is both increased demand for these substrates and an impaired delivery system, thereby subjecting the brain to increased risk for an energy crisis. Consequently, many authors now think that CPP is a parameter as or more important than ICP. CPP is physiologically defined as the difference between mean arterial pressure and ICP. Early work showed significant improvement in the outcomes of adult patients with severe TBI when treatment was directed toward maintaining CPP at a level higher than 70 mm Hg.43–45 One study reviewed CPP and outcomes in pediatric TBI patients and stratified patients by those with CPP of less than 40 mm Hg and then in deciles from 40 to 49 mm Hg, 50 to 59 mm Hg, 60 to 69 mm Hg, and finally, higher than 70 mm Hg and found no significant difference in mortality or Glasgow outcome score (GOS) distribution in all patients with CPP higher than 40. However, no patient with a CPP lower than 40 mm Hg survived.46 This has helped support the need to maintain CPP at a level greater than 40 mm Hg in all pediatric patients.47
The 2003 guidelines state as a treatment option that ICP above 20 mm Hg is considered a pathologic elevation and treatment should be corroborated with the findings on clinical examination, physiologic monitoring, and cranial imaging.37,48 Although specific thresholds of ICP at which to initiate treatment have not been established, data support treating ICP elevated above 20 to 25 mm Hg for a prolonged period.42,49–51 Further investigation is needed to define age-specific ranges for both ICP and CPP thresholds to guide treatment, but persistent elevations in ICP above 20 mm Hg should be considered pathologic.
Intracranial Pressure Monitoring Technology
The accuracy of fiberoptic monitors in comparison to ventricular catheters has been evaluated in both children and adults and was reviewed in the 2003 guidelines.52 The Camino fiberoptic, in general, ranged 1 to 4 mm Hg higher than the ICP recorded in comparable patients measured with a ventricular catheter, which was corroborated by a study in adult patients that also showed slightly higher readings with the fiberoptic monitor.53,54
Typically, most monitors and drains are placed in the ED or ICU and tend to be used for relatively short durations of 7 days or less. One study reviewed complications associated with ICP monitors and found no difference in the incidence of colonization of ventricular catheters with hospital location of insertion or duration of monitoring, with a Staphylococcus epidermis colonization rate of 7% regardless of the location placed. No patients had clinical features of central nervous system infection.55
Because of the relatively low complication rate and high potential survival and outcome benefit in patients with severe TBI, the 2003 guidelines support the use of ICP monitoring as a treatment option in infants and children with severe TBI.52 As mentioned earlier, an open fontanelle or open sutures (or both) do not preclude the development of intracranial hypertension and should not be considered a contraindication to placement of a monitor.
Diversion of Cerebrospinal Fluid
Cerebrospinal fluid (CSF) diversion is an excellent treatment option to lower ICP by decreasing CSF fluid volume. This can be accomplished by external ventricular drainage alone or with the addition of a lumbar drain. Several studies in pediatric TBI patients evaluated the effectiveness of CSF diversion in lowering ICP.42 Other studies reported on lumbar drainage, in addition to ventricular drainage, for control of ICP with the theoretical benefit that it can reach subarachnoid fluid spaces not affected by the ventricular drain and therefore further lower ICP.56,57
The 2003 guidelines recommend ventriculostomy and CSF drainage as a treatment option for refractory intracranial hypertension. Lumbar drainage may be added if there are open cisterns and no mass lesions on imaging.58
Hyperosmolar Therapy
Hyperosmolar therapy has a long tradition in modern medicine. These agents were first shown to reduce ICP at the turn of the 20th century, and mannitol was introduced into clinical practice in 1961. Over the next 20 years, a variety of hyperosmolar agents, including mannitol, glycerol, and urea, were used clinically, with mannitol gradually replacing most other agents for the management of intracranial hypertension by the late 1970s.59
Several studies have investigated the use of mannitol versus other agents and found mixed success, with a 1.75 relative risk for death with mannitol versus saline placebo or hypertonic saline.60–62 A final study compared the use of mannitol as treatment based on ICP monitoring (with goals of ICP < 25 mm Hg) versus the use of neurological or physiologic indications for administration of mannitol. This study found a 0.83 relative risk for death with ICP-directed mannitol administration, thus indicating a small benefit for ICP-directed administration of mannitol.63
There has been recent interest in hypertonic saline as the agent of choice in hyperosmolar therapy for head injury. Webb and McKibben first used hypertonic saline as their hyperosmolar agent in 1919 to lower ICP, but it later fell out of favor as an osmotic agent. Many studies in animals and humans have investigated the mechanism of action of hypertonic saline solutions. Hypertonic saline, unlike mannitol, expands the plasma volume and also has a higher osmotic reflection coefficient. Consequently, it may be more efficient in creating an osmotic gradient even in damaged tissue and may be less likely to induce rebound edema. Hypertonic saline could also have beneficial effects by modulating inflammation, reducing serum viscosity, restoring normal cellular membrane potential, augmenting the cardiac index, and improving gas exchange and cerebral oxygenation.64–69 Several studies have shown that fluctuations in ICP vary inversely with the serum concentrations of sodium.70–72
