CHAPTER 37 Management of postoperative complications following glaucoma surgery
The clinical examination
Intraocular pressure
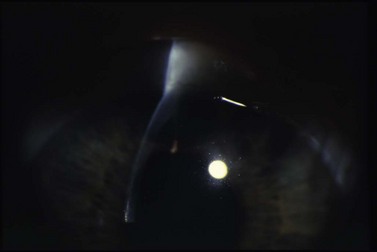
Finally, evaluation of the IOP is important to assess surgical complications. Often the eye is soft following filtering surgery, thus making IOP measurement difficult, especially if there is significant lid edema or chemosis. Additionally, accurate pressure reading may be particularly challenging with a shallow or flat AC. If there is lens or intraocular lens (IOL)–corneal touch, the applanation pressure may appear falsely elevated, as one may be essentially applanating the lens of the eye. In these circumstances, one may need to rely on assessment of the pressure by tactile tension to determine if the globe is soft or firm. See Figure 37.1.
Complications associated with a shallow or flat anterior chamber
Flat AC/low bleb/low IOP
Bleb leak
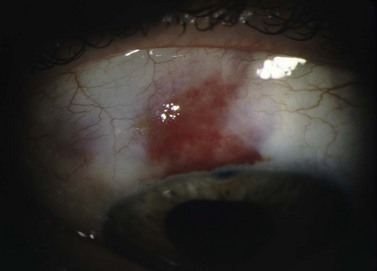
Formation of a healthy, well-functioning bleb is essential for a successful outcome following trabeculectomy. A complete, watertight closure of the conjunctival incision at the time of surgery is imperative. If the closure is incomplete or there is a defect in the overlying conjunctiva, the bleb will leak aqueous uncontrollably. Brisk leaks can cause failure bleb formation or lead to its collapse. Bleb leaks in the late postoperative period may result from conjunctival erosion from an underlying suture or perforation of a thin, avascular cystic bleb. In addition to a flat AC and hypotony, complications of untreated bleb leaks include formation of adhesions between intraocular structures, cataracts, corneal edema, hypotony maculopathy, blebitis, bleb failure, and endophthalmitis1,2.
Clinical features
Location of the surgical incision and bleb morphology may also contribute to the risk of developing bleb leaks. Limbal incisions are more likely to leak and have a positive Seidel test when exposed to positive pressure during the short-term postoperative period. During the long-term postoperative period, many surgeons feel that blebs with a limbal incision are flatter, more diffuse, and thus less prone to develop a late bleb leak. However, bleb leak incidence is not solely incision dependent. The additional surgical steps required to create a trabeculectomy can also contribute to bleb leakage. For example, the use of anti-metabolites intra- and/or postoperatively increases the risk of bleb leaks1,2.
Leaks are best diagnosed at the slit lamp using fluoroscein and the Seidel test. Using the Indiana Bleb Appearance Grading Scale, a S0 bleb has no leak, S1 has multiple pinpoint leaks, and an S2 bleb has a brisk, streaming leak. The incidence of late bleb leaks ranges from 1 to 10%3,4. Bleb leaks are more common today secondary to anti-metabolite use, which produces thinner, more avascular blebs5,6. On presentation, patients may complain of mild blurring of vision, tearing, and light sensitivity. Frequently, a small bleb leak with only moderately decreased IOP can be managed conservatively in the short term with antibiotics, aqueous suppressants, and/or patching7. Larger leaks put the eye at risk for hypotony-related complications and failure of the bleb. The presence of a significantly shallow or flat AC, corneal edema, or deep stromal/Descemet’s folds indicates aggressive management is required.
Management
The treatment of a bleb leak is determined by bleb appearance, IOP levels, and presence of associated complications. A slow, pinpoint leak (S1), can be managed conservatively if the IOP is not too low. In addition to prophylactic broad-spectrum antibiotic drops, non-invasive measures include placement of a large diameter bandage contact lens (22–24 mm), aqueous suppressants, and possibly pressure patching to reduce aqueous flow through the defect, thus allowing conjunctival healing. If these measures fail, cyanoacrylic glue, autologous fibrin, and other tissue adhesives can be attempted to patch the defect8–10. Argon laser and Nd : YAG laser can cause a localized contraction of the overlying conjunctiva and potentially seal the defect11,12.
More invasive treatments include the placement of an ‘X’ suture (9-0 or 10-0 Prolene, ‘Palmberg’ suture) and an autologous blood patch13,14. Bleb needling redirects aqueous flow posteriorly and stimulates an inflammatory response. If the bleb leak is unresponsive to conservative or minimally invasive treatment, surgical intervention is warranted. In chronic or rapid leaks, the deficient conjunctiva is excised and the bleb is reformed by advancing adjacent conjunctiva15,16. In eyes with conjunctival scarring or insufficient healthy adjacent conjunctiva, an autologous conjunctival graft (usually from the inferior fornix or the other eye) may be used16. Alternatively, the bleb conjunctiva can be mechanically or chemically de-epithelialized (64 Beaver blade or denatured alcohol) followed by posterior conjunctival advancement during bleb revision17. See Figure 37.2.
Choroidal effusion
Clinical features
Choroidal detachment or effusion usually occurs in the setting of significant postoperative hypotony. Serous fluid from the choriocapillaries accumulates in the suprachoroidal space secondary to the difference in hydrostatic pressure between the hypotonous eye and the choroidal vessels18,19. Patients typically present with blurred vision, reduced peripheral vision, and occasionally with a headache or nausea. On exam, the IOP is usually low and the AC is shallow due to an anterior shift of the lens–iris diaphragm. Small choriodal effusions appear as shallow elevations with the loss of choroidal markings. Large detachments are dome-shaped elevations that have increased transillumination (Hagen’s sign).
Despite the increased bleb survival rate, the incidence of post-trabeculectomy effusions has risen secondary to anti-metabolite use. Various studies found the incidence of choroidal effusion with 5-FU was 3–33% and 10–25% with MMC20–27. Overall, the incidence of choroidal effusion requiring surgical draining has been reported to be as high as 14%20. Other factors that are associated with choroidal effusion include significant postoperative hypotony, inflammation, increased episcleral venous pressure and increased scleral rigidity28–34.
The risk of choroidal effusions can be reduced by minimizing the use (dosage and application/contact time) of anti-fibrotic agents, proper surgical closure of the sclerostomy and conjunctival incision and timely treatment of wound/bleb leaks and hypotony. If a patient is a low risk for extensive bleb scarring (primary trabeculectomy, thin conjunctiva, elderly patient), a single application of 5-FU for 5 minutes may be sufficient35. In higher risk groups (eyes with a history of uveitis, neovascularization, prior scarring or of African, West Indian, or Latino descent), MMC with multiple postoperative subconjunctival injections of 5-FU may be required36.
Management
The management of choroidal effusion in the early postoperative period includes addressing causes of hypotony, topical cycloplegia, topical corticosteroids, and avoiding physical activities that elevate intrathoracic pressure. Cycloplegia will rotate the lens–iris diaphragm posteriorly to minimize angle shallowing. If the AC is extremely shallow or flat, viscoelastic can be injected through the paracentesis to reform the chamber37,38. Most effusions will resolve spontaneously as the preoperative effects of glaucoma medications decrease, early wound healing occurs, and bleb formation begins. Indications for surgical intervention and drainage of an effusion include: (i) flat AC with persistent lens–corneal contact, (ii) flat AC with significant intraocular inflammation, (iii) appositional choroidal detachment (‘kissing choroidals’), (iv) combined serous retinal–choroidal detachment, (v) persistent bleb leak with hypotony, and (vi) persistent choroidal detachment39. Effusions can be drained via inferiorly located (3.5–4.5 mm posterior to the limbus), full-thickness scleral incisions placed either parallel or radial to the limbus. The retrieval of straw-colored fluid confirms that the effusion cavity was successfully accessed. The anterior chamber can be filled with fluid or viscoelastic during drainage of the effusion to facilitate more complete drainage, to reform the anterior chamber, and to elevate the bleb.
Visual prognosis following choroidal effusion is largely based on size, location relative to the macula, severity, chronicity, and other ocular complications of hypotony or a flat AC. Most small to moderate sized effusions resolve spontaneously without surgical intervention, and the visual outcome is good. The most common complication is cataract formation (4–54% in phakic eyes)40,41. Large choroidal effusions with severe hypotony and a flat AC are at a higher risk for developing corneal scaring from iris–cornea or lens–corneal touch42.
Flat AC/low bleb/high IOP
Malignant glaucoma/aqueous misdirection/ciliary block
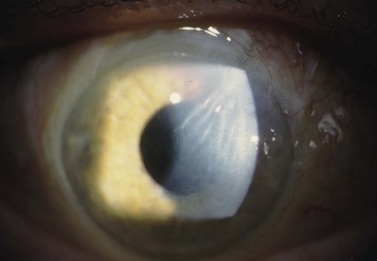
Aqueous misdirection, also know as ‘malignant’ or ciliary block glaucoma, is an uncommon and challenging complication of intraocular surgery. Although malignant glaucoma has a complex and not fully understood pathophysiology, it is believed to occur when aqueous flow is diverted posteriorly into the vitreous cavity. Chandler proposed choroidal swelling causes a forward movement of the lens and an accumulation of fluid in the vitreous cavity. A ‘vicious’ cycle then ensues with increasing pressure posteriorly holding the lens in a forward position43. More recently, Quigley has proposed that malignant glaucoma is caused by poor vitreal fluid conductivity and a substantial posterior–anterior pressure gradient44. Choroidal expansion, documented by UMB, can elevate IOP, increase aqueous outflow from the AC, and develop a significant posterior–anterior pressure gradient. In this setting, the vitreous condenses and, along with the lens and iris, moves forward due to fluid expansion from the compressed vitreous45,46.
Clinical features
IOP will be elevated, the AC will shallow and flatten, and the bleb is poorly formed. Malignant glaucoma is difficult to distinguish from angle closure secondary to pupillary block. Typically in malignant glaucoma, the AC is uniformly shallow, whereas iris bombe is more typical with pupillary block. In addition, an iridotomy will relieve pupillary block, but will not break angle closure secondary to malignant glaucoma. Vision is typically decreased secondary to corneal edema, but may be normal. Although 20–54% of cases will have an IOP > 40 mmHg, up to 21% of patients will present with a normal or only slightly elevated IOP47–49. In addition, a B-scan ultrasound and a complete dilated fundus exam must be performed to rule out an annular choroidal detachment or suprachoroidal hemorrhage.
