Chapter 142 Management of Penetrating Brain Injury
Epidemiology
In the United States, the incidence of PBI is estimated to be 12 per 100,000, higher than in any other developed nation.1 Approximately 15% to 20% of trauma-related noncombatant deaths are gunshot wounds to the head—the most common PBI in the civilian sector.1,1a Of the 178,876 traumatic brain injury (TBI) cases among U.S. military personnel in Iraq between 2000 and 2010, approximately 2% (more than 3500 cases) were PBIs—the majority due to explosions from improvised explosive devices combining blast injury and shrapnel that penetrate the cranium.2
History
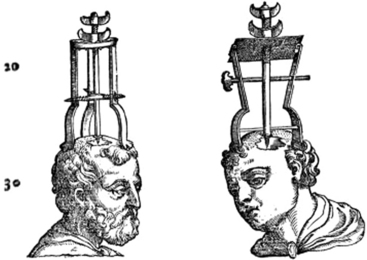
Since early human history, healers have treated PBIs. Military combat provided the highest concentration of PBIs; therefore, the majority of advances in PBI management were conceived on or near the battlefield. The earliest recorded case series dates to 1700 BC, when Egyptians recorded treatment of four depressed skull fractures by free drainage of the intracranial cavity and dressing of the wound with grease. In the 5th century BC, the Greeks utilized trephination (Fig. 142-1) for injuries to the skull. In the 2nd century AD, Galen published his observations correlating motor deficits in gladiators to contralateral brain injury. In the 17th century, Richard Wiseman managed subdural hematomas and blunt trauma by evacuation of hematomas and foreign bodies. The advent of germ theory in the 19th century and Joseph Lister’s aseptic technique were first put to use for PBI during the Second Boer War. During World War I, as senior consultant to the American Expeditionary Force,3 Harvey Cushing brought mortality down from 54.4% to 28.8% by establishing the meticulous technique of craniectomy, débridement, and dural closure. He brought Wilhelm Roentgen’s (Röntgen’s) novel x-ray to the operating theater for the localization and extraction of foreign bodies—which he believed served as nidi for future infection. Cushing’s techniques remained the standard of care, unchanged for more than 50 years. Survival was doubled in World War II after the introduction of antibiotics and Hugh Cairns’ mobile neurosurgical units. The Korean War also saw improvement with the advent of early evacuation of head injury patients. It was not until after the Vietnam War that aggressive pursuit of all bone and shrapnel fragments came into question. Results of the Vietnam Head Injury Study, as well as data from the Lebanon conflict,4 indicated that there is no added benefit in removing deep-seated metallic fragments or bone chips, introducing the doctrine in use today that only accessible fragments should be surgically removed. Current practice continues to develop in the battlefield, as addressed further later in this chapter.
Pathophysiology
Primary Injury
The injury sustained by the brain upon impact with a projectile depends upon four factors: (1) the nature of the projectile (blunt or sharp), (2) the approach of the projectile through the cranium, (3) the kinetic and thermal energy transferred by the projectile, and (4) the susceptibility factors of the victim. The skull has been shown to withstand impacts exceeding 5000 N/cm2 over the frontal bone and more than 2000 N/cm2 over the temporal bone.5 The cranium is often penetrated due to the sharpness and/or the kinetic energy of the foreign body. Bone fragments can act as additional projectiles, causing their own tracts of injury. Kinetic energy (KE) of a projectile is calculated by the following formula:
Here, m represents mass, and V represents velocity. Vinitial represents the velocity immediately before impact. At impact, the skull absorbs most of the energy of the projectile, but the brain parenchyma must absorb the remainder. A penetrating bullet usually causes a narrow, carrot-shaped tract. Higher-velocity projectiles transfer additional energy to the parenchyma in the form of expansile–contractile cavitation waves, leaving wide, turnip-shaped tracts.6 Biophysicists have used high-speed digital video and pressure transducers in brain parenchyma simulant to demonstrate that a 9-mm round (379 m/sec) transfers 6 times the energy and 1.5 times the cavitation volume of a 25-caliber round (238 m/sec). Clinically, the type of round is less important, because the speed of the missile after leaving the weapon rarely informs Vinitial due to unknown friction losses during trajectory.
Secondary Injury
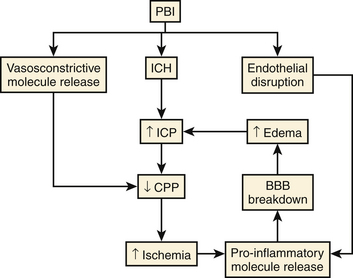
The parenchymal damage mediated by direct laceration and indirect pressure waves, or primary injury, is instantaneous and irreversible. The events that follow—ischemia, neuroinflammation, excitotoxicity, and oxidative stress—combine to create a self-perpetuating cycle of blood–brain barrier (BBB) breakdown, cerebral edema, increased intracranial pressure (ICP), and ultimately decreased cerebral perfusion pressure (CPP) (Fig. 142-2). This is often referred to as secondary injury, and though most of it is thought to occur within the first 12 to 24 hours postinjury, it has been observed to persist for days, possibly weeks.
Animal models have demonstrated the effects of PBI on mean arterial blood pressure, ICP, and CPP. Initially, PBI is followed by a rapid rise in ICP, peaking within a few minutes and decreasing thereafter.7 This is due in part to the reexpansion of the missile cavity by extravasation of blood. In multiple models, CPP was shown to decrease by 50% within the first minute postinjury as measured by intracarotid xenon 133 injections.8 When the pressure waves created by a missile rise sufficiently high, they may pass through the fourth ventricle, affecting the medullary respiratory center and causing apnea. Experiments in the feline model suggest that there is a narrow window between the energy required to penetrate the skull and the level causing fatal apnea,9 indicating that airway protection and rapid management of elevated ICP are crucial.
Extracranial pathology secondary to PBI, especially myocardial dysfunction, coagulopathy,10 and acute lung injury,11 also contributes to hypoxemia and brain ischemia. Therefore, the clinical management of PBI requires a comprehensive approach, as discussed in the next section. The primary aim is to halt or slow the cycle of secondary brain injury in an attempt to preserve the neurovascular unit (neuronal circuit, microvasculature, and astrocyte), with the ultimate goal of preserving cerebral function.