Outcomes in pediatric TBI patients in whom hypertonic saline has been used either as fluid resuscitation or as treatment of refractory increased ICP have been evaluated in several studies. When compared with lactated Ringer’s solution, patients receiving hypertonic saline required fewer interventions for elevated ICP, including mannitol, and also had significantly shorter ICU stays and fewer days on mechanical ventilation; however, there was no significant difference in survival.70 In another study, continuous 3% hypertonic saline solution was titrated to control ICP higher than 20 mm Hg after other treatments had failed (on average, 3 days after injury). Although two patients required short-term dialysis for renal failure, there were no long-term complications noted and the average GCS in these patients was 4.71 In another study, the authors compared 3% hypertonic saline with 0.9% saline boluses for the treatment of intracranial hypertension and found that patients receiving hypertonic saline had lower ICP and less need for interventions, which included thiopental, mannitol, dopamine, and hyperventilation.73
Several recent studies have now compared mannitol with hypertonic saline for the treatment of intracranial hypertension. Two adult studies found an improved response of ICP to hypertonic saline alone versus mannitol or in conjunction with mannitol and also found that hypertonic saline improved cerebral oxygenation, as well as CPP and cardiac output, in comparison to mannitol alone.74,75 This was supported by another study in which it was shown that hypertonic saline appears to be more effective than mannitol in controlling cerebral edema.76
With regard to the 2003 guidelines, both mannitol and hypertonic saline are considered acceptable for use as hyperosmolar therapy after brain injury. Hypertonic saline (3%) can be used to control elevated ICP by administering between 0.1 and 1.0 mL/kg and using the lowest rate needed to control ICP. Mannitol is effective for control of ICP in bolus doses ranging from 0.25 to 1 g/kg. Serum osmolarity should be maintained below 320 mOsm/L with mannitol, but levels up to 360 mOsm/L are tolerated with hypertonic saline, even when used in conjunction with mannitol.59
Hyperventilation
For many years, hyperventilation was one of the mainstays of the treatment of severe TBI. Standard therapy for all patients would include routine hyperventilation and dehydration. Hyperventilation was used as a treatment modality based on the assumption that it could relieve diffuse hyperemia in pediatric TBI patients. A prospective cohort study compared severe TBI patients with a PaCO2 greater than 35 mm Hg, a PaCO2 of 25 to 35 mm Hg, or a PaCO2 lower than 25 mm Hg and found that as PaCO2 decreased, there was a decrease in ICP and increase in CPP. However, more critically, CBF was decreased.77 In more recent studies, it has been found that hyperemia is less common in pediatric TBI patients than formerly believed, which has led to concern that hyperventilation could lead to worse outcomes by depriving the brain of needed blood flow.78 Another study found that although hyperventilation led to a change in CBF in TBI patients, this did not correlate with a decrease in ICP.79 Most importantly, it was found that pediatric patients with severe TBI hyperventilated to PaCO2 values lower than 25 mm Hg in the first 5 days had worse outcomes at 3 and 6 months than did normocapnic controls (PaCO2 > 35 mm Hg).80 A small case series found that hyperventilation even induced ischemia in both injured and intact brain tissue.81
The 2003 guidelines state that prophylactic hyperventilation (PaCO2 < 35 mm Hg) should be avoided but that mild hyperventilation (PaCO2 of 30 to 35 mm Hg) may be considered as a treatment option for patients with elevated ICP refractory to other treatments, including sedation, hyperosmolar therapy, and CSF diversion. Aggressive hyperventilation (PaCO2 < 30 mm Hg) may be considered a second-tier option for refractory intracranial hypertension, but CBF or tissue oxygen monitoring is recommended in this situation to prevent cerebral ischemia. Finally, aggressive hyperventilation titrated to clinical effect may be needed for brief periods in the setting of acute deterioration or impending herniation.82
Sedation and Barbiturates
Both sedation and analgesia, as well as barbiturates, have been used to control elevated ICP. Interestingly, despite their widespread application, data to support their use are somewhat lacking, but they are a treatment option in the brain trauma guidelines.83,84 Some data suggest that sedation can decrease secondary damage by reducing metabolic demand, especially since routine ICU care such as suctioning has been shown to increase ICP and decrease cerebral oxygenation.85,86 The studies evaluating sedation in TBI patients do not reach class III evidence, with eight small case reports or nonstratified pediatric data.
The benefits of barbiturates appear to be multifactorial. They can decrease ICP through suppression of metabolism, alter vascular tone to improve the blood supply to brain regions that need it most, and provide neuroprotection both through inhibition of lipid peroxidation and membrane stabilization. The goal of treatment is generally cerebral burst suppression, and studies have found that measuring burst suppression is more effective than monitoring serum drug levels to determine clinical effectiveness.87,88 Several studies have been conducted to evaluate the use of pentobarbital in controlling ICP in both pediatric and adult TBI patients. In general, they found a significant decrease in mortality if pentobarbital was effective in lowering ICP, with mortality ranging from 8% to 37% in responders versus 83% in nonresponders.89–92 The major complication in these studies was hypotension requiring pressor support to maintain CPP at greater than 40 mm Hg.