The incidence of malignant glaucoma after trabeculectomy is 0.2–4% in the general population and is higher in patients with narrow angles50–52. Malignant glaucoma typically occurs following intraocular surgery: 2.8–3.7% after a glaucoma drainage device (GDD) procedure, 0.03% after cataract surgery, 2–4.0% after iridectomy, and 10% after combined cataract extraction and penetrating keratoplasty43,50,53–56. However, single cases in non-operated eyes have been reported57,58. Smaller eyes (shorter axial length), eyes with narrow angles and eyes with a history of angle closure, pseudo-exfoliation, and chronic miotic use are at an increased risk for malignant glaucoma45,47,48,58. Malignant glaucoma in the fellow eye is a significant risk factor. This indicates an inherent predisposition for the development of malignant glaucoma43,44.
Management
The treatment of malignant glaucoma aims to lower IOP, to restore anterior chamber depth, and to re-establish the proper flow of aqueous. Cycloplegics widen the ciliary body diameter and pull the lens posteriorly by tightening the zonules59. Osmotic medications remove fluid from the eye and reduce the vitreous volume. Topical anti-inflammatory and anti-hypertensive medications are also used to control IOP and to reduce the formation of synechiae between intraocular structures. Anterior chamber reformation with fluid or viscoelastics may restore normal aqueous dynamics by re-establishing normal anatomic relationships.
Surgical management of malignant glaucoma is indicated when medical treatment fails. Argon laser causes a contraction of the ciliary processes to relieve ciliolenticular block60. Nd : Yag laser hyaloidotomy in aphakic and pseudophakic eyes produces communication between the anterior and posterior segments and allows the anterior flow of fluid60–66. Incisional interventions include transcorneal needling to disrupt the anterior hyaloids face, posterior sclerostomy with air injection (Chandler procedure), pars plana vitrectomy, lens extraction with posterior capsulotomy and anterior vitrectomy, cyclodestruction, and zonulo-hyaloido-vitrectomy48,50,51,62,67–72. The goal of all of these surgical approaches is to create a unicameral eye without the potential for competing anterior and posterior spaces.
The prognosis of malignant glaucoma depends on the duration of elevated IOP and angle closure. Prompt diagnosis and treatment optimize the visual outcome. However, despite good IOP control and early treatment, many patients with malignant glaucoma will experience a significant loss of vision. See Figure 37.3.
Flat AC/high bleb/high IOP
Suprachoroidal hemorrhage
Clinical features
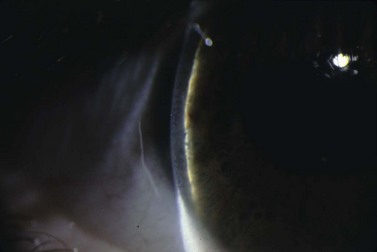
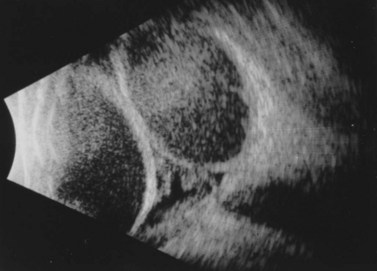
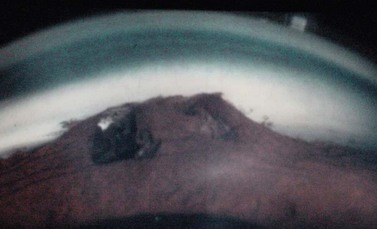
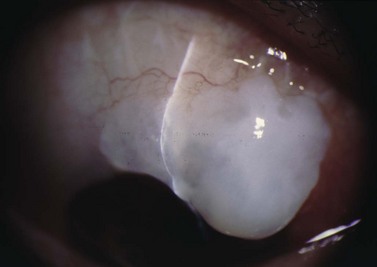
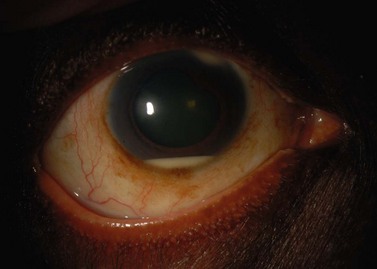
Suprachoroidal hemorrhage (SCH) is a feared surgical complication of intraocular surgery (Fig. 37.4). A SCH occurs when the short posterior ciliary vessels rupture and bleed into the suprachoroidal space. SCH usually occurs after a sudden drop in IOP, which creates a change in the vascular gradient. When a SCH occurs intraoperatively along with loss of red reflex, iris and vitreous prolapse, and hardening of the globe, it’s known as an ‘expulsive hemorrhage’. Extrusion of ocular contents bodes for a poor prognosis. A delayed SCH occurs hours to days following surgery. In most delayed SCH there is preceding serous choroidal effusion with subsequent hemorrhage into the effusion. Patients will complain of sudden loss of vision and pain, which can develop after straining. On examination, the AC is shallow, the IOP is elevated, and the bleb is high secondary forced fluid expulsion in the anterior chamber through the sclerostomy and into the subconjunctival space73,74. A dilated fundus exam shows a dark red or brown choroidal elevation suggestive of blood, and a B-scan ultrasonography will show high internal reflectivity in the suprachoridal space.
The reported incidence of delayed SCH following glaucoma surgery ranges from 1.6% to 6.1% depending on the type of surgery performed49. Ocular risk factors for delayed SCH include aphakia, myopia, a high preoperative IOP, a sudden decrease in IOP in the immediate postoperative period (<24 hours), early postoperative hypotony (IOP ≤3 mmHg) and prior intraocular surgery. Prior history of SCH in the fellow eye is also a risk factor. The use of anti-metabolites may also be associated with a higher incidence of delayed SCH. Systemic risk factors for SCH include hypertension, preoperative anticoagulation, ischemic heart disease and respiratory disease. SCH has also been associated with a sudden increased in intrathoracic pressure, which can occur with vigorous coughing, vomiting, straining, and intraoperative ‘bucking’ while under general anesthesia75–81. These and all other types of Valsalva maneuvers should be avoided in the early postoperative period.
Management
The management of intraoperative SCH is directed at prompt closure of the eye and pressurization of the globe. Pain management and IOP control are vital to treat both intraoperative and delayed SCH. Indications for surgical intervention include intractable pain, flat AC, uncontrolled IOP, retinal detachment, central apposition (kissing choroidals), displacement of vitreous into the anterior chamber, and incarceration of vitreous or other intraocular contents into the wound. Surgical drainage of a SCH is performed through a full-thickness sclerostomy, 3–4 mm posterior to the limbus, and anterior segment infusion of balanced salt solution82. The timing of surgical intervention of SCH is debatable. Although many surgeons prefer nearly complete clot lysis prior to surgical drainage (confirmed by B-scan ultrasound), delaying intervention until complete liquefaction has not fully been elucidated. Some studies have shown improved visual outcome with early surgical intervention75,83,84. Surgical drainage often requires 3–5 days until greater than 50% of the blood has liquefied within the suprachoroidal space by B-scan, which often requires 3–5 days.
SCH has a guarded prognosis and can lead to vision-threatening complications, such as retinal detachment, hypotony-associated maculopathy, RPE disruption, keratopathy, and phthisis bulbi77. Small SCHs may resolve without compromising the vision; however, expulsive or large SCHs typically have poor outcomes83,84. Poor prognostic factors include an afferent pupillary defect, poor visual acuity at presentation, coexisting uveitis or macular degeneration, retinal detachment, and prior extracapsular cataract extraction75,83. Prognosis is also worse with breakthrough bleeding into the vitreous cavity.
Flat AC/high bleb/low IOP
Over-filtration
Over-filtration, with resultant ocular hypotony, can be seen with overly diffuse blebs, loose suturing of the scleral flap at the time of surgery, and thin, avascular blebs. The rise in use of intraoperative anti-metabolites has increased the incidence of ocular hypotony following trabeculectomy85. Clinically, IOP will be low and the AC depth normal or shallow. Bleb morphology is an important to diagnose the underlying cause of over-filtration. A diffuse bleb, a very high bleb, or a thin, cystic bleb along with a low IOP, normal or shallow AC, and a negative Seidel test can indicate over-filtration.
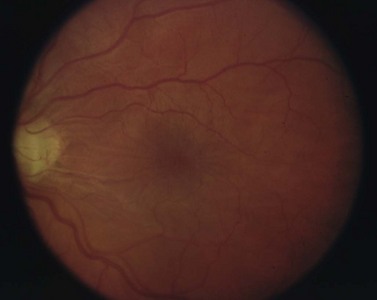
Long-standing hypotony may lead to a number of serious, sight-threatening complications. Loss of vision is typically secondary to choroidal effusion, choroidal hemorrhage, cataract development, and hypotony maculopathy. Hypotony maculopathy is one of the more serious and common complications associated with over-filtration. Chronic hypotony causes chorioretinal folds, choroidal thickening, loss of photoreceptors, and dilated retinal vessels. If left untreated, these can lead to permanent retinal changes and loss of vision. See Figure 37.5.
Given the potential long-term complications with persistent hypotony, several techniques have been developed to reduce the outflow of aqueous humor through an overfunctioning filtering bleb. Treatment options include the placement of an oversized bandage contact lenses or compressive Simmons shell, intra-bleb autologous blood injection (Fig. 37.6), trichloroacetic acid, cryotherapy, laser therapy (argon, Nd : YAG), compression mattress sutures, AC injection of viscoelastic, and surgical bleb revision. The placement of a large diameter contact lens is a safe and effective way of compressing the bleb and restricting flow. After 1–2 weeks, cellular remodeling occurs and aqueous outflow slows. Cryotherapy can shrink larger blebs, and tricholoracetic acid can induce inflammation with subsequent bleb fibrosis. However, a high incidence of corneal toxicity and conjunctival breakdown has been also reported following tricholoracetic acid. Shirato et al. and Letartre et al. describe transconjunctival suturing of the scleral flap as a safe and effective over-filtration treatment86,87. Transient leakage at the site of suturing points was occasionally seen; however, these spontaneously closed without intervention.