Initial Management: Basic Advanced Trauma Life Support Guidelines
As in all trauma situations, initial management of the patient begins with well-trained prehospital personnel and a trauma team that is experienced in the practice of advanced trauma life support guidelines. In the military, this is of particular importance due to time, safety, and resource limitations in the field of combat. Initial stabilization of the PBI patient requires constant surveillance of the airway, breathing, and circulation, with simultaneous initial neurologic evaluation and assessment for other life- or limb-threatening injuries. Specifically, the Glasgow Coma Scale (GCS)12 is useful for neurologic assessment, making decisions about intubation in the field, and predicting outcomes. The trauma team, including a general surgeon, neurosurgeon, and anesthesiologist, should be notified of information related to the mechanism of injury, vital signs, and initial GCS, as well as a report on interventions performed at the scene and en route to hospital. Careful survey of the body, including nasopharynx and external auditory canals, uncovers hemorrhage, cerebrospinal fluid (CSF) leaks, and additional entrance and exit wounds. Shaving of the scalp may be necessary to expose wounds concealed behind hair clotted with blood. Immediately upon arrival, pulse and blood pressure monitoring, as well as pulse oximetry, should be initiated. If not already done, large-bore intravenous access for colloid or crystalloid volume resuscitation should be established immediately. Simultaneously, arterial blood gases are taken, and venous blood is drawn and sent for complete blood count, electrolytes, glucose, coagulation profile, type, and cross match.
PBI-Specific Medical Management
Intracranial Pressure
All patients with suspected brain injury should have the head of bed elevated to 30 degrees once the cervical spine has been cleared. This allows gravity to facilitate venous and CSF drainage from the cranium. Changes in ICP can occur rapidly, and it is important to perform regular pupillary examinations. If a fixed and dilated pupil is noted and herniation is suspected, osmotic therapy should be initiated immediately in any hemodynamically stable patient. Osmotic agents, first described in a neurosurgical context by Weed and McKibben in 1919,13 create an osmotic gradient that draws free water out of the brain and lowers ICP.
Mannitol is the agent most commonly used, and its proposed mechanisms of action include dehydration of extravascular spaces and augmentation of blood volume while decreasing viscosity, which can lead to increased CPP, cerebral blood flow, and oxygen delivery.14 The diuretic effect of mannitol is efficacious in patients with good renal function and systolic blood pressure of more than 90 mm Hg. Recent retrospective analysis demonstrates a dose-dependent efficacy for mannitol, where every 0.1 g/kg increased over the 50-g dose achieved a 1-mm Hg drop in ICP. The data are still not sufficient to establish a standard dose for mannitol.14
Hypertonic saline (HS) also creates an osmotic gradient to decrease ICP; similarly, it induces a biphasic rheologic and osmotic response in patients. Other proposed mechanisms of ICP reduction with HS include anti-inflammatory activity and downregulation of the molecular water channel, aquaporin-4,15 theoretically decreasing water transport across the BBB. Administration of HS is even less standardized than mannitol. Some studies report the administration of a 3% infusion,16 while others utilize a bolus dose of concentrations ranging from 7% to 23.4%.17 Adverse effects of HS include the possibility of inducing central pontine myelinolysis in patients who are hyponatremic, a tendency to induce a persistent hyperchloremic metabolic acidosis, and the risk of pulmonary edema in patients with underlying cardiac or pulmonary disease.
Hyperventilation has long been known to decrease ICP, likely due to the elevated pH created by eliminating carbonic acid, which causes cerebral arteriole vasoconstriction and decreased cerebral blood volume. This effect is short lived, and vessels have been shown to retrain to a newer, elevated pH. Upon returning the patient to a normal PaCO2, these retrained vessels dilate, thereby elevating ICP even further. Not surprisingly, routine use of hyperventilation was shown to lead to poorer outcomes in randomized trials.18 The Guidelines for the Management of Severe Traumatic Brain Injury recommend avoidance of hyperventilation during the first 24 hours after trauma to preserve cerebral blood flow to at-risk brain tissue. The use of hyperventilation for brief temporizing measures to reduce ICP is acceptable, but it is recommended that brain oxygenation be monitored to reduce the risk of inducing brain tissue hypoxia.
Seizure Prophylaxis
It is reported that 30% to 50% of PBI patients have seizures, and up to 10% of these occur within the first week after injury. PBI is clearly associated with a higher rate of seizures than nonpenetrating TBI.19,20 Therefore, all patients with PBI should receive a loading dose of antiepileptic medications upon arrival to hospital, and maintenance doses should be administered for at least 7 days postinjury. An intravenous bolus of diazepam or lorazepam is used to treat any witnessed seizure. Although the Brain Trauma Foundation guidelines do not recommend prophylaxis beyond 1 week, this recommendation is not based on a study designed to test the efficacy of anticonvulsant therapy specifically in a PBI population, so some authors recommend a longer course of prophylaxis. Phenytoin has long been the first-line drug for short-term seizure prophylaxis.21 Disadvantages of phenytoin include effects on drug metabolism via cytochrome P450 induction, hypersensitivity reactions, and the requirement for serum level monitoring. These have caused some groups to consider alternatives. Since it became available in intravenous form in 2006, levetiracetam has become a popular alternative because it requires no monitoring of drug concentration in the serum.
Infection Prophylaxis
Broad-spectrum antibiotic therapy is recommended in the setting of PBI due to high likelihood of foreign body contamination.22 Although infection with gram-positive organisms is most common, increasing rates of infection with gram-negative organisms, especially Acinetobacter species, is being reported.23 Prophylactic antibiotics were first introduced at the dawn of the penicillin era, during World War II, effectively dropping infection rates from 60% to 6% in PBI patients.24 Current infection rates have been reported to be as low as 1% in the civilian setting, with higher rates of 12% associated with PBI in the military setting.25,26 In addition, tetanus toxin is administered with any foreign body penetration. In recent years, the use of antibiotic-impregnated ventricular catheters has been shown to dramatically decrease the rates of ventriculostomy infections in general neurosurgery patients,27 though no study has been performed specifically in PBI patients.
Imaging and Endovascular Intervention
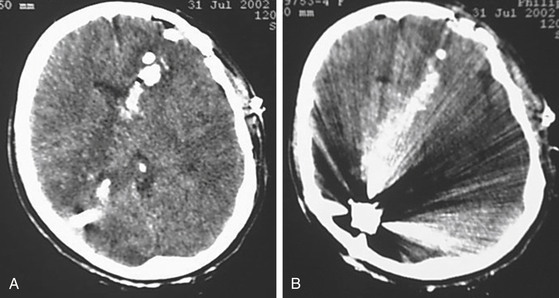
Upon stabilization of the patient, the initial imaging study is computed tomography (CT). The extent of brain injury, wound tract, presence of pneumocephalus, hematomas, and location of bone chips or foreign bodies can be identified rapidly.4 Today, volume acquisition is the preferred modality.28 Although advances in technology have made computed tomography angiography (CTA) increasingly popular, digital subtraction angiography (DSA) remains the gold standard for detection of traumatic aneurysms, and a recent comparative analysis suggests that CTA only detects half as many vascular injuries as DSA.29 More importantly, DSA allows the opportunity for rapid, less-invasive endovascular intervention with coil occlusion, liquid embolic agents, and stent-buttressed coil embolization to treat traumatic intracranial vessel injuries (Fig. 142-3).