Decompressive Craniectomy
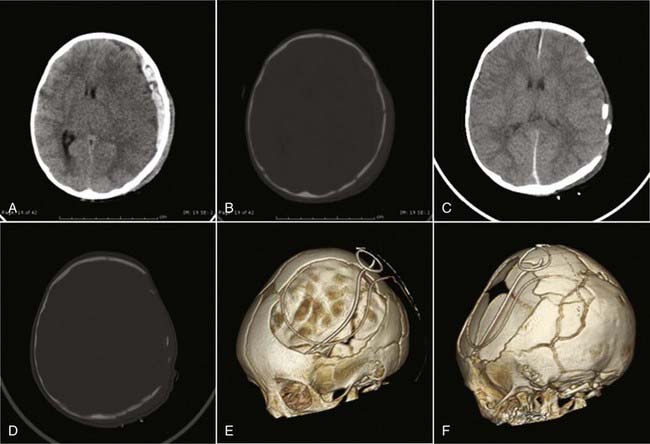
Decompressive craniectomy can be accomplished in several ways. In the acute setting, a portion of the cranium can be removed at the time of evacuating an intracranial lesion such as SDH. In this case, the decompression is being done expectantly in advance of an ICP problem (Fig. 209-2). Alternatively, decompressive craniectomy can be performed in response to elevated ICP that is refractory to medical treatment. In this case, the surgery can be unilateral if the pathology is predominantly unilateral or bilateral if the brain swelling is diffuse. It is important to realize that decompressive craniectomy is really only effective when done before severe secondary brain injury occurs. If malignant intracranial hypertension has existed for a long period and the brain has suffered diffuse bilateral hemispheric injury as a result, the decompression may prevent death but cannot really lead to any satisfactory quality of life. However, the poor prognosis of both pediatric and adult patients with severe TBI and diffuse cerebral injury, as confirmed by the Traumatic Coma Data Bank (34% mortality rate and 16% rate of having a good or moderate outcome), has established a possible role for this aggressive procedure.93,94
Many studies have shown that decompressive craniectomy is effective in lowering ICP, with two studies focusing on pediatric patients.38,40,95–97
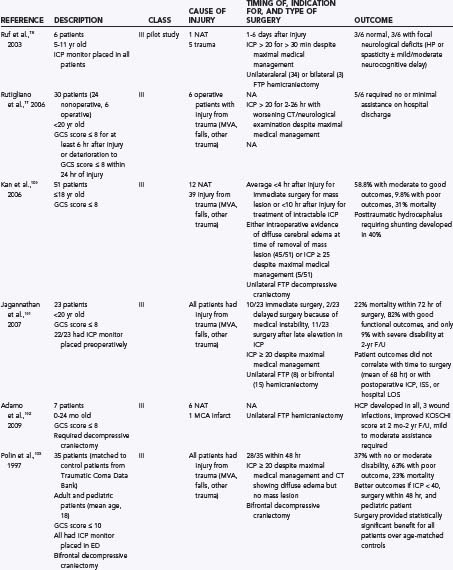
Although decompressive craniectomy can reduce ICP, its effect on outcome has been less clearly established. A number of studies have investigated outcomes of children after decompressive craniectomy for intractable intracranial hypertension and are summarized in Table 209-1.96,98–104
To summarize, decompressive craniectomy has been shown to lower ICP in patients with severe TBI and may improve outcomes in these patients, but complication rates and mortality remain high. The 2003 guidelines offer decompressive craniectomy as an option, the weakest level of recommendation, in patients who meet all or some of the following criteria: diffuse cerebral swelling on imaging, less than 48 hours after injury, no episodes of sustained ICP higher than 40 mm Hg before surgery, GCS score higher than 3 at some point after injury, and secondary clinical deterioration or clinical evidence of herniation.105 Further studies that compare decompressive craniectomy with other second-tier therapies may elucidate the indications and timing for surgery to achieve better outcomes.
Hypothermia
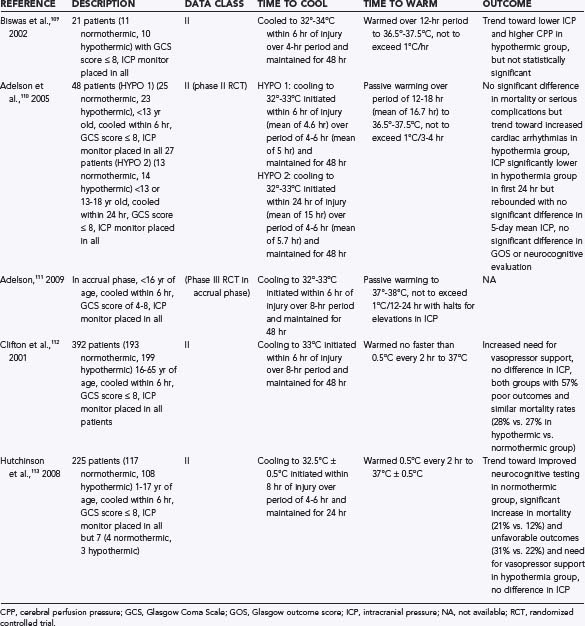
Hypothermia is an area of some controversy. It is well established that hyperthermia is associated with poor outcomes in TBI patients in both experimental and clinical studies.106 However, the effectiveness of hypothermia in preventing secondary injury and improving outcomes is still under investigation in the pediatric population. Several studies have offered conflicting data, and all adult studies have shown a negative impact of hypothermia after TBI. The National Acute Brain Injury Study: Hypothermia (NABIS-H) found that in adults, hypothermia may be contributing to increased mortality in older patients, and it was halted.107 The pediatric data are possibly more hopeful and are summarized in Table 209-2.108–112
Because of the data available, the 2003 guidelines could not endorse hypothermia as a treatment option. Instead, it recommended that hyperthermia (temperature > 38.5°C) be avoided.113 With further research, these recommendations may change over the next several years.