Complications associated with deep anterior chamber
Deep AC/low bleb/high IOP
Obstruction of outflow
Resistance at the internal sclerostomy
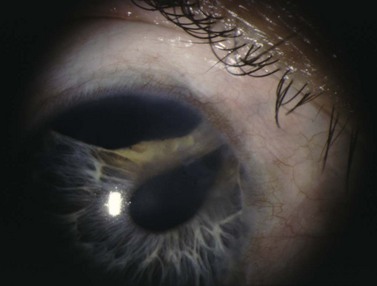
Blood, fibrin, viscoelastic, ocular structure (iris, vitreous, ciliary body), or implant material can block the internal sclerostomy or tip of a drainage device. Increased resistance to outflow deepens the AC and increases IOP. The bleb height will be low. Gonioscopy directly visualizes the internal ostomy and is essential for diagnosis and proper treatment. Blood occluding the sclerostomy will appear as a reddish discoloration around or in the sclerostomy track. Digital pressure to the globe may dislodge the blood88. If the IOP is not too high, conservative management with close monitoring is appropriate as most clots resolve within 24–72 hours. If the IOP is significantly elevated or the integrity of the bleb is in question, additional treatment modalities can be used. Intracameral tissue plasminogen activator (tPA) can lyse the clot successfully89,90. However, significant complications have been reported: hyphema, band keratopathy, corneal clouding, and vitreous hemorrhage90–94. Early transconjunctival suture lysis or loosening of releasable sutures can help to dislodge the clot. Once the obstruction has been cleared, hypotony is common. Therefore, in the absence of vision threatening pressure spikes, conservative management is advised.
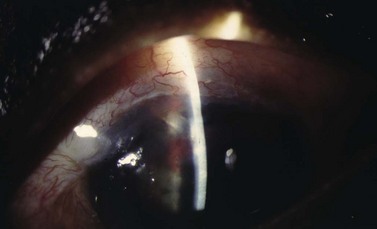
Peripheral iris tissue can become incarcerated into the internal sclerostomy (Fig. 37.7). Pupillary constriction with topical mitotics may occasionally relieve the occlusion. Argon laser to adjacent iris stroma can contract the iris away from the internal ostium; Nd : YAG laser can remove iris blocking the opening. Injection of viscoelastic at the site of blockage can separate the two opposing structures. If surgical treatment is required, an iris spatula can sweep the incarcerated tissue away from the sclerostomy. If vitreous is occluding the internal sclerostomy, an anterior vitrectomy can be performed.
Resistance at the level of the sclera
If outflow obstruction is suspected, but no blockage is seen gonioscopically, the resistance is typically at the level of the sclera or more superficial structures. Tight flap sutures, adhesions at the flap margins, and subconjunctival hemorrhage are common causes of early postoperative outflow obstruction. All management aims to restore flow, and to cause bleb formation. Digital massage or focal pressure may stretch tight sutures or break adhesions. Early suture lysis with argon laser or suture release with a 30-gauge needle can be performed. Digital pressure or subconjunctival tPA can be attempted for subconjunctival hemorrhage (Fig. 37.8).
Superficial resistance
Early episcleral fibrosis causes early bleb failure following an exuberant inflammatory response. Untreated, this causes bleb fibrosis and permanent scarring. Frequent topical steroids in the early postoperative period can inhibit bleb scarring and failure95. Postoperative subconjunctival 5-FU injections can inhibit fibroblast-induced scarring96. If all non-surgical treatments fail, the patient may need bleb needling, with or without anti-metabolites.
Retained viscoelastic
Viscoelastics (VEs) help maintain AC depth intraoperatively, as well as in the early postoperative period. Retained VEs can obstruct aqueous outflow and elevate IOP97. Typically, VEs are aspirated from the AC at the end of intraocular surgery. During trabeculectomy, the surgeon may leave VE material in the AC to avoid hypotony, to reduce bleeding, and to maintain the AC. Molecular characteristics of different VEs affect the outflow restriction and the likelihood of IOP elevation. Higher molecular weight VEs are more effective at maintaining AC depth, but may cause larger IOP spikes98,99. Many surgeons will treat possible postoperative IOP spikes prophylactically with an oral carbonic anhydrase inhibitor. In most cases, medical treatment will provide adequate IOP stabilization while the VE is reabsorbed from the eye.
Deep AC/high bleb/high IOP
Encapsulated bleb
An encapsulated bleb, also known as ‘Tenon’s cyst’, is dome shaped, elevated and tense. Aqueous flows through the sclera, but remains localized. The incidence of bleb encapsulation is as high as 3.6–28% in the first few weeks following trabeculectomy100–103. Increased risks are male gender, congenital and juvenile glaucoma, and chronic beta-adrenergic therapy100,102–104. Pathology of tissue samples from eyes after chronic topical glaucoma therapy shows increased concentrations of inflammatory cells within Tenon’s capsule105.
Similar to bleb fibrosis or scarring, topical steroids and ocular massage are used to prevent and to treat formation of early bleb encapsulation106,107. Alternative treatments include oral steroids, colchicine or other non-steroidal anti-inflammatories, and anti-vascular endothelial growth factor108. If encapsulation occurs, consider bleb needling with 5FU109. In refractory cases, surgical excision of the pseudo-capsule followed by bleb revision is recommended. Encapsulated blebs may become thin and avascular, and are thus prone to bleb leaks. A significant percentage of these blebs will ultimately fail, while some cases of fibrous encapsulation spontaneously resolve.
Glaucoma drainage device considerations
Tube-related complications
Tube obstruction
The tip of a tube can become obstructed by iris or fibrovascular membranes (30.8%), neovascular or fibrinous membranes (15.4%), vitreous, fibrin, silicone oil, blood, and pigmentary debris110. A hyphema can cause early tube obstruction52,110–114. Tube occlusion typically occurs at the internal ostium, but can occur at the external sclerostomy ostium or along the course of the tube (e.g. a kink in tube typically at the sclerostomy site). The incidence of tube occlusion is 2–12% in the early postoperative period52,110–113,115.
Conservative management of tube blockage includes aqueous suppressants and subconjunctival or oral steroids. Intracameral tissue plasminogen activator (tPA) has been used to treat an internal ostium blocked by blood. Since tPA acts quickly, a large drop in IOP within 24 hours indicates success: this may be as high as 89%; however, complications may occur in 22% (hyphemas, hypotony, and anterior chamber complications)116.
Argon and/or Nd : YAG laser may remove pigmented tissue, fibrin, iris, and vitreous from the internal ostium. In one study, the Nd : YAG laser successfully opened 84% of blocked tubes but at 11 weeks only 36% of tubes were patent. The laser was more successful when treating iris or fibrin plugs compared with neovascular plugs. There was no correlation between laser success and postoperative occlusion time (usually 18 +/– 11 months). When the laser fails intracameral tube irrigation, consider sweeping iris or a membrane out of the tube orifice, tube repositioning, repeat implantation, or cyclodestruction to lower IOP110.
Peritubular filtration
Early postoperative hypotony and over-filtration can result from aqueous outflow around the tube. Typically, a 23-gauge needle (or smaller) is used to create the scleral tunnel, through which the tube is inserted into the AC. To avoid overzealous peritubular filtration a watertight seal between tube and sclera is important. Since short needle track (<3 mm) is associated with increased risk of peritubular outflow, aim for a track length of least 3 mm117. Intraoperatively, a fluouroscein strip is used to detect leakage. The management options of peritubular over-filtration include additional scleral sutures around the tube, repositioning the tube, or applying fibrin glue to seal the leak117.
Device-related complications
Valve failure
Early or late failure of valved GDDs is secondary to valve leaflet adhesion. Surgical manipulation of the GDD can damage the device and attract inflammatory cells. Grasping the device along its center line can indent the valve cover and create a gap between the cover and plate. Fibrovascular ingrowth through the gap at the valve cover/valve body junction can create adhesions of the silicone leaflets and obstruct outflow118. Surgical handling of the plate laterally or posteriorly does not produce a gap; consequently, there is a no-touch zone (posterior to the rivets) on the Ahmed valve118.
Exposure complications
Exposure and extrusion of device
Tube erosion and subsequent GDD exposure can occur early or many years after surgery. The superior conjunctiva, under which most GDDs are placed, may be more susceptible to breakdown because of mechanical irritation from the upper eyelid. Excessive tissue tension when closing the conjunctival incision and thin, scarred conjunctiva predispose to erosion. If breakdown over the tube extends posteriorly to expose the plate, the risk of endophthalmitis and extrusion increase dramatically. The placement of a patch graft over the tube is used to prevent or to delay erosion119. Materials used include banked sclera, pericardium, fascia lata, and other fibrous tissue. Some surgeons feel that scleral patch grafts are less prone to thinning and erosion than other materials. Other surgeons use a folded, double layer of pericardium to reduce the risk of erosion. One study reported a 16% rate of tube erosion with single-thickness grafts, while no erosions were seen with double-thickness grafts120. A longer scleral tunnel may also decrease the risk of tube exposure121.
Conjunctival erosion can be repaired using a conjunctival autograft, pedicle flaps or additional patch grafts122. Recurrent tube exposure can be seen with incomplete excision of diseased tissue and epithelial downgrowth at the site of exposure123. Therefore, complete excision of unhealthy conjunctiva and scar tissue at the time of revision is recommended. Due to the risk of bacterial contamination, an extruded tube or plate often requires removal of the entire implant. The scleral tunnel should be sutured closed to decrease the risk of infection and hypotony.
Motility and orbital complications
Motility disorders and strabismus
Persistent strabismus and motility disturbances are uncommon complications following GDD surgery. Large plate size, plate encapsulation, lack of plate fenestrations, superonasal placement, and proximity to muscle insertions are associated with motility complications. GDDs can directly impinge upon muscles or create adhesions between the muscle and adjacent structures. Persistent concomitant binocular diplopia has been reported in up to 5% of patients with Baerveldt 350 mm2 implants52. An acquired Brown’s syndrome pattern has been described with superonasal quadrant GDD implantation. Ocular motility disturbances following GDD implantation are more frequent in children than in adults. A recent study reported secondary strabismus occurred in 37% of children postoperatively120. Restriction is typically largest in the quadrant containing the implant. Early postoperative peribulbar edema can also cause transient binocular diplopia, especially in people with a latent hyperphoria.