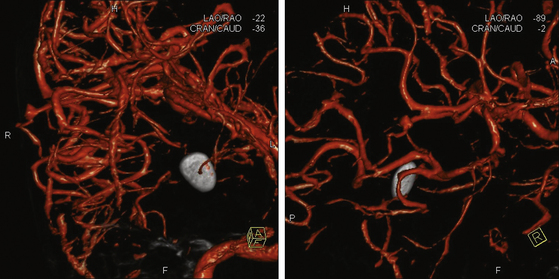
The reported incidence of traumatic intracranial aneurysms (TICAs) varies widely.28 Whether this is due to true variation in incidence or to different rates of lesion detection in various series is unknown. The indications for performing angiography have varied among series. Patients with wound tracts through multiple dural compartments or through faciocranial, orbitocranial, and pterional regions with concomitant hematoma have been reported to have 4 to 10 times the risk of developing an aneurysm (Figs. 142-4 and 142-5).30 However, limiting angiographic studies to these patients may miss important vascular compromise, leading to increased ICP and scarring, making future surgery more difficult, and possibly worsening outcome.31 During Operation Iraqi Freedom and Operation Enduring Freedom, Bell and colleagues33 utilized DSA in all patients with PBI, as well as in those with increased velocities detected by transcranial Doppler ultrasonography. The incidence of TICAs was surprisingly higher than in previous reports: more than 64 of 187 injured combatants (34%) had PBI.
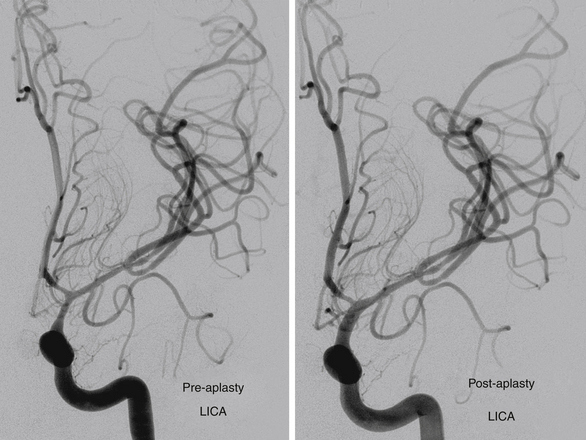
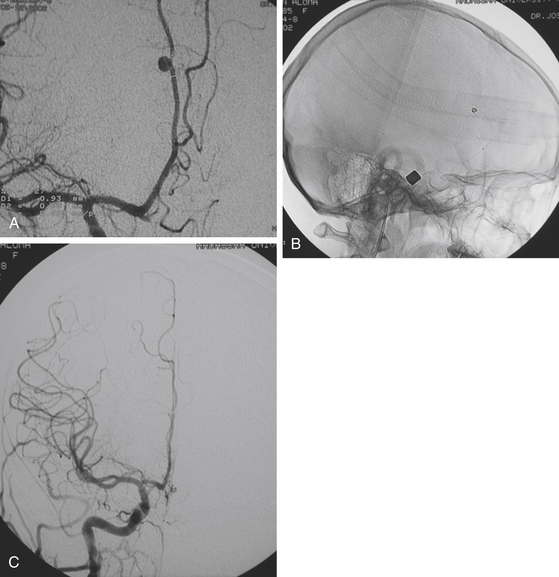
FIGURE 142-4 Vasospasm with associated traumatic psuedoaneurysm enlargement, treated with endovascular stenting.
Traumatic cerebral vasospasm (TCV) is another important complication of PBI that is a major cause of delayed ischemia.32 A recent case series demonstrates TCV occurring in more than half of combatants suffering PBI during Operations Iraqi and Enduring Freedom.33 Treatment of this condition remains controversial, and current modalities include medical management with hypertension, hemodilution, and hypervolemia, as well as systemic or intra-arterial nicardipine or intra-arterial papaverine.33 As the field of neuroendovascular therapy continues to expand, further studies will greatly enhance our understanding of these pathologies and our ability to intervene.
Surgical Management
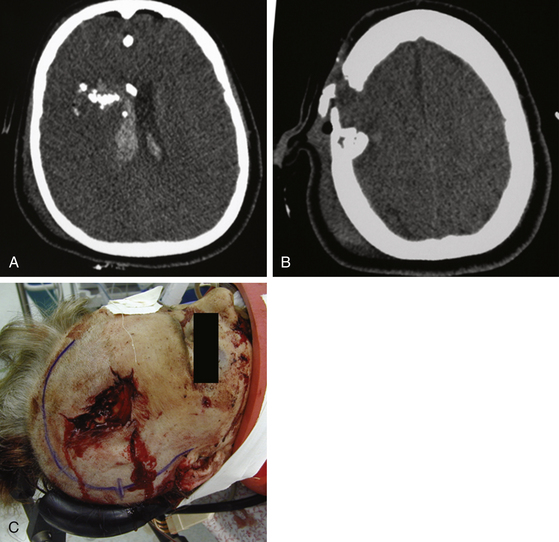
All patients with PBI should receive aggressive initial resuscitation and imaging evaluation by CT scan. When stabilization and imaging are complete, decisions concerning further therapy often take into account the patient’s neurologic status as determined by the GCS score. Poor survival and outcome are reported in patients with GCS scores between 3 and 5 points.34,35 Most neurosurgeons agree that a patient with a postresuscitation GCS score of 3 points with two dilated nonreactive pupils but without a mass lesion on CT should not receive surgical intervention.36 It has long been known that bihemispheric and transventricular injuries have poor prognosis (Fig. 142-6).37 Kim et al. used vector analysis on bullet trajectories in a series of penetrating head injuries, identifying other anatomic regions that correlated with fatal outcome. This fatal region, or zona fatalis, is located 4 cm above the dorsum sella and involves the midbody of the ventricle, body of the corpus callosum, and cingulum. Other indications of a particularly poor prognosis include midline shift of more than 10 mm, ICP of more than 45 mm Hg, onset of diabetes insipidus on the day of injury, and a tram–track sign in the wound track—likely a sign of secondary cavitation.37
In the civilian sector, gunshot wound victims make up the majority of PBIs. Patients with a postresuscitation GCS score of 3 to 5 points make up nearly half of the civilian PBI population. Although only 8% are likely to survive,38 Stone et al. recommend surgery for any patient with reactive pupils and hemodynamic stability.39 Moreover, they suggest that patients who have a GCS score of 4 to 7 points without hypotension should receive surgery—even if pupils are nonreactive. Indeed, many authors feel that evidence of motor or brain stem function is all that is needed to move forward with aggressive care.40
In the military, the distribution of PBI mechanisms is substantially different and has changed over time. As modern conflicts have become asymmetrical, the proportion of injuries caused by gunshot wound has declined in Western forces and the proportion of injuries caused by blast injury and shrapnel has increased. In recent series, the proportion of PBI caused by gunshot wounds is reported as 20%, while those caused by blast injury and shrapnel is 62%.41 Many factors, including transport time, death in the field, and uniform application of neurosurgical intervention protocols, account for the differences in mortality between military and civilian settings (5.6% vs. 47.9%).41 No evidence-based recommendation can apply to all situations, and deciding whether the costs of aggressive treatment of all patients in these categories are justified is an ethical dilemma beyond the scope of this chapter. Neurosurgeons treating PBI continue to face this difficult decision. Prognosis, family, societal, and religious factors all need to be considered when making such difficult decisions.