Nutrition
Although the nutritional status of pediatric TBI patients may be an integral component of the recovery process, there are few data on nutritional formulations, metabolism, timing, or location of feeding. It is generally accepted that the metabolic demands of a brain-injured patient is increased despite sedation and paralysis, although much of this data is from the adult literature. Two small studies of 7 and 12 pediatric TBI patients found that they had 180% resting oxygen consumption and 130% to 173% resting energy expenditure.114,115 According to the 2003 guidelines, it is an option to replace 130% to 160% of resting metabolic expenditure by weight in TBI patients.116 In one study, patients who did not receive nutrition until day 5 or 7 after injury had a twofold and fourfold increased risk for death, respectively.117
Hyperglycemia has been correlated with worse outcomes in many studies. Several animal models have shown significantly worse outcomes with hyperglycemia after ischemic brain injury, and this has been corroborated in reviews of outcomes after head injury, which found that hyperglycemia led to worse outcomes in TBI patients.118–122 Three studies evaluated persistent elevations in serum glucose over the first week after injury and found that persistent hyperglycemia was related to increased mortality and worse outcomes and that no pediatric TBI patients survived if the admission glucose was higher 300 mg/dL.123–125
Seizure Prophylaxis
TBI significantly increases a patient’s risk for the development of seizures. Mild TBI can increase seizure risk by 50%, with moderate and severe TBI carrying an increased risk for seizures of 3- and 17-fold.126 Posttraumatic epilepsy is reported to have developed in 15% of patients with severe TBI, and the prophylactic use of antiseizure medication has been shown to improve survival.127,128 One third of TBI patients have seizures within the first 3 to 4 months, and two thirds have had seizures by 2 years, with anywhere from 58% to 95% of pediatric patients reported as having a seizure within the first 24 hours after injury.126,129–131 Several risk factors have been found that significantly increase the risk for seizures: depressed skull fractures, SDH, an early (provoked) seizure, or severe head injury, with posttraumatic epilepsy developing in 20% to 35% of this patient population.129,131,132
Regarding the routine use of anticonvulsants after TBI, one study found a 15% versus 53% incidence of early seizures with phenytoin prophylaxis versus placebo.133 However, a more recent study found that 7% of phenytoin-treated patients had a seizure within the first 48 hours as compared with 5% given a placebo. Thus, the rate of posttraumatic seizures may be lower than previously presumed.128
The current pediatric guidelines recommend a 7-day course of prophylactic antiepileptics after head injury to decrease the risk for early seizures.134 Although this evidence comes from studies with phenytoin, levetiracetam (Keppra) has gained popularity as an antiepileptic since its introduction because of its lower side effect profile and lack of hepatic metabolism. At least one study has shown that it has efficacy similar to that of phenytoin.135
Steroids
The 2003 guidelines do not recommend the use of steroids in patients with TBI because of the lack of evidence supporting improved outcome with steroid use.136 There were many studies in the 1970s and 1980s that evaluated steroid use in TBI patients, both adults and children, with no significant difference in mortality or outcome in seven studies and increased infection rates seen in two other studies. The CRASH study was an international randomized, double-blind placebo-controlled study that evaluated glucocorticoid use after TBI. Approximately 10,000 adult patients with a GCS score of 14 or lower were randomized to receive 48 hours of methylprednisolone within 8 hours of injury or placebo. Mortality was 25.7% in the treatment group versus 22.3% in controls (relative risk of 1.15), and poor outcomes were noted in 38.1% versus 36.3%, respectively (relative risk of 1.05).137,138 Because of the potential risks and lack of support for any clinical benefit, there is no recommendation for steroid use in patients with TBI.137
Adelson PD, Cratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 12. Use of hyperventilation in the acute management of severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4:S45-S48.
Adelson PD, Cratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 6. Threshold for treatment of intracranial hypertension. Pediatr Crit Care Med. 2003;4:S25-S27.
Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 4. Resuscitation of blood pressure and oxygenation and prehospital brain-specific therapies for the severe pediatric traumatic brain injury patient. Pediatr Crit Care Med. 2003;4:S12-S18.
Anderson V, Catroppa C, Morse S, et al. Functional plasticity or vulnerability after early brain injury? Pediatrics. 2005;116:1374-1382.
Christensen J, Pederson MG, Pedersen CB, et al. Long-term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet. 2009;373:1105-1110.
Clifton GL, Miller ER, Choi SC, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344:556-563.
Cochran A, Scaife ER, Hansen KW, et al. Hyperglycemia and outcomes from pediatric traumatic brain injury. J Trauma. 2003;55:1035-1038.
Davis DP, Dunford JV, Poste JC, et al. The impact of hypoxia and hyperventilation on outcome after paramedic rapid sequence intubation of severely head-injured patients. J Trauma. 2004;57:1-8.
Downard C, Hulka F, Mullins RJ, et al. Relationship of cerebral perfusion pressure and survival in pediatric brain-injured patients. J Trauma. 2000;49:654-658.
Edwards P, Arango M, Balica L, et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury—outcomes at 6 months. Lancet. 2005;365:1957-1959.
Gausche M, Lewis RJ, Stratton SJ, et al. Effect of out-of-hospital pediatric endotracheal intubation on survival and neurological outcome: a controlled clinical trial. JAMA. 2000;283:783-790.
, 2007 Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(suppl 1):S1-S106.
Khanna S, Davis D, Peterson B, et al. Use of hypertonic saline in the treatment of severe refractory posttraumatic intracranial hypertension in pediatric traumatic brain injury. Crit Care Med. 2000;28:1144-1151.
Peterson B, Khanna S, Fisher B, et al. Prolonged hypernatremia controls elevated intracranial pressure in head-injured pediatric patients. Crit Care Med. 2000;28:1136-1143.
Potoka DA, Schall LC, Gardner MJ, et al. Impact of pediatric trauma centers on mortality in a statewide system. J Trauma. 2000;49:237-245.
Simma B, Burger R, Falk M, Sacher P, et al. A prospective, randomized, and controlled study of fluid management in children with severe head injury: lactated Ringer’s solution versus hypertonic saline. Crit Care Med. 1998;26:1265-1270.
Wakai A, Roberts I, Schierhout G. Mannitol for acute traumatic brain injury. Cochrane Database Syst Rev. 2007;(1):CD001049.
Young KD, Okada PJ, Sokolove PE, et al. A randomized, double-blinded, placebo-controlled trial of phenytoin for the prevention of early posttraumatic seizures in children with moderate to severe blunt head injury. Ann Emerg Med. 2004;43:435-446.
1 Luerssen TG, Klauber MR, Marshall LF. Outcome from head injury related to patient’s age. A longitudinal prospective study of adult and pediatric head injury. J Neurosurg. 1988;68:409-416.
2 Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States: differences by race. J Head Trauma Rehabil. 2005;20:229-238.
3 Kraus JF, Fife D, Cox P, et al. Incidence, severity, and external causes of pediatric brain injury. Am J Dis Child. 1986;140:687-693.
4 Tiret L, Hausherr E, Thicoipe M, et al. The epidemiology of head trauma in Aquitaine (France), 1986: a community-based study of hospital admissions and deaths. Int J Epidemiol. 1990;19:133-140.