Orbital complications
Proptosis, lid protrusion, bleb extension, and postoperative ptosis have all been reported with GDD implantation124. With superior quadrant placement of a GDD, the eye is infraducted and the lid is raised for sufficient surgical exposure. Similar to superior limbus-based trabeculectomy, ptosis occurs secondary to intraoperative stretching of the levator muscle. Lid complications may be more common with Baerveldt implants, which require more globe traction as the wings are placed underneath the rectus muscles. Postoperative ptosis can be minimized by using a smaller GDD, placing the implant inferiorly (which increases the risk of vertical diplopia), and limiting traction on the upper lid.
Proptosis and delayed retrobulbar hemorrhage have also been reported125,126. Postoperative proptosis can be caused by a large bleb/plate posterior to the equator. Although extremely rare, compression of the optic nerve has been seen with an Ahmed valve placed too posteriorly. Definitive treatment requires removal, exchange, or repositioning of the GDD.
Corneal complications
Corneal decompensation
Glaucoma itself predisposes the cornea to endothelial loss when compared with age-matched controls127. Nearly all glaucoma surgical treatment modalities can cause postoperative corneal complications. Corneal complications following trabeculectomy and shunting procedures range from mild endothelial loss to complete corneal decompensation. Underlying corneal disease, in addition to intra operative and postoperative complications, predispose the eye to significant complications. Corneal complications after GDD implantation are more frequent than with trabeculectomy and have been reported to occur in 8–29% of patients128. The incidence of corneal decompensation requiring corneal transplantation after trabeculectomy or GDD implantation in patients without preexisiting corneal pathology is 10–16%129–132.
Although the use of anti-metabolite agents during trabeculectomy has increased the success rate of filtering surgery, ocular surface toxicity has increased. Both mitomycin C (MMC) and 5-fluorouracil (5FU) are toxic to replicating corneal epithelium. Exposure of high concentrations of topical MMC has been associated with iritis, scleritis, secondary glaucoma, corneal edema, mature cataracts, scleral calcification, and corneal melt. Greater endothelial cell loss has also been found in trabeculectomy with anti-metabolites when compared with standard trabeculectomy127. Severe corneal complications such as regional bullous keratopathy, adjacent to the site of MMC application, have also been reported. The most common side effects of postoperative subconjunctival 5FU injection are corneal and conjunctival epithelial toxicity, corneal epithelial defects, abrasion, and primary conjunctival wound leaks52. Using the lowest effective concentration of anti-metabolites and minimizing contact time will reduce the risk of corneal complications54,133.
Even without the use of anti-metabolites, corneal decompensation is a common complication. Postoperative inflammation and tube–endothelial contact compromise the corneal endothelium and increase the risk of corneal decompensation134. Preoperatively, AC depth should be assessed for tube placement. As stated previously, there should be at least 2–3 mm between the endothelium and tube to allow for movement of the tube without posterior corneal surface contact. Keeping the needle parallel to the iris plane during scleral tunnel formation keeps the tube away from the endothelium. Patients with pre-existing corneal disease or a previous corneal graft should have the tube inserted into the vitreous cavity (via the pars plana) or through the sulcus to reduce the risk of corneal complications126. Additionally, in eyes with shallow chambers, a pars plana tube may be the safest approach. For pars plana insertion to control IOP, an adequate anterior vitrectomy is required.
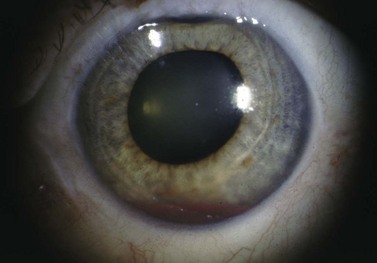
Corneal decompensation can be controlled medically with topical steroids and with IOP control (Fig. 37.9). Tube repositioning is recommended when corneal decompensation is secondary to tube–cornea contact. Deep stromal endothelial keratoplasty (DSEK) or penetrating keratoplasty can also be performed. Treating ocular surface disease such as dry eye and blepharitis along with utilizing the lowest dose of anti-metabolite reduce the risk of damage to the ocular surface. Copious irrigation of the surgical site following the application of anti-metabolites such as MMC minimizes epithelial toxicity. See also Figure 37.10.
Corneal graft failure
In eyes with pre-existing corneal transplants, the highest reported rate of corneal decompensation is 70%135. One study showed graft survival to be 58% at 1 year and 25% at 2 years. In eyes with significant corneal disease, GDD placement prior to having a PKP was associated with a 4-fold increase in subsequent graft failure. Risk factors for graft failure following GDD placement include advanced glaucoma, surgery-related complications (inflammation, shallow AC with iris, lens or tube–endothelial touch), and elevated postoperative IOP. Secondary glaucomas, such as traumatic, uveitic, and neovascular, have a 3.4-fold greater rate of graft failure136.
In addition to direct injury to the endothelium, immunologic processes put the eye at risk for more severe decompensation and graft rejection most likely from chronic postoperative blood–aqueous barrier breakdown. The retrograde flow of postoperative inflammatory cells into the AC after Molteno implantation has been reported to contribute to corneal graft rejection113.
Other corneal complications
Intracorneal bleb dissection and an overhanging bleb are late complications contributing to ‘bleb dysesthesia’ (Fig. 37.11). Both complications can change corneal topography and induce astigmatism. Superior corneal steepening and flattening and changes in axial length can cause significant changes in a patient’s refractive status postoperatively137,138. Large, overhanging blebs can form dellen and induce superficial punctate keratopathy and chronic epithelial defects. A Ferry line or iron deposition in the surperficial cornea may occur just anterior to the filtering bleb. Frequent lubrication of the ocular surface may protect the corneal epithelium and promote wound healing. If conservative management fails, a bleb revision may be required. Exposed releasable sutures may cause irritation, windshield-wiper keratopathy, and possible infectious keratitis132,135,136,139.
Intraoperative detachment of Descemet’s membrane during glaucoma surgery has also been reported140. Treatment of resulting corneal edema includes topical steroids and hypertonic saline. If needed, a descmetopexy with SF6 gas or a sterile air bubble can be performed113. Corneal blood staining with a postoperative hyphema can occur if the blood is not cleared and IOP is significantly elevated (Fig. 37.12). If a postoperative hyphema with an elevated IOP does not resolve within a few days, or if the patient has sickle cell trait or disease, a surgical evacuation or AC wash-out may be indicated.
Cataract
Glaucoma surgery causes cataract formation and progression141,142. The incidence of visually significant cataract formation after filtering surgery ranges from 2–53%143. According to the Collaborative Initial Glaucoma Treatment Study (CIGTS), the risk of requiring cataract extraction at 3 years is 11.6% in surgically controlled glaucoma patients, whereas the risk is 2.7% in medically controlled glaucoma patients. At 5 years, the incidence is 17.3% in the surgical group versus 6.2% in the medical group144. Other risk factors for cataract formation following glaucoma surgery include female gender, African ancestry, diabetes, preoperative lens opacification, chronic pilocarine use, exposure to benzalkonium chloride and long-term (>3 months) corticosteroid use145–152. Surgical risks include intraoperative complications, postoperative shallow or flat anterior chamber, and significant inflammation146. Overall, the cumulative probability for cataract formation following trabeculectomy is reported as 78% at 5 years145.
Causes of lens opacification following glaucoma surgery are multifactorial. The timing of cataract development can help determine the underlying cause. Lens opacification secondary to intraoperative trauma develops shortly after surgery. Formation or worsening of cataracts in the first 6 months after surgery may be caused by inflammation, hypotony, shallow AC or changes in aqueous humor (altering lens nutrition)153,154. Cataracts formed during the late postoperative period are associated with chronic inflammation, topical corticosteroids, or may be independent of the surgery155.
Fortunately, cataract extraction following glaucoma surgery has a relatively high success rate. When the cataract becomes visually significant, cataract extraction should be offered. Changes in visual field testing results should be expected after cataract surgery and, at times, can be challenging for the clinician monitoring glaucomatous progression. Visual field testing is a very sensitive and frequently used way to monitor glaucomatous progression. Visual field testing can become increasingly problematic with decreasing visual acuity secondary to lens opacification. For mild to moderate glaucoma, mean deviation improves while pattern standard deviation and corrected pattern standard deviation worsen after cataract extraction156,157. A new visual field baseline should be established following cataract surgery.
A few studies have demonstrated worsening of IOP control after cataract surgery in eyes with previous glaucoma surgery. A mean increase in IOP of 1.6 mmHg following cataract surgery was noted in one study, with 4% of the study’s patients requiring additional topical glaucoma medications and 9.6% undergoing bleb needling or revision158. A second study suggested an increase in IOP of 2 mmHg in eyes with previous trabeculectomy, whereas glaucomatous eyes without previous surgery had an overall decrease in IOP by 2 mmHg159,160. A recent study examined whether postoperative subconjunctival (5FU) use reduces the risk of bleb failure after cataract extraction and found that subconjunctival 5FU injections at 2, 4, and 12 weeks surgery does not reduce the risk of trabeculectomy failure.
Infectious complications
Bleb-related
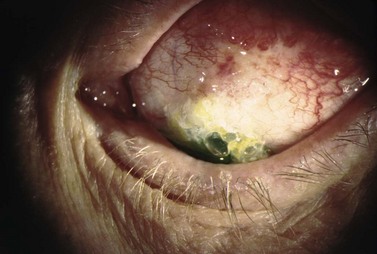
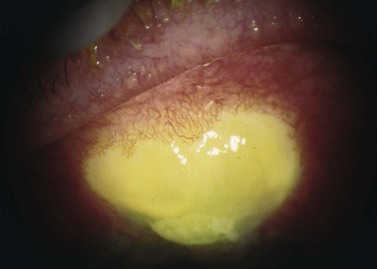
Blebitis or bleb-related endophthalmitis can occur at any time after glaucoma surgery. Infectious complications have been reported in both the early postoperative period and several years following filtering and GDD surgery. Visual outcome with blebitis is typically good with prompt diagnosis and treatment. However, if left untreated, blebitis can quickly progress to endophthalmitis, which can cause blindness161–167. Bleb-related infectious complications can be classified as early onset or late onset, with an arbitrary cut-off point of 4 weeks after surgery. Late-onset endophthalmitis is associated with a significantly worse visual outcome despite aggressive treatment.