Surgical Principles: Débridement and Closure
1. Attain adequate débridement of devitalized tissue.
3. Remove accessible in-driven bone fragments and foreign bodies while preserving viable brain tissue.
All operative procedures for PBI should begin with wide prepping and draping of the injured area to allow adequate exposure. Thorough but gentle irrigation is used to flush all tissues extruding from the wound site. Two strategies are acceptable in planning the initial scalp incision. A large question mark–shaped skin flap may be utilized, especially above large hematomas, as determined by CT. In puncture-entry sites, the skin flap may encompass it, allowing necrotic edges to be excised and closed primarily. Large lacerations at the entry sites can be extended in lieu of the question mark–shaped flap (Fig. 142-7)—particularly when flap vascularity is jeopardized. Consultation with plastic surgery may be helpful when extensive scalp damage makes wound closure difficult.
Adequate exposure of the bony defect is essential, and the neurosurgeon must be aware that underlying dural injury often extends beyond the margins of the bone injury. Therefore, the craniotomy should be large enough to expose the intact dura and allow adequate closure. The same principle is utilized when an underlying hematoma is present, but this may require the elevation of a larger bone flap if the clot is extensive. At the site of penetration, adequate débridement of the bony edges should be performed. At this point, hemostasis can become difficult due to epidural bleeding—particularly when opening the cranium near the midline. Placement of closely spaced epidural tack-up sutures around the bone edges, as well as utilization of absorbable hemostatic agents such as Surgicel (Johnson & Johnson, Arlington, TX), Gelfoam (Pharmacia/Upjohn, Kalamazoo, MI), or fibrillar collagen underneath the bony edge, may provide bulk for adequate tamponade. Once the dura is exposed, herniating necrotic brain tissue is often noted extruding from the dural defect. This can be removed by irrigating with warm saline and suctioning. The tract of penetration through the brain is flushed gently through the dural defect, removing necrotic tissue, as well as any loose foreign objects within the necrotic cavity. At this point, the dural defect may need to be extended to allow adequate débridement of the injured brain. This must be performed slowly—despite the time-critical nature of the procedure. Slow dural opening may allow gradual reduction in ICP, possibly reducing the likelihood of sudden, massive herniation through the craniotomy.42 Dural opening can be accomplished by utilizing a cruciate opening with four incisions, allowing clot evacuation and rapid closure. Alternatively, some neurosurgeons make a reverse U incision, leaving the dura near the midline intact by incising anteriorly, inferiorly, and posteriorly.43 This was reported by Alves and Bullock to be helpful in avoiding of massive brain swelling and herniation through the durotomy.43 Next, a thorough exploration of the resulting cavity is undertaken. Accessible bone fragments and foreign bodies are removed. We recommend not continuing the exploration beyond this point despite preoperative CT demonstrating deeper retained fragments. This is consistent with the approach advocating preservation of viable brain tissue over the removal of all retained fragments.4
Meticulous hemostasis is accomplished using bipolar coagulation and caution while opening the dura. Leaving hemostatic agents in the cavity is typically avoided to the extent possible. It is estimated that approximately 50% of TBI victims develop a coagulopathy. Once hemostasis has been achieved, dural repair is undertaken. Any necrotic edges should be removed. Commonly, primary closure of the dura is not possible. Autologous grafts should be utilized to obtain a watertight closure. Temporalis fascia is probably the best option if accessible, followed by pericranium. If these are not available, a graft needs to be harvested, either fascia lata from the thigh or transversalis fascia from the abdomen. Allographic dura or cadaveric pericardium may be used only when the previously mentioned options are not practical. Artificial dural substitutes are increasingly used in closure of decompressive craniotomies, because the infection rate has decreased to equal that of the natural tissue.44 Once dural closure is obtained, a decision regarding bone replacement needs to be made. When severe brain swelling cannot be controlled despite all operative and medical measures, the bone flap should not be replaced.