5 Bazarian JJ, McClung J, Shah MN, et al. Mild traumatic brain injury in the United States, 1998-2000. Brain Inj. 2005;19:85-91.
6 Goldstrohm SL, Arffa S. Preschool children with mild to moderate traumatic brain injury: an exploration of immediate and post-acute morbidity. Arch Clin Neuropsychol. 2005;20:675-695.
7 Ewing-Cobbs L, Barnes M, Fletcher JM, et al. Modeling of longitudinal academic achievement scores after pediatric traumatic brain injury. Dev Neuropsychol. 2004;25:107-133.
8 Ewing-Cobbs L, Prasad MR, Landry SH, et al. Executive functions following traumatic brain injury in young children: a preliminary analysis. Dev Neuropsychol. 2004;26:487-512.
9 Keenan HT, Bratton SL. Epidemiology and outcomes of pediatric traumatic brain injury. Dev Neurosci. 2006;28:256-263.
10 Max JE, Koele SL, Castillo CC, et al. Personality change disorder in children and adolescents following traumatic brain injury. J Int Neuropsychol Soc. 2000;6:279-289.
11 Schwartz L, Taylor HG, Drotar D, et al. Long-term behavior problems following pediatric traumatic brain injury: prevalence, predictors, and correlates. J Pediatr Psychol. 2003;28:251-263.
12 Anderson V, Catroppa C, Morse S, et al. Functional plasticity or vulnerability after early brain injury? Pediatrics. 2005;116:1374-1382.
13 Seelig JM, Becker DP, Miller JD, et al. Traumatic acute subdural hematoma: major mortality reduction in comatose patients treated within four hours. N Engl J Med. 1981;304:1511-1518.
14 Wilberger JEJr, Harris M, Diamond DL. Acute subdural hematoma: morbidity, mortality, and operative timing. J Neurosurg. 1991;74:212-218.
15 Johnson DL, Krishnamurthy S. Send severely head-injured children to a pediatric trauma center. Pediatr Neurosurg. 1996;25:309-314.
16 Potoka DA, Schall LC, Gardner MJ, et al. Impact of pediatric trauma centers on mortality in a statewide system. J Trauma. 2000;49:237-245.
17 Diamond IR, Parkin PC, Wales PW, et al. Preventable pediatric trauma deaths in Ontario: a comparative population-based study. J Trauma. 2009;66:1189-1194.
18 Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(Suppl 1):S1-S106.
19 Stocchetti N, Furlan A, Volta F. Hypoxemia and arterial hypotension at the accident scene in head injury. J Trauma. 1996;40:764-767.
20 Davis DP, Dunford JV, Poste JC, et al. The impact of hypoxia and hyperventilation on outcome after paramedic rapid sequence intubation of severely head-injured patients. J Trauma. 2004;57:1-8.
21 Cooper A, DiScala C, Foltin G, et al. Prehospital endotracheal intubation for severe head injury in children: a reappraisal. Semin Pediatr Surg. 2001;10:3-6.
22 Gausche M, Lewis RJ, Stratton SJ, et al. Effect of out-of-hospital pediatric endotracheal intubation on survival and neurological outcome: a controlled clinical trial. JAMA. 2000;283:783-790.
23 Meyer G, Orliaguet G, Blanot S, et al. Complications of emergency tracheal intubation in severely head-injured children. Paediatr Anaesth. 2000;10:253-260.
24 Suominen P, Baillie C, Kivioja A, et al. Intubation and survival in severe paediatric blunt head injury. Eur J Emerg Med. 2000;7:3-7.
25 Helm M, Hauke J, Lampl L. A prospective study of the quality of pre-hospital emergency ventilation in patients with severe head injury. Br J Anaesth. 2002;88:345-349.
26 Helm M, Schuster R, Hauke J, et al. Tight control of prehospital ventilation by capnography in major trauma victims. Br J Anaesth. 2003;90:327-332.
27 Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 3. Prehospital airway management. Pediatr Crit Care Med. 2003;4:S9-S11.
28 Kirkpatrick PJ, Tseng MY, Hutchinson PJ. How SAFE is albumin for fluid resuscitation in critically ill patients with traumatic brain injury? Nat Clin Pract Neurol. 2008;4:248-249.
29 Vassar MJ, Perry CA, Holcroft JW. Prehospital resuscitation of hypotensive trauma patients with 7.5% NaCl versus 7.5% NaCl with added dextran: a controlled trial. J Trauma. 1993;34:622-632.
30 Cooper DJ, Myles PS, McDermott FT, et al. Prehospital hypertonic saline resuscitation of patients with hypotension and severe traumatic brain injury: a randomized controlled trial. JAMA. 2004;291:1350-1357.
31 Mayer TA, Walker ML. Pediatric head injury: the critical role of the emergency physician. Ann Emerg Med. 1985;14:1178-1184.
32 Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 4. Resuscitation of blood pressure and oxygenation and prehospital brain-specific therapies for the severe pediatric traumatic brain injury patient. Pediatr Crit Care Med. 2003;4:S12-S18.
33 Chan HC, Aasim WA, Abdullah NM, et al. Characteristics and clinical predictors of minor head injury in children presenting to two Malaysian accident and emergency departments. Singapore Med J. 2005;46:219-223.
34 Halley MK, Silva PD, Foley J, et al. Loss of consciousness: when to perform computed tomography? Pediatr Crit Care Med. 2004;5:230-233.
35 Massagli TL, Michaud LJ, Rivara FP. Association between injury indices and outcome after severe traumatic brain injury in children. Arch Phys Med Rehabil. 1996;77:125-132.
36 McCabe CF, Donahue SP. Prognostic indicators for vision and mortality in shaken baby syndrome. Arch Ophthalmol. 2000;118:373-377.
37 Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 5. Indications for intracranial pressure monitoring in pediatric patients with severe traumatic brain injury. Pediatr Crit Care Med. 2003;4:S19-S24.