The incidence of surgery-related endophthalmitis increased after the introduction of trabeculectomy in 1968168. In the 1980s, spikes of bleb-related infections occurred following the introduction of 5FU and MMC161,169–173. Today, the cumulative reported incidence of bleb-related infection ranges from 0.05% to 8.33%174. For inferiorly placed blebs, the incidence increases to 7.8-9.4% versus superiorly located blebs with a reported incidence of 1.1–3%. The increased incidence of endophthalmitis in inferiorly placed blebs may be due to bacterial flora exposure in the tear lake and lower lid margin162,175.
Use of anti-metabolites in glaucoma surgery is an important risk factor for bleb-related infection. The risk ratios for bleb-related infection have been calculated to be 1.31 with 5FU and 2.48 with MMC174. Other risk factors for bleb-related infection include full-thickness filtering procedures, bleb leaks, cystic blebs, frequent bleb manipulation, autologous blood injection, previous blebitis, and exposed sutures161–165,167,176–185. Other ocular factors include blepharitis, conjunctivitis, contact lens wear, increased axial length, nasolacrimal duct obstruction, abnormal lid position, tear insufficiency, lagophthalmos, cicatricial ocular pemphigoid, and atopy164,165,173,177,185,186. Systemic factors include male gender, young age, African ancestry, upper respiratory tract infection, winter season, diabetes, drug abuse, neoplastic disease, malnutrition, and intermittent or constant use of antibiotics after surgery162–164,173,181,183,187.
Patients with blebitis may report visual loss, purulent discharge, and severe ocular pain. With mild infection, symptoms may be absent or minimal. The bleb appears milky white from the mucopurulent infiltrate with injection of surrounding conjunctiva and a purulent discharge. The bleb may be Seidel positive with a mild AC inflammatory reaction without vitreous involvement173,176. If not treated appropriately, blebitis can progress to endophthalmitis188.
Bleb-related endophthalmitis (Fig. 37.13) typically follows blebitis and has vitreous involvement. Clinically, the bleb has a mucopurulent infiltrate with both AC and vitreous inflammation, with or without hypopyon190. Early-onset endophthalmitis is rare191. In typical bleb-related endophthalmitis, the loss of the conjunctival physical and immunologic barrier function allows common surface pathogen exposure192,193. More virulent organisms, such as Streptococcal spp., produce exotoxins which penetrate intact conjunctiva and access intraocular structures. The most common causative organisms in pediatric patients are Haemophilus influenzae and Streptococcus pneumonia. In adults, common organisms include coagulase negative and positive Staphylococcus species, Streptococcus pneumonia, and Pseudomonas aeruginosa. With increased antifibrotic usage, Staphylococcus epidermis and Staphylococcus aureus are becoming more common. Haemophilus influenzae has recently been reported to be an increasingly common cause in both adults and pediatric populations.
In patients with a suspicious AC inflammation, aqueous samples should be sent for culture and polymerase chain reaction (PCR) to identify the infectious agent. A vitreous biopsy is the most reliable test to identify the causative agent. If endophthalmitis is diagnosed or suspected, fortified, broad-spectrum antibiotics (cephalosporins, vancomycin, gentamicin, and amikacin) that cover both Gram-positive and Gram-negative species should be started immediately184,194. Topical fluoroquinolones (with or without another antibiotics) have been used), and oral fluoroquinolones such as ofloxacin or ciprofloxacin have been suggested as effective adjunct treatment184,191.
If no improvement is seen within 24–48 hours of initiating topical antibiotic treatment, intravitreal injection of antibiotic is indicated182. Topical treatment with hourly fortified antibiotics and possibly an oral third or fourth generation fluoroquinolone with good vitreous penetration should be continued165,188,195. Other physicians, depending upon the clinical severity, may opt to perform an intravitreal injection of antibiotics, a vitreous biopsy, and initiate topical antibiotics upon presentation. With clinical improvement, topical corticosteroids can be started 24–48 hours after initiation of antibiotic treatment to control intraocular inflammation. An early vitrectomy has not been found to improve visual outcome in bleb-related endophthalmitis172,173,196. Despite topical, systemic, and intravitreal antibiotics with vitrectomy, bleb-related endophthalmitis carries a poor visual prognosis171,172. Bleb-related inflammation and infection frequently lead to bleb failure and, later, progression of glaucomatous optic neuropathy.
GDD-related
Although uncommon, infectious complications can occur after GDDs (Fig. 37.14). Due to the insertion of a foreign body, eyes with GDDs are at risk for endophthalmitis, panophthalmitis, and orbital cellulitis54,197,198. Conjunctival erosion with exposure of the tube and/or the drainage plate allows bacteria to travel in a retrograde fashion from the site of exposure, to the plate, through the tube and into the eye. Any eye with an exposed tube should be started immediately on prophylactic antibiotic therapy and have prompt repair or removal of the GDD. Once infected, some suggest that the GDD should be removed because of the low likelihood of sterilizing the implant in situ. As with bleb-related endophthalmitis, endophthalmitis following GDD implantation usually has a poor visual outcome despite medical and surgical treatment.
1 Khaw PT, Chang L, Wong TT, et al. Modulation of wound healing after glaucoma surgery. Curr Opin Ophthalmol. 2001;12(2):143-148. Review. Erratum in: Curr Opin Ophthalmol 2001;12(3):235
2 Soltan JB, Rollman RF, Budenz DL, et al. Risk factors for glaucoma filtering bleb infections. Arch Ophthalmol. 2000;118:338-342.
3 Loane ME, Galanopoulos A. The surgical management of leaking filtering blebs. Curr Opin Ophthalmol. 1999;10(2):121-125.
4 Greenfield DS, Liebmann JM, Jee J, et al. Late-onset bleb leaks after glaucoma filtering surgery. Arch Ophthalmol. 1998;116(4):443-447.
5 Wilensky JT. Management of late bleb leaks following glaucoma filtering surgery. Trans Am Ophthalmol Soc. 1992;90:161-168. discussion 168–70
6 Belyea DA, Dan JA, Stamper RL, et al. Late onset of sequential multifocal bleb leaks after glaucoma filtration surgery with 5-fluorouracil and mitomycin C. Am J Ophthalmol. 1997;124(1):40-45.
7 Parrish RK2nd, Schiffman JC, Feuer WJ, et al. Prognosis and risk factors for early postoperative wound leaks after trabeculectomy with and without 5-fluorouracil. Am J Ophthalmol. 2001;132(5):633-640.
8 Zalta AH, Wieder RH. Closure of leaking filtering blebs with cyanoacrylate tissue adhesive. Br J Ophthalmol. 1991;75(3):170-173.
9 Seligsohn A, Moster MR, Steinmann W, et al. Use of Tisseel fibrin sealant to manage bleb leaks and hypotony: case series. J Glaucoma. 2004;13(3):227.
10 Asrani SG, Wilensky JT. alternative. Ophthalmology. 1996;103(2):294-298.
11 Baum M, Weiss HS. Argon laser closure of conjunctival bleb leak. Arch Ophthalmol. 1993;111(4):438.
12 Geyer O. Management of large, leaking, and inadvertent filtering blebs with the neodymium:YAG laser. Ophthalmology. 1998;105(6):983-987.
13 Palmberg P. Late complications after glaucoma filtering surgery: peril to the nerve. In: Leader BJ, Calkwood JC, editors. Glaucoma and Clinical Neuro-Ophthalmology Proceedings of the 45 Annual Symposium of the New Orleans Academy of Ophthalmology, New Orleans, 1996:25–28. The Hague: Kiegler Publications; 1998. p. 183–93.
14 Leen MM, Moster MR, Katz LJ, et al. Management of overfiltering and leaking blebs with autologous blood injection. Arch Ophthalmol. 1995;113(8):1050-1055.
15 Myers JS, Yang CB, Herndon LW, et al. Excisional bleb revision to correct over-filtration or leakage. J Glaucoma. 2000;9(2):169-173.
16 Tannenbaum DP, Hoffman D, Greaney MJ, et al. Outcomes of bleb excision and conjunctival advancement for leaking or hypotonous eyes after glaucoma filtering surgery. Br J Ophthalmol. 2004;88(1):99-103.
17 Catoira Y, Wudunn D, Cantor LB. Revision of dysfunctional filtering blebs by conjunctival advancement with bleb preservation. Am J Ophthalmol. 2000;130(5):574-579.
18 Brubaker RF, Pederson JE. Ciliochoroidal detachment. Surv Ophthalmol. 1983;27(5):281-289.
19 Brubaker RF. The physiology of aqueous humor formation. In: Drance SM, Newufeld AH, editors. Glaucoma: Applied Pharmacology in Medical Treatment. Orlando: Grune and Stratton, 1984.
20 Lamping K, Bellows R, Hutchinson T, et al. Long-term evaluation of initial filtration surgery. Ophthalmology. 1986;93:91-101.
21 Fluorouracil Filtering Study Group. Fluorouracil filterning surgery study, one year follow up. Am J Ophthalmol. 1989;108:623-635.
22 Lieberman M, Stamper R, McMenemy M, et al. Initial 5-fluorouracil trabeculectomy in uncomplicated glaucoma. Ophthalmology. 1991;98:1036-1041.
23 Lamping K, Belkin J. 5 fluorouracil and mitomycin C in pseudophakic patients. Ophthalmology. 1995;102:70-75.
24 Montenegro M, Murcia A, Kashara N, et al. High dose intraoperative mitomycin C trabeculectomy. Invest Ophthalmol Vis Sci. 1995;36:86.
25 Skuta GL, Beeson CC, Higginbotham EJ, et al. Intraoperative mitomycin versus postoperative 5-fluorouracil in high-risk glaucoma filtering surgery. Ophthalmology. 1992;99(3):438-444.
26 Zacharia PT, Deppermann SR, Schuman JS. Ocular hypotony after trabeculectomy with mitomycin C. Am J Ophthalmol. 1993;116(3):314-326.
27 Bindlish R, Condon GP, Schlosser JD, et al. Efficacy and safety of mitomycin-C in primary trabeculectomy: five-year follow-up. Ophthalmology. 2002;109(7):1336-1341. discussion 1341–2
28 Moshfeghi DM, Kim BY, Kaiser PK, et al. Appositional suprachoroidal hemorrhage: a case–control study. Am J Ophthalmol. 2004;138(6):959-963.