Decompressive Craniectomy
DC has been employed with increasing frequency in both the military and the civilian sectors over the last decade. Many studies have shown that DC decreases ICP substantially,45 but a clear benefit on outcome has yet to be demonstrated in a randomized prospective study. DC is used either initially, when the treating surgeon feels the degree of brain injury and swelling indicate the need for the procedure, or later in the course of injury, when maximal medical management has failed to control elevated ICP. In either case, a large cranial defect and wide dural opening are essential to achieve adequate reduction of ICP and to prevent injury of the swollen brain from the bony edges of the craniectomy. Both the recently completed Decompressive Craniectomy Trial (DECRA)45a of bifrontal DC for diffuse brain swelling and the ongoing Randomised Evaluation of Surgery with Craniectomy for Uncontrollable Elevation of Intra-Cranial Pressure of unilateral or bifrontal DC address only the issue of surgical intervention when maximal medical management has failed to control elevated ICP. The great majority of DCs performed for PBI are done initially due to severe brain injury with accompanying edema. In a recent large U.S. military series of patients from the Iraq and Afghanistan wars, only 13 of 188 DCs (7%) were performed in a delayed fashion to treat intractably elevated ICP.46 Therefore, current randomized, controlled studies of DC to treat medically intractable ICP are of limited relevance to PBI patients. It seems unlikely that a randomized, well-controlled study of DC in the setting of PBI will be done in the near future. Therefore, in treating the individual patient, neurosurgeons need to take into account the benefit of likely ICP reduction against the known complications of this surgical intervention.47
Possibly the most important feature of a DC is the size.42,48,49 Craniotomies that are too small to accommodate the swelling brain can cause herniation through the bony defect, strangulation, infarction, and subsequent increased edema. Theoretical work has indicated that a cranial diameter defect of at least 14 cm is essential to achieve adequate volume gain by this operation.50 Another feature to consider is the type of incision. Three commonly used incisions are the midline sagittal incision with a “T-bar” extension (Kempe’s hemispherectomy incision), the standard large reverse question mark, and the bicoronal incision.51 Ragel et al. utilized DC extensively in the military setting, and they recommend the T-bar extension over more posterior incisions due to the excessive dependent swelling often encountered in the posterior portion of the scalp flap, putting the occipital and posterior auricular arterial supplies at risk for ischemia.48
Dural closure is another area of variation in the DC procedure. Dural substitutes, although less ideal in most situations, are important due to their nonadhesive nature. When the bone flaps are removed and stored for future repair, the dura is at risk of adhering to the closed scalp. Overlaying the dural defect with dural substitutes42,48 effectively keeps scalp tissue separate from dural tissue. Nagy et al. report an alternative closure technique.52 They utilize absorbable sutures wrapped around sponges that serve as pillars on either side of the major superficial vasculature. This is meant to form protective tunnels that theoretically prevent vessel compression and venous infarction. They demonstrate that patency is maintained 2 months postoperatively.53
Dural Sinus Repair
Injury to the dural sinus remains one of the most concerning injuries to the neurosurgeon treating PBI. When an injury occurs in proximity to the midline of the cranial vault or torcular herophili, a high level of suspicion for dural sinus injury must be maintained. Digital exploration of the wound should be avoided, as it can exacerbate bleeding by dislodging incidental tamponades. When a venous sinus is injured, profuse bleeding may emanate, and it should be treated immediately by compressive bandage and elevation of the head to 30 degrees. Indeed, as in any serious trauma, blood products such as fresh frozen plasma and packed red blood cells should be on hand. Good exposure without disturbance of fragments that might be maintaining tamponade is helpful. Hemostasis of the bleeding sinus can be accomplished with the aforementioned absorbable hemostatic agents such as Gelfoam or Surgicel and/or Cottonoids. Larger bleeds can be damaged by clipping the sinus with a temporary vascular clip proximally and distally to allow identification and repair of the defect—either by primary closure or with periosteal or muscle graft. Injury to the anterior third of the superior sagittal sinus can be ligated; however, the injured posterior two thirds should be preserved when possible via stent, graft, or bypass.54
Postoperative Management
Treatment of elevated ICP in postoperative PBI patients is similar to that in the acute preoperative setting described earlier in detail. CSF drainage, mannitol, HS, moderate hyperventilation, and in cases of refractory hypertension, barbiturate coma are used in a tiered fashion as dictated by the ICP. These measures are the same as in nonpenetrating TBI, although the underlying physiologic causes responsible for ICP elevation may not be identical. It has been suggested that failure of cerebral autoregulation plays an important role in the etiology of elevated ICP early after PBI.55 Studies on the efficacy of treatment regimens for elevated ICP specifically in PBI patients are yet to be done.
Coagulopathy is a concern following penetrating head injury, as it has been estimated to occur in about half of all PBI patients. Risk factors for development of coagulopathy include the extent of brain injury,10,56 massive blood loss in the multisystem-injured patient, acidosis, and hypothermia. Patients at risk should have fibrinogen levels, fibrin split products, and D-dimers checked, in addition to routine studies of prothrombin time, activated partial thromboplastin time, and platelet count. The presence of disseminated intravascular coagulation (DIC) has been shown to be associated with risk of delayed intracranial hemorrhage, and it may impair hemostasis intraoperatively. In the patient with DIC, plasma factors should be replaced until correction of the coagulation parameters is achieved. Recent reports have suggested that recombinant factor VIIa may be efficacious in stabilizing patients with exsanguinating hemorrhage.57,58 However, the published literature regarding the use of recombinant factor VIIa to correct coagulopathy in the neurosurgical population is limited. Concerns exist, including thrombosis (most ominously, of the venous sinuses),59 and caution is indicated until further studies are carried out.
CSF Leak
The variable most highly correlated with infection is acute or delayed CSF leakage. This has been corroborated by several authors, including Meirowsky et al.’s evaluation of casualties from the Korean War.60,60a Brandvold et al. reported the same finding in analyzing data from the Israeli experience in Lebanon,4 as did Aarabi in reported experience with casualties from the Iran–Iraq War.61 Meirowsky et al., analyzing data from the Vietnam conflict, documented a 49.5% incidence of infection in patients who leaked CSF, as opposed to a rate of 4.6% in those who did not.60 Only half of the studied patients experienced leaks from the wound site; the remainder had rhinorrhea or otorrhea from base-of-skull fractures or air sinus injury. Mortality in patients who leaked CSF was 22.8%, compared with 4.6% in those who did not leak. A majority (72%) of leaks appeared within 2 weeks after injury. The prompt treatment of CSF fistula is imperative. CSF drainage by ventricular or lumbar catheter is the first option. If there is a persistent leak from the skull base, craniotomy may be required. Preoperative imaging with thin-slice CT and with intrathecal contrast media and coronal reconstructions is often helpful in defining the site of bony defect.62 Endonasal and intrathecal fluorescein dye have been utilized in leak detection with moderate success.63,64 Furthermore, closure technique alone has recently been shown to decrease the incidence of wound complications such as CSF leaks.65
When not contraindicated by retained ferromagnetic fragments, magnetic resonance imaging may help in identifying the site of CSF leakage. A recent prospective open-label study demonstrated that intrathecal gadolinium can be used to detect CSF leaks.66 An extradural search for the site of dural injury and bony defect is then undertaken. Primary dural repair is often not feasible; when it is not, options include placing a vascularized periosteal flap; plugging the bony defect with fat or muscle, together with fibrin glue; and oversewing the dura with a fascial graft. Unless contraindicated, patients should have a lumbar drain maintained for several days postoperatively.