38 Taylor A, Butt W, Rosenfeld J, et al. A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Childs Nerv Syst. 2001;17:154-162.
39 Peterson B, Khanna S, Fisher B, et al. Prolonged hypernatremia controls elevated intracranial pressure in head-injured pediatric patients. Crit Care Med. 2000;28:1136-1143.
40 Cho DY, Wang YC, Chi CS. Decompressive craniotomy for acute shaken/impact baby syndrome. Pediatr Neurosurg. 1995;23:192-198.
41 Bruce DA, Raphaely RC, Goldberg AI, et al. Pathophysiology, treatment and outcome following severe head injury in children. Childs Brain. 1979;5:174-191.
42 Shapiro K, Marmarou A. Clinical applications of the pressure-volume index in treatment of pediatric head injuries. J Neurosurg. 1982;56:819-825.
43 Rosner MJ, Daughton S. Cerebral perfusion pressure management in head injury. J Trauma. 1990;30:933-940.
44 Rosner MJ. Introduction to cerebral perfusion pressure management. Neurosurg Clin N Am. 1995;6:761-773.
45 Rosner MJ, Rosner SD, Johnson AH. Cerebral perfusion pressure: management protocol and clinical results. J Neurosurg. 1995;83:949-962.
46 Downard C, Hulka F, Mullins RJ, et al. Relationship of cerebral perfusion pressure and survival in pediatric brain-injured patients. J Trauma. 2000;49:654-658.
47 Adelson PD, Cratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 8. Cerebral perfusion pressure. Pediatr Crit Care Med. 2003;4:S31-S33.
48 Adelson PD, Cratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 6. Threshold for treatment of intracranial hypertension. Pediatr Crit Care Med. 2003;4:S25-S27.
49 Sharples PM, Stuart AG, Matthews DS, et al. Cerebral blood flow and metabolism in children with severe head injury. Part: 1: relation to age, Glasgow Coma Score, outcome, intracranial pressure, and time after injury. J Neurol Neurosurg Psychiatry. 1995;58:145-152.
50 Esparza J, M-Portillo J, Sarabia M, et al. Outcome in children with severe head injuries. Childs Nerv Syst. 1985;1:109-114.
51 Pfenninger J, Kaiser G, Lütschg J, et al. Treatment and outcome of the severely head injured child. Intensive Care Med. 1983;9:13-16.
52 Adelson PD, Cratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 7. Intracranial pressure monitoring technology. Pediatr Crit Care Med. 2003;4:S28-S30.
53 Gambardella G, Zaccone C, Cardia E, et al. Intracranial pressure monitoring in children: comparison of external ventricular device with the fiberoptic system. Childs Nerv Syst. 1993;9:470-473.
54 Schickner DJ, Young RF. Intracranial pressure monitoring: fiberoptic monitor compared with the ventricular catheter. Surg Neurol. 1992;37:251-254.
55 Jensen RL, Hahn YS, Ciro E. Risk factors of intracranial pressure monitoring in children with fiberoptic devices: a critical review. Surg Neurol. 1997;47:16-22.
56 Baldwin HZ, Rekate HL. Preliminary experience with controlled external lumbar drainage in diffuse pediatric head injury. Pediatr Neurosurg. 1991;17:115-120.
57 Levy DI, Rekate HL, Cherny WB, et al. Controlled lumbar drainage in pediatric head injury. J Neurosurg. 1995;83:453-460.
58 Adelson PD, Cratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 10. The role of cerebrospinal fluid drainage in the treatment of severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4:S38-S39.
59 Adelson PD, Cratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 11. Use of hyperosmolar therapy in the management of severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4:S40-S44.
60 Schwartz ML, Tator CH, Rowed DW, et al. The University of Toronto head injury treatment study: a prospective, randomized comparison of pentobarbital and mannitol. Can J Neurol Sci. 1984;11:434-440.
61 Sayre MR, Daily SW, Stern SA, et al. Out-of-hospital administration of mannitol to head-injured patients does not change systolic blood pressure. Acad Emerg Med. 1996;3:840-848.
62 Vialet R, Albanése J, Thomachot L, et al. Isovolume hypertonic solutes (sodium chloride or mannitol) in the treatment of refractory posttraumatic intracranial hypertension: 2 mL/kg 7.5% saline is more effective than 2 mL/kg 20% mannitol. Crit Care Med. 2003;31:1683-1687.
63 Wakai A, Roberts I, Schierhout G. Mannitol for acute traumatic brain injury. Cochrane Database Syst Rev. 2007(1)CD001049.
64 Hartl R, Ghajar J, Hochleuthner H, et al. Hypertonic/hyperoncotic saline reliably reduces ICP in severely head-injured patients with intracranial hypertension. Acta Neurochir Suppl. 1997;70:126-129.
65 Hartl R, Medary MB, Ruge M, et al. Hypertonic/hyperoncotic saline attenuates microcirculatory disturbances after traumatic brain injury. J Trauma. 1997;42:S41-S47.
66 Mazzoni MC, Borgström P, Arfors KE, et al. Dynamic fluid redistribution in hyperosmotic resuscitation of hypovolemic hemorrhage. Am J Physiol. 1988;255:H629-H637.
67 Nakayama S, Kramer GC, Carlsen RC, et al. Infusion of very hypertonic saline to bled rats: membrane potentials and fluid shifts. J Surg Res. 1985;38:180-186.
68 Munar F, Ferrer AM, de Nadal M, et al. Cerebral hemodynamic effects of 7.2% hypertonic saline in patients with head injury and raised intracranial pressure. J Neurotrauma. 2000;17:41-51.
69 Taylor G, Myers S, Kurth CD, et al. Hypertonic saline improves brain resuscitation in a pediatric model of head injury and hemorrhagic shock. J Pediatr Surg. 1996;31:65-70.