29 Chu TG, Cano MR, Green RL. Massive suprachoroidal hemorrhage with a central retinal apposition: a clinical and echographic study. Arch Ophthalmol. 1991;109:1575-1581.
30 Cantor LB, Katz LJ, Spaeth GL. Complications of surgery in glaucoma: suprachoroidal expulsive hemorrhage in glaucoma patients undergoing intraocular surgery. Ophthalmology. 1998;105:428-431.
31 Givens K, Shields MB. Suprachoroidal after glaucoma filtering surgery. Am J Ophthalmol. 1987;103:689-694.
32 Straatsma BR, Khwarg SG, Rajacich GM. Cataract surgery after expulsive choroidal hemorrhage in the fellow eye. Ophthalmic Surg. 1986;17:400-403.
33 Speaker MG, Guerriero PN, Met JA, et al. A case control study of risk factors for intraoperative suprachoroidal hemorrhage. Ophthalmology. 1991;98:202-210.
34 Ruderman HM, Harbin TSJr, Campbell DG. Postoperative suprachoroidal hemorrhage following filtration procedures. Arch Ophthalmol. 1986;104:201-205.
35 Weinreb RN. Adjusting the dose of 5-fluorouracil after filtration surgery to minimize side effects. Ophthalmology. 1987;94(5):564-570.
36 Robin AL, Ramakrishnan R, Krishnadas R, et al. A long-term dose–response study of mitomycin in glaucoma filtration surgery. Arch Ophthalmol. 1997;115(8):969-974.
37 Salvo ECJr, Luntz MH, Medow MD. Use of viscoelastics post-trabeculectomy: a survey of members of the American Glaucoma Society. Ophthalmic Surg Lasers. 1999;30(4):271-275.
38 Osher RH, Cionni RJ, Cohen JS. Reforming the anterior chamber with Healon. J Cataract Refract Surg. 1996;22:411-415.
39 Bellows AR, Chylack LTJr, Hutchinson BT. Choroidal detachment. Clinical manifestation, therapy and mechanism of formation. Ophthalmology. 1981;88(11):1107-1115.
40 Clarcke MP, Vernon SA, Shledrick JH. The development of cataract after trabeculectomy. Eye. 1990;4:577-583.
41 Popovic V, Sjostrand J. Long term outcome of trabeculectomy: I. Retrospective analysis of intraocular pressure regulation and cataract formation. Acta Ophthalmol. 1991;69:299-304.
42 Stewart WC, Shields MB. Management of anterior chamber depth after trabeculectomy. Am J Ophthalmol. 1988;106(1):41-44. 15
43 Chandler PA. Malignant glaucoma. Trans Am Ophthalmol Soc. 1951:128-143.
44 Quigley HA. Malignant glaucoma and fluid flow rate. Am J Ophthalmol. 1980;89(6):879-880.
45 Trope GE, Pavlin CJ, Bau A, et al. Malignant glaucoma. Clinical and ultrasound biomicroscopic features. Ophthalmology. 1994;101(6):1030-1035.
46 Tello C, Chi T, Shepps G, et al. Ultrasound biomicroscopy in pseudophakic malignant glaucoma. Ophthalmology. 1993;100(9):1330-1334. Erratum in: Ophthalmology 1993;100(12):1747
47 Harbour JW, Rubsamen PE, Palmberg P. Pars plana vitrectomy in the management of phakic and pseudophakic malignant glaucoma. Arch Ophthalmol. 1996;114(9):1073-1078.
48 Tsai JC, Barton KA, Miller MH, et al. Surgical results in malignant glaucoma refractory to medical or laser therapy. Eye (Lond). 1997;11(Pt 5):677-681.
49 Edmunds B, Thompson JR, et al. The National Survey of Trabeculectomy. III. Early and late complications. Eye. 2002;16(3):297-303.
50 Chandler PA, Simmons RJ, et al. Malignant glaucoma. Medical and surgical treatment. Am J Ophthalmol. 1968;66(3):495-502.
51 Simmons RJ. Malignant glaucoma. Br J Ophthalmol. 1972;56(3):263-272.
52 Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the tube versus trabeculectomy study after one year of follow-up. Am J Ophthalmol. 2007;143:9-22.
53 Jampel HD, Musch DC, Gillespie BW, et al. Perioperative complications of trabeculectomy in the Collaborative Initial Glaucoma Treatment Study. Am J Ophthalmol. 2005;140(1):16-22.
54 Nyugen QH, Budenz DL, Parrish RK2nd. Complications of Baerveldt glaucoma drainage implants. Arch Ophthalmol. 1998;116(5):571-575.
55 Von Graefe A. Bietrage zur pathologie und therapie de glaucoms. Arch Ophthalmol. 1869;15:108.
56 Byrnes GA, Leen MM, Wong TP, et al. Vitrectomy for ciliary block (malignant) glaucoma. Ophthalmology. 1995;102(9):1308-1311.
57 Fanous S, Brouillette G. Ciliary block glaucoma: malignant glaucoma in the absence of a history of surgery and of miotic therapy. Can J Ophthalmol. 1983;18(6):302-303.
58 Schwartz AL, Anderson DR. Malignant glaucoma in an eye with no antecedent operation or miotics. Arch Ophthalmol. 1975;93(5):379-381.
59 Chandler PA, Grant WM. Mydriatic–cycloplegic treatment in malignant glaucoma. Arch Ophthalmol. 1962;68:353-359.
60 Herschler J. Laser shrinkage of the ciliary processes. A treatment for malignant (ciliary block) glaucoma. Ophthalmology. 1980;87(11):1155-1159.
61 Epstein DL, Steinert RF, Puliafito CA. Neodymium-YAG laser therapy to the anterior hyaloid in aphakic malignant (ciliovitreal block) glaucoma. Am J Ophthalmol. 1984;98(2):137-143.
62 Little BC, Hitchings RA. Pseudophakic malignant glaucoma: Nd:YAG capsulotomy as a primary treatment. Eye. 1993;7:102-104.
63 Brown RH, Lynch MG, et al. Neodymium-YAG vitreous surgery for phakic and pseudophakic malignant glaucoma. Arch Ophthalmol. 1986;104(10):1464-1466.
64 Halkias A, Magauran DM, et al. Ciliary block (malignant) glaucoma after cataract extraction with lens implant treated with YAG laser capsulotomy and anterior hyaloidotomy. Br J Ophthalmol. 1992;76(9):569-570.
65 Lockie P. Ciliary-block glaucoma treated by posterior capsulotomy. Aust NZ J Ophthalmol. 1987;15(3):207-209.
66 Risco JM, Tomey KF, Perkins TW. Laser capsulotomy through intraocular lens positioning holes in anterior aqueous misdirection. Case report. Arch Ophthalmol. 1989;107(11):1569.
67 Shaffer RN, Hoskins HDJr. Ciliary block glaucoma. Ophthalmology. 1978;85(3):215-221.
68 Francis BA, Wong RM, et al. Slit-lamp needle revision for aqueous misdirection after trabeculectomy. J Glaucoma. 2002;11(3):183-188.
69 Sharma A, Sii F, Shah P, et al. Vitrectomy-phacoemulsification – vitrectomy for the management of aqueous misdirection syndromes in phakic eyes. Ophthalmology. 2006;113(11):1968-1973.
70 Benedikt O. A new operative method for the treatment of malignant glaucoma. Klin Montasblenheilkd. 1977;170(5):665-672.
71 Carassa RG, Bettin P, Fiori M, et al. Treatment of malignant glaucoma with contact transscleral cyclophotocoagulation. Arch Ophthalmol. 1999;117(5):688-690.
72 Sengupta R, Austin M, Morgan J. Treatment of aqueous misdirection by trans-scleral diode laser photocoagulation. Eye. 2000;14:808-810.
73 Gressel MG, Parrish RK2nd, Heuer DK. Delayed nonexpulsive suprachoroidal hemorrhage. Arch Ophthalmol. 1984;102(12):1757-1760.
74 Givens K, Shields MB. Suprachoroidal hemorrhage after glaucoma filtering surgery. Am J Ophthalmol. 1987;103(5):689-694.
75 Moshfeghi DM, Kim BY, Kaiser PK, et al. Appositional suprachoroidal hemorrhage: a case–control study. Am J Ophthalmol. 2004;138(6):959-963.
76 Chu TG, Cano MR, Green RL. Massive suprachoroidal hemorrhage with a central retinal apposition: a clinical and echographic study. Arch Ophthalmol. 1991;109:1575-1581.
77 Cantor LB, Katz LJ, Spaeth GL. Complications of surgery in glaucoma: suprachoroidal expulsive hemorrhage in glaucoma patients undergoing intraocular surgery. Ophthalmology. 1998;105:428-431.
78 Givens K, Shields MB. Suprachoroidal after glaucoma filtering surgery. Am J Ophthalmol. 1987;103:689-694.
79 Straatsma BR, Khwarg SG, Rajacich GM. Cataract surgery after expulsive choroidal hemorrhage in the fellow eye. Ophthalmic Surg. 1986;17:400-403.
80 Speaker MG, Guerriero PN, Met JA, et al. A case control study of risk factors for intraoperative suprachoroidal hemorrhage. Ophthalmology. 1991;98:202-210.
81 Ruderman HM, Harbin TSJr, Campbell DG. Postoperative suprachoroidal hemorrhage following filtration procedures. Arch Ophthalmol. 1986;104:201-205.
82 Healey PR, Herdon L, Smiddy W. Cases in controversy: management of suprachoroidal hemorrhage. J Glaucoma. 2007;16(6):577-579.
83 Scott IU, Flynn HWJr, Schiffman J, et al. Visual acuity outcomes among patients with appositional suprachoroidal hemorrhage. Ophthalmology. 1997;104(12):2039-2046.
84 Lambrou FHJr, Meredith TA, Kaplan HJ. Secondary surgical management of expulsive choroidal hemorrhage. Arch Ophthalmol. 1987;105(9):1195-1198.
85 Suñer IJ, Greenfield DS, Miller MP, et al. Hypotony maculopathy after filtering surgery with mitomycin C. Incidence and treatment. Ophthalmology. 1997;104(2):207-214. discussion 214–15
86 Shirato Suyama K, Haneda M. Resuturing the scleral flap through conjunctiva for treatment of excess filtration. Am J Ophthalmol. 2004;137(1):173-174.