Cranioplasty
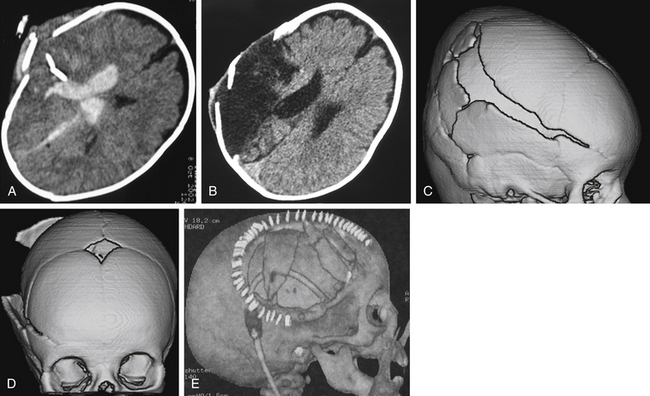
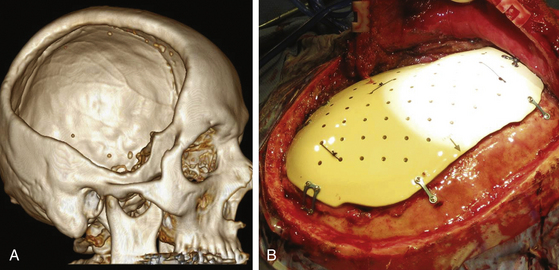
The patient with penetrating head injury often has a bony deficit, ranging from a small entry site wound to a large skull defect. When the defect is large or presents a cosmetic deformity, a cranioplasty needs to be performed after the patient has recovered from the initial injury. In young children and infants, a growing skull fracture may result from a penetrating or tangential wound (Fig. 142-8). In general, we recommend waiting at least 3 months from the time of injury before repair of a bony deficit to reduce the risk of infectious complications. By this time, the patient usually has recovered from the initial injury and is in better medical condition to undergo repeat surgery. When a large bone flap is removed during initial surgery, it may be sterilized and preserved in the patient’s abdomen or frozen at −80°C. When the patient is ready for cranioplasty, the original bone flap, after repeat sterilization, often achieves the best biologic and cosmetic result. Even when bone has been fragmented, a good result can usually be attained with fixation of the fragments. When the autologous bone is unavailable, an adequate prosthesis can be made to fit the defect. Cranioplasty prostheses have developed substantially over the last 10 years. Computer-designed implants are becoming the standard of care.67,68 For years, methyl methacrylate has been used for prosthesis reconstruction, likely due to its contourable surfaces and long history of use. It has some drawbacks, however, such as its exothermic reaction, when hardening, with the production of toxic monomer. This can lead to both local wound and systemic reactions. Also, when methyl methacrylate is placed near the paranasal sinuses, there is significantly increased risk of secondary infection. It is also not strong enough for larger frontal defects. Computer-designed titanium plates, polyethylene, and hydroxyapatite blocks have also been tried with some success.69 Polyetheretherketone, which was first used in spine and hip implants due to its heat and radiation resistance; inert, nontoxic chemistry; and low propensity for infection, has been used in recent years to repair large cranial defects70 (Fig. 142-9).
Armonda R.A., Bell R.S., Vo A.H., et al. Wartime traumatic cerebral vasospasm: recent review of combat casualties. Neurosurgery. 2006;5(6):1215-1225.
Bell R.S., Ecker R.D., Severson M.A.3rd, et al. The evolution of the treatment of traumatic cerebrovascular injury during wartime. Neurosurg Focus. May 2010;28(5):E5.
Brain Trauma Foundation, et al. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(suppl 1):S14-S20.
Brandvold B., Levi L., Feinsod M., George E.D. Penetrating craniocerebral injuries in the Israeli involvement in the Lebanese conflict, 1982-1985: Analysis of a less aggressive approach. J Neurosurg. 1990;72(1):15-21.
Chim H., Schantz J.T. New frontiers in calvarial reconstruction: integrating computer-assisted design and tissue engineering in cranioplasty. Plast Reconstr Surg. Nov 2005;116(6):1726-1741.
Cooper D.J., Rosenfeld J.V., Murray L. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011, Apr 21;364(16):1493-1502.
Dubose J.J., Barmparas G., Inaba K., et al. Isolated severe traumatic brain injuries sustained during combat operations: demographics, mortality outcomes, and lessons to be learned from contrasts to civilian counterparts. J Trauma. Jan 2011;7(1):11-16.
Faul M., Xu L, Wald M.M., Coronado V.G. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002-2006. In National Center for Injury Prevention and Control. Atlanta, GA: Centers for Disease Control and Prevention; 2010.
Fischer H. U.S. Military Casualty Statistics: Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom. Washington, D.C.: Congressional Research Service; 2010.
Forsyth L.L., Liu-DeRyke X., Parker D.Jr., Rhoney D.H. Role of hypertonic saline for the management of intracranial hypertension after stroke and traumatic brain injury. Pharmacotherapy. April 2008;28(4):469-484.
Kazim S.F., Shamim M.S., Tahir M.Z., et al. Management of penetrating brain injury. J Emerg Trauma Shock. 2011, Jul;4(3):395-402.
Kim K.A., Wang M.Y., McNatt S.A., et al. Vector analysis correlating bullet trajectory to outcome after civilian through-and-through gunshot wound to the head: using imaging cues to predict fatal outcome. Neurosurgery. Oct 2005;57(4):737-747.
Ling G.S., Ecklund J.M. Traumatic brain injury in modern war. Current opinion in anaesthesiology. 2011;24(2):124-130.
Mayer S.A., Brun N.C., Begtrup K. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352(8):777-785.
Paredes-Andrade E., Solid C.A., Rockswold S.B., et al. Hypertonic saline reduces intracranial hypertension in the presence of high serum and cerebrospinal fluid osmolalities. Neurocrit Care. Jul 2011. [Epub ahead of print]
Ragel B.T., Klimo P.Jr., Martin J.E., et al. Wartime decompressive craniectomy: technique and lessons learned. Neurosurg Focus. May 2010;28(5):E2.
Sorani M.D., Morabito D., Rosenthal G., et al. Characterizing the dose–response relationship between mannitol and intracranial pressure in traumatic brain injury patients using a high-frequency physiological data collection system. J Neurotrauma. April 2008;25(4):291-298.
Sughrue M.E., Bloch O.G., Manley G.T., Stiver S.I. Marked reduction in wound complication rates following decompressive hemicraniectomy with an improved operative closure technique. J Clin Neurosci. Sep 2011;18(9):1201-1205.
Sun Y., Wang J., Wu X., et al. Validating the incidence of coagulopathy and disseminated intravascular coagulation in patients with traumatic brain injury: analysis of 242 cases. Br J Neurosurg. June 2011;25(3):363-368.
Valadka A.B., Robertson C.S. Surgery of cerebral trauma and associated critical care. Neurosurgery. Jul 2007;61(suppl 1):203-220.
1. Vinas F.C., Pilitsis J.. Medscape Reference, 2011, June http://emedicine.medscape.com/article/247664-overview#showall [Online].
1a. Faul M., Xu L., Wald M.M., Coronado V.G.. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, 2010
2. Fischer H. U.S. Military Casualty Statistics: operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom. Washington, D.C., 2010.