70 Simma B, Burger R, Falkm, et al. A prospective, randomized, and controlled study of fluid management in children with severe head injury: lactated Ringer’s solution versus hypertonic saline. Crit Care Med. 1998;26:1265-1270.
71 Khanna S, Davis D, Peterson B, et al. Use of hypertonic saline in the treatment of severe refractory posttraumatic intracranial hypertension in pediatric traumatic brain injury. Crit Care Med. 2000;28:1144-1151.
72 Bentsen G, Breivik H, Lundar T, et al. Hypertonic saline (7.2%) in 6% hydroxyethyl starch reduces intracranial pressure and improves hemodynamics in a placebo-controlled study involving stable patients with subarachnoid hemorrhage. Crit Care Med. 2006;34:2912-2917.
73 Fisher B, Thomas D, Peterson B. Hypertonic saline lowers raised intracranial pressure in children after head trauma. J Neurosurg Anesthesiol. 1992;4:4-10.
74 Kerwin AJ, Schinco MA, Tepas JJ3rd, et al. The use of 23.4% hypertonic saline for the management of elevated intracranial pressure in patients with severe traumatic brain injury: a pilot study. J Trauma. 2009;67:277-282.
75 Oddo M, Levine JM, Frango S, et al. Effect of mannitol and hypertonic saline on cerebral oxygenation in patients with severe traumatic brain injury and refractory intracranial hypertension. J Neurol Neurosurg Psychiatry. 2009;80:916-920.
76 Yildizdas D, Altunbasak S, Celik U, et al. Hypertonic saline treatment in children with cerebral edema. Indian Pediatr. 2006;43:771-779.
77 Skippen P, Seear M, Poskitt K, et al. Effect of hyperventilation on regional cerebral blood flow in head-injured children. Crit Care Med. 1997;25:1402-1409.
78 Zwienenberg M, Muizelaar JP. Severe pediatric head injury: the role of hyperemia revisited. J Neurotrauma. 1999;16:937-943.
79 Muizelaar JP, Ward JD, Marmarou A, et al. Cerebral blood flow and metabolism in severely head-injured children. Part: 2: autoregulation. J Neurosurg. 1989;71:72-76.
80 Muizelaar JP, Marmarou A, Ward JD, et al. Adverse effects of prolonged hyperventilation in patients with severe head injury: a randomized clinical trial. J Neurosurg. 1991;75:731-739.
81 Stringer WA, Hasso AN, Thompson JR, et al. Hyperventilation-induced cerebral ischemia in patients with acute brain lesions: demonstration by xenon-enhanced CT. AJNR Am J Neuroradiol. 1993;14:475-484.
82 Adelson PD, Cratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 12. Use of hyperventilation in the acute management of severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4:S45-S48.
83 Adelson PD, Cratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 13. The use of barbiturates in the control of intracranial hypertension in severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4:S49-S52.
84 Adelson PD, Cratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 9. Use of sedation and neuromuscular blockade in the treatment of severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4:S34-S37.
85 Kerr ME, Weber BB, Sereika SM, et al. Effect of endotracheal suctioning on cerebral oxygenation in traumatic brain-injured patients. Crit Care Med. 1999;27:2776-2781.
86 Fortune JB, Feustel PJ, Weigle CG, et al. Continuous measurement of jugular venous oxygen saturation in response to transient elevations of blood pressure in head-injured patients. J Neurosurg. 1994;80:461-468.
87 Piatt JHJr, Schiff SJ. High dose barbiturate therapy in neurosurgery and intensive care. Neurosurgery. 1984;15:427-444.
88 Kassell NF, Hitchon PW, Gerk MK, et al. Alterations in cerebral blood flow, oxygen metabolism, and electrical activity produced by high dose sodium thiopental. Neurosurgery. 1980;7:598-603.
89 Marshall LF, Smith RW, Shapiro HM. The outcome with aggressive treatment in severe head injuries. Part I: the significance of intracranial pressure monitoring. J Neurosurg. 1979;50:20-25.
90 Kasoff SS, Lansen TA, Holder D, et al. Aggressive physiologic monitoring of pediatric head trauma patients with elevated intracranial pressure. Pediatr Neurosci. 1988;14:241-249.
91 Pittman T, Bucholz R, Williams D. Efficacy of barbiturates in the treatment of resistant intracranial hypertension in severely head-injured children. Pediatr Neurosci. 1989;15:13-17.
92 Eisenberg HM, Frankowski RF, Contant CF, et al. High-dose barbiturate control of elevated intracranial pressure in patients with severe head injury. J Neurosurg. 1988;69:15-23.
93 Levin HS, Aldrich EF, Saydari C, et al. Severe head injury in children: experience of the Traumatic Coma Data Bank. Neurosurgery. 1992;31:435-443.
94 Chesnut RM, Marshall LF. Management of head injury. Treatment of abnormal intracranial pressure. Neurosurg Clin N Am. 1991;2:267-284.
95 Polin RS, Shaffrey ME, Bogaev CA, et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 1997;41:84-92.
96 Kunze E, Meixensberger J, Janka M, et al. Decompressive craniectomy in patients with uncontrollable intracranial hypertension. Acta Neurochir Suppl. 1998;71:16-18.
97 Gaab MR, Rittierodt M, Lorenz M, et al. Traumatic brain swelling and operative decompression: a prospective investigation. Acta Neurochir Suppl. 1990;51:326-328.
98 Ruf B, Heckmann M, Schroth I, et al. Early decompressive craniectomy and duraplasty for refractory intracranial hypertension in children: results of a pilot study. Crit Care. 2003;7:R133-R138.
99 Rutigliano D, Egnor MR, Priebe CJ, et al. Decompressive craniectomy in pediatric patients with traumatic brain injury with intractable elevated intracranial pressure. J Pediatr Surg. 2006;41:83-87.