87 Letartre L, Basheikh A, Anctil JL, et al. Transconjunctival suturing of the scleral flap for over-filtration with hypotony maculopathy after trabeculectomy. Can J Ophthalmol. 2009;44(5):567-570.
88 Traverso CE, Greenidge GL, Spaeth KC, et al. Focal pressure: a new method to encourage filtration after trabeculectomy. Ophthalmic Surg. 1984;15:62-65.
89 Tripathi RC, Tripathi BJ, Pak JK, et al. Intracameral tissue plasminogen activator for resolution of fibrin clots after glaucoma filtering procedures. Am J Ophthalmol. 1991;111(2):247-248.
90 Smith MF, Doyle JW. Use of tissue plasminogen activator to revive blebs following intraocular surgery. Arch Ophthalmol. 2001;119(6):809-812.
91 Lundy DC, Sidoti P, Loffler KU, et al. Intracameral tissue plasminogen activator after glaucoma surgery. Indications, effectiveness, and complications. Ophthalmology. 1996;103(2):274-282.
92 Wollensak G, Meyer JH, Loffler KU, et al. [Band-like keratopathy after treatment of postoperative fibrin reaction with tissue plasminogen activator]. Klin Monatsblenheilkd. 1996;209(1):43-46.
93 Loffler KU, Meyer JH, Wollensak G, et al. [Success and complications of rTPA treatment of the anterior eye segment]. Ophthalmologe. 1997;94(1):50-52.
94 Kim MH, Koo TH, Sah WJ, et al. Treatment of total hyphema with relatively low-dose tissue plasminogen activator. Ophthalmic Surg Lasers. 1998;29(9):762-766.
95 Molteno AC, Straughan JL, Ancker E. Control of bleb fibrosis after glaucoma surgery by anti-inflammatory agents. S Afr Med J. 1976;50(23):881-885.
96 Pederson JE, Smith SG. Surgical management of encapsulated filtering blebs. Ophthalmology. 1985;92(7):955-995.
97 Liebmann JM, Ritch R, DiSclafani M, et al. Early intraocular pressure rise after trabeculectomy. Arch Ophthalmol. 1990;108(11):1549-1552.
98 Salvo ECJr, Luntz MH, Medow NB. Use of viscoelastics post-trabeculectomy: a survey of members of the American Glaucoma Society. Ophthalmic Surg Lasers. 1999;30(4):271-275.
99 Michielsens A, Hennekes R. The use of hyaluronic acid of different viscosities in preventing postoperative hypotony after trabeculectomy. Bull Soc Belge Ophthalmol. 1993;250:17-23.
100 Feldman RM, Gross RL, Spaeth GL, et al. Risk factors for the development of Tenon’s capsule cysts after trabeculectomy. Ophthalmology. 1989;96(3):336-341.
101 Sherwood MB, Spaeth GL, Simmons ST, et al. Cysts of Tenon’s capsule following filtration surgery. Medical management. Arch Ophthalmol. 1987;105(11):1517-1521.
102 Richter CU, Shingleton BJ, Bellows AR, et al. The development of encapsulated filtering blebs. Ophthalmology. 1988;5(9):1163-1168.
103 Schwartz AL, Van Veldhuisen PC, Gaasterland DE, et al. The Advanced Glaucoma Intervention Study (AGIS): 5. Encapsulated bleb after initial trabeculectomy. Am J Ophthalmol. 1999;127(1):8-19.
104 Campagna JA, Munden PM, Alward WL. Tenon’s cyst formation after trabeculectomy with mitomycin C. Ophthalmic Surg. 1995;26(1):57-60.
105 Sherwood MB, Grierson I, Millar L, et al. Long-term morphologic effects of antiglaucoma drugs on the conjunctiva and Tenon’s capsule in glaucomatous atients. Ophthalmology. 1989;96(3):327-335.
106 Shingleton BJ, Richter CU, Bellows AR, et al. Management of encapsulated filtration blebs. Ophthalmology. 1990;97(1):63-68.
107 Costa VP, Correa MM, Jose NK, et al. Needling versus medical treatment in encapsulated blebs. A randomized, prospective study. Ophthalmology. 1997;104(8):1215-1220.
108 Molteno AC, Straughan JL, Ancker E. Control of bleb fibrosis after glaucoma surgery by anti-inflammatory agents. S Afr Med J. 1976;50(23):881-885.
109 Pederson JE, Smith SG. Surgical management of encapsulated filtering blebs. Ophthalmology. 1985;92(7):955-958.
110 Singh K, Eid TE, Katz LJ, et al. Evaluation of Nd:YAG laser membranectomy in blocked tubes after glaucoma tube-shunt surgery. Am J Ophthalmol. 1997;124(6):781-786.
111 Tong L, Frazao K, LaBree L, et al. Intraocular pressure control and complications with two-stage insertion of the Baerveldt implant. Ophthalmology. 2003;110(2):353-358.
112 Harbick KH, Sidoti PA, Budenz DL, et al. Outcomes of inferonasal Baerveldt glaucoma drainage implant surgery. J Glaucoma. 2006;15(1):7-12.
113 Topouzis F, Coleman AL, Choplin N, et al. Follow-up of the original cohort with the Ahmed glaucoma valve implant. Am J Ophthalmol. 1999;128(2):198-204.
114 Wilson MR, Mendis U, Paliwal A, et al. Long-term follow-up of primary glaucoma surgery with Ahmed glaucoma valve implant versus trabeculectomy. Am J Ophthalmol. 2003;136(3):464-470.
115 Ishida K, Netland PA. Ahmed glaucoma valve implantation in African American and white patients. Arch Ophthalmol. 2006;124(6):800-806.
116 Zalta AH, Sweeney CP, Zalta AK, et al. Intracameral tissue plasminogen activator use in a large series of eyes with valved glaucoma drainage implants. Arch Ophthalmol. 2002;120(11):1487-1493.
117 Välimäki J. Fibrin glue for preventing immediate postoperative hypotony following glaucoma drainage implant surgery. Acta Ophthalmol Scand. 2006;84(3):372-374.
118 Hill RA, Pirouzian A, Liaw L. Occlusion. Am J Ophthalmol. 2000;129(5):608-612.
119 Fechter HP, Parrish RK2nd. Preventing and treating complications of Baervledt glaucoma drainage device surgery. Int Ophthalmol Clin. 2004;44(2):107-136.
120 Sarkisian SRJr. Tube shunt complications and their prevention. Curr Opin Ophthalmol. 2009;20(2):126-130. Review
121 Sidoti PA, Minckler DS, Baerveldt G, et al. Epithelial ingrowth and glaucoma drainage implants. Ophthalmology. 1994;101(5):872-875.
122 Godfrey DG, Merritt JH, Fellman RL, et al. Interpolated conjunctival pedicle flaps for the treatment of exposed glaucoma drainage devices. Arch Ophthalmol. 2003;121(12):1772-1775.
123 Sidoti PA, Minckler DS, Baerveldt G, et al. Epithelial ingrowth and glaucoma drainage implants. Ophthalmology. 1994;101(5):872-875.
124 Younger JR, Kooner KS. Upper eyelid extension of a filtering bleb following glaucoma shunt surgery. Ophthalmic Surg Lasers Imaging. 2006;37(4):324-326.
125 Chan CH, Lai JS, Shen SY. Delayed retrobulbar haemorrhage after Ahmed glaucoma implant: a case report. Eye (Lond). 2006;20(4):494-495.
126 Danesh-Meyer HV, Spaeth GL, Maus M. Cosmetically significant proptosis following a tube shunt procedure. Arch Ophthalmol. 2002;120(6):846-847.
127 Hau S, Barton K. Corneal complications of glaucoma surgery. Curr Opin Ophthalmol. 2009;20(2):131-136. Review
128 Erie JC, Baratz KH, Mahr MA, et al. Phacoemulsification in patients with Baerveldt tube shunts. J Cataract Refract Surg. 2006;32(9):1489-1491.
129 Tong L, Frazao K, LaBree L, et al. Intraocular pressure control and complications with two-stage insertion of the Baerveldt implant. Ophthalmology. 2003;110(2):353-358.
130 Fechter HP, Parrish RK2nd. Preventing and treating complications of Baerveldt Glaucoma Drainage Device surgery. Int Ophthalmol Clin. 2004;44(2):107-136.
131 Harbick KH, Sidoti PA, Budenz DL, et al. Outcomes of inferonasal Baerveldt glaucoma drainage implant surgery. J Glaucoma. 2006;15(1):7-12.
132 Broadway DC, Iester M, Schulzer M, et al. Survival analysis for success of Molteno tube implants. Br J Ophthalmol. 2001;85(6):689-695.
133 Gedde SJ, Schiffman JC, Feuer WJ, et al. Three-year follow-up of the tube versus trabeculectomy study. Am J Ophthalmol. 2009;148(5):670-684.
134 Sa HS, Kee C. Effect of temporal clear corneal phacoemulsification on intraocular pressure in eyes with prior Ahmed glaucoma valve insertion. J Cataract Refract Surg. 2006;32(6):1011-1014.
135 Alvarenga LS, Mannis MJ, Brandt JD, et al. The long-term results of keratoplasty in eyes with a glaucoma drainage device. Am J Ophthalmol. 2004;138(2):200-205.
136 Kwon YH, Taylor JM, Hong S, et al. Long-term results of eyes with penetrating keratoplasty and glaucoma drainage tube implant. Ophthalmology. 2001;108(2):272-278.
137 Cantor L, Burgoyne J, Sanders S, et al. The effect of mitomycin C on Molteno implant surgery: a 1-year randomized, masked, prospective study. J Glaucoma. 1998;7(4):240-246.
138 Hwang JM, Kee C. The effect of surface area expansion with pericardial membrane (preclude) in Ahmed glaucoma valve implant surgery. J Glaucoma. 2004;13(4):335-339.
139 Al-Torbak A, Edward DP. Transcorneal tube erosion of an Ahmed valve implant in a child. Arch Ophthalmol. 2001;119(10):1558-1559.
140 Greenfield DS, Tello C, Budenz DL, et al. Aqueous misdirection after glaucoma drainage device implantation. Ophthalmology. 1999;106(5):1035-1040.