3. McCallum J.E. Military medicine: from ancient times to the 21st century. Santa Barbara, CA: ABC-CLIO, Inc.; 2008.
4. Brandvold B., Levi L., Feinsod M., George E.D., et al. Penetrating craniocerebral injuries in the Israeli involvement in the Lebanese conflict, 1982-1985: analysis of a less aggressive approach. J Neurosurg. 1990;72(1):15-21.
5. Raymond D., Van Ee C., Crawford G., Bir C. Tolerance of the skull to blunt ballistic temporo-parietal impact. Journal of Biomechanics. November 2009;42(15):2479-2485.
6. Ling G.S., Ecklund J.M. Traumatic brain injury in modern war. Current opinion in anaesthesiology. 2011;24(2):124-130.
7. Crockard H., Brown F., Calica A., et al. Physiological consequences of experimental cerebral missile injury and use of data analysis to predict survival. Journal of Neurosurgery. 1977;46:784-794.
8. Levett J.M., Johns L.M., Replogle R.L., et al. Cardiovascular effects of experimental cerebral missile injury in primates. Surgical Neurology. January 1980;13(1):59-64.
9. Carey M.. Experimental missile wounding of the brain, Apuzzo M Levy M., editor. Neurosurgery Clinics of North America, Penetrating Craniocerebral Injuries: spectrum of Injury and Methods of Salvageability, 6. Philadelphia: WB Saunders, 1995.
10. Sun Y., Wang J., Wu X., et al. Validating the incidence of coagulopathy and disseminated intravascular coagulation in patients with traumatic brain injury: analysis of 242 cases. Br J Neurosurg. June 2011;25(3):363-368.
11. Nduom E., Frangos S., MacKenzie L., et al. Acute lung injury is an independent risk factor for brain hypoxia after severe traumatic brain injury. Neurosurgery. Aug 2010;67(2):338-344.
12. Teasdale G., Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81-84.
13. Weed L.H., McKibben P.S. Experimental alteration of brain bulk. Am J Physiol. May 1919;48(4):531-558.
14. Sorani M.D., Morabito D., Rosenthal G., et al. Characterizing the dose–response relationship between mannitol and intracranial pressure in traumatic brain injury patients using a high-frequency physiological data collection system. J Neurotrauma. April 2008;25(4):291-298.
15. Liu-DeRyke X., Parker D.Jr., Rhoney D.H., Forsyth L.L. Role of hypertonic saline for the management of intracranial hypertension after stroke and traumatic brain injury. Pharmacotherapy. April 2008;28(4):469-484.
16. Brain Trauma Foundation, et al. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(suppl 1):S14-S20.
17. Paredes-Andrade E., Solid C.A., Rockswold S.B., et al. Hypertonic saline reduces intracranial hypertension in the presence of high serum and cerebrospinal fluid osmolalities. Neurocrit Care. Jul 2011. [Epub ahead of print]
18. Marmarou A., Ward J.D., Kontos H.A., et al. Adverse effects of prolonged hyperventilation in patients with severe head injury: a randomized clinical trial. J Neurosurg. 1991;75:731-739.
19. Salazar A.M., Jabbari B., Vance S.C., et al. Epilepsy after penetrating head injury. I: clinical correlates: a report of the Vietnam Head Injury Study. Neurology. 1985;35(10):1406-1414.
20. Caveness W.F., Meirowsky A.M., Rish B.L., et al. The nature of posttraumatic epilepsy. J Neurosurg. 1998;50(5):545-553.
21. Temkin N.R., Dikmen S.S., Wilensky A.J., et al. N Engl J Med. 1990;323:497-502.
22. Haines S.J. Efficacy of antibiotic prophylaxis in clean neurosurgical operations. Neurosurgery. 1989;24:401-405.
23. de Louvois J., Brown E.M., Johnston R.A., et al. Use of antibiotics in penetrating craniocerebral injuries. “Infection in Neurosurgery” Working Party of British Society for Antimicrobial Chemotherapy. Lancet. May 2000;355(9217):1813-1817.
24. Selmon H. Forward neurosurgery in Italy. J Neurosurg. 1945;2:332-339.
25. Carey M., Young H., Mathis J., et al. A bacteriological study of cranial cerebral missile wounds from Vietnam. J Neurosurg. 1971;34:145-154.
26. Mossop C.M., Bell R.S., Tigno T.Jr., et al. Cranioplasty complications following wartime decompressive craniectomy. Neurosurg Focus. May 2010;28(no. 5):E3.
27. Whiting D., Darouiche R.O., Horner T.G., et al. Efficacy of antimicrobial-impregnated external ventricular drain catheters: a prospective, randomized, controlled trial. J Neurosurg. Apr 2003;98(4):725-730.
28. Twigg S., Offiah C. Imaging assessment of penetrating craniocerebral and spinal trauma. Clin Radiol. Dec 2009;64(12):1146-1157.
29. Emmett K.P., Fabian T.C., Zarzaur B.L., et al. Blunt cerebrovascular injury screening with 32-channel multidetector computed tomography: more slices still don’t cut it. Ann Surg. Mar 2011;253(3):444-450.
30. Pruitt B.A. Neuroimaging in the management of penetrating brain injury. J Trauma. 2001;51:S7-S11.
31. Bell R.S., Ecker R.D., Severson M.A.3rd, et al. The evolution of the treatment of traumatic cerebrovascular injury during wartime. Neurosurg Focus. May 2010;28(no. 5):E5.
32. Kataoka K., Akai F., Asai T., et al. Traumatic subarachnoid hemorrhage as a predictable indicator of delayed ischemic symptoms. J Neurosurg. 1996;84:762-768.
33. Armonda G., Bell R.S., Vo A.H., et al. Wartime traumatic cerebral vasospasm: recent review of combat casualties. Neurosurgery. 2006;59:1215-1225.
34. Graham T.W., Wiliams F.C., Harrington T., et al. Civilian gunshot wounds to the head: a prospective study. Neurosurgery. 1990;27(5):696-700.
35. Kdolsky R., Figl M., Grünauer J., et al. Predictive factors influencing the outcome after gunshot injuries to the head: a retrospective cohort study. J Trauma. October 2010;69(4):770-775.
36. Clark W.C., Mulhbauer M.S., Watridge C.B., et al. Analysis of 76 civilian craniocerebral gunshot wounds. J Neurosurg. 1986;65(1):9-14.
37. Kim K.A., Wang M.Y., McNatt S.A., et al. Vector analysis correlating bullet trajectory to outcome after civilian through-and-through gunshot wound to the head: using imaging cues to predict fatal outcome. Neurosurgery. Oct 2005;57(4):737-747.