100 Kan P, Amini A, Hansen K, et al. Outcomes after decompressive craniectomy for severe traumatic brain injury in children. J Neurosurg. 2006;105:337-342.
101 Jagannathan J, Okonkwo DO, Dumost AS, et al. Outcome following decompressive craniectomy in children with severe traumatic brain injury: a 10-year single-center experience with long-term follow up. J Neurosurg. 2007;106:268-275.
102 Adamo MA, Drazin D, Waldman JB. Decompressive craniectomy and postoperative complication management in infants and toddlers with severe traumatic brain injuries. J Neurosurg Pediatr. 2009;3:334-339.
103 Polin RS, Shaffrey ME, Bogaev CA, et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 1997;41:84-92.
104 Gaab MR, Rittierodt M, Lorenz M, et al. Traumatic brain swelling and operative decompression: a prospective investigation. Acta Neurochir Suppl. 1990;51:326-328.
105 Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 15. Surgical treatment of pediatric intracranial hypertension. Pediatr Crit Care Med. 2003;4:S56-S59.
106 Jones PA, Andrews PJ, Midgley S, et al. Measuring the burden of secondary insults in head-injured patients during intensive care. J Neurosurg Anesthesiol. 1994;6:4-14.
107 Clifton GL, Miller ER, Choi SC, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344:556-563.
108 Biswas AK, Bruce DA, Sklar FH, et al. Treatment of acute traumatic brain injury in children with moderate hypothermia improves intracranial hypertension. Crit Care Med. 2002;30:2742-2751.
109 Adelson PD, Ragheb J, Kanev P, et al. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery. 2005;56:740-754.
110 Adelson PD. Hypothermia following pediatric traumatic brain injury. J Neurotrauma. 2009;26:429-436.
111 Clifton GL, Miller ER, Choi SC, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344:556-563.
112 Hutchison JS, Ward RE, Lacroix J, et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358:2447-2456.
113 Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 14. The role of temperature control following severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4:S53-S55.
114 Moore R, Najarian MP, Konvolinka CW. Measured energy expenditure in severe head trauma. J Trauma. 1989;29:1633-1666.
115 Phillips R, Ott L, Young B, et al. Nutritional support and measured energy expenditure of the child and adolescent with head injury. J Neurosurg. 1987;67:846-851.
116 Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 18. Nutritional support. Pediatr Crit Care Med. 2003;4:S68-S71.
117 Hartl R, Gerber LM, Ni Q, et al. Effect of early nutrition on deaths due to severe traumatic brain injury. J Neurosurg. 2008;109:50-56.
118 Cherian L, Goodman LC, Robertson CS. Hyperglycemia increases brain injury caused by secondary ischemia after cortical impact injury in rats. Crit Care Med. 1997;25:1378-1383.
119 Cherian L, Hannay HJ, Vagner G, et al. Hyperglycemia increases neurological damage and behavioral deficits from post-traumatic secondary ischemic insults. J Neurotrauma. 1998;15:307-321.
120 Hovda DA, Yoshino A, Kawamata T, et al. The increase in local cerebral glucose utilization following fluid percussion brain injury is prevented with kynurenic acid and is associated with an increase in calcium. Acta Neurochir Suppl. 1990;51:331-333.
121 Lam AM, Winn HR, Cullen BF, et al. Hyperglycemia and neurological outcome in patients with head injury. J Neurosurg. 1991;75:545-551.
122 Rovlias A, Kotsou S. The influence of hyperglycemia on neurological outcome in patients with severe head injury. Neurosurgery. 2000;46:335-342.
123 Cochran A, Scaife ER, Hansen KW, et al. Hyperglycemia and outcomes from pediatric traumatic brain injury. J Trauma. 2003;55:1035-1038.
124 Jeremitsky E, Omert LA, Dunham CM, et al. The impact of hyperglycemia on patients with severe brain injury. J Trauma. 2005;58:47-50.
125 Salim A, Hadjizacharia P, Dubose J, et al. Persistent hyperglycemia in severe traumatic brain injury: an independent predictor of outcome. Am Surg. 2009;75:25-29.
126 Temkin NR. Preventing and treating posttraumatic seizures: the human experience. Epilepsia. 2009;50:10-13.
127 Tilford JM, Simpson PM, Yeh TS, et al. Variation in therapy and outcome for pediatric head trauma patients. Crit Care Med. 2001;29:1056-1061.
128 Young KD, Okada PJ, Sokolove PE, et al. A randomized, double-blinded, placebo-controlled trial of phenytoin for the prevention of early posttraumatic seizures in children with moderate to severe blunt head injury. Ann Emerg Med. 2004;43:435-446.
129 Hahn YS, Fuchs S, Flannery AM, et al. Factors influencing posttraumatic seizures in children. Neurosurgery. 1988;22:864-867.
130 Wang HC, Chang WN, Chang HW, et al. Factors predictive of outcome in posttraumatic seizures. J Trauma. 2008;64:883-888.
131 Temkin NR. Risk factors for posttraumatic seizures in adults. Epilepsia. 2003;44:18-20.
132 Christensen J, Pederson MG, Pedersen CB, et al. Long-term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet. 2009;373:1105-1110.
133 Lewis RJ, Yee L, Inkelis SH, et al. Clinical predictors of post-traumatic seizures in children with head trauma. Ann Emerg Med. 1993;22:1114-1118.
134 Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 19. The role of anti-seizure prophylaxis following severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4:S72-S75.
135 Jones KE, Puccio AM, Harshman KJ, et al. Levetiracetam versus phenytoin for seizure prophylaxis in severe traumatic brain injury. Neurosurg Focus. 2008;25(4):E3.
136 Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 16. The use of corticosteroids in the treatment of severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4:S60-S64.
137 Edwards P, Arango M, Balica L, et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury—outcomes at 6 months. Lancet. 2005;365:1957-1959.
138 Roberts I, Yates D, Sandercock P, et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet. 2004;364:1321-1328.