141 Mills KB. Trabeculectomy: a retrospective long-term follow-up of 444 cases. Br J Ophthalmol. 1981;65(11):790-795.
142 Ridgway AE. Trabeculectomy. A follow-up study. Br J Ophthalmol. 1974;58(7):680-686.
143 Liebmann JM, Ritch B. Complications of glaucoma filtering surgery. Glaucomas. St Louis: Mosby; 1996. p. 1703–36
144 Feiner L, Piltz-Seymour JR. Collaborative Initial Glaucoma Treatment Study. Collaborative Initial Glaucoma Treatment Study: a summary of results to date. Curr Opin Ophthalmol. 2003;14(2):106-111.
145 AGIS (Advanced Glaucoma Intervention Study) Investigators. The Advanced Glaucoma Intervention Study: 8. Risk of cataract formation after trabeculectomy. Arch Ophthalmol. 2001;119(12):1771-1779.
146 Costa VP, Smith M, Spaeth GL, et al. Loss of visual acuity after trabeculectomy. Ophthalmology. 1993;100(5):599-612.
147 Brandt JD. Does benzalkonium chloride cause cataract? Arch Ophthalmol. 2003;121(6):892-893.
148 Husain R, Aung T, Gazzard G, et al. Effect of trabeculectomy on lens opacities in an East Asian population. Arch Ophthalmol. 2006;124(6):787-792.
149 Leske MC, Wu SY, Nemesure B, et al. Risk factors for incident nuclear opacities. Barbados Eye Studies Group. Ophthalmology. 2002;109(7):1303-1308.
150 Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268-1279.
151 Herman DC, Gordon MO, Beiser JA, et al. Topical ocular hypotensive medication and lens opacification: evidence from the ocular hypertension treatment study. Am J Ophthalmol. 2006;142(5):800-810.
152 Araujo SV, Spaeth GL, Roth SM, et al. A ten-year follow-up on a prospective, randomized trial of postoperative corticosteroids after trabeculectomy. Ophthalmology. 1995;102(12):1753-1759.
153 Razzak A, al Samarrai A, Sunba MS. Incidence of posttrabeculectomy cataract among Arabs in Kuwait. Ophthalmic Res. 1991;23(1):21-23.
154 Pillai S, Mahmood MA, Limaye SR. Transient lenticular opacification following trabeculectomy. Ophthalmic Surg. 1988;19(7):508-509.
155 Sugar HS. Postoperative cataract in successfully filtering glaucomatous eyes. Am J Ophthalmol. 1970;69(5):740-746.
156 Musch DC, Gillespie BW, Niziol LM, et al. Cataract extraction in the collaborative initial glaucoma treatment study. Arch Ophthalmol. 2006;124(12):1694-1700.
157 Chen PP, Budenz DL. The effects of cataract extraction on the visual field of eyes with chronic open-angle glaucoma. Am J Ophthalmol. 1998;125(3):325-333.
158 Chen PP, Weaver YK, Budenz DL, et al. Trabeculectomy function after cataract extraction. Ophthalmology. 1998;105(10):1928-1935.
159 Klink J, Schmitz B, Lieb WE, et al. Filtering bleb function after clear cornea phacoemulsification: a prospective study. Br J Ophthalmol. 2005;89(5):597-601.
160 Rebolleda G, Muñoz-Negrete FJ. Phacoemulsification in eyes with functioning filtering blebs: a prospective study. Ophthalmology. 2002;109(12):2248-2255.
161 Higginbotham EJ, Stevens RK, Musch DC, et al. Bleb-related endophthalmitis after trabeculectomy with mitomycin C. Ophthalmology. 1996;103(4):650-656.
162 Soltau JB, Rothman RF, Budenz DL, et al. Risk factors for glaucoma filtering bleb infections. Arch Ophthalmol. 2000;118(3):338-342.
163 Wolner B, Liebmann JM, Sassani JW, et al. Late bleb-related endophthalmitis after trabeculectomy with adjunctive 5-fluorouracil. Ophthalmology. 1991;98(7):1053-1060.
164 Ashkenazi I, Melamed S, Avni I, et al. Risk factors associated with late infection of filtering blebs and endophthalmitis. Ophthalmic Surg. 1991;22(10):570-574.
165 Greenfield DS, Suñer IJ, Miller MP, et al. Endophthalmitis after filtering surgery with mitomycin. Arch Ophthalmol. 1996;114(8):943-949.
166 Waheed S, Ritterband DC, Greenfield DS, et al. New patterns of infecting organisms in late bleb-related endophthalmitis: a ten year review. Eye (Lond). 1998;12(Part 6):9101-9105.
167 Mochizuki K, Jikihara S, Ando Y, et al. Incidence of delayed onset infection after trabeculectomy with adjunctive mitomycin C or 5-fluorouracil treatment. Br J Ophthalmol. 1997;81(10):877-883.
168 Cairns JE. Trabeculectomy. Preliminary report of a surgical method of reducing intra-ocular pressure in chronic simple glaucoma without sub-conjunctival drainage of aqueous humor. Bibl Ophthalmol. 1970;81:143-153.
169 Shields MB, Scroggs MW, Sloop CM, et al. Clinical and histopathologic observations concerning hypotony after trabeculectomy with adjunctive mitomycin C. Am J Ophthalmol. 1993;116(6):673-683.
170 Parrish R, Minckler D. ‘Late endophthalmitis’ – filtering surgery time bomb? Ophthalmology. 1996;103(8):1167-1168.
171 Song A, Scott IU, Flynn HWJr, et al. Delayed-onset bleb-associated endophthalmitis: clinical features and visual acuity outcomes. Ophthalmology. 2002;109(5):985-991.
172 Busbee BG, Recchia FM, Kaiser R, et al. Bleb-associated endophthalmitis: clinical characteristics and visual outcomes. Ophthalmology. 2004;111(8):1495-1503.
173 Brown RH, Yang LH, Walker SD, et al. Treatment of bleb infection after glaucoma surgery. Arch Ophthalmol. 1994;112(1):57-61.
174 Ang GS, Varga Z, Shaarawy T. Postoperative infection in penetrating versus non-penetrating glaucoma surgery. Br J Ophthalmol. 2009:5.
175 Wolner B, Liebmann J, Ritch R, et al. Treatment of bleb infection after glaucoma surgery. Arch Ophthalmol. 1994;112(10):1277-1278.
176 Hattenhauer JM, Lipsich MP. Late endophthalmitis after filtering surgery. Am J Ophthalmol. 1971;72(6):1097-1101.
177 Tabbara KF. Late infections following filtering procedures. Ann Ophthalmol. 1976;8(10):1228-1231.
178 Freedman J, Gupta M, Bunke A. Endophthalmitis after trabeculectomy. Arch Ophthalmol. 1978;96(6):1017-1018.
179 Ayyala RS, Bellows AR, Thomas JV, et al. Bleb infections: clinically different courses of ‘blebitis’ and endophthalmitis. J Ophthalmic Nurs Technol. 1997;16(6):292-300.
180 Mills KB. Trabeculectomy: a retrospective long-term follow-up of 444 cases. Br J Ophthalmol. 1981;65(11):790-795.
181 Lehmann OJ, Bunce C, Matheson MM, et al. Risk factors for development of post-trabeculectomy endophthalmitis. Br J Ophthalmol. 2000;84(12):1349-1353.
182 Waheed S, Liebmann JM, Greenfield DS, et al. Recurrent bleb infections. Br J Ophthalmol. 1998;82(8):926-929.
183 Jampel HD, Quigley HA, Kerrigan-Baumrind LA, et al. Risk factors for late-onset infection following glaucoma filtration surgery. Arch Ophthalmol. 2001;119(7):1001-1008.
184 Chen PP, Gedde SJ, Budenz DL, et al. Outpatient treatment of bleb infection. Arch Ophthalmol. 1997;115(9):1124-1128.
185 Bellows AR, McCulley JP. Endophthalmitis in aphakic patients with unplanned filtering blebs wearing contact lenses. Ophthalmology. 1981;88(8):839-843.
186 Gupta N, Weinreb RN. Filtering bleb infection as a complication of orthokeratology. Arch Ophthalmol. 1997;115(8):1076.
187 Leopold IH, Apt L. Postoperative intraocular infections. Am J Ophthalmol. 1960;50:1225-1247.
188 Mandelbaum S, Forster RK, Gelender H, et al. Late onset endophthalmitis associated with filtering blebs. Ophthalmology. 1985;92(7):964-972.
189 Kanski JJ. Treatment of late endophthalmitis associated with filtering blebs. Arch Ophthalmol. 1974;91(5):339-343.
190 Katz LJ, Cantor LB, Spaeth GL. Complications of surgery in glaucoma. Early and late bacterial endophthalmitis following glaucoma filtering surgery. Ophthalmology. 1985;92(7):959-963.
191 Ciulla TA, Beck AD, Topping TM, et al. Blebitis, early endophthalmitis, and late endophthalmitis after glaucoma-filtering surgery. Ophthalmology. 1997;104(6):986-995.
192 Beck AD, Grossniklaus HE, Hubbard B, et al. Pathologic findings in late endophthalmitis after glaucoma filtering surgery. Ophthalmology. 2000;107(11):2111-2114.
193 Gollamudi SR, Hodapp EA, Cubillas A, et al. Photographically documented access of tear film to the anterior chamber through a leaky filtering bleb. Arch Ophthalmol. 1993;111(3):394-395.
194 Azuara-Blanco A, Katz LJ. Dysfunctional filtering blebs. Surv Ophthalmol. 1998;43(2):93-126.
195 Busbee BG. Update on treatment strategies for bleb-associated endophthalmitis. Curr Opin Ophthalmol. 2005;16(3):170-174.
196 Sharma T, Chen SD, Salmon JF. Bleb-associated endophthalmitis. Ophthalmology. 2005;112(6):1168. author reply 1168–9
197 Laviña AM, Creasy JL, Tsai JC. Orbital cellulitis as a late complication of glaucoma shunt implantation. Arch Ophthalmol. 2002;120(6):849-851.
198 Marcet MM, Woog JJ, Bellows AR, et al. Orbital complications after aqueous drainage device procedures. Ophthal Plast Reconstr Surg. 2005;21(1):67-69.