38. Rosenfeld J.V. Gunshot injury to the head and spine. J Clin Neurosci. Jan 2002;9(1):9-16.
39. Stone J.L., Lichtor T., Fitzgerald L.F. Gunshot wounds to the head in civilian practice. Neurosurgery. 1995;37:1104-1112.
40. Kelly D., Nikas D., Becker D. Diagnosis and treatment of moderate and severe head injuries in adults. In: Youmans J., editor. Neurological Surgery. Philadelphia: WB Saunders, 1996.
41. Dubose J.J., Barmparas G., Inaba K., et al. Isolated severe traumatic brain injuries sustained during combat operations: demographics, mortality outcomes, and lessons to be learned from contrasts to civilian counterparts. J Trauma. Jan 2011;7(1):11-16.
42. Valadka A.B., Robertson C.S. Surgery of cerebral trauma and associated critical care. Neurosurgery. Jul 2007;61(suppl 1):203-220.
43. Alves O.L., Bullock R. “Basal durotomy” to prevent massive intra-operative traumatic brain swelling. Acta Neurochir (Wien). 2003;145:583-586.
44. Lee T.C., Chen W.F., Wang Y.M., Huang Y.H. Safety of the nonabsorbable dural substitute in decompressive craniectomy for severe traumatic brain injury. J Trauma. Jul 2011. Epub ahead of print
45. Arikan F., Sahuquillo J. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 364(16), Jan 2011. CD003983
45a. Cooper D.J., Rosenfeld J.V., Murray L. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364(16):1493-1502.
46. Mossop C.M., Dirks M.S., Stephens F.L., et al. Early decompressive craniectomy for severe penetrating and closed head injury during wartime. Neurosurg Focus. May 2010;28(no. 5):E1.
47. Stiver S.I. Complications of decompressive craniectomy for traumatic brain injury. Neurosurg Focus. Jun 2009;26(no. 6):E7.
48. Ragel B.T., Klimo P.Jr., Martin J.E., et al. Wartime decompressive craniectomy: technique and lessons learned. Neurosurg Focus. May 2010;28(no. 5):E2.
49. Xu W., Li W.P., Xu W.H., et al. Efficacy of standard trauma craniectomy for refractory intracranial hypertension with severe traumatic brain injury: a multicenter, prospective, randomized controlled study. J Neurotrauma. 2005;22(6):623-628.
50. Steiner T., Aschoff A., Schwab S., et al. Hemicraniectomy with dural augmentation in medically uncontrollable hemispheric infarction. Neurosurg Focus. May 1997;2(no. 5):E3.
51. Kempe L.G. Hemispherectomy. Operative Neurosurgery. 1968;1:179-189.
52. Csókay A., Nagy L., Novoth B. Avoidance of vascular compression in decompressive surgery for brain edema caused by trauma and tumor ablation. Neurosurg Rev. Dec 2001;24(4):209-213.
53. Csókay A., Láng J., Lajgut A., et al. In vitro and in vivo surgical and MRI evidence to clarify the effectiveness of the vascular tunnel technique in the course of decompressive craniectomy. Neurol Res. Sept 2011;33(7):747-749.
54. Sindou M., Auque J., Jouanneau E. Neurosurgery and the intracranial venous system. Acta Neurochir Suppl. 2005;94:167-175.
55. Crockard H.A. Early intracranial pressure studies in gunshot wounds of the brain. J Trauma. 1975;15(4):339-347.
56. Kaufman H.H., Makela M.E., Lee K.F., et al. Gunshot wounds to the head: a perspective. Neurosurgery. 1986;27(5):696-700.
57. Menendez J.A., Diringer M.N., Aiyagari V. Treatment of severe coagulopathy after gunshot injury to the head using recombinant activated factor VII. J Crit Care. Jun 2005;20(2):176-179.
58. Mayer S.A., Brun N.C., Begtrup K. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352(8):777-785.
59. Siegel L.J., Gerigk L., Tuettenberg J., et al. Cerebral sinus thrombosis in a trauma patient after recombinant activated factor VII infusion. Anesthesiology. 2004;100(2):441-443.
60. Meirowsky A.M., Caveness W.F., Dillon J.D., et al. Cerebrospinal fluid fistulas complicating missile wounds of the brain. J Neurosurg. 1981;54(1):44-48.
60a. Arendall R.E., Meirowsky A.M., et al. Air sinus wounds: an analysis of 163 consecutive cases incurred in the Korean War, 1950–1952. Neurosurgery. 1983;13(4):377-380.
61. Aarabi B. Comparative study of bacteriological contamination between primary and secondary exploration of missile head wounds. Neurosurgery. 1987;20(4):610-616.
62. Ozgen T., Tekkok I.H., Cila A., et al. CT cisternography in evaluation of cerebrospinal fluid rhinorrhea. Neuroradiology. 1990;32(6):481-484.
63. Reino T., Gnoy A., Guillory S., et al. Identification of intranasal cerebrospinal fluid leaks by topical application with fluorescein dye. Am J Rhinol. Mar-Apr 2000;14(2):93-96.
64. Rajasekaran K., Benninger M.S., Batra P.S., Seth R. The utility of intrathecal fluorescein in cerebrospinal fluid leak repair. Otolaryngol Head Neck Surg. 2010;143(5):626-632.
65. Sughrue M.E., Bloch O.G., Manley G.T., Stiver S.I. Marked reduction in wound complication rates following decompressive hemicraniectomy with an improved operative closure technique. J Clin Neurosci. Sep 2011;18(9):1201-1205.
66. Dedeken P., Casselman J.W., Vlaminck S.A., Vanopdenbosch L.J. MRI with intrathecal gadolinium to detect a CSF leak: a prospective open-label cohort study. J Neurol Neurosurg Psychiatry. Apr 2011;82(4):456-458.
67. Frodel J.L.Jr. Computer-designed implants for fronto-orbital defect reconstruction. Facial Plast Surg. Jan 2008;24(1):22-34.
68. Chim H., Schantz J.T. New frontiers in calvarial reconstruction: integrating computer-assisted design and tissue engineering in cranioplasty. Plast Reconstr Surg. Nov 2005;116(6):1726-1741.
69. Sittitavornwong S., Waite P.D., Lai J.B. Computer-assisted designed and computer-assisted manufactured polyetheretherketone prosthesis for complex fronto-orbito-temporal defect. J Oral Maxillofac Surg. Apr 2011;69(4):1175-1180.
70. Andereggen L., Fandino J., Lukes A., Marbacher S. Combined bone and soft-tissue augmentation surgery in temporo-orbital contour reconstruction. J Craniofac Surg. Jan 2011;22(1):266-